Significance
The origin of modern amphibians remains controversial, and especially the fossil record of salamanders remains poor. Their tiny, feeble skeletons are rarely preserved in rocks of the early Mesozoic era, the time frame in which they are believed to have originated. Here we report 230 million-year-old fossils from Kyrgyzstan, Inner Asia, providing the most ancient evidence of salamanders. They enable us to reconstruct crucial steps in the evolution of the salamander body plan, sharing numerous features with ancient amphibians, the temnospondyls. These finds push back the rock record of salamanders by 60 to 74 Ma and at the same time bridge the wide anatomic gap among salamanders, frogs, and temnospondyls.
Keywords: evolution, phylogeny, amphibians
Abstract
The origin of extant amphibians remains largely obscure, with only a few early Mesozoic stem taxa known, as opposed to a much better fossil record from the mid-Jurassic on. In recent time, anurans have been traced back to Early Triassic forms and caecilians have been traced back to the Late Jurassic Eocaecilia, both of which exemplify the stepwise acquisition of apomorphies. Yet the most ancient stem-salamanders, known from mid-Jurassic rocks, shed little light on the origin of the clade. The gap between salamanders and other lissamphibians, as well as Paleozoic tetrapods, remains considerable. Here we report a new specimen of Triassurus sixtelae, a hitherto enigmatic tetrapod from the Middle/Late Triassic of Kyrgyzstan, which we identify as the geologically oldest stem-group salamander. This sheds light not only on the early evolution of the salamander body plan, but also on the origin of the group as a whole. The new, second specimen is derived from the same beds as the holotype, the Madygen Formation of southwestern Kyrgyzstan. It reveals a range of salamander characters in this taxon, pushing back the rock record of urodeles by at least 60 to 74 Ma (Carnian–Bathonian). In addition, this stem-salamander shares plesiomorphic characters with temnospondyls, especially branchiosaurids and amphibamiforms.
The problem of modern amphibian origin(s) remains controversial and hotly debated (1–3). In the last 50 y, only few significant fossils have been reported that helped reduce the morphological gap between Paleozoic tetrapods and Mesozoic lissamphibians. For instance, the Early Permian Doleserpeton revealed pedicellate teeth and cylindrical vertebrae (4), whereas coeval Gerobatrachus had a shortened trunk, rearranged carpus, and skull with batrachian characters (5). While these Permian taxa narrow the gap from a bottom-up perspective, discoveries in the Early Triassic of Poland (Czatkobatrachus; ref. 6) and reexamination of Early Triassic Triadobatrachus (7) from Madagascar have provided insight into the early evolution of stem-anurans (salientians). These represent top-down approaches toward the batrachian ground pattern from the salientian branch. However, to more profoundly understand the batrachian diversification, as well as dispersal, the fossil record of stem-salamanders (urodeles) clearly requires more fossils, especially from rocks older than the Middle Jurassic.
The findings that we report here fill this gap by adding a very ancient and basal stem-salamander from the Triassic of Madygen in Kyrgyzstan, Inner Asia. The taxon was described and named by Ivakhnenko (8), who considered it a urodele, as Triassurus sixtelae. However, that referral was not given much attention, and the urodele affinities were either doubted (9) or considered inconclusive (10) because of the small size, immature nature, and relatively poor preservation of the specimen. Instead, the oldest undisputed stem-salamanders were the mid-Jurassic genera Kokartus (Kyrgyzstan) (11) and Marmorerpeton (England) (12), the most completely known taxon forming late Jurassic Karaurus from the Karatau lake deposit of Kazakhstan (8). Rich finds of early stem-salamanders (13) and crown taxa (caudates) (14, 15) have recently been reported from the Middle Jurassic to Early Cretaceous in northwestern China, providing much data on the early evolution and diversification of the clade.
Recently, a German team excavating in the Kyrgyz Madygen Formation (16) recovered a second find of Triassurus that is not only larger and better preserved, but also adds significantly more data on this taxon. Reexamination of the type has revealed shared apomorphic features between the two Madygen specimens, some of which turned out to be stem-salamander (urodele) autapomorphies. The present findings demonstrate not only that Triassurus is a valid tetrapod taxon, but also, and more importantly, that it forms a very basal stem-salamander, combining plesiomorphic temnospondyl features with salamander apomorphies. Moreover, these findings add to our understanding of the sequence by which apomorphic characters were acquired in the salamander stem lineage.
