Abstract
The anti-malarial drug Chloroquine (CQ) and its derivative hydroxychloroquine have shown antiviral activities in vitro against many viruses, including coronaviruses, dengue virus and the biosafety level 4 Nipah and Hendra paramyxoviruses. The in vivo efficacy of CQ in the treatment of COVID-19 is currently a matter of debate. CQ is a lysosomotrophic compound that accumulates in lysosomes, as well as in food vacuoles of Plasmodium falciparum. In the treatment of malaria, CQ impairs the digestion and growth of the parasite by increasing the pH of the food vacuole. Similarly, it is assumed that the antiviral effects of CQ results from the increase of lysosome pH and the inhibition of acidic proteases involved in the maturation of virus fusion protein. CQ has however other effects, among which phospholipidosis, characterized by the accumulation of multivesicular bodies within the cell. The increase in phospholipid species particularly concerns bis(monoacylglycero)phosphate (BMP), a specific lipid of late endosomes involved in vesicular trafficking and pH-dependent vesicle budding. It was shown previously that drugs like progesterone, the cationic amphiphile U18666A and the phospholipase inhibitor methyl arachidonyl fluoro phosphonate (MAFP) induce the accumulation of BMP in THP-1 cells and decrease cell infection by human immunodeficiency virus. HIV viral particles were found to be retained into large endosomal-type vesicles, preventing virus spreading. Since BMP was also reported to favour virus entry through hijacking of the endocytic pathway, we propose here that BMP could play a dual role in viral infection, with its antiviral effects triggered by lysosomotropic drugs like CQ.
Graphical abstract
Highlights
-
•
A known effect of Chloroquine (CQ) is phospholipidosis.
-
•
The intracellular accumulation of phospholipids is marked by a large increase in BMP, an endosomal specific-lipid.
-
•
Other antiviral drugs like progesterone induce BMP accumulation and viral particles sequestration in the cell.
-
•
BMP could be associated with the antiviral effects of CQ, at least in vitro.
1. Introduction
The current outbreak of the COVID-19 has triggered various strategies to fight against the infection by SARS-CoV-2 virus, including the screening and repurposing of already available approved drugs [1]. Among these drugs, the antimalarial drug chloroquine (CQ; Fig. 1 A) and its derivative hydroxychloroquine (HCQ; Fig. 1B), have attracted much interest [[2], [3], [4]]. CQ had already been tested in vitro against several coronaviruses [3] as well as many other viruses [2] and was found to be effective against SARS-CoV-2 with an EC50 of around 1 μM [5]. A subsequent chinese study found that HCQ is even more efficient than CQ in preventing SARS-CoV-2 replication in vitro in Vero cells [6]. A recent preprint reported however that HCQ showed antiviral activity in this latter cellular model but not in a model of reconstituted human airway epithelium [7]. Chinese studies have then reported that CQ could reduce the length of hospitalization and improve the evolution of COVID-19 pneumonia [[8], [9], [10]]. In spite of the in vitro efficiency of CQ in preventing the replication of several viruses [3,[11], [12], [13]], the in vivo efficacy in infected patients was not always confirmed [2] and CQ was ineffective in the prevention of influenza [14]. Efficacy in SARS-CoV-2-infected patients has been the topic of a virulent debate after the recent claim that HCQ, in association with azithromycin, a macrolide antibiotic, accelerates virus clearance [15]. This study has been criticized in light of the biases it suffered, that rendered its conclusions poorly reliable for a large part of the scientific community. A randomized chinese study also provided support for the favorable clinical evolution of patients treated with HCQ [10], while subsequent clinical studies performed in both France and the US on hospitalized patients showed no clinical benefit [16,17]. Altogether, these studies advocate at best for a possible benefit of the treatment provided that it is administered early after the appearance of symptoms. More recently, a retrospective study on 96 032 patients from 671 hospitals in six continents could not confirm a benefit of HCQ or CQ, when used alone or with a macrolide, on in-hospital outcomes for COVID-19. These drug treatments were found to be associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias [18]. This study had immediately a considerable impact on public health practice and ongoing trials but concerns regarding the statistical analysis and data integrity are already raised.
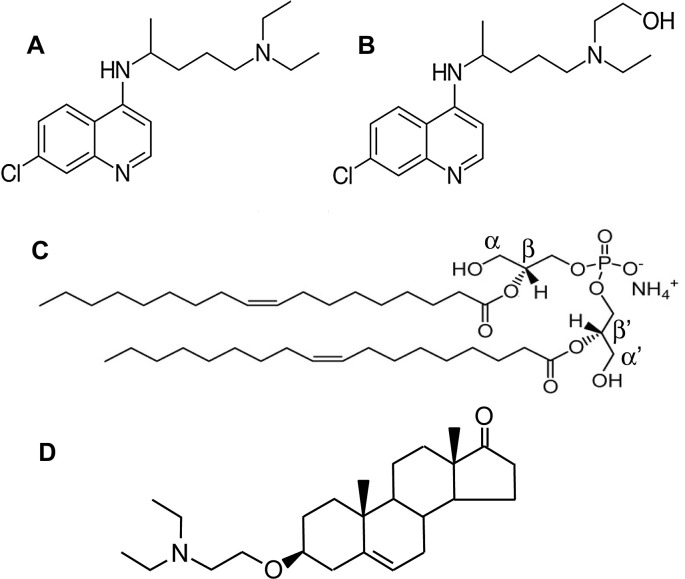
Fig. 1.
Chemical structures of chloroquine (A), hydroxychloroquine (B),bis(monoacylglycero)phosphate (BMP; C) and of the cationic sterol amphiphile U18666A (D). BMP is a polyglycerophospholipid, consisting of two monoacylated glycerol molecules, combined together through a single phosphate group. BMP is presented here with its two acyl chains (18:1) bound to the β and β′ positions of the glycerol units, a structure that is well accepted for intracellular BMP [75,76,86,87,106] and has been deduced from the resistance of intracellular BMP to the action of a phospholipase A1 [76]. The cationic sterol amphiphile U18666A is known to increase BMP levels in THP-1 cell cutures [103].
In these times of emergency, it is worth exploring quickly this track and the mechanism of action underlying the anti-viral effect of CQ. Here we highlight a possible connection between phospholipidosis (Fig. 2 ), a known effect of CQ and other lysosomotropic drugs leading to the accumulation of phospholipids in endocytic organelles, the enrichment of these organelles in the unconventional phospholipid bis-(monoacylglycero)-phosphate (BMP; Fig. 1C) and the inhibition of viral particle endosomal trafficking and dissemination.
Fig. 2.
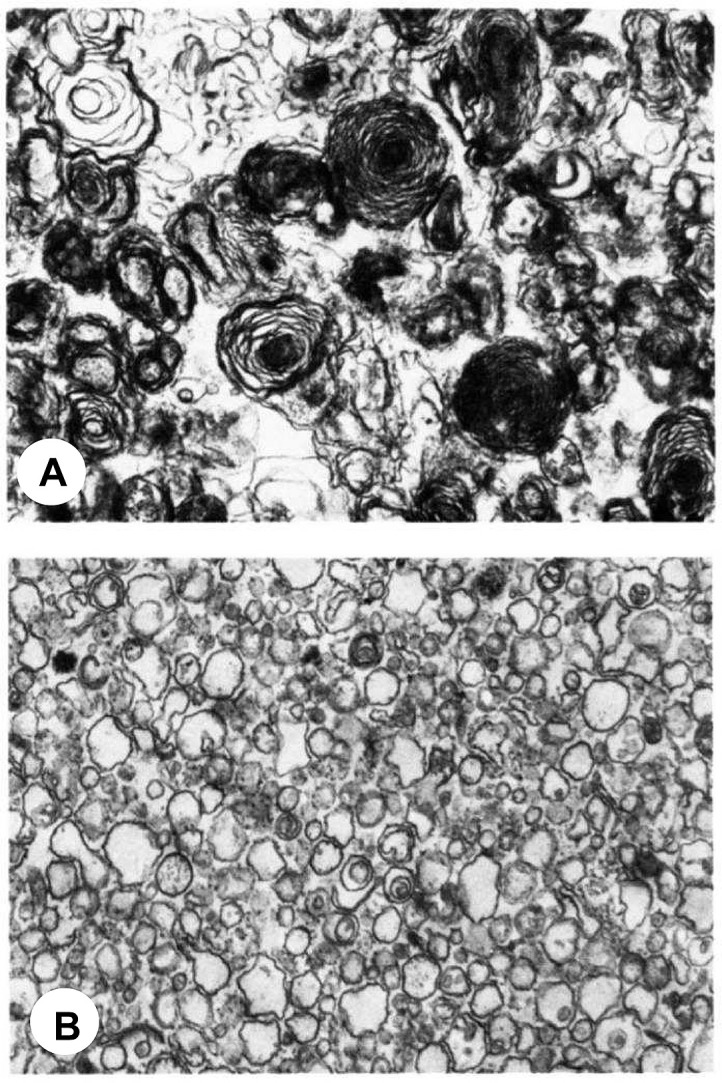
Electron microscopy of two different lysosomal fractions (A and B) isolated from chloroquine-treated rat liver. ( × 20.000). Reprinted with permission from Refs. [52].
2. Antiviral effects of CQ on several viruses and presumed mode of action
The in vitro antiviral effect of chloroquine was first reported 50 years ago [19,20] and since then CQ was found to be effective in vitro against many viruses including coronaviruses [2], dengue virus [13], the biosafety level 4 Nipah (NiV) and Hendra (HeV) paramyxoviruses [11,12,21], rabies virus [22], poliovirus [23], HIV [24,25], hepatitis A virus [26], hepatitis C virus [27], influenza A and B viruses [[28], [29], [30]], Sendai virus [28], Semliki Forest virus [28], Chikungunya virus [[31], [32], [33]], Zika virus [34], Pichinde, Mopeia and Lassa arenaviruses [35], Crimean–Congo hemorrhagic fever virus [36], Ebola virus [37], as well as a DNA virus like herpes simplex virus [38]. In the case of HIV, an in vitro antiviral activity was also reported for HCQ [39].
