Abstract
Coronavirus disease 2019 (COVID-19) is an infectious disease with fast spreading all over the world caused by the SARS-CoV-2 virus which can culminate in a severe acute respiratory syndrome by the injury caused in the lungs. However, other organs can be also damaged. SARS-CoV-2 enter into the host cells using the angiotensin-converting enzyme 2 (ACE2) as receptor, like its ancestor SARS-CoV. ACE2 is then downregulated in lung tissues with augmented serum levels of ACE2 in SARS-CoV-2 patients. Interestingly, ACE2+ organs reveal the symptomatic repercussions, which are signals of the infection such as dry cough, shortness of breath, heart failure, liver and kidney damage, anosmia or hyposmia, and diarrhea. ACE2 exerts a chief role in the renin-angiotensin system (RAS) by converting angiotensin II to angiotensin-(1–7) that activates Mas receptor, inhibits ACE1, and modulates bradykinin (BK) receptor sensitivity, especially the BK type 2 receptor (BKB2R). ACE2 also hydrolizes des-Arg9-bradykinin (DABK), an active BK metabolite, agonist at BK type 1 receptors (BKB1R), which is upregulated by inflammation. In this opinion article, we conjecture a dialogue by the figure of Sérgio Ferreira which brought together basic science of classical pharmacology and clinical repercussions in COVID-19, then we propose that in the course of SARS-CoV-2 infection: i) downregulation of ACE2 impairs the angiotensin II and DABK inactivation; ii) BK and its metabolite DABK seems to be in elevated levels in tissues by interferences in kallikrein/kinin system; iii) BK1 receptor contributes to the outbreak and maintenance of the inflammatory response; iv) kallikrein/kinin system crosstalks to RAS and coagulation system, linking inflammation to thrombosis and organ injury. We hypothesize that targeting the kallikrein/kinin system and BKB1R pathway may be beneficial in SARS-CoV-2 infection, especially on early stages. This route of inference should be experimentally verified by SARS-CoV-2 infected mice.
Keywords: Covid-19, Kallikrein, Kinin, Inflammation
Introduction
Background
Current evidence demonstrates that the virus SARS-CoV-2, like its relative SARS-CoV, invades target cells through the interaction of its spike proteins with an enzyme called angiotensin-converting enzyme 2 (ACE2) [1]. In this context, ACE2 is the receptor for SARS-CoV-2 and the result of the interaction between them is the development of a severe acute respiratory syndrome, a highly contagious disease known as coronavirus disease 19 (COVID-19). The major aim of this article is to propose a hypothesis concerning the potential mechanism by which the interaction between the virus and ACE2 promotes pathological phenomena, all this through a conjecture on the history of pharmacology, Sérgio Ferreira’s contributions from basic science to clinical ambit and the kallikrein/kinin system.
First, ACE2 is part of at least two physiological systems involved with a pleiad of functions (Fig. 1 ). On the one hand, its physiological role started to be unmasked in the 1940 s when Rocha e Silva, Beraldo and Rosenfeld described that bradykinin exists as a hypotensive and smooth muscle stimulating factor released by trypsin and snake venoms from a plasma “bradykininogen” [2]. Additional studies of Rocha e Silva, Ferreira and Vane revealed that certain peptides found in the Bothrops jararaca snake venom potentiated the effects of bradykinin by inhibiting its degradation especially in the lungs [3], [4]. These peptides were named bradykinin-potentiating factor or BPF.
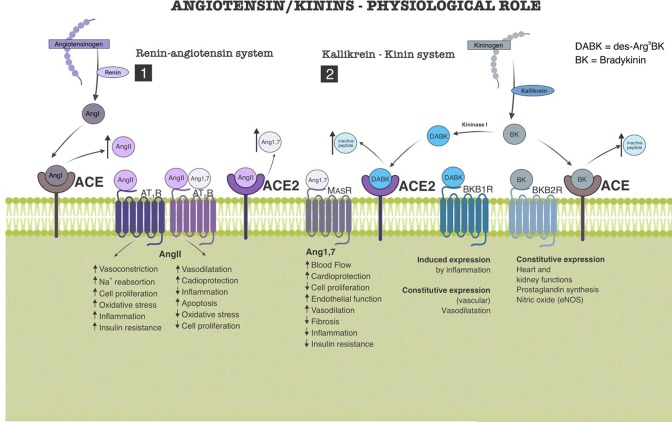
Fig. 1.
