Abstract
Recently, a mini-review was published in the Medical Hypotheses journal by Usul Afsar entitled 2019-nCoV-SARS-CoV-2 (COVID-19) infection: Cruciality of Furin and relevance with cancer. Previous studies have pointed out that disruption of the proteolytic cleavage of proteins can promote infectious and non-infectious diseases. The last few weeks have been marked by an important revelation concerning the pathophysiology of SARS-CoV-2. This new coronavirus disease (COVID-19) is a highly contagious and transmissible acute respiratory infectious disorder. SARS-CoV-2 is composed of RNA-dependent RNA polymerase and structural proteins including Spike protein (S protein). Interestingly, the FURIN, one of the proproteins of the convertase family, plays a crucial role in the maturation of viral glycoproteins. In addition, many viruses including coronaviruses, exploit FURIN for the activation of their glycoproteins. Recent data indicate that SARS-CoV-2 enters human cells by binding to angiotensin-converting enzyme 2. Subsequently, the S protein is cleaved by transmembrane protease serine 2 with the help of FURIN which facilitates the entry of the virus into the cell after binding. Furthermore, it seems that FURIN is implicated in the pathogenesis of SARS-CoV-2 and potentially in the increased rates of human-to-human transmission.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, FURIN, Protease, Pathogenesis, ACE2
Dear Editor,
Recently, a mini-review was published in the Medical Hypotheses journal by Usul Afsar entitled 2019-nCoV-SARS-CoV-2 (COVID-19) infection: Cruciality of Furin and relevance with cancer [1]. Previous studies have pointed out that disruption of a simple event in proteolytic cleavage of proproteins can promote infectious and non-infectious diseases [2]. The FURIN, one of the proproteins of the convertase family, plays a crucial role in the maturation of viral glycoproteins [1], [2]. Interestingly, many viruses including coronaviruses exploit FURIN for the activation of their glycoproteins [2].
The last few weeks have been marked by an important revelation concerning the pathophysiology of SARS-CoV-2 (Severe Acute Respiratory Syndrome with Coronavirus 2). This new coronavirus disease (COVID-19) is a highly contagious and highly transmitted acute respiratory infectious disorder, which by the end of April 2020 has caused more than 200,000 deaths worldwide (according to World Health Organization data). SARS-CoV-2 is composed of RNA-dependent RNA polymerase (RdRp), structural proteins including spike protein (S protein), envelope and membrane proteins and nucleocapsid phosphoprotein, and a set of non-structural proteins (ORFs) [3].
FURIN plays a role in the cleavage of SARS-CoV-2 and its entry into the host cell. Previous studies have shown that coronaviruses use different mechanisms of cell entry in terms of membrane fusion activities [4], by binding to the angiotensin-converting enzyme 2 (ACE2), via viral glycoprotein (S protein) [5], [6]. Thus, ACE2 can be considered as a functional receptor for coronavirus to enter host cells [7]. ACE2 is a chimeric monocarboxypeptidase protein; homologous to ACE that converts angiotensin II (Ang II) into angiotensin 1–7 [8], [9]. ACE2 plays a key role in the regulation of the renin-angiotensin system (RAS), which is implicated in coronavirus-induced lung injury [10].
Recently, SARS-CoV-2 has been shown to enter human cells by binding to ACE2. Subsequently, the S protein is cleaved by transmembrane protease serine 2 (TMPRSS2) [11], with the help of FURIN which facilitates the entry of the virus into the cell after binding [12], [13]. Theoretically, other human’s proteases (cathepsin L and B, elastase, trypsin and factor X) may be involved in this process [14].
The protein cleavage between the S1 and S2 units is the most important step for virus entry into host cells, which is performed by the protease TMPRSS2. This cleavage is a crucial step because after S1 detachment, the remaining viral S2 unit undergoes a conformational reconfiguration that completes fusion between the viral and cell membrane, with subsequent entry of the virus into the cell, release of its contents, replication and infection of other cells [15]. This role is confirmed by the fact that the use of camostat mesylate, a TMPRSS2 inhibitor, partially blocks the entry of SARS-CoV-2 into host cells [11].