CQ was also found to be effective in vivo against dengue virus replication in monkeys [40] and avian influenza A H5N1 virus infection in mice [41], but was ineffective in the prevention of influenza [14] and treatment of acute chikungunya infections [31] in humans, as well as in the protection against Ebola virus infection and disease in a guinea pig model [37]. CQ did not protect hamsters against infection by NiV and HeV when administered either individually or in combination with ribavirin [21]. In vitro, however, NiV and HeV replication is impaired by CQ with an EC50 of 1 μM [12]. It was proposed that the mechanism of action of CQ could be the indirect inhibition of cathepsin L, a lysosomal enzyme acting at low pH, that is essential for the processing of the viral fusion glycoprotein NiV–F. Indeed, NiV entry, believed to occur only via fusion with the host cell’s plasma membrane, can also occur through the endocytic pathway of macropinocytosis that brings the virus to lysosomes. There, CQ would impair the cleavage of the viral fusion glycoprotein by cathepsin L by raising the pH of the lysosome [11].
CQ is effective in the treatment of malaria because it raises the pH of Plasmodium falciparum food vacuoles, impairs the proteolytic digestion of haemoglobin and thus prevents growth of the parasite [42]. Similarly, it is assumed that the antiviral effect of CQ mainly results from the alkalization of the phagolysosome or endolysosome [29], a cytoplasmic body formed in the process of phagocytosis of a virus or a bacterium by the cell. Phagolysosomes are essential for the intracellular destruction of pathogens, a process that results from the fusion with lysosomes and the action of lysosomal hydrolytic enzymes. A characteristic of phagolysosomes and lysosomes is their acidic pH at which lysosomal enzymes preferentially act. It thus appears counter intuitive to use a drug that increases the pH [43,44] and impairs the activity of lysosomal enzymes. Nevertheless, the alkalization of intracellular bodies can globally impact many cellular functions, including vesicular/endosomal trafficking [45], endocytosis, secretion, autophagy, apoptosis, innate and adaptative immunity [46] and various signaling pathways [47] as well as the late fusion of viral envelop with the lysosome membrane that can be mediated by acidic pH [29] and/or the cleavage of surface envelop proteins by acid lysosomal proteases like cathepsin L [48]. It is largely admitted that these two latter effects are the basis of the antiviral activity of CQ and other drugs inducing a pH increase in lysosomes and late endosomes. This may explain why a proton pump inhibitor like omeprazole has also been identified in vitro as a drug candidate against SARS-CoV-2 [49].
3. CQ, a drug with multiple effects
CQ, like azithromycin, is a weakly basic compound that accumulates in acidic organelles due to pH-partitioning and interaction with negatively charged phospholipids. These kinds of drugs are referred to as lysosomotropic [50,51]. Studies on the effects of azithromycin and CQ on lysosomes and lysosome-dependent processes have shown that both drugs accumulate in lysosomes, increase lysosomal pH, reduce lysosomal enzyme activities, trigger the accumulation of autophagic vacuoles, induce phospholipidosis [52,53], i. e accumulation of phospholipids in cells and tissues, and enlargement of lysosomes [3,4]. They also have an impact on fluid phase and receptor-mediated endocytosis of ligands like ovalbumin and low-density lipoproteins (LDL) that accumulate inside the cells [54,55]. Therefore, these compounds can have a broad impact on cellular processes. The biological activity of lysosomotropic drugs can be partly due to their effects on lysosomal functions, regardless of specific interactions with proteins or other targets. However, it was also proposed that CQ could act through other mechanisms. It could inhibit virus entry through the clathrin-dependent pathway [36]. Very recently, it has been proposed that both CQ and HCQ could inhibit SARS-CoV-2 infection through their ability to bind to sialic acids linked to host cell surface gangliosides, thereby preventing interaction between these co-receptors and the S protein [56]. CQ could also block virus cell entry by impairing the terminal glycosylation of the cellular receptor angiotensin converting enzyme 2 (ACE2) thereby negatively affecting virus-receptor binding as shown with SARS-CoV-1 [57]. Since ACE2 is also a receptor for SARS-CoV-2 [58], CQ may negatively influence the virus-receptor binding and prevent the infection. Besides these effects, CQ and azithromycin show anti-inflammatory activities in a number of cellular systems, with these effects likely resulting from the inhibition of arachidonic acid release by cytosolic phospholipase A2 (cPLA2) and the subsequent inhibition of prostaglandin E2 synthesis [[59], [60], [61], [62]]. The mechanisms of these inhibitory activities have however not been clarified unequivocally yet. Finally, CQ could have an inhibitory effect on innate immunity sensor activation via the endosomal nucleic acid-sensing Toll-like receptors (TLR7 and TLR9), that are responsible for initiating the antiviral response of plasmacytoïd dendritic cells (pDC) by triggering type 1 interferon-α (IFN–I) expression, a mechanism that can further lead to the induction of cytokine storm if it is not controlled [63]. Indeed, CQ is known to suppress endosomal TLR activation [64] and to impair BAD-LAMP (brain and DC-associated, lysosomal-associated membrane protein) control of TLR9 trafficking to late endosomes and production of pro-inflammatory cytokines in human plasmacytoid dendritic cells [65]. CQ can therefore display two different effects: a direct antiviral one and an indirect immunomodulatory one through the inhibition of endosomal TLRs [66]. The latter effect is targeted in the treatment by CQ and HCQ of autoimmune diseases like systemic lupus erythematosus [64].
CQ can increase lysosomal/endosomal pH in two different ways. First, it is a weak base that causes alkalization of endosome/lysosome lumen where its protonated forms are trapped [67]. Secondly, CQ was found to be a competitive inhibitor of ion transporting ATPases (Na+,K + -, Ca2+- and Ca2+,Mg2+−ATPases) from enriched microsomal membranes [68], which could lead to additional alkalization. CQ can thus alter the gradient of pH existing in the lumen of organelles along the endocytotic pathway from early endosomes (pH 6.0–6.6) through late endosomes (pH 5.5) to lysosomes (pH 4.5–5.0) [69] or along the secretory pathway from endoplasmic reticulum (pH 7.4) through trans-Golgi network (pH 6.2) to secretory granules (pH 5.5) [70,71]. The vast majority of articles describe the effects of CQ on lysosomal pH. Those of HCQ are generally inferred based on the high structural similarity between the two drugs and on the similarity of the observed effects in vitro and in clinical trials. Few studies have experimentally addressed the ability of HCQ to trigger an increase in lysosomal pH. One such a study [72] reports that HCQ has a lower interference on lysosomal degradation capacity and induces less intensive vacuolization of the cytoplasm compared to CQ. This may be the basis for the lower toxicity of HCQ.
4. CQ-induced phospholipidosis and BMP accumulation
Alkalization and the subsequent inhibition of lysosomal phospholipases A was proposed as one of the mechanisms by which lysosomotropic drugs induce the intracellular accumulation of phospholipids, e.g. phospholipidosis [73]. It has also been proposed that cationic amphiphilic agents like CQ bind tightly to phospholipids forming complexes which may be resistant to hydrolysis by phospholipase A [74]. Oral administration of CQ to rats (100 mg/kg for 1 week) results in up to 50% increase in liver phospholipid content [52,73]. This dose is in the same order of magnitude of that used to treat Covid-19 patients in clinical trials [15]. The increase in liver phospholipids can be entirely accounted for by the presence of secondary lysosomes and multivesicular (MVB) or multilamellar bodies in liver cells (Fig. 2). Among all phospholipids, the accumulation of the unconventional phospholipid BMP is particularly pronounced with a 20-fold increase compared to controls [52,73], while CQ-induced variations in major phospholipids are much lower. Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG) and phosphatidylinosito1 (PI) are only increased 1.4, 1.7, 5 and 1.7 times, respectively, compared to controls [52]. The highest content in BMP is found in the liver lysosomal fraction from CQ-treated rats, which agrees with the fact that BMP is mostly found in lysosomes and late endosomes. In particular, BMP accumulates in MVB and in certain subpopulations of intraluminal vesicles (ILV or exosomes), where it accounts for 15% and 70% of the phospholipid contents (mass %), respectively, under normal conditions [75,76]. Thus, CQ treatment induces a clear increase in BMP, a phospholipid involved in protein and lipid trafficking through late endosomes, and which normally represents less than 1% of total intracellular phospholipids [75,76]. BMP is today recognized as a biomarker of phospholipidosis, either induced by drugs like CQ and amiodarone [77] or associated with lysosomal lipid storage diseases like Niemann-Pick disease type C [78]. It has been hypothesized that BMP is involved in phospholipidosis-induction and the subsequent cholesterol traffic jam that leads to the formation of typical multilamellar bodies (Fig. 2) [79].
The intracellular localization of CQ was determined in subcellular fractions of treated rat liver and its distribution was found to be similar to that of acid phosphatase and other enzymes that could be involved in BMP synthesis [52]. Therefore CQ accumulates where large amounts of BMP are found and where the enzymes presumably involved in its synthesis are found.
CQ was also found to induce phospholipidosis in various cell cultures, including 3T3-L1K murine adipocytes [80], primary cultures of rat hepatocytes [81], in mice J774A.1 cells [54] and MDCK kidney cells [82], as well as in renal cells in which a phenotype resembling Fabry’s disease is observed [83,84].
