Abstract
The SARS-CoV-2-caused COVID-19 pandemic has resulted in a devastating threat to human society in terms of health, economy, and lifestyle. Although the virus usually first invades and infects the lung and respiratory track tissue, in extreme cases, almost all major organs in the body are now known to be negatively impacted often leading to severe systemic failure in some people. Unfortunately, there is currently no effective treatment for this disease. Pre-existing pathological conditions or comorbidities such as age are a major reason for premature death and increased morbidity and mortality. The immobilization due to hospitalization and bed rest and the physical inactivity due to sustained quarantine and social distancing can downregulate the ability of organs systems to resist to viral infection and increase the risk of damage to the immune, respiratory, cardiovascular, musculoskeletal systems and the brain. The cellular mechanisms and danger of this “second wave” effect of COVID-19 to the human body, along with the effects of aging, proper nutrition, and regular physical activity, are reviewed in this article.
Keywords: Aging, Brain, Cardiovascular, COVID-19, Immune, Muscle, Nutrition, Physical inactivity, Respiratory
Introduction
Coronavirus disease (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first detected in December 2019 in the city of Wuhan, China.1, 2, 3 Currently, this pandemic has infected more than 15 million people in nearly 210 countries around the world resulting in nearly 600,000 deaths. A pandemic of this scale has never been seen since the Spanish Influenza during WWI, and has already created dramatic challenges all over the world in terms of economy, social interactions, and individual lifestyles. Coronaviruses are one of the largest (27–34 kilobase) positive-stranded non-segmented RNA viruses, named after the ∼120 nm diameter envelop (resembles of solar corona) around the nucleic acid-protein complex. The foremost damage of the virus is on human health, including direct injury to the respiratory system, compromise of the immune system, exacerbation of the underlying medical conditions, and eventually systematic failure and death.4 Due to the COVID-19 attack, tens of thousands of patients have been hospitalized, with additional thousands of millions of people forced to stay in limited space. Conceivably, this dramatic change in lifestyle, resulting from immobilization (hospitalization and bed rest), quarantine, and physical inactivity can cause a second-wave attack on the health and wellbeing of the infected as well as general population.5
As a major journal of sport medicine and health in the world, the Editor-in-Chiefs and the Editorial Board share a strong sense of obligation to provide an overview on the impact of COVID-19 and related physical inactivity on human health, and to offer some physical activity guidelines to individuals suffering from the adverse outcomes during the pandemic and those recovering from an infection. Thus, the goal of this review article is three-fold: 1) to highlight the COVID-19 threats and damages to the various human physiological systems; 2) to address the harm of physical inactivity associated with the virus outbreak to the body; and 3) to recommend some practical strategies to mitigate the potential damage. Specifically, we will first give a brief overview on the pathology of COVID-19 and its impact on the immune system. We will then review the impacts of the COVID-19 outbreak and physical inactivity on the respiratory, cardiovascular, and musculoskeletal systems. Special sections will be devoted to how the virus may specifically devastate the aged population and compromise the psychological and mental wellbeing. Finally, we will provide some practical suggestions as to how good nutrition and exercise training can protect against and help recovery from the virus attack. Ultimately, the harm and suffering that the coronavirus can cause to an individual is determined by not only the endowed factors such as age, sex, race, medical conditions, but also the lifestyle of the individual during the pandemic.
Impact of COVID-19 and physical inactivity on the immune system
SARS-CoV-2 causes COVID-19 characterized by the major symptoms of fever, dry cough, myalgia, and fatigue.6 Currently, there are neither vaccines nor clinically proven effective therapeutics. Convalescent plasma and anti-viral drugs (e.g., Remdesivir) have shown some promise in treating COVID-19 patients,7 but their widespread use await statistical rigor. Behavioral strategies of social distancing and hygiene are currently the best and only methods to limit the spread and reduce morbidity and mortality. As this virus strain is novel to the human immune system, we are dependent on aspects of our innate immunity to deal with the initial infection. Like most viral infections, if we survive the infection, over the course of weeks we develop antibody and cell-mediated immune responses specific to the virus. In most instances, this exposure-related ‘training’ of our immune systems offers us long-lasting protection from re-infection or, if we are re-infected, disease symptoms are much milder. However, we currently do not know if our response to SARS-CoV-2 is sufficient to be protective and long lasting. Along with tests for the presence of viral particles and plasma antibodies, a clear need exists for research related to vaccine development and research to determine whether our immune response is adequate to protect us.
The public health recommendations (i.e., stay-at-home orders, closures of parks, gymnasiums, and fitness centers) to prevent SARS-CoV-2 spread have the potential to reduce daily physical activity (PA). These recommendations are unfortunate because daily exercise may help combat the disease by boosting our immune systems and counteracting some of the co-morbidities like obesity, diabetes, hypertension, and serious heart conditions that make us more susceptible to severe COVID-19 illness.8
Exercise affects the immune system and its anti-viral defenses.9,10 Animal experiments administering influenza and herpes simplex viruses 1 (HSV-1) in the respiratory tract, have shown that moderate exercise, performed before (i.e. training) or after infection (for a few days before symptom onset), improves morbidity and mortality to the infection.11, 12, 13 Conversely, preclinical studies have also shown that intense exercise leads to poorer outcomes in response to respiratory viral infections.14,15 Follow-up studies have elucidated some understanding of the mechanisms responsible for these observations.16, 17, 18, 19
An early epidemiological study suggested that intense, prolonged exercise was associated with an increase in upper respiratory tract infections.20 This work led to the concept of the inverted J theory, where moderate exercise reduces, and prolonged, high intensity exercise increases susceptibility to infection.21 Many studies since have supported the theory with respect to individual immune parameters including those specific to viral defense. For example, salivary lactoferrin and its secretion rate increased for up to 2 hours after moderate exercise.22 Mucosal lactoferrin is important because it can prevent DNA and RNA viruses form infecting cells by binding and blocking host receptors. Conversely, low levels or secretion rates of salivary immunoglobulin A, which can bind to viruses and inactivate them, has been shown to be associated with upper respiratory tract infection in some athletes undergoing intense training.23 In addition, because PA and exercise results in profound movement of leukocytes in blood and tissues,24 many researchers theorize that being physically active increases immunosurveillance against infectious pathogens including viruses.
Despite this, whether exercise-induced changes in the immune system affect respiratory virus susceptibility in people is unclear.25 Indeed, controversy remains whether intense, prolonged exercise can alter immunity that leads to infectious disease risk25 or whether moderate exercise-induced improvements in immune response reduces it. Definitive studies where both exercise and infection are manipulated and controlled are needed and yet scarce due to ethical concerns. In one such study, moderate exercise training (40 min at 70% heart rate reserve every other day for 10 days) was initiated after nasal rhinovirus administration to determine its effects on the severity and duration of infection.26 No differences were found in self-reported symptoms or mucus weight (collected from provided facial tissues) and concluded that PA and exercise moderately are safe during a rhinovirus-induced upper respiratory tract infection. Of special note is these subjects were young, healthy college students and (other than mucus weight) and no measurement of viral infection or subsequent antibody responses were completed.