Role of renin/angiotensin system (RAS) and kallikrein/kinin system (KKS) in body homeostasis. 1) Renin cleaves angiotensinogen producing angiotensin I (AngI), which is quickly converted to angiotensin II (AngII) by the angiotensin-converting enzyme (ACE). AngII binds to two membrane receptors (AT1 and AT2) with antagonistic effects on homeostasis. AT1 activation promotes proinflammatory and increase of blood pressure and AT2 activation produces cardioprotective and anti-inflammatory effects. AT2 is also the target of Angiotensin1-7 (Ang1-7), product of ACE2 enzymatic activity upon AngII, which in turn decreases the concentration of AngII. MasR, another Ang1-7 binding site, produces anti-inflammatory, cardioprotective and hypotensive effects. (2) The crosstalk between the two pathways takes place through the ACEs which have also catabolic activities on bradykinin (BK) and its analog, des-Arg9BK (DABK). BK and DABK are products of KKS. Through kallikrein, kininogen is cleaved generating BK which is, metabolized to DABK by kininase I. The BKB1R receptor (activated by DABK), in physiological conditions, has basal activity (constitutive) and promotes vasodilation, but is upregulated in inflammatory conditions, with important effects in this scenario. BKB2R (activated by BK) is constitutive and participates in the homeostasis of organs such as heart and kidney.
On the other hand, the enzyme named angiotensin-converting enzyme (ACE) is a key component of the renin-angiotensin system (RAS), a chief and well-known system for hydroelectrolytic and blood pressure control that was initially unveiled by Tigerstedt and Bergman with the description of renin [5]. Skeggs and collaborators revealed that ACE converts angiotensin I to angiotensin II by removing from the angiotensin I the peptide histidylleucine [6], [7]. The study of Erdös and Wohler showed the inactivation of bradykinin in plasma in response to a carboxypeptidase [8]. Bakhle, Reynard and Vane demonstrated that beyond the influence with the effects of bradykinin, the BPF inhibited the conversion of angiotensin I to angiotensin II [9]. Altogether, it was evident that ACE is an endogenous enzyme whose function is shared by both angiotensin and bradykinin [10]. Finally, the studies of Ferreira, Greene and other collaborators constituted the hallmark for the development of non-peptide ACE inhibitors, e.g. captopril, one of the most common therapies against arterial hypertension [11], [12]. Most of these steps and concepts are ingrained in Ferreira's thesis and in his scientific journey [13].
Lentz and collaborators suggested that a second carboxypeptidase was responsible for the inactivation of the angiotensin II by the liberation of phenylalanine [14]. Such enzyme differed from ACE because it cleaved a single amino acid from its substrates, whereas ACE functions as a peptidyl dipeptidase [6], [7]. In fact, Tipnis and colleagues, almost simultaneously than Donoghue and collaborators, described an angiotensin-converting enzyme homolog able to cleave angiotensin I and angiotensin II but not bradykinin [15], [16]. It was named ACE2 and it is expressed constitutively in the lungs, as well as in other tissues [17], [18]. Although ACE2 is unable to cleave bradykinin, it has the bradykinin metabolite des-Arg9-bradykinin as substrate, here named DABK. The production of DABK results from the action of the kininase I on the bradykinin [16]. At this point, the crosslink between the angiotensin and the bradykinin pathway has a potential influence in the pathogenesis of COVID-19.
The hypothesis
Like other virus, SARS-CoV-2 has the ability to enter into the host cell. It has been reported that the entry of its relative SARS-CoV resulted from a SARS-S induced TNF-α-converting enzyme (TACE)-dependent shedding of the ectodomain of ACE2 and the consequent ACE2 down-regulation, a phenomenon that facilitates viral entry but also induces tissue damage via TNF-α production [19]. It has been demonstrated that the recruitment of ACE2 is protectant against lung injury [17]. A similar pathway may be adopted by SARS-CoV-2, although with a dependence of the cellular serine protease TMPRSS2 for S protein priming (virus spike proteins). The interaction between SARS-CoV-2 and ACE2 promotes viral attachment to the surface of the target cells and priming of the viral S protein by a target cell-associated transmembrane serine protease (TMPRSS2), steps that allow the fusion between the viral and the target cell membranes, the virus entry into the cell and the down-regulation of ACE2 [1]. ACE2 down-regulation is also a result of the proteolysis of the its ectodomain by TMPRSS2, which cleaves ACE2 in amino acid residues in a different manner than TACE/ADAM17 [20]. It is probable that a putative SARS-CoV-2-induced ACE2 down-regulation may be involved in the molecular basis of the severe respiratory distress caused by SARS-CoV-2. We advocate that the key event in the COVID-19 may be the interruption of the degradative pathway for angiotensin II and DABK in virtue of this down-regulated ACE2 (Fig. 2 ), which generates a triad of factors including (i) the increased levels of angiotensin II, (ii) a decreased production of angiotensin 1–7 and (iii) an augmented availability of DABK. Other mechanisms that could be involved in the sequence of these events should be the TMPRSS2 activity (kallikrein-like effect) upon plasmatic kininogen, since serine proteases has proteolytic activity upon kininogen, thus enhancing BK and DABK production [21]. SARS-COV cysteine protease can also have the same action upon kininogen and requires low pH for this activity, suggesting these enzymes as candidates for kinin production in a pathophysiological environment as shown in Fig. 2 [21], [22].