Systematic Paleontology
Tetrapoda Haworth, 1825; Lissamphibia Haeckel, 1866; Urodela Dumeril, 1806; Triassurus Ivakhnenko, 1978; Triassurus sixtelae Ivakhnenko, 1978.
Holotype.
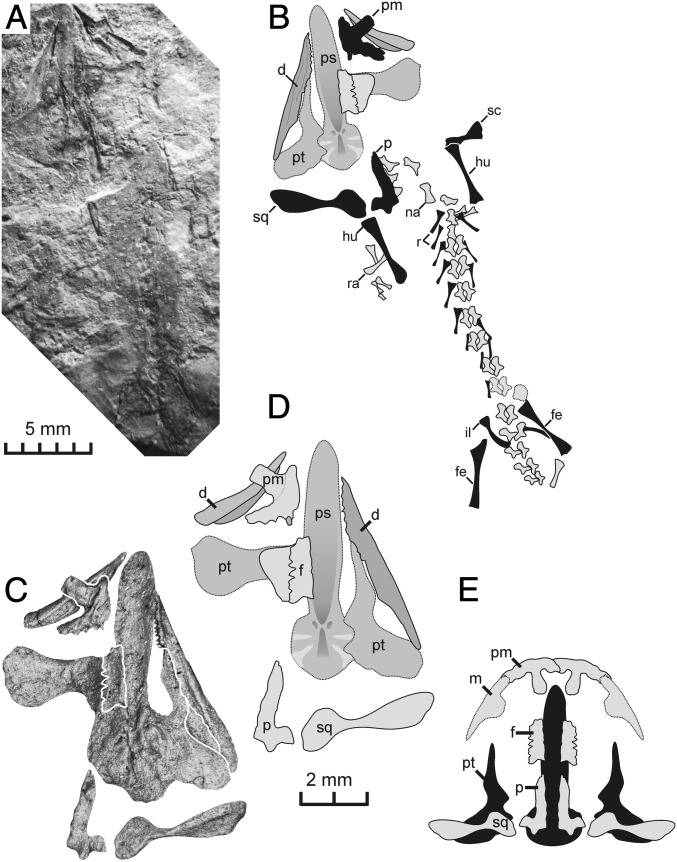
PIN-2584/10, a fairly complete skeleton of very small immature specimen (skull length 3.8 mm) mostly in ventral view, with some elements preserved as imprints, but most as bone (collected by T. A. Sixtel in 1961) (Fig. 1).
Fig. 1.
T. sixtelae type specimen (PIN-2584/10). (A) Close-up of skull (B and C) Complete skeleton. Salamander apomorphies are in black. b.d, branchial denticles; d, dentary; e, ectopterygoid; f, frontal; fe, femur; fi, fibula; hy, hypobranchial; hu, humerus; il, ilium; m, maxilla; na, neural arch; pl, palatine; p, parietal; pm, premaxilla; ps, parasphenoid; pt, pterygoid; r, rib; ra, radius; sc, scapula; sq, squamosal; sr, sacral rib; ti, tibia; ul, ulna. PIN, Paleontological Institute of the Russian Academy of Sciences Moscow.
Type Locality.
Madygen, Turkestan Range, southwestern Kyrgyzstan.
Type Horizon.
Madygen Formation, Ladinian/Carnian (Middle/Late Triassic).
Referred Specimen.
FG 596/V/20, a skeleton including the skull and postcranium up to the pelvis (skull length 11 mm, Fig. 2), preserved mostly as natural mold, from Urochishche Madygen, also from the Madygen Formation, collected by Khairill Sakhidov, a local girl.
Fig. 2.
T. sixtelae new, larger specimen (FG 596/V/20). (A and B) Entire skeleton, preserved as natural mold. (C and D) Mirror-imaged close-up of skull as derived from casting natural mold. (E) Restoration of skull (dorsal view). Salamander apomorphies are in black. FG, Technische Universität Bergakademie Freiberg.
