Significance
Recent studies have implicated LTB4 in inflammation in adipose tissue and in the subsequent development of insulin resistance. The mechanism by which LTB4 is produced in adipose tissue has remained unknown, however. We now reveal that insulin regulates LTB4 production in adipocytes via a PDK1–FoxO1–5-LO pathway and thereby maintains systemic insulin sensitivity. Whereas signaling pathways of insulin and LTB4 have been extensively investigated, a cross-talk between these two signaling pathways has not been reported. We also reveal that LTB4 exists in plasma at concentrations sufficient to activate its receptor BLT1 and that the plasma concentration of LTB4 correlated with insulin resistance in human, implying that LTB4 is a mediator of organ cross-talk.
Keywords: insulin resistance, leukotriene B4, 5-lipoxygenase, PDK1, FoxO1
Abstract
Although adipocytes are major targets of insulin, the influence of impaired insulin action in adipocytes on metabolic homeostasis remains unclear. We here show that adipocyte-specific PDK1 (3′-phosphoinositide–dependent kinase 1)-deficient (A-PDK1KO) mice manifest impaired metabolic actions of insulin in adipose tissue and reduction of adipose tissue mass. A-PDK1KO mice developed insulin resistance, glucose intolerance, and hepatic steatosis, and this phenotype was suppressed by additional ablation of FoxO1 specifically in adipocytes (A-PDK1/FoxO1KO mice) without an effect on adipose tissue mass. Neither circulating levels of adiponectin and leptin nor inflammatory markers in adipose tissue differed between A-PDK1KO and A-PDK1/FoxO1KO mice. Lipidomics and microarray analyses revealed that leukotriene B4 (LTB4) levels in plasma and in adipose tissue as well as the expression of 5-lipoxygenase (5-LO) in adipose tissue were increased and restored in A-PDK1KO mice and A-PDK1/FoxO1KO mice, respectively. Genetic deletion of the LTB4 receptor BLT1 as well as pharmacological intervention to 5-LO or BLT1 ameliorated insulin resistance in A-PDK1KO mice. Furthermore, insulin was found to inhibit LTB4 production through down-regulation of 5-LO expression via the PDK1−FoxO1 pathway in isolated adipocytes. Our results indicate that insulin signaling in adipocytes negatively regulates the production of LTB4 via the PDK1−FoxO1 pathway and thereby maintains systemic insulin sensitivity.
Adipocytes are a major cellular target of insulin. Many cell-based studies have implicated insulin in the regulation of various biological processes in adipocytes, including promotion of energy storage, secretion of adipokines, and cell differentiation and maintenance of cell maturity (1–3). The relevance of these various effects of insulin to systemic metabolic homeostasis has remained unclear, however. Mice with adipocyte-specific deficiency of the insulin receptor (IR) generated with the use of a floxed allele of the IR gene and a Cre recombinase transgene under the control of the adipocyte protein 2 gene promoter (aP2-Cre) manifested a slight reduction in the amount of adipose tissue but no substantial deteriorations in whole-body metabolism (4). A different line of mice with adipocyte-specific IR deficiency established with the use of a floxed allele of the IR gene and a Cre transgene controlled by the adiponectin gene promoter (Adipoq-Cre) (5) manifested a pronounced reduction in adipose tissue mass as well as insulin resistance, glucose intolerance, and hepatic steatosis (6, 7), all of which are common metabolic abnormalities associated with lipodystrophic diabetes in both mice and humans (8). Whereas these findings underscore the importance of insulin action in regulation of the quantity of adipose tissue and of metabolic changes induced by a decline in the amount of such tissue, the molecular mechanism underlying the phenomena has not been well understood.
Chronic inflammation in adipose tissue is a key contributor to insulin resistance (9). Leukotriene B4 (LTB4) is a proinflammatory lipid mediator produced from arachidonic acids. Recent evidence suggests that the inhibition of the production of or the blockade of the action of LTB4 resulted in the amelioration of obesity-induced insulin resistance (10–12), suggesting the importance of the lipid mediators in the pathogenesis of insulin resistance. Mechanism of how the lipid mediator is produced during the development of insulin resistance is unclear, however.
The 3′-phosphoinositide–dependent kinase 1 (PDK1) is a serine-threonine kinase that plays a central role in insulin signal transduction by phosphorylating and activating downstream kinases such as Akt and ribosomal protein S6 kinase (13). FoxO1 is a transcription factor whose activity is negatively regulated via the PDK1–Akt pathway (14), and it mediates various biological actions of insulin. The physiological relevance of the PDK1–FoxO1 pathway in adipocytes to systemic metabolic control has remained unknown. To provide insight into the physiological role of insulin action in adipocytes, we have now generated mice with adipocyte-specific PDK1 deficiency (A-PDK1KO mice) and with adipocyte-specific PDK1 and FoxO1 deficiency (A-PDK1/FoxO1DKO mice) and investigated their metabolic phenotypes. We here show that the PDK1–FoxO1 pathway in adipocytes plays an important role in the regulation of systemic insulin sensitivity and glucose metabolism, which is mediated, at least partly, by the regulation of the production of LTB4 via the control of the expression of 5-lipoxygenase (5-LO), a rate-limiting enzyme for production of LTB4.
Results
Insulin Action in Adipocytes Is Attenuated in A-PDK1KO Mice.
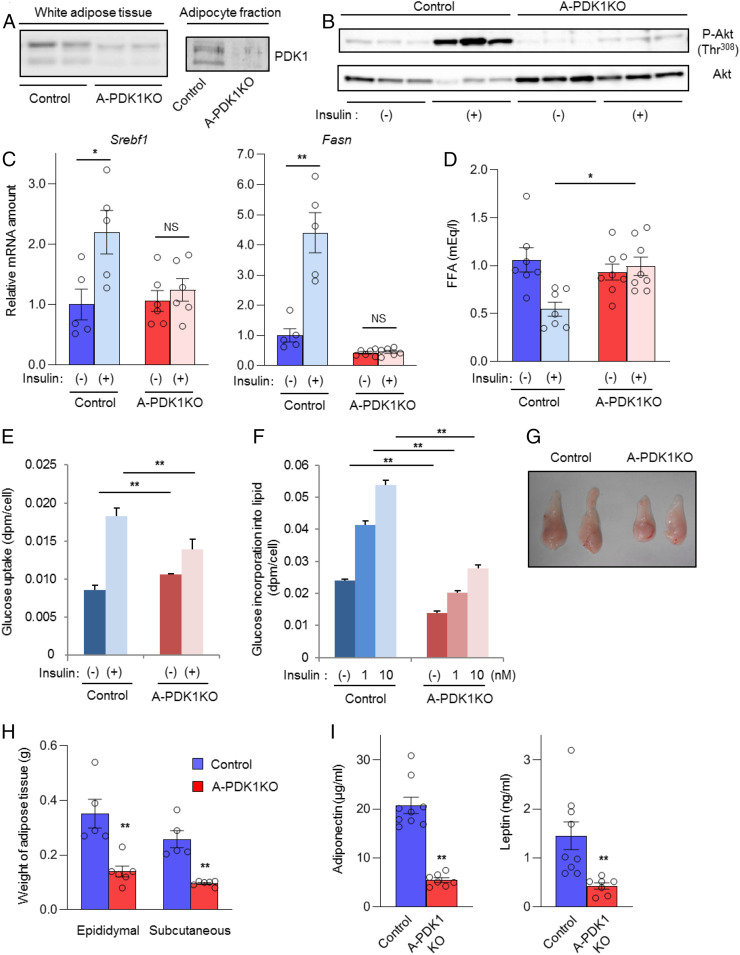
We generated A-PDK1KO mice by crossing PDK1-floxed mice (15) with Adipoq-Cre mice (5). All data shown are from male mice at 10 wk to 12 wk of age unless indicated otherwise. The expression of PDK1 protein was markedly reduced in whole adipose tissue of A-PDK1KO mice and was virtually abolished in the mature adipocyte fraction of the tissue (Fig. 1A). The insulin-induced phosphorylation of Akt at Thr308 (Fig. 1B), which is mediated by PDK1 and essential for Akt activity (13), as well as the insulin-induced expression of genes related to lipogenesis including Srebf1 and Fasn (Fig. 1C) were also greatly attenuated in adipose tissue of the mutant mice compared with those apparent in control (PDK1-floxed) mice. Moreover, the insulin-induced decrease in the plasma concentration of free fatty acids (FFA), which reflects the antilipolytic action of insulin, was inhibited in the mutant mice (Fig. 1D), as was insulin-induced glucose uptake (Fig. 1E) and lipogenesis (Fig. 1F) in adipocytes isolated from these mice.
