Significance
In humans, a key class of natural killer (NK) receptors are termed killer cell immunoglobulin-like receptors (KIRs), which interact with human leukocyte antigen (HLA) class I molecules. This KIR–HLA axis plays an important role in immunity, including, for example, HIV control. Nevertheless, how HLA polymorphism shapes KIR recognition was unknown. We show how buried polymorphic residues within the peptide-binding cleft of HLA impact KIR recognition by modulating the conformation of the bound peptide. Accordingly, we provide a mechanistic basis for the differing clinical associations between KIR and HLA polymorphism.
Keywords: natural killer cells, HLA, KIR
Abstract
Micropolymorphisms within human leukocyte antigen (HLA) class I molecules can change the architecture of the peptide-binding cleft, leading to differences in peptide presentation and T cell recognition. The impact of such HLA variation on natural killer (NK) cell recognition remains unclear. Given the differential association of HLA-B*57:01 and HLA-B*57:03 with the control of HIV, recognition of these HLA-B57 allomorphs by the killer cell immunoglobulin-like receptor (KIR) 3DL1 was compared. Despite differing by only two polymorphic residues, both buried within the peptide-binding cleft, HLA-B*57:01 more potently inhibited NK cell activation. Direct-binding studies showed KIR3DL1 to preferentially recognize HLA-B*57:01, particularly when presenting peptides with positively charged position (P)Ω-2 residues. In HLA-B*57:01, charged PΩ-2 residues were oriented toward the peptide-binding cleft and away from KIR3DL1. In HLA-B*57:03, the charged PΩ-2 residues protruded out from the cleft and directly impacted KIR3DL1 engagement. Accordingly, KIR3DL1 recognition of HLA class I ligands is modulated by both the peptide sequence and conformation, as determined by the HLA polymorphic framework, providing a rationale for understanding differences in clinical associations.
The genes encoding human leukocyte antigen (HLA) class I molecules are among the most polymorphic in the human genome. This polymorphism impacts both the nature of the peptide-binding cleft and the surface features displayed to both innate and adaptive immune receptors. While major allotypic groupings of HLA class I may differ by 20 to 30 amino acids in these regions (1), there are numerous closely related HLA allotypes that exhibit micropolymorphisms, differing by as few as one or two amino acids (2, 3). While these closely related HLA allotypes frequently bind overlapping sets of peptides, these subtle polymorphisms can nevertheless profoundly impact T cell receptor (TCR) recognition, tapasin dependence, deletional tolerance during T cell development, T cell alloreactivity, and drug hypersensitivity reactions (3–9).
Polymorphic variations within the peptide-binding cleft can alter the depth, size, electrostatic and hydrophobic properties of the HLA pocket architecture, which subsequently impact the peptide repertoire or the conformation in which these peptides are held. For example, in the HLA-B35 family, HLA-B*35:01 (Leu156) and HLA-B*35:08 (Arg156) both bind the same viral determinant yet the positioning of the position (P)4-P6 residues is dramatically different in the two allotypes (4). Peptide conformation also varied in HLA-B*44:02 and HLA-B*44:03 (Asp116), compared to HLA-B*44:05 (Tyr116), where greater peptide flexibility in the context of HLA-B*44:05 allowed for a reshaping of the peptide for improved TCR binding (3).
While the impact of micropolymorphism in HLA class I genes on T cell responses is well studied, its impact on natural killer (NK) cell recognition is less well understood. Receptors of the killer cell immunoglobulin (Ig)-like receptor (KIR) family specifically recognize HLA class I proteins. Unlike the unique peptide-HLA specificity of TCR, individual KIRs typically recognize multiple HLA class I allotypes that share structural motifs. For example, KIR2DL1 recognizes C2 allotypes, a group of HLA-C allomorphs that possess Asn77 and Lys80, while KIR3DL1 binds HLA molecules that possess the Bw4 motif, a subset of polymorphic residues spanning positions 77 to 83 of the heavy chain. Despite these broad groupings, KIRs display hierarchical recognition of HLA class I molecules, including between closely related HLA class I allotypes. Indeed, in assessing KIR3DL1 binding to a series of HLA-bound beads, KIR3DL1 was observed to bind HLA-B*57:01 better than HLA-B*57:03 (10, 11). Other closely related HLA allomorphs, such as HLA-B*51:01 (His171) and HLA-B*51:02 (Tyr171), were similarly recognized by KIR3DL1 while HLA-B*44:02 (Asp156) was more poorly bound than HLA-B*44:03 (Leu156), particularly by KIR3DL1*005 (10). Although structural studies have shown that the HLA class I residues contacted by individual KIRs are essentially conserved across the entire HLA class I group (12, 13), the molecular mechanisms by which this class I micropolymorphism impacts KIR recognition remain unclear.
The biological significance of this capacity of KIR to discriminate between closely related HLA class I allotypes was recently highlighted in analyses of HIV-infected individuals where allotypic variation in KIR3DL1 in HLA-B*57:01+ individuals, but not HLA-B*57:03+ individuals, was associated with better control of viral replication and elevated CD4+ T cell numbers (14). The positioning of the polymorphic residues within the cleft of the two HLA class I molecules indicated a possible role for differences in peptide presentation in contributing to their varying association with HIV. Consequently, KIR3DL1 recognition of HLA-B*57:01 and HLA-B*57:03 allotypes was compared, with preferential KIR3DL1 recognition of HLA-B*57:01 over HLA-B*57:03 observed. Given that the two HLA class I molecules share overlapping peptide repertoires, the ability of KIR3DL1 to discriminate between HLA-B*57:01 and HLA-B*57:03 when presenting identical peptides was examined. The interaction was found to be highly sensitive to the PΩ-2 residue of the bound peptide, with structural analyses revealing the potential for amino acid residues to be positioned more favorably for KIR3DL1 interaction in the context of HLA-B*57:01. Thus HLA-centric factors can impact not only which peptides bind a given HLA class I allotype, but also how they are presented within the peptide-binding groove and ultimately their capacity to interact with KIR3DL1. Thus, the data provide mechanistic insight as to how micropolymorphism within HLA class I can impact KIR recognition to drive differential associations with disease outcomes.
Results
HLA-B*57:01 Mediates More Robust Inhibition of KIR3DL1+ NK Cells than HLA-B*57:03.
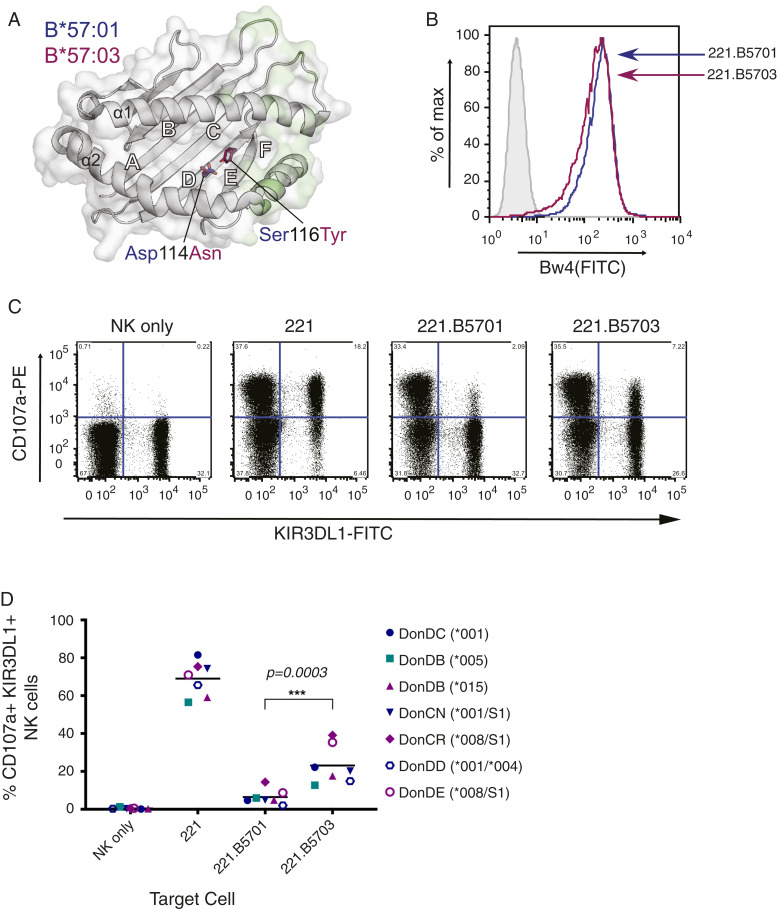
Both HLA-B*57:01 and HLA-B*57:03 possess the Bw4 motif and share >99% amino acid identity, differing at only two residues located in the floor of the peptide-binding cleft (Fig. 1A). To determine whether this difference was functionally significant, the ability of these two HLA class I allotypes to inhibit activation of primary KIR3DL1+ NK cells was compared; 721.221 (221) cells were transfected with HLA-B*57:01 or HLA-B*57:03 to generate stable cell lines expressing similar levels of cell surface HLA class I (Fig. 1B). Recognition of wild-type (wt) and transfected 221 cells by primary NK cells was then assessed by flow cytometry. Both KIR3DL1+ and KIR3DL1− NK cells expressed high levels of CD107a following culture with untransfected 221 cells, which express no HLA-A or HLA-B (Fig. 1C). In contrast, KIR3DL1+, but not KIR3DL1−, NK cells were inhibited in the presence of the HLA-B57 ligands, with HLA-B*57:01 being associated with more marked inhibition than HLA-B*57:03 (Fig. 1 C and D). When donors were considered on the basis of their KIR3DL1 allotype (Fig. 1D, KIR3DL1*001, blue symbols; *005, teal symbols; or *008/*015, purple symbols), the difference in HLA-B57 recognition was most notable for donors with KIR3DL1*015-like allotypes (purple) while the KIR3DL1*005 donor showed the most similar response to the two HLA molecules. Since both HLA-B*57:01 and HLA-B*57:03 were expressed at equivalent levels on the surface of transfected cells (Fig. 1B), this suggested that the overall difference in the extent of inhibition was likely driven by the polymorphic differences in the peptide-binding groove of the two HLA class I allotypes and/or their associated peptide repertoires.