At this time, we know very little about how PA or exercise might interact with the immune system to affect SARS-CoV-2 infectivity and COVID-19 disease susceptibility. As the pandemic proceeds, it will be important to perform retrospective studies to determine whether PA status had any bearing on SARS-CoV-2 infection or COVID-19 outcome; valid virus and antibody testing protocols will aid such studies. In addition, animal models determining the effect of PA and exercise on coronavirus infection and subsequent immune responses would also be informative. Current practical advice dictates that people follow social distancing and hygiene practices, and we propose exercise can be safely incorporated. Disruption of PA and exercise routines and reducing physical fitness may increase susceptibility to infection and certainly increase some comorbidities associated with poor COVID-19 outcomes if protracted. As animal studies have documented that intense training or intense, prolonged single exercise bouts can lead to reduced immune responses, it is not prudent to begin an intense training regimen or perform highly intense prolonged exercise if you are not accustomed to such activities. A good practice is to start exercising at lower intensities and durations and build up slowly. For example, walking is the most natural and practical form of exercise and beneficial to many organ systems. For those who have underlying health conditions, consultation with a primary care provider is warranted before beginning an exercise program.
COVID-19, physical activity, and the respiratory system
While the clinical course of the COVID-19 pandemic continues to be investigated, many COVID-19 patients develop respiratory failure and require mechanical ventilation (MV) to maintain adequate pulmonary gas exchange. In this regard, a recent report reveals that ∼54% of patients hospitalized due to COVID-19 experience respiratory failure and >30% require MV.27 Although MV is often a life-saving intervention, an unwanted consequence of prolonged MV is the rapid development of respiratory muscle weakness due to diaphragm muscle atrophy and contractile dysfunction (collectively termed ventilator-induced diaphragm dysfunction, VIDD). VIDD is clinically significant because diaphragmatic weakness is a major contributor to the inability to wean patients from the ventilator.28 Many COVID-19 patients often require prolonged time on the ventilator that increases the risk of weaning problems. Patients who experience difficult weaning suffer higher morbidity and mortality than patients weaned quickly on their first attempts to separate from the ventilator29 and unfortunately, many COVID-19 patients succumb to ICU-related complications (e.g., sepsis).27 Given that respiratory muscle weakness is a primary risk factor for failure to wean from the ventilator, developing strategies to protect the diaphragm against MV-induced weakness has become a priority in critical care medicine. Interestingly, studies into the effects of endurance exercise training on the respiratory system have led the way. Details about this story follow.
Although many organ systems adapt in response to endurance exercise training, the structural and functional properties of the lung and airways are not altered due to exercise training.30 Nonetheless, while the gas-exchange side of the respiratory system does not adapt to exercise training, “the pump” side of the respiratory system does undergo adaptive changes in response to endurance exercise. Specifically, endurance exercise training promotes numerous biochemical alterations in diaphragm muscle resulting in a phenotype that is protected against several challenges including prolonged MV.31 Indeed, as few as 10 consecutive days of endurance exercise training results in significant protection against VIDD.32, 33, 34 Therefore, it is predicted that endurance trained individuals that develop COVID-19 and require ventilator support will benefit from the exercise-induced preconditioning of the diaphragm.
Unfortunately, many patients that develop COVID-19 are not endurance trained prior to infection. Nonetheless, studies into the mechanism(s) responsible for endurance training preconditioning of the diaphragm are a powerful tool in the pursuit of pharmacological treatments to prevent VIDD and reduce weaning problems in patients exposed to long-duration ventilator support. In this regard, preclinical investigations reveal that endurance exercise training alters the abundance of ∼70 cytosolic proteins and ∼25 mitochondrial proteins in the diaphragm.35 Studies investigating which of these proteins contribute to protection of the diaphragm against VIDD reveal that exercise-induced changes in both mitochondrial proteins (e.g., superoxide dismutase 2) and cytosolic proteins (e.g., heat shock protein 72) contribute to exercise preconditioning of the diaphragm.32, 33, 34, 35 This vital information has been used to develop successful pharmacological treatments to protect the diaphragm against MV-induced diaphragmatic weakness.34,36 Importantly, these preclinical studies provide an example of how exercise physiology research leads to improved health-care.
Impact of COVID-19 and physical inactivity on cardiovascular system
PA is critical to cardiovascular health and deemed essential during the pandemic. Part of the strategy to reduce the spread of the virus is through social isolation, but social isolation runs the risk of reduced PA with potential long-term consequences. Humans evolved as physically active animals and regular PA is in our genes.37,38 The effects of inactivity promote genes that are detrimental to health. Inactivity for any reason reduces heart health and increases the long-term risk of coronary artery disease and sudden cardiac death. The positive impact of PA on the prevention of coronary artery disease and sudden cardiac death reduction is well known dating back to the London Bus Drivers study.39,40 Current studies on steps per day and other measures of exercise show that regular PA promotes cardiovascular health and those who have higher levels of fitness have better exercise stress testing outcomes.41,42
The muscle aches that accompany influenza and corona viral infections are a well-known symptom and a result of direct and indirect harm to the tissue. Muscle soreness is likely due to a combination of direct tissue infection and the inflammatory response of cytokines released to fight the viral invasion. Excessive cytokine release (cytokine storm) is the dark side of the immune response that is responsible for tissue damage beyond that of the direct viral infection. While both heart and peripheral muscle are infected by viruses, heart muscle infection has both short- and long-term consequences. COVID-19 is no different and may, as a novel virus, trigger more extensive tissue damage in the heart. Heart muscle infection leads to myocarditis with the potential for acute myocardial infarction, heart failure, and/or arrhythmia.43, 44, 45 In the acute infection phase, the adrenergic release can trigger acute coronary syndrome or fatal arrhythmias.46 Systemic viral infections also cause an inflammatory reaction that irritates the lining of the arteries. In the coronary arteries, inflammation allows tears in the tissue holding plaques in place, leading to plaque rupture with clot formation and hence either fatal arrhythmia or local hypoxia and cardiac tissue death. Plaque rupture is a common cause of sudden cardiac arrest and death both at rest and during exercise. Muscle scarring induced by viral infection can trigger potentially fatal post infection and exertion related arrhythmias, which can be fatal.47 The cardiac effects of COVID-19 can be present in concert with or after the respiratory symptoms have abated in some patients.
During the COVID-19 pandemic, PA and exercise will play both a positive and a negative role in individual health outcomes. On the negative side COVID-19 infection increases risk of cardiac damage and cardiac death during exercise and the increased risk may extend into the post infection time period. PA during any systemic viral disease is not recommended because the inflammatory reaction within the muscle cells and coronary artery walls put an affected individual at risk for sudden cardiac death during and after the infection. Data from post mortem analysis is showing this to be true for COVID-19 patients also.43,45 The accompanying myocardial scarring leaves individuals at risk for sudden cardiac death for a lifetime. Non-steroidal anti-inflammatory drugs (NSAIDs) are often used to relieve muscle discomfort but increase risk of heart events under normal circumstances. The risk is accentuated during concurrent viral infections like COVID-19, so NSAIDs are not the choice for muscle pain control during a COVID 19 viral infection.