Interestingly, Lukassen and colleagues have shown that TMPRSS2 is highly expressed in lung tissue and sub-segmental bronchial branches [12]. In addition, their study revealed that ACE2 is mainly expressed in transient secretory cells. These cells show enrichment of pathways related to RHO GTPase function and viral processes, suggesting an increased susceptibility to SARS-CoV-2 infection [12]. The RHO GTPase family regulates many aspects of intracellular dynamics and activates viral processes regulating pathways. These processes are related to membrane remodelling and the immune system [16].
It is interesting to note that protease-dependent non-endosomal replication of the SARS coronavirus is much more frequent (up to 1000 times) than endosomal replication [17]. Therefore, according to the experimental results previously reported [12], [16], SARS-CoV-2 can also enter the cell by the non-endosomal route, either through the involvement of proteases including FURIN, or through the membrane remodelling way in the transient secretory cells, which include RHO GTPases.
FURIN is implicated in the pathogenesis of SRS-CoV-2 and in the increased rates of human-to-human transmission. The SARS-CoV-2 S protein contains four redundant FURIN cleavage sites (PRRA motif) [12]. The involvement of this protease during S protein priming could increase the entry of SARS-CoV-2 into cells. FURIN can be secreted as an active isoform [18], and therefore can act in neighbouring cells that do not express it. These arguments supported the hypothesis of a role for FURIN in increasing human-to-human transmission rates of SARS-CoV-2. FURIN may also play a role in the higher pathogenicity of SARS-CoV-2 due to increased affinity for the ACE2 receptor [12], [19], [20], [21].
These experimental studies were supported a few days ago by important data from single-cell RNA sequencing (scRNA-seq), revealing that the cells that co-express ACE2 and TMPRSS2 are type II pneumocytes (lungs), absorbent ileal enterocytes (small intestine) and nasal secretory cells (nasal cavity) [22]. Moreover, ACE2 is expressed in the heart, vessels, kidney, testis and brain. The scRNA-seq showed that les interferons (IFNs) regulate ACE2 and promote the ability of SARS-CoV-2 to maintain cellular targets in neighbouring human upper airway epithelial cells. Even more striking, the authors found that the ACE2 gene is stimulated by human INF in vitro, and they were able to extend their discovery to viral infections in vivo [22]. These data suggest that SARS-CoV-2 may exploit the positive regulation of ACE2, to enhance infection.
Consequently, this broad expression of ACE2 associated with TMPRSS2 and FURIN may explain the high pathogenic potential of SARS-CoV-2 described in some COVID-19 patients with severe multi-organ dysfunction. Obviously, the confirmation of such a hypothesis requires analyses of the level of FURIN expression in different tissues, especially those with high levels of ACE2 expression.
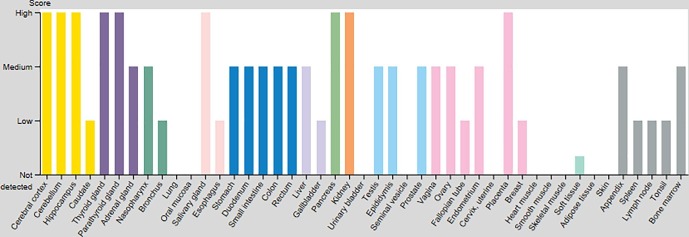
In silico analysis shows that FURIN is expressed in several cell types and in different organs. By consulting the human protein atlas site (https://www.proteinatlas.org/ENSG00000140564-FURIN/tissue), we can observe that the protein encoded by the FURIN gene is mainly localized in the Golgi apparatus and in the nucleoplasm. This protein is expressed in many human tissues, including neuroendocrine system (thyroid, parathyroid and pancreatic tissues), central nervous system (cerebral cortex and cerebellar tissues), kidneys, salivary glands and placenta (Fig. 1 ). Moderate levels of expression were found in the female and male reproductive tracts, liver, small intestine and colon. Interestingly, moderate expression was found in the nasopharynx with a slightly lower level in the branchial tissue corresponding to the first site of contact for SARS-CoV-2 infection. According to this curve (Fig. 1), no significant expression of FURIN protein was detected in the lung by antibody-antigen reaction using immunohistochemical technique. Nevertheless, by looking closely at the histological sections of tissue microarrays we could identify a weak to moderate intra-cytoplasmic stain in some pneumocytes lining the alveolar spaces as well as in some macrophages floating in the alveolar lumen (https://www.proteinatlas.org/ENSG00000140564-FURIN/tissue/lung). This expression may have been overlooked due to its weakness compared to the high protein expression found in other tissues. Nevertheless, the RNA expression of FURIN in lung specimens obtained by RNA-seq has the mean pTPM (protein-transcripts per million) value of 94.1 and 58,4 in GTEx and HPA datasets respectively (based on The Human Protein Atlas version 19.3 and Ensembl version 92.38).