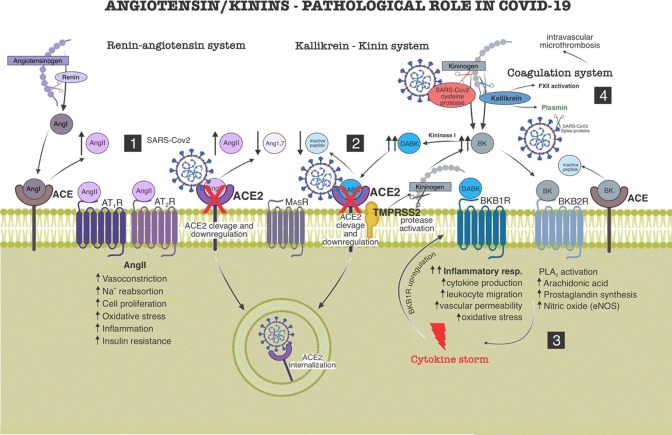
Fig. 2.
Role of renin/angiotensin system (RAS) and kallikrein/kinin system (KKS) in pathogenesis of COVID-19. (1) SARS-CoV2 (through spike proteins) binds to ACE2, mechanism by which it enters the target cell. This action promotes a downlregulation of ACE2 by sequestration and internalization, as well as by cleavage of its extracellular domain. ACE2′s downregulation causes RAS imbalance, angiotensin II (AngII) accumulation, plus Angiotensin-(1–7) (Ang1-7) decrease, which shifts AngII binding toward the AT1, leading to pro-inflammatory and cardiovascular injury mechanisms. (2) ACE2 downregulation causes imbalance of the KKS, des-Arg9BK (DABK) accumulation and BKB1R activation leading to pro-inflammatory repercussions. SARS-CoV2 invasion on the target cell also depends on a TMPRSS2 membrane protease, necessary for the cleavage of its spike protein. Active TMPRSS2 additionally cleaves the kininogen, activating the production of bradykinin (BK). The virus itself also expresses a cysteine protease which could activate the kinin pathway by interacting with kininogen. BK via BKB2R also contributes to the pro-inflammatory pathway by activating nitric oxide (NO) and prostaglandins (PGs) synthesis. (3) BKB1R is strongly upregulated by inflammatory mediators (mainly cytokines), rising endothelial permeability and leukocyte migration. A positive feedback loop between BKB1R and cytokines generates cytokines storm, that in turn, lead the body to a sepsis-like scenario. (4) Kallikrein (from KKS), additionally, causes imbalance of coagulation system by activating factor 12 (FXII) and plasmin. The two mechanisms contribute to the formation of intravascular microthrombi, observed mainly in the lung tissue. The presence of plasmin increases cleavage of spikes proteins which boosts SARS-CoV2 virulence.
Evaluation of the hypothesis
As angiotensin II degradation occurs via ACE2, a presumed downregulation of this enzyme afterwards the infection with SARS-CoV-2 may result in a potential augment in the angiotensin II levels. As depicted in Fig. 1, angiotensin II is the major biologically active peptide belonging the RAS. By acting on AT1 receptors, it promotes effects such as vasoconstriction and release of the anti-natriuretic hormone aldosterone [23]. However, angiotensin II has also AT1-mediated pro-inflammatory properties that may contribute to a detrimental role in the function of the organs in disease [24]. Free radical generation and mitochondrial dysfunction may constitute part of the potential events involved with such a detrimental effect. In lungs, angiotensin II acting on AT1 receptors induces pulmonary edema and impairs lung function [17]. Neutrophil infiltration in the lung has been associated with the angiotensin II/AT1 receptor axis, especially concerning the role of TGF-β1 as a potent profibrotic cytokine downstream angiotensin II (JIA).
In contrast, binding of angiotensin II to AT2 receptors produce, for instance, vasodilation and decreased cell proliferation [22]. The study of Imai and collaborators revealed the protector role of angiotensin II receptor 2 (AT2) by protecting mice from severe acute lung injury induced by acid aspiration or sepsis [17]. By occasion of the SARS-CoV-2 infection (Fig. 2), the protective effects mediated by AT2 receptors appear surmountable by the pro-inflammatory effects of AT1 receptors in response to the increased levels of angiotensin II. Nevertheless, the clinical scenario showed that one third of patients affected by SARS-CoV-2 had arterial hypertension disease as comorbidity [25]. The use of AT1 receptor blockers, such as losartan, is a common therapeutic option against hypertension in these patients, and Gurwitz proposed losartan as a therapeutic tool for reducing the aggressiveness and mortality from SARS‐CoV‐2 virus infections [26]. However, Marin raised the concern that the increased levels of ACE2 in patients treated with drugs that inhibit this enzyme may favor infection or aggravation of COVID-19 symptoms [27]. Thus, it is probable that other factors of the triad further contribute to the inflammatory scenario of COVID-19.