Fig. 1.
Impaired insulin action in adipocytes of A-PDK1KO mice. (A) Immunoblot analysis of PDK1 in epididymal adipose tissue and in the adipocyte fraction of this tissue from control (PDK1flox/flox) and A-PDK1KO mice. (B) Immunoblot analysis of total and Thr308-phosphorylated (P-) forms of Akt in epididymal adipose tissue isolated from control or A-PDK1KO mice (n = 3) at 10 min after i.p. injection of insulin (5 U/kg) or vehicle. (C) RT and real-time PCR analysis of Srebf1 and Fasn expression in epididymal adipose tissue isolated from control or A-PDK1KO mice (n = 5 or 6) at 6 h after i.p. injection of insulin (1 U/kg) or vehicle. (D) Plasma FFA concentration in control or A-PDK1KO mice (n = 7 or 8) measured 1 h after i.p. injection of insulin (1 U/kg) or vehicle. (E) Basal and insulin (1 nM)-stimulated glucose uptake in isolated adipocytes from control or A-PDK1KO mice (n = 3). (F) Basal and insulin-stimulated lipogenesis in isolated adipocytes from control or A-PDK1KO mice (n = 3). (G) Representative images of epididymal adipose tissue and (H) weight of epididymal or subcutaneous (s.c.) adipose tissue from control or A-PDK1KO mice (n = 5 or 6). (I) Plasma adiponectin and leptin concentrations in control or A-PDK1KO mice (n = 7 to 9). All quantitative data are means ± SEM; *P < 0.05, **P < 0.01 for the indicated comparisons or versus corresponding control value (Student’s t test); NS, not significant.
In association with the impairment of these metabolic actions of insulin, adipose tissue mass was significantly reduced in A-PDK1KO mice (Fig. 1 G and H). Histological analysis revealed an increase in the number of small adipocytes in adipose tissue of the mutant mice, a finding that was confirmed by Coulter Counter analysis showing that the mean and mode of adipocyte diameter were smaller in A-PDK1KO mice than in control animals (SI Appendix, Fig. S1 A–C). The plasma concentrations of the adipokines leptin and adiponectin were also decreased in A-PDK1KO mice (Fig. 1I). These results thus indicated that depletion of PDK1 in adipocytes markedly impaired various metabolic actions of insulin in adipocytes, with this impairment resulting in a reduction in adipose tissue mass.
Body mass and food intake were increased, whereas locomotor activity and energy expenditure were unaltered, in A-PDK1KO mice (SI Appendix, Fig. S2), suggesting that the increase in body mass is attributable to increased food intake.
Metabolic Abnormalities of A-PDK1KO Mice.
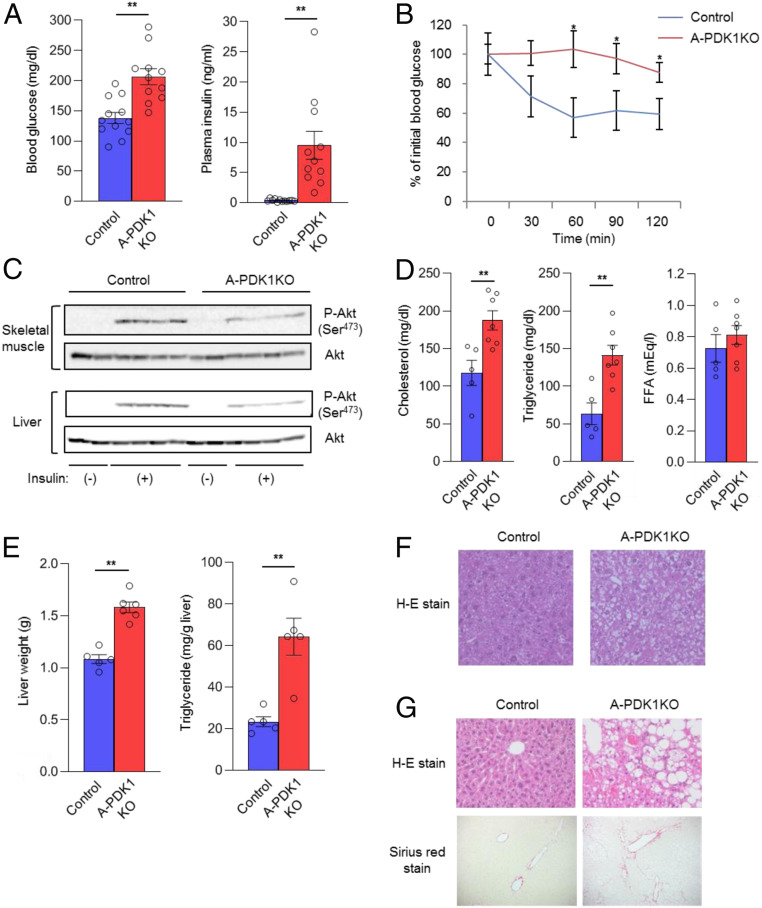
Blood glucose and plasma insulin concentrations were increased (Fig. 2A) and the glucose-lowering effect of exogenous insulin was impaired (Fig. 2B) in A-PDK1KO mice compared with control mice, indicative of insulin resistance and glucose intolerance in the mutant animals. Whereas PDK1 was specifically depleted in adipocytes, insulin-induced phosphorylation of Akt was inhibited in both liver and skeletal muscle of A-PDK1KO mice (Fig. 2C). Plasma concentrations of cholesterol and triglycerides, but not that of FFA, were increased in A-PDK1KO mice (Fig. 2D). Liver mass was also increased in A-PDK1KO mice (Fig. 2E), which may account, at least in part, for the increased body mass of these animals (SI Appendix, Fig. S2A). In addition, hepatic triglyceride content (Fig. 2E) and lipid accumulation in hepatocytes (Fig. 2F) were greater in the mutant mice than in control animals. Histological features—including ballooning degeneration, infiltration of inflammatory cells, and fibrosis (Fig. 2G)—as well as changes in gene expression (up-regulation of mRNAs related to lipogenesis, inflammation, and fibrosis) (SI Appendix, Fig. S3) consistent with nonalcoholic steatohepatitis (NASH) were also apparent in the liver of aged (35- to 37-wk-old) A-PDK1KO mice. These results thus indicate that depletion of PDK1 in adipocytes led to insulin resistance, glucose intolerance, dyslipidemia, and hepatic steatosis in association with impaired insulin action in remote organs, including the liver and skeletal muscle.
Fig. 2.
