Abstract
Objective
The objective of this systematic review was to evaluate the key imaging manifestations of COVID-19 on chest CT in adult patients by providing a comprehensive review of the published literature.
Methods
We performed a systematic literature search from the PubMed, Google Scholar, Embase, and WHO databases for studies mentioning the chest CT imaging findings of adult COVID-19 patients.
Results
A total of 45 studies comprising 4410 patients were included. Ground glass opacities (GGO), in isolation (50.2%) or coexisting with consolidations (44.2%), were the most common lesions. Distribution of GGOs was most commonly bilateral, peripheral/subpleural, and posterior with predilection for lower lobes. Common ancillary findings included pulmonary vascular enlargement (64%), intralobular septal thickening (60%), adjacent pleural thickening (41.7%), air bronchograms (41.2%), subpleural lines, crazy paving, bronchus distortion, bronchiectasis, and interlobular septal thickening. CT in early follow-up period generally showed an increase in size, number, and density of GGOs, with progression into mixed areas of GGOs plus consolidations and crazy paving, peaking at 10–11 days, before gradually resolving or persisting as patchy fibrosis. While younger adults more commonly had GGOs, extensive/multilobar involvement with consolidations was prevalent in the older population and those with severe disease.
Conclusion
This review describes the imaging features for diagnosis, stratification, and follow-up of COVID-19 patients. The most common CT manifestations are bilateral, peripheral/subpleural, posterior GGOs with or without consolidations with a lower lobe predominance. It is pertinent to be familiar with the various imaging findings to positively impact the management of these patients.
Key Points
• Ground glass opacities (GGOs), whether isolated or coexisting with consolidations, in bilateral and subpleural distribution, are the most prevalent chest CT findings in adult COVID-19 patients.
• Follow-up CT shows a progression of GGOs into a mixed pattern, reaching a peak at 10–11 days, before gradually resolving or persisting as patchy fibrosis.
• Younger people tend to have more GGOs. Older or sicker people tend to have more extensive involvement with consolidations.
Electronic supplementary material
The online version of this article (10.1007/s00330-020-06975-7) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, Diagnostic imaging, Radiology
Introduction
In December 2019, a highly infectious disease emerged in the city of Wuhan in Hubei province, China, which was later proven to have been caused by a novel coronavirus (2019-nCoV or SARS-CoV-2) [1]. The WHO christened the disease as COVID-19 and declared it as a pandemic on March 11, 2020. In the following weeks, the disease has swept rapidly across most of the countries of the world causing a global health emergency. As of April 14, 2020, the total number of cases has exceeded 1,800,000 with more than 110,000 deaths [2]. Chest CT has proven to be a useful supplement to RT-PCR and has been shown to have high sensitivity to diagnose this condition. Apart from being a diagnostic tool, CT can also potentially help in prognostication, evaluating the disease progression and monitoring the response to therapy [3, 4]. With the global health emergency that we are currently facing, a thorough knowledge of the chest imaging findings of COVID-19 is essential so as to make accurate and early diagnosis of this deadly disease.
The information on the wide variety of imaging manifestations of this disease on CT is constantly evolving every day and is scattered in numerous publications to date; however, there is a lack of an up-to-date comprehensive compilation of these studies. This systematic review, which is the largest to date, was performed to identify the key imaging manifestations, myriad of atypical manifestations, distribution, and temporal evolution of the lesions in the patients with COVID-19 to help the radiologists and the frontline workers to ensure proper clinical management. We also aim to describe the changes in the imaging findings according to the age of the patients, disease severity, and follow-up.
Material and methods
Search strategy
The search strategy was developed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist [5]. The study was also registered with PROSPERO (Registration number: CRD42020176900). We performed an electronic search of 4 databases including PubMed, Embase, Google Scholar, and World Health Organization Library, using the keywords “covid” or “covid-19” or “coronavirus” or “SARS-CoV-2” or “2019-nCoV” or “n-CoV” on April 7, 2020. The search was limited to articles published from January 1, 2020, until the time of search. Additional search of the reference lists of the studies extracted via the above process was done to find any other potentially relevant studies. Duplicates were removed.
Study selection
The selection criteria for articles to be shortlisted for the final review included research papers/case series with a sample size of 5 or more, and mentioning the chest CT findings of adult patients with COVID-19 infection confirmed on RT-PCR. Additional inclusion criteria were articles published in English and conducted on humans, and those which had a full text extractable. No restrictions were applied on the country of the original research. Case series with < 5 patients, case reports, editorials, systematic and pictorial reviews, and meta-analyses were excluded. The titles and abstracts of the included articles were screened by two independent reviewers based on the abovementioned inclusion criteria. Disagreements were resolved by consensus and subsequently by a senior reviewer.
Risk of bias (quality assessment)
The studies were rated for their quality based on the National Institutes of Health (NIH) Quality Assessment Tool for Case Series Studies by two independent reviewers [6].
Data extraction
The full texts of the articles included for review were retrieved and further screened for their eligibility. After scrutiny, the final list of the articles to be included for the systematic review was arrived at. Data extraction was done by two independent reviewers from full texts into a Microsoft Excel database using the following fields: study design, country, demographics, sample size, chest CT imaging features, and follow-up. Various subfields were also used to classify the granular data, especially with regard to CT imaging features, distribution of the lesions, and pattern and temporal changes in the lesions. Any discrepancies were resolved by discussion between the reviewers. Data was stored and analyzed using Excel for Microsoft Office. We intended to primarily conduct a narrative synthesis of the findings (synthesis without meta-analysis (SWiM)).