As a negative regulator of many actions of angiotensin II on AT1 receptors, the conversion of angiotensin II into angiotensin 1–7 by ACE2 (Fig. 1) produces anti-inflammatory and vasodilatory effects when angiotensin 1–7 binds to its functional receptor Mas (MasR) as proposed by Santos and collaborators [28]. Evidence exist to suggest that binding of angiotensin 1–7 to MasR counteracts angiotensin II-mediated apoptosis, angiogenesis, vasoconstriction, and inflammation in many tissues and organs including the lung. In support of this context, Gheblawi and collaborators highlighted the critical role of ACE2 as the negative regulator of the RAS by shifting the RAS peptide balance away from angiotensin II towards angiotensin 1–7 [29]. Contrarily, treatment with D-Ala-Ang-(1–7), an antagonist of the angiotensin 1–7 on Mas receptor, attenuated the protective effect of ACE2 in mice subjected to LPS-induced acute lung injury [30].
The third factor is the augmented availability of DABK (Fig. 2). As cited before, the major role in RAS is the conversion of angiotensin I to angiotensin II, but ACE is also the enzyme responsible for the degradation of bradykinin, a peptide formed by the activation of the kinin cascade [31]. The breakdown of bradykinin produces an inactive peptide, a relevant step to cease the actions of bradykinin. Once drugs such as the antihypertensive drug captopril inhibit ACE, the subsequent accumulation of bradykinin is involved with dry cough events, one of the common side effects of ACE inhibitor therapy [32]. Dry cough was reported in almost 60% of the patients with COVID-19 in Wuhan [26]; and bradykinin is potential candidate to be involved in this signal.
A putative accumulation of bradykinin in COVID-19 by an impaired degradation by ACE is unlikely, but we hypothesize that SARS-CoV-2 contributes to an augmented production of bradykinin. Niemeyer and collaborators showed that catalytic activity of papain-like cysteine proteases are critical to viral replication and may determine the virulence among SARS-coronavirus species [33]. Interestingly, papain-family cysteine proteases known as falcipains are found in Plasmodium falciparum and they have the ability to generate kinins from proteolysis of high molecular weight kininogen [34]. Such capability to release kinins suggests that these enzymes may contribute for malaria pathophysiology and we speculate whether papain-like protease (PLpro) detected in SARS-CoV-2 by Wu and collaborators may be the source of the increased levels of bradykinin in COVID-19 [35].
Ferreira and collaborators demonstrated that BK production is an important step in the activation of a cascade of cytokines that participate in the inflammatory hyperalgesia [36]. In response to the production of bradykinin, release of cytokines such as TNF-α, IL-1β, IL-6 and IL-8 occurs, which would then precede the release of prostaglandins, products of the cyclooxygenase [36], [37], [38], [39]. In addition, bradykinin is important in the pathophysiology of respiratory diseases such as asthma and acute respiratory distress syndromes by upregulating inflammatory mediators, mainly phlogistic cytokines [40].
The major constituent of the third factor of our hypothesis, however, is the high availability of DABK in virtue of its decreased degradation by a down-regulated ACE2, a metallomonocarboxypeptidase expressed not only along the respiratory tract in lung alveolar epithelial cells and in the smooth muscle of blood vessels, but also in the intestine and kidney (LI). DABK is an active metabolite of bradykinin and possesses a G protein-coupled receptor B1 (BK1) as the major site of its action [41]. Sodhi and collaborators reported a similar place of distribution for BK1 and ACE2, the apical surface of airway epithelial cells, arrange that would constitute a microenvironment for ACE2 the relationship between DABK/BK1R axis [42]. BK1 receptor has a low expression under native conditions but may be strongly upregulated by tissue offense or inflammatory mediators [43]. Sodhi and collaborators also demonstrated that lacking ACE2 function in an experimental model of lung injury activated the DABK/BK1 receptor axis with release of pro-inflammatory chemokines from airway epithelia, neutrophil infiltration, lung inflammation and injury [42].
The fourth factor is that probably kininogen/kinin system induction, both by interaction of SARS-CoV-2 cysteine protease and TMPRSS2 with kininogen, or by early inflammatory response activation of kallikrein, additionally can disturb the coagulation system (Fig. 2) [21], [35], [44], [45]. Indeed, activated factor 12 (FXIIa) from coagulation cascade induces pre-kallikrein forms plasma kallikrein that reciprocally activates FXII, generating a positive feedback loop between KKS and coagulation factors, pushing the system toward thrombus formation [46]. Miles and Cols (1983) have demonstrated that plasmin (from fibrinolytic cascade) is also activated by kallikrein, which, in turns collaborates to thrombus formation (via hyperfibrinolys with prolonged prethrombin time) together with a burst in SARS-CoV2 virulence by virus S proteins cleavage, recently reviewed by Ji and Cols (2020) [47], [48]. This could explain the evolution of COVID-19, in some subjects, to an aggressive disease with intravascular microthrombi formation that aggravates lung injury [49]. Non survivors of COVID-19 presented severe decrease in platelet count and 71.4% of them meet the criteria from International Society on Thrombosis and Hemostasis (ISTH) for disseminate intravascular coagulation, suggesting the coexistence of coagulation activation and hyperfibrinolysis in patients with severe COVID-19 infection [48], [50].
