Abstract
The coronavirus SARS-CoV-2 pandemia is infecting millions of people and some studies relate conditions that might increase the risk of developing a fatal course for the disease, such as diabetes, cardiovascular diseases and obesity. In COVID-19 physiopathology, one of the main inflammation mechanisms is the “cytokine storm”, causing a pro-inflammatory state, related to cardiac and pulmonary damage. There is also a less effective role of lymphocyte B and T in the humoral immunity due to the reduction of their proliferative response. The physiopathology of ASD (Autism Spectrum Disorder) involves several modifications at the genetic and at the immune level, such as the increase of inflammatory cytokines and abnormal immune response in several levels. We hypothesize that ASD could be a risk-factor as the other conditions are.
Keywords: Coronavirus, SARS-CoV-2, COVID-19, Autism Spectrum Disorder, Risk Factor
Introduction
The coronavirus SARS-CoV-2 infection surprised the world with the spread of a new pandemia, spreading itself through over 200 countries and infecting millions of people. Its main manifestation is a flu-like syndrome that can evolve rapidly into an atypical pneumonia and affect other important systems, such as cardiovascular, digestive and immune systems [1], [2].
Some studies started relating conditions that might increase the risk of developing a fatal course for the disease [3], [4]. Are included diabetes, hypertension, cardiovascular and coronary diseases, and obesity. These comorbidities present a large inflammatory component, which directly modulates the individual’s immune system, increasing its vulnerability to the virus [5].
Autism Spectrum Disorder (ASD) is a brain-based neurodevelopmental disorder characterized by impairment in social communication as well as the presence of repetitive behaviors and restricted interests [6]. As one of the most frequent and serious neurodevelopmental conditions, ASD accounts for significant burden in public health, with an estimated annual total cost of $250 billion in the United States [7].
Approximately 1.6% of American children aged 8 years old had an ASD and it is estimated an international prevalence of 0,76% [8], [9]. Epidemiology also estimate that more patients are men, with the rate of 3:1 [9]. There has been a worldwide tendency of increase in ASD prevalence in the last few years, which could be explained by changes in diagnostic practice, coding tendency and community awareness. However, ASD prevalence could be increasing due to changes in true risk factors [10]. Furthermore, data analysis regarding the rise of frequency in this condition should be cautious.
The physiopathology of ASD (Autism Spectrum Disorder) involves several modifications at the genetic and at the immune level, such as the increase of inflammatory cytokines and abnormal immune response in several levels [11]. Some of these modifications are common in the conditions that are considered risk-factor to symptomatic COVID-19 and its worse outcome, and it is possible to stablish a correlation between them.
Since ASD is a disorders that affects a small, but growing and expressive portion of world’s population, and the fact that we do not yet fully understand the entire physiopathology of COVID-19, we would like with this article bring known data that can support the hypothesis of ASD being a risk-factor as the other conditions are.
Coronavirus disease 2019 (COVID-19)
Clinically, the infection and the immune response manifest in two phases. Initially there is an endogenous immune response, which depends on individual health and genetic characteristics, which prevents the virus from spreading through organism. It is believed that has close relation to specific HLA and histocompatibility complex to activate the immunity. The response is linked to the destruction level of the virus, the patient’s innate response, and it determines its inflammation status and symptomatology [12].
Studies that investigate COVID-19 physiopathology demonstrate that there is a second phase of hyper-activation of cytokines (known as “cytokine storm”), especially in respiratory epithelium [13]. The main cytokines activated are IL-1-beta, IL-6 and TNF-alfa. They are related with aggravation of respiratory symptoms, particularly severe pneumonia and fatal acute lung injury. These cytokines act causing damage to the pulmonary microvasculature, while affecting apoptosis and chemotaxis, decreasing epithelial barriers and causing alveolar edema.
A correlation was also found between a secretory increase of ACE-2 (angiotensin-converting enzyme 2) and COVID-19 patients [14]. ACE-2 is a regulator in angiotensin-2 transformation into angiotensin-[1], [2], [3], [4], [5], [6], [7], metabolite that has pro-inflammatory effects, causing vasodilatation, anti-proliferation and apoptosis, being a common way with cardiovascular diseases [15]. In COVID-19 infection, there seem to exist an ACE-2 super-expression leading to a pro-inflammatory state, related to cardiac and pulmonary damage [14].
Coronavirus also have neuroinvasive ability, presenting as febrile seizures, encephalitis, convulsions and change in mental status [16]. Main neurological symptoms found in COVID-19 patients are non-specific, such as headache, dizziness and confusion. It is known that neurological symptoms were directly related to the severity of the patients [17]. Thereby, we can hypothesize that its physiopathology might have a strict relation with the nervous system.