Fig. 1.
Expression profile of FURIN in human tissues based on immunohistochemisty using tissue microarrays. The data represented here includes data available in the Human Protein Atlas version 19.3 (Figure adapted from the Human Protein Atlas: https://www.proteinatlas.org/ENSG00000140564-FURIN/tissue).
This in silico analysis confirms the presence of FURIN in different human tissues at varying levels, particularly in the cells lining the nasopharyngeal tract and the intestine. In addition, these data prove that the expression of FURIN is not limited to a specific tissue or cell type, as it can be expressed by tissues of different differentiation and functions (Fig. 2 ).
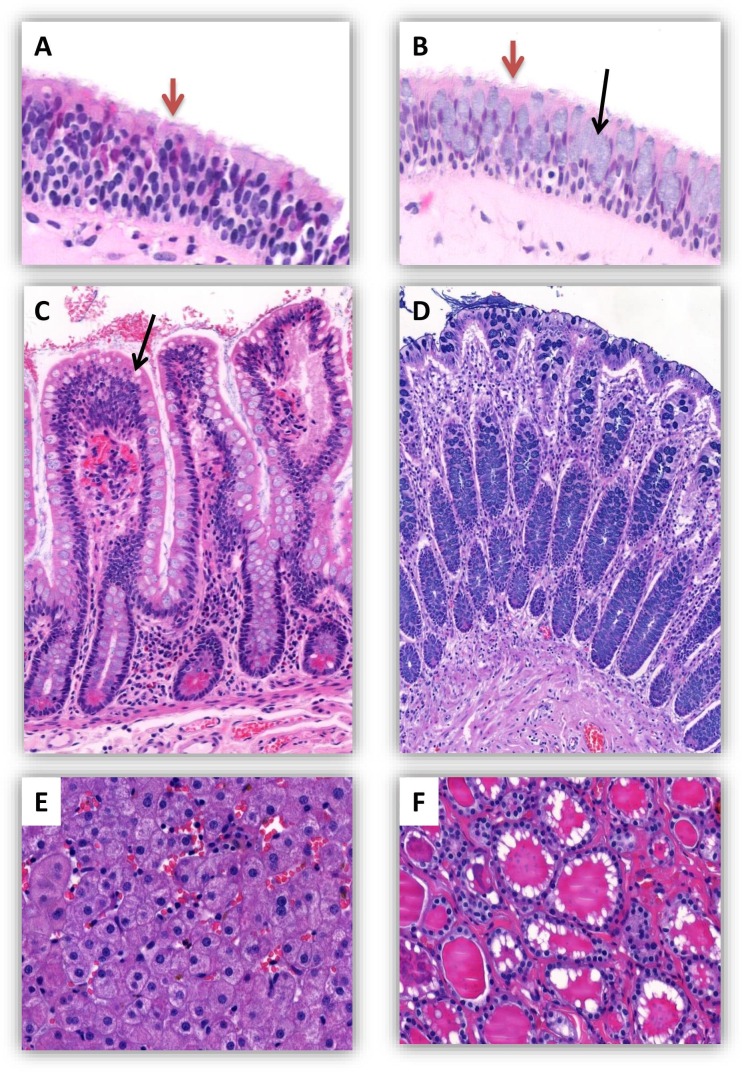
Fig. 2.