Maturity of Specimens.
While the holotype was evidently a tiny larva, it shares well-established apomorphies with the larger specimen, by which it can be distinguished from all other urodeles (see below). FG 596/V/20 shows numerous additional features because of a more complete ossification and differentiation of skull bones. It lacks larval traits, such as branchial denticles and hyobranchial elements, and also lacks fully adult features, such as vertebral centra and an ossified carpus and tarsus, and thus likely forms a large larva close to metamorphosis or paedomorph. Thus, all morphological differences between the two specimens are consistent with the hypothesis of ontogenetic changes.
Diagnosis.
Premaxilla with straight anterior margin; medial five premaxilla teeth enlarged; maxilla short with hardly offset alary process and much smaller teeth than premaxilla; L-shaped parietal with rounded posterior buttress; squamosal proportionately large but slender with a rounded medial portion and asymmetrically expanded lateral end; parasphenoid with a relatively slender knife-shaped cultriform process and rounded anterior end; one pair of elongated hyobranchial ossifications; 16 presacrals; straight scapula with expanded ends; long and gently curved ilium; only neural arches ossified; and elongated humerus (40% of skull length).
Locality and Paleoenvironment.
The skeletons of Triassurus were found in an epicontinental setting, in which lake sediments alternate with those of a densely vegetated floodplain (16). The skeletons were embedded in fine silty mudstones occurring at the base of a transgressional sequence. Triassurus is likely to have inhabited a shallow lake, as both specimens were found in the same lacustrine facies (16).
T. sixtelae is evidently a stem-salamander based on four apomorphic characters: parasphenoid shape and dorsal surface, with a V-shaped anterior depression, an unpaired posteromedial crest, and a radial arrangement of furrows; parietal not plate-like and rectangular but L-shaped; squamosal forming a straight transverse strut with slightly expanded lateral end and well-expanded medial end, without squamosal embayment; and straight scapula with expanded ends. Three further characters are apomorphic for lissamphibians: very elongated and curved ilium; extremely short and thin trunk ribs, distally unexpanded but with greatly expanded proximal ends; and long, delicate limb bones.
Despite a general resemblance, T. sixtelae is clearly not a branchiosaurid temnospondyl. The limb elements are longer and more slender even at very small growth stages. At comparable stages, especially of the holotype of T. sixtelae, branchiosaurids have short and undifferentiated humeri and femora, as well as much wider trunk ribs (17, 18). The outline of the squamosal is unlike that in any temnospondyl, with a straight distal strut-like portion and an offset medial rounded portion. The lateral rather than posterior orientation of the squamosal correlates with the anterior position of the jaw articulation, which is of a urodele character. Finally, the presacral count is markedly lower than that of branchiosaurids with 19 to 22 (19), amphibamids with 18 to 24 (19), or even the stem-batrachian Gerobatrachus with as few as 17 (5).
In combination, these features indicate a phylogenetic position of T. sixtelae above the level of Amphibamidae and Branchiosauridae, within Batrachia, and on the stem of Caudata, which is corroborated by the phylogenetic analysis. To our knowledge, there is no conflicting evidence suggesting a different position.
Description and Comparison
The premaxillae are clearly paired and have tall and slender alary processes similar to those of caudates (20) and the enigmatic dissorophoid Tungussogyrinus (21). In the type, it bears eight or nine teeth, with the medial five teeth enlarged. These teeth are conical and slender, with a single cusp, and not pedicellate; in the larger specimen, only one partial tooth and several sockets are preserved on the premaxilla. In the larger specimen, the element is ornamented with pits and short grooves and massive, consistent with Karaurus. The maxilla is short with a marked alary process. Teeth are exposed only in the type specimen, in which they are much smaller than in the premaxilla. The mandible has a short tooth row, consistent with the length of the maxilla.