Fig. 1.
Greater inhibition of KIR3DL1+ NK cells by HLA-B*57:01 than HLA-B*57:03. (A) Location of the two HLA-B57 polymorphisms (Asp114Asn and Ser116Tyr) which distinguish HLA-B*57:01 and HLA-B*57:03. The HLA binding groove is represented as a gray schematic, and the surface with KIR3DL1 contact regions is highlighted in light green. Polymorphic residues, represented as sticks with carbon atoms colored by allele (HLA-B*57:01, blue; HLA-B*57:03, magenta) and by atom (O, red; N, blue), are buried in the D and E pockets away from KIR3DL1 contact. Generated using PDB accession codes 3VH8 (12) and 5VWF (2). (B) Surface expression of transfected HLA-B*57:01 and HLA-B*57:03 molecules on 221 cells, as detected with anti-Bw4 supernatant followed by anti-mouse Ig-FITC. Shaded and light gray histograms represent unstained and 221 cells stained with anti-Bw4, respectively. Blue lines correspond to 221.B5701, and magenta lines to 221.B5703. (C) NK cells were purified from healthy donor PBMCs and expanded with IL-2 before being incubated with 221 and transfected 221 targets. Following incubation with monensin and anti–CD107a-PE, degranulation was assessed by flow cytometry, staining for CD56 and NKB1 (anti-KIR3DL1). Representative plots depict the percentage of KIR3DL1+ and KIR3DL1− NK cells (gated on CD56+ cells) expressing CD107a with given targets (donor CN: KIR3DL1*001/S1). (D) Percentage expression of CD107a on CD56+, KIR3DL1+ NK cells following incubation with 221 targets from six donors (where KIR3DL1 high and low-expressing cells from donor DB were sorted and assessed separately) across two independent experiments (open versus closed symbols), and analyzed by a one-way ANOVA using a Tukey’s multiple comparison test (***P = 0.0003). Horizontal lines represent the mean. Teal, KIR3DL1*005; blue, KIR3DL1*001; purple, KIR3DL1*008/*015 (note that KIR3DL1*004 is a null allele that is not expressed at the cell surface).
KIR3DL1*005 Is less Sensitive to Peptide Variation in HLA-B57.
To examine the role of peptide in KIR3DL1 discrimination between closely related HLA class I allotypes, a panel of tetrameric HLA-B*57:01 and HLA-B*57:03 molecules refolded with five peptides previously identified in the peptide repertoires of HLA-B*57:01 and HLA-B*57:03 (2) were generated (SI Appendix, Table S1). This included the LF9 peptide (LSSPVTKSF), which has been previously solved in complex with HLA-B*57:01 and KIR3DL1*001, and is known to be permissive for KIR3DL1 binding (12). Four additional shared peptides were selected to provide a variety of amino acid characteristics toward the C terminus of the peptide (PΩ), particularly at residues PΩ-1 and PΩ-2, but still with the potential to mediate KIR3DL1 binding. AW10 (ASLNLPAVSW) contained a P8-Ser, which was favorable in the LF9 peptide (12), while KF9 (KSFDFHFGF) possessed a hydrophobic P7-Phe and P8-Gly that may be permissive as previously reported for a P7-Tyr with P8-Gly in HLA-B*57:03 (15). Likewise, P8-Thr (15) and P8-His (12) have been reported to allow KIR3DL1 binding and were thus selected in RW11 (RVLPPSHRVTW) and LW10 (LALSPVPSHW). The peptides LY9 (LTVQVARVY), a peptide only found in the repertoire of HLA-B*57:01, and LF9(W9) (LSSPVTKSW), a substitution in LF9 which slightly enhanced the stability of both HLA-B*57:01 and HLA-B*57:03 complexes, were also chosen following recent structural analyses that revealed disparate peptide conformations when bound to the two HLA-B57 allotypes (2). AW10, LW10, RW11, and LY9 were also previously assessed for their relative contribution to the peptide repertoires of HLA-B*57:01 and HLA-B*57:03, with AW10 and LW10 showing similar contribution to the repertoire of both allotypes, RW11 showing greater relative contribution to the HLA-B*57:01 repertoire than to that of HLA-B*57:03, and LY9 undetected in the repertoire of HLA-B*57:03 (2). As a negative control, both HLA-B57 proteins were also refolded with an LF9 peptide carrying a S8E mutation, which permits binding to both HLA-B57 allotypes but has previously been shown to abrogate binding to KIR3DL1 (12).
The integrity of each of the tetramers was first addressed by measuring their binding to an LILRB1-expressing Ba/F3 cell line (SI Appendix, Fig. S1). LILRB1 binds to HLA class I molecules via the highly conserved HLA α3 domain and monomorphic β2m subunit and thus should not be markedly impacted by differences in the peptide cargo (16). Indeed, each pair of HLA-B57 tetramers with a given peptide bound to LILRB1 similarly, with the exception of LY9 in the context of HLA-B*57:03 that consistently bound to LILRB1 more efficiently than HLA-B*57:01/LY9, possibly reflecting intrinsic differences in the stability of this HLA/peptide complex. Indeed, HLA-B*57:03/LY9 is reportedly less stable than HLA-B*57:01/LY9 whereas the thermostabilities of LF9 and LF9(W9) bound to the two HLA-B57 molecules are more similar (2). Thus, the data suggest that, despite potential differences in the intrinsic affinity of each peptide for the HLA class I allotypes, both HLA-B57 allotypes could nevertheless be refolded with each peptide allowing for generation of tetramers of sufficient stability to allow us to compare their binding to KIR3DL1.