Histological sections of normal human tissues in which FURIN is expressed (Hematoxylin and Eosin stain). (A) Nasal cavity: a stratified respiratory-type epithelium with superficial cells having a ciliated border (red arrow). (B) Inferior concha: a stratified respiratory-type epithelium with superficial ciliated cells (red arrow) and mucus producing goblet cells (black arrow). (C) Ileal mucosa (small intestine): intestinal villi with goblet cells (black arrow). (D) Colon: colonic mucosa with numerous mucosecretory cells. (E) Liver: histological appearance of normal hepatocytes. (F) Thyroid: numerous thyroid follicles lined with vesicular cells, the lumen of which is filled with colloid containing thyroid hormones. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
FURIN expressed by T cells is essential for the maintenance of peripheral immune tolerance and the regulation of cell-mediated immunity.
Previous analyses have identified numerous FURIN cleavage sites in proteins including cytokines, chemokines (CXCL10) and growth factors (IGF1 and 2, PDGFα, NGF, TGFβ and VEGF-C), hormones (PTH, TRH), collagens, metalloproteases, and adhesion molecules (integrins and vitronectin) [2], [23].
In the case of SARS-CoV-2 infection, the Gene Set Enrichment Analysis (GSEA) analysis showed that the high expression of ACE2 was related to innate and adaptive immune responses, cytokine secretion, and increased inflammatory response induced by interleukins (IL-1, IL-10, IL-6 and IL-8) [21]. Moreover, characteristic clinical and immunological analyses found that highly increased levels of cytokines “cytokine storm” (IL-1β, IL-6, IL-8, IL-10 and TNFα), lymphopenia (decreased CD4 + and CD8 + T lymphocytes) and decreased IFNγ expression in CD4 + T cells are associated with severe COVID-19 [24], [25]. Thus, it can be hypothesized that the immune system dysfunction involved in high ACE2 expression is related to the symptoms of a cytokine storm.
Interestingly, the FURIN is a direct target of the Interleukin/STAT pathway, and upregulated in T helper 1 (Th1) cells. FURIN expressed by T lymphocytes is essential for host resistance against certain pathogen, and for the generation of pathogen-specific lymphocytes, including Th1 cells. Thus, FURIN is also considered to be a central regulator of cell-mediated immunity [26]. Accordingly, the GSEA analysis of SARS-CoV specimens revealed that T-cell cytokine secretion was increased and T-cell activation was stimulated [21]. Moreover, the level of ACE2 in SARS-CoV infected epithelial cells was significantly correlated with TNFα and Th1, Th2 and Th17 cells [21], [22].
In addition, FURIN is induced in activated T-cells and is believed to transform various substrates, including TGFβ1. Previous experimental study revealed that inhibition of FURIN could activate immune responses leading to a disruption of the peripheral immune tolerance. These results indicate that FURIN is essential for the maintenance of peripheral immune tolerance [27].
Furthermore, as several bacterial exotoxins are activated by FURIN-mediated cleavage [2], we can speculate that the FURIN may be involved in the bacterial coinfection in COVID-19 patients. The FURIN and ACE2 may also be indirect indicators of poor prognosis of COVID-19 severe cases via metalloproteinases mechanisms. Metalloproteinase 17 upregulated by Ang II has been shown to cleave membrane-anchored ACE2, releasing an active form of circulating ACE2 with loss of catalytic activity of the remaining part of the membrane-anchored enzyme [28]. A high level of circulating ACE2 is a marker of a disease state indicating increased RAS activity and associated with a poorer prognosis [29].