On the positive side, regular PA and exercise promote cardiorespiratory fitness and longevity. Our recommendation for healthy individuals during and following the COVID-19 pandemic is to remain physically active and exercise while socially distanced when you are well, stop exercise when you develop symptoms or signs of an infection, and return to PA and exercise slowly following recovery. Social distancing requires some changes in perspective while exercising. Recent models suggest the 2-m diameter “bubble” of safety changes shape with movement. The slipstream of dirty air created by running or biking requires 5–20 m of spacing for a person following directly behind an infected person to stay in clean air.48 The 2-m safety zone may also be broken by forced breathing that comes with vigorous exercise based on the spread of the virus amongst church choir members who met for practice and maintained social spacing during the rehearsal; approximately 75% of those in attendance contracted the disease.
Once completely well, it is reasonable for mildly infected individuals to gradually resume PA and exercise with a goal of returning to pre-infection fitness. For people with more severe COVID-19 illness, return to PA may require testing or imaging prior to exercise. If exertion-related symptoms like palpitations, chest pain, exercise intolerance, or dyspnea occur during the return to exercise, evaluation with cardiac imaging and stress testing may be indicated to rule out COVID-19 cardiac damage before progressing to higher PA levels.
Impact of COVID-19 and physical inactivity on musculoskeletal system
Staying healthy requires daily PA. Our body is constantly sensing internal environment and responding to these changes.49 The increased demands from the contracting skeletal muscles during exercise represent a major challenge to the body homeostasis provoking a plethora of responses in several organs. The metabolic rate of the skeletal muscle can rise even 100-fold on activation when compared to resting conditions.50 In order to support the energy demand of the working muscle fibers, temporary acute responses occur in our organism to meet the PA and exercise challenge. As a result of the accumulation of activity sessions, the organism adapts to the metabolic demands. PA and exercise adaptations refer to the long-term changes that occur in our body as a consequence of PA and training. Heart hypertrophy or resting bradycardia is two well-known examples of these adaptations.50 However, the musculoskeletal system, one of the largest tissues in the body, is the main target of exercise training. Plasticity describes the ability of our muscles to adapt to variations in activity and in working demand. The adaptive event involves the whole muscle fiber structure from the sarcolemma to the mitochondria, including the myofibrils, the extracellular matrix, as well as capillaries surrounding the muscle fibers.51
Exercise is one of the most frequently prescribed therapies in both health and diseases.52 However, western societal lifestyle behaviors promote physical inactivity and sedentariness.53 This situation is greatly aggravated by the containment measures imposed by the countries to control the expansion of the recent pandemic of COVID-19. A large number of people have been asked by health authorities to stay home in quarantine for an extended period of time, and this recommendation poses a significant challenge for remaining physically active. Several models have given us information on the effects of inactivity in the musculoskeletal system: bed rest and limb immobilization are extreme experimental models. Reduction of daily walking steps may indicate a more physiological model of reduced PA that reflects the risk of long-term confinement. In terms of steps walked, ∼10,000 steps/day is generally considered as a high level of PA, while ∼1500 steps/day is classified as a low level of PA.53
Physical inactivity is associated with many detrimental effects, including loss of aerobic fitness (∼7% reduction in V̇O2 peak in healthy young adults), musculoskeletal, and cognitive decline.53 It is also accompanied with metabolic effects that include alterations in insulin signaling that leads to increased peripheral insulin resistance, an increase in inflammation, as well as alterations in adipose tissue lipolysis and mitochondrial pathways.53 In skeletal muscle physical inactivity-induced reduction in insulin sensitivity contributes to the distribution of energy substrates into other tissues, which increases central fat accumulation.54 The body needs regular muscular activity during the day, whereas some of the most powerful mechanisms regulating disease susceptibility such as the mitochondrial function and the lipoprotein metabolism are being downregulated during physical inactivity.50
PA and exercise are essential in preserving muscle mass through the activation of muscle protein synthesis.53 On the contrary, the lack of muscle contractile activity during inactivity, especially in old individuals, is a leading cause of anabolic resistance and muscle atrophy.55 Significant muscle atrophy (1–4% losses) has been reported with only 14 days of step reduction in both young and older adults.53 Skeletal muscle adapts to a prolonged physical inactivity by decreasing not only muscle fiber size (atrophy) but also muscle function and quality.56 Mechanosensor proteins, such as costameres, titin, filamin-C, and Bag3, which allow muscle fibers to sense mechanical forces, are also involved in the regulation of skeletal muscle mass. Their activation during muscle contraction regulates protein turnover through interaction with the mammalian target of rapamycin complex 1 (mTORC1) and with the main proteolytic pathways: the autophagic-lysosomal and the ubiquitin-proteasome systems.57
Mitochondrion, conventionally regarded as the “power house” of muscle energy generation, plays an important role in not only controlling the proliferation and generation of the new organelle (mitochondrial biogenesis),58 but also regulating the elimination of dysfunctional mitochondria via mitophagy and the morphological dynamics via fusion and fission.59, 60, 61 Studies in human subjects with long-term bedrest and other forms of confinements reveal that mitochondrial homeostasis is disrupted by muscle immobilization resulting in decreased protein synthesis and enhanced protein degradation.62, 63, 64 Research in rodents demonstrates that muscle immobilization for a period of 2–3 weeks dramatically reduces mitochondrial quantity and quality because of the downregulation of mitochondrial biogenesis, the upregulation of the ubiquitin-proteolysis, and the overexpression of mitophagic genes.65
Research shows that deterioration of mitochondrial homeostasis due to muscle immobilization can lead to organic and systemic inflammation, an important mechanism for COVID-19 pathogenesis. Decreased mTOR activity unleashes forkhead box O (FoxO) family transcription factor activation, which is an important reason for enhanced proteolysis and mitophagy61,62 Increased ROS generation also activates nuclear factor kappa B (NFκB) signaling to produce pro-inflammatory cytokines such as TNFα, interleukin IL)-1 and IL-6 in the muscle and exacerbates muscle atrophy and functional decline.
COVID-19 infection and the brain function
Does SARS-CoV-2 infection threat and damage brain?
Although the main risks of COVID-19 is to cause injuries to the upper and lower respiratory track and lung, other organs are not necessarily void of this viral infection. It is believed that the entry of SARS-CoV-2 in the human tissues is facilitated via angiotensin-converting enzyme 2 (ACE-2), however the poor absence of ACE-2 receptors in central nervous system (CNS) does not mean that CNS is resistant against this type of viruses.66 Indeed, it has been shown that when SARS-CoV-2 types of virus was given through intra-nasally to mice, the virus translocated into thalamus and brainstem and were significantly lethal suggesting that CNS could be one of the targets of SARS-CoV-2. It is suggested that the virus can reach the CNS via neural circuits through trans-synaptic pathways.67 The relatively long latency period of the virus of 5–12 days would allow the virus to significantly damage medullary neurons, and indeed, patients infected by SARS-CoV-2 reported severe neurologic symptoms manifested as acute cerebrovascular diseases, consciousness impairment and skeletal muscle symptoms.68 Therefore, these observations suggest that SARS-CoV-2s could belong to the group of neuroinvasive viruses.
