Significance
Polycomb group (PcG) proteins are employed by a wide variety of eukaryotes for the maintenance of gene repression. Polycomb repressive complex 2 (PRC2), a multimeric complex of PcG proteins, catalyzes the methylation of histone H3 lysine 27 (H3K27). In the filamentous fungus, Neurospora crassa, H3K27 methylation represses scores of genes, despite the absence of canonical H3K27 methylation effectors that are present in plants and animals. We report the identification and characterization of an H3K27 methylation effector, EPR-1, in N. crassa and demonstrate its widespread presence and early eukaryotic origins with phylogenetic analyses. These findings indicate that an ancient EPR-1 may have been part of a nascent Polycomb repression system in eukaryotes.
Keywords: epigenetics, facultative heterochromatin, H3K27 methylation, Polycomb repressive complex, histone reader
Abstract
Methylation of histone H3 lysine 27 (H3K27) is widely recognized as a transcriptionally repressive chromatin modification but the mechanism of repression remains unclear. We devised and implemented a forward genetic scheme to identify factors required for H3K27 methylation-mediated silencing in the filamentous fungus Neurospora crassa and identified a bromo-adjacent homology (BAH)-plant homeodomain (PHD)-containing protein, EPR-1 (effector of polycomb repression 1; NCU07505). EPR-1 associates with H3K27-methylated chromatin, and loss of EPR-1 de-represses H3K27-methylated genes without loss of H3K27 methylation. EPR-1 is not fungal-specific; orthologs of EPR-1 are present in a diverse array of eukaryotic lineages, suggesting an ancestral EPR-1 was a component of a primitive Polycomb repression pathway.
The establishment and maintenance of transcriptionally repressive chromatin is critical for the development of multicellular organisms (1–4). Polycomb group (PcG) proteins, originally discovered in Drosophila melanogaster (5), form multiple complexes that maintain such chromatin repression (6). Although the composition of PcG complexes varies, a few core constituents define two major classes of chromatin-modifying complexes, namely Polycomb repressive complex 1 (PRC1) and PRC2 (7). According to the “classic model,” PcG-mediated gene silencing is initiated by targeting of PRC2 to chromatin (8), which catalyzes methylation of H3K27 (9). Canonical PRC1, which contains a chromodomain protein (e.g., Polycomb in D. melanogaster and CBX2/4/6–8 in mammals), recognizes trimethylated H3K27 (10), catalyzes monoubiquitination of neighboring histone H2A lysine 119 by RING1A/B (11), and promotes chromatin compaction (12, 13). In reality, this hierarchical recruitment model is an oversimplification, as PRC1 can be recruited to PcG targets irrespective of PRC2 activity (14), and PRC1 presence is required for stable PRC2 association at many Polycomb response elements in D. melanogaster (15). Interdependence of these complexes has limited our understanding of their respective roles and the function of their associated chromatin “marks” on gene repression.
While plants and animals utilize distinct sets of accessory proteins to recognize methylated H3K27 (10, 16–18), they are generally thought to mediate repression in the context of a canonical PRC1 complex (7, 19–21). In fungal lineages that employ H3K27 methylation as a repressive chromatin mark, however, core PRC1 components are notably absent (7). This raises the question of how H3K27 methylation mediates repression in the absence of PRC1. It suggests that either 1) H3K27 methylation per se may be repressive, or 2) there is a “reader” of H3K27 methylation that functions outside the context of canonical PRC1.
To elucidate the repressive mechanism of H3K27 methylation in fungi, we developed and employed a forward genetics approach to identify effectors of Polycomb repression using Neurospora crassa. H3K27 methylation covers ∼7% of the N. crassa genome and is responsible for the repression of scores of genes (22, 23). We found four mutant alleles of an undescribed gene (NCU07505) that we show is critical for H3K27 methylation-mediated silencing and therefore named it effector of Polycomb repression 1 (epr-1). It encodes a protein with a bromo-adjacent homology (BAH) domain and plant homeodomain (PHD) finger. Although epr-1 mutants display phenotypic and gene-expression changes similar to strains lacking PRC2 components, H3K27 methylation is essentially unaffected. We demonstrate that EPR-1 forms nuclear foci, reminiscent of Polycomb bodies (24), and its genomic distribution is limited to, and dependent upon, H3K27-methylated chromatin, which may be recognized through its BAH domain. Finally, we discovered that EPR-1 orthologs are widely distributed across eukaryotes, contrary to previous reports (21, 25, 26), suggesting an ancient role of EPR-1 homologs in Polycomb repression that was then lost on multiple occasions in certain lineages.
Results
Genetic Selection for Factors Necessary for H3K27 Methylation-Mediated Repression.
In an effort to identify factors required for H3K27 methylation-mediated repression, we engineered a strain of N. crassa in which we replaced the open reading frames (ORFs) of two PRC2-repressed genes (23), NCU05173 and NCU07152, with the antibiotic-resistance genes hph and nat-1, respectively (Fig. 1A). Strains that bear these gene replacements and lack the H3K27 methyltransferase (SET-7) are resistant to Hygromycin B and Nourseothricin, whereas a wild-type strain with these gene replacements is sensitive to these drugs (Fig. 1C). We subjected conidia collected from such an antibiotic-sensitive, otherwise wild-type strain to UV mutagenesis and selected for mutants that derepressed both the hph and nat-1 genes (Fig. 1B). One mutant isolated in this manner and characterized here is epr-1 (Fig. 1C).
Fig. 1.
Forward genetics identifies a gene, epr-1, required for H3K27 methylation-mediated repression. (A) mRNA-seq results for two genes repressed by the N. crassa H3K27 methyltransferase, encoded by set-7. (B) Selection scheme, utilizing reporter genes illustrated in A, to identify factors required for H3K27 methylation-mediated silencing. (C) Serial dilution spot test silencing assay for the indicated strains plated on the indicated media. All strains harbor PNCU05173::hph and PNCU07152::nat-1. (D) Scheme for genetic mapping of critical mutation in epr-1UV1. (E) Whole-genome sequencing of pooled epr-1UV1 mutant genomic DNA identified a region on the left arm of linkage group (LG) I that is enriched for Oak Ridge SNPs and contains a premature stop codon in the BAH domain of NCU07505 (BAH domain, light blue; PHD finger [split], dark blue; no annotated domains, gray). Each translucent point represents a running average of SNPs (window size = 10 SNPs, step size = 1 SNP). (F) Serial dilution spot test silencing assay for the indicated strains. epr-1UV1 + NCU07505WT has a wild-type copy of NCU07505 at the his-3 locus. All strains harbor PNCU05173::hph.
Mapping and Identification of epr-1 as NCU07505.
In order to map and identify the causative mutation in the epr-1UV1 mutant, we crossed epr-1UV1, which is in an Oak Ridge genetic background, to a highly polymorphic wild-type strain named “Mauriceville” (27). We then pooled the genomic DNA from Hygromycin B-resistant progeny and subjected it to whole-genome sequencing (∼15× coverage) (Fig. 1D). When we scored the percentage of Oak Ridge single-nucleotide polymorphisms (SNPs) across the genome (28), we found a region on linkage group I that was enriched for Oak Ridge SNPs and included an early stop mutation (Q206*, CAG→TAG) in NCU07505 (Fig. 1E).
To verify that the early stop in NCU07505 is the causative mutation in epr-1UV1, we targeted a wild-type copy of NCU07505 to the his-3 locus in the epr-1UV1 mutant background. This ectopic copy of NCU07505 complemented the mutation (i.e., it restored drug sensitivity) (Fig. 1F). In addition, we found that deletion of NCU07505 resulted in resistance to Hygromycin B, similar to the epr-1UV1 strain (Fig. 1F). We subsequently isolated and characterized three additional alleles of epr-1 generated in the mutagenesis, further supporting the notion that mutations in NCU07505 support drug resistance (SI Appendix, Fig. S1).