In the skull roof, paired frontals and parietals are present, forming slender bars in the holotype and more broadened elements in the larger specimen; they fail to meet in the midline in both stages. The frontal has a serrated lateral margin and an ornamented dorsal surface in FG 596/V/20. The parietal is L-shaped as in Karaurus (8), Kokartus (11), and Chunerpeton (14) and bears a rounded posterior buttress at about the midlevel of its occipital margin. The squamosal is proportionately very large, indicating a broad cheek and overall wide skull. It is elongate, consistent in both specimens in its strut-like shape and the broadened medial portion, consistent with Kokartus and Karaurus (8, 11). The long axis of the bone is straight, reaching one-third the length of the parasphenoid in the type but one-half that length in the larger specimen. This indicates that the skull became wider during ontogeny, a feature found in many caudates (20).
The palate is dominated by a huge parasphenoid that is more slender than that in all urodeles, most closely resembling the outline in very small larval branchiosaurids (17, 18). The anterior end is convex, in contrast to the concave shape in other urodeles. The dorsal surface of the cultriform process bears a deep V-shaped depression that is not found in branchiosaurids but is known from Mesozoic urodeles (11) and cryptobranchids (22). Together with the medial sagittal crest at the base of the basal plate, this forms a distinctive urodele feature of Triassurus. A small strut-like palatine is exposed in the type specimen, but its proper outline and the existence of teeth cannot be ascertained. A further, tiny element is present posterior to the palatine but cannot be unequivocally identified as ectopterygoid. The pterygoid is short and semilunar in the type, reaching only approximately one-half the length of the parasphenoid, again without evidence of teeth or denticles. In FG 596/V/20, the pterygoid measures two-thirds of the parasphenoid, documenting proportional changes consistent with late larvae in caudates (23). The quadrate ramus of the pterygoid is short but very wide, as in Kokartus (9); the basipterygoid ramus is poorly differentiated; and the palatine branch is elongate and tapering. The mandible is poorly preserved, and only the dentary is exposed, which is delicate and smooth.
Two hyobranchial elements are preserved in the holotype, both of which are slender and straight with poorly expanded ends. These might represent a pair of hypobranchials and are absent in the larger specimen. Likewise, conical branchial denticles are present only in the tiny type specimen, where they are exposed on the left side in somewhat disarticulated fashion. These are consistent with those in some Mesozoic caudates, Chunerpeton (14) and Beiyanerpeton (15), as well as those of branchiosaurids, in which they are much larger (24). In the larger specimen, branchial denticles are absent, a common feature of larger neotenic branchiosaurids and in temnospondyls that are close to metamorphosis (17, 19).
The axial skeleton is weakly ossified in both specimens. The neural arches are unequivocally preserved, resembling those of branchiosaurid larvae as well as early larval stages of hynobiids, the only caudates in which neural arches ossify before the centra. Ossified centra are absent in both specimens, but the neural arches are more well differentiated with well-established zygapophyses in the larger specimen. The ribs are short and straight, with a very thin shaft, unexpanded distal ends, and much broadened, bicapitate proximal ends. Despite the wide size range, the ribs are very similar in both specimens. In the type, ribs 2 to 11 are present, and the large specimen has a full complement of presacral ribs. The sacral rib is well preserved in the type, more robust and curved than the anterior trunk ribs, and overlies the neural arch of the 17th vertebra.
The scapula is straight and slender, reaching approximately two-thirds the length of the humerus and having a well-expanded dorsal end. It differs markedly from the semilunar element of temnospondyls, irrespective of ontogenetic age (17). In FG 596/V/20, the scapula is more robust with broadened ends, especially dorsally. The humerus is elongate with a slender shaft and weakly expanded ends, reaching approximately 40% of the skull length. The radius and ulna are minute in the type but well expanded in the large specimen, having only half the humeral length. The manus has elongate metacarpals but lacks an ossified carpus.
The ilium is gracile and curved, with an extensive dorsal process, exceeding the length of all dissorophoid ilia. The femur is no longer than the humerus but is twice the length of the tibia, which in turn is longer and more massive than the fibula. The foot is preserved in the type but is incomplete, and the tarsus is unossified. Consistent with batrachians, T. sixtelae lacks bony scales.