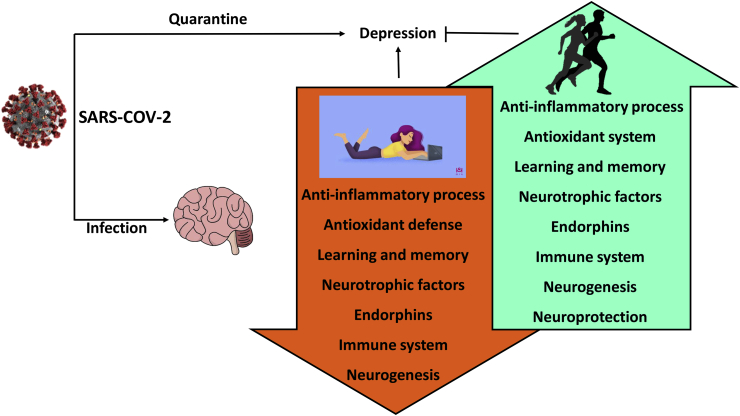
One of the most common protections against virus infections is quarantine. However, social isolation often causes psychological and mental disorders including acute stress disorder, exhaustion, detachment from others, irritability, insomnia, poor concentration indecisiveness, fear, and anxiety. Data suggest that depression, anxiety, and post traumatic disorders have significant effects on the immune system, resulting in mast cell activation, increased generation of cytokines like IL-1, IL-37, TNFα, IL-6, and C-reactive protein.69 Traumatic events activate the hypothalamic–pituitary–adrenal axis (HPA) and acute inflammation via activation of NFkB and cytokine production. Apparently quarantine-associated mental and psychological disorders weaken the protective capacity of the immune system against diseases making individuals more vulnerable. Overall, it is suggested that SARS-CoV-2 virus directly or, with associated conditions like quarantine-induced mental and psychological disorders, can damage or impact the CNS negatively.
Can physical fitness protect or attenuate the consequences of infection?
There is currently no proven medicine to treat the viral infection, however the progress and severity of virus-induced diseases could vary greatly. The general observation is that under the age of 60 years, mortality rates and severity of symptoms of SARS-CoV-2 infections are much less then in advanced age. To date, no data is available whether the level of physical fitness affects the progress of SARS-CoCV-2 infections. However, it is well documented that regular exercise induced-adaptations enhance the effectiveness of immune system,70 which actual level could affect the severity of SARS-CoV-2 infection.
However, quarantine-associated decline in the immune system as a result of the development of depression or traumatic disorders can be prevented and/or attenuated. Indeed, the inflammatory process generated by ROS can be more effectively detoxified by antioxidant systems in various organs including the brain of well-trained individuals from adaptations to exercise training.71 In addition, exercise training can efficiently decrease depression, and is one of the power modulator of the neuroprotective and anti-depressive effects of PA and exercise is the brain derived neurotrophic factor (BDNF).72 Present data suggest depression is closely linked to structural abnormalities and dysregulation of some neuroplastic mechanisms. Many brain regions are affected by depression, but the most consistently affected area in individuals with depression is the hippocampus, which is implicated in memory, emotion processing, and stress regulation.
The exercise effect on the brain can elicit systemic influences on the entire body, as exercise-induced euphoria is associated with the release of endogenous opioids (endorphins). Endorphins are identified as three distinct peptides termed alpha-endorphins, beta-endorphins, and gamma-endorphins. Euphoria is significantly increased after running and is inversely correlated with opioid binding in prefrontal/orbitofrontal cortices, the anterior cingulate cortex, bilateral insula, parainsular cortex, and temporoparietal regions (region-specific effects in frontolimbic brain areas that are involved in the processing of affective states and mood).73 Thus, regular exercise can attenuate the symptoms and consequences of quarantine-induced depression and traumatic disorders with the systemic, complex, and powerful neuroprotective effects.
Recommendation to fight against COVID-19-associated neurological and mental disorders
Since vaccination is not an available option against SARS-CoCV-2 infection at present, one alternative feasible option is to increase the effectiveness of the immune system. Research data suggest that higher level of physical fitness improves immune responses to vaccination, lowers chronic low-grade inflammation, and improves various immune markers in several disease states including cancer, acquired human immunodeficiency syndrome, cardio-vascular diseases, diabetes, cognitive impairments, and obesity.74 The adaptive effects of exercise are dependent upon the intensity and duration of exercise sessions. Available information suggests that to boost the power of the immune system, moderate intensity exercise up to 45 min is best. On the other hand, strenuous exercise can suppress immune system function leading to upper respiratory tract infections and appearance of latent viral reactivation.75 Although a debate exists regarding possible suppressing effects of severe exercise training on immune system, moderate intensity exercise clearly up-grades the power of immune system.
Since the aged population has higher risk to suffer from SARS-CoV-2 infections and generally this group benefits the most from regular PA and exercise, moderate-intensity aerobic exercise with 2–3 sessions/week lasting not less than 30 minute is suggested to as the lowest exercise dose to exert beneficial brain effects. Few studies aimed to investigate the relationship between exercise intensity and relief of depression via endorphin secretion exist. In subjects with moderate level of depression, it appears that the moderate- and high-intensity exercise can attenuate depression levels, whereas very-low intensity exercise has no effect, and the β-endorphin results are inconclusive.75 It appears that exercise-associated-dose response is individual and could be dependent on the type of exercise. Nevertheless, daily aerobic exercise is highly recommended for all individuals at all ages (Fig. 1).
Fig. 1.
. SARS-CoV-2 can directly attack central nervous system. The quarantine which is used to prevent the spreading of SARS-CoV-2 readily can cause depression, which has negative effects on CNS and immune system. Regular exercise with moderate intensity curbs the quarantine-associated harmful effects on the brain.
Impact of COVID-19 on the older adults
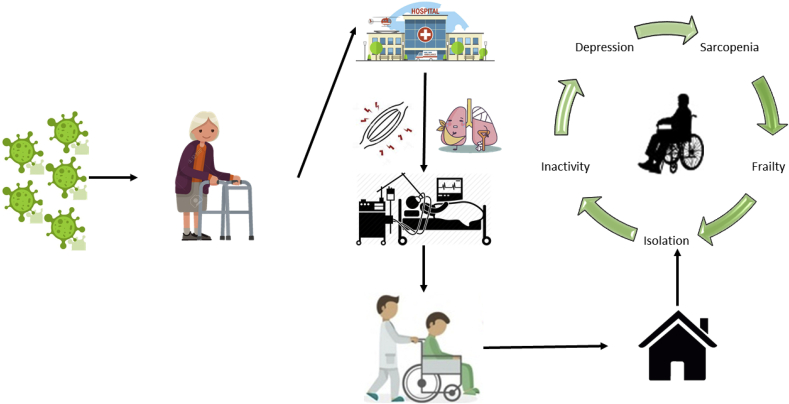
COVID-19 is having a major impact on people's lives by causing hospitalizations and deaths, but also through reducing quality of life resulting from social isolation, depression, fear, and financial crisis.1 Older adults are experience the most burdensome from COVID-1968. Indeed, the constellation of changes in cellular and physiological function that accompany the aging process make older people especially vulnerable to COVID-19. Hence, the identification of health-related parameters predisposing older adults to the negative outcomes associated with COVID-19 is of utmost importance. Here, we provide a brief description of the mechanisms through which SARS-CoV-2 infection might contribute to the development or progression of frailty and sarcopenia in older ages.