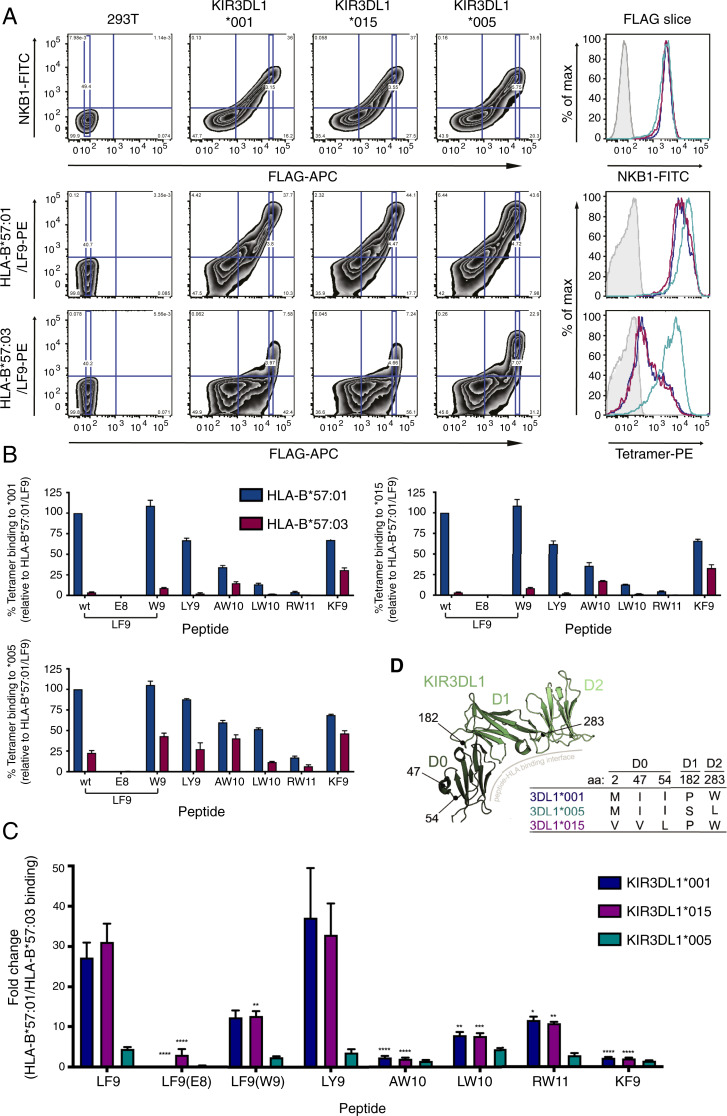
Given that KIR3DL1*005 demonstrates broader HLA-Bw4 specificity and peptide tolerance (17), the extent to which allotypic variation in KIR3DL1 impacted recognition of HLA-B*57:01 and HLA-B*57:03 was further assessed. 293T cells transfected with FLAG-tagged KIR3DL1*001, *015, or *005 allotypes were stained with the panel of HLA-B*57:01 and HLA-B*57:03 tetramers, which were used at concentrations that resulted in equivalent staining intensities on LILRB1-expressing cells. To control for differences in KIR3DL1 surface expression and/or transfection efficiency, the mean fluorescence intensity (MFI) of the tetramer staining was taken at a given FLAG intensity, correlating to equivalent surface KIR3DL1 expression (Fig. 2A). Consistent with previous observations, the LF9 peptide in the context of HLA-B*57:01 allowed robust binding to KIR3DL1 allotypes while the S8E substitution [LF9(E8)] abrogated KIR3DL1 binding (12). Overall, HLA-B*57:01 tetramers bound more strongly than HLA-B*57:03 tetramers to KIR3DL1*001 and *015 (Fig. 2B). This was most noticeable for LF9(wild-type), LF9(W9), and LY9, which bound strongly to both KIR3DL1 allotypes in the context of HLA-B*57:01. When assessed relative to one another, the LF9 and LY9 peptides in the context of HLA-B*57:01 stained cells expressing KIR3DL1*001 and *015 with MFI’s approximately 30 times greater than the same peptides in the context of HLA-B*57:03 whereas those for LF9(W9) reagents were 12 times greater, albeit the magnitude of these differences may be augmented by low binding of the HLA-B*57:03 tetramers to KIR3DL1*015 and *001 (Fig. 2C). In contrast, the peptides AW10 and KF9 supported weak staining of the KIR3DL1 allotypes when presented by HLA-B*57:03, which was only 2.5-fold less than HLA-B*57:01. Both LW10 and RW11 mediated very weak or negligible binding to KIR3DL1*001 and *015, even when presented by HLA-B*57:01, consistent with observations that the capacity of individual peptides to facilitate the interaction between KIR and HLA class I allotypes varies markedly (18–20).
Fig. 2.
KIR3DL1*005 displays broader binding to HLA-B*57:03 regardless of peptide. 293T cells were transfected with FLAG-tagged KIR3DL1*001, *015, or *005 constructs, and the binding of HLA-B*57:01 and HLA-B*57:03 tetramers loaded with the peptides LF9(wild-type; wt), LF9(E8), LF9(W9), LY9, AW10, LW10, RW11, and KF9 was assessed 48 h later. Surface expression of KIR3DL1 at equal FLAG intensities (FLAG slice) was confirmed by staining with NKB1-FITC. Transfectants were stained with tetramers at equivalent MFI values (based on LILRB1 binding) and for FLAG. Tetramer binding values were obtained at a given FLAG-APC MFI (FLAG slice). (A) Representative flow cytometry plots for NKB1-FITC, HLA-B*57:01/LF9-PE tetramer, and HLA-B*57:03/LF9-PE staining versus FLAG-APC staining, with the corresponding MFI of the FLAG-slice shown in histogram form (unstained, filled; SA-PE only, light gray; *001, blue; *015, purple; and *005 teal). (B) Staining of HLA-B*57:01 and HLA-B*57:03 tetramers with given peptides to KIR3DL1*001, *015, or *005–expressing 293T cells at the selected FLAG MFI. Tetramer staining is normalized to HLA-B*57:01 with wt LF9 as a known positive control from three independent experiments (error bars represent SEM). (C) The fold change between the MFI of HLA-B*57:01 and HLA-B*57:03 tetramer binding at the given FLAG intensity. Error bars represent the SEM, and asterisks indicate a statistical difference from the wt LF9 peptide for each respective KIR3DL1 allotype as calculated with a two-way ANOVA with Tukey’s multiple comparison test (*P < 0.1, **P < 0.01, ***P < 0.001, ****P < 0.0001). (D) Overview of KIR3DL1 polymorphisms in alleles tested (*001, *005, and *015) for HLA-B57 tetramer binding. Amino acid position 283 is proximal to the peptide-HLA binding interface in contrast to positions 47, 54, and 182. Amino acid usage in each position and their domain positions are indicated by the Inset table. Generated using PDB accession code 3VH8 (12).
This clear distinction between peptides was not the same for HLA-B57 tetramer binding to KIR3DL1*005, which differs from KIR3DL1*001 and *015 at residues 182 and 283 (Fig. 2D). Although HLA-B*57:03 bound more weakly to KIR3DL1*005 than HLA-B*57:01 across the peptide panel (Fig. 2B), there was no significant difference in the degree to which KIR3DL1*005 differentiated between peptides when presented by HLA-B*57:01 verses HLA-B*57:03 (Fig. 2C). These findings further emphasize the capacity of polymorphisms in KIR3DL1 to impact ligand binding, with KIR3DL1*005 better able to tolerate differences between HLA-peptide complexes.
HLA-B57 Polymorphisms Alter Peptide Preferences for KIR3DL1 Binding.
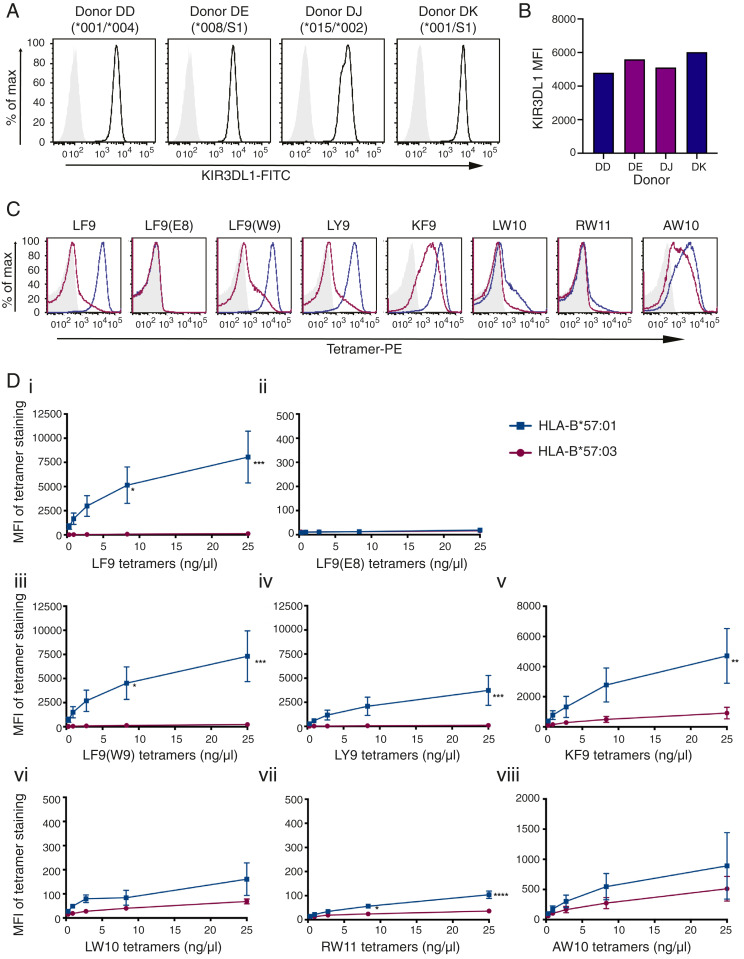
The ability of KIR3DL1*001 and *015 to differentiate between peptides when loaded onto HLA-B*57:01 compared to HLA-B*57:03 was next tested by staining primary NK cells expressing physiological levels of KIR3DL1. NK cells expressing KIR3DL1*001, *002, *008, or *015 were sorted and expanded, and receptor expression was confirmed (Fig. 3 A and B). The KIR3DL1+ NK cells were then stained with the panel of HLA-B57 tetramers (serially diluted threefold) revealing similar patterns of binding as observed in the 293T transfection system (Fig. 3 C and D). While HLA-B*57:01 tetramers loaded with LF9, LF9(W9), and LY9 efficiently bound to KIR3DL1+ NK cells, there was negligible staining of the NK cells by HLA-B*57:03 tetramers complexed with these peptides (Fig. 3 D, i, iii, and iv). Notably, LY9 and LF9(W9) both contain a basic residue at position PΩ-2 (Table 1), which is buried in the cleft of HLA-B*57:01 and solvent exposed in HLA-B*57:03 (2). The difference in KIR3DL1 binding was particularly marked for LY9, given the more intense staining of LILRB1 by HLA-B*57:03/LY9 tetramers compared to HLA-B*57:01/LY9 complexes (SI Appendix, Fig. S1).