Systemic insulin resistance and metabolic abnormalities in A-PDK1KO mice. (A) Blood glucose and plasma insulin concentrations for control or A-PDK1KO mice (n = 11 or 12) in the randomly fed state. (B) Insulin tolerance test for control or A-PDK1KO mice (n = 7 or 8). (C) Immunoblot analysis of total and Ser473-phosphorylated forms of Akt in liver and skeletal muscle isolated from control or A-PDK1KO mice at 10 min after i.p. injection of insulin (5 U/kg) or vehicle. (D) Plasma cholesterol, triglyceride, and FFA concentrations in control or A-PDK1KO mice (n = 5 to 7). (E) Weight and triglyceride content of the liver from control or A-PDK1KO mice (n = 5 or 6). (F) H&E staining of liver sections from 11-wk-old control or A-PDK1KO mice. (Original magnification: 200×.) (G) H&E (Upper) and Sirius red (Lower) staining of liver sections from 35- to 37-wk-old control or A-PDK1KO mice. (Original magnification: 200×.) All quantitative data are means ± SEM; *P < 0.05, **P < 0.01 for the indicated comparisons or versus corresponding control value (Student’s t test).
Additional Depletion of FoxO1 in Adipocytes Ameliorates Metabolic Abnormalities of A-PDK1KO Mice.
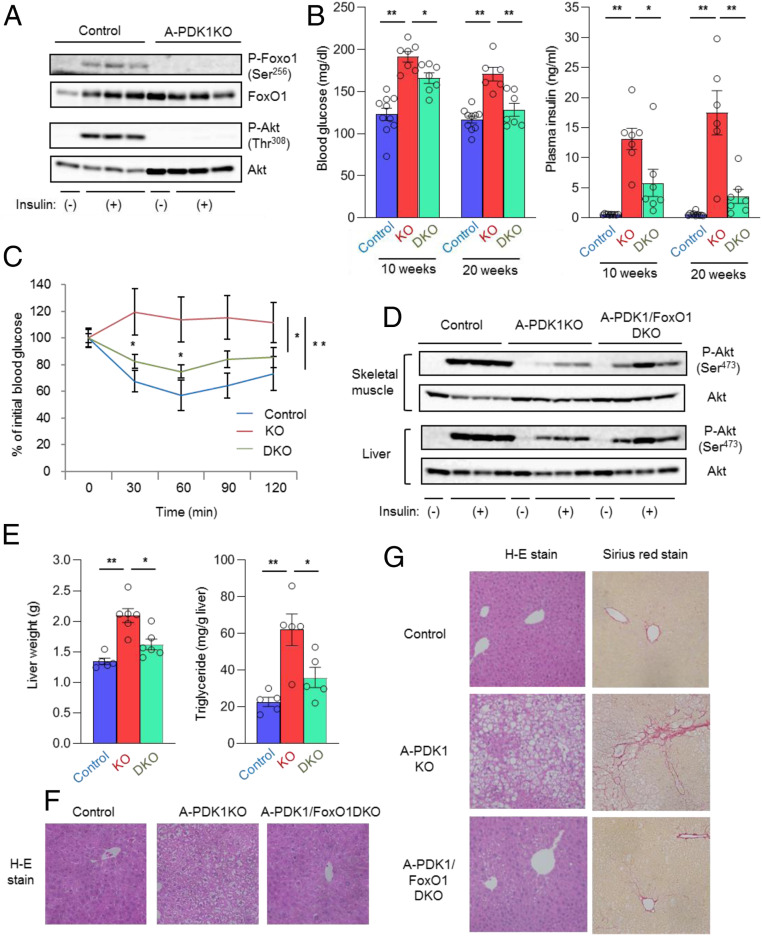
FoxO1 is a transcription factor whose activity is negatively regulated by insulin (14). Insulin-induced phosphorylation of FoxO1, which results in its translocation from the nucleus to the cytosol (14), was not apparent in adipose tissue of A-PDK1KO mice (Fig. 3A), suggesting that FoxO1 remained in the nucleus and activated its target genes even in the presence of insulin. To investigate whether the persistent activation of FoxO1 is responsible for the metabolic abnormalities of A-PDK1KO mice, we generated mice lacking both PDK1 and FoxO1 selectively in adipocytes (A-PDK1/FoxO1DKO mice).
Fig. 3.
Additional ablation of FoxO1 in adipocytes ameliorates metabolic abnormalities of A-PDK1KO mice. (A) Immunoblot analysis of total and Ser256-phosphorylated forms of FoxO1 as well as of total and Thr308-phosphorylated forms of Akt in epididymal adipose tissue of control and A-PDK1KO mice at 10 min after i.p. injection of insulin (5 U/kg) or vehicle. (B) Blood glucose and plasma insulin concentrations in control, A-PDK1KO, or A-PDK1/FoxO1DKO mice at 10 or 20 wk of age (n = 6 to 10). (C) Insulin tolerance test in control, A-PDK1KO, or A-PDK1/FoxO1DKO mice (n = 6 to 8). (D) Immunoblot analysis of Akt phosphorylation in skeletal muscle and liver isolated from control, A-PDK1KO, or A-PDK1/FoxO1DKO female mice at 10 min after i.p. injection of insulin (5 U/kg) or vehicle. (E) Weight and triglyceride content of the liver of control, A-PDK1KO, or A-PDK1/FoxO1DKO mice (n = 5 or 6). (F) H&E staining of liver sections from control, A-PDK1KO, or A-PDK1/FoxO1DKO mice. (Original magnification: 200×.) (G) H&E and Sirius red staining of liver sections from control, A-PDK1KO, or A-PDK1/FoxO1DKO mice at 35 wk to 37 wk of age. (Original magnification: 200×.) All quantitative data are means ± SEM; *P < 0.05, **P < 0.01 for the indicated comparisons or versus corresponding control value (Student’s t test, or ANOVA for comparison of overall time courses in C).
The hyperglycemia and hyperinsulinemia apparent in A-PDK1KO mice were markedly ameliorated in A-PDK1/FoxO1DKO mice (Fig. 3B). The glucose-lowering effect of exogenous insulin (Fig. 3C) as well as insulin-induced phosphorylation of Akt in liver and skeletal muscle (Fig. 3D) were also substantially, although not completely, restored in A-PDK1/FoxO1DKO mice. In addition, hepatomegaly and hepatic steatosis (Fig. 3 E and F) as well as age-related NASH-like histology (Fig. 3G) were ameliorated in A-PDK1/FoxO1DKO mice compared with A-PDK1KO mice. These results suggest that the persistent activation of FoxO1 is largely responsible for the metabolic alterations induced by the lack of PDK1 in adipocytes. The increases in the plasma levels of cholesterol and triglycerides apparent in A-PDK1KO mice were not significantly affected by FoxO1 ablation (Fig. 4A), however, suggesting that FoxO1 is not responsible for the dyslipidemia of A-PDK1KO mice.
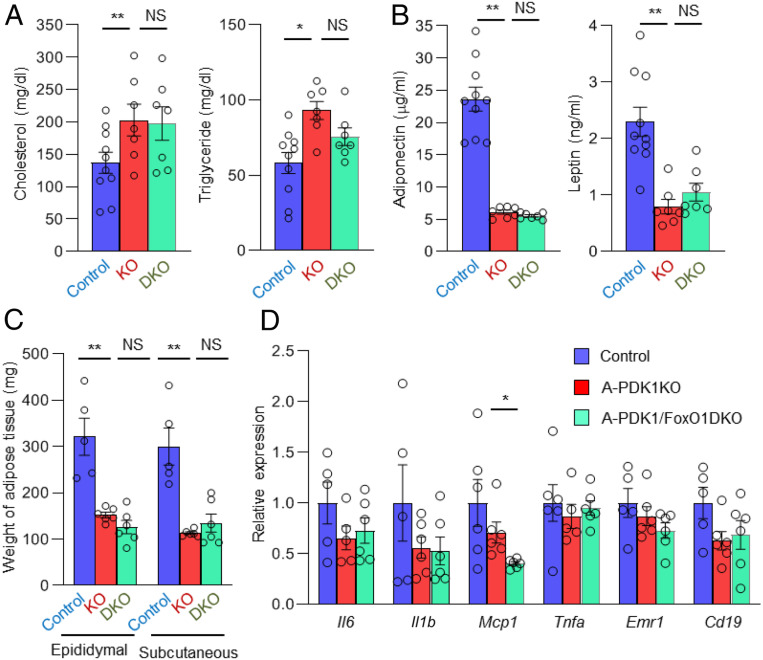
Fig. 4.