5. BMP and vesicular/endosomal trafficking
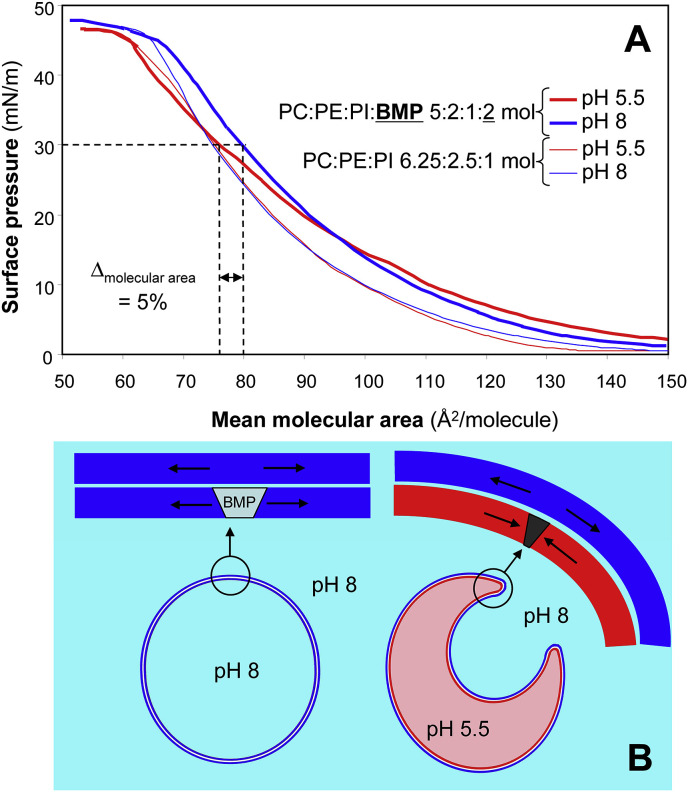
BMP is involved in the inward budding of ILVs into the acidic lumen of late endosomes, a process that leads to the formation of MVB [85]. Indeed, BMP is capable of inducing spontaneous inward vesiculation using model membranes (large unilamellar liposomes of 600–800 nm) with a phospholipid composition similar to that of late endosomes (PC:PE:PI:BMP, 5:2:1:2 mol) [86]. Because this inward budding was found to be pH-dependent and triggered at acidic pH, it was proposed that it results from the alteration of membrane curvature caused by transmembrane flipping of the protonated BMP molecules. So far, however, no BMP flippase has been identified [87]. Based on monomolecular film experiments with individual phospholipids and a mixture of PC, PE and PI ± BMP, we proposed another hypothesis based on the pH-dependent surface occupancy of phospholipids in the presence of BMP [88]. Indeed, at a surface pressure of 30 mN/m corresponding to the lateral pressure in natural membranes [89], the presence of BMP induces a decrease in the mean molecular area of phospholipids at pH 5.5 compared to pH 8 (Fig. 3 ). Since BMP is enriched in the membrane of late endosomes [90] and preferentially found on the inner leaflet of this membrane [75] facing the acid lumen, the differential molecular area of phospholipids on each leaflet of the late endosome membrane could trigger membrane curvature toward the acidic inside, budding of ILVs and formation of MVB without the need for a transmembrane flipping of BMP molecules.
Fig. 3.
pH-dependent effects of BMP in a model membrane. Panel A: compression isotherms (surface pressure versus mean molecular area) of mixed monomolecular films of phospholipids at pH 5.5 and 8. All films formed at the air-water interface contained PC, PE and PI at relative molar ratios of 6.25:2.5:1. When BMP was added to these films, the relative molar ratios were 5:2:1:2 for PC, PE, PI and BMP (molar fraction of 20%) which is similar to the relative proportions of the main phospholipids in the membrane of late endosomes [75]. Other membrane lipids (phosphatidylserine, sphingomyelin and cardiolipin) present at lower levels were omitted; Panel B, schematic representation of membrane curvature and inward budding in liposomes [86] and late endosomes, induced by BMP and the inner acidic pH of late endosomes. The latter are enriched in BMP [90] and BMP is preferentially found on the inner leaflet of the late endosome membrane [75]. Adapted from Ref. [88], in which experimental details are provided. NB: It is worth noting that these experiments were performed with the α, α′-BMP isomer that differs from the intracellular β, β′-BMP isomer used for demonstrating vesicular budding in lisosomes [86].
Whether the budding of ILVs is dependent on specific proteins in vivo is not fully elucidated. It was observed that BMP-containing liposomes mixed with cell homogenates could specifically recruit apoptosis-linked gene-2-interacting protein X (Alix) [86], a cytosolic protein also found in exosomes [91] and phagosomes [92]. Alix was found to control the ILV invagination process in vitro using liposomes [86] and the formation of BMP-containing endosomes in vivo [86]. It was postulated that ILV and the delimiting membrane of MVB interact dynamically by means of membrane fission and fusion events, and that this process is controlled, at least in part, by pH and transient interactions between BMP-containing membranes and Alix [86]. Noteworthy Alix contains a BMP binding site in the Bro domain of the protein [93]. The fusogenic properties of BMP are known to vary with pH (Fig. 4 ). They are maximum at acidic pH and are very low above pH 6.5 [76]. Therefore, CQ, which normalizes to 7.4 the pH of all acidic compartments in cells after a 30-min incubation at a concentration of 500 μM [94], might impair BMP and pH-dependent fusion processes and probably disrupt the endocytic pathway.
Fig. 4.
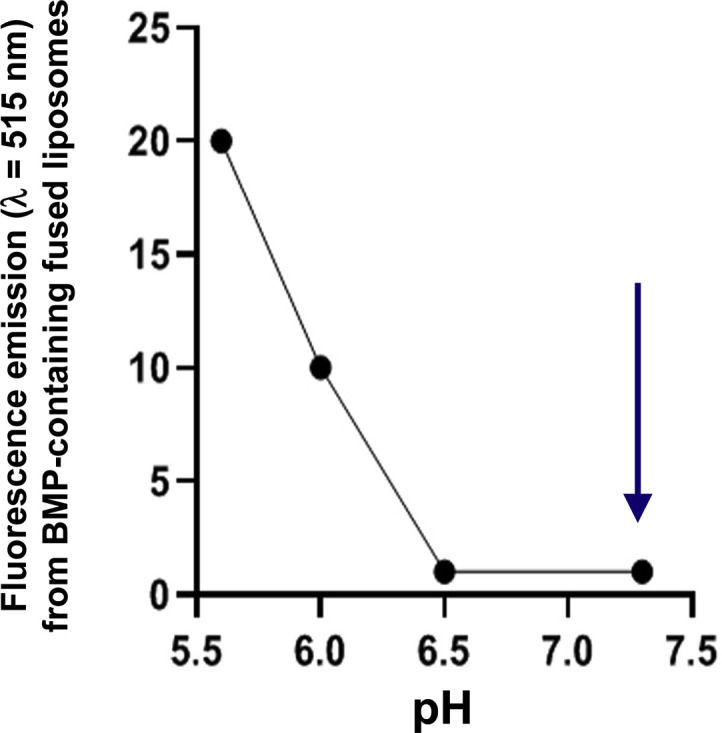
pH-dependent fusogenic capacity of BMP measured from fluorescence emission (λ = 515 nm) upon fusion of liposomes containing BMP and a fluorescent phospholipid analog. Donor liposomes were prepared by mixing 74% DOPC, 21% BMP, and 5% of the fluorescent β-BODIPY FLC12, 1-hexadecanoylphosphatidylcholine and acceptor liposomes containing 67% DOPC and 33% BMP. Fusion was started by mixing donor and acceptor liposomes at various pH, and analyzed by fluorescence emission at 515 nm after excitation at 500 nm. The time course increase of fluorescence emission at 515 nm was recorded as an indicator of liposome fusion. The arrow shows pH 7.4, the pH at which all acidic compartments in cells are normalized by incubation with CQ at 500 μM for 30min [94]. Adapted from Fig. 3 in Ref. [76].
6. Dual behaviour of BMP in viral infection and antiviral effects
There are several in vitro studies establishing a link between BMP, viral infection and virus entry through hijacking of the endocytic pathway. A correlation between BMP and viral infection was shown in the case of HeLa cells infected by Vesicular stomatitis virus (VSV). VSV infects cells through the endocytic pathway, with the release of its nucleocapsid occurring at the level of late endosomes [95,96]. The acidic late endosome pH triggers fusion of the VSV envelope with endosomal membranes, allowing nucleocapsid release into the cytoplasm and ensuing virus replication. It was shown that siRNA-mediated down-expression of Alix reduces VSV infection of HeLa cells, the number of MVB, as well as BMP levels by around 50% [86]. It was proposed that cell infection by VSV is reduced upon down-expression of Alix presumably because the number of acidic late endosomes is decreased, as well as Alix-dependent dynamics of late endosomal membranes, required for efficient nucleocapsid release [86]. Other studies also suggested that BMP, together with Alix, could favour viral infection and play a key role in the early phase of dengue virus (DENV) replication. Indeed, the levels of Alix, colocalized with BMP, were found to be increased in human endothelial EA. hy926 cells infected by DENV [97]. From these findings, it was proposed that the Alix-BMP complex could be involved in the export of DENV proteins from late endosomes to the cytoplasm. In line with this hypothesis, DENV infection was found to be decreased after cell treatment with an anti-BMP monoclonal antibody [97]. This antibody is known to be internalized and to accumulate in late endosomes [98] where it could impair the fusogenic effects of BMP and virus nucleocapsid released into the cytosol. More generally, BMP and its partner protein Alix are connected with the endosomal sorting complexes required for transport (ESCRT) machinery that controls membrane deformation and fission processes. This machinery is involved in sorting of downregulated signaling receptors and other proteins destined for late endosomes and lysosomes, via the formation of ILVs [87]. ILVs can be targeted to lysosomes for degradation, or undergo back-fusion with the limiting membrane, a process analogous to the budding of enveloped viruses. These processes allow to understand how enveloped viruses can hijack the endocytic pathway in various ways [87]: 1) virus entry via its fusion with endosome membranes leading to the release of the nucleocapsid (Fig. 6), 2) virus replication via the formation of double-membrane vesicles (DMVs) that provide an anchoring scaffold for viral replication/transcription complexes, a process shown for instance in the case of Middle East respiratory syndrome coronavirus (MERS-CoV) replication with viral protein palmitoylation serving as the membrane anchor [99] and 3) viral particle budding and dissemination outside the cell. The precise role of BMP in these viral processes is not fully elucidated but it has been shown for instance that the fusion of DENV [100] and VSV [101,102] depends on anionic phospholipids including BMP.