Fig. 3.
Identical peptides loaded onto HLA-B*57:01 and HLA-B*57:03 molecules demonstrate disparate binding to KIR3DL1+ NK cells. (A) NK cells were sorted and expanded from four KIR3DL1+ donors and stained with NKB1-FITC (anti-KIR3DL1). Donors were KIR3DL1 subtyped as High1(*001)/Null(*004), High2(*008)/S1, High2(*015/*002), and High1(*001)/S1. (B) MFI of NKB1-FITC staining on KIR3DL1+ NK cells from each donor is compared (blue, KIR3DL1*001; purple, KIR3DL1*015-like). (C) HLA-B*57:01 and HLA-B*57:03 tetramers loaded with eight different peptides were used to stain KIR3DL1+ NK cells (representative plots for donor DK with 25 ng/μL tetramer: unstained/SA-PE only, light gray; HLA-B*57:01-PE tetramers, blue; HLA-B*57:03 tetramers, magenta). (D) KIR3DL1+ NK cells were stained with HLA-B*57:01 and HLA-B*57:03 PE-tetramers loaded with peptides as follows: (i) LF9; (ii) LF9(E8); (iii) LF9(W9); (iv) LY9; (v) KF9; (vi) LW10; (vii) RW11; or (viii) AW10. Threefold serial dilutions were performed, and the MFI of tetramer binding was plotted. HLA-B*57:01 and HLA-B*57:03 tetramer binding on KIR3DL1+ NK cells across two independent experiments (with two donors each) was compared using a two-way ANOVA with Sidak’s multiple comparison test [LF9: ***P = 0.0001, *P = 0.0152; LF9(W9): ***P = 0.0003, *P = 0.0344; LY9: ***P = 0.0010; KF9: **P = 0.046; RW11: ****P < 0.0001, *P = 0.0188]. The SEM is depicted.
Table 1.
Alignment of peptides tested
| Peptide | PΩ-10 | PΩ-9 | PΩ-8 | PΩ-7 | PΩ-6 | PΩ-5 | PΩ-4 | PΩ-3 | PΩ-2 | PΩ-1 | PΩ |
| LF9 | L | S | S | P | V | T | K | S | F | ||
| LF9(E8) | L | S | S | P | V | T | K | E | F | ||
| LF9(W9) | L | S | S | P | V | T | K | S | W | ||
| LY9 | L | T | V | Q | V | A | R | V | Y | ||
| AW10 | A | S | L | N | L | P | A | V | S | W | |
| KF9 | K | S | F | D | F | H | F | G | F | ||
| LW10 | L | A | L | S | P | V | P | S | H | W | |
| RW11 | R | V | L | P | P | S | H | R | V | T | W |
The KF9 peptide (phenylalanine at PΩ-2) in the context of HLA-B*57:03 was able to mediate weak binding to KIR3DL1+ NK cells (Fig. 3 D, v). In contrast, both HLA-B*57:01 and HLA-B*57:03 presenting the RW11 peptide showed little to no binding to KIR3DL1+ NK cells, behaving much like the nonpermissive negative control peptide, LF9(E8). When complexed with HLA-B*57:01, the LW10 peptide displayed very weak binding to KIR3DL1+ NK cells whereas no binding was evident in the context of HLA-B*57:03 (Fig. 3 D, vi and vii). The AW10 peptide complexes showed weak binding to KIR3DL1+ NK cells, but this did not differ significantly as a function of the HLA-B57 allotype (Fig. 3 D, viii). Notably, the AW10 peptide shares identical PΩ-1 and PΩ residues to LF9(W9) yet contains a small valine at PΩ-2. When the hierarchy of KIR3DL1 binding to each HLA-B57 peptide complex was broadly considered, HLA-B*57:01 peptides were favored in the order LF9/LF9(W9) > KF9 > LY9 > AW10 > LW10/RW11 > LF9(E8) while peptides presented by HLA-B*57:03 were preferred in the order KF9 > AW10 > LF9(W9) > LF9/LY9 > LW10/RW11/LF9(E8).
In HIV-infected individuals, a protective association of HLA-B*57:01, but not HLA-B*57:03, has been observed with position 47 of KIR3DL1, a dimorphic residue, with the presence of a valine being more protective than an isoleucine (14). To determine whether this related to differences in binding, the data were further analyzed on the basis of a KIR3DL1 residue at position 47. KIR3DL1Ile47+ NK cells (KIR3DL1*001+) were found to bind HLA-B*57:01 tetramers more strongly than KIR3DL1Val47+ NK cells (KIR3DL1*002/*008/*015) across the peptide panel, particularly for HLA-B*57:01 bound to the LF9 peptide as previously observed (SI Appendix, Fig. S2) (14). This trend was less evident for HLA-B*57:03 due to the overall poor binding of HLA-B*57:03 tetramers, and, indeed, HLA-B*57:03 was previously found to display no correlation between KIR3DL1 position 47 and HIV control (14). Importantly, however, there were no differences in the preferred peptide hierarchies observed between KIR3DL1Ile47+ and KIR3DL1Val47+ NK cells.
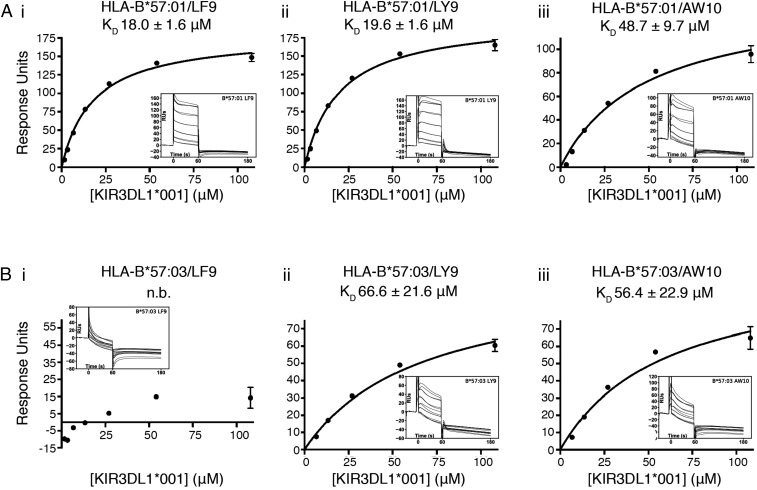
To confirm the observed effects of peptide and HLA allotype on KIR3DL1 engagement, the ability of soluble KIR3DL1*001 to bind to HLA-B*57:01 and HLA-B*57:03 complexed to three peptides (LF9, LY9, and AW10) was assessed by surface plasmon resonance (SPR). KIR3DL1*001 bound similarly to HLA-B*57:01 refolded with LF9 (dissociation constant [KD] = 18.0 ± 1.6 μM) and LY9 (KD = 19.6 ± 1.6 μM), while exhibiting weaker binding to HLA-B*57:01/AW10 (KD = 48.7 ± 9.7 μM), consistent with the tetramer staining analyses (Fig. 4A). KIR3DL1*001 binding to HLA-B*57:03/LF9 could not be detected, and the interaction between KIR3DL1*001 with HLA-B*57:03/LY9 was markedly weaker than that with HLA-B*57:01/LY9 (Fig. 4B). Consistent with tetramer staining, KIR3DL1*001 binding to HLA-B*57:01/AW10 was comparable to that for HLA-B*57:03/AW10. Together, these results suggest that the differences in KIR3DL1 binding to HLA-B*57:01 and HLA-B*57:03 are attributable to HLA-centric factors beyond the primary peptide sequence that impact the permissiveness of given peptides for KIR3DL1 binding.
Fig. 4.