CT data analysis
Because of heterogeneity of data, the assessment of major CT findings was done in accordance with the standard definitions recommended by the Fleischner Society, as previously published [7].
Results
Characteristics of included studies
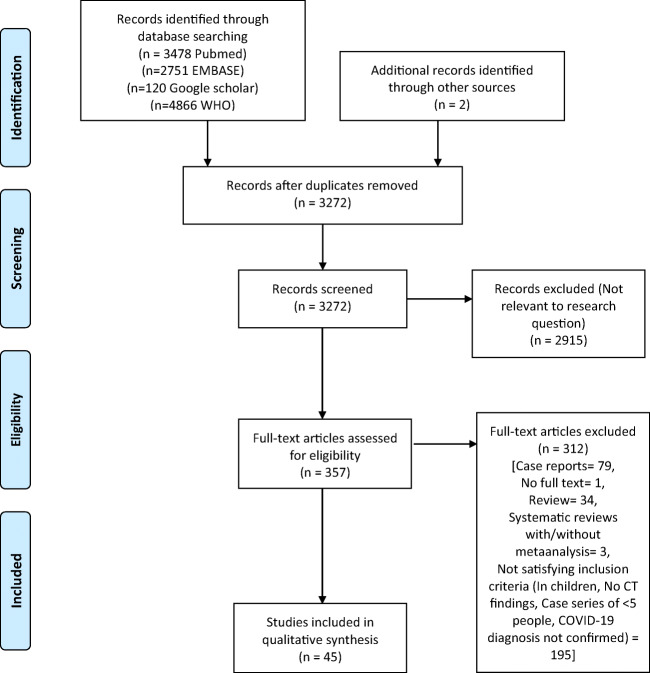
A total of 3272 unique articles were identified from the searches of 4 databases, after removing the duplicates (Fig. 1). Out of these, 357 records met the criteria for full-text review after initial screening. After these records were scrutinized, 45 studies, which met the inclusion criteria, were included in the final analysis. The list of included studies is given in Supplementary Table 1 and the demographic characteristics of the included population are given in Table 1. These 45 studies included a total of 4733 CT scans in a total of 4410 patients, since few patients underwent multiple CT scans. We excluded those studies which solely mentioned CT findings on children and not adults. In the included studies, findings on children were rarely described (Table 1). All the included studies were case series. Most of the studies were reported from China, except for 3 from Japan and 1 each from South Korea and Italy. Methodologic quality of the studies was assessed using NIH Quality Assessment Tool for Case Series Studies and was fair for most of the studies (Supplementary Table 2).
Fig. 1.
PRISMA 2009 flowchart describing selection of studies included in the systematic review (adapted from Moher et al [5])
Table 1.
Demographic characteristics of included studies
| Study | Date of publication in mm-yy | Country of study | Number of patients with confirmed COVID-19 on RT-CR | Males | Females | Age (in years) (mean or median) | Age range/IQR |
|---|---|---|---|---|---|---|---|
| Huang et al [8] | Jan-20 | China | 41 | 30 | 11 | 49 | IQR = 41–58 |
| Xie et al [9] | Feb-20 | China | 167 | NP | NP | NP | NP |
| Song et al [10] | Feb-20 | China | 51 | 25 | 26 | 49 ± 16 | 16–76 |
| Chung et al [11] | Feb-20 | China | 21 | 13 | 8 | 51 ± 14 | 29–77 |
| Wang et al [12] | Feb-20 | China | 138 | 75 | 63 | Median = 56 (IQR, 42–68) | 22–92 |
| Chen et al [13] | Jan-20 | China | 99 | 67 | 32 | 55.5 ± 13.1 | Not provided |
| Chan et al [14] | Feb-20 | China | 5 | 3 | 3 | 48.6 | 10–66 (1 patient was 10 years old) |
| Chang et al [15] | Feb-20 | China | 9 | 10 | 3 | Median = 34 | IQR = 34–48 |
| Pan et al [16] | Feb-20 | China | 63 | 33 | 30 | 44.9 ± 15.2 | 25–63 |
| Pan et al [17] | Feb-20 | China | 21 | 6 | 15 | 40 | 25–63 |
| Diao et al [18] | Feb-20 | China | 6 | 3 | 3 | 47.5 | 19–59 |
| Chen et al [19] | Feb-20 | China | 9 | 0 | 9 | NP | 26–40 |
| Ng et al [20] | Feb-20 | China | 21 | 13 | 8 | Median = 56 | IQR = 37–65 |
| Wang et al [3] | Mar-20 | China | 90 | 33 | 57 | 45 | |
| Inui et al [21] | Mar-20 | Japan | 104 | 54 | 50 | 62 ± 16 | 31–87 |
| Lei et al [22] | Mar-20 | China | 49 | 27 | 22 | 41 | 25–70 |
| Zhu et al [23] | Mar-20 | China | 6 | 0 | 6 | 44 | 27–63 |
| Xu et al [24] | Mar-20 | China | 50 | 29 | 21 | 43.9 ± 16.8 | 3–85 (five (10%) patients were below the age of 18 years) |
| Bernheim et al [25] | Feb-20 | China | 121 | 61 | 60 | 45 ± 15.6 | 18–80 |
| Wu et al [26] | Mar-20 | China | 80 | 42 | 38 | 44 ± 11 | 15–79 |
| Li and Xia [27] | Feb-20 | China | 51 | 28 | 23 | 58 | 26–83 |
| Zhou et al [28] | Feb-20 | China | 62 | 39 | 23 | 52.8 ± 12.2 | 30–77 |
| Cheng et al [29] | Mar-20 | China | 11 | 8 | 3 | 50.3 ± 15.5 | Not provided |
| Shi et al [30] | Feb-20 | China | 81 | 42 | 39 | 49.5 ± 11 | 25–81 |