EPR-1 and SET-7 Repress an Overlapping Set of H3K27-Methylated Genes.
Although our selection was designed to isolate mutants with specific defects in Polycomb repression, we could conceivably recover mutants that globally altered transcription or led to antibiotic resistance independent of hph or nat-1 up-regulation. To determine if EPR-1 was specifically required for repression of H3K27-methylated genes, we performed mRNA sequencing (mRNA-seq) on ∆epr-1 siblings and compared the gene-expression profile to previously published wild-type and ∆set-7 datasets (23): 632 genes were up-regulated and 974 genes were down-regulated greater than twofold in ∆epr-1 strains compared to wild-type strains (P < 0.05) (SI Appendix, Fig. S2A). The up-regulated gene set in ∆epr-1 was significantly enriched for H3K27-methylated genes [χ2(1, n = 632) = 40.8, P = 1.684 × 10−10] (SI Appendix, Fig. S2A), and H3K27-methylated genes up-regulated in both ∆epr-1 and ∆set-7 significantly overlapped (P = 1.436 × 10−16) (Fig. 2A). To verify our mRNA-seq results, we performed reverse transcription followed by quantitative PCR (RT-qPCR) on RNA isolated from biological triplicates of wild-type, ∆set-7, and ∆epr-1 strains. Five of six examined H3K27-methylated genes found up-regulated in both ∆set-7 and ∆epr-1 strains by mRNA-seq were confirmed by RT-qPCR (Fig. 2B and SI Appendix, Fig. S2B). In contrast, only one of six H3K27-methylated genes found exclusively up-regulated in ∆set-7 or ∆epr-1 by mRNA-seq was confirmed by RT-qPCR (SI Appendix, Fig. S2 C and D). Thus, these data show that loss of EPR-1 derepresses a significant number of H3K27-methylated genes that are also up-regulated in strains lacking H3K27 methylation.
Fig. 2.
∆epr-1 and ∆set-7 strains share defects in transcriptional silencing and sexual development. (A) Venn diagram depicting H3K27-methylated genes that appear up-regulated by mRNA-seq in both ∆epr-1 and ∆set-7 strains, only in ∆epr-1 strains, or only in ∆set-7 strains, using a significance cutoff of log2(mutant/wild type) > 1 and P < 0.05 using the Benjamin–Hochberg correction for multiple comparisons. Significance of genes up-regulated in both ∆epr-1 and ∆set-7 strains was determined using a hypergeometric test. (B) RT-qPCR of H3K27-methylated genes that were replaced with antibiotic resistance genes (NCU07152, NCU05173) and used for initial selection of mutants, and H3K27-methylated genes that appeared up-regulated in both ∆epr-1 and ∆set-7 strains by mRNA-seq (NCU09640, NCU07624, NCU09178, NCU08570, NCU010038, NCU08097). Each value was normalized to expression of actin gene (act) and presented relative to wild type. Filled bars represent the mean from biological triplicates and error bars show SD. (***P < 0.001, **P < 0.01, *P < 0.05, and ns, not significant; all relative to wild type by two-tailed, unpaired t test). (C) Quantification of false perithecia developed in a Petri dish (85-mm diameter) after 2 wk of unfertilized growth are shown for the indicated strains. Horizontal lines and numbers indicate the mean of two biological replicates (open circles).
∆epr-1 and ∆set-7 Strains Share a Sexual Development Defect.
Since ∆epr-1 and ∆set-7 strains exhibit similar transcriptional profiles, we wondered if they also shared vegetative growth and sexual development phenotypes as well. To assess if ∆epr-1 strains have an altered vegetative growth rate, we measured linear growth rates of wild-type, ∆set-7, and ∆epr-1 strains with “race tubes” (29). We confirmed that ∆set-7 strains do not have a linear growth defect (22) and found that ∆epr-1 strains also grow at wild-type rates (SI Appendix, Fig. S2E). Loss of SET-7 has been implicated in promoting sexual development in mutants that are homozygous sterile (30). To determine if ∆set-7 and ∆epr-1 strains aberrantly promote sexual differentiation in the absence of a mating partner, we singly inoculated crossing plates (31) with wild-type, ∆set-7, or ∆epr-1 strains. After 2 wk of unfertilized growth at 25 °C, we observed the development of few false perithecia with wild-type controls, whereas ∆epr-1 and ∆set-7 developed ∼10- and 100-fold more false perithecia than wild type, respectively (Fig. 2C and SI Appendix, Fig. S2F). These data suggest that EPR-1, and SET-7 to a greater extent, repress premature sexual development, which is reminiscent of fertilization-independent seed development observed in plant Polycomb mutants (32, 33).
H3K27 Methylation Is Essentially Normal in ∆epr-1.
As a first step to assess if the transcriptional silencing and sexual development defects shared between ∆epr-1 and ∆set-7 strains were due to a common global loss of H3K27 methylation, we performed a Western blot on whole-cell lysates to detect H3K27me3 in wild-type, ∆set-7, and ∆epr-1 strains. The total levels of H3K27me3 in ∆epr-1 strains were comparable to that in wild type (Fig. 3A and SI Appendix, Fig. S3 A–C). However, because only a subset of H3K27-methylated genes are derepressed in both ∆epr-1 and ∆set-7 strains, we wanted to know if H3K27 methylation might be specifically lost at up-regulated genes in ∆epr-1 strains. To examine this possibility, we performed H3K27me2/3 chromatin immunoprecipitation followed by sequencing (ChIP-seq) on two ∆epr-1 siblings and compared the data to that for wild type (34). We found that the global distribution of H3K27me2/3 in ∆epr-1 appeared to mirror that of wild type (Fig. 3B). Comparison of H3K27me2/3 levels associated with individual genes showed good agreement between the averaged wild-type and ∆epr-1 datasets (R2 = 0.9105), and within replicate data for wild-type (R2 = 0.9272) and ∆epr-1 (R2 = 0.8941) strains (Fig. 3C and SI Appendix, Fig. S3 D and E). We did, however, identify 29 genes with a greater than twofold decrease and nine genes with a greater than twofold increase in H3K27me2/3 levels in ∆epr-1 compared to wild type. Interestingly, none of these 38 genes with altered H3K27 methylation were classified among the up-regulated or down-regulated gene sets in the mRNA-seq analysis of ∆epr-1. To validate the H3K27me2/3 ChIP-seq results, we performed H3K27me2/3 ChIP followed by quantitative PCR (ChIP-qPCR) on wild-type, ∆set-7, and ∆epr-1 strains in biological triplicate. These data confirmed wild-type levels of H3K27me2/3 at a subtelomere (Tel IL) and at the genes replaced by the antibiotic-resistance markers (NCU07152 and NCU05173), and also corroborated the loss (NCU08834) and gain (NCU02856) of H3K27me2/3 observed in the ChIP-seq of ∆epr-1 strains (Fig. 3D). Altogether, these data show that the derepression of H3K27-methylated genes in ∆epr-1 strains is not due to concomitant loss of H3K27 methylation.