Consequences of the hypothesis and discussion
Manifestations of Covid-19 include other sites with symptoms at the digestive tract, kidney, cardiovascular system, sensory perceptions and at the central nervous system. Several studies have shown that bradykinin causes activation of peristalsis and diarrhea by activation of intestinal chloride channels [41], [51], [52], [53], [54]. Murugesan and colleagues (2016) showed in an experimental model of sepsis that a nonpeptide BK1 antagonist reduced systemic and tissue inflammatory responses in the liver and kidneys, and improved overall survival [55].
ACE2 is also expressed in cardiomyocytes, cardiac fibroblasts, and coronary endothelial cells. Overexpression of ACE2 can prevent or even reverse the heart failure phenotype, while loss of ACE2 can promote the development of heart failure [56]. In BK1 receptor knockout mice, attenuation of the cardiac inflammation and fibrosis with improved systolic and diastolic functions was seen during the development of experimental diabetic cardiomyopathy when compared to wild type mice [57]. Deficiency in ACE2 may explain BK and its metabolites such as DABK surplus on inflammatory intensification instead.
Activation of BK receptors in the medulla oblongata appears to be related to the appearance of disorders in respiratory rate, and also disturbances on taste and smell sensation caused by the accumulation of bradykinin, probably caused by ACE impairment associated with the use of ACE inhibitors captopril and enalapril [58], [59]. Such observations are in accordance with manifestations related by patients with COVID-19 [60], [61].
Indeed, there is ample evidence of BK's participation in multifaceted functions over diverse systems (Fig. 3 ). The symptoms of Covid-19 involve, in some cases, anosmia, in addition to dry cough, diarrhea, breathing difficulties (possibly aggravated by the effects of ACE2 inactivation on the CNS) and a pulmonary inflammation that leads to a dangerous triggered systemic inflammation by a cytokine storm [62]. With all these symptoms, we can make an association between ACE2 inactivation by downregulation and the accumulation of BK, even in the most dangerous phase of the disease where inflammatory reaction spreads, leading the patient to death.
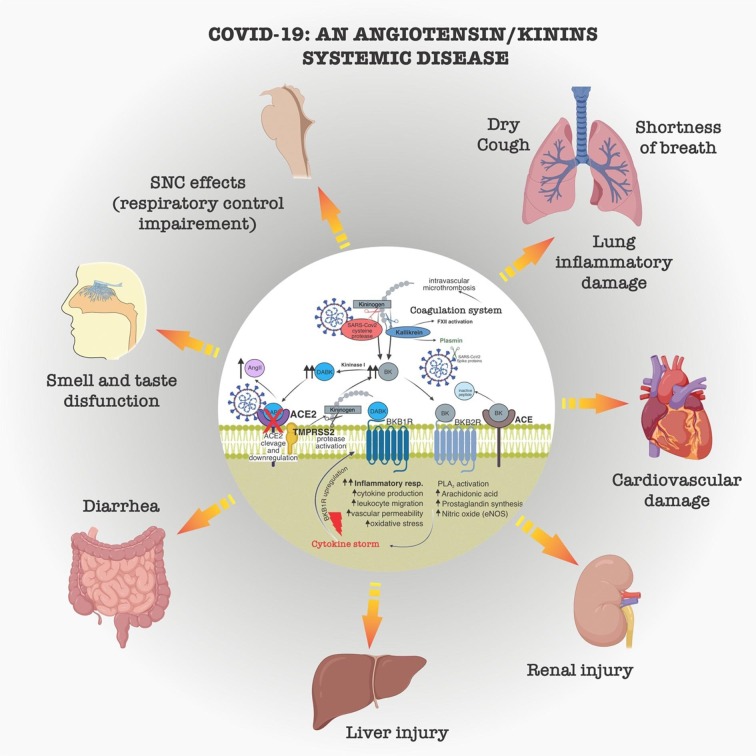
Fig. 3.
Systemic repercussion of angiotensin/kinins systems disfunctions in COVID-19. Dysfunction of the renin/angiotensin and kallikrein/kinin systems by SARS-CoV2 mainly due to downregulation of angiotensin converting enzyme (ACE2), activation of the kinin cascade, with increased angiotensin II (AngII), bradykinin (BK) and des-Arg9BK (DABK), leads to pathological repercussions in the most diverse organs. Sustained inflammation, oxidative stress, and intravascular microthrombi causes symptoms of inflammatory lung disease, shortness of breath, dry cough, dysfunction of smell and taste, changes in central breathing control, cardiovascular damage, kidney injury, steatosis and liver injury, inflammation, pain and diarrhea in the gastrointestinal tract. Cytokine storm and intravascular microthrombi may be late events related to COVID-19 mortality.
Just going back to the 80/90s, reviewing Professor Sérgio Ferreira's publications on the acute inflammatory potential of bradykinin, we can visualize this peptide as a robust molecular target for Covid-19. He would probably look very closely at the relationships compiled here. Possibly, preventing the increase in DABK (substrate of the notorious ACE2) or its action on BK1 receptors is a promising route to reverse the effects of SARS-CoV-2 as soon as possible, avoiding the stage of respiratory failure, pulmonary inflammation and the unpleasant cytokine storm.