Differential binding of KIR3DL1 to HLA-B*57:01 and HLA-B*57:03-presented peptides. SPR measurements of soluble KIR3DL1*001 binding to HLA-B*57:01 (A) or HLA-B*57:03 (B) with the peptides LF9 (i), LY9 (ii) or AW10 (iii). Equilibrium analysis of duplicate concentration series (108.0 to 1.69 μM) from which affinity (KD) was calculated (Inset value ± SE). Data are shown as mean ± range of duplicate injections (n.b., no binding). Inset graphs show reference subtracted sensograms of each injection. Data are representative of two independent experiments.
Micropolymorphisms in HLA-B57 Alter KIR3DL1 Ligands in a Peptide-Specific Manner.
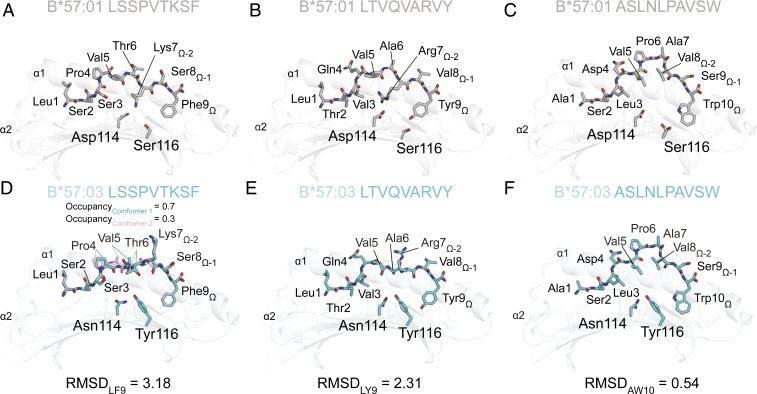
To understand how KIR3DL1 distinguished between the same peptides presented by HLA-B*57:01 and HLA-B*57:03, the crystal structures of the peptide-HLA complexes measured by SPR (those containing the LF9, LY9, and AW10 peptides) were compared. AW10 in complex with both HLA-B*57:01 and HLA-B*57:03 was solved to 1.3 and 1.2 Å, respectively, and LF9 with HLA-B*57:03 to 1.6 Å (data collection and refinement statistics summarized in SI Appendix, Table S2). This enabled a comparison of AW10 and LF9 presentation between the two HLA-B57 molecules, as well as to the previously published structures of HLA-B*57:01/LF9 (21), and HLA-B*57:01/LY9 and HLA-B*57:03/LY9 (2) (Fig. 5 A–F).
Fig. 5.
Lysine and Arginine at PΩ-2 are differentially presented by HLA-B*57:01 and HLA-B*57:03. Structural comparison of HLA-B*57:01 presenting LF9 (PDB accession code 2RFX) (21) (A), LY9 (PDB 5VUF) (2) (B), and AW10 (C) peptides, as well as HLA-B*57:03 presenting LF9 (D), LY9 (PDB 5VWF) (2) (E), and AW10 (F) peptides. In each panel, the HLA-B57 peptide-binding groove is shown in schematic format (HLA-B*57:01, light gray; HLA-B*57:03, light cyan) with peptides and HLA-B57 micropolymorphisms (Asp114Asn and Ser116Tyr) shown as sticks with carbon atoms colored by presenting allele (HLA-B*57:01, gray; HLA-B*57:03, cyan) and by atom (O, red; N, blue). All-atom alignments of peptides between alleles are indicated by the provided RMSD value. For HLA-B*57:03/LF9, two peptide conformations were observed, which refined with the following occupancies: conformer 1, light cyan, occupancy = 0.7; conformer 2, light pink, occupancy = 0.3.
As detailed previously, the substitutions D114N and S116Y that distinguish HLA-B*57:01 from HLA-B*57:03 create a deep, electronegative E pocket in HLA-B*57:01 that accommodates long, positively charged arginine and lysine peptide residues (2). Thus, as observed in the structures containing the peptides LF9(W9) and LY9 (2), the lysine residue at PΩ-2 (P7) of LF9 and arginine residue at PΩ-2 (P7) of LY9 were significantly different between the two HLA-B57 allomorphs and were coincident with structural differences across the P4–P7 region of the peptide. For LF9, the all-atom root-mean-square deviation (RMSD) between allomorphs (RMSDLF9) was 3.18 Å (Fig. 5 A and D) while, for LY9, the RMSDLY9 was 2.31 Å (Fig. 5 B and E). This resulted in the lysine/arginine residue at PΩ-2 of LF9 and LY9 being in a “flipped” orientation in HLA-B*57:01 compared to HLA-B*57:03, with a rotation of the side chain of 148°. The positively charged residue was therefore buried in the E pocket of HLA-B*57:01 while solvent exposed in HLA-B*57:03 (Fig. 5 A, B, D, and E). In HLA-B*57:03, P5 instead partially occupied the E pocket and consequently pushed P4 into the C pocket. Notably, in the HLA-B*57:03/LF9 complex, a low occupancy alternate conformation (0.3 occupancy) for the peptide was observed across P4–P7 (Fig. 5D). This was the result of the inversion of backbone dihedrals that placed P5-Val into the D pocket and P6-Thr into the C pocket yet, for both conformations, the Lys at PΩ-2 remained solvent exposed (Fig. 5D).
In contrast to LF9 and LY9, AW10 has an aliphatic valine residue at PΩ-2 and maintained a highly similar conformation regardless of HLA-B57 allotype, as reflected by the small RMSDAW10 of 0.59 (Fig. 5 C and F). These structural observations correlate with the SPR, in which LF9 and LY9 permitted KIR3DL1*001 binding to HLA-B*57:01, yet showed reduced binding to HLA-B*57:03, whereas the binding of AW10-containing complexes to KIR3DL1*001 was comparable for both HLA-B57 allotypes. Taken together, positively charged P7 residues are differentially presented by HLA-B*57:01 and HLA-B*57:03 and suggest that HLA-B57 polymorphisms can alter peptide conformation of individual KIR3DL1 peptide-HLA ligands.
Solvent Exposure of P7-Lys Causes a Steric Barrier to KIR3DL1*001 Recognition.
To understand how these conformational differences between HLA-B*57:01 and HLA-B*57:03-presented peptides impacted KIR3DL1 recognition, the structure of the KIR3DL1*001–HLA-B*57:03/LF9 complex was determined and compared to the KIR3DL1*001–HLA-B*57:01/LF9 complex previously published (Protein Data Bank [PDB] accession code 3VH8) (12). The KIR3DL1*001–HLA-B*57:03/LF9 complex was crystallized from the same conditions and space group as the previous HLA-B*57:01–containing complex and determined to a high resolution of 2.0 Å (SI Appendix, Table S2), enabling detailed comparison to be made. Overall, the docking of KIR3DL1 onto HLA-B*57:03/LF9 closely resembled that of HLA-B*57:01/LF9 (Fig. 6A). Indeed, all of the KIR3DL1 contacts to the HLA are conserved between the complexes. The primary point of difference is thus the conformation of the peptide presented by the HLA.
Fig. 6.
Solvent exposure of Lysine at PΩ-2 by HLA-B*57:03 impedes KIR3DL1*001 binding. (A) Structural overlay of KIR3DL1*001-HLA-B*57:03/LF9 and KIR3DL1*001-HLA-B*57:01/LF9 (PDB accession code 3VH8) (12). The HLA-B*57:01 complex is shown in gray. The HLA-B*57:03 complex is colored by chain (KIR3DL1*001, green; HLA-B*57:03, light cyan; β2m, gray; and LF9 peptide, teal). (B) Comparison of contacts between KIR3DL1 and the LF9 peptide in HLA-B*57:01 (gray) and HLA-B*57:03 (KIR3DL1*001, green; peptide, teal). The water-mediated network of contacts in the HLA-B*57:01 complex is not conserved in HLA-B*57:03 due to a shift in the position of P8-Ser. Dashed lines represent water-mediated hydrogen bonds in KIR3DL1*001-HLA-B*57:03/LF9 (black) and KIR3DL1*001-HLA-B*57:01/LF9 (pink). Water molecules are represented as pink spheres. (C) Comparison of HLA-B*57:03/LF9 peptide conformation unligated (peptide, light brown) and bound to KIR3DL1*001 (peptide, teal). Clash of Lys7apo position with Pro199 of KIR3DL1*001 in complex structure is concurrent with conformational differences at P4-Pro to P7-Lys. Directional arrows represent differences in peptide conformation from unligated to ligated form.