| Xu et al [31] | Feb-20 | China | 90 | 39 | 51 | 50 | 18–86 |
| Liu et al [32] | Mar-20 | China | 73 | 41 | 32 | 41.6 ± 14.5 | Not provided |
| Zhao et al [33] | Feb-20 | China | 101 | 56 | 45 | 44.44 | 21–50 |
| Guan et al [34] | Mar-20 | China | 53 | NP | NP | NP | NP |
| Zhou et al [35] | Mar-20 | China | 62 | 34 | 28 | 47.5 ± 13 | 20–91 |
| Li et al [36] | Apr-20 | China | 131 | 63 | 68 | 47 ± 15 | 20–90 |
| Wang et al [37] | Apr-20 | China | 1012 | NP | NP | Median = 50 | IQR = 39–58 |
| Gao et al [38] | Mar-20 | China | 6 | 1 | 5 | 40 ± 10 | Not provided |
| Caruso et al [39] | Apr-20 | Italy | 62 | NP | NP | 57 | 18–89 |
| Iwasawa et al [40] | Mar-20 | Japan | 6 | 2 | 4 | 57.16 | 63–71 |
| Zhu et al [41] | Mar-20 | China | 72 | 42 | 30 | 55.6 ± 12.8 | 30–83 |
| Himoto et al [42] | Mar-20 | Japan | 6 | 5 | 1 | 58.5 | 45–81 |
| Long et al [43] | Mar-20 | China | 36 | 20 | 16 | 44.8 ± 18.2 | Not provided |
| Han et al [44] | Mar-20 | China | 17 | 6 | 11 | 40 ± 10 | 27–60 |
| Zhang et al [45] | Mar-20 | China | 28 | 17 | 11 | Median = 65 | IQR = 56.0–70.0 |
| Chen et al [46] | Mar-20 | China | 5 | 0 | 5 | 28.8 | 25–31 |
| Yang et al [47] | Mar-20 | China | 44 | 25 | 19 | Median = 48.5 | 20–76 |
| Wang et al [48] | Mar-20 | China | 114 | 56 | 58 | Median = 53 | 23–78 |
| Ai et al [49] | Feb-20 | China | 1014 (601 positive on RT-PCR) | 467(46%) | 51 ± 15 | 2 to 95 | |
| Yoon et al [50] | Mar-20 | S. Korea | 9 | 4 | 5 | Median = 54 | Not provided |
| Fang et al [51] | Feb-20 | China | 51 | 29 | 22 | Median = 45 | IQR = 39–55 |
IQR, interquartile range; NP, not provided
Common imaging findings on chest CT
A wide gamut of imaging findings has been described across the various studies included for review (Supplementary Table 3). The combined incidence of various imaging findings after pooling the data from the studies is given in Table 2. Since many of the studies describe multiple CTs in the given population, the data is presented as the cumulative percentage of the imaging findings out of the total number of CTs rather than the total number of patients. Ground glass opacities (GGOs) (50.2%; 2383/4738) were the most common major pattern across all the CT scans performed, followed by mixed GGOs plus consolidation pattern (44.4%; 854/1922) and consolidation (24.2%; 1021/4221) (Fig. 2). Reticular pattern was seen in 9.9% (170/1712) of all the CT scans.
Table 2.
Pooled incidence of various radiological findings on chest CT in Covid-19 patients
| Number of studies | Pooled incidence(as per total number of CT performed) | |
|---|---|---|
| Major patterns of increased attenuation | ||
| GGO | 43 | 2383/4738 (50.2%) |
| Consolidation | 33 | 1021/4221 (24.2%) |
| GGO + consolidation (mixed) | 20 | 854/1922 (44.4%) |
| Reticular pattern | 11 | 170/1712 (9.9%) |
| Ancillary findings | ||
| Interlobular septal thickening | 12 | 310/2053 (15.1%) |
| Intralobular septal thickening | 1 | 30/50 (60%) |
| Crazy paving | 18 | 266/1364 (19.5%) |
| Vascular enlargement | 7 | 353/550 (64%) |
| Reversed halo sign | 6 | 10/417 (2.4%) |
| Air bronchogram | 16 | 572/1388 (41.2%) |
| Bronchial wall thickening | 8 | 74/509 (14.5%) |
| Subpleural line | 6 | 98/388 (25%) |
| Nodule | 17 | 139/1769 (7.8%) |
| Pleural effusion | 27 | 105/2084 (5%) |
| Pleural thickening | 6 | 213/511 (41.7%) |
| Lymphadenopathy | 24 | 98/1825 (5.4%) |
| Pericardial effusion | 5 | 11/300 (3.6%) |
| Cavitation | 14 | 1/944 (0.1%) |
| Pulmonary fibrosis | 10 | 121/694 (17.4%) |
| Bronchus distortion | 2 | 21/113 (18.6%) |
| Bronchiectasis | 6 | 69/385 (18%) |
Fig. 2.