Fig. 3.
epr-1 is not required for H3K27 methylation. (A) Western blot showing H3K27me3 and total histone H3 (hH3) in the indicated strains. Biological replicates are shown. The same lysate was run on separate gels, and hH3 was used as a sample processing control. (B) ChIP-seq track showing average levels of H3K27me2/3 from two biological replicates of wild-type and ∆epr-1 strains on LG VI. Open circle indicates the middle of the centromere region. Gray bar represents 500 kb. The y axis is 0 to 800 RPKM for wild type and 0 to 1,200 RPKM for ∆epr-1. (C) Scatter plot showing the correlation of H3K27me2/3 levels at all genes (black dots) in wild-type and ∆epr-1 strains based on biological replicates of ChIP-seq data. Line of best fit displayed in red (R2 = 0.9105). Representative genes that gained (NCU02856) or lost (NCU08834) H3K27me2/3 in ∆epr-1 are indicated. (D) H3K27me2/3 ChIP-qPCR to confirm ChIP-seq data at six regions in wild-type, ∆set-7 and ∆epr-1 strains: hH4 (negative control), Tel IL (unchanged H3K27me2/3 in ∆epr-1), NCU07152 (unchanged H3K27me2/3 in ∆epr-1), NCU05173 (unchanged H3K27me2/3 in ∆epr-1), NCU08834 (loss of H3K27me2/3 in ∆epr-1), and NCU02856 (gain of H3K27me2/3 in ∆epr-1). Filled bars represent the mean of biological triplicates and error bars show SD (**P < 0.01, *P < 0.05, and ns, not significant; all relative to wild type by two-tailed, unpaired t test).
EPR-1 Forms Telomere-Associated Foci Dependent on EED.
To localize EPR-1 in vivo, we used the ccg-1 promoter to drive expression of wild-type EPR-1 fused with green fluorescent protein (GFP) at its N terminus (EPR-1WT) (35) in a strain that had fluorescent markers for the nuclear membrane (ISH1), telomeres (TRF1), and centromeres (CenH3) (23). We found that EPR-1WT was restricted to the nucleus and formed distinct foci that were typically closely associated with TRF1 foci (Fig. 4A). EPR-1WT foci were significantly closer to telomeres as compared to centromeres (negative control; P = 0.0403) (SI Appendix, Fig. S4A), and the number of EPR-1WT and TRF1 foci per nucleus were not statistically different (P = 0.7422) (SI Appendix, Fig. S4B), although the majority of nuclei examined had more EPR-1WT than TRF1 foci (SI Appendix, Fig. S4C). Considering that H3K27 methylation is predominantly present near chromosome ends in N. crassa (22), it is not unexpected that a putative PcG protein, such as EPR-1, would colocalize with the telomere marker, TRF1.
Fig. 4.
EPR-1 forms telomere-associated foci that are dependent on EED and the BAH domain of EPR-1. Maximum-intensity projection images of fluorescence microscopy z-stacks showing EPR-1 (GFP-EPR-1, green) for epr-1WT (A), epr-1WT; ∆eed (B), epr-1BAH (C), and epr-1PHD (D). Telomeres (TRF1-TagRFP-T, red), centromeres (CenH3-iRFP670, magenta), the nuclear membrane (ISH1-TagBFP2, blue), and merged images are shown for reference. Each image shows a single conidium with multiple nuclei. (Scale bars, 2 µm.)
To determine if the formation of EPR-1WT foci was dependent on H3K27 methylation, we crossed our fluorescent marker strain to a strain lacking eed, a gene encoding a component of PRC2 necessary for catalytic activity (22). We could not easily introduce a deletion of the H3K27 methyltransferase gene, set-7, due to its tight genetic linkage to epr-1. Strains bearing a deletion of eed lacked distinct nuclear foci of EPR-1WT and instead displayed a diffuse nuclear distribution of EPR-1WT (Fig. 4B). Thus, an intact PRC2 complex or H3K27 methylation is required for proper EPR-1WT subnuclear localization.
An Intact BAH Domain Is Required for Normal Nuclear Distribution of EPR-1.
EPR-1 is predicted (36) to have a BAH domain and PHD finger, protein modules implicated in chromatin engagement (37, 38). To determine if these domains are necessary for the formation of the EPR-1WT foci, we created GFP–EPR-1 constructs in which a previously identified critical tryptophan in either the BAH domain (EPR-1BAH) or PHD finger (EPR-1PHD) was replaced with an alanine (39, 40). We found that EPR-1BAH displayed the same diffuse nuclear distribution as EPR-1WT in a ∆eed background, consistent with the possibility that the BAH domain mediates interaction with H3K27-methylated chromatin (Fig. 4C). In contrast, EPR-1PHD still formed nuclear foci that were equivalent to EPR-1WT in number and proximity to TRF1 foci (Fig. 4D and SI Appendix, Fig. S4D), demonstrating the nonessential nature of this conserved tryptophan residue for the normal nuclear distribution of EPR-1.
EPR-1 Localizes to H3K27 Methylation Genome-Wide.
Since proper subnuclear localization of EPR-1WT appeared to require H3K27 methylation, we performed ChIP-seq to determine if EPR-1WT genomic targets coincided with H3K27me2/3 throughout the genome (Fig. 5A). Results of EPR-1WT ChIP-seq appeared to match the distribution of H3K27me2/3 in wild type (Fig. 5A) and we found good correlation between EPR-1WT and H3K27me2/3 relative sequencing coverage over each gene (R2 = 0.8675) (SI Appendix, Fig. S5A). We also examined the genomic distribution of EPR-1PHD and EPR-1BAH mutant alleles (Fig. 5A), which were expressed at comparable levels (SI Appendix, Fig. S5 B and C). The ChIP-seq coverage of EPR-1PHD was still enriched at H3K27-methylated genes, albeit less so than EPR-1WT, while the EPR-1BAH ChIP-seq did not show enrichment (SI Appendix, Fig. S5D). To validate the ChIP-seq of GFP–EPR-1–expressing strains, we performed ChIP-qPCR for representative regions (Fig. 5B). Consistent with the ChIP-seq data, EPR-1WT—and EPR-1PHD to a lesser degree—were enriched at examined regions bearing H3K27 methylation. In addition, the ChIP-qPCR confirmed that EPR-1BAH, as well as EPR-1WT in a ∆eed background, lack such enrichment.
Fig. 5.
BAH domain of EPR-1 required for localization to H3K27-methylated chromatin. (A) ChIP-seq and DamID-seq tracks showing average levels from two biological replicates for the indicated genotypes on LG III. The y axis is 0 to 800 RPKM for H3K9me3 and H3K27me2/3 ChIP-seq, 0 to 500 RPKM for all GFP ChIP-seq, and 0 to 3,500 RPKM for all DamID-seq. Gray bar represents 500 kb. (B) GFP ChIP-qPCR to validate GFP–EPR-1 ChIP-seq data at four genomic regions: Tel IL (H3K27-methylated), NCU05173 promoter (H3K27-methylated), NCU07152 promoter (H3K27-methylated), and H3K9me3 region (negative control, LG VI centromere). All data are normalized to a negative, euchromatic control, hH4. Filled bars represent the mean and error bars show SD from three biological replicates (***P < 0.001, **P < 0.01, and ns, not significant; all relative to wild type by two-tailed, unpaired t test). (C) Serial dilution spot test silencing assay for the indicated strains. All strains harbor PNCU05173::hph and PNCU07152::nat-1.
As an orthogonal approach to ChIP, we determined the chromatin targets of EPR-1 by fusing an Escherichia coli DNA adenine methyltransferase (Dam) (41) to the C terminus of endogenous EPR-1 and assayed adenine-methylated DNA fragments by sequencing (DamID-seq) (42) (Fig. 5A). Using DamID-seq of EPR-1WT–Dam and methyl-sensitive restriction enzyme Southern blots, we found that EPR-1WT–Dam localizes to H3K27-methylated genes and this is dependent upon EED (Fig. 5A and SI Appendix, Fig. S5 E and F). Mutation of the PHD finger in the EPR-1–Dam fusion did not abolish its targeting to H3K27-methylated chromatin (Fig. 5A). These results were consistent with our ChIP-seq findings. We conclude that EPR-1 localizes to H3K27-methylated regions of the genome and that proper recruitment of EPR-1 to chromatin requires both an intact BAH domain and the integral PRC2 component, EED.