Despite the exposure of the P7-Lys in HLA-B*57:03, it formed no direct contacts with KIR3DL1*001. The lysine residue at PΩ-2 (P7) of LF9 is in a “flipped” orientation in HLA-B*57:01 compared with HLA-B*57:03, with a rotation of the side chain of 103°. Concomitant with the shift at P7, there were substantial changes to the orientation of the P6 and P8 residues (Fig. 6B). A single difference in the contacts between KIR3DL1 and the peptide was also observed. In KIR3DL1*001-HLA-B*57:01/LF9, the P8-Ser formed a direct contact to Leu166 of KIR3DL1*001, and a network of water-mediated contacts that incorporated Tyr200, Glu282, and the C terminus of the peptide were also formed (Fig. 6B). In KIR3DL1*001-HLA-B*57:03/LF9, the P8-Ser formed direct contacts to both Leu166 and Glu282 and hence only conserved the water-mediated contact to Tyr200 (Fig. 6B). Consequently, the solvent exposed Lys at PΩ-2 in HLA-B*57:03/LF9 is accommodated by KIR3DL1 without any direct contacts and with subtle changes to the KIR3DL1/peptide contacts, compared with the KIR3DL1*001–HLA-B*57:01/LF9 complex.
The binding of KIR3DL1*001 to HLA-B*57:03/LF9 was unmeasurable by SPR, and yet the complex was successfully crystallized. Comparison of the ligated KIR3DL1*001–HLA-B*57:03/LF9 complex with unligated HLA-B*57:03/LF9 highlighted a requirement for LF9 peptide plasticity in order to accommodate KIR3DL1*001 binding (Fig. 6C). In the unligated conformation, Lys at PΩ-2 would clash with Pro199 of the KIR3DL1 (Fig. 6C). As observed in the KIR3DL1*001–HLA-B*57:03/LF9 complex, the Lys at PΩ-2 is shifted 3.1 Å away from KIR3DL1. Concurrently, in the ligated structure P4-Pro to P6-Thr similarly changed conformation to accommodate movement of the P7-Lys. Thus, KIR3DL1*001 engagement of HLA-B*57:03/LF9 was impeded by the solvent exposure of P7-Lys and required the remodeling of the peptide or the selection of a favorable LF9 peptide conformation to enable binding. For HLA-B*57:01, there is no such energetic requirement as the P7-Lys is buried. Accordingly, the differences in KIR3DL1 binding observed to HLA-B*57:01/LF9 and HLA-B*57:03/LF9 are likely driven by the differential accommodation of the positively charged P7 residue in the E pocket.
Discussion
While micropolymorphism in HLA class I molecules can impact T cell responses through a diverse array of mechanisms that include subtle changes in peptide repertoire and the repositioning of peptides within the binding cleft, its impact on recognition of HLA class I by the innate immune system is not well-defined. Although HLA class I allotypes in possession of the signature motif required for recognition by a particular KIR (e.g., C1, C2, Bw4) are frequently treated as functional equivalents, these HLA class I molecules display hierarchical binding to KIR (10, 11, 22). Here, we have assessed the impact of HLA micropolymorphism as a potential factor impacting such hierarchical KIR3DL1 recognition, focusing on two closely related HLA-Bw4 allotypes, HLA-B*57:01 and HLA-B*57:03. The two HLA allotypes display different population distributions, with HLA-B*57:01 being found at moderate frequencies throughout Europe and Asia, whereas HLA-B*57:03 is found at high frequency (up to 0.05) in sub-Saharan Africa (23). While the differences between these two closely related HLA allotypes have been shown to drive divergent T cell responses, as well as hypersensitivity reactions (6, 24), these HLAs have also been differentially associated with KIR3DL1-dependent control of HIV replication (14). While these allotypes differ at only two positions, residues 114 and 116, HLA-B*57:01 was better able to inhibit the activation of primary NK cells expressing KIR3DL1 than HLA-B*57:03. Positions 114 and 116 lie at the floor of the peptide-binding groove and are therefore not solvent exposed, nor do they form part of the Bw4 motif. Rather, these residues contribute to the structure of both the E and F pockets of the HLA class I molecule and hence have the potential to impact the repertoire and conformation of peptides associated with each allotype.
The primary structure of the peptide can impact the capacity of an HLA class I allotype to interact with KIR3DL1 (12, 15, 20, 25). Indeed, the observation that HLA-B57/LF9(E8) tetramers bound poorly to KIR3DL1-expressing cells was consistent with previous analyses suggesting that acidic residues at the PΩ-1 position were not permissive for KIR3DL1 binding (12, 15, 20). There was nevertheless considerable variation in KIR3DL1 binding of tetramers with different peptides, such that HLA-B*57:01/LF9, LF9(W9), and LY9 tetramers all bound strongly to KIR3DL1 whereas tetramers containing the longer LW10, RW11, and AW10 peptides all bound relatively weakly.
Although the differences in KIR3DL1 recognition of HLA-B*57:01 compared with HLA-B*57:03 could be attributable to peptide repertoire differences, the data here suggest that the sequence of the peptide does not solely determine its capacity to promote or inhibit KIR3DL1/HLA class I interactions. Rather, the tertiary structure of a given peptide could differ markedly as a result of HLA class I micropolymorphism. For example, in the LF9 and LY9 peptides, the lysine and arginine at PΩ-2 were fully accommodated in the E pocket of HLA-B*57:01 but were surface exposed when complexed with HLA-B*57:03. This same switch in the conformation of the PΩ-2 residue in peptides bound to HLA-B*57:01 and HLA-B*57:03 has also been observed for the LTVQVARVW and LSSPVTKSW [LF9(W9)] peptides, indicative of characteristic differences between these closely related HLA class I allotypes (2). Critically, this altered conformation of the PΩ-2 side chain markedly impacted the capacity of KIR3DL1 to bind each HLA-B57 molecule as assessed by both tetramer-binding analyses and SPR. Specifically, the presence of an exposed, charged PΩ-2 side chain was invariably associated with a weak interaction with KIR3DL1. Lysine and arginine were both permissive residues at PΩ-2 in the context of HLA-B*57:01 but impaired recognition when bound to HLA-B*57:03. The degree of conformational difference between HLA-B*57:01 and HLA-B*57:03 presenting the LF9 and LY9 peptides compared to their presentation of the AW10 peptide was further evident in the lower RMSD value for AW10. Structural analyses showed that the AW10 peptide adopted a similar conformation in both HLA-B*57:01 and HLA-B*57:03 but that, in both cases, the peptide assumed a bulged conformation, in part driven by the presence of P6-Pro, that potentially impacted the capacity of KIR3DL1 to dock onto HLA-B57. Thus, while the contribution of individual amino acids at defined positions, and in particular PΩ-1, has been assessed extensively with respect to KIR binding, the conformation of the peptide itself, which is a product of the intricate byplay between variation in both peptide and the HLA class I, may well preclude clear definition of amino acids at positions PΩ-2 and PΩ-1 that support KIR3DL1 engagement.
The data here also demonstrate that allelic variation in KIR3DL1 markedly impacts the capacity of the receptor to interact with HLA-B57/peptide complexes. Consistent with previous observations, KIR3DL1*005 demonstrated greater tolerance to peptide variation than KIR3DL1*001 or *015 irrespective of HLA-B57 allotype (17). Thus, KIR3DL1*005, which is identical to KIR3DL1*001 at position 47 but differs at positions 182 and 283, was better able to accommodate the altered conformation of peptides such as LF9 and LY9 when complexed with HLA-B*57:03 compared with HLA-B*57:01. Both Ile47 and Val47 allotypes of KIR3DL1 demonstrated identical hierarchies of peptide preferences and were highly sensitive to the positioning of charged peptide residues at PΩ-2, which impacted their capacity to distinguish between HLA-B*57:01 and HLA-B*57:03. These observations suggest that, much like the necessity to consider the influence of micropolymorphisms between HLA-B*57:01, HLA-B*57:03, and HLA-B*58:01 on HIV epitope selection and control (26), so too should closely related HLA class I molecules be individually considered as KIR3DL1 ligands.
Here, we have shown that, beyond influencing peptide repertoire, micropolymorphism in HLA class I can alter the display of this repertoire by HLA-B57 to profoundly impact its recognition by the innate immune system. These observations have clear relevance for the interaction between KIR3DL1 and other HLA-Bw4 allotypes, but also more broadly for the interactions between other KIR and their HLA class I ligands. Moreover, the polymorphism within KIR3DL1 itself results in marked differences in tolerance to varied peptide conformation and ultimately recognition of HLA class I by NK cells. This has the potential to impact both NK cell education and the capacity of NK cells to sense subtle decreases in HLA class I expression (11, 27, 28). The poorer recognition of HLA-B*57:03 by KIR3DL1+ NK cells provides functional support for the observed association between KIR3DL1 and HLA-B*57:01 and the control of HIV replication (14). Similar mechanisms may govern the recognition of other groups of closely related Bw4 allotypes, such as those in the HLA-B44 family which, like HLA-B*57:01 and HLA-B*57:03, can differ at positions 114 and116. The KIR3DL1/S1 genotype, along with certain HLA-B27 family members, has been associated with the development of ankylosing spondylitis (29). Interestingly HLA-B*27:04 and HLA-B*27:05, both disease-associated alleles, have been shown to permit greater peptide conformational flexibility than alleles such as HLA-B*27:06, which are not associated with the disease (30). The data here suggest that this allotypic variation in peptide conformation has the potential to impact KIR recognition of HLA class I, which could conceivably play a role in the development of pathology. Nevertheless, the extent to which this impacts clinical outcomes will require further investigation. Ultimately, however, our data suggest that such associations may be dependent on specific allotypic combinations of both KIR3DL1 and HLA class I and imply that genetic association studies linking KIR and HLA class I with clinical outcomes may be highly dependent on high resolution typing of both the HLA and KIR alleles.