Possible effects of COVID-19 on muscle atrophy and physical function
The pathophysiological mechanisms underlying COVID-19 are under intense investigation. Evidence is accumulating that SARS-CoV-2 invades and damage multiple organs, such as the respiratory system, cardiovascular system, central nervous system, kidneys, and liver. Yet, no studies have investigated whether the virus directly damages skeletal muscle. However, information about the time-course of COVID-19 and related hospital outcomes allows speculating the disease may affect muscle homeostasis.
Notably, acute respiratory distress syndrome (ARDS), the most worrisome consequence of SARS-CoV-2 infection, seems to develop mainly in older adults with multimorbidity.69,76 ARDS involves bilateral pulmonary infiltration limiting hematosis and reducing oxygen supply for mitochondrial bioenergetics.77 Patients with ARDS are transferred to intensive care unit (ICU) to receive adequate oxygen supplementation through non-invasive or mechanical ventilation.78 The combination of ARDS and ICU-related procedures may cause a major insult to muscle by increasing protein breakdown(rhabdomyolysis) and reducing protein synthesis, thereby establishing a catabolic environment leading to severe muscle atrophy.79 Muscle wasting is experienced by 50% of ICU patients involving diaphragmatic and lower limb muscle, causing serious respiratory and physical complications that may remain for years after hospital discharge.80 Observational studies have shown that ARDS survivors have substantially lower performance on mobility tests relative to healthy age- and sex-matched people.79
Are frailty and sarcopenia possible outcomes of COVID-19
Muscle atrophy and declining physical function are key features of frailty and sarcopenia.81,82 Frailty is a geriatric syndrome characterized by reduced capacity to reach physiological homeostasis after a stressful event,81 while sarcopenia is a neurodegenerative disease that involves muscle atrophy, loss of muscle strength and power, and physical dysfunction.81 Based on the aforementioned possible complications of COVID-19, the plausibility that SARS-CoV-2 infection might promote the development of frailty and sarcopenia and accelerate their progression. Furthermore, extreme home isolation and increased physical inactivity, combined with depression and anxiety,83 could increase the susceptibility to falls or other prominent geriatric conditions (Fig. 2). These topics are certainly areas for future investigation.
Fig. 2.
The detrimental effect of COVID-19 on the development of sarcopenia and frailty among people of old age. Potential influences of physical inactivity and social isolation on the pathogenesis are illustrated.
These premises have important implications during and after hospitalization since both sarcopenia and frailty are associated with ICU complications and mortality, as well as negative outcomes after hospital discharge.84,85 Health professionals responsible for the care of older adults with COVID-19 should assess the presence of frailty and/or sarcopenia at patient admission to identify those individuals at higher risk of negative outcomes an again at discharge. Indeed, older patients who survive COVID-19 might present many conditions associated with the progression of frailty and sarcopenia, including cardiovascular, respiratory, metabolic, muscular, cognitive, psychological, and social complications. Hence, health professionals responsible for post-acute rehabilitation need to be prepared to manage weak patients with extreme fatigue when performing simple movements (e.g., sitting).
Fighting COVID-19 with proper nutrition
Nutrition is an important factor for human health, including maintaining a strong immune system. However, research up to date indicates that no single nutrient or dietary supplement can prevent or treat COVID-19. On the contrary, inappropriate intake, especially overdoses of dietary supplements might be more harmful than beneficial. Clinical data demonstrate that patients dying from COVID-19 are mostly elder people with complication from other diseases and malnutrition problem due to aging.86 Also, development of COVID-19 from mild to serious symptoms is closely related to the nutritional status.87 Therefore, assessing nutrition status is necessary and important during COVID-19 infection.
SARS-CoV-2 virus, like other coronaviruses, causes rapidly generation of free radicals and the release of cytokines (cytokine storm), leading to oxidative stress which promotes cell death and ultimately results in organ failure.88,89 Patients with COVID-19 have increased pro-inflammatory cytokines, high-sensitivity C-reactive protein (hsCRP) and increased risk for sepsis and ARDS.90 Experiences from treating SARS, MERS, and other virus infectious diseases and from clinical trials in COVID-19 patients show the beneficial effects of nutritional support against COVID-19. Through reducing oxidative stress and enhancing immunity, nutritional support helps people to lower the risk of virus infection or to alleviate the symptom of COVID-1986.
In plasm from COVID-19 patients, hsCRP, a marker of inflammation and oxidative stress, is markedly elevated.88 Therefore, increasing antioxidant status and reducing pro-inflammatory cytokine release along with regular treatments is likely an effective strategy for lowering ARDS and COVID-19 risk. Vitamin C is a commonly used antioxidant to scavenge ROS and to protect cells from oxidative stress. Intravenous (i.v.) or oral administration of high-dose vitamin C has been reported to be safe and protects against viral infection without major adverse events.91 In addition, high-dose vitamin C supplementation by i.v. administration shortened the intensive care unit (ICU) stay by 7.8% and significantly reduced mortality rate.91 Several registered clinical trials are presently being performed to investigate the effects of vitamin C in treatment of COVID-19 in several countries such as NCT04264533, NCT04357782 and NCT04335084 (ClinicalTrials.gov).
Enhancing immunity is an important means for preventing and managing viral infections. Nutritional status affects immune homeostasis while malnutrition will impair immune response to pathogens.92 Vitamins and trace elements are crucial to maintain the function of immune system.86 Therefore, supplementation of proper amounts of vitamins and trace elements may enhance immunity against COVID-19.
Vitamin D is a group of steroids responsible for absorption of calcium. Vitamin D not only plays the vital role in maintaining proper bone structure, but also a modulate s immune response. Several studies claimed that vitamin D induces antimicrobial peptides to kill the invading pathogens, including bacteria, virus, and fungi.90 Vitamin D can reduce the cytokine storm by inhibiting expression of pro-inflammatory cytokines, such as TNFα and interferon γ (IFNγ) while stimulating anti-inflammatory cytokines expression by macrophages.90 In addition, vitamin D supplementation may reduce hsCRP in diabetic patients in whom vitamin D deficiency increases the risk of ARDS.90 Therefore, vitamin D supplementation is highly recommended to reduce the risk of COVID-19. Currently, several clinical trials are underway to investigate the potential protective effects of vitamin D supplementation at different doses and durations against COVID-19 such as NCT04344041 and NCT04326725 (ClinicalTrials.gov).
Vitamin A is a group of retinoids including retinol, retinal and retinoic acid, and is one of the most important factors in maintaining immune system function. Vitamin A supplementation has been shown to reduce morbidity and mortality of measles, pneumonia, diarrhea, malaria, and HIV infection.93 Vitamin A supplementation also enhances immune response after vaccination for measles and influenza.94
Vitamin B is a group of water-soluble vitamins having different functions in the human body. Vitamin B2 could decrease the titer of MERS virus in human plasm.95 During lung injury induced by the ventilator, vitamin B3 treatment significantly inhibited the infiltration of neutrophils into the lungs and elicited a strong anti-inflammatory effect.84 In addition, vitamin B6 deficiency is known to weaken host immune response.84
Vitamin E is an antioxidant and its deficiency impairs humoral and cellular immunity.96 Vitamin E supplementation is particularly effective in improving age-related immunity. Protective effects of vitamin E supplementation on hepatic B virus infection and bacterial pneumonia infection have been reported, however, vitamin E supplementation apparently has no protective effects on acute respiratory tract infections.85 In view of the protective effect of these vitamins on viral infection, supplementation with multiple vitamins is recommended to reduce COVID-19 risk.