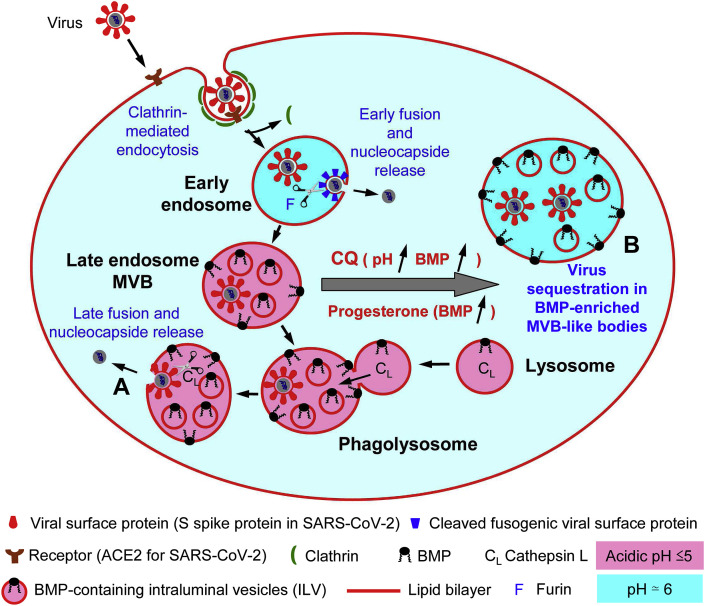
Fig. 6.
Schematic representation of the cell entry of an enveloped virus through the endo-/lysosomal pathway and possible mode of action of chloroquine and progesterone towards SARS-CoV-2 infection. It has been shown that coronaviruses can enter cells via clathrin-mediated endocytosis and their replication relies on trafficking through the endosomal pathway to lysosomes. After interaction with the target cell via a receptor (angiotensin conversion enzyme 2 (ACE2) in the case of SARS-CoV-2 [132,133]), virus internalization proceeds through clathrin-mediated endocytosis [48](not yet demonstrated for SARS-CoV-2 to our knowledge, but speculated [112]). Then, the release of the virus nucleocapsid into the cytosol for replication to occur depends on proteolytic cleavage of the virus envelop protein (S spike protein in the case of SARS-CoV-2). Upon cleavage the envelop protein acquires fusogenic properties that allow membrane fusion between the virus envelop and endosome/lysosome membrane. This process can occur at different steps of the endo-/lysosomal pathway depending on the virus and protease cleavage sites present in the envelop protein [48,134]. It was shown that the proteolytic cleavage site in the S protein of different coronaviruses is an essential determinant of the intracellular site of fusion. This allows the virus to escape the endo/lysosomal system from different compartments. Early fusion can occur in early endosomes through the cleavage of S by a furin. It can also results from the cleavage of S by an acidic lysosomal protease, Cathepsin L after fusion of late endosomes with lysosomes [48]. In (A) the basal content of BMP in late endosome/MVB would facilitate at low pH the fusion between virus envelope and the endosomal membrane. In (B) CQ, known to increase pH in late endosomes/lysosomes and to induce an accumulation of BMP in late endosomes and multivesicular bodies (MVB) [52,73] would lead to the sequestration of SARS-CoV-2 viral particles in MVB-like bodies. Progesterone is also known to induce the accumulation of BMP in cells infected by HIV and HIV viral particles are found to be sequestred in MVB [103]. Moreover, progesterone was recently found to display some antiviral activity in vitro against SARS-CoV-2 [126]. Thus, the combined effects of CQ on endosomal/lysosomal pH and BMP accumulation may result in the impairment of SARS-CoV-2 endosomal:lysosomal trafficking and possibly its sequestration in MVB.
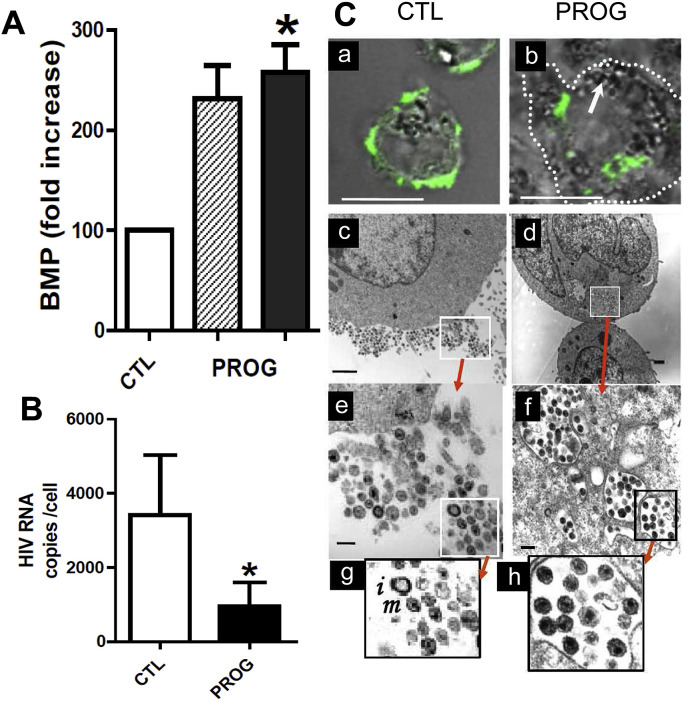
BMP is however also associated with opposite effects on viral infection. Indeed, we previously reported that increasing the endosomal content in BMP by various approaches, including treatment of human monocytic cell line THP-1 by progesterone (Fig. 5 A), the cationic sterol amphiphile U18666A (Fig. 1D) and the phospholipase inhibitor methyl arachidonyl fluoro phosphonate (MAFP), correlated with a strong decrease in HIV cell infection (Fig. 5B) [103]. HIV viral particles were found to be retained into large endosomal-type vesicles and no more viruses were visible in the cell periphery, preventing virus spreading (Fig. 5C). Although the fate of BMP-enriched endosomes in cells is not documented, one can conceive that trapped viruses would be further degraded. Another effect of progesterone was to inhibit macropinocytosis i.e. fluid phase endocytosis-mediated virus entry [103]. It is also worth noting that the cationic sterol U18666A shown to increase BMP levels and to inhibit HIV production in THP-1 cells [103], was also found to inhibit Ebola virus entry and infection in SNB19 human glioblastoma cells at micromolar concentrations, while inducing a Niemann-Pick C phenotype, i.e. the accumulation of cholesterol in late endosomes and lysosomes [104]. On the contrary to other lysosomotropic amines, U18666A has no impact on the acidity of late endosomes/lysosomes and does not inhibit cathepsin L [104], but it was shown to bind to the Niemann-Pick C1 (NPC1) lysosomal membrane protein involved in both the export of cholesterol and the cellular entry of Ebola virus [105]. The possible role of BMP was not highlighted in these studies, but since BMP is tightly associated with cholesterol transport [87,106] and is increased by U18666A [103], it is likely that it is also associated with the antiviral effects on Ebola virus. Cholesterol accumulation in membranes increases membrane rigidity and prevents for instance fusion between endosomes and lysosomes. This increase in endosome membrane rigidity might also impair the fusion of viruses with the endosomal membrane for releasing the nucleocapsid in the cytosol independently of any change in endosomal pH, and it appears as a distinct mechanism compared that of BMP. A protective function of late endosome/lysosome cholesterol accumulation against influenza A virus (IAV) has been established and suggested that the endosomal cholesterol balance could be a possible antiviral target [107]. Since BMP is involved in cholesterol homeostasis [90], these findings also support a role for BMP in the antiviral response.
Fig. 5.
Effects of progesterone on BMP levels and viral replication in THP-1 cells infected by HIV. Panel A: Relative increase in BMP cellular content upon treatment of THP-1 infected cells by progesterone versus control (ethanol as vehicle, 0.1% v/v final). Hatched bars corresponds to BMP quantified by its fatty acid content determined following HPLC purification and gas chromatography analysis from 50 × 106 cells. Black bars: BMP content was measured by flow cytometry from 0.5 × 106 cells labeled using the purified anti-BMP antibody 6C4 as primary antibody, Phycoerythrin (PE)-labeled goat anti-mouse IgG as secondary antibody and the Mean Fluorescence Intensity (MFI) as quantification unit. In both cases, the amounts in the control were normalized to 100. Panel B: Inhibition of HIV production in THP-1 cells treated with progesterone (initial concentration at day 0 = 10 μM). Residual HIV-1 RNA in the culture supernatant was quantified at day 4 post-infection. Data in panels A and B are mean ± SEM; ∗ indicates a significant difference vs. control (p < 0.05). Adapted from Ref. [103]. Panel C: Confocal microscopy images of HIV-infected cells, untreated (a; control), or treated with progesterone (b) were taken from cells infected at 1:1000 ratio, stained with the FITC-labeled KC57 anti-HIV-1 antibody and examined using a Zeiss 510 confocal microscope. For transmission electron microscopy (c, untreated infected cells; d, progesterone-treated infected cells), cells were infected at a ratio of 1:2 to visualize enough viral particles. The images in panels e, g, and f, h are successive enlargements of areas outlined in panels c and d, respectively. Bar: 10 μm (a, b); 1 μm (c, d); 200 nm (e, f). Immature (i) and mature (m) viral particles are shown in panel g.