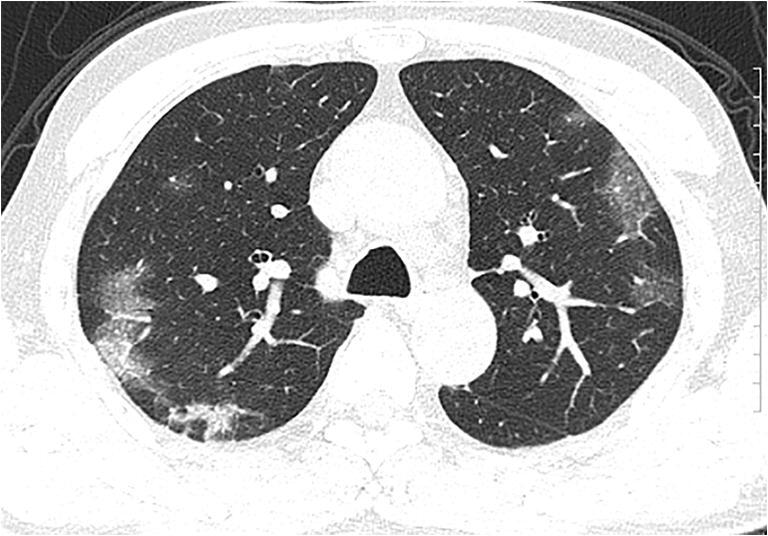
CT images of a 56-year-old man show multiple peripheral areas of organizing pneumonia with some areas of interlobular/intralobular septal thickening and multiple patchy areas of consolidation which are focal and mainly subpleural-associated architectural distortion (reproduced with permissions from Song et al [10])
The important ancillary findings described in some studies were pulmonary vascular enlargement (64%; 353/550), intralobular septal thickening (60%; 310/2053), adjacent pleural thickening (41.7%; 213/511), air bronchograms (41.2%; 572/1388), subpleural lines (25%; 98/388), crazy paving (19.5%; 266/1364), bronchus distortion (18.6%; 21/113), bronchiectasis (18%; 69/385), and interlobular septal thickening (15.1%; 310/2053). Of note, the incidence of interlobular septal thickening as described here is that excluding crazy paving (which also has an element of interlobular septal thickening). Other ancillary CT manifestations included pulmonary fibrosis, bronchial wall thickening, and nodules with incidence as described in Table 2. Of note, cavitation (0.1%; 1/944), pleural effusions (5%; 105/2084), mediastinal lymphadenopathy (5.4%; 98/1825), pericardial effusions (3.6%; 11/300), and reversed halo sign (2.4%; 10/417) were less common manifestations of COVID-19 in our pooled review.
Less common imaging signs on CT
Several studies have described some less common findings (Supplementary Table 3). In the study by Chen et al, the incidence of pneumothorax has been described to be 1% among 99 patients [13]. However, several authors have mentioned that none of their patients had a pneumothorax [3, 28]. Zhou et al have also mentioned a few other signs that they came across in their study population [28]. Vacuolar sign (a transparent attenuation of length less than 5 mm within the lesion), pleural retraction sign (presence of subpleural lesions which pull the visceral pleura), and a subpleural transparent line (a thin hypoattenuating line in between the visceral pleura and the lesion) were seen in 34/62 (54.8%), 35/62 (56.5%), and 33/62 (53.2%) respectively. Halo sign (an area of consolidation/nodule surrounded by GGOs) was seen in approximately 11% of the patients in two studies comprised of 62 and 60 patients respectively [35, 39]. Other findings that were described in a few studies were interlobar fissure displacement, pleural traction, cystic changes, and emphysema as described in Supplementary Table 3.
Spatial distribution
The distributions of lesions across the lungs are described in Supplementary Table 4. The pooled incidence of various patterns of distribution across all the abnormal CTs is given in Table 3. Bilateral distribution of the opacities (84%; 1185/1407) (mostly GGOs or mixed lesions) in a peripheral/subpleural distribution (68.8%; 570/828), mainly located posteriorly in the lungs (77.7%; 178/229), was the hallmark feature of distribution of lesions due to COVID-19 in our review. Twelve studies described the lobar distribution of the lesions. The lesions were most commonly seen in lower lobes with an incidence of approximately 84% in each of the lower lobes in the pooled analysis (Table 3).
Table 3.
Distribution of findings in COVID-19 patients with abnormal CT
| Distribution | Number of studies where the corresponding data has been provided for all the patterns | Pooled incidence (according to abnormal CT) |
|---|---|---|
| Laterality | ||
| Unilateral | 25 | 223/1407 (16%) |
| Bilateral | 25 | 1185/1407 (84%) |
| Anteroposterior distribution | ||
| Anterior distribution | 4 | 47/229 (20.5%) |
| Posterior distribution | 4 | 178/229 (77.7%) |
| Axial distribution | ||
| Central/perihilar | 15 | 44/828 (5.3%) |
| Peripheral/subpleural | 15 | 570/828 (68.8%) |
| Central+peripheral | 15 | 245/828 (29.5%) |
| Lobar distribution | ||
| Right upper lobe | 12 | 335/506 (66.2%) |
| Right medial lobe | 12 | 293/506 (58%) |
| Right lower lobe | 12 | 427/506 (84.3%) |
| Left upper lobe | 12 | 348/506 (68.7%) |
| Left lower lobe | 12 | 422/506 (83.4%) |
Temporal progression
Few studies have systematically described the serial changes in the pulmonary parenchymal abnormalities over the course of time in their patient population. In the study by Wang et al in 90 patients, GGOs were the predominant pattern just after symptom onset (65% on illness days 0–5) which gradually progressed to a mixed pattern of GGOs plus consolidations (38% in the third week). The percentage of pure GGOs again showed a rising trend during the fourth week (approximately 70%). The extent of CT lesions peaked at 6–11 days after symptom onset. A total of 94% of the patients had residual lesions at the time of discharge, of which a majority were GGOs [3]. Pan et al also mentioned similar findings, with GGOs (75%) being the most common imaging manifestations at 0–4 days from the onset of symptoms, changing to crazy-paving pattern (53%) at 5–8 days. Consolidations (91%) were the most common manifestation at 9–13 days, with gradual resolution of consolidations (75%) at more than 14 days of symptom onset. The findings peaked at 10 days in this study [17].