Results and Discussion
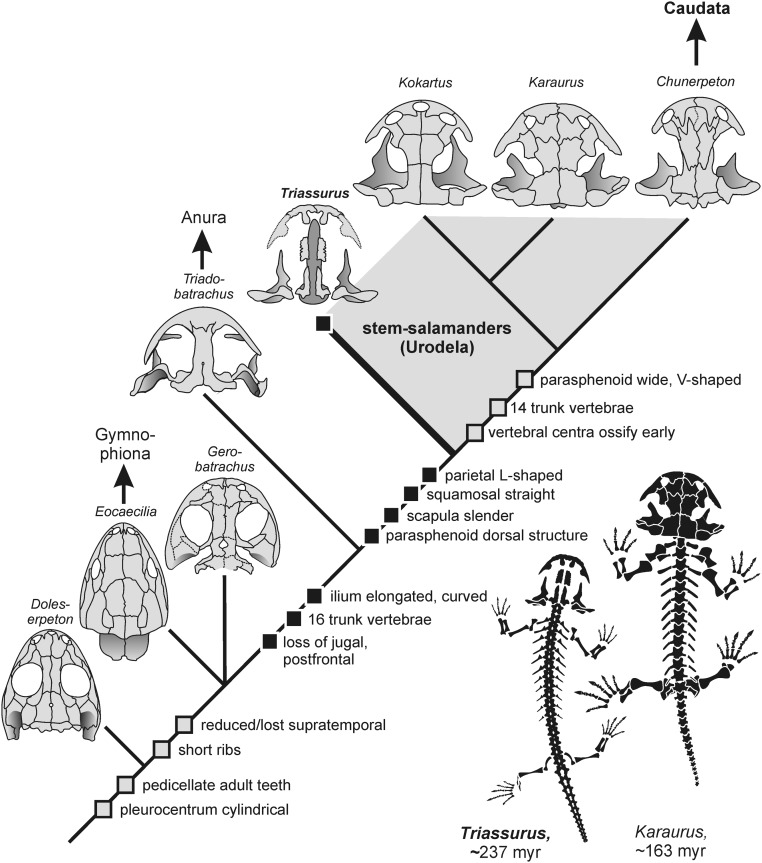
The position of Triassurus was tested in a phylogenetic analysis that contained microsaurs, lysorophians, temnospondyls, and extant and fossil lissamphibians, including the extinct albanerpetontids. Within this framework, extant amphibians were found to be monophyletic, nesting within amphibamiform temnospondyls, with Amphibamus, Doleserpeton, and Gerobatrachus forming successive, ever more closely related sister groups of Lissamphibia. Although our analysis unequivocally found Gerobatrachus below Lissamphibia, we consider the batrachian hypothesis (5) as a plausible alternative, based on several key features shared with batrachians (Fig. 3).
Fig. 3.
Evolutionary history of lissamphibians, with Triassurus at the base of the salamander stem lineage. Squares denote important synapomorphies, with all those shared by Triassurus mapped in black. The positions of Gerobatrachus and Eocaecilia are considered equivocal, due to substantial conflicting evidence.
Our analyses invariably found Triassurus to nest well above Branchiosauridae and other amphibamiforms, within Batrachia, and forming an unresolved polytomy with urodeles (Kokartus and Karaurus) and extant caudates (Hynobius, Cryptobranchus, and Ambystoma). This result was not affected by the omission of selected taxa.
The morphology of Triassurus is consistent with the temnospondyl hypothesis on the origin of Batrachia (1, 4, 5). First, the number of trunk vertebrae was reduced in batrachians relative to outgroups such as Amphibamus and Doleserpeton (each with 24 presacrals), Apateon (19 to 22 presacrals), and the albanerpetontid Celtedens (22 presacrals). In Eocaecilia and caecilians, the higher presacral count is likely derived, whereas the stem-batrachian Gerobatrachus had a reduced count of 17 (5). Second, the loss of dermal skull bones (postfrontal, postorbital, postparietal, and tabular) arguably occurred in parallel in caecilians and batrachians, as indicated by the plesiomorphic retention of most of these elements in Eocaecilia (25). Irrespective of the exact relationship among Gerobatrachus, Eocaecilia, and Batrachia, Triassurus is consistent with the basal salientian Triadobatrachus in the lack of all these bones. Gerobatrachus retained these elements but already had an emargination in the supratemporal region, probably for the attachment of adductor musculature (5). The possession of nonpedicellate teeth by Triassurus is consistent with its larval morphology, as urodele larvae usually have peg-like or slender conical rather than pedicellate teeth (26), and is consistent with albanerpetontids and some urodeles that also lack pedicely (27).