Other nutrients involved in strengthening immunity are trace elements such as selenium and zinc. Selenium status is correlated with the cure rate and death rate of COVID-19 patients.97 High hair selenium level has been shown to correlate positively with treatment outcomes of COVID-19 patients. The mechanism for the protective effect of selenium is likely related to the selenium-dependent enzyme glutathione peroxidases, which is an important antioxidant enzyme to reduce ROS and oxidative stress.86
Zinc is another important trace element for developing and maintaining immune system function. Previous research on the SARS coronavirus (SARS-CoV) pandemic in 2003 reported that combination of low concentrations of zinc and pyrithione inhibited coronavirus replication.98 Since the SARS-CoV-2 virus belongs to the same family of coronavirus as SARS-CoV, zinc supplementation has high potential for prevention of COVID-19. Considering the potential effect of zine against COVID-19, adding zinc along with chloroquine and hydroxychloroquine may improve the treatment outcome of patients with COVID-1998. Clinical trials are presently being conducted to estimate the synergistic action of zinc and chloroquine as therapy for COVID-19 (NCT04326725, ClinicalTrials.gov).
In summary, although definite proof for the potential effectiveness of various nutrients in alleviating harmful effects of COVID-19 is still forthcoming, supplementation of sufficient vitamins and proper trace elements is recommended to help prevent lung infection and alleviate COVID-19 symptom. Importantly, all nutritional supplements only reduce the possibility of infection and are only adjuvant therapies, whereas the only strategies for COVID-19 prevention and treatment are in the development of vaccine and drugs.
PA and exercise programming during a pandemic
Infectious and non-communicable diseases have always beset humans, but the recent appearance of COVID-19 has refocused public health perspectives to infectious disease. In the early part of the 20th century, advances in the prevention and treatment of infectious disease was primary, but deaths caused by noncommunicable disease continued to rise.99 During the last part of the 20th century, higher global death rates shifted this focus from infectious to noncommunicable diseases and the scientific community sought to better understand prevention and treatment of these diseases.100,101 The impact of PA and exercise on noncommunicable disease are well-documented53,102,103 and also impact the immune system and thus affects the bodies anti-virale defenses.8,9
Unfortunately, modern lifestyle behaviors promote physical inactivity and sedentariness.53,101, 102, 103 These poor lifestyle behaviors are intensified by social distancing and self-imposed or government mandated quarantine measures intended to reduce COVID-19 spread. These circumstances pose significant challenges for remaining physically active. During periods of isolation, all socioeconomic groups, ethnicities, and ages should maintain good health by following the WHO PA recommendations of 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity PA per week, or a combination of both. Muscle-strengthening activities involving major muscle groups are recommended on two or more days a week.105 In children/adolescents the recommendations include at least 60 minutes per day of vigorous or moderate intensity PA.106
To exercise or not to exercise when flu or COVID-19 symptoms are observed
Common COVID-19 symptoms are fever, cough, shortness of breath, and breathing difficulties. In severe cases, infection causes pneumonia, ARDS, organ failure, and even death. Symptoms usually appear within two to 14 days and are difficult for the non-health professional to differentiate between flu or COVID-19. In either case, the PA or exercising individual should seek medical diagnosis and discontinue PA and exercise immediately. Present data suggest the median time from onset to clinical recovery for mild COVID-19 cases is approximately two weeks and is three to six weeks or longer for patients with severe or critical disease.
When body aches, fatigue, fever or symptoms such as a stomachache or a hacking cough are present, bed rest is recommended until symptoms subside. Even at this point, taking a break from PA or exercise for a few days is sensible for the body to regain full function. Using the body as a guide to determine when to resume PA or exercise is always useful but be careful not to over-exert. If one is not sure whether or when to exercise, talking with your doctor is vital. When becoming PA or starting exercise after an illness, reduce PA and exercise intensity and duration for several days or even weeks. Complete recovery depends on the severity and the length of time of illness. Each individual responds and recovers differently to illnesses. Attempting PA or exercise at regular exercise intensity and duration before completely recovered, increases risk for more-serious injury or illness.
Starting an exercise program during a pandemic
When starting a PA or exercise program while in the midst of a pandemic, public health recommendations for social distancing and hygiene practices are paramount considerations when starting a PA or exercise program. Becoming physically active and reducing sedentary behavior is easily accomplished by avoiding sitting for long time periods, taking short movement or activity breaks, utilizing online exercise classes, and using mobile technologies such as telephone applications and wearable sensors to encourage movement. Examples of home exercises not requiring large spaces or equipment while easily practiced at all times of the day include walking, stair climbing, lifting and carrying groceries, chair squats, pushups, sit-ups, rope jumping, yoga, Pilates, and Tai Chi. A beginning exercise program should start at low intensities for short durations and progress slowly to more intense PA or exercise periods of longer durations. Because these activities are easily performed at home, difficulties in finding facilities with proper space and specific equipment is reduced or eliminated.
A goal of any beginning PA or exercise program is to progressively work toward completing at least one-half hour of moderate PA every day or at least twenty minutes of vigorous PA every other day of the week. Ideally, strengthening-type activities are included in daily activities at least twice a week.104 Individuals susceptible to chronic diseases such as cardiovascular or pulmonary disease should seek advice from health care providers regarding safe exercises106,107. Recommendations for children and youth aged five to 17 years are the accumulation of at least 60 minutes of moderate - to vigorous-intensity daily PA. In addition, vigorous-intensity activities that strengthen muscle and bone are recommended at least three times per week.107
If engaged in regular PA or exercise and wanting to further enhance cardiovascular and muscle fitness, suddenly beginning an intense aerobic and resistance exercise training program or performing unaccustomed highly intense prolonged exercise is not prudent, because such PA or exercise training can lead to reduced immune function. Thus, if you are already physically active or a regular exerciser but want to become more physically active, adjust exercise programming slowly and progressively to obtain new fitness goals to reduce the likelihood of any negative impact on the immune system.
Submission statement
The manuscript has not been published and is not under consideration for publication elsewhere.
Authors’ contributions
All coauthors contributed to the writing of the manuscript. Junzhi Sun, J.Larry Durstine and Li Li Ji contributed to the writing and editing of the manuscripts.
Conflict of interest
The authors have no conflict of interest to report.