At the beginning of this century, there was an important manifestation of patent deposits aimed at the development of pharmacological candidates aimed at antagonism of BK1 receptors as a beneficial application for pain and inflammation [63], [64]. Since the discovery of BK with Rocha e Silva up to now, it has come a long highway in which made the connection between basic and clinical science, also has led to countless findings about the role of this peptide in health and disease [65]. Lately, Qadri and Bader (2018) summarized where were drugs whose aim is the kinin BK1 receptors as a therapeutic target for inflammation. They related in most cases the pharmacological inhibition of BK1 receptor or its genetic deletion was useful for the denouement of disease in non-clinical models [66]. Then, many companies have developed BK1 receptor antagonists and tested them in phase I and II clinical trials. However, none of the developed BK1 receptor antagonists was further developed for clinical use.
Kallikrein seem to be another target to be explored, since its impairment can prevent BK and DABK formation and thus BKB1R/inflammatory loop activation, as well as the coagulation system disturbs. In 2009 was approved, by FDA, the kallikrein inhibitor DX-88 (Ecallantide, Dyax Corp.). Ecallantide is a highly specific recombinant plasma kallikrein inhibitor that halts the production of bradykinin and analogues. Actually, is one of the few drugs used to treat hereditary angioedema, a pathological condition provoked by genetic inability to express C1 protein from Complement System [67].
In the same thinking line, it is known that heparin binds to high molecular weight kininogen (HK). Binding of heparin to HK is a complex function of Zn2+ interacting with histidine to create high-affinity binding sites [68]. Thus, heparin administration, plus zinc, could be a strategy in lowering KKS activation and kinins receptors derived inflammatory effects. Heparin could also interfere with the imbalance of coagulation system related to KKS dysfunction caused by SARS-CoV2 infection. Indeed, autopsies of lung tissue from COVID-19 victims showed disseminated intravascular coagulation, which has been supposed to aggravate respiratory impairment in advanced disease [49].
In conclusion, our postulation hitched a ride on the history of Brazilian pharmacology through a hypothetical dialogue between Ferreira and his physician to try to understand what happens on the backstage of SARS-CoV-2 infection. Greater attention should be given to the participation of kallikrein, bradykinin and its metabolite des-Arg9-BK via BK1 receptors to be verified experimentally by mice infected with SARS-CoV-2 through non-clinical pharmacological tools and drugs approved for human use.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Piauí (FAPEPI).
References
- 1.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva M.R., Beraldo W.T., Rosenfeld G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am J Physiol-Legacy Content. 1949;156:261–273. doi: 10.1152/ajplegacy.1949.156.2.261. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira S.H., Rocha e Silva M. Potentiation of bradykinin and eledoisin by BPF (bradykinin potentiating factor) from Bothrops jararaca venom. Experientia. 1965;21:347–349. doi: 10.1007/bf02144709. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira S.H., Vane J.R. The disappearance of bradykinin and eledoisin in the circulation and vascular beds of the cat. Br J Pharmacol Chemotherapy. 1967;30:417–424. doi: 10.1111/j.1476-5381.1967.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tigerstedt R., Bergman P.Q. Niere und Kreislauf1. Skandinavisches Archiv Für Physiologie. 1898;8:223–271. doi: 10.1111/j.1748-1716.1898.tb00272.x. [DOI] [Google Scholar]
- 6.Skeggs L.T., Jr, Kahn J.R., Shumway N.P. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skeggs L.T., Jr, Lentz K.E., Kahn J.R., Shumway N.P., Woods K.R. The amino acid sequence of hypertensin II. J Exp Med. 1956;104:193–197. doi: 10.1084/jem.104.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdös E.G., Wohler J.R. Inhibition in vivo of the enzymatic inactivation of bradykinin and kallidin. Biochem Pharmacol. 1963;12:1193–1199. doi: 10.1016/0006-2952(63)90094-7. [DOI] [PubMed] [Google Scholar]
- 9.Bakhle Y.S., Reynard A.M., Vane J.R. Metabolism of the angiotensins in isolated perfused tissues. Nature. 1969;222:956–959. doi: 10.1038/222956a0. [DOI] [PubMed] [Google Scholar]
- 10.Schmaier A.H. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am J Physiol Regulatory, Integrative and Comparative Physiology. 2003;285:R1–R13. doi: 10.1152/ajpregu.00535.2002. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira S.H., Bartelt D.C., Greene L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9:2583–2593. doi: 10.1021/bi00815a005. [DOI] [PubMed] [Google Scholar]