Triassurus in particular sheds light on the character evolution in the salamander stem lineage. After the loss of dermal bones in the cheek and skull table, the squamosal was rearranged in the skull, which produced a shorter gape; furthermore, it lacks an otic notch and also has a characteristic offset medial portion. The parietal assumed its L-shaped morphology, likely permitting the further dorsomedial attachment of adductor musculature (28). The trunk was shortened in a stepwise fashion from the batrachian ground pattern to the crown caudate condition (17 > 16 > 14). The parasphenoid evolved its characteristic dorsal morphology before it became the much wider element of later urodeles. The scapula attained its straight symmetrical shape, and the neural arches ossified before the centra, a feature shared with anurans and temnospondyls that has already been suggested as the primitive condition for caudates (29).
Triassurus also retained plesiomorphic temnospondyl features: the short maxilla, branchial denticles, shape of the parasphenoid, and the absence of ossified centra are shared especially with small branchiosaurid larvae, lending further support to the hypothesis that paedomorphosis is a common pattern not only in the evolution of salamanders (30), but also in their origin (31). However, when comparing Triassurus and branchiosaurids, it also must be considered that larvae of other amphibamiforms are largely unknown and probably were similar to branchiosaurids.
Finally, the identification of Triassurus as a urodele also raises the issue of paleobiogeography and vicariance. The most ancient stem-salamanders have all been reported from Eurasia: Triassurus and Kokartus from Kyrgyzstan, Karaurus from Kazakhstan, Urupia from Siberia (32), and Marmorerpeton plus an unnamed taxon from England. The first evidence from outside Eurasia is the substantially younger (155 Ma) caudate Iridotrition from Utah (33). The Yehol deposits of Liaoning have yielded a range of early caudates (Beiyanerpeton, Chunerpeton, and Liaoxitriton), which document a fast radiation of the crown clade during the Jurassic (14, 15). Considering the complicated, largely insular-marine paleogeography of Europe during the Jurassic and Cretaceous, the land bridges required for the dispersal of urodeles might have existed only during the latest Triassic time.
The identification of Triassurus as a urodele predates the latest time window for the dispersal of stem-salamanders before the marine ingression into Europe and currently indicates Inner Asia as the place of origin of the salamander lineage, which is consistent with earlier vicariance hypotheses (34). Batrachian stem taxa are known from North America and Europe, whereas salientians evidently dispersed rapidly to Europe (6), North America (35), and southern Gondwana (Madagascar). Thus, batrachians are likely to have originated in the Early Permian somewhere in the Variscian mountain belt, with frog ancestors extending southward into Gondwana and salamander ancestors eastward into Inner Asia.
Materials and Methods
The dataset was analyzed with the software package TNT 1.0 (36). Both Traditional Search and New Technology Search options were used, and both nonadditive and additive character sets were calculated. A total of 62 taxa were analyzed, including 57 of the taxa considered by Pardo et al. (3) plus the lepospondyls Rhynchonkos stovalli, Batropetes fritschi, and Brachydectes elongatus; the albanerpetontid Celtedens ibericus; and T. sixtelae. As in the original matrix (37), phylogenetic polarity was assessed using two outgroups, the embolomere Proterogyrinus and the colosteid Greererpeton. The dataset comprised 360 characters, including 345 stemming from Pardo et al. (3) and 15 additional characters defined here (SI Appendix). Cladistic analysis (both Traditional Search and New Technology Search options performed with TNT 1.0) gave two alternative most parsimonious trees, which required 1,392 steps (CI = 0.3; RI = 0.697).
The topology of the obtained trees contains a basal dichotomy between the amniote stem (represented by three lepospondyls) and temnospondyls plus Lissamphibia. Within Lissamphibia, Apoda form the basalmost taxon, followed by Celtedens and finally Batrachia (Salientia and Urodela). As for Urodela, Triassurus is found to form an unresolved trichotomy with a clade containing Kokartus and Karaurus and the crown-group salamanders (Caudata).
