Figure 1. Rerouting of glucose utilization in RA and SLE T cells.
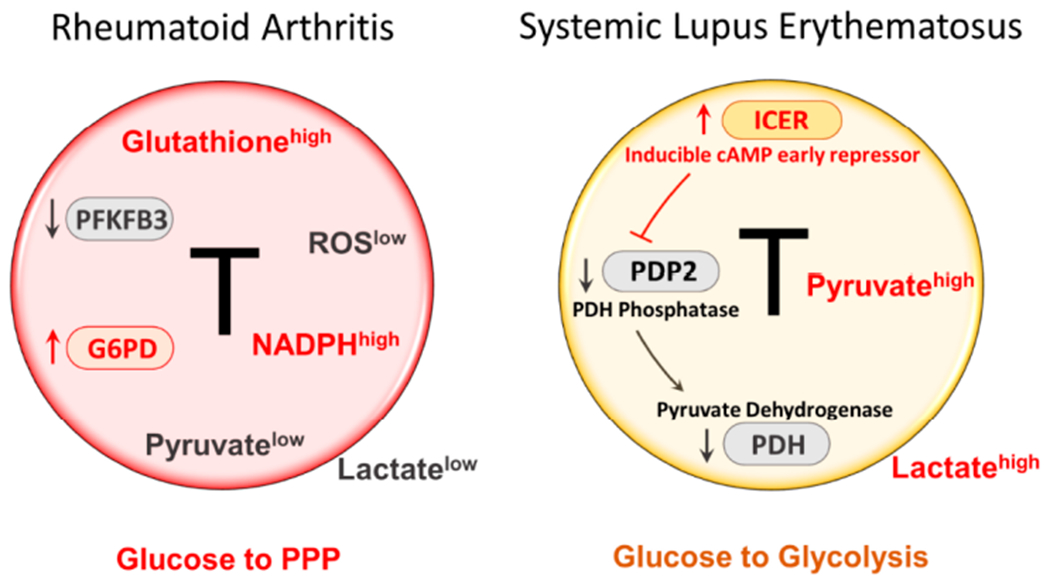
RA T cells fail to upregulate PFKFB3, a key enzyme in the glycolytic pathway, leading to low production of pyruvate and lactate. Instead, RA T cells upregulate expression and function of G6PD, the enzyme that controls the entry to the pentose phosphate pathway (PPP). As a result, RA T cells produce more NADPH and shift the cellular redox balance towards a reductive environment (low ROS, high glutathione). T cells from SLE patients increase glycolytic breakdown of glucose and produce copious amounts of pyruvate and lactate. The intracellular environment is biased towards oxidative stress. One mechanism underlying the preferential routing of glucose towards glycolysis relates to the suppression of pyruvate dehydrogenase (PDH). An upstream event is the high expression of the transcription factor inducible cAMP early repressor (ICER), which, in turn, leads to inhibition of PDH, and suppression of PDH. ICER is considered a lineage-promoting transcription factor promoting Th17 commitment. Th17 cells are recognized as key effector cells in SLE. Overall, RA T cells are programmed towards anabolic activity, whereas SLE T cells favor catabolic metabolism. PPP, pentose phosphate pathway; ROS, reactive oxygen species; PDP2, pyruvate dehydrogenase phosphatase catalytic subunit 2.