Materials and Methods
Cell Lines.
The HLA-A and -B deficient lymphoblastoid cell line 721.221 (221) transfected with HLA-B*57:01 has been described previously (31). The pcDNA3.1(-).B5703 construct was gratefully obtained from Lars Kjer-Nielsen, Peter Doherty Institute for Infection and Immunity, Department of Microbiology and Immunology, The University of Melbourne, Melbourne, Australia, and transfected into 221 cells via electroporation at 200 V and 975 μF, and selected with 0.5 mg/mL geneticin (Life Technologies) as described previously (31). The 221 cells and transfectants were maintained in RPMI media 1640, with 10% fetal calf serum (FCS) and supplements. LILRB1-expressing Ba/F3 lines were generously provided by Geraldine O’Connor, Peter Doherty Institute for Infection and Immunity, Department of Microbiology and Immunology, The University of Melbourne, Melbourne, Australia, and were maintained with 10 ng/mL murine IL-3 (17). Human embryonic kidney 293T (HEK293T) cells were maintained in Dulbecco’s modified Eagle’s medium with 10% FCS and supplements and were transfected with FLAG-tagged pEF6.KIR3DL1*001/*015/*005 constructs (17) using Fugene6 (Promega) as per the manufacturer’s instructions. Expression of transfected HLA class I molecules was confirmed by indirect flow cytometry, staining with specific anti-Bw4 supernatant (RM7.9.63) (7) followed by anti-mouse IgG- fluorescein isothiocyanate (FITC). Expression of LILRB1 was confirmed with anti–ILT2-APC (clone HP-F1; Life Research/Jomar), and KIR3DL1 expression with anti–NKB1-FITC (clone DX9; anti-KIR3DL1; BD Biosciences).
NK Cell Purification, KIR3DL1 Subtyping, and CD107a Assay.
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy blood donors via Ficoll/Hypaque density gradient centrifugation, and NK cells were purified using the EasySep Human NK Cell Enrichment Kit (Stemcell Technologies). NK cells (some donors KIR3DL1+ sorted) were expanded with irradiated feeder cells in the presence of 100 U/mL recombinant human IL-2 (rhIL-2) (Miltenyi) and 1.5 ng/mL phytohemagglutinin (Gibco Ltd.), as previously described (10). The KIR3DL1 subtype of each donor was determined using the multiplex PCR established in ref. 32, dividing donors into High1 (*001-like), High2 (*015-like), and Low1 (*005-like) allotypes, while the Null allotype (*004) is not surface expressed, and KIR3DS1 represents an activating allele (32). The D0 and D2 regions of the High2 alleles were further sequenced as KIR3DL1*002, *008, or *015 using previously published primers: 3DL1 Exon3 (D0) F 5′…CTTCTGGGCACTGGGAGT…3′; 3DL1 Exon3 (D0) R 5′…ACAGTGAGAAGCCCAGACR…3′; 3DL1 Exon5 (D2) F 5′…AAAGGTAGAAGGAGGAAACAGAT…3′; 3DL1 Exon5 (D2) R 5′…GGAAGCTCCTTAGCTAAGGATT…’; 3DL1-SEQ-E5F1 5′…GGTCATAGAGCAGGGGAGTG…3′; and 3DL1-SEQ-E5R 5′…TGCATCTGTCCATGCTTTTC…3′ (33, 34). The expression of CD107a on NK cells in response to targets was assessed as described previously (10). Briefly, NK cells and 221 transfectants were incubated at a 1:1 ratio in the presence of anti-CD107a (clone H4A3; PE or PE-Cy5; BD Biosciences) for 1 h before the addition of monensin (BD). Three hours later, cells were stained for KIR3DL1 (clone DX9; anti-NKB1; FITC; BD Biosciences) and CD56 (clone NCAM16.2; APC; BD Biosciences) and fixed with 2% paraformaldehyde. Cells were analyzed by flow cytometry, and the percentage of CD107a+, CD56+, and KIR3DL1+ NK cells was assessed.
Tetramers and Staining.
HLA-B*57:01 in the pET.30 vector was mutated at residues 114 and 116 using the primers B57-D114NS116Y-F: 5′ CCTCCTCCG‐CGGGCATAACCAGTACGCCT ACGACGGCAAGG 3′ and B57-D114NS116Y-R: 5′ CCTTGCCGTCGTAGGC GTACTGGTTATGCCCGCGGAGGAGG 3′ to generate HLA-B*57:03 (where base pairs to introduce mutations into HLA-B*57:01 to generate HLA-B*57:03 are underlined). Tetramers were generated as previously described (21). Briefly, soluble HLA-B*57:01 or HLA-B*57:03 (residues 1 to 276; with BirA tags) and β2-microglobulin (residues 1 to 99) were isolated from inclusion bodies and refolded together with peptide (Genscript) in 0.1 M Tris (pH 8), 2 mM ethylenediaminetetraacetic acid, 0.4 M l-arginine-HCl, 0.5 mM oxidized glutathione, 1.5 mM reduced glutathione, and 4 M urea. Following dialysis, refolded HLA-peptide complexes were purified via anion exchange chromatography and fast protein liquid chromatography and then biotinylated overnight. HLA were tetramerized with streptavidin-PE (Life Technologies) and titrated on LILRB1-expressing Ba/F3 cells (17). Tetramers were used to stain expanded KIR3DL1+ NK cells or 293T cells transfected with FLAG-tagged KIR3DL1, followed by FLAG-APC (clone L5; Biolegend). For 293T transfectants, the MFI of tetramer staining was taken at a given FLAG-APC MFI.
SPR.
HLA-B*57:01 and HLA-B*57:03 (without BirA tag) were refolded in the presence of relevant peptide as described for tetramer production. KIR3DL1*001 (residues 1 to 299) containing an N-terminal 6xHis tag was produced using the pFastBac insect cell expression system in Hi-5 insect cells (Thermo Fisher Scientific) as described previously (12). Soluble protein was purified from culture media using loose Ni-NTA resin (Qiagen) and further purified by size exclusion chromatography. KIR3DL1*001 analyte binding was analyzed on a Biacore T200 instrument (GE Healthcare Life Sciences) using HLA capture to an amine coupled anti–HLA-A,B,C (W6/32 clone) (35) CM5 surface chip as described previously (12, 14). KIR3DL1*001 binding (60 s association, 120 s dissociation) was analyzed at 5 μL/min at 298 K in a buffer composed of 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-HCl (pH 7.4), 300 mM NaCl, and 0.005% surfactant P20. Duplicate injections of seven twofold dilutions (∼100 to 1.56 μM) were analyzed over surfaces captured with HLA (∼400 response units) prior to each analyte injection. Chip surfaces were regenerated between each analyte injection using ActiSep (Sterogene). Resulting sensograms were reference subtracted against a flow cell coupled with W6/32 in the absence of HLA ligand capture. Binding affinities were determined by equilibrium analysis by implementing the one-site specific binding model using Graphpad Prism v7.
Crystallization and Structure Determination.
KIR3DL1*001 was expressed in mammalian HEK 293S GnTI− cells and purified as described previously (12, 36). The receptor was concentrated to 10 mg/mL and combined with HLA-B*57:03/LF9 at a 1:1 molar ratio and crystallized at 294 K by the hanging-drop vapor-diffusion method from a solution comprising 14% PEG 3350, 2% tacsimate, pH 5.0, and 0.1 M trisodium citrate, pH 5.6. HLA-B*57:03/LF9, HLA-B*57:01/AW10, and HLA-B*57:03/AW10 were buffer exchanged into 10 mM Tris, 150 mM NaCl, pH 8.0, and crystallized at 4 to 10 mg/mL in 12 to 24% PEG 4000, 0.2 M ammonium acetate, 0.1 M trisodium citrate, pH 5.4, at 294 K in the presence of crystal microseeds of HLA-B57. Prior to data collection, the crystals were equilibrated in crystallization solution with 35% PEG 3350 (for KIR3DL1*001-HLA-B*57:03/LF9) or 10% glycerol (for HLA-B57 binary complexes) added as a cryoprotectant and then flash-cooled in a stream of liquid nitrogen at 100 K.