BMP can therefore show opposite effects on viral infection. In the studies on VSV and DENV, in which BMP favoured viral infection, its cellular content was however not as high as in cells treated with CQ, which induces around 20 times higher levels than in control cells [52,73]. The different effects of BMP might rely on its concentration in late endosomes with subsequent rearrangements of membrane lipids which would modify vesicular trafficking within the cells. For instance, BMP associated with gangliosides can form pH-dependent multilamellar bodies [108] which can recall the multilamellar bodies observed in rat treated with CQ. The late endosomal pH may be the key parameter that underlies the opposite effects of BMP, which can favour virus entry at low pH on one hand because of the fusogenic property of BMP, and block virus vesicular trafficking on the other hand when a higher pH value impairs BMP fusogenic capability (Fig. 4). Whatever the contribution of BMP, all these studies highlight BMP as a key marker of viral infection via the endocytic pathway. Since treatment with CQ and other drugs has a strong impact on BMP levels, it would be worth investigating further the possible role of BMP in the antiviral activity of these drugs.
7. Possible relationship between CQ and BMP accumulation
Besides its historical use in the treatment of malaria, CQ and its analogs have demonstrated beneficial effects in many dermatological, immunological, rheumatological and infectious diseases [109]. Therefore, it is likely that more than one mechanism of action is involved in all these therapeutic effects. CQ and its analogs have been shown to act on lipid metabolism and homeostasis, with plasma lipid-lowering effects in patients with systemic lupus erythematosus, rheumatoid arthritis, dyslipidaemia and diabetes mellitus. HCQ also reduces the plasma levels of cholesterol, triglycerides and LDL and their clearance rate would be increased by the up-regulation of LDL receptors [109]. Since the cellular internalization of LDL is one of the possible pathways associated with phospholipidosis and the accumulation of MVB and BMP in cells, CQ and its analogs could thus trigger the uptake of phospholipids and BMP precursors necessary for the assembly of MVB membranes. It is possible that the various effects observed with CQ, including the increase in lysosome/late endosome pH and the accumulation of MVB and BMP are tightly linked. In response to the increase in late endosome pH and its deleterious impact on BMP-mediated membrane curvature and vesicular budding, the cell could produce more BMP to maintain vesicular trafficking. Indirectly, this would impact the virus course and retention within the cell as seen with HIV and progesterone treatment [103] (Fig. 6).
In addition, CQ was shown to reduce expression of phosphatidylinositol binding clathrin assembly protein (PICALM), a cargo-selecting clathrin adaptor that is involved in endocytosis rate regulation [110]. Depletion of PICALM was previously shown to inhibit clathrin-mediated endocytosis [110], i.e. the predominant pathway for synthetic nanoparticle internalization [111]. In these studies, CQ was shown to reduce nanoparticle uptake in macrophages by suppressing endocytosis. Based on the similarity in size and shape between SARS-CoV-2 and commonly studied synthetic nanoparticles, it has been recently proposed that one of the mechanisms responsible for chloroquine-mediated effects against SARS-CoV-2 may be a decreased cell ability to perform clathrin-mediated endocytosis of nanosized structures due to PICALM suppression [112]. The latter mechanism might enhance/potentiate the BMP-mediated mechanism that we herein propose.
8. Other drugs inducing BMP accumulation
CQ is not the only drug leading to phospholipidosis and accumulation of BMP in lysosomes and MVB. Treatments of rats with diazacholesterol [73] and 4,4′-bis(diethylaminoethoxy)α,β-diethyldiphenylethane [52] were also found to induce as much accumulation of BMP as CQ. We have shown that progesterone (Fig. 5A), the cationic sterol amphiphile U18666A and the phospholipase inhibitor MAFP increase BMP levels 2.5, 1.7 and 1.25 times, respectively, in endosomes of THP-1 monocytes [103]. The molecular basis for the accumulation of phospholipids and especially BMP in lysosomes and MVB is not fully understood. First, phospholipid and cholesterol can reach the lysosomes either by (1) the uptake of extracellular material such as lipoproteins which enter the cell by adsorptive endocytosis [113] or surface transport [114], or by (2) interaction with intracellular membranes (autophagy) [115]. Examination of liver tissue following treatment with CQ has shown evidence for increased autophagy [[116], [117], [118], [119]], and it is likely that the phospholipids that accumulate in lysosomes are mostly of intracellular origin. Secondly, phospholipid modification and degradation by lysosomal enzymes may be impaired. For instance, MAFP could induce BMP accumulation in THP-1 monocytes [103], presumably through the inhibition of lysosomal phospholipases, including pancreatic-lipase related protein 2 (PLRP2) [120], an enzyme also found in lysosomes [121], displaying phospholipase A1 activity and active in vitro on α, α′-BMP and BMP precursors like PG and bis(diacylglycero)phosphate (BDP) [88,103]. CQ might also inhibit lysosomal phospholipase A activities by its ability to raise the intralysosomal pH [43,44]. It has also been proposed that cationic amphiphilic agents bind tightly to phospholipids and form complexes which may be resistant to hydrolysis by phospholipase A [74].
9. Conclusion
We propose that a mechanism involving BMP accumulation in late endosomes and impairing vesicular trafficking of viral particles (Fig. 6) could account for CQ reported antiviral activity against SARS-COV-2 [5,15,122] and other viruses [2,[11], [12], [13]], at least in vitro. In addition, the observed effects of progesterone on BMP accumulation and reduction of HIV intercellular transmission [103], at a concentration found in human placenta (10 μM), i. e about a thousand times higher than in blood (1–50 nM) [123], might explain why SARS-COV-2 has not been observed so far in amniotic fluid (WHO sources; April 6, 2020). It also suggests that progesterone could provide to women a higher resistance to viral infections. Progesterone was previously reported to protect adult female mice from influenza A virus, but another mechanism involving the epidermal growth factor amphiregulin (AREG) was proposed [124]. Hence, the mechanism we suggest here may also be associated with the fact that women show less mortality due to COVID-19, in spite of a higher susceptibility to the infection then men [125]. Since we submitted this article, a study published in Nature reported that progesterone had some antiviral effects on SARS-CoV-2 [126], which supports our hypothesis. Moreover, it is worth mentioning that sex-dependent differences in the outcomes of mice infection by SARS-CoV-1 were also reported previously with a higher susceptibility for infection in males [127]. Furthermore, ovariectomy or treating female mice with an estrogen receptor antagonist increased mortality, indicating a protective effect for estrogen receptor signaling in mice infected with SARS-CoV-1 [127].
Our hypothesis remains speculative in the absence of experimental evidence showing that BMP is increased upon CQ treatment of cells or patients infected by SARS-COV-2, but we hope that it will trigger further studies for a better understanding of the mode of action of drug candidates to treat COVID-19 and for targeting the endocytic pathway as a therapeutic strategy to fight against viral infections by enveloped viruses [128]. To explore further the BMP track, it is worth mentioning that BMP is considered as a biomarker of drug-induced phospholipidosis [78,129], with di-docosahexanoyl BMP (di-22:6 BMP) being the molecular species with the highest increase in sera where it is released by exosomes and can be measured by mass spectrometry [130]. Similarly, di-22:6 BMP is also high in Nieman-Pick disease, the most analogous inherited lysosomal lipid storage disorder to drug induced phospholipidosis [129]. Therefore, available assays could be implemented in clinical trials.
Note from the authors: While this article was in revision, an article raising similar hypothesis on the in vitro antiviral activity of HCQ against SARS-CoV-2 was published in FASEB Journal. In that case, the authors highlighted the Niemann-Pick disease type C phenotype induced by HCQ and the accumulation of cholesterol that could contribute to changes in membrane dynamics and sequestration of viral particles in the endocytic pathway [131].
Contributors
Frédéric Carrière, Sonia Longhi and Michel Record performed literature search, analyzed articles and wrote jointly all the sections of this article.
Aknowledgements
This hypothesis paper is a contribution to the initiative of GERLI (Groupe d’Etude et de Recherche en Lipidomique, the French Lipidomics Society), the lipid division of the French Society for Biochemistry and Molecular Biology (SFBBM), for supporting research on COVID-19 that could involve lipids and lipid metabolism. We are grateful to Dr Brigitte Gontero (CNRS, UMR7281, Marseille) for her critical reading of the manuscript and to Dr Evelina Gatti (Centre d’Immunologie de Marseille-Luminy, Aix Marseille Université, CNRS, Marseille) for her comments on the manuscript and her suggestions on the immunomodulatory effect of CQ via the inhibition of endosomal Toll-like receptors.