Both the BAH Domain and PHD Finger of EPR-1 Are Necessary for Gene Repression.
Our localization studies of EPR-1PHD and EPR-1BAH, while suggestive, did not directly test the role of the PHD finger and BAH domain of EPR-1 in H3K27 methylation-mediated silencing. We therefore utilized our antibiotic-resistance reporters, used in the initial selection, to test more directly their possible involvement in gene repression. We targeted ectopic copies of epr-1WT, epr-1BAH, or epr-1PHD to the his-3 locus in an epr-1UV1 strain bearing the antibiotic-resistance genes and scored drug resistance. Whereas epr-1WT restored sensitivity to Hygromycin B and Nourseothricin, epr-1BAH remained resistant to both drugs (Fig. 5C). In contrast, introduction of epr-1PHD apparently resilenced the hph, but not the nat-1, antibiotic-resistance gene (Fig. 5C). This suggests that while the PHD finger of EPR-1 is not essential for recruitment to H3K27 methylated chromatin, it is not entirely dispensable for gene silencing.
EPR-1 Is a Homolog of Plant EBS/SHL and Widely Distributed across Eukaryotes.
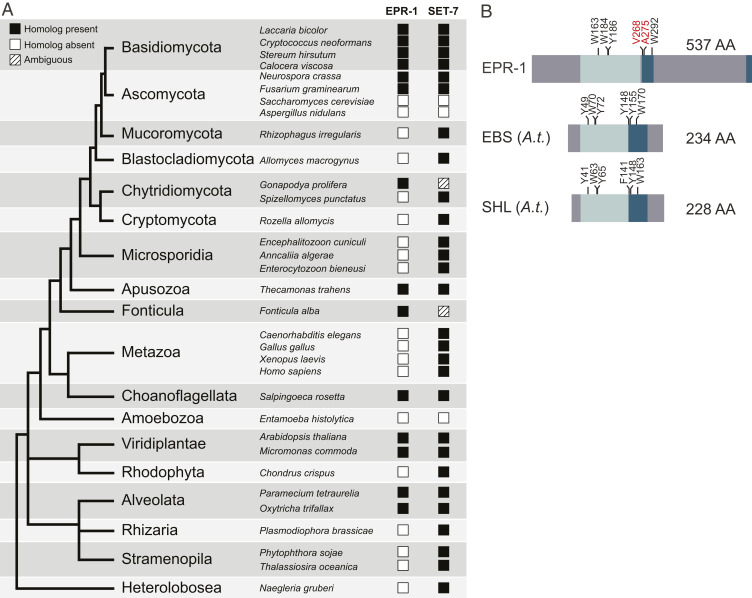
To determine if EPR-1 orthologs exist outside of N. crassa, we performed sequence similarity searches to identify homologs, followed by phylogenetic and domain architectural analysis of those to identify genuine orthologs. Consequently, we were able to identify orthologs in various fungal species as well as a wide range of other eukaryotes (Fig. 6A). Notably, we determined the Arabidopsis thaliana paralogs EBS and SHL as orthologs of EPR-1 in our analyses, which have been erroneously reported as plant-unique proteins (21, 25, 26). Similar to EPR-1, the plant paralogs, EBS and SHL, bind H3K27 methylation and have been implicated in gene repression (17, 18, 21). As one approach to investigate if other EPR-1 orthologs may have roles independent of H3K27 methylation, we checked if any species has an EPR-1 ortholog but lacks a SET-7 (H3K27 methyltransferase) ortholog. With the exception of Chytridiomycota and Fonticula lineages, in which the presence of SET-7 homologs was deemed ambiguous due to lack of a definitive pre-SET domain, all examined species with EPR-1 homologs had clear SET-7 homologs (Fig. 6A). This result is consistent with EPR-1 orthologs mediating H3K27 methylation-based repression in a wide variety of species.
Fig. 6.
EPR-1 homologs are present in species beyond fungi. (A) The presence and absence of EPR-1 and SET-7 protein homologs across major species divisions is depicted in a representative tree of eukaryotes. The leaves of the tree are labeled with the names of the divisions. Representative species are featured and their associated squares in the EPR-1 and SET-7 columns indicate the presence or absence of homologs, as indicated. (B) Protein domain structure of EPR-1, as well as EBS and SHL from A. thaliana (A.t.). BAH domains are indicated by light blue, the PHD fingers by dark blue, and regions with no known domains are gray. Aromatic amino acid residues involved in methylated histone recognition in the BAH domain and PHD finger are indicated above the protein structure diagram (black text). Red text above the PHD finger in EPR-1 highlights the absence of aromatic residues at these amino acid positions.
In contrast to EPR-1, A. thaliana EBS is not entirely restricted to H3K27-methylated genes. Indeed, the majority of EBS-bound genes are devoid of H3K27me3 and instead are associated with an “active” chromatin mark, H3K4me3 (43), via the PHD finger of EBS (17). To understand this discrepancy, we examined the underlying protein sequence of the PHD finger of EPR-1. Whereas aromatic residues implicated in methylated histone recognition in the BAH domain (44) are present in both EPR-1 and the plant paralogs, comparable residues in the PHD finger (18, 45, 46) are present in EBS and SHL, but lacking in EPR-1 (Fig. 6B, highlighted in red). Furthermore, a single amino acid substitution replacing an aromatic tyrosine residue with an alanine residue in the PHD finger of a plant SHL was sufficient to diminish in vitro H3K4me3-binding greater than twofold (18). Intriguingly, this residue corresponds to the naturally occurring alanine 275 in EPR-1 (Fig. 6B). This suggests that while many species have EPR-1 homologs, they may not necessarily be bivalent histone readers like plant EBS and SHL.
Discussion
Deciphering the basic mechanisms of Polycomb repression has been difficult, in part, due to the diversity (47), redundancy (48), as well as interdependence (15) of protein players involved. For this reason, the model organism N. crassa, which employs H3K27 methylation catalyzed by PRC2 for gene repression (22) yet conspicuously lacks PRC1 components (7), represents an ideal organism to uncover fundamental aspects of Polycomb silencing. Here we have identified an effector of H3K27 methylation in fungi, EPR-1. This provides insight into how Polycomb silencing can function in the absence of PRC1.
Our ChIP and DamID results demonstrated that EPR-1 colocalizes with H3K27 methylation genome-wide and cytological examination revealed that GFP–EPR-1 forms approximately three to five foci per nucleus. This implies that domains of H3K27 methylation within and between the seven N. crassa chromosomes generally self-associate. This is consistent with the observed intra- and interchromosomal contacts among H3K27-methylated regions of the genome in Hi-C experiments (49). Similar, and perhaps equivalent, higher-order chromatin structures, referred to as Polycomb bodies (24), are known to be mediated by PRC1 components in both plant and animal cells (50–53). Our group has previously shown that SET-7, the catalytic component of PRC2, is required for normal three-dimensional genome organization (23). It would be interesting to learn if EPR-1, the only known effector of H3K27 methylation in N. crassa, is also essential for this wild-type chromatin organization.