References
- 1.Pan A., Liu L., Wang C., et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. J Am Med Assoc. 2020;323(19):1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond). 2020;20(2):124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabutti G., d'Anchera E., Sandri F. Coronavirus: update related to the current outbreak of COVID-19. Infect Dis Ther. 2020;8:1–13. doi: 10.1007/s40121-020-00295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasmi A., Noor S., Tippairote T., Dadar M. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020;7:108409. doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scavone C., Brusco S., Bertini M. Current pharmacological treatments for COVID-19: what's next? Br J Pharmacol. 2020 doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siordia J.A., Jr. Epidemiology and clinical features of COVID-19: a review of current literature. J Clin Virol. 2020;127:104357. doi: 10.1016/j.jcv.2020.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh N.P., Gleeson M., Shephard R.J., et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. doi: 10.1002/eji.201040296. [DOI] [PubMed] [Google Scholar]
- 10.Martin S.A., Pence B.D., Woods J.A. Exercise and respiratory tract viral infections. Exerc Sport Sci Rev. 2009;37(4):157–164. doi: 10.1097/JES.0b013e3181b7b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowder T., Padgett D.A., Woods J.A. Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun. 2005;19(5):377–380. doi: 10.1016/j.bbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Warren K.J., Olson M.M., Thompson N.J. Exercise improves host response to influenza viral infection in obese and non-obese mice through different mechanisms. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0129713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sim Y.J., Yu S., Yoon K.J., Loiacono C.M. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis. 2009;200(9):1434–1442. doi: 10.1086/606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis J.M., Kohut M.L., Colbert L.H. Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J Appl Physiol. 1985;83(5):1461–1466. doi: 10.1152/jappl.1997.83.5.1461. 1997. [DOI] [PubMed] [Google Scholar]
- 15.Murphy E.A., Davis J.M., Carmichael M.D. Exercise stress increases susceptibility to influenza infection. Brain Behav Immun. 2008;22(8):1152–1155. doi: 10.1016/j.bbi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Lowder T., Padgett D.A., Woods J.A. Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev. 2006;12:97–111. doi: 10.1016/j.bbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Murphy E.A., Davis J.M., Brown A.S., et al. Role of lung macrophages on susceptibility to respiratory infection following short-term moderate exercise training. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1354–R1358. doi: 10.1016/j.bbi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Kohut M.L., Davis J.M., Jackson D.A., et al. The role of stress hormones in exercise-induced suppression of alveolar macrophage antiviral function. J Neuroimmunol. 1998;81(1-2):193–200. doi: 10.1016/s0165-5728(97)00179-3. [DOI] [PubMed] [Google Scholar]
- 19.Kohut M.L., Martin A.E., Senchina D.S., Lee W. Glucocorticoids produced during exercise may be necessary for optimal virus-induced IL-2 and cell proliferation whereas both catecholamines and glucocorticoids may be required for adequate immune defense to viral infection. Brain Behav Immun. 2005;19(5):423–435. doi: 10.1016/j.bbi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Nieman D.C., Johanssen L.M., Lee J.W., Arabatzis K. Infectious episodes in runners before and after the Los Angeles marathon. J Sports Med Phys Fit. 1990;30(3):316–328. doi: 10.1249/00005768-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Nieman D.C. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc. 1994;26(2):128–139. doi: 10.1249/00005768-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Svendsen I.S., Hem E., Gleeson M. Effect of acute exercise and hypoxia on markers of systemic and mucosal immunity. Eur J Appl Physiol. 2016;116(6):1219–1229. doi: 10.1007/s00421-016-3380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleeson M., Bishop N., Oliveira M., McCauley T., Tauler P., Muhamad A.S. Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2012;22(3):410–417. doi: 10.1111/j.1600-0838.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 24.Rooney B.V., Bigley A.B., LaVoy E.C., et al. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: a detailed temporal analysis of leukocyte extravasation. Physiol Behav. 2018;194:260–267. doi: 10.1016/j.physbeh.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Simpson R.J., Campbell J.P., Gleeson M., et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- 26.Weidner T.G., Cranston T., Schurr T., et al. The effect of exercise training on the severity and duration of a viral upper respiratory illness. Med Sci Sports Exerc. 1998;30(11):1578–1583. doi: 10.1097/00005768-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dres M., Demoule Diaphragm dysfunction during weaning from mechanical ventilation: an underestimated phenomenon with clinical implications. Crit Care. 2018;22(1):73. doi: 10.1186/s13054-018-1992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vassilakopoulos T., Petrof B.J. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169(3):336–341. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie D.C. Respiratory physiology: adaptations to high-level exercise. Br J Sports Med. 2012;46(6):381–384. doi: 10.1136/bjsports-2011-090824. [DOI] [PubMed] [Google Scholar]
- 31.Powers S.K., Bomkamp M., Ozdemir M., et al. Mechanisms of exercise-induced preconditioning in skeletal muscles. Redox Biol. 2020:101462. doi: 10.1016/j.redox.2020.101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morton A.B., Smuder A.J., Wiggs M.P., et al. Increased SOD2 in the diaphragm contributes to exercise-induced protection against ventilator-induced diaphragm dysfunction. Redox Biol. 2019;20:402–413. doi: 10.1016/j.redox.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smuder A.J., Min K., Hudson M.B., et al. Endurance exercise attenuates ventilator-induced diaphragm dysfunction. J Appl Physiol. 2012;112(3):501–510. doi: 10.1152/japplphysiol.01086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smuder A.J., Morton A.B., Hall S.E., et al. Effects of exercise preconditioning and HSP72 on diaphragm muscle function during mechanical ventilation. J Cachexia Sarcopenia Muscle. 2019;10(4):767–781. doi: 10.1002/jcsm.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sollanek K.J., Burniston J.G., Kavazis A.N., et al. Global proteome changes in the rat diaphragm induced by endurance exercise training. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0171007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powers S.K., Hudson M.B., Nelson W.B., et al. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011;39(7):1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth F.W., Gordon S.E., Carlson C.J., et al. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–787. doi: 10.1111/j.1600-0838.2000.100509.x. [DOI] [PubMed] [Google Scholar]
- 38.Current Lightfoot. Understanding of the genetic basis for physical activity. J Nutr. 2011;141(3):526–530. doi: 10.3945/jn.110.127290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norman L.G. The health of bus drivers a study in London transport. Lancet. 1958;272(7051):807–812. doi: 10.1016/S0140-6736(58)90373-8. [DOI] [PubMed] [Google Scholar]
- 40.Morris J.N., Crawford M.D. Coronary heart disease and physical activity of work: evidence of a national necropsy survey. Br Med J. 1958;20(12):1445–1496. doi: 10.1136/bmj.2.5111.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandsager K., Harb S., Cremer P., et al. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1(6) doi: 10.1001/jamanetworkopen.2018.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee I.M., Shiroma E.J., Kamada M., et al. Association of step volume and intensity with all-cause mortality in older women. JAMA Int Med. 2019;179(8):1105. doi: 10.1001/jamanetworkopen.2018.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. Published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonow RO, Fonarow GC, O'Gara PT, et al. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. Published online March 27. DOI:10.1001/jamacardio.2020.1105. [DOI] [PubMed]
- 45.Yang C., Jin Z. An acute respiratory infection runs into the most common noncommunicable epidemic—COVID-19 and cardiovascular diseases. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0934. Published online March 25. [DOI] [PubMed] [Google Scholar]
- 46.Sribhutorn A., Phrommintikul A., Wongcharoen W., et al. The modification effect of influenza vaccine on prognostic indicators for cardiovascular events after acute coronary syndrome: observations from an influenza vaccination trial. Cardiol Res Pract. 2016;2016:4097471. doi: 10.1155/2016/4097471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson P.D., Dec G.W. We need better data on how to manage myocarditis in athletes. Eur J Prev Cardiol. 2020:1–3. doi: 10.1177/2047487320915545. 0(0) [DOI] [PubMed] [Google Scholar]
- 48.Blocken B., Malizia F., van Druenen T., et al. Towards aerodynamically equivalent COVID19 1.5 m social distancing for walking and running. 2020. http://www.urbanphysics.net/Social%20Distancing%20v20_White_Paper.pdf Preprint at.