Imaging findings according to the severity/stage of disease
Two studies have classified the patients into 4 groups according to the fifth edition of the Guidelines for the Diagnosis and Treatment of New Coronavirus Pneumonia endorsed by the National Health Commission of China: (i) mild (few symptoms but no imaging manifestation); (ii) common (fever, dyspnea, and imaging features consistent with pneumonia); (iii) severe type (respiratory distress and hypoxia at rest); (iv) critically severe (respiratory failure which needs mechanical ventilation, shock, or organ failure needing intensive care) [52]. As per the criteria, there were no imaging features in the mild type in both these studies. In the study by Liu et al on 73 patients, patients with common type (59%) had peripheral GGOs, with/without interlobular septal thickening. The common imaging manifestations in the severe type (29%) were extensive GGOs and consolidations and those in critically severe types (4%) were extensive “white lung” with atelectasis and pleural effusions. In the resolution phase, few patients showed residual linear opacities [32]. Xu et al also described the findings according to these disease severity groups [24]. In their study, out of 50 patients, severe and critically severe patients (n = 13) had more common multilobar (4–5 lobes) involvement and significantly more bilateral involvement compared with common type.
Imaging findings in different age groups among adults
Age-wise predilection of imaging findings in the initial CT was noted in two studies. In the study by Song et al, extensive involvement and consolidations were significantly more predominant in patients who were older than 50 years than in those who were 50 years or younger. A younger population tended to have more GGOs [10]. In another study of 72 symptomatic patients, the authors compared the chest CT findings between younger (< 60 years) and older (> 60 years) adults. They concluded that extensive multilobar involvement of the lung lobes was more likely to happen in elderly patients (71.4% vs. 36.4%, p = 0.009) when compared with younger subjects. Also, the occurrence of subpleural lines and pleural thickening was significantly more common in the older age group [41].
Follow-up
The studies which have described the detailed follow-up of the lesions on the CT apart from those describing temporal progression (mentioned above) are described in Table 4. In general, progression of the lesions was associated with increase in the number and size of the lesions, previous GGOs changing into consolidations, and increase in the extent of involvement. Improvement was generally described as resolution/absorption of GGOs and consolidations with decrease in their sizes and number, with the appearance of residual fibrotic areas/stripes. In general, most of the studies showed that the lesions showed mild to moderate progression along the initial 2 weeks from symptom onset, except for a few as highlighted in Table 4.
Table 4.
Follow-up of chest CT findings
| Study | Number of patients with confirmed COVID-19 (total number of CTs if multiple CTs performed) | Follow-up data available (n) | Mean duration of follow-up CT | Follow-up (n = number of patients (% of total patients on follow-up)) |
|---|---|---|---|---|
| Huang et al [8] | 41 | NP | NP | Bilateral GGO persisted, consolidations were resolved |
| Song et al [10]* | 51 | 13 | NP | Progression in size or number of consolidations = 4/13 (31%), decrease in size = 7/13 (54%) |
| Chung et al [11] | 21 | 8 | 2.5 days | Mild progression = 5/8 (63%), Moderate progression =2/8 (25%), normal initial and follow-up CT = 1/8 (13%), improvement or severe progression = 0 |
| Pan et al [16] | 63 | 63 | 3–14 days | Progression (increase in number and size of lesions) = 54/63 (85.7%) |
| Ng et al [20] | 21 | 4 | 3–4 days | Improvement (reduction in the consolidation) = 1, normal = 1, progression = 2 (new GGO, consolidation) |
| Zhu et al [23]* | 6 | 3 | NP | Progression = 1, resorption = 2 |
| Xu et al [24]* | 50 | 30 | 3-13 days | Improvement = 28, no marked improvements = 2 |
| Li and Xia [27] | 51 | 24 | 5 | Progression = 18/24 (75.0%); improvement = 5/24 (21%) |
| Shi et al [30] | 81 | 57 | NP | Initial progression to peak level, followed by radiographic improvement (type 1) = 26 (46%), radiographic deterioration (type 2) = 18 (32%), radiographic improvement (type 3) = 8 (14%), and unchanged radiographic appearance (type 4) = 5 (9%) |
| Xu et al [31] | 90 | 52 | 3.5 days | No change = 10 (19%), disease resolution = 4 (8%), disease progression = 38 (73%) |
| Liu et al [32] | 73 | NP | NP | Improvement =12 (16%). Residual interstitial abnormalities = 4 (5.4%) |
| Guan et al [34] | 53 | 33 | 6.2 ± 2.9 days | Lesions increased = 25 (75.8%), lesions resorbed = 8 (24.2%) |
| Li et al [36] | 131 | 91 | 3.5 | Lesions absorbed = 25 (27%); no significant changes = 25 (27%), disease progression = 41 (46%) |
| Zhang et al [45]* | 28 | 28 | 7-14 days | Improvement = 13 (46.4%), unchanged appearance = 5 (17.9%), and deterioration = 6 (21.4%) |
| Yang et al [47] | 44 | NP | median = 4 days | Lesions progression = 81.82%, lesion absorption = 13.64% |
| Wang et al [48] | 114 | 16 | NP | No change = 0 (0%); lesion disappeared = 0 (0%); lesion mitigated slightly = 4 (25%); mild disease progression = 7 (43.8%); moderate disease progression = 5 (31.2%); severe disease progression = 0 (0%) |
NP, not provided
*Studies showing a greater number of disease improvements rather than progression
Discussion
CT imaging can demonstrate typical patterns of imaging manifestations that could be used to diagnose COVID-19. In this systematic review, we describe the key imaging findings for diagnosis, stratification, and early follow-up of patients with COVID-19 after compiling the data from published literature. The quality of most of the studies included in this review was rated as fair denoting some risk of bias. However, this is unavoidable as, because of the emergent pandemic situation, most of the studies are descriptive and non-blinded and have presented the preliminary data of chest CT findings on COVID-19. However, our objective was to systematically review the common findings on CT and these flaws were not considered sufficient to invalidate the results of the same.