Despite the loss of transcriptional silencing observed in epr-1 mutants, they do not exhibit appreciably altered H3K27 methylation; this is striking for a few reasons. First, it suggests that H3K27 methylation-mediated silencing is a unidirectional pathway in N. crassa, in which EPR-1 acts downstream of PRC2. This is in contrast to findings in plants and animals, in which PRC1 components can affect the recruitment or activity of PRC2 (21, 54, 55), a fact that has hampered the elucidation of the direct role of PRC components in gene repression. Second, since ∆epr-1 strains de-repress H3K27-methylated genes without loss of H3K27 methylation, it suggests that active transcription does not necessarily preclude PRC2 activity. This was surprising since transcriptional shut-off is thought to precede PRC2 activity during normal animal development (56) and because artificial gene repression can be sufficient to recruit PRC2 (57, 58). Finally, the presence of H3K27 methylation on de-repressed genes in ∆epr-1 strains demonstrates that H3K27 methylation per se is not sufficient for effective gene repression, consistent with previous reports (59, 60). It is noteworthy, however, that while ∆set-7 and ∆epr-1 strains share transcriptional and sexual defects, the phenotype of ∆set-7 strains is generally more pronounced. This suggests that PRC2 or H3K27 methylation may have additional roles in gene repression that go beyond recruitment of EPR-1 to chromatin.
Our investigation into the phylogenetic distribution of EPR-1 homologs indicates that an ancestral EPR-1 emerged prior to the divergence of plants, animals, and fungi. This ancestral EPR-1 may have been an integral component of an early eukaryotic Polycomb silencing system. While animals are a notable exception, regarding the absence of EPR-1 homologs with a BAH–PHD structure, a human BAH domain-containing protein, BAHD1, has been reported to “read” H3K27me3 (39) and promote gene silencing (61), although apparently not interact with known PRC1 components (62). It is therefore conceivable that BAHD1 homologs present in animal lineages are actually divergent orthologs of EPR-1 that lack a PHD finger. Regardless of their ancestry, both human BAHD1 and N. crassa EPR-1 represent forms of H3K27 methylation-mediated repression that do not rely on PRC1 components.
Materials and Methods
Strains, Media and Growth Conditions.
All N. crassa strains used in this study are listed in SI Appendix, Table S1. Liquid cultures were grown with shaking at 32 °C in Vogel’s minimal medium (VMM) with 1.5% sucrose (63). Crosses were performed at 25 °C on modified VMM with 0.1% sucrose (31). Spot tests were performed at 32 °C on VMM with 0.8% sorbose, 0.2% fructose, and 0.2% glucose (FGS). When appropriate, plates included 200 µg/mL Hygromycin B Gold (InvivoGen) or 133 µg/mL Nourseothricin (Gold Biotechnology). Linear growth rates were determined as previously described except 25-mL serological pipettes were used in place of glass tubes (29). Genomic DNA was isolated as previously described (64). Nutritional supplements required for auxotrophic strains were included in all growth media when necessary.
Selection for Mutants Defective in H3K27 Methylation-Mediated Silencing.
Ten-thousand conidia of strain N6279 (created with primers in SI Appendix, Table S2) were plated on VMM supplemented with FGS and 500 µg/mL histidine and subjected to 0, 3, 6, or 9 s of UV light (Spectrolinker XL-1500 UV Crosslinker, Spectronics Corporation) in a dark room and plates were wrapped in aluminum foil to prevent photoreactivation (65). Plates were incubated at 32 °C for 16 h before being overlaid with 1% top agar containing VMM, FGS, 500 µg/mL histidine, Hygromycin B, and Nourseothricin. Drug-resistant colonies were picked after an additional 48 to 72 h at 32 °C. Initial mutant strains were crossed to a Sad-1 mutant (66) strain (N3756) and resultant progeny were germinated on Hygromycin B- and Nourseothricin-containing medium to obtain homokaryotic mutants.
Whole-Genome Sequencing, Mapping, and Identification of epr-1 Alleles.
Homokaryotic mutants resistant to Hygromycin B and Nourseothricin were crossed to the genetically polymorphic Mauriceville strain (FGSC 2225) (27) and resultant progeny were germinated on medium containing Hygromycin B and/or Nourseothricin to select for strains bearing the causative mutation. Genomic DNA from ∼15 to 20 progeny per mutant were pooled and sequencing libraries were prepared with a Nextera kit (Illumina, FC-121-1030). All whole-genome sequencing data are available in National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) (accession no. PRJNA526508). To map the approximate location of a particular causative mutation, the fraction of Oak Ridge (versus Mauriceville) SNPs across the N. crassa genome was determined as previously described (28) and visualized as a moving average (window size = 10 SNPs, step size = 1 SNP) with Matplotlib (67). We utilized FreeBayes and VCFtools to identify genetic variants present in our pooled mutant genomic DNA but absent in the original mutagenized strain (N6279) and the Mauriceville strain (68, 69). Only genetic variants of high probability that were consistent with the mapping data were considered further.
Western Blotting.
N. crassa tissue from a 16-h liquid culture of germinated conidia was collected by filtration, washed with 1× phosphate-buffered saline (PBS) (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.5), and suspended in 500 μL of ice-cold lysis buffer (50 mM Hepes [pH 7.5], 150 mM NaCl, 10% glycerol, 0.02–0.2% Nonidet P-40, 1 mM ethylenediaminetetraacetic acid [EDTA]) supplemented with 1× Halt Protease Inhibitor Cocktail (Thermo Scientific). Tissue was sonicated (Branson Sonifier-450) for three sets of 10 pulses (output = 2, duty cycle = 80), keeping the sample on ice between sets. Insoluble material was pelleted by centrifugation at 14,000 rpm at 4 °C for 10 min and the supernatant used as the Western sample. Anti-H3K27me3 (Cell Signaling Technology, 9733) and anti-hH3 (Abcam, ab1791) primary antibodies were used with IRDye 680RD goat anti-rabbit secondary (LI-COR, 926-68071). Anti-GFP (Thermo Fisher, A10262) primary antibody was used with goat anti-chicken horseradish peroxidase (HRP)-conjugated secondary antibody (Abcam, 6877). Images were acquired with an Odyssey Fc Imaging System (LI-COR) and analyzed with Image Studio software (LI-COR).
ChIP.
Liquid cultures were grown for ∼18 h with shaking at 32 °C. Tissue samples for H3K27me2/3 ChIP were cross-linked with 0.5% formaldehyde for 10 min and GFP ChIP samples were cross-linked with 1% formaldehyde for 10 min in 1× PBS. Cross-linking was quenched with 125 mM glycine, tissue was washed with 1× PBS and collected. Cells were lysed in ChIP lysis buffer (50 mM Hepes [pH 7.5], 90 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% Deoxycholate) supplemented with 1× Halt Protease Inhibitor Cocktail (Thermo Scientific) using a Branson Sonifier 450. Chromatin was sheared using a Bioruptor (Diagenode) and 2 µL of appropriate antibody (H3K27me2/3, Active Motif 39536, or GFP, MBL 598) was added and incubated with rotation at 4 °C overnight. Protein A/G agarose (40 µL; Santa Cruz Biotechnologies) was added to H3K27me2/3 ChIP samples and Protein A agarose (40 µL; Sigma) was added to GFP ChIP samples and incubated for 3 h, rotating at 4 °C. Beads were washed twice with ChIP lysis buffer supplemented with 140 mM NaCl, once with ChIP lysis buffer with 0.5 M NaCl, once with LiCl wash buffer (10 mM Tris⋅HCl [pH 8.0], 250 mM LiCl, 0.5% Nonidet P-40, 0.5% Deoxycholate, 1 mM EDTA), and once with TE (10 mM Tris⋅HCl, 1 mM EDTA), all rotating at 4 °C for 10 min each. DNA was eluted from beads by incubation in TES (10 mM Tris⋅HCl, 1 mM EDTA, 1% SDS) at 65 °C. Cross-linking was reversed by incubation at 65 °C for 16 h and then samples were treated with proteinase K for 2 h at 50 °C. DNA was purified using Minelute columns (Qiagen) and subsequently used for qPCR with the PerfeCTa SYBR Green FastMix (QuantBio, 95071-012) on a Step One Plus Real Time PCR System (Life Technologies) using primer pairs in SI Appendix, Table S3, or prepared for sequencing using the NEBNext DNA Library Prep Master Mix Set for Illumina (New England BioLabs). ChIP-seq data are available in the NCBI Gene Expression Omnibus (GEO) database (accession no. GSE128317).