References
- 1.Weston S., Haupt R., Logue J., Matthews K., Frieman M.B. bioRxiv; 2020. FDA Approved Drugs with Broad Anti-coronaviral Activity Inhibit SARS-CoV-2 in Vitro. 2020.2003.2025.008482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105938. 105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105932. 105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colson P., Rolain J.M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105923. 105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. ciaa237, Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maisonnasse P. Preprint under review; 2020. Hydroxychloroquine in the Treatment and Prophylaxis of SARS-CoV-2 Infection in Non-human Primates. [Google Scholar]
- 8.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 9.The multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia - expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chin. J. Tuberc. Respir. Dis. 2020;43 (Epub ahead of print) [Google Scholar]
- 10.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. medRxiv; 2020. Efficacy of Hydroxychloroquine in Patients with COVID-19: Results of a Randomized Clinical Trial. 2020.2003.2022.20040758. [Google Scholar]
- 11.Pernet O., Pohl C., Ainouze M., Kweder H., Buckland R. Nipah virus entry can occur by macropinocytosis. Virology. 2009;395:298–311. doi: 10.1016/j.virol.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Porotto M., Orefice G., Yokoyama C.C., Mungall B.A., Realubit R., Sganga M.L., Aljofan M., Whitt M., Glickman F., Moscona A. Simulating henipavirus multicycle replication in a screening assay leads to identification of a promising candidate for therapy. J. Virol. 2009;83:5148–5155. doi: 10.1128/JVI.00164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farias K.J., Machado P.R., de Almeida Junior R.F., de Aquino A.A., da Fonseca B.A. Chloroquine interferes with dengue-2 virus replication in U937 cells. Microbiol. Immunol. 2014;58:318–326. doi: 10.1111/1348-0421.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paton N.I., Lee L., Xu Y., Ooi E.E., Cheung Y.B., Archuleta S., Wong G., Wilder-Smith A. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect. Dis. 2011;11:677–683. doi: 10.1016/S1473-3099(11)70065-2. [DOI] [PubMed] [Google Scholar]
- 15.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 Mar 20:105949. doi: 10.1016/j.ijantimicag.2020.105949. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Mahevas M. MedRxiv; 2020. No Evidence of Clinical Efficacy of Hydroxychloroquine in Patients Hospitalized for COVID-19 Infection with Oxygen Requirement: Results of a Study Using Routinely Collected Data to Emulate a Target Trial. [Google Scholar]
- 17.Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J.W., Sutton S.S., Ambati J. MedRxiv; 2020. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with Covid-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 May 22 doi: 10.1016/S0140-6736(20)31180-6. S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Inglot A.D. Comparison of the antiviral activity in vitro of some non-steroidal anti-inflammatory drugs. J. Gen. Virol. 1969;4:203–214. doi: 10.1099/0022-1317-4-2-203. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu Y., Yamamoto S., Homma M., Ishida N. Effect of chloroquine on the growth of animal viruses. Arch. Gesamte Virusforsch. 1972;36:93–104. doi: 10.1007/BF01250299. [DOI] [PubMed] [Google Scholar]
- 21.Freiberg A.N., Worthy M.N., Lee B., Holbrook M.R. Combined chloroquine and ribavirin treatment does not prevent death in a hamster model of Nipah and Hendra virus infection. J. Gen. Virol. 2010;91:765–772. doi: 10.1099/vir.0.017269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsiang H., Superti F. Ammonium chloride and chloroquine inhibit rabies virus infection in neuroblastoma cells. Brief report. Arch. Virol. 1984;81:377–382. doi: 10.1007/BF01310010. [DOI] [PubMed] [Google Scholar]
- 23.Kronenberger P., Vrijsen R., Boeye A. Chloroquine induces empty capsid formation during poliovirus eclipse. J. Virol. 1991;65:7008–7011. doi: 10.1128/jvi.65.12.7008-7011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boelaert J.R., Piette J., Sperber K. The potential place of chloroquine in the treatment of HIV-1-infected patients. J. Clin. Virol. 2001;20:137–140. doi: 10.1016/s1386-6532(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsai W.P., Nara P.L., Kung H.F., Oroszlan S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res. Hum. Retrovir. 1990;6:481–489. doi: 10.1089/aid.1990.6.481. [DOI] [PubMed] [Google Scholar]
- 26.Bishop N.E. Examination of potential inhibitors of hepatitis A virus uncoating. Intervirology. 1998;41:261–271. doi: 10.1159/000024948. [DOI] [PubMed] [Google Scholar]
- 27.Mizui T., Yamashina S., Tanida I., Takei Y., Ueno T., Sakamoto N., Ikejima K., Kitamura T., Enomoto N., Sakai T., Kominami E., Watanabe S. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. J. Gastroenterol. 2010;45:195–203. doi: 10.1007/s00535-009-0132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller D.K., Lenard J. Antihistaminics, local anesthetics, and other amines as antiviral agents. Proc. Natl. Acad. Sci. U. S. A. 1981;78:3605–3609. doi: 10.1073/pnas.78.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata M., Aoki H., Tsurumi T., Sugiura Y., Nishiyama Y., Suzuki S., Maeno K. Mechanism of uncoating of influenza B virus in MDCK cells: action of chloroquine. J. Gen. Virol. 1983;64:1149–1156. doi: 10.1099/0022-1317-64-5-1149. [DOI] [PubMed] [Google Scholar]
- 30.Ooi E.E., Chew J.S., Loh J.P., Chua R.C. In vitro inhibition of human influenza A virus replication by chloroquine. Virol. J. 2006;3:39. doi: 10.1186/1743-422X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Lamballerie X., Boisson V., Reynier J.C., Enault S., Charrel R.N., Flahault A., Roques P., Le Grand R. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8:837–839. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- 32.Khan M., Santhosh S.R., Tiwari M., Lakshmana Rao P.V., Parida M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J. Med. Virol. 2010;82:817–824. doi: 10.1002/jmv.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delogu I., de Lamballerie X. Chikungunya disease and chloroquine treatment. J. Med. Virol. 2011;83:1058–1059. doi: 10.1002/jmv.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delvecchio R., Higa L.M., Pezzuto P., Valadao A.L., Garcez P.P., Monteiro F.L., Loiola E.C., Dias A.A., Silva F.J., Aliota M.T., Caine E.A., Osorio J.E., Bellio M., O’Connor D.H., Rehen S., de Aguiar R.S., Savarino A., Campanati L., Tanuri A. Chloroquine, an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses. 2016;8 doi: 10.3390/v8120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glushakova S.E., Lukashevich I.S. Early events in arenavirus replication are sensitive to lysosomotropic compounds. Arch. Virol. 1989;104:157–161. doi: 10.1007/BF01313817. [DOI] [PubMed] [Google Scholar]
- 36.Ferraris O., Moroso M., Pernet O., Emonet S., Ferrier Rembert A., Paranhos-Baccala G., Peyrefitte C.N. Evaluation of Crimean-Congo hemorrhagic fever virus in vitro inhibition by chloroquine and chlorpromazine, two FDA approved molecules. Antivir. Res. 2015;118:75–81. doi: 10.1016/j.antiviral.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowall S.D., Bosworth A., Watson R., Bewley K., Taylor I., Rayner E., Hunter L., Pearson G., Easterbrook L., Pitman J., Hewson R., Carroll M.W. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo Guinea pig model. J. Gen. Virol. 2015;96:3484–3492. doi: 10.1099/jgv.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyama A.H., Uchida T. Inhibition of multiplication of herpes simplex virus type 1 by ammonium chloride and chloroquine. Virology. 1984;138:332–335. doi: 10.1016/0042-6822(84)90356-8. [DOI] [PubMed] [Google Scholar]
- 39.Romanelli F., Smith K.M., Hoven A.D. Chloroquine and hydroxychloroquine as inhibitors of human immunodeficiency virus (HIV-1) activity. Curr. Pharmaceut. Des. 2004;10:2643–2648. doi: 10.2174/1381612043383791. [DOI] [PubMed] [Google Scholar]
- 40.Farias K.J., Machado P.R., Muniz J.A., Imbeloni A.A., da Fonseca B.A. Antiviral activity of chloroquine against dengue virus type 2 replication in Aotus monkeys. Viral Immunol. 2015;28:161–169. doi: 10.1089/vim.2014.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan Y., Zou Z., Sun Y., Li X., Xu K.F., Wei Y., Jin N., Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Homewood C.A., Warhurst D.C., Peters W., Baggaley V.C. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972;235:50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- 43.de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 44.Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. U. S. A. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naslavsky N., Caplan S. The enigmatic endosome - sorting the ins and outs of endocytic trafficking. J. Cell Sci. 2018;131 doi: 10.1242/jcs.216499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watts C. The endosome-lysosome pathway and information generation in the immune system. Biochim. Biophys. Acta. 2012;1824:14–21. doi: 10.1016/j.bbapap.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Zastrow M., Sorkin A. Signaling on the endocytic pathway. Curr. Opin. Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Touret F., Gilles M., Barral K., Nougairède A., Decroly E., de Lamballerie X., Coutard B. bioRxiv; 2020. In Vitro Screening of a FDA Approved Chemical Library Reveals Potential Inhibitors 1 of SARS-CoV-2 Replication. 2020.2004.2003.023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duvvuri M., Krise J.P. A novel assay reveals that weakly basic model compounds concentrate in lysosomes to an extent greater than pH-partitioning theory would predict. Mol. Pharm. 2005;2:440–448. doi: 10.1021/mp050043s. [DOI] [PubMed] [Google Scholar]