- 49.Hawley J.A., Hargreaves M., Joyner M.J., et al. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton M.T. The role of skeletal muscle contractile duration throughout the whole day: reducing sedentary time and promoting universal physical activity in all people. J Physiol. 2018;596:1331–1340. doi: 10.1113/JP273284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pette D. Historical Perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol. 2001;90:1119–1124. doi: 10.1152/jappl.2001.90.3.1119. [DOI] [PubMed] [Google Scholar]
- 52.Vina J., Sanchis-Gomar F., Martinez-Bello V., et al. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167(1):1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowden Davies K.A., Pickles S., Sprung V.S., et al. Reduced physical activity in young and older adults: metabolic and musculoskeletal implications. Ther Adv Endocrinol Metab. 2019;10 doi: 10.1177/2042018819888824. 2042018819888824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabøl R., Petersen K.F., Dufour S., Flannery C., Shulman G.I. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci U S A. 2011;108:13705–13709. doi: 10.1073/pnas.1110105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nascimento C.M., Ingles M., Salvador-Pascual A., et al. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. 2019;132:42–49. doi: 10.1016/j.freeradbiomed.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 56.Arc-Chagnaud C., Py G., Fovet T., Roumanille R., et al. Evaluation of an antioxidant and anti-inflammatory cocktail against human hypoactivity-induced skeletal muscle deconditioning. Front Physiol. 2020;11:71. doi: 10.3389/fphys.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wackerhage H., Schoenfeld B.J., Hamilton D.L., et al. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J Appl Physiol. 2019;126:30–43. doi: 10.1152/japplphysiol.00685.2018. [DOI] [PubMed] [Google Scholar]
- 58.Hood D.A., Tryon L.D., Carter H.N., et al. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J. 2016;473:2295–2314. doi: 10.1042/BCJ20160009. [DOI] [PubMed] [Google Scholar]
- 59.Chan D.C. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–996. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 60.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji, L. L., D. Yeo and C. Kang. Muscle disuse atrophy caused by discord of intracellular signaling. Antioxidants Redox Signal. DOI: 10.1089/ars.2020.8072. [DOI] [PubMed]
- 62.Jackman R.W., Kandarian S.C. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 63.Timmons J.A., Norrbom J., Scheele C., et al. Expression profiling following local muscle inactivity in humans provides new perspective on diabetes-related genes. Genomics. 2006;87:165–172. doi: 10.1016/j.ygeno.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Schiaffino S., Dyar K.A., Ciciliot S., et al. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 65.Kang C., Goodman C.A., Hornberger T.A., et al. PGC-1α over-expression by in vivo transfection attenuates mitochondrial deterioration of skeletal muscle caused by immobilization. Faseb J. 2015;29:4092–4106. doi: 10.1096/fj.14-266619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu J., Gong E., Zhang B., et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y.C., Bai W.Z., Hirano N., et al. Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J Comp Neurol. 2013;521(1):203–212. doi: 10.1002/cne.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dowlati Y., Herrmann N., Swardfager W., et al. A meta-analysis of cytokines in major depression. Biol Psychiatr. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 70.Kruger K., Mooren F.C., Pilat C. The immunomodulatory effects of physical activity. Curr Pharmaceut Des. 2016;22(24):3730–3748. doi: 10.2174/1381612822666160322145107. [DOI] [PubMed] [Google Scholar]
- 71.Radak Z., Taylor A.W., Ohno H., et al. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001;7:90–107. doi: 10.1007/s004210000352. [DOI] [PubMed] [Google Scholar]
- 72.Kandola A., Ashdown-Franks G., Hendrikse J., et al. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. 2019;107:525–539. doi: 10.1007/s004210000352. [DOI] [PubMed] [Google Scholar]
- 73.Boecker H., Sprenger T., Spilker M.E., et al. The runner's high: opioidergic mechanisms in the human brain. Cerebr Cortex. 2008;18(11):2523–2531. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- 74.Balchin R., Linde J., Blackhurst D., et al. Sweating away depression? The impact of intensive exercise on depression. J Affect Disord. 2016;200:218–221. doi: 10.1016/j.jad.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 75.Li L.Q., Huang T., Wang Y.Q., et al. Novel coronavirus patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2019 doi: 10.1002/jmv.25757. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang R., Pan M., Zhang X., et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. April 2020 doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 78.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herridge M.S., Tansey C.M., Matté A., et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 80.Herridge M.S., Moss M., Hough C.L., et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42(5):725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 81.Hoogendijk E.O., Afilalo J., Ensrud K.E., et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 82.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 83.Jawaid A. Protecting older adults during social distancing. Science. 2020;368(6487):141–145. doi: 10.1126/science.abb7885. [DOI] [PubMed] [Google Scholar]
- 84.Flaatten H., De Lange D.W., Morandi A., et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years) Intensive Care Med. 2017;43(12):1820–1828. doi: 10.1007/s00134-017-4940-8. [DOI] [PubMed] [Google Scholar]
- 85.Ferrante L.E., Pisani M.A., Murphy T.E., Gahbauer E.A., Leo-Summers L.S., Gill T.M. The association of frailty with post-ICU disability, nursing home admission, and mortality: a longitudinal study. Chest. 2018;153(6):1378–1386. doi: 10.1016/j.chest.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74(6):110834 1–2. doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li T., Zhang Y., Gong C., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020 doi: 10.1038/s41430-020-0642-3. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang D., Hu B., Hu C., Zhu F., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grant W.B., Lahore H., McDonnell S.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boretti A., Banik B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020;12(6):1–8. doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jayawardena R., Sooriyaarachchi P., Chourdakis M., et al. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Semba, R. D. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 58(3): 719-727. DOI: 10.1017/s0029665199000944. [DOI] [PubMed]
- 94.Huang Z., Liu Y., Qi G., et al. Role of vitamin A in the immune system. J Clin Med. 2018;7(9):258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lewis E.D., Meydani S.N., Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71(4):487–494. doi: 10.1002/iub.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J.E., Taylor W., Bennett K., et al. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020 doi: 10.1093/ajcn/nqaa095. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.te Velthuis A.J., van den Worm S.H., et al. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176 1–e100117610. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fauci A.S. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32(5):675–685. doi: 10.1086/319235. [DOI] [PubMed] [Google Scholar]
- 100.Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 101.Naghavi M., Abajobir T., Bettcher D., et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piercy K.L., Troiano R.P., Ballard R.M., et al. The physical activity guidelines for Americans. J Am Med Assoc. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson E., Durstine J.L. Physical activity, exercise, and chronic diseases: a brief review. Sports Med Health Sci. 2019;1(1):3–10. doi: 10.1016/j.smhs.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.World Health Organization . 2011. Global Strategy on Diet, Physical Activity and Health.https://www.who.int/dietphysicalactivity/factsheet_adults/en/ [Google Scholar]
- 105.World Health Organization . 2011. Global Strategy on Diet, Physical Activity and Health.https://www.who.int/dietphysicalactivity/publications/physical-activity-recommendations-5-17years.pdf?ua=1 [Google Scholar]
- 106.Norton S., Matthews F.E., Barnes D.E., et al. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 107.Liguori G. eleventh ed. Wolters Kluwer; Philadelphia, PA: 2017. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]