Additional ablation of FoxO1 in adipocytes does not restore adipokine levels and adipose tissue mass in A-PDK1KO mice. (A) Plasma cholesterol and triglyceride as well as (B) adiponectin and leptin concentrations in control, A-PDK1KO, or A-PDK1/FoxO1DKO mice (n = 7 to 10). (C) Weight of epididymal and s.c. adipose tissue in control, A-PDK1KO, or A-PDK1/FoxO1DKO mice (n = 5 or 6). (D) RT and real-time PCR analysis of the expression of genes related to inflammation in epididymal adipose tissue of control, A-PDK1KO, or A-PDK1/FoxO1DKO mice (n = 5 or 6). All data are means ± SEM; *P < 0.05, **P < 0.01 (Student’s t test).
Given that Adipoq-Cre mice express Cre recombinase in both white and brown adipocytes (5), the gene for PDK1 was deleted also in brown adipose tissue (BAT) in A-PDK1KO mice. Although the mass of BAT was unaltered (SI Appendix, Fig. S4A), the size of lipid droplets in BAT was increased in A-PDK1KO mice, and this alteration was partially restored in A-PDK1/FoxO1DKO mice (SI Appendix, Fig. S4B). Among genes related to the function of BAT, the expression of Ucp1, Elovl3, and Cidea was significantly decreased and that of Ppargc1a tended to be decreased in A-PDK1KO mice (SI Appendix, Fig. S4C). Moreover, the changes in the expression of Ucp1 and Ppargc1a were restored in A-PDK1/FoxO1DKO mice (SI Appendix, Fig. S4C). These results suggest that PDK1–FoxO1 pathway contributes to the characteristics of brown adipocyte, and the metabolic phenotypes of A-PDK1KO mice and A-PDK1/FoxO1DKO mice might be affected by such alteration in BAT.
Increase in food intake observed in A-PDK1KO mice was partially restored in A-PDK1/FoxO1DKO mice (SI Appendix, Fig. S4D), suggesting that the amelioration of metabolic abnormalities in A-PDK1/FoxO1DKO mice is also attributable, at least in part, to the decrease in food intake.
Adiponectin and leptin possess insulin-sensitizing activity (16, 17), and genetically engineered mice with reduced adipose tissue mass manifest metabolic abnormalities, including insulin resistance, that are, at least partly, attributable to the reduced circulating levels of these hormones (18, 19). The reduced plasma levels of adiponectin and leptin apparent in A-PDK1KO mice were unaltered in A-PDK1/FoxO1DKO mice (Fig. 4B). The decreased adipose tissue mass in A-PDK1KO mice was also unchanged in A-PDK1/FoxO1DKO mice (Fig. 4C), which is consistent with a finding that the impairment of antilipolytic action observed in A-PDK1KO mice was not recovered in A-PDK1/FoxO1DKO mice (SI Appendix, Fig. S4E). These results suggest that the recovery of insulin sensitivity in A-PDK1/FoxO1DKO mice is independent of these adipokines or of adipose tissue mass. Chronic inflammation in adipose tissue contributes to the development of insulin resistance (9). Immunohistochemistry with the use of antibody to F4/80, a marker of macrophage, revealed no increase in cells that were positive for this antibody in adipose tissue of A-PDK1KO mice (SI Appendix, Fig. S1D). The expression of inflammatory cytokine genes including Il6, Il1b, Mcp1, and Tnfa as well as that of marker genes for macrophages (Emr1) or B lymphocytes (Cd19) was not significantly increased in adipose tissue of A-PDK1KO mice (Fig. 4D). Furthermore, with the exception of Mcp1, the expression of these various genes was similar in A-PDK1KO and A-PDK1/FoxO1DKO mice. Inflammation in adipose tissue thus does not appear to explain the development of insulin resistance in A-PDK1KO mice.
Insulin Signaling Regulates the 5-LO–LTB4 Axis in Adipocytes.
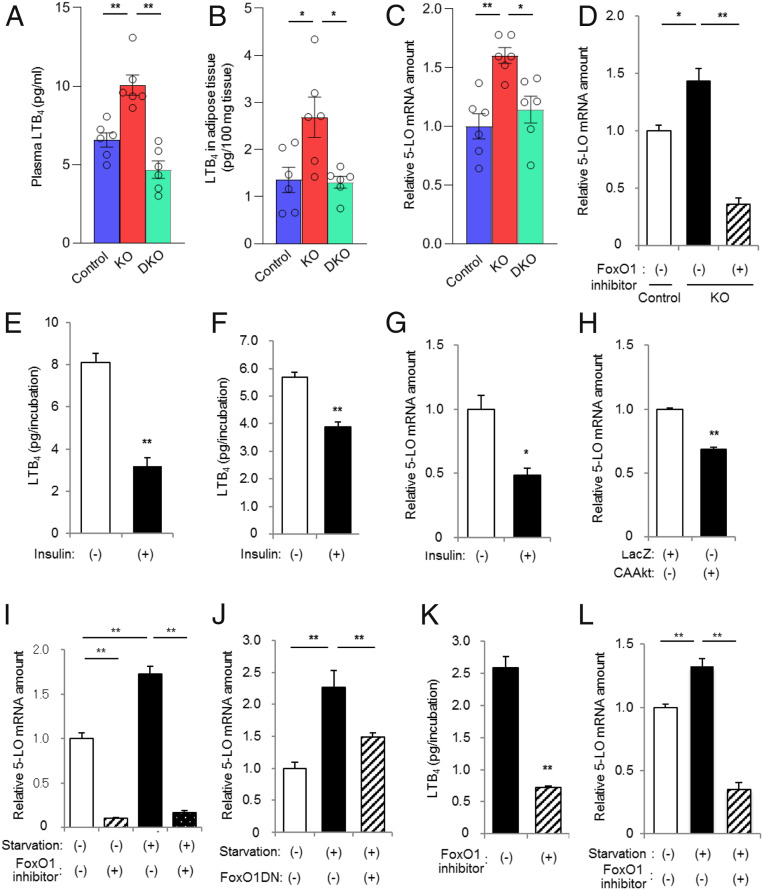
Given that neither circulating levels of adiponectin and leptin nor inflammation in adipose tissue appeared to account for the development of or recovery from insulin resistance in A-PDK1KO and A-PDK1/FoxO1DKO mice, respectively, we examined the possible role of other factors or pathways in such insulin resistance. Microarray analysis of adipose tissue revealed that the expression of various genes was altered and restored in A-PDK1KO and A-PDK1/FoxO1DKO mice, respectively (SI Appendix, Tables S1 and S2). Liquid chromatography (LC) and tandem mass spectrometry (MS/MS) allows the comprehensive measurement of proinflammatory and proresolving lipid mediators, with high sensitivity (20). Application of this system to plasma of the mutant mice revealed that, among the >70 lipid mediators examined (SI Appendix, Table S3), 20 compounds were detected. However, only the concentration of LTB4, a compound that has been implicated in the pathogenesis of insulin resistance (11, 12), was increased and normalized in A-PDK1KO and A-PDK1/FoxO1DKO mice, respectively (Fig. 5A and SI Appendix, Fig. S5). The plasma concentration of 5-HETE, a pathway marker for the pathway of LTB4 production, exhibited a similar pattern to that of LTB4, although the changes did not reach to statistical significance (SI Appendix, Fig. S5). LC-MS/MS analysis also revealed that the content of LTB4 in adipose tissue was increased and normalized in A-PDK1KO and A-PDK1/FoxO1DKO mice, respectively (Fig. 5B). The 5-LO is a rate-limiting enzyme in the production of LTB4 (21). Our microarray data showed that the abundance of 5-LO messenger RNA (mRNA) in adipose tissue was increased and normalized in A-PDK1KO and A-PDK1/FoxO1DKO mice, respectively (SI Appendix, Table S1), and this finding was confirmed by RT and real-time PCR analysis (Fig. 5C). These results thus suggested that insulin might regulate the production of LTB4 at the level of 5-LO expression in adipose tissue.