Risk factors for COVID-19
According to Wu et al [5], the main factors associated with the development of acute respiratory distress syndrome and death were neutrophilia, organ dysfunction, inflammation and older age. Neutrophils and inflammation are strictly related due its function of being a source of chemokines and cytokines, contributing to the “cytokine storm”. Older age, contrarywise, presents a less vigorous immune response, exposing the organism to an inadequate protection against viral destruction.
A recent study demonstrated that a significant amount of patients (about 40%) of pneumonia due to COVID-19 in the city of Wuhan, China, presented cardiovascular and cerebrovascular diseases, such as diabetes and coronary disease [4]. They were also more related to the development of acute respiratory distress syndrome. It is also revealed in the study that male patients are epidemiologically more related to the infection and with the severity of the pathological process.
There is a theory that male genetics are more vulnerable thanks to major expression on androgen receptors, considered to be primary factors in the genetic transcription of virus proteins (spike protein) [18]. This relationship is also enhanced by the presence of polymorphically relation to an X chromosome-linked inheritance between the loci of androgen receptor genes and ACE-2 receptor gene. This way, it is believed that the hyperandrogenic phenotype is correlated to an increased viral load and viral spread, and with the severity of lung involvement.
Badawi and Ryoo had shown in their study that similar respiratory syndrome caused by coronavirus have a worse prognostic when associated with hypertension, diabetes and obesity [3].
In diabetes and obesity, occurs a chronic activation of neutrophils and macrophages – considering them as chronic inflammatory diseases [19]. The increase of natural-killer cells and pro-inflammatory cytokines is related with higher lethality due to sepsis and multiple organ failure. There is also a metabolic disfunction associated with glucose, glutamine and fats, with their less to generate energy to combat acute events.
An additional mechanism causes the decrease of the immune system in these patients: a less effective role of lymphocyte B and T in the humoral immunity due to the reduction of their proliferative response [19], [20]. It is also common the disfunction of chemotaxis and phagocytosis in the cellular immunity [20].
Coronaviruses have neuroinvasion and neurotropism characteristics, also causing demyelination and central nervous system (CNS) inflammation with a possible pathway through the olfactory nerve due to its nasal inoculation [16], [17]. It is known that viral nervous dissemination is possible due to histopathological alterations in neurons located in cortex and hypothalamus, facilitated by proteins dinein and kinesin, affecting brain stem areas which regulate the respiratory rhythm [17]. This could likewise be found in SARS-CoV-2 infection, although the data in literature are still insufficient to establish an association.
Autism spectrum disorder and co-morbidity
ASD is a highly heterogeneous disturb, presumably with multiple underlying causes ranging from genetic to environmental factors, especially those influencing prenatal and early life development [21]. Congenital infections, maternal immune activation and transplacental antibodies likely play an important role in ASD physiopathology [22].
Furthermore, ASD has been correlated with abnormal immune function, including cytokine dysregulation, inflammation and the presence of autoantibodies. Several studies have reported the presence of anti-brain immunoglobulins in ASD patients [22]. ASD status could also be directly correlated with patients’ cytokine and chemokine levels, contributing for the chronic pro-inflammatory environment noticed in their organism [23].
A large amount of patients with ASD shares co-occurring medical conditions. Attention Deficit Hyperactivity Disorder (ADHA) is commonly present within these patients, as well as others neuropsychiatric conditions such as intellectual disability, sleeping problems, anxiety, irritability, language and motor impairments [24]. Gastrointestinal symptoms are seen in 47% of people with ASD [25]. Epilepsy has a reported prevalence of 8–6% in autistic patients [26]. The variety of other clinical conditions, which usually affects the immune system as well, should be considered when analyzing the role of chronic neuroinflammation among these patients.
Autism spectrum disorder and immune system
The concept of persistent inflammation of the CNS among patients with ASD and its role in neurodevelopmental outcome has been gaining increasing credibility. Proliferation of glial cells and subsequent release of multiple pro-inflammatory mediators, markedly cytokines and chemokines, lead to abnormal immune profiles and altered neural signaling and cognitive function [27].
Immune system dysregulation in ASD determines abnormal innate and adaptive immunity responses. Natural Killer (NK) cells from individuals with ASD present higher resting but reduced stimulated cytolytic activity, leading to an impaired response following injury stimulus and an excessive baseline level of activity [22].
Regarding humoral adaptive role in ASD pathogenesis, the presence of brain autoantibodies, as well as the correlation between severity of ASD and serum antineuronal and anti-ganglioside M1 antibodies, sustains the hypothesis of immunoglobulins contribution to neuroinflammation and behavioral changes [28]. Furthermore, shifts in IgG into lower affinity classes possibly play an important part in ASD manifestations [29].
Numerous studies in animal models established that a maternal immune activation could lead to macrophage inflammatory state, up-regulation of interferon-gamma (IFN-γ) and systemic deficits of T regulatory cells [27].