- 12.Stewart J.M., Ferreira S.H., Greene L.J. Bradykinin potentiating peptide PCA-Lys-Trp-Ala-Pro. Biochem Pharmacol. 1971;20:1557–1567. doi: 10.1016/0006-2952(71)90284-x. [DOI] [PubMed] [Google Scholar]
- 13.Downey P. Profile of Sergio Ferreira. Proc Natl Acad Sci. 2008;105:19035–19037. doi: 10.1073/pnas.0811464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lentz K.E., Skeggs L.T., Jr, Woods K.R., Kahn J.R., Shumway N.P. The amino acid composition of hypertensin ii and its biochemical relationship to hypertensin I. J Exp Med. 1956;104:183–191. doi: 10.1084/jem.104.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.m002615200. [DOI] [PubMed] [Google Scholar]
- 16.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000:87. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T. Modulation of TNF- -converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF- production and facilitates viral entry. Proc. Natl. Acad. Sci. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2013;88:1293–1307. doi: 10.1128/jvi.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalmanach G., Naudin C., Lecaille F., Fritz H. Kininogens: More than cysteine protease inhibitors and kinin precursors. Biochimie. 2010;92:1568–1579. doi: 10.1016/j.biochi.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Solowiej J., Thomson J.A., Ryan K., Luo C., He M., Lou J. Steady-state and pre-steady-state kinetic evaluation of severe acute respiratory syndrome coronavirus (SARS-CoV) 3CLproCysteine protease: development of an ion-pair model for catalysis. Biochemistry. 2008;47:2617–2630. doi: 10.1021/bi702107v. [DOI] [PubMed] [Google Scholar]
- 23.Lumbers E.R. Angiotensin and aldosterone. Regul Pept. 1999;80:91–100. doi: 10.1016/s0167-0115(99)00026-9. [DOI] [PubMed] [Google Scholar]
- 24.Benigni A., Cassis P., Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin GH. Facts and reflections on COVID-19 and anti-hypertensives drugs, Drug Discov Ther. 2020;26. doi: 10.5582/ddt.2020.01017. [DOI] [PubMed]
- 28.Santos RAS, e Silva ACS, Maric C, Silva DMR, Machado RP, de Buhr I, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci 2003;100:8258–63. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed]
- 29.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res. 2020 doi: 10.1161/circresaha.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye R., Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol. 2020;113 doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- 31.Abbas A., Gorelik G., Carbini L.A., Scicli A.G. Angiotensin-(1–7) induces Bradykinin-mediated hypotensive responses in anesthetized rats. Hypertension. 1997;30:217–221. doi: 10.1161/01.hyp.30.2.217. [DOI] [PubMed] [Google Scholar]
- 32.Wood R. Bronchospasm and cough as adverse reactions to the ACE inhibitors captopril, enalapril and lisinopril. A controlled retrospective cohort study. Br J Clin Pharmacol. 1995;39:265–270. doi: 10.1111/j.1365-2125.1995.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niemeyer D., Mösbauer K., Klein E.M., Sieberg A., Mettelman R.C., Mielech A.M. The papain-like protease determines a virulence trait that varies among members of the SARS-coronavirus species. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotrin S.S., Gouvêa I.E., Melo P.M.S., Bagnaresi P., Assis D.M., Araújo M.S. Substrate specificity studies of the cysteine peptidases falcipain-2 and falcipain-3 from Plasmodium falciparum and demonstration of their kininogenase activity. Mol Biochem Parasitol. 2013;187:111–116. doi: 10.1016/j.molbiopara.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira S.H., Lorenzetti B.B., Poole S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol. 1993;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole S., Lorenzetti B.B., Cunha J.M., Cunha F.Q., Ferreira S.H. Bradykinin B1 and B2 receptors, tumour necrosis factorαand inflammatory hyperalgesia. Br J Pharmacol. 1999;126:649–656. doi: 10.1038/sj.bjp.0702347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tonussi C.R., Ferreira S.H. Bradykinin-induced knee joint incapacitation involves bradykinin B2 receptor mediated hyperalgesia and bradykinin B1 receptor-mediated nociception. Eur J Pharmacol. 1997;326:61–65. doi: 10.1016/s0014-2999(97)00153-2. [DOI] [PubMed] [Google Scholar]
- 39.Pan Z.K., Zuraw B.L., Lung C.C., Prossnitz E.R., Browning D.D., Ye R.D. Bradykinin stimulates NF-kappaB activation and interleukin 1beta gene expression in cultured human fibroblasts. J Clin Invest. 1996;98:2042–2049. doi: 10.1172/jci119009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes PJ. Effect of bradykinin on airway function, Agents Actions Suppl. 1992 Pt3:432-8. Review. PubMed PMID: 1462877. [PubMed]
- 41.Manning D.C., Snyder S.H., Kachur J.F., Miller R.J., Field M. Bradykinin receptor-mediated chloride secretion in intestinal function. Nature. 1982;299:256–259. doi: 10.1038/299256a0. [DOI] [PubMed] [Google Scholar]