GGOs, whether in isolation or coexisting with other lesions like consolidations, interlobular septal thickening, or crazy paving, were uniformly the commonest lesions among all the studies, according to our review. In one of the large case series by Wang et al, all the 138 patients included in the study had GGOs [12]. Bilateral, peripheral/subpleural, posterior distribution of the opacities with a lower lobe predominance was the most common distribution pattern, which is in agreement with previous studies [3, 10, 11, 25]. These findings are similar to a previous systematic review which described the findings published as of February 19, 2020, in which GGOs were present in 88% of the patients [53]. However, it is to be noted that the pooled incidence in our review revealed that the prevalence of GGOs was 50% and that of mixed lesions was 44% with mixed lesions being reported more commonly than before, in contrast to the previous review [53]. These CT findings in COVID-19 are attributed to infection of type II alveolar epithelial cells via ACE2 receptors by the SARS-CoV virus. The virus replicates and triggers cellular apoptosis in alveolar cells. Pathological changes in COVID-19 resemble those of severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS) [54]. The viral particles released from the cell infect other cells, inducing the release of proinflammatory cytokines and causing widespread alveolar epithelial damage. This leads to exudation into the alveolar space with diffuse alveolar damage and fibrin-rich hyaline membranes. This produces the ground glass opacities and consolidations encountered on CT. Aberrant healing mechanisms are triggered which cause scarring of the epithelium causing fibrosis at resolution [55].
Multiple newer articles have been published since the systematic review by Salehi et al which have described many atypical CT imaging features in patients with COVID-19 such as pulmonary vascular enlargement, intralobular and interlobular septal thickening, adjacent pleural thickening, air bronchograms, subpleural lines, crazy paving, bronchiectasis, and few imaging signs like vacuolar retraction sign, halo sign, pleural retraction sign, etc., as described in the previous section. Of note, pleural effusion (5%) and pericardial effusion (3.6%) are being recognized more commonly now than previously, especially in patients in advanced stages of the disease [53].
As regards the temporal trends in the imaging pattern, follow-up CT in the early period (up to 14 days) generally shows an increase in size, number, and density of GGOs, with progression into mixed areas of consolidations and GGOs, crazy paving, or multifocal consolidations. The peak of the CT lesions is reached at around 10–11 days of symptom onset before gradually resolving or persisting as patchy fibrosis up to 4 weeks. The persistence of residual lesions at long-term follow-up of COVID-19 patients is unknown and yet to be published. In some of the critically ill patients, the lesions may progress to “white out” lung or acute respiratory distress syndrome (ARDS) leading to death.
There is variability in the CT manifestations according to the age groups and disease severity stages with GGOs being commoner in younger adults and consolidations as well as multilobar involvement in the older individuals. The patients who have severe or critically severe pneumonias have a more extensive involvement with consolidations, effusions, atelectasis, “white out” lungs, and ARDS. However, the data available at this time is insufficient to characterize the clinical significance of this stratification due to lack of longer follow-ups.
The imaging findings in COVID-19 closely resemble those of MERS and SARS leading to a notion that it is a part of the acute lung injury caused by viral pneumonia. The mortality rate of COVID-19 is lower (2–4%) than that of these previous viral pneumonias. RT-PCR from the samples collected from the sputum or throat swab currently serves as the gold standard for the diagnosis of COVID-19. However, the reports take hours to arrive and it also has a high false negative rate. The sensitivity of RT-PCR may be as low as 60–70%, mainly attributed to insufficient samples. Therefore, patients with pneumonia due to COVID-19 may have lung abnormalities on chest CT but an initially negative RT-PCR [25]. Chest CT has proven to be a useful supplement to RT-PCR and has been shown to have high sensitivity to diagnose this condition early [49, 51]. However, some authors have shown that as many as 50% of the patients with positive RT-PCR can have a normal CT at 0–2 days after symptom onset [25]. Keeping all these considerations, Fleischer Society has released its recommendations for the use of imaging during this pandemic [56]. Imaging is not routinely indicated in asymptomatic patients for screening or in low-risk patients with mild clinical symptoms. Imaging is now indicated in a patient with COVID-19 only if he/she has moderate to severe pneumonia or worsening respiratory status or is at risk of progression or those with functional impairment or hypoxemia after recovery. In these subsets, CT in combination with laboratory testing should be used judiciously to diagnose and monitor the response to treatment.