Data Availability.
All data used in this paper are available in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dieter Seegis, Ronald Böttcher, Erin Maxwell, and Ilya Kogan for helpful discussions and David Wake, Nadia Fröbisch, an anonymous reviewer, and the handling editor for suggestions that helped improve the manuscript. The field work was supported by the German Research Foundation (DFG grant VO 1466-1/1).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001424117/-/DCSupplemental.
References
- 1.Anderson J. S., Focal review: The origin(s) of modern amphibians. Evol. Biol. 35, 231–247 (2008). [Google Scholar]
- 2.Marjanovic D., Laurin M., The origin(s) of extant amphibians: A review with emphasis on the “lepospondyl hypothesis”. Geodiversitas 35, 207–272 (2013). [Google Scholar]
- 3.Pardo J. D., Small B. J., Huttenlocker A. K., Stem caecilian from the Triassic of Colorado sheds light on the origins of Lissamphibia. Proc. Natl. Acad. Sci. U.S.A. 114, E5389–E5395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolt J. R., Lissamphibian origins: Possible protolissamphibian from the lower permian of Oklahoma. Science 166, 888–891 (1969). [DOI] [PubMed] [Google Scholar]
- 5.Anderson J. S., Reisz R. R., Scott D., Fröbisch N. B., Sumida S. S., A stem batrachian from the Early Permian of Texas and the origin of frogs and salamanders. Nature 453, 515–518 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Evans S. E., Borsuk-Białynicka M., A stem-group frog from the Early Triassic of Poland. Acta Palaeontol. Pol. 43, 573–580 (1998). [Google Scholar]
- 7.Ascarrunz E., Rage J. C., Legreneur P., Laurin M., Triadobatrachus massinoti, the earliest known lissamphibian (Vertebrata: Tetrapoda) re-examined by µCT-scan, and the evolution of trunk length in batrachians. Contrib. Zool. 58, 201–234 (2016). [Google Scholar]
- 8.Ivakhnenko M., Urodeles from the Triassic and Jurassic of Soviet inner Asia. Paleontol. J. 12, 362–368 (1978). [Google Scholar]
- 9.Estes R., “Gymnophiona, Caudata” in Encyclopedia of Paleoherpetology 2, Wellnhofer P., Ed. (Gustav Fischer, 1981), pp. 1–115. [Google Scholar]
- 10.Milner A. R., “Mesozoic and tertiary Caudata and Albanerpetontidae” in Amphibian Biology 4, Heatwole H., Carroll R. L., Eds. (Surrey Beatty & Sons, 2000), pp. 1412–1444. [Google Scholar]
- 11.Skutschas P., Martin T., Cranial anatomy of the stem-salamander Kokartus honorarius (Amphibia: Caudata) from the Middle Jurassic of Kyrgyzstan. Zool. J. Linn. Soc. 161, 816–838 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans S. E., Milner A. R., Mussett F., The earliest known salamanders (Amphibia, Caudata): A record from the Middle Jurassic of England. Geobios 21, 539–552 (1988). [Google Scholar]
- 13.Gao K. Q., Shubin N. H., Late Jurassic salamanders from northern China. Nature 410, 574–577 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Gao K. Q., Shubin N. H., Earliest known crown-group salamanders. Nature 422, 424–428 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Gao K. Q., Shubin N. H., Late Jurassic salamandroid from western Liaoning, China. Proc. Natl. Acad. Sci. U.S.A. 109, 5767–5772 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voigt S., et al. , Terrestrial Conservation Lagerstätten, Fraser N., Sues H. D., Eds. (Dunedin Academic Press, 2017), pp. 65–104. [Google Scholar]
- 17.Schoch R. R., Comparative ontogeny of early Permian branchiosaurid amphibians from southwestern Germany. Developmental stages. Palaeontographica Abt. A 222, 43–83 (1992). [Google Scholar]
- 18.Werneburg R., Apateon dracyiensis—Eine frühe Pionierform der Branchiosaurier aus dem Europäischen Rotliegend, Teil 1: Morphologie. Veröff. Naturhist. Mus. Schleusingen. 16, 17–36 (2001). [Google Scholar]
- 19.Schoch R. R., Milner A. R., Temnospondyli I, Encyclopedia of Paleoherpetology, Sues H. D., Ed. (Verlag Friedrich Pfeil, 2014), pp. 1–150. [Google Scholar]
- 20.Larsen J. H., “The cranial osteology of neotenic and transformed salamanders and its bearing on intrafamilial relationships”, PhD thesis, University of Washington, Seattle, 205 (1963).