ChIP-Seq Mapping and Analysis.
The suite of tools available on the open-source platform Galaxy (70) was used to map ChIP-seq reads (71) against the corrected N. crassa OR74A (NC12) genome (49) and to create bigWig coverage files normalized to reads per kilobase per million mapped reads (RPKM) (72). ChIP-seq tracks were visualized with the Integrative Genomics Viewer (73). MAnorm was used to compare H3K27me2/3 ChIP-seq coverage on all genes (designated as “peaks”) between samples and data were visualized with Matplotlib (67). Genes were scored by their normalized H3K27me2/3 ChIP-seq coverage in wild type and the top-ranking genes (873) were designated as H3K27-methylated.
RNA Isolation, mRNA-seq Library Prep, and RT-qPCR.
RNA was extracted from germinated conidia grown for 16 to 18 h with a 1:1:1 glass beads, NETS (300 mM NaCl, 1 mM EDTA, 10 mM Tris⋅HCl, 0.2% SDS), acid phenol:chloroform mixture (5:1; [pH 4.5]) using a bead beater and ethanol precipitated. RNA was treated with DNase I (Amplification grade; Thermo Fisher Scientific). DNase I-treated RNA was used for RNA-seq library preparation as previously described (23) or cDNA was synthesized using the SuperScript III First Strand-Synthesis System (Thermo Fisher Scientific) with poly-dT primers. cDNA was used for qPCR using the PerfeCTa SYBR Green FastMix (QuantBio) on a Step One Plus Real Time PCR System (Life Technologies) using primer pairs in SI Appendix, Table S4. mRNA-seq data are available in the NCBI GEO database (accession no. GSE128317).
mRNA-seq Mapping and Analysis.
Tools available on Galaxy (70) were used to map mRNA-sequencing reads (intron size < 1 kb) (74) against the corrected N. crassa OR74A (NC12) genome (49), to count the number of reads per gene (74) and to identify differentially expressed genes (75). P values reported are adjusted for false-discovery rates (75). The χ2 test was used to determine if up-regulated genes in ∆epr-1 strains were enriched for genes marked with H3K27 methylation. Significance of gene set intersections were calculated with a hypergeometric distribution (http://nemates.org/MA/progs/overlap_stats_prog.html).
DamID Southern Hybridizations and Sequencing.
Southern hybridizations were carried out as previously described (76), except probes were made with PCR products amplified from wild-type N. crassa genomic DNA (NCU05173, Tel VIIL) or plasmid pBM61 (his-3) using primer pairs from SI Appendix, Table S5. Preparation of N6-methyladenine-containing DNA for sequencing was performed using a previously reported procedure (42) using primers in SI Appendix, Table S6 with the following modifications: 5 μg of genomic DNA from N. crassa strains expressing a Dam fusion was digested with 1 μL of DpnI (New England Biolabs, 20 units/μL); ligation to primer 5050 was carried out overnight at 16 °C; amplification reactions of ligated DNA with primer 5051 were performed in triplicate with 5 μL dNTPs, and an additional PCR cycle was added to the second and third phase of the PCR protocol (4 and 18 cycles, respectively); 3 μg of pooled, amplified DNA was sheared using a Bioruptor (Diagenode) twice on high for 10 min (30 s on/off) at 4 °C; biotinylated DNA was purified using 250 μL slurry of streptavidin-agarose beads (Sigma). DNA was cleaved from the beads with DpnII and libraries were prepared for sequencing using the NEBNext DNA Library Prep Master Mix Set for Illumina (New England Biolabs).
False Perithecia Assay and Image Analysis.
To assay of the development of false perithecia, strains were grown on modified VMM (31) as described above without a fertilizing strain. Images of plates were acquired after 2 wk of growth at 25 °C and false perithecia were detected and quantified using the Laplacian of Gaussian blob detection algorithm from scikit-image (77).
Microscopy Image Acquisition and Analysis.
Live conidia were suspended in water and placed on a poly-l-lysine (Sigma) coated coverslip (No. 1.5; VWR) and mounted on a glass slide. Single-plane images for distance measurements were captured with the ELYRA S.1 system (Zeiss) mounted on an AXIO Observer Z1 inverted microscope stand (Zeiss) equipped with a 63×, 1.4 NA Plan-Apochromat oil-immersion lens (Zeiss) and analyzed using Imaris (v9.2.1). Images for volume renderings and max projections were collected with the DeltaVision Ultra microscope system (GE) equipped with a 100×, 1.4 NA UPlanSApo objective (Olympus). Three-dimensional z-stack wide-field fluorescent (eGFP, TagRFP, iRFP, and TagBFP) images were captured with an sCMOS camera controlled with Acquire Ultra software. Images were processed using 10 cycles of enhanced ratio deconvolution and max projections were made using softWoRx (GE, v7.1.0). Imaris (v9.2.1) was used to make volume renderings and TFR1 and EPR1 foci were counted by hand.
Bioinformatic Identification of EPR-1 and SET-7 Homologs.
PSI-BLAST (78) was used to initiate sequence similarity searches with representative sequences of EPR-1 (accession no. XP_965052.2) and SET-7 (accession no. XP_965043.2, residues 577 to 833) from N. crassa against the NCBI nonredundant (NR) database and locally maintained databases of proteins from representative species from different branches of the tree of life. HHpred (79) was used to perform profile-profile comparisons against the Protein Data Bank (PDB), Pfam, and locally maintained protein sequence profiles. Sequences were clustered using BLASTCLUST (ftp://ftp.ncbi.nih.gov/blast/documents/blastclust.html). Multiple sequence alignments were generated using Kalign (80) and then adjusted manually. FastTree (81) was used to assess phylogenetic relationships among the proteins retrieved after sequence similarity searches. Customized PERL scripts were used for the analysis of domain architectures and other contextual information about protein sequences. Stringent criteria of domain-architectural concordance were used to retrieve only orthologs of all proteins under study along with grouping in phylogenetic tree analysis. In the case of SET-7, only those protein sequences that have a complete pre-SET domain followed by a SET domain were considered for analysis.
Replacement of NCU05173 and NCU07152 ORFs with hph and nat-1.
To delete NCU07152, the 5′ and 3′ regions flanking the ORF were amplified from wild-type genomic DNA with primers 6385 to 6388 (SI Appendix, Table S2). The nat-1 gene was amplified from plasmid 3237 with primers 6269 and 6270. The resulting three pieces of DNA were stitched by overlap extension using primers 6385 and 6388 and the final product was transformed into N4840. Primary transformants were selected on Nourseothricin-containing medium and crossed to a wild-type strain (N3753) to remove ∆set-7 and ∆mus-52 from the genetic background, resulting in strain N5808. To delete NCU05173, the 5′ and 3′ regions flanking the ORF were amplified from wild-type genomic DNA with primers 6605 to 6608. The hph gene was amplified from plasmid 2283. The 5′ flank was stitched to the 5′ portion of hph by overlap extension using primers 6605 and 2955, and the 3′ flank was stitched to the 3′ portion of hph using primers 6608 and 2954. The resulting two pieces of “split marker” DNA were transformed into strain N4840. Primary transformants were selected on Hygromycin B-containing medium and crossed to N5808 to generate a homokaryotic strain with both marker genes (N6233). N6233 was crossed to N623 to introduce his-3, resulting in the strain used for the mutant hunt (N6279).