Limitations
Our inclusion criteria were limited to studies published in English, which is a major limitation since the initial epicenter of the outbreak was in China. Chinese literature should be reviewed for a more comprehensive assessment of the imaging findings. We also have not reviewed the articles which mention the manifestations solely in children, as these might be different from what are seen in adults. We have also excluded the individual case reports which could represent some of the atypical manifestations. Since a number of included studies were limited as well as heterogeneous in terms of sample size, methodologic quality, and data availability, findings from this study should be interpreted with caution and in appropriate clinical context. Some of the results such as temporal progression and findings by age and stage were based on only 2 studies. We also agree that presence of different scanners, acquisition parameters, and the experience of the interpreting radiologists may induce some variability in the reported CT findings. However, we understand that learning the common imaging features is what the situation demands. Still, the data is continuously evolving with newer studies being published, which will demand a more updated systematic review in the future. We strive to do so.
In conclusion, CT imaging can help in early diagnosis, stratification, and initial follow-up of patients with COVID-19. The most common CT manifestations are bilateral, peripheral/subpleural, posterior GGOs with/without consolidations with a lower lobe predominance. It is pertinent for the radiologists and the clinicians to be familiar with various manifestations of COVID-19 on CT, so that they are able to impact the clinical decision making and management for these patients, which is the need of the hour, more than ever.
Electronic supplementary material
(DOCX 67 kb)
(DOCX 69 kb)
(DOCX 16 kb)
(DOCX 33 kb)
Abbreviations
- COVID-19
Corona virus disease 2019
- n-CoV
Novel coronavirus
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- PROSPERO
International Prospective Register of Systematic Reviews
- RT-PCR
Real-time reverse transcriptasepolymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Funding information
The authors state that this work has not received any funding.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Dr. Sanjeev Kumar, Department of Cardiovascular Radiology and Endovascular Interventions, AIIMS.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Statistics was done using Microsoft Excel. No complex statistical methods were necessary for this paper.
Informed consent
Not applicable (since this is a systematic review of previously published literature).
Ethical approval
Institutional Review Board approval was not necessary. PROSPERO registration was obtained; registration number is mentioned in the manuscript.
Study subjects or cohorts overlap
This is a systematic review of published data.
Methodology
• Systematic review
• Narrative synthesis without meta-analysis
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vineeta Ojha and Avinash Mani contributed equally to this work.
References
- 1.Zhu N, Zhang D, Wang W J et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733 [DOI] [PMC free article] [PubMed]
- 2.Novel coronavirus (2019-nCoV) situation reports. [cited 2020 Apr 8]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 3.Wang Y, Dong C, Hu Y et al (2020) Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 19:200843 [DOI] [PMC free article] [PubMed]
- 4.Tabatabaei SMH, Talari H, Moghaddas F, Rajebi H. Computed tomographic features and short-term prognosis of coronavirus disease 2019 (COVID-19) pneumonia: a single-center study from Kashan, Iran. Radiology: Cardiothoracic Imaging. 2020;2(2):e200130. doi: 10.1148/ryct.2020200130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Study quality assessment tools | National Heart, Lung, and Blood Institute (NHLBI). [cited 2020 Apr 14]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 7.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506 [DOI] [PMC free article] [PubMed]
- 9.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;12:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song F, Shi N, Shan F et al (2020) Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 295(1):210–217 [DOI] [PMC free article] [PubMed]
- 11.Chung M, Bernheim A, Mei X et al (2020) CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 295(1):202–207 [DOI] [PMC free article] [PubMed]
- 12.Wang D, Hu B, Hu C J et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069 [DOI] [PMC free article] [PubMed]
- 13.Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513 [DOI] [PMC free article] [PubMed]
- 14.Chan JF-W, Yuan S, Kok K-H J et al (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395(10223):514–523 [DOI] [PMC free article] [PubMed]
- 15.Chang D, Lin M, Wei L et al (2020) Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA 323(11):1092–1093 [DOI] [PMC free article] [PubMed]
- 16.Pan Y, Guan H, Zhou S et al (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 30(6):3306–3309 [DOI] [PMC free article] [PubMed]
- 17.Pan F, Ye T, Sun P et al (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 13:200370 [DOI] [PMC free article] [PubMed]
- 18.Diao K, Han P, Pang T, Li Y, Yang Z. HRCT imaging features in representative imported cases of 2019 novel coronavirus pneumonia. Prec Clin Med. 2020;3(1):9–13. doi: 10.1093/pcmedi/pbaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Guo J, Wang C et al (2020) Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395(10226):809–815 [DOI] [PMC free article] [PubMed]
- 20.Ng M-Y, Lee EY, Yang J et al (2020) Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardiothorac Imaging 2(1):e200034 [DOI] [PMC free article] [PubMed]
- 21.Inui S, Fujikawa A, Jitsu M et al (2020) Chest CT findings in cases from the cruise ship “diamond princess” with coronavirus disease 2019 (COVID-19). Radiol Cardiothorac Imaging 2(2):e200110 [DOI] [PMC free article] [PubMed]