- 21.Werneburg R., The Permotriassic branchiosaurid Tungussogyrinus Efremov, 1939 (Temnospondyli, Dissorophoidea) from Siberia restudied. Foss. Rec. 12, 105–120 (2009). [Google Scholar]
- 22.Osawa G., Beiträge zur Anatomie des japanischen Riesensalamanders (Mitt med Fac Kais Japan University Tokio, 1901), vol. 5, pp. 1–205. [Google Scholar]
- 23.Lebedkina N. S., The Evolution of the Amphibian Skull (Nauka, Moscow, 1979). [Google Scholar]
- 24.Boy J. A., Die Branchiosaurier (Amphibia) des saarpfälzischen Rotliegenden (Perm, SW-Deutschland) (Hessisches Landesamt für Bodenforschung, 1972), vol. 65, pp. 1–137. [Google Scholar]
- 25.Jenkins F. A., Walsh D. M., Carroll R. L., Anatomy of Eocaecilia micropodia, a limbed caecilian of the early Jurassic. Bull. Mus. Comp. Zool. 158, 285–366 (2007). [Google Scholar]
- 26.Berkovitz B., Shellis P., The Teeth of Non-Mammalian Vertebrates (Academic Press, 2017). [Google Scholar]
- 27.Gardner J. D., Böhme M., Vertebrate Microfossil Assemblages: Their Role in Paleoecology and Paleobiogeography, Sankey J. T., Baszio S., Eds. (Indiana University Press, 2008), pp. 178–218. [Google Scholar]
- 28.Schoch R. R., Amphibian skull evolution: The developmental and functional context of simplification, bone loss and heterotopy. J. Exp. Zoolog. B Mol. Dev. Evol. 322, 619–630 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Boisvert C. A., Vertebral development of modern salamanders provides insights into a unique event of their evolutionary history. J. Exp. Zoolog. B Mol. Dev. Evol. 312, 1–29 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Wake D. B., What salamanders have taught us about evolution. Annu. Rev. Ecol. Evol. Syst. 40, 333–352 (2009). [Google Scholar]
- 31.Milner A. R., The Phylogeny and Classification of the Tetrapods, Volume 1: Amphibians, Reptiles, Birds, Benton M. J., Ed. (Clarendon Press, 1988), pp 59–102. [Google Scholar]
- 32.Skutchas P. P., Krasnolutskii S. A., A new genus and species of basal salamanders from the Middle Jurassic of Western Siberia, Russia. Proc. Zool. Inst. RAS 315, 167–175 (2011). [Google Scholar]
- 33.Evans S. E., Lally C., Chure D. C., Maisano J. A., A late Jurassic salamander (Amphibia: Caudata) from the Morrison Formation of North America. Zool. J. Linn. Soc. 143, 599–616 (2005). [Google Scholar]
- 34.Milner A. R., The biogeography of salamanders in the Mesozoic and early Caenozoic: A cladistic-vicariance model (Urodela). Syst. Assoc. Spec. Vol. 23, 431–468 (1983). [Google Scholar]
- 35.Stocker M. R., et al. , The earliest equatorial record of frogs from the Late Triassic of Arizona. Biol. Lett. 15, 20180922 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goloboff P. A., Farris J. S., Nixon K. C., TNT, a free program for phylogenetic analysis. Cladistics 24, 1–13 (2008). [Google Scholar]
- 37.Schoch R. R., The major clades of temnospondyls: An inclusive phylogenetic analysis. J. Syst. Palaeontology 11, 673–705 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this paper are available in SI Appendix.