- 42.Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol-Lung Cell Mol Physiol. 2018;314:L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhr F., Lowry J., Zhang Y., Brovkovych V., Skidgel R.A. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides. 2010;44:145–154. doi: 10.1016/j.npep.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagnaresi P., de Barros N.M., Assis D.M., Melo P.M., Fonseca R.G., Juliano M.A. Intracellular proteolysis of kininogen by malaria parasites promotes release of active kinins. Malar J. 2012;11:156. doi: 10.1186/1475-2875-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davie E.W., Ratnoff O.D. Waterfall Sequence for Intrinsic Blood Clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 47.Miles L.A., Greengard J.S., Griffin J.H. A comparison of the abilities of plasma kallikrein, β-factor XIIa, factor XIa and urokinase to activate plasminogen. Thromb Res. 1983;29:407–417. doi: 10.1016/0049-3848(83)90244-x. [DOI] [PubMed] [Google Scholar]
- 48.Ji H.-L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100:1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fishlock D.J. Effect of bradykinin on the human isolated small and large intestine. Nature. 1966;212:1533–1535. doi: 10.1038/2121533a0. [DOI] [PubMed] [Google Scholar]
- 52.Gaginella T.S., Kachur J.F. Kinins as mediators of intestinal secretion. Am J Physiol Gastrointestinal Liver Physiol. 1989;256:G1–G15. doi: 10.1152/ajpgi.1989.256.1.g1. [DOI] [PubMed] [Google Scholar]
- 53.Hu H.-Z., Gao N., Liu S., Ren J., Wang X., Xia Y. Action of bradykinin in the submucosal plexus of guinea pig small intestine. J Pharmacol Exp Ther. 2004;309:320–327. doi: 10.1124/jpet.103.059188. [DOI] [PubMed] [Google Scholar]
- 54.Baird A.W., Skelly M.M., O’Donoghue D.P., Barrett K.E., Keely S.J. Bradykinin regulates human colonic ion transport in vitro. Br J Pharmacol. 2009;155:558–566. doi: 10.1038/bjp.2008.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murugesan P., Jung B., Lee D., Khang G., Doods H., Wu D. Kinin B1 receptor inhibition with BI113823 reduces inflammatory response, mitigates organ injury, and improves survival among rats with severe sepsis. J Infect Dis. 2015;213:532–540. doi: 10.1093/infdis/jiv426. [DOI] [PubMed] [Google Scholar]
- 56.Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID‐19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin‐Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Journal of the American Heart Association 2020;9. doi: 10.1161/jaha.120.016219. [DOI] [PMC free article] [PubMed]
- 57.Westermann D., Walther T., Savvatis K., Escher F., Sobirey M., Riad A. Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. 2009;58:1373–1381. doi: 10.2337/db08-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ackerman BH, Kasbekar N. Disturbances of taste and smell induced by drugs. Pharmacotherapy, 1997;482-96. Review. PubMed PMID: 9165552. [PubMed]
- 59.Fior D.R., Martins D.T., Lindsey C.J. Localization of central pressor action of bradykinin in medulla oblongata. Am J Physiol-Heart and Circulatory Physiol. 1993;265:H1000–H1006. doi: 10.1152/ajpheart.1993.265.3.h1000. [DOI] [PubMed] [Google Scholar]
- 60.Vetter P., Vu D.L., L’Huillier A.G., Schibler M., Kaiser L., Jacquerioz F. Clinical features of Covid-19. BMJ. 2020: doi: 10.1136/bmj.m1470. [DOI] [PubMed] [Google Scholar]
- 61.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurology 2020. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- 62.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395:1033–1034. doi: 10.1016/s0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fincham C.I., Bressan A., Paris M., Rossi C., Fattori D. Bradykinin receptor antagonists – a review of the patent literature 2005–2008. Expert Opin Ther Pat. 2009;19:919–941. doi: 10.1517/13543770902994389. [DOI] [PubMed] [Google Scholar]
- 64.Bozó É., Éles J., Keserű G.M. Bradykinin B1 receptor antagonists: a patent update 2009–2012. Expert Opin Ther Pat. 2012;22:1443–1452. doi: 10.1517/13543776.2012.730521. [DOI] [PubMed] [Google Scholar]
- 65.Sharma JN. Basic and Clinical Aspects of Bradykinin Receptor Antagonists. Recent Developments in the Regulation of Kinins, Springer International Publishing; 2014, p. 1–14. doi: 10.1007/978-3-319-06683-7_1.
- 66.Qadri F., Bader M. Kinin B1 receptors as a therapeutic target for inflammation. Expert Opinion on Therapeutic Targets. 2017;22:31–44. doi: 10.1080/14728222.2018.1409724. [DOI] [PubMed] [Google Scholar]
- 67.Stolz L.E., Horn P.T. Ecallantide: a plasma kallikrein inhibitor for the treatment of acute attacks of hereditary angioedema. Drugs of Today. 2010;46:547. doi: 10.1358/dot.2010.46.8.1507205. [DOI] [PubMed] [Google Scholar]
- 68.Lin Y., Pixley R.A., Colman R.W. Kinetic analysis of the role of zinc in the interaction of domain 5 of high-molecular weight kininogen (HK) with heparin†. Biochemistry. 2000;39:5104–5110. doi: 10.1021/bi992048z. [DOI] [PubMed] [Google Scholar]