- 51.Kaufmann A.M., Krise J.P. Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J. Pharmacol. Sci. 2007;96:729–746. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzawa Y., Hostetler K.Y. Studies on drug-induced lipidosis: subcellular localization of phospholipid and cholesterol in the liver of rats treated with chloroquine or 4,4’-bis (diethylaminoethoxy)alpha, beta-diethyldiphenylethane. J. Lipid Res. 1980;21:202–214. [PubMed] [Google Scholar]
- 53.Van Bambeke F., Gerbaux C., Michot J.M., d’Yvoire M.B., Montenez J.P., Tulkens P.M. Lysosomal alterations induced in cultured rat fibroblasts by long-term exposure to low concentrations of azithromycin. J. Antimicrob. Chemother. 1998;42:761–767. doi: 10.1093/jac/42.6.761. [DOI] [PubMed] [Google Scholar]
- 54.Nujic K., Banjanac M., Munic V., Polancec D., Erakovic Haber V. Impairment of lysosomal functions by azithromycin and chloroquine contributes to anti-inflammatory phenotype. Cell. Immunol. 2012;279:78–86. doi: 10.1016/j.cellimm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Tyteca D., Van Der Smissen P., Mettlen M., Van Bambeke F., Tulkens P.M., Mingeot-Leclercq M.P., Courtoy P.J. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp. Cell Res. 2002;281:86–100. doi: 10.1006/excr.2002.5613. [DOI] [PubMed] [Google Scholar]
- 56.Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020 May;55(5):105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020 May 26;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bondeson J., Sundler R. Antimalarial drugs inhibit phospholipase A2 activation and induction of interleukin 1beta and tumor necrosis factor alpha in macrophages: implications for their mode of action in rheumatoid arthritis. Gen. Pharmacol. 1998;30:357–366. doi: 10.1016/s0306-3623(97)00269-3. [DOI] [PubMed] [Google Scholar]
- 60.Banjanac M., Munic Kos V., Nujic K., Vrancic M., Belamaric D., Crnkovic S., Hlevnjak M., Erakovic Haber V. Anti-inflammatory mechanism of action of azithromycin in LPS-stimulated J774A.1 cells. Pharmacol. Res. 2012;66:357–362. doi: 10.1016/j.phrs.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Loffler B.M., Bohn E., Hesse B., Kunze H. Effects of antimalarial drugs on phospholipase A and lysophospholipase activities in plasma membrane, mitochondrial, microsomal and cytosolic subcellular fractions of rat liver. Biochim. Biophys. Acta. 1985;835:448–455. doi: 10.1016/0005-2760(85)90114-6. [DOI] [PubMed] [Google Scholar]
- 62.Nosal R., Jancinova V. Cationic amphiphilic drugs and platelet phospholipase A(2) (cPLA(2)) Thromb. Res. 2002;105:339–345. doi: 10.1016/s0049-3848(02)00036-1. [DOI] [PubMed] [Google Scholar]
- 63.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.C., Perret M., Villard M., Brengel-Pesce K., Lina B., Mezidi M., Bitker L., Belot A. Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 2020 Apr 29 doi: 10.1016/j.jaci.2020.04.029. S0091-6749(20)30578-9, Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuznik A., Bencina M., Svajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 65.Combes A., Camosseto V., N’Guessan P., Arguello R.J., Mussard J., Caux C., Bendriss-Vermare N., Pierre P., Gatti E. BAD-LAMP controls TLR9 trafficking and signalling in human plasmacytoid dendritic cells. Nat. Commun. 2017;8:913. doi: 10.1038/s41467-017-00695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yasuda H., Leelahavanichkul A., Tsunoda S., Dear J.W., Takahashi Y., Ito S., Hu X., Zhou H., Doi K., Childs R., Klinman D.M., Yuen P.S., Star R.A. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008;294:F1050–F1058. doi: 10.1152/ajprenal.00461.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Neill P.M., Bray P.G., Hawley S.R., Ward S.A., Park B.K. 4-Aminoquinolines--past, present, and future: a chemical perspective. Pharmacol. Ther. 1998;77:29–58. doi: 10.1016/s0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 68.Bhattacharyya D., Sen P.C. The effect of binding of chlorpromazine and chloroquine to ion transporting ATPases. Mol. Cell. Biochem. 1999;198:179–185. doi: 10.1023/a:1006902031255. [DOI] [PubMed] [Google Scholar]
- 69.Mukherjee S., Ghosh R.N., Maxfield F.R. Endocytosis, Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 70.Wu M.M., Grabe M., Adams S., Tsien R.Y., Moore H.P., Machen T.E. Mechanisms of pH regulation in the regulated secretory pathway. J. Biol. Chem. 2001;276:33027–33035. doi: 10.1074/jbc.M103917200. [DOI] [PubMed] [Google Scholar]
- 71.Hullin-Matsuda F., Taguchi T., Greimel P., Kobayashi T. Lipid compartmentalization in the endosome system. Semin. Cell Dev. Biol. 2014;31:48–56. doi: 10.1016/j.semcdb.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Sundelin S.P., Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;110:481–489. doi: 10.1034/j.1600-0463.2002.100606.x. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto A., Adachi S., Matsuzawa Y., Kitani T., Hiraoka A., Seki K. Studies on drug-induced lipidosis: VII. Effects of bis-beta-diethyl-aminoethylether of hexestrol, chloroquine, homochlorocyclizine, prenylamine, and diazacholesterol on the lipid composition of rat liver and kidney. Lipids. 1976;11:616–622. doi: 10.1007/BF02532875. [DOI] [PubMed] [Google Scholar]
- 74.Seydel J.K., Wassermann O. NMR-studies on the molecular basis of drug-induced phospholipidosis--II. Interaction between several amphiphilic drugs and phospholipids. Biochem. Pharmacol. 1976;25:2357–2364. doi: 10.1016/0006-2952(76)90028-9. [DOI] [PubMed] [Google Scholar]
- 75.Kobayashi T., Stang E., Fang K.S., de Moerloose P., Parton R.G., Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi T., Beuchat M.H., Chevallier J., Makino A., Mayran N., Escola J.M., Lebrand C., Cosson P., Gruenberg J. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- 77.Mesens N., Desmidt M., Verheyen G.R., Starckx S., Damsch S., De Vries R., Verhemeldonck M., Van Gompel J., Lampo A., Lammens L. Phospholipidosis in rats treated with amiodarone: serum biochemistry and whole genome micro-array analysis supporting the lipid traffic jam hypothesis and the subsequent rise of the biomarker BMP. Toxicol. Pathol. 2012;40:491–503. doi: 10.1177/0192623311432290. [DOI] [PubMed] [Google Scholar]
- 78.Tengstrand E.A., Miwa G.T., Hsieh F.Y. Bis(monoacylglycerol)phosphate as a non-invasive biomarker to monitor the onset and time-course of phospholipidosis with drug-induced toxicities. Expet Opin. Drug Metabol. Toxicol. 2010;6:555–570. doi: 10.1517/17425251003601961. [DOI] [PubMed] [Google Scholar]
- 79.Liscum L. Niemann-Pick type C mutations cause lipid traffic jam. Traffic. 2000;1:218–225. doi: 10.1034/j.1600-0854.2000.010304.x. [DOI] [PubMed] [Google Scholar]
- 80.Kagebeck P., Nikiforova V., Brunken L., Easwaranathan A., Ruegg J., Cotgreave I., Munic Kos V. Lysosomotropic cationic amphiphilic drugs inhibit adipocyte differentiation in 3T3-L1K cells via accumulation in cells and phospholipid membranes, and inhibition of autophagy. Eur. J. Pharmacol. 2018;829:44–53. doi: 10.1016/j.ejphar.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Ferslew B.C., Brouwer K.L. Identification of hepatic phospholipidosis inducers in sandwich-cultured rat hepatocytes, a physiologically relevant model, reveals altered basolateral uptake and biliary excretion of anionic probe substrates. Toxicol. Sci. 2014;139:99–107. doi: 10.1093/toxsci/kfu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng N., Zhang X., Rosania G.R. Effect of phospholipidosis on the cellular pharmacokinetics of chloroquine. J. Pharmacol. Exp. Therapeut. 2011;336:661–671. doi: 10.1124/jpet.110.175679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller-Hocker J., Schmid H., Weiss M., Dendorfer U., Braun G.S. Chloroquine-induced phospholipidosis of the kidney mimicking Fabry’s disease: case report and review of the literature. Hum. Pathol. 2003;34:285–289. doi: 10.1053/hupa.2003.36. [DOI] [PubMed] [Google Scholar]
- 84.Costa R.M., Martul E.V., Reboredo J.M., Cigarran S. Curvilinear bodies in hydroxychloroquine-induced renal phospholipidosis resembling Fabry disease. Clin Kidney J. 2013;6:533–536. doi: 10.1093/ckj/sft089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bohdanowicz M., Grinstein S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 2013;93:69–106. doi: 10.1152/physrev.00002.2012. [DOI] [PubMed] [Google Scholar]
- 86.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Faure J., Blanc N.S., Matile S., Dubochet J., Sadoul R., Parton R.G., Vilbois F., Gruenberg J. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 87.Gruenberg J. Life in the lumen: the multivesicular endosome. Traffic. 2020;21:76–93. doi: 10.1111/tra.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Record M., Amara S., Subra C., Jiang G., Prestwich G.D., Ferrato F., Carrière F. Bis (monoacylglycero) phosphate interfacial properties and lipolysis by pancreatic lipase-related protein 2, an enzyme present in THP-1 human monocytes. Biochim. Biophys. Acta. 2011;1811:419–430. doi: 10.1016/j.bbalip.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 89.Marsh D. Lateral pressure in membranes. Biochim. Biophys. Acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi T., Beuchat M.H., Lindsay M., Frias S., Palmiter R.D., Sakuraba H., Parton R.G., Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 91.Thery C., Boussac M., Veron P., Ricciardi-Castagnoli P., Raposo G., Garin J., Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 92.Garin J., Diez R., Kieffer S., Dermine J.F., Duclos S., Gagnon E., Sadoul R., Rondeau C., Desjardins M. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bissig C., Lenoir M., Velluz M.C., Kufareva I., Abagyan R., Overduin M., Gruenberg J. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev. Cell. 2013;25:364–373. doi: 10.1016/j.devcel.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tycko B., Maxfield F.R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982;28:643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- 95.Daro E., Sheff D., Gomez M., Kreis T., Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J. Cell Biol. 1997;139:1747–1759. doi: 10.1083/jcb.139.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Whitney J.A., Gomez M., Sheff D., Kreis T.E., Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 97.Pattanakitsakul S.N., Poungsawai J., Kanlaya R., Sinchaikul S., Chen S.T., Thongboonkerd V. Association of Alix with late endosomal lysobisphosphatidic acid is important for dengue virus infection in human endothelial cells. J. Proteome Res. 2010;9:4640–4648. doi: 10.1021/pr100357f. [DOI] [PubMed] [Google Scholar]