Finally, there is considerable evidence that the expression or aberrant activity of FURIN can lead to a variety of disorders such as infectious and non-infectious diseases, metabolic disorders and even cancer [2]. In addition, the data cited above have shown evidence of the involvement of FURIN in the cleavage and pathogenicity of SARS-CoV-2. Current therapeutic approaches propose targeting FURIN either at the protein level or by focusing on its RNA [30]. Although FURIN is already considered a potential therapeutic target in infectious diseases, however, the use of host protease inhibitors, particularly those directed against FURIN, as a therapeutic strategy in COVID-19 seems premature and requires further clarification of its role in the cleavage and pathogenesis of SARS-CoV-2.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
No funding to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.109893.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Afsar Usul. C2019-nCoV-SARS-CoV-2 (COVID-19) infection: cruciality of Furın and relevance wıth cancer. Med Hypotheses. 2020 Apr;22(140):109770. doi: 10.1016/j.mehy.2020.109770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun E., Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin Transl Immunol. 2019;8:e1073. doi: 10.1002/cti2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020;S1684–1182(20):30082–30087. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White J.M., Whittaker G.R. Fusion of enveloped viruses in endosomes. Traffic. 2016;17:593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons G., Zmora P., Gierer S., Heurich A., Pohlmann Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danilczyk U. Essential role for collectrin in renal amino acid transport. Nature. 2006;444:1088–1091. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- 9.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/Angiotensin 1–7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuba K. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020 Apr;4:e105114. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res 2020; 8;126(10): 10.1161/CIRCRESAHA.120.317015. doi: 10.1161/CIRCRESAHA.120.317015. Epub ahead of print. PMID: 32264791; PMCID: PMC7188049. [DOI] [PMC free article] [PubMed]
- 15.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;S0953–6205(20):30151–30155. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Broeke C., Jacob T., Favoreel H.W. Rho’ing in and out of cells: viral interactions with Rho GTPase signaling. Small GTPases. 2014;5:e28318. doi: 10.4161/sgtp.28318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuyama S., Ujike M., Morikawa S., Tashiro M., Taguchi F. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc Natl Acad Sci USA. 2005;102:12543–12547. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidricaire G., Denault J.B., Leduc R. Characterization of a secreted form of human furinendoprotease. Biochem Biophys Res Commun. 1993;195:1011–1018. doi: 10.1006/bbrc.1993.2145. [DOI] [PubMed] [Google Scholar]
- 19.Kido H., Okumura Y., Takahashi E., Pan H.Y., Wang S., Yao D. Role of host cellular proteases in the pathogenesis of influenza and influenza-induced multiple organ failure. Biochim Biophys Acta. 2012;1824:186–194. doi: 10.1016/j.bbapap.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10:e1004502. doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G., He X., Zhang L., Ran Q., Wang J., Xiong A. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J Autoimmun. 2020 Apr;13:102463. doi: 10.1016/j.jaut.2020.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N. HCA lung biological network, SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020 doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian S., Huang Q., Fang Y. FurinDB: a database of 20-residue furin cleavage site motifs, substrates and their associated drugs. Int J Mol Sci. 2011;12:1060–1065. doi: 10.3390/ijms12021060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oksanen A., Aittomäki S., Jankovic D. Proprotein convertase FURIN constrains Th2 differentiation and is critical for host resistance against Toxoplasma gondii. J Immunol. 2014;193(11):5470–5479. doi: 10.4049/jimmunol.140162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pesu M., Watford W., Wei L. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–250. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J., Sriramula S., Xia H., Moreno-Walton L., Culicchia F., Domenig O. Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ Res. 2017;121:43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bitker L., Burrell L.M. Classic and nonclassic renin-angiotensin systems in the critically Ill. Crit Care Clin. 2019;35:213–227. doi: 10.1016/j.ccc.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiryaev S.A. Targeting host cell furin proprotein convertases as a therapeutic strategy against bacterial toxins and viral pathogens. J Biol Chem. 2007;282:20847–20853. doi: 10.1074/jbc.M703847200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.