Fig. 5.
The insulin–PDK1–FoxO1 pathway regulates the 5-LO–LTB4 axis in adipocytes. (A) LTB4 concentration in plasma and (B) LTB4 content in epididymal adipose tissue of control, A-PDK1KO, or A-PDK1/FoxO1DKO mice (n = 6) as determined by LC-MS/MS analysis. (C) RT and real-time PCR analysis of 5-LO mRNA in epididymal adipose tissue of control, A-PDK1KO, or A-PDK1/FoxO1DKO mice (n = 6). (D) Real-time PCR analysis of 5-LO mRNA in primary adipocytes isolated from control or A-PDK1KO mice and incubated in the absence or presence of the FoxO1 inhibitor AS1842856 (10 μM) for 24 h. (E and F) LTB4 production by (E) primary adipocytes and (F) SV cells isolated from C57BL/6 mice and incubated in the absence or presence of 100 nM insulin for 24 h. (G–J) Real-time PCR analysis of 5-LO mRNA in SV cells isolated from C57BL/6 mice and either (G) incubated in the absence or presence of 100 nM insulin for 24 h, (H) infected with adenoviruses encoding LacZ (control) or a constitutively active form (CAAkt) of Akt, (I) deprived of serum and incubated in the absence or presence of AS1842856 (10 μM) for 24 h, or (J) infected with an adenovirus encoding a dominant negative mutant of FoxO1 (FoxO1DN) and then deprived of serum for 24 h. (K) LTB4 production by SV cells isolated from C57BL/6 mice and then deprived of serum during incubation with or without AS1842856 (10 μM) for 24 h. (L) Real-time PCR analysis of 5-LO mRNA in Raw264.7 cells that had been deprived of serum during incubation with or without AS1842856 (10 μM) for 24 h. All data are means ± SEM, and those in D–L are from three or four samples. *P < 0.05, **P < 0.01 for the indicated comparisons or versus corresponding control (Student’s t test).
To examine this notion further, we first performed experiments with isolated adipocytes. RT and real-time PCR analysis showed that the abundance of 5-LO mRNA was increased in adipocytes isolated from A-PDK1KO mice and that this increase was attenuated by exposure of the cells to AS1842856, a pharmacological inhibitor of FoxO1 (Fig. 5D). We also found that insulin down-regulated LTB4 production by adipocytes isolated from wild-type mice (Fig. 5E). Given that the efficiency of exogenous gene transfer is low in isolated adipocytes, we next isolated cells from the stromal vascular (SV) fraction of adipose tissue from wild-type mice in order to further analyze regulation of the 5-LO–LTB4 axis. Insulin reduced LTB4 production (Fig. 5F) and the abundance of 5-LO mRNA (Fig. 5G) in SV cells, whereas adenovirus-mediated expression of an active form of Akt also reduced the amount of 5-LO mRNA in these cells (Fig. 5H). Serum deprivation down-regulated the phosphorylation of FoxO1 in SV cells (SI Appendix, Fig. S6), likely resulting in an increase in FoxO1 activity. Serum deprivation also increased the abundance of 5-LO mRNA in SV cells in a manner sensitive to inhibition by AS1842856 (Fig. 5I) or by forced expression of a dominant negative form of FoxO1 (Fig. 5J). Moreover, AS1842856 treatment reduced LTB4 production by serum-deprived SV cells (Fig. 5K). Chromatin immunoprecipitation (ChIP) assay demonstrated that the binding of FoxO1 to the promoter region of the 5-LO gene was augmented by serum starvation, which is restored by insulin treatment in 3T3L1 adipocytes (SI Appendix, Fig. S7), suggesting that the 5-LO gene is a direct target of FoxO1. Together, these results suggested that insulin inhibits the production of LTB4 by down-regulating the expression of 5-LO, and that this action of insulin is mediated by the PDK1–FoxO1 pathway.
Macrophages are a key source of LTB4 during inflammatory responses (22). Serum deprivation of cultured Raw264.7 macrophages increased the abundance of 5-LO mRNA, and this effect was blocked by the FoxO1 inhibitor (Fig. 5L), suggesting that the regulation of 5-LO expression by the FoxO1 pathway might be a general phenomenon.
Inhibition of the 5-LO–LTB4–BLT1 Axis Ameliorates Insulin Resistance in A-PDK1KO Mice.
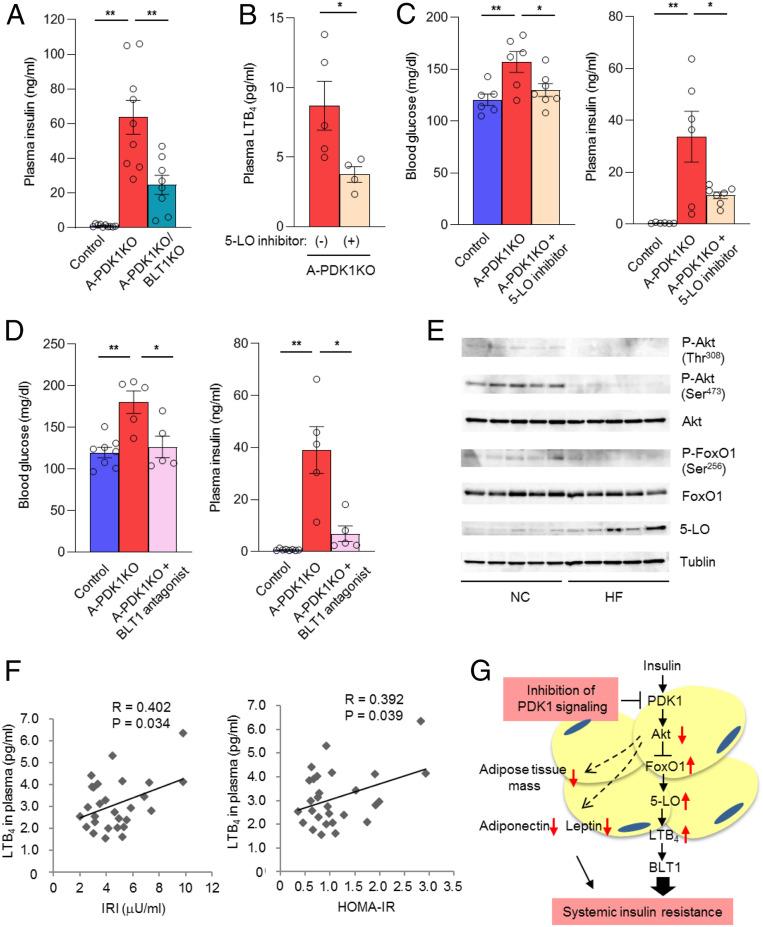
To confirm whether LTB4 is involved in the pathogenesis of insulin resistance in A-PDK1KO mice, we crossbred A-PDK1KO mice with mice lacking BLT1 (23), a specific receptor for LTB4 (24). Whereas blood glucose level was unaltered, the plasma concentration of insulin was significantly decreased by the deletion of BLT1 in A-PDK1KO mice (Fig. 6A). We also performed the experiment with pharmacological tools for the intervention of the LTB4 pathway in A-PDK1KO mice. Administration of the 5-LO inhibitor zileuton resulted in a significant decrease in the plasma concentration of LTB4 (Fig. 6B) as well as markedly attenuating hyperglycemia and hyperinsulinemia (Fig. 6C) in A-PDK1KO mice. Administration of the BLT1 antagonist CP-105696 (25) also ameliorated both hyperglycemia and hyperinsulinemia in A-PDK1KO mice to extents similar to those apparent with zileuton (Fig. 6D). Neither zileuton nor CP-105696 administration affected body and adipose tissue mass in A-PDK1KO mice (SI Appendix, Fig. S8). The results obtained with genetic or pharmacological interventions thus indicated that the 5-LO–LTB4–BLT1 axis contributes to the development of insulin resistance in A-PDK1KO mice. To investigate the mechanism by which LTB4 triggers insulin resistance, we examined the effect of LTB4 on insulin signaling in mouse primary hepatocytes. Insulin-induced phosphorylation of Akt was suppressed by the treatment of the cells with LTB4 (SI Appendix, Fig. S9), which is consistent with a previous finding (12). These results suggest the possibility that LTB4 triggers insulin resistance by the inhibition of insulin signaling.