- 22.Lei P, Fan B, Yuan Y (2020) The evolution of CT characteristics in the patients with COVID-19 pneumonia. J Infect. 2020 Mar 19 [cited 2020 Mar 27];0(0). Available from: https://www.journalofinfection.com/article/S0163-4453(20)30143-2/abstract [DOI] [PMC free article] [PubMed]
- 23.Zhu Y, Liu Y-L, Li Z-P et al (2020) Clinical and CT imaging features of 2019 novel coronavirus disease (COVID-19). J Infect. 10.1016/j.jinf.2020.02.022 [DOI] [PMC free article] [PubMed]
- 24.Xu Y-H, Dong J-H, An W-M et al (2020) Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect 80(4):394–400 [DOI] [PMC free article] [PubMed]
- 25.Bernheim A, Mei X, Huang M et al (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 20:200463 [DOI] [PMC free article] [PubMed]
- 26.Wu J, Wu X, Zeng W et al (2020) Chest CT findings in patients with corona virus disease 2019 and its relationship with clinical features. Invest Radiol 55(5):257–261 [DOI] [PMC free article] [PubMed]
- 27.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;4:1–7. doi: 10.2214/AJR.19.22372. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. Am J Roentgenol. 2020;5:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Z, Lu Y, Cao Q et al (2020) Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol 14:1–6 [DOI] [PubMed]
- 30.Shi H, Han X, Jiang N et al (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 20(4):425–434 [DOI] [PMC free article] [PubMed]
- 31.Xu X, Yu C, Qu J et al (2020) Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging 47(5):1275–1280 [DOI] [PMC free article] [PubMed]
- 32.Liu K-C, Xu P, Lv W-F et al (2020) CT manifestations of coronavirus disease-2019: a retrospective analysis of 73 cases by disease severity. Eur J Radiol 126:108941 [DOI] [PMC free article] [PubMed]
- 33.Zhao W, Zhong Z, Xie X, Yu Q, Liu J (2020) Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol 214(5):1072–1077 [DOI] [PubMed]
- 34.Guan CS, Lv ZB, Yan S et al (2020) Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Acad Radiol 27(5):609–613 [DOI] [PMC free article] [PubMed]
- 35.Zhou Z, Guo D, Li C et al (2020) Coronavirus disease 2019: initial chest CT findings. Eur Radiol. 10.1007/s00330-020-06816-7 [DOI] [PMC free article] [PubMed]
- 36.Li X, Zeng W, Li X et al (2020) CT imaging changes of corona virus disease 2019 (COVID-19): a multi-center study in Southwest China. J Transl Med 18(1):154 [DOI] [PMC free article] [PubMed]
- 37.Wang X, Fang J, Zhu Y et al (2020) Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect S1198-743X(20)30177-4 [DOI] [PMC free article] [PubMed]
- 38.Gao L, Zhang J. Pulmonary high-resolution computed tomography (HRCT) findings of patients with early-stage coronavirus disease 2019 (COVID-19) in Hangzhou, China. Med Sci Monit. 2020;26:e923885. doi: 10.12659/MSM.923885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caruso D, Zerunian M, Polici M et al (2020) Chest CT features of COVID-19 in Rome, Italy. Radiology 3:201237 [DOI] [PMC free article] [PubMed]
- 40.Iwasawa T, Sato M, Yamaya T et al (2020) Ultra-high-resolution computed tomography can demonstrate alveolar collapse in novel coronavirus (COVID-19) pneumonia. Jpn J Radiol 31 [DOI] [PMC free article] [PubMed]
- 41.Zhu T, Wang Y, Zhou S, Zhang N, Xia L (2020) A comparative study of chest computed tomography features in young and older adults with corona virus disease (COVID-19). J Thorac Imaging 10.1097/RTI.0000000000000513 [DOI] [PMC free article] [PubMed]
- 42.Himoto Y, Sakata A, Kirita M et al (2020) Diagnostic performance of chest CT to differentiate COVID-19 pneumonia in non-high-epidemic area in Japan. Jpn J Radiol. 2020;38(5):400–406 [DOI] [PMC free article] [PubMed]
- 43.Long C, Xu H, Shen Q et al (2020) Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol 126:108961 [DOI] [PMC free article] [PubMed]
- 44.Han X, Cao Y, Jiang N et al (2020) Novel coronavirus pneumonia (COVID-19) progression course in 17 discharged patients: comparison of clinical and thin-section CT features during recovery. Clin Infect Dis 2020:ciaa271 [DOI] [PMC free article] [PubMed]
- 45.Zhang L, Zhu F, Xie L et al (2020) Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol S0923-7534(20)36383–3 [DOI] [PMC free article] [PubMed]
- 46.Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y (2020) Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol 10.1002/jmv.25789 [DOI] [PMC free article] [PubMed]
- 47.Yang S, Shi Y, Lu H et al (2020) Clinical and CT features of early-stage patients with COVID-19: a retrospective analysis of imported cases in Shanghai, China. Clin Radiol 75(5):341–347 [DOI] [PMC free article] [PubMed]
- 48.Wang K, Kang S, Tian R, Zhang X, Zhang X, Wang Y (2020) Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID-19) in the Xiaogan area. Clin Radiol 23 [DOI] [PMC free article] [PubMed]
- 49.Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 26:200642 [DOI] [PMC free article] [PubMed]
- 50.Yoon SH, Lee KH, Kim JY et al (2020) Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol 21(4):494 [DOI] [PMC free article] [PubMed]
- 51.Fang Y, Zhang H, Xie J et al (2020) Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 19:200432 [DOI] [PMC free article] [PubMed]
- 52.Lin L, Li TS. Interpretation of “Guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the National Health Commission (Trial Version 5)”. Zhonghua Yi Xue Za Zhi. 2020;100:E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. [DOI] [PubMed] [Google Scholar]
- 53.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;14:1–7. doi: 10.2214/AJR.20.24425. [DOI] [PubMed] [Google Scholar]
- 54.Xu Z, Shi L, Wang Y et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8(4):420–422 [DOI] [PMC free article] [PubMed]
- 55.Mason RJ (2020) Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J 55(4):2000607 [DOI] [PMC free article] [PubMed]
- 56.Rubin GD, Ryerson CJ, Haramati LB et al (2020) The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Radiology 201365 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 67 kb)
(DOCX 69 kb)
(DOCX 16 kb)
(DOCX 33 kb)