Studies suggest an alteration in cytokines profile in patients with ASD. Elevation of prenatal levels of IL-1 beta and IL-4 could be associated with cognitive development in autistic patients. Moreover, an increase of pro-inflammatory cytokines such as IL-1 beta, IL-1, IL-5, IL-6, IL-8, IL-12, IL-13, IL-17, 1L-23 and TNF-alfa is often seen in ASD patients, even in postnatal period [11].
Although uncertain, most studies support the theory of an increased Th1 response leading to a pro-inflammatory environment in ASD [30]. Repeatedly, ASD individuals were reported to have lower concentration of anti-inflammatory or higher concentrations of proinflammatory cytokines. However, some results are contradictory [28]. Further studies are necessary to better understand the Th1/Th2 balance among ASD patients.
Some alleles of human leukocyte antigens (HLA) were also associated with ASD. Higher frequencies of HLA-DRB1*03 and HLA-DRB1*11 were found in ASD children. This evidence support the theory of autism as an autoimmune disorder, leading to chronic neuroinflammation [31].
Autism spectrum disorder and COVID-19
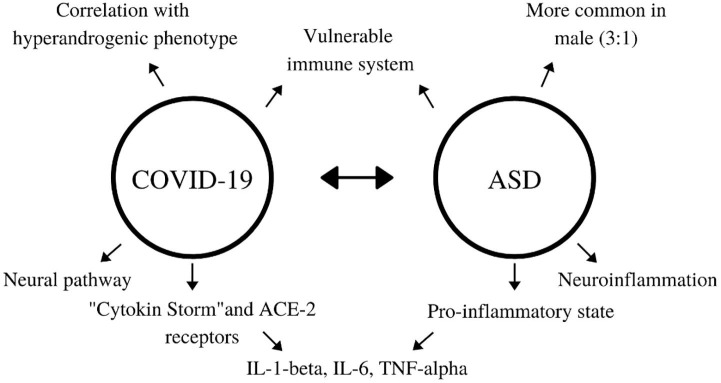
People with low-grade chronic inflammation conditions, such as diabetes or chronic heart failure are at high risk of developing Acute Respiratory Distress Syndrome (ARDS) due to COVID-19. This may be explained by a massive inflammatory activation – “cytokine storm” – facilitated by an already pro-inflammatory environment observed in these conditions [32]. This supposition raises the question that patients with ASD, a chronic neuroinflammatory disorder, are at risk of poor outcome in COVID-19. The relation of factors is resumed in Fig. 1 .
Fig. 1.
Common aspects between COVID-19 and ASD.
Pro-inflammatory cytokines, markedly IL-6 and IL-1 beta, are highly increased in patients with ARDS due to COVID-19 [33]. In effect, suppressors of these cytokines, such as tocilizumab, are been tested as therapeutical weapons among these patients. Elevated IL-beta and IL-6 levels are often present in ASD patients [11]. Therefore, autistic patients could be at higher risk of developing a cytokine storm due to the already increased levels of the implied cytokines.
The neural pathway of coronavirus through the olfactory nerve [17] could lead to a major immune response on CNS, leading to an important state of neuroinflammation. This state is also seen in ASD patients and could be related directly to the organism capacity of defense and to the functionality of the respiratory tract.
Although no major mutation has been identified as a genetic etiology for ASD, epidemiology shows that it is more common in male patients and some genetic syndromes, as the Fragile X, have some clinical resemble [34], straightening the possibility of a correlation with hyperandrogenic phenotype, as seen in SARS-CoV-2 infection.
It is important to make a parallel with the vulnerability of the older patients in COVID-19. Some studies have shown that ASD patients present higher levels of viral and bacterial seromarkers, as well as increased diagnosis of numerous infections, including common cold, being also considered more vulnerable, especially when they in the first three years of life [35]. Although the mechanism is yet unclear, there can be similarities between the exposure and the risk in both conditions.
Conclusion
ASD patients present several comorbidities that usually potentialize their inflammatory system. Even though there are blank spaces in COVID-19 physiopathology, we know that patients who manifest endogenous inflammation tend to present a worst prognostic.
Given the complexity of COVID-19 and ASD, it is possible to notice a coincidence in immune and genetic factors in their physiopathology, particularly when it comes to comorbidities considered risk-factor for worse prognostic of the viral infection. Thus, further studies are necessary for the confirmation of this correlation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the Study Group on Neuroinflammation and Neurotoxicology of Autistic Spectrum Disorder – GENIT/UECE.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.109899.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhu N, Zhang D, Wang W, et al. China Novel Coronavirus I and Research T. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. 2. http://doi.org/10.1056/NEJMoa2001017.