X-ray diffraction data were collected at the MX2 beamline (Australian Synchrotron) (37). The ternary complex was scaled using MOSFLM and SCALA from the CCP4 program suite (38). HLA-B57 binary complexes were processed using XDS (39) and scaled using AIMLESS (38). Details of the data processing statistics are summarized in SI Appendix, Table S2. Each structure was determined by molecular replacement, as implemented in PHASER (40) using the following search models: KIR3DL1*001-HLA-B*57:03/LF9: PDB ID 3VH8 (12); HLA-B*57:01/AW10: PDB ID 5VUD (2); and HLA-B*57:03 complexes: PDB ID 5VVP (2) with peptide atoms omitted. Refinement of the models proceeded with iterative rounds of manual building in Coot (41), refinement in PHENIX (42), and validation with MOLPROBITY (43). Refinement statistics are summarized in SI Appendix, Table S2. Coordinates and structure factors were deposited in the PDB.
Data Availability.
The protein structural data that support the findings of this study have been deposited in the Research Collaboratory for Structural Bioinformatics PDB with the accession codes 6V2O, 6V2P, 6V2Q, and 6V3J.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Health and Medical Research Council (to J.R., A.G.B., and J.P.V.), the Australian Research Council (to A.G.B.), and the worldwide cancer research organization. J.R. is supported by an Australian Research Council Laureate Fellowship, and A.W.P. by a National Health and Medical Research Council Principal Research Fellowship. This research was undertaken in part using the MX2 beamline at the Australian Synchrotron, part of the Australian Nuclear Science and Technology Organisation, and made use of the Australian Cancer Research Foundation detector. We thank the staff at the Monash Macromolecular Crystallization Facility.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. P. Parham is a guest editor invited by the Editorial Board.
Data deposition: Protein Data Bank codes for the three binaries and the ternary are as follows: 6V2O (B5701-AW10), 6V2P (B5703-AW10), 6V2Q (B5703-LF9), and 6V3J (KIR3DL1-B5703-LF9).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920570117/-/DCSupplemental.
References
- 1.Bade-Döding C., et al. , The impact of human leukocyte antigen (HLA) micropolymorphism on ligand specificity within the HLA-B*41 allotypic family. Haematologica 96, 110–118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Illing P. T., et al. , HLA-B57 micropolymorphism defines the sequence and conformational breadth of the immunopeptidome. Nat. Commun. 9, 4693 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archbold J. K., et al. , Natural micropolymorphism in human leukocyte antigens provides a basis for genetic control of antigen recognition. J. Exp. Med. 206, 209–219 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tynan F. E., et al. , The immunogenicity of a viral cytotoxic T cell epitope is controlled by its MHC-bound conformation. J. Exp. Med. 202, 1249–1260 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrows S. R., et al. , Human leukocyte antigen phenotype imposes complex constraints on the antigen-specific cytotoxic T lymphocyte repertoire. Eur. J. Immunol. 27, 178–182 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Illing P. T., et al. , Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 486, 554–558 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Zernich D., et al. , Natural HLA class I polymorphism controls the pathway of antigen presentation and susceptibility to viral evasion. J. Exp. Med. 200, 13–24 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macdonald W. A., et al. , A naturally selected dimorphism within the HLA-B44 supertype alters class I structure, peptide repertoire, and T cell recognition. J. Exp. Med. 198, 679–691 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macdonald W. A., et al. , T cell allorecognition via molecular mimicry. Immunity 31, 897–908 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Saunders P. M., et al. , Killer cell immunoglobulin-like receptor 3DL1 polymorphism defines distinct hierarchies of HLA class I recognition. J. Exp. Med. 213, 791–807 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudreau J. E., Mulrooney T. J., Le Luduec J. B., Barker E., Hsu K. C., KIR3DL1 and HLA-B density and binding calibrate NK education and response to HIV. J. Immunol. 196, 3398–3410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivian J. P., et al. , Killer cell immunoglobulin-like receptor 3DL1-mediated recognition of human leukocyte antigen B. Nature 479, 401–405 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saunders P. M., et al. , A bird’s eye view of NK cell receptor interactions with their MHC class I ligands. Immunol. Rev. 267, 148–166 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Martin M. P., et al. , Killer cell immunoglobulin-like receptor 3DL1 variation modifies HLA-B*57 protection against HIV-1. J. Clin. Invest. 128, 1903–1912 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brackenridge S., et al. , An early HIV mutation within an HLA-B*57-restricted T cell epitope abrogates binding to the killer inhibitory receptor 3DL1. J. Virol. 85, 5415–5422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willcox B. E., Thomas L. M., Bjorkman P. J., Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat. Immunol. 4, 913–919 (2003). [DOI] [PubMed] [Google Scholar]
- 17.O’Connor G. M., et al. , Mutational and structural analysis of KIR3DL1 reveals a lineage-defining allotypic dimorphism that impacts both HLA and peptide sensitivity. J. Immunol. 192, 2875–2884 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malnati M. S., et al. , Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science 267, 1016–1018 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Zappacosta F., Borrego F., Brooks A. G., Parker K. C., Coligan J. E., Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis. Proc. Natl. Acad. Sci. U.S.A. 94, 6313–6318 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peruzzi M., Parker K. C., Long E. O., Malnati M. S., Peptide sequence requirements for the recognition of HLA-B*2705 by specific natural killer cells. J. Immunol. 157, 3350–3356 (1996). [PubMed] [Google Scholar]
- 21.Chessman D., et al. , Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity 28, 822–832 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Hilton H. G., et al. , Polymorphic HLA-C receptors balance the functional characteristics of KIR haplotypes. J. Immunol. 195, 3160–3170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solberg O. D., et al. , Balancing selection and heterogeneity across the classical human leukocyte antigen loci: A meta-analytic review of 497 population studies. Hum. Immunol. 69, 443–464 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart-Jones G. B., et al. , Structural features underlying T-cell receptor sensitivity to concealed MHC class I micropolymorphisms. Proc. Natl. Acad. Sci. U.S.A. 109, E3483–E3492 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart-Jones G. B., et al. , Crystal structures and KIR3DL1 recognition of three immunodominant viral peptides complexed to HLA-B*2705. Eur. J. Immunol. 35, 341–351 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Kloverpris H. N., et al. , HLA-B*57 Micropolymorphism shapes HLA allele-specific epitope immunogenicity, selection pressure, and HIV immune control. J. Virol. 86, 919–929 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S., et al. , HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc. Natl. Acad. Sci. U.S.A. 105, 3053–3058 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altfeld M., Gale M. Jr., Innate immunity against HIV-1 infection. Nat. Immunol. 16, 554–562 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Larrea C., et al. , Contribution of KIR3DL1/3DS1 to ankylosing spondylitis in human leukocyte antigen-B27 Caucasian populations. Arthritis Res. Ther. 8, R101 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loll B., et al. , Increased conformational flexibility of HLA-B*27 subtypes associated with ankylosing spondylitis. Arthritis Rheumatol. 68, 1172–1182 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Saunders P. M., et al. , The interaction of KIR3DL1*001 with HLA class I molecules is dependent upon molecular microarchitecture within the Bw4 epitope. J. Immunol. 194, 781–789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudreau J. E., Le Luduec J. B., Hsu K. C., Development of a novel multiplex PCR assay to detect functional subtypes of KIR3DL1 alleles. PLoS One 9, e99543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norman P. J., et al. , Unusual selection on the KIR3DL1/S1 natural killer cell receptor in Africans. Nat. Genet. 39, 1092–1099 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Hou L., et al. , Killer cell immunoglobulin-like receptors (KIR) typing by DNA sequencing. Methods Mol. Biol. 882, 431–468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F., Monoclonal antibodies for analysis of the HLA system. Immunol. Rev. 47, 3–61 (1979). [DOI] [PubMed] [Google Scholar]
- 36.Aricescu A. R., Lu W., Jones E. Y., A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 62, 1243–1250 (2006). [DOI] [PubMed] [Google Scholar]
- 37.McPhillips T. M., et al. , Blu-ice and the distributed control system: Software for data acquisition and instrument control at macromolecular crystallography beamlines. J. Synchrotron Radiat. 9, 401–406 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Collaborative Computational Project, Number 4 , The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Kabsch W., Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCoy A. J., et al. , Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Adams P. D., et al. , PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen V. B., et al. , MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protein structural data that support the findings of this study have been deposited in the Research Collaboratory for Structural Bioinformatics PDB with the accession codes 6V2O, 6V2P, 6V2Q, and 6V3J.