Creation and Targeting of his-3+::pCCG::N-GFP::EPR-1 Plasmids.
The ORF and 3′ UTR of epr-1 were PCR-amplified from wild-type genomic DNA with primers 6416 and 6417 (SI Appendix, Table S8) and cloned into plasmid 2406 (35) using PacI and XbaI restriction sites. For the BAH point mutant, epr-1W184A, two PCR products amplified from wild-type genomic DNA with primers pairs 6416 and 6368, and 6367 and 6417 were PCR-stitched together with primers 6416 and 6417, and similarly cloned into plasmid 2406. For the PHD point mutant, epr-1W292A, two PCR products amplified from wild-type genomic DNA with primer pairs 6416 and 6400, and 6399 and 6417 were PCR-stitched together with primers 6416 and 6417, and cloned into plasmid 2406. Plasmids were linearized with NdeI and targeted to his-3 in either N7451 (for complementation) or N7567 (for microscopy), as previously described (82). Primary transformants were then crossed to N7549 or N7552, respectively.
Replacement of epr-1 with trpC::nat-1.
The 5′ and 3′ flanks of epr-1 were PCR-amplified from wild-type genomic DNA with primer pairs 6401 and 6402, and 6350 and 6351, respectively (SI Appendix, Table S9). The 5′ and 3′ flanks were separately PCR-stitched with plasmid 3237 (source of nat-1) using primer pairs 6401 and 4883, and 4882 and 6351, respectively. These two “split-marker” PCR products were transformed into strain N7537 and epr-1 replacements were selected on Nourseothricin-containing medium. Primary transformants were crossed to N3752 (to generate N7567) and N6234 (to generate N7576).
Endogenous C-terminal–tagging of EPR-1 with 10xGly::Dam.
The regions immediately upstream (5′) and downstream (3′) of epr-1’s stop codon were PCR-amplified from wild-type genomic DNA with primer pairs 6348 and 6349, and 6350 and 6351, respectively (SI Appendix, Table S10). The 5′ and 3′ regions were separately PCR-stitched with plasmid 3131 (source of 10xGly::Dam::trpC::nat-1) using primer pairs 6348 and 4883, and 4882 and 6351, respectively. These two split-marker PCR products were transformed into N2718 and knockins were selected on Nourseothricin-containing medium. Primary transformants were crossed to N3752 to obtain homokaryons. To make epr-1W292A fusions with Dam, the same approach was taken except the 5′ region was a PCR product stitched from two PCR products amplified from wild-type genomic DNA with primer pairs 6346 and 6400, and 6399 and 6349.
Data Availability.
All ChIP-seq, DamID-seq, and mRNA-seq data have been submitted to the NCBI GEO database (accession no. GSE128317). All whole-genome sequencing data have been submitted to the NCBI SRA (accession no. PRJNA526508).
Supplementary Material
Acknowledgments
We thank S. De Silva and C. Musselman (University of Colorado Anschutz Medical Campus), and C. Petell and B. Strahl (University of North Carolina School of Medicine) for their significant efforts to characterize the binding specificity of EPR-1 in vitro; S. Honda (University of Fukui) for providing Neurospora crassa strains utilized in fluorescence microscopy experiments; J. Lyle, A. Leiferman, and N. Meyers for help mapping the UV-generated mutants; A. Harvey for help with preliminary microscopy experiments; and A. Zemper for chicken anti-GFP and goat anti-chicken HRP antibodies. This work was funded by National Institutes of Health Grants GM127142 and GM093061 (to E.U.S.) and HD007348 (for partial support of K.J.M.); and the American Heart Association Grant 14POST20450071 (for partial support of E.T.W.). G.K. and L.A. were supported by the Intramural Research Program of the National Library of Medicine.
Footnotes
The authors declare no competing interest.
Data deposition: All ChIP-seq, DamID-seq, and mRNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE128317). All whole-genome sequencing data have been deposited in the NCBI Sequence Read Archive (SRA), https://www.ncbi.nlm.nih.gov/sra/ (accession no. PRJNA526508).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918776117/-/DCSupplemental.
References
- 1.He Y., Control of the transition to flowering by chromatin modifications. Mol. Plant 2, 554–564 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Ahringer J., Gasser S. M., Repressive chromatin in Caenorhabditis elegans: Establishment, composition, and function. Genetics 208, 491–511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jégu T., Aeby E., Lee J. T., The X chromosome in space. Nat. Rev. Genet. 18, 377–389 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Freitag M., Histone methylation by SET domain proteins in fungi. Annu. Rev. Microbiol. 71, 413–439 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Lewis E. B., A gene complex controlling segmentation in Drosophila. Nature 276, 565–570 (1978). [DOI] [PubMed] [Google Scholar]
- 6.Kassis J. A., Kennison J. A., Tamkun J. W., Polycomb and trithorax group genes in Drosophila. Genetics 206, 1699–1725 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuettengruber B., Bourbon H.-M., Di Croce L., Cavalli G., Genome regulation by polycomb and trithorax: 70 Years and counting. Cell 171, 34–57 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Kassis J. A., Brown J. L., Polycomb group response elements in Drosophila and vertebrates. Adv. Genet. 81, 83–118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller J., et al. , Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111, 197–208 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Min J., Zhang Y., Xu R.-M., Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev. 17, 1823–1828 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H., et al. , Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Grau D. J., et al. , Compaction of chromatin by diverse Polycomb group proteins requires localized regions of high charge. Genes Dev. 25, 2210–2221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheutin T., Cavalli G., Loss of PRC1 induces higher-order opening of Hox loci independently of transcription during Drosophila embryogenesis. Nat. Commun. 9, 3898 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavares L., et al. , RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell 148, 664–678 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn T. G., et al. , Interdependence of PRC1 and PRC2 for recruitment to polycomb response elements. Nucleic Acids Res. 44, 10132–10149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turck F., et al. , Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 3, e86 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z., et al. , EBS is a bivalent histone reader that regulates floral phase transition in Arabidopsis. Nat. Genet. 50, 1247–1253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian S., et al. , Dual recognition of H3K4me3 and H3K27me3 by a plant histone reader SHL. Nat. Commun. 9, 2425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L., Shen W.-H., Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr. Biol. 18, 1966–1971 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Bratzel F., López-Torrejón G., Koch M., Del Pozo J. C., Calonje M., Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr. Biol. 20, 1853–1859 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Li Z., Fu X., Wang Y., Liu R., He Y., Polycomb-mediated gene silencing by the BAH-EMF1 complex in plants. Nat. Genet. 50, 1254–1261 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Jamieson K., Rountree M. R., Lewis Z. A., Stajich J. E., Selker E. U., Regional control of histone H3 lysine 27 methylation in Neurospora. Proc. Natl. Acad. Sci. U.S.A. 110, 6027–6032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klocko A. D., et al. , Normal chromosome conformation depends on subtelomeric facultative heterochromatin in Neurospora crassa. Proc. Natl. Acad. Sci. U.S.A. 113, 15048–15053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirrotta V., Li H.-B., A view of nuclear Polycomb bodies. Curr. Opin. Genet. Dev. 22, 101–109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-González L., et al. , Chromatin-dependent repression of the Arabidopsis floral integrator genes involves plant specific PHD-containing proteins. Plant Cell 26, 3922–3938 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y., et al. , Evolution and conservation of polycomb repressive complex 1 core components and putative associated factors in the green lineage. BMC Genomics 20, 533 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzenberg R. L., Stevens J. N., Selker E. U., Morzycka-Wroblewska E., Identification and chromosomal distribution of 5S rRNA genes in Neurospora crassa. Proc. Natl. Acad. Sci. U.S.A. 82, 2067–2071 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pomraning K. R., Smith K. M., Freitag M., Bulk segregant analysis followed by high-throughput sequencing reveals the Neurospora cell cycle gene, ndc-1, to be allelic with the gene for ornithine decarboxylase, spe-1. Eukaryot. Cell 10, 724–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis R. H., de Serres F. J., Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 17, 79–143 (1970). [Google Scholar]