- 2.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Inf Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA internal medicine, 2020. https://doi.org/10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 6.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, (p. 81). Arlington: American Psychiatric Association. 2013.
- 7.Buescher A.V., Cidav Z., Knapp M., Mandell D.S. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA pediatrics. 2014;168(8):721–728. doi: 10.1001/jamapediatrics.2014.210. [DOI] [PubMed] [Google Scholar]
- 8.Christensen DL, Braun KVN, Baio J, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveillance Summaries. 2018;65(13):1. https://doi.org/10.15585/mmwr.ss6513a1. [DOI] [PMC free article] [PubMed]
- 9.Holly H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Translational Pediatrics, 2020. 9,l1: S55. http://doi.org/ 10.21037/tp.2019.09.09. [DOI] [PMC free article] [PubMed]
- 10.Lyall K., Croen L., Daniels J. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. 2017;38:81–102. doi: 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ormstad H., Bryn V., Saugstad O.D., Skjeldal O., Maes M. Role of the Immune System in Autism Spectrum Disorders (ASD) CNS & Neurological Disorders-Drug Targets. 2018;17(7):489–495. doi: 10.2174/1871527317666180706123229. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y., Wang Y., Shao C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;2020(27):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Q., Wang B., Mao J. Cytokine Storm in COVID-19 and Treatment. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci. 2020 doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conde G., Pájaro L.D.Q., Marzola I.D.Q., Villegas Y.R., Salazar L.R.M. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J Neurol Sci. 2020 doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wambier C.G., Goren A. SARS-COV-2 infection is likely to be androgen mediated. J Am Acad Dermatol. 2020 doi: 10.1016/j.ecoenv.2020.110598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frydrych L.M., Bian G., O'Lone D.E., Ward P.A., Delano M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol. 2018;104(3):525–534. doi: 10.1002/JLB.5VMR0118-021RR. [DOI] [PubMed] [Google Scholar]
- 20.Dryden M., Baguneid M., Eckmann C. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21:S27–S32. doi: 10.1016/j.cmi.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Madore C., Leyrolle Q., Lacabanne C. Neuroinflammation in autism: plausible role of maternal inflammation, dietary omega 3, and microbiota. Neural plasticity. 2016 doi: 10.1155/2016/3597209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology. 2017, 42(1):284-98. https://doi.org/10.1038/npp.2016.158. [DOI] [PMC free article] [PubMed]
- 23.Depino A.M. Peripheral and central inflammation in autism spectrum disorders. Mol Cell Neurosci. 2013;53:69–76. doi: 10.1016/j.mcn.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Lord C., Elsabbagh M., Baird G., Veenstra-Vanderweele J. Autism spectrum disorder. The Lancet. 2018;392(10146):508–520. doi: 10.1016/S0140-6736(18)31129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaidez V., Hansen R.L., Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44(5):1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas S., Hovinga M.E., Rai D., Lee B.K. Brief report: prevalence of co-occurring epilepsy and autism spectrum disorder: the US National Survey of Children’s Health 2011–2012. J Autism Dev Disord. 2017;47(1):224–229. doi: 10.1016/j.rasd.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Matta SM, Hill-Yardin EL, Crack PJ. The influence of neuroinflammation in Autism Spectrum Disorder. Brain, behavior, and immunity. 2019. https://doi.org/10.1016/j.bbi.2019.04.037. [DOI] [PubMed]
- 28.Gładysz D., Krzywdzińska A., Hozyasz K.K. Immune abnormalities in autism spectrum disorder—could they hold promise for causative treatment? Mol Neurobiol. 2018;55(8):6387–6435. doi: 10.1007/s12035-017-0822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaman S., Yazdani U., Deng Y. A search for blood biomarkers for autism: peptoids. Sci Rep. 2016;6:19164. doi: 10.1038/srep19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haroon E., Raison C.L., Miller A.H. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostafa G.A., Shehab A.A., Al-Ayadhi L.Y. The link between some alleles on human leukocyte antigen system and autism in children. J Neuroimmunol. 2013;255(1–2):70–74. doi: 10.1155/2014/242048. [DOI] [PubMed] [Google Scholar]
- 32.Maddaloni E., Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes/Metabolism Res Rev. 2020 doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of biological regulators and homeostatic agents. 2020, 34(2). https://doi.org/10.23812/CONTI-E. [DOI] [PubMed]
- 34.Steinman G., Mankuta D. Molecular Biology of Autism’s Etiology –An Alternative Mechanism. Med Hypotheses. 2019;130 doi: 10.1016/j.mehy.2019.109272. [DOI] [PubMed] [Google Scholar]
- 35.Sabourin K.R., Reynolds A., Schendel D. Infections in children with autism spectrum disorder: Study to Explore Early Development (SEED) Autism Res. 2018;12(1):136–146. doi: 10.1002/aur.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.