Fig. 6.
The 5-LO–LTB4 axis is associated with systemic insulin resistance in both mice and humans. (A) Plasma insulin concentrations for control, A-PDK1KO mice, and A-PDK1KO/BLT1KO mice (n = 8 to 10) in the randomly fed state at 24 wk of age. (B) Plasma LTB4 concentration in female A-PDK1KO mice (n = 4 or 5) treated with the 5-LO inhibitor zileuton for 2 wk. (C) Blood glucose and plasma insulin concentrations in control or A-PDK1KO mice (n = 6 or 7) treated (or not) with zileuton for 7 wk. (D) Blood glucose and plasma insulin concentrations in control or A-PDK1KO mice (n = 5 to 8) treated (or not) with the BLT1 antagonist CP-105696 for 5 wk. Quantitative data in A–D are means ± SEM; *P < 0.05, **P < 0.01. (E) Immunoblot analysis of total and Thr308 or Ser473-phosphorylated forms of Akt, total, and Ser256-phosphorylated forms of FoxO1, 5-LO, and tublin in epididymal adipose tissue of mice fed a normal chow (NC) or a high-fat diet (HF) at 1 h after refeeding. (F) Correlation between plasma LTB4 concentration and either serum immunoreactive insulin (IRI) concentration or HOMA-IR in human subjects (n = 28). R, Pearson’s correlation coefficient. (G) Model for the regulation of systemic insulin sensitivity by insulin–PDK1 signaling in adipocytes. Red arrows indicate effects of PDK1 ablation in adipocytes.
LTB4 has been shown to be up-regulated in adipose tissue of obese animals (10, 12). Phosphorylation of Akt as well as FoxO1 was inhibited, and, reciprocally, protein abundance of 5-LO was up-regulated in adipose tissue of mice fed a high-fat diet (Fig. 6E). These results are consistent with a notion that the abundance of 5-LO is regulated by the PDK1-FoxO1 pathway.
Plasma LTB4 Levels Correlate with Insulin Resistance in Humans.
The plasma concentration of LTB4 determined by LC-MS/MS analysis in human subjects was positively correlated with both the serum insulin level and homeostasis model assessment of insulin resistance (HOMA-IR) (Fig. 6F), a widely applied clinical index of insulin resistance, suggesting that LTB4 contributes to the pathogenesis of human insulin resistance.
Discussion
We have here shown that A-PDK1KO mice manifest impairment of metabolic actions of insulin in adipose tissue and a reduction in adipose tissue mass. These animals develop insulin resistance, glucose intolerance, and hepatic steatosis, all of which were markedly attenuated by additional ablation of FoxO1 in adipocytes without an effect on adipose tissue mass. Our results thus indicate that insulin action in adipocytes plays an important role in systemic metabolic homeostasis through a mechanism independent of the regulation of adipose tissue mass, and that suppression of FoxO1 activity is largely responsible for such metabolic effects of insulin.
With the application of high-sensitivity, LC-MS/MS–based analysis of lipid mediators, we found that the plasma concentration of LTB4 was increased in A-PDK1KO mice and that this effect was abolished in PDK1/FoxO1DKO mice. We also showed that, in primary cells derived from adipose tissue, insulin inhibits the production of LTB4 via down-regulation of 5-LO expression in a manner dependent on the FoxO1 pathway. Furthermore, inhibition of the production or action of LTB4 markedly ameliorated insulin resistance in A-PDK1KO mice. These results uncover a previously unrecognized action of insulin in adipocytes that contributes to the control of systemic metabolism. The observed correlation of plasma LTB4 concentration with indexes of insulin resistance in human subjects also suggests that this biological pathway plays a role in the regulation of insulin sensitivity in humans.
Immune cells such as granulocytes and monocytes–macrophages play a major role in the production of LTB4. Nonimmune cells have been thought to participate in the production of LTB4 only through “transcellular biosynthesis,” in which infiltrated immune cells expressing 5-LO produce LTA4 that is then converted to LTB4 in nonimmune cells (26). However, we found that even nonimmune cells such as adipocytes produce LTB4 in a cell-autonomous manner via the up-regulation of 5-LO. It remains to be determined how widespread this ability is among nonimmune cell types as well as which biological events are regulated by LTB4 produced by such a mechanism.
In obesity-induced insulin resistance, immune cells including macrophages are infiltrated in adipose tissue, and the infiltration of such cells is thought to trigger insulin resistance. Thus, in obesity-induced insulin resistance, it is likely that LTB4 is produced by both adipocytes and immune cells. Given that the impairment of insulin action results in the exaggerated production of LTB4 and that LTB4 promotes insulin resistance, it is possible that a vicious cycle between the two processes contributes to the development of insulin resistance.
Mice lacking either 5-LO (10) or BLT1 (11, 12) were previously shown to be protected against the development of insulin resistance, and treatment of wild-type mice with the 5-LO inhibitor zileuton (10) or the BLT1 antagonist CP-105696 (12) ameliorated insulin resistance induced by a high-fat diet. Moreover, LTB4 was shown to be produced during chronic inflammation associated with diet-induced obesity and to contribute to the recruitment of macrophages and B2 lymphocytes to adipose tissue (12, 27), implicating LTB4 in insulin resistance triggered by such inflammation in adipose tissue. However, we found that neither the expression of markers for inflammation, including those for macrophages and B lymphocytes, nor that of proinflammatory cytokines was increased in adipose tissue of A-PDK1KO mice. Our results thus suggest that impaired insulin action in adipocytes results in the production of LTB4 in a manner independent of inflammation (Fig. 6G). The mechanism by which the production of LTB4 is triggered in adipose tissue during the development of high-fat diet-induced obesity remains unknown. It is possible that impaired insulin action elicits the production of LTB4, which, in turn, contributes to the recruitment of inflammatory cells into adipose tissue. Given that A-PDK1KO mice did not manifest inflammation in adipose tissue, a “second hit” might be required for the development of chronic inflammation in high-fat diet-fed models.
The precise mechanism by which LTB4 induces insulin resistance in A-PDK1KO mice remains to be determined. We found that the plasma concentration of LTB4 in mice and humans ranges from ∼1 pg/mL to 10 pg/mL, levels that are sufficient to activate BLT1 (24). It is thus possible that LTB4 produced in adipocytes is transported, via the circulation, to remote organs, where it directly affects insulin action. In this regard, LTB4 was previously shown to inhibit insulin action in cultured hepatocytes and muscle cells (12). However, the current study does not provide sufficient information to understand the exact mechanism how LTB4 triggers insulin resistance. Although BLT1-floxed mice have not yet been established, such a genetic tool would be expected to shed light on the mechanism of LTB4-induced insulin resistance.
A-PDK1KO mice exhibit the alterations in gene expression and histology of BAT, which are partially restored in A-PDK1/FoxO1DKO mice, suggesting the PDK1–FoxO1 pathway controls the characteristics of BAT. Since recent advances in brown adipocyte biology suggest that these cells play pivotal roles in energy homeostasis (28, 29), the PDK1–FoxO1 pathway in BAT might affect metabolic phenotypes of A-PDK1KO and A-PDK1/FoxO1DKO mice.