- 98.Delton-Vandenbroucke I., Bouvier J., Makino A., Besson N., Pageaux J.F., Lagarde M., Kobayashi T. Anti-bis(monoacylglycero)phosphate antibody accumulates acetylated LDL-derived cholesterol in cultured macrophages. J. Lipid Res. 2007;48:543–552. doi: 10.1194/jlr.M600266-JLR200. [DOI] [PubMed] [Google Scholar]
- 99.Yuan S., Chu H., Chan J.F., Ye Z.W., Wen L., Yan B., Lai P.M., Tee K.M., Huang J., Chen D., Li C., Zhao X., Yang D., Chiu M.C., Yip C., Poon V.K., Chan C.C., Sze K.H., Zhou J., Chan I.H., Kok K.H., To K.K., Kao R.Y., Lau J.Y., Jin D.Y., Perlman S., Yuen K.Y. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019;10:120. doi: 10.1038/s41467-018-08015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaitseva E., Yang S.T., Melikov K., Pourmal S., Chernomordik L.V. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matos P.M., Marin M., Ahn B., Lam W., Santos N.C., Melikyan G.B. Anionic lipids are required for vesicular stomatitis virus G protein-mediated single particle fusion with supported lipid bilayers. J. Biol. Chem. 2013;288:12416–12425. doi: 10.1074/jbc.M113.462028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roth S.L., Whittaker G.R. Promotion of vesicular stomatitis virus fusion by the endosome-specific phospholipid bis(monoacylglycero)phosphate (BMP) FEBS Lett. 2011;585:865–869. doi: 10.1016/j.febslet.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 103.Chapuy-Regaud S., Subra C., Requena M., de Medina P., Amara S., Delton-Vandenbroucke I., Payre B., Cazabat M., Carriere F., Izopet J., Poirot M., Record M. Progesterone and a phospholipase inhibitor increase the endosomal bis(monoacylglycero)phosphate content and block HIV viral particle intercellular transmission. Biochimie. 2013;95:1677–1688. doi: 10.1016/j.biochi.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 104.Shoemaker C.J., Schornberg K.L., Delos S.E., Scully C., Pajouhesh H., Olinger G.G., Johansen L.M., White J.M. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. PloS One. 2013;8 doi: 10.1371/journal.pone.0056265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu F., Liang Q., Abi-Mosleh L., Das A., De Brabander J.K., Goldstein J.L., Brown M.S. Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife. 2015;4 doi: 10.7554/eLife.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chevallier J., Chamoun Z., Jiang G., Prestwich G., Sakai N., Matile S., Parton R.G., Gruenberg J. Lysobisphosphatidic acid controls endosomal cholesterol levels. J. Biol. Chem. 2008;283:27871–27880. doi: 10.1074/jbc.M801463200. [DOI] [PubMed] [Google Scholar]
- 107.Kühnl A., Musiol A., Heitzig N., Johnson D.E., Ehrhardt C., Grewal T., Gerke V., Ludwig S., Rescher U. Late endosomal/lysosomal cholesterol accumulation is a host cell-protective mechanism inhibiting endosomal escape of influenza A virus. mBio. 2018;9 doi: 10.1128/mBio.01345-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hayakawa T., Makino A., Murate M., Sugimoto I., Hashimoto Y., Takahashi H., Ito K., Fujisawa T., Matsuo H., Kobayashi T. pH-dependent formation of membranous cytoplasmic body-like structure of ganglioside G(M1)/bis(monoacylglycero)phosphate mixed membranes. Biophys. J. 2007;92:L13–L16. doi: 10.1529/biophysj.106.098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Al-Bari M.A. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015;70:1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller S.E., Mathiasen S., Bright N.A., Pierre F., Kelly B.T., Kladt N., Schauss A., Merrifield C.J., Stamou D., Honing S., Owen D.J. CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev. Cell. 2015;33:163–175. doi: 10.1016/j.devcel.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wolfram J., Nizzero S., Liu H., Li F., Zhang G., Li Z., Shen H., Blanco E., Ferrari M. A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci. Rep. 2017;7:13738. doi: 10.1038/s41598-017-14221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu T.Y., Frieman M., Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat. Nanotechnol. 2020;15:247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goldstein J.L., Brown M.S. The lowdensity lipoprotein pathway and its relation to atherosclerosis. Annu. Rev. Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 114.Rothblat G.H., Arbogast L.Y., Ray E.K. Stimulation of esterified cholesterol accumulation in tissue culture cells exposed to high density lipoproteins enriched in free cholesterol. J. Lipid Res. 1978;19:350–358. [PubMed] [Google Scholar]
- 115.Osman C., Voelker D.R., Langer T. Making heads or tails of phospholipids in mitochondria. J. Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seki K., Simji Y., Nishikawa M. Studies on drug-induced lipidosis 11. Light and electron microscopic observations on the liver biopsy specimens. Acta Hepatol Jap. 1971;12:226–232. [Google Scholar]
- 117.De la Iglesia F.A., Feuer G., Takada A., Matsuda Y. Morphologic studies on secondary phospholipidosis in human liver. Lab. Invest. 1974;30:539–549. [PubMed] [Google Scholar]
- 118.Abraham R., Hendy R., Grasso P. Formation of myeloid bodies in rat liver lysosomes after chloroquine administration. Exp. Mol. Pathol. 1968;9:212–229. doi: 10.1016/0014-4800(68)90037-3. [DOI] [PubMed] [Google Scholar]
- 119.Tashiro Y. An electron microscopic observation on the cytological changes in the experimental drug-induced lipidosis. Keio J. Med. 1975;24:115–143. doi: 10.2302/kjm.24.115. [DOI] [PubMed] [Google Scholar]
- 120.Amara S., Delorme V., Record M., Carriere F. Inhibition of phospholipase A1, lipase and galactolipase activities of pancreatic lipase-related protein 2 by methyl arachidonyl fluorophosphonate (MAFP) Biochim. Biophys. Acta. 2012;1821:1379–1385. doi: 10.1016/j.bbalip.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 121.Gilleron M., Lepore M., Layre E., Cala-De Paepe D., Mebarek N., Shayman J.A., Canaan S., Mori L., Carriere F., Puzo G., De Libero G. Lysosomal lipases PLRP2 and LPLA2 process mycobacterial multi-acylated lipids and generate T cell stimulatory antigens. Cell Chem Biol. 2016;23:1147–1156. doi: 10.1016/j.chembiol.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 122.Travel Med Infect Dis . 2020 May 5;101738. doi: 10.1016/j.tmaid.2020.101738. Online ahead of print. Early Treatment of COVID-19 Patients With Hydroxychloroquine and Azithromycin: A Retrospective Analysis of 1061 Cases in Marseille, France Matthieu Million 1, Jean-Christophe Lagier 1, Philippe Gautret 2, Philippe Colson 1, Pierre-Edouard Fournier 2, Sophie Amrane 1, Marie Hocquart 3, Morgane Mailhe 3, Vera Esteves-Vieira 3, Barbara Doudier 3, Camille Aubry 3, Florian Correard 4, Audrey Giraud-Gatineau 5, Yanis Roussel 1, Cyril Berenger 2, Nadim Cassir 1, Piseth Seng 1, Christine Zandotti 3, Catherine Dhiver 3, Isabelle Ravaux 3, Christelle Tomei 3, Carole Eldin 2, Hervé Tissot-Dupont 3, Stéphane Honoré 4, Andreas Stein 1, Alexis Jacquier 6, Jean-Claude Deharo 7, Eric Chabrière 1, Anthony Levasseur 1, Florence Fenollar 2, Jean-Marc Rolain 1, Yolande Obadia 3, Philippe Brouqui 1, Michel Drancourt 1, Bernard La Scola 1, Philippe Parola 2, Didier Raoult. [DOI] [PMC free article] [PubMed]
- 123.Carmina E., Lobo R.A. Chapter 32 - evaluation of hormonal status. In: Strauss J., Barbieri R., editors. Yen & Jaffe’s Reproductive Endocrinology. Elsevier Health Sciences; Amsterdam: 2009. pp. 801–823. [Google Scholar]
- 124.Hall O.J., Limjunyawong N., Vermillion M.S., Robinson D.P., Wohlgemuth N., Pekosz A., Mitzner W., Klein S.L. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wenham C., Smith J., Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Huettenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O’Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d’Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., Garcia-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang N., Shen H.M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chackalamannil S., Rotella D., Ward S. Elsevier Science; Amsterdam: 2017. Bis(monoacylglycero)phosphate as a New Biomarker of Drug-Induced Phospholipidosis, Comprehensive Medicinal Chemistry III; pp. 277–283. [Google Scholar]
- 130.Thompson K.L., Zhang J., Stewart S., Rosenzweig B.A., Shea K., Mans D., Colatsky T. Comparison of urinary and serum levels of di-22:6-bis(monoacylglycerol)phosphate as noninvasive biomarkers of phospholipidosis in rats. Toxicol. Lett. 2012;213:285–291. doi: 10.1016/j.toxlet.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 131.Ballout R.A., Sviridov D., Bukrinsky M.I., Remaley A.T. The lysosome: a potential juncture between SARS-CoV-2 infectivity and Niemann-Pick disease type C, with therapeutic implications. Faseb. J. 2020 May 5 doi: 10.1096/fj.202000654R. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Cell; 2020. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. 104742. [DOI] [PMC free article] [PubMed] [Google Scholar]