Previous studies have shown that genetic or pharmacological inhibition of the LTB4 signaling is associated with the amelioration of hepatic steatosis in mice (11, 12) and that LTB4 stimulates lipogenesis in hepatocytes (12), suggesting that the lipid mediator is involved in the pathogenesis of fatty liver. However, it is unclear to what extent LTB4 contributes to the development of the liver condition in A-PDK1KO mice. It is possible that the increase in food intake or the alteration of characteristics of BAT of A-PDK1KO mice might be involved in the pathogenesis of hepatic steatosis. In any case, the finding that hepatic steatosis triggered by adipocyte-specific disruption of PDK1 was ameliorated by the additional disruption of FoxO1 suggests that pathological factors regulated by the PDK1–FoxO1 pathway in adipocytes trigger the development of the hepatic disease.
In summary, we have shown that insulin signaling via the PDK1–FoxO1 pathway negatively regulates the production of LTB4 in adipocytes and thereby maintains systemic insulin sensitivity. The mechanism uncovered in this study may serve as a therapeutic target for insulin resistance and associated conditions.
Methods
Animal Studies.
PDK1-floxed mice (15), Adipoq-Cre mice (5), FoxO1-floxed mice (30), and BLT1 knockout mice (23) were described previously. A-PDK1/FoxO1DKO mice were generated by crossing A-PDK1KO mice and FoxO1-floxed mice. All experiments were performed with male mice at 10 wk to 12 wk of age unless indicated otherwise. C57BL/6J mice (CLEA) at 5 wk of age received either a normal diet or a high-fat died (CLEA) for 12 wk. Mice were housed at 21 °C to 25 °C and maintained on a 12-h-light, 12-h-dark cycle in the animal facility at Kobe University Graduate School of Medicine, Juntendo University School of Medicine, and Gunma University.
Analysis of Metabolic Parameters.
Blood glucose concentration was measured with a glucometer (Sanwa Kagaku Kenkyusho). Plasma insulin, leptin (Morinaga Institute of Biological Science), and adiponectin (Otsuka Pharmaceutical) concentrations were measured with enzyme-linked immunosorbent assay kits. Plasma cholesterol, triglyceride, and FFA concentrations were measured with assay kits (Wako).
Materials.
The FoxO1-selective inhibitor AS1842856 was obtained from Calbiochem, the 5-LO inhibitor zileuton was from Combi-Blocks, LTB4 was from Cayman Chemical, and the BLT1 antagonist CP-105696 was kindly provided by Pfizer.
Insulin Tolerance Test.
Mice were deprived of food for 4 h and then injected intraperitoneally (i.p.) with insulin (human regular insulin; Eli Lilly) at a dose of 0.8 U/kg. Blood glucose level was measured at indicated time points after insulin injection.
In Vivo Insulin Stimulation Assay.
For the evaluation of antilipolytic action of insulin, mice were deprived of food for 5 h and then injected i.p. with insulin at a dose of 1 U/kg. Plasma FFA concentration was measured 1 h after insulin injection. For immunoblot analysis of insulin signaling in tissues, mice were deprived of food for 6 h and then injected i.p. with insulin at a dose of 5 U/kg. Tissues were removed 10 min after insulin injection. For real-time PCR analysis of Srebf1 and Fasn, mice were deprived of food for 15 h and then injected i.p. with insulin at a dose of 1 U/kg. Epididymal adipose tissue was removed 6 h after insulin injection.
LC-MS/MS–Based Metabololipidomics.
Lipidomics was performed as described previously (20, 31). Briefly, deuterated internal standards (500 pg each, d4-LTB4, d8-5-hydroxyeicosatetraenoic acid, d4–prostaglandin E2, and d5-resolvin D2) representing each chromatographic region of identified lipid mediators were added to samples to facilitate quantification. The samples were extracted by automated solid phase extraction system on C18 columns and were then subjected to LC-MS/MS analysis with a Qtrap 6500 (Sciex) connected with a Shimadzu LC-30AD high-pressure liquid chromatography system. A ZORBAX Eclipse Plus C18 column (100 by 4.6 mm, 3.5 μm; Agilent Technologies) was subjected to elute lipid mediators at a flow rate of 0.4 mL/min. For monitoring and quantification of the target, a multiple reaction monitoring method was developed with signature ion pairs Q1 (parent ion)/Q3 (characteristic fragment ion) for each molecule.
Zileuton and CP-105696 Administration.
Zileuton (0.12% [wt/wt] in standard chow) was administered orally to A-PDK1KO mice from the age of 13 wk. Blood glucose and plasma insulin concentrations were measured after treatment for 7 wk. CP-105696 (0.04% [wt/wt] in standard chow) was administered orally to A-PDK1KO mice from the age of 15 wk. Blood glucose and plasma insulin concentrations were measured after treatment for 5 wk.
Indirect Calorimetry and Locomotor Activity Measurement.
Oxygen consumption and CO2 production were measured for individual mice with the use of an Oxymax apparatus (Columbus Instruments) as described previously (32). The O2 and CO2 measurements were performed every 18 min for each mouse over a 3-d period, and the data from the final day were analyzed. Locomotor activity was measured with an ACTIMO-100 system (Shinfactory). For normalization of VO2 and heat production by lean mass, mice were anesthetized and subjected to a whole-body scan with a computed tomography system for experimental animals (LaTheta LCT-200; Hitachi Aloka Medical) as described previously (33). Lean mass, fat mass, and adiposity of the mice were calculated with the LCT-200 software.
Histology.
Tissues were fixed with 10% neutral buffered formalin and processed for staining of paraffin-embedded sections with hematoxylin/eosin (H&E) or Sirius red.
Cell Culture.
Adipocytes and SV cells were isolated from epididymal adipose tissue by digestion with collagenase (0.5 mg/mL), as described previously (34). Raw264.7 cells were cultured as previously described (35). Mouse primary hepatocytes were prepared as described previously (36)
Adenovirus Infection.
Adenovirus vectors for LacZ or a constitutively active form of Akt (37) or for a dominant negative form of FoxO1 (38) were described previously. Isolated SV cells were infected with the indicated adenovirus.
LTB4 Production in Isolated Cells.
Isolated adipocytes or SV cells were added with a 2× volume of ice-cold methanol after the indicated treatment, and LTB4 production from cells was quantified by LC-MS/MS analysis.
Immunoblot Analysis.
Tissues or cells were homogenized on ice in lysis buffer [20 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 2 mM (ethylenedinitrilo)tetraacetic acid, 1% Nonidet P-40, 10% glycerol] supplemented with protease and phosphatase inhibitors. Lysates were fractionated by sodium dodecyl sulfate (SDS)/polyacrylamide gel electrophoresis, and the separated proteins were transferred to a nitrocellulose membrane for immunoblot analysis with antibodies to PDK1 (39) or with those to total, Thr308-phosphorylated, or Ser473-phosphorylated forms of Akt or to total or Ser256-phosphorylated forms of FoxO1 (Cell Signaling Technology).
Gene Expression Analysis.
Total RNA was extracted from tissues or cells with the use of an RNeasy Minikit (Qiagen). The purity of total RNA was determined with a Bioanalyzer (Agilent Technologies) before microarray analysis with a Mouse Gene 2.0 ST Array (Affymetrix). Expression data were processed with a Gene Expression Consol (Affymetrix). For real-time PCR analysis, complementary DNA was synthesized with a high-capacity RT kit (Applied Biosystems) and then quantitated with SYBR Green PCR Master Mix in an ABI StepOne Plus Real-Time PCR system (Applied Biosystems). Relative expression levels were determined with the standard curve method and with the use of 18S ribosomal RNA or 36B4 mRNA for normalization of target mRNA abundance. Sequences of PCR primers are listed in SI Appendix.
Measurement of Adipocyte Size and Number.
Adipocyte size and number were measured after fixation of isolated adipocytes with osmium tetroxide, as described previously (40).
Lipid Synthesis and Glucose Uptake in Isolated Adipocytes.
Lipogenesis was assayed as previously described, with slight modification (5). In brief, isolated adipocytes were incubated first with the indicated concentrations of insulin for 10 min and then in the additional presence of 5 mM [14C]glucose for 60 min, after which the incorporation of radioactivity into triglycerides was measured by liquid scintillation counting. Glucose uptake was also assayed as previously described, again with slight modification (4). In brief, isolated adipocytes were incubated first in the absence or presence of 1 nM insulin for 10 min and then in the additional presence of 0.5 mM 2-deoxy-D-[3H]glucose for 30 min. Cells were washed three times with ice-cold phosphate-buffered saline and solubilized with 1% SDS, and the incorporated radioactivity was measured by liquid scintillation counting.
ChIP Assays.
ChIP assays were performed using ChIP assay kit (Merck Millipore) following the manufacturer’s instructions. Nuclear lysates from 3T3L1 adipocytes were immunoprecipitated with Anti-FoxO1 antibody (Abcam) or control IgG. The mouse 5-LO promoter region was searched for potential binding sites for FoxO1 using JASPAR database. The 3′ untranslated region (UTR) of mouse 5-LO genomic locus was used as a nontarget control. Target and nontarget regions of genomic DNA were amplified by qPCR in both the immunoprecipitates and input samples. The amount in immunoprecipitates was normalized by that in input samples and was presented as fold enrichment relative to normal IgG sample. ChIP primer sequences are as follows: 5-LO promoter forward 5′-TCAGCATATGCTGAGATGACCAGG-3′, reverse 5′-ACTTGCAACCTGAATCCAACTTGC-3′, 3′ UTR forward 5′- ATCACAGAGCCTCAGCATG-3′, reverse 5′-CTGATGCTACCGAGTGACG-3′.
Human Studies.
A total of 28 subjects (19 men, 9 women; aged 31 y to 81 y) who underwent health examinations at Aijinkai Total Health Care Center between December 2016 and March 2017 were recruited. Blood samples were collected after overnight fasting for measurement of plasma LTB4, glucose, and serum insulin concentrations.
Statistics.
Quantitative data are presented as means ± SEM and were compared between or among groups with the two-tailed Student’s t test or ANOVA. The relation between the plasma LTB4 concentration and either the serum concentration of immunoreactive insulin or HOMA-IR in human subjects was assessed with Pearson’s correlation coefficient. A P value of <0.05 was considered statistically significant.
Study approval.
The clinical study conformed to the principles of Declaration of Helsinki, was approved by the Ethics Committee of Chibune General Hospital, and was registered in the clinical trial registry (20161114A). All participants provided written informed consent to analyze and publish their data for scientific purposes. All animal experiments were approved by and performed in accordance with the guidelines of the animal ethics committees of Kobe University Graduate School of Medicine, Juntendo University School of Medicine, and Gunma University.
Data Availability.
All data are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We thank E. D. Rosen and J. Eguchi for generating and providing Adipoq-Cre mice. We are also grateful to M. Murakami for technical assistance. This study was supported, in part, by Japan Society for the Promotion of Science KAKENHI Grants JP19H03709 (to W.O.), JP15K09389, and JP18K08478 (to T.H.) as well as by grants from Yamaguchi Endocrine Research Foundation (to T.H.), from Suzuken Memorial Foundation (to T.H.), and from Novartis (to W.O. and T.H.). T.H. was supported by the grant “Collaborative Research Program” between Kobe University and Eli Lilly, Japan (to S.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921015117/-/DCSupplemental.
References
- 1.Giorgino F., Laviola L., Eriksson J. W., Regional differences of insulin action in adipose tissue: Insights from in vivo and in vitro studies. Acta Physiol. Scand. 183, 13–30 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Fasshauer M., Paschke R., Regulation of adipocytokines and insulin resistance. Diabetologia 46, 1594–1603 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Fève B., Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 19, 483–499 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Blüher M., et al. , Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 3, 25–38 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Eguchi J., et al. , Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 13, 249–259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher J., et al. , Differential roles of insulin and IGF-1 receptors in adipose tissue development and function. Diabetes 65, 2201–2213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Softic S., et al. , Lipodystrophy due to adipose tissue-specific insulin receptor knockout results in progressive NAFLD. Diabetes 65, 2187–2200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang-Doran I., Sleigh A., Rochford J. J., O’Rahilly S., Savage D. B., Lipodystrophy: Metabolic insights from a rare disorder. J. Endocrinol. 207, 245–255 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil G. S., Inflammation and metabolic disorders. Nature 444, 860–867 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Mothe-Satney I., et al. , Adipocytes secrete leukotrienes: Contribution to obesity-associated inflammation and insulin resistance in mice. Diabetes 61, 2311–2319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spite M., et al. , Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J. Immunol. 187, 1942–1949 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P., et al. , LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 21, 239–247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce L. R., Komander D., Alessi D. R., The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Accili D., Arden K. C., FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Inoue H., et al. , Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 3, 267–275 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Spiegelman B. M., Flier J. S., Obesity and the regulation of energy balance. Cell 104, 531–543 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Kadowaki T., et al. , Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimomura I., Hammer R. E., Ikemoto S., Brown M. S., Goldstein J. L., Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401, 73–76 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T., et al. , The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Colas R. A., Shinohara M., Dalli J., Chiang N., Serhan C. N., Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307, C39–C54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rådmark O., Werz O., Steinhilber D., Samuelsson B., 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 32, 332–341 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Stables M. J., Gilroy D. W., Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 50, 35–51 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Terawaki K., et al. , Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J. Immunol. 175, 4217–4225 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T., A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 387, 620–624 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Griffiths R. J., et al. , Leukotriene B4 plays a critical role in the progression of collagen-induced arthritis. Proc. Natl. Acad. Sci. U.S.A. 92, 517–521 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osher E., Weisinger G., Limor R., Tordjman K., Stern N., The 5 lipoxygenase system in the vasculature: Emerging role in health and disease. Mol. Cell. Endocrinol. 252, 201–206 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Ying W., et al. , Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J. Clin. Invest. 127, 1019–1030 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajimura S., Spiegelman B. M., Seale P., Brown and beige fat: Physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidossis L., Kajimura S., Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest. 125, 478–486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paik J. H., et al. , FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada K., Hosooka T., Shinohara M., Ogawa W., Modulation of lipid mediator profile may contribute to amelioration of chronic inflammation in adipose tissue of obese mice by pioglitazone. Biochem. Biophys. Res. Commun. 505, 29–35 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Sasaki T., et al. , Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia 57, 819–831 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki T., et al. , Overexpression of insulin receptor partially improves obese and diabetic phenotypes in db/db mice. Endocr. J. 62, 787–796 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Hosooka T., et al. , Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-gamma phosphorylation. Nat. Med. 14, 188–193 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Norseen J., et al. , Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell. Biol. 32, 2010–2019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto M., et al. , Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signaling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes 51, 1672–1680 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Kitamura T., et al. , Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol. Cell. Biol. 19, 6286–6296 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakae J., Kitamura T., Silver D. L., Accili D., The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Invest. 108, 1359–1367 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wick M. J., et al. , Substitution of the autophosphorylation site Thr516 with a negatively charged residue confers constitutive activity to mouse 3-phosphoinositide-dependent protein kinase-1 in cells. J. Biol. Chem. 277, 16632–16638 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Sakai T., et al. , Skp2 controls adipocyte proliferation during the development of obesity. J. Biol. Chem. 282, 2038–2046 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and SI Appendix.