- 30.Basenko E. Y., et al. , Genome-wide redistribution of H3K27me3 is linked to genotoxic stress and defective growth. Proc. Natl. Acad. Sci. U.S.A. 112, E6339–E6348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo V., Sommer T., Chambers J., A modified Vogel’s medium for crossings, mating-type tests and the isolation of female-sterile mutants of Neurospora crassa. Fungal Genet. Rep. 32, 10 (1985). [Google Scholar]
- 32.Luo M., et al. , Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 96, 296–301 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohad N., et al. , Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell 11, 407–416 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bicocca V. T., Ormsby T., Adhvaryu K. K., Honda S., Selker E. U., ASH1-catalyzed H3K36 methylation drives gene repression and marks H3K27me2/3-competent chromatin. eLife 7, 1455 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honda S., Selker E. U., Tools for fungal proteomics: Multifunctional neurospora vectors for gene replacement, protein expression and protein purification. Genetics 182, 11–23 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchler-Bauer A., et al. , CDD: NCBI’s conserved domain database. Nucleic Acids Res. 43, D222–D226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Callebaut I., Courvalin J. C., Mornon J. P., The BAH (bromo-adjacent homology) domain: A link between DNA methylation, replication and transcriptional regulation. FEBS Lett. 446, 189–193 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Schindler U., Beckmann H., Cashmore A. R., HAT3.1, a novel Arabidopsis homeodomain protein containing a conserved cysteine-rich region. Plant J. 4, 137–150 (1993). [DOI] [PubMed] [Google Scholar]
- 39.Zhao D., et al. , The BAH domain of BAHD1 is a histone H3K27me3 reader. Protein Cell 7, 222–226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez R., Zhou M.-M., The PHD finger: A versatile epigenome reader. Trends Biochem. Sci. 36, 364–372 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Steensel B., Henikoff S., Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat. Biotechnol. 18, 424–428 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Zhou V. W., Methods for Global Characterization of Chromatin Regulators in Human Cells, (Harvard University Press, 2012). [Google Scholar]
- 43.Hyun K., Jeon J., Park K., Kim J., Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 49, e324 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du J., et al. , Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell 151, 167–180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H., et al. , Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature 442, 91–95 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boamah D., et al. , Characteristics of a PHD finger subtype. Biochemistry 57, 525–539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hauri S., et al. , A high-density map for navigating the human polycomb complexome. Cell Rep. 17, 583–595 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Scelfo A., et al. , Functional landscape of PCGF proteins reveals both RING1A/B-Dependent-and RING1A/B-Independent-Specific activities. Mol. Cell 74, 1037–1052.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galazka J. M., et al. , Neurospora chromosomes are organized by blocks of importin alpha-dependent heterochromatin that are largely independent of H3K9me3. Genome Res. 26, 1069–1080 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berry S., Rosa S., Howard M., Bühler M., Dean C., Disruption of an RNA-binding hinge region abolishes LHP1-mediated epigenetic repression. Genes Dev. 31, 2115–2120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veluchamy A., et al. , LHP1 regulates H3K27me3 spreading and shapes the three-dimensional conformation of the Arabidopsis genome. PLoS One 11, e0158936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kundu S., et al. , Polycomb repressive complex 1 generates discrete compacted domains that change during differentiation. Mol. Cell 65, 432–446.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wani A. H., et al. , Chromatin topology is coupled to Polycomb group protein subnuclear organization. Nat. Commun. 7, 10291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derkacheva M., et al. , Arabidopsis MSI1 connects LHP1 to PRC2 complexes. EMBO J. 32, 2073–2085 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blackledge N. P., et al. , Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157, 1445–1459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steffen P. A., Ringrose L., What are memories made of? How Polycomb and trithorax proteins mediate epigenetic memory. Nat. Rev. Mol. Cell Biol. 15, 340–356 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Riising E. M., et al. , Gene silencing triggers polycomb repressive complex 2 recruitment to CpG islands genome wide. Mol. Cell 55, 347–360 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Hosogane M., Funayama R., Shirota M., Nakayama K., Lack of transcription triggers H3K27me3 accumulation in the gene body. Cell Rep. 16, 696–706 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Eskeland R., et al. , Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol. Cell 38, 452–464 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao Z., et al. , PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell 45, 344–356 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bierne H., et al. , Human BAHD1 promotes heterochromatic gene silencing. Proc. Natl. Acad. Sci. U.S.A. 106, 13826–13831 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lakisic G., et al. , Role of the BAHD1 chromatin-repressive complex in placental development and regulation of steroid metabolism. PLoS Genet. 12, e1005898 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis R. H., Neurospora: Contributions of a Model Organism, (Oxford University Press, 2000). [Google Scholar]
- 64.Pomraning K. R., Smith K. M., Freitag M., Genome-wide high throughput analysis of DNA methylation in eukaryotes. Methods 47, 142–150 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Kilbey B. J., De Serres F. J., Quantitative and qualitative aspects of photoreactivation of premutational ultraviolet damage at the ad-3 loci of Neurospora crassa. Mutat. Res. 4, 21–29 (1967). [DOI] [PubMed] [Google Scholar]
- 66.Shiu P. K. T., Raju N. B., Zickler D., Metzenberg R. L., Meiotic silencing by unpaired DNA. Cell 107, 905–916 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Hunter J. D., Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007). [Google Scholar]
- 68.Garrison E., Marth G., Haplotype-based variant detection from short-read sequencing. arXiv:1207.3907 (20 July 2012).
- 69.Danecek P. et al.; 1000 Genomes Project Analysis Group , The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Afgan E., et al. , The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 46, W537–W544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Langmead B., Salzberg S. L., Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramírez F., et al. , deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160-5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thorvaldsdóttir H., Robinson J. T., Mesirov J. P., Integrative genomics viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dobin A., et al. , STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miao V. P. W., Freitag M., Selker E. U., Short TpA-rich segments of the ζ-η region induce DNA methylation in Neurospora crassa. J. Mol. Biol. 300, 249–273 (2000). [DOI] [PubMed] [Google Scholar]
- 77.van der Walt S. et al.; scikit-Image Contributors , scikit-image: Image processing in Python. PeerJ 2, e453 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Altschul S. F., et al. , Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Söding J., Biegert A., Lupas A. N., The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244-8 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lassmann T., Frings O., Sonnhammer E. L. L., Kalign2: High-performance multiple alignment of protein and nucleotide sequences allowing external features. Nucleic Acids Res. 37, 858–865 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Price M. N., Dehal P. S., Arkin A. P., FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5, e9490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Margolin B. S., Freitag M., Selker E. U., Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Rep. 44, 34–36 (1997). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All ChIP-seq, DamID-seq, and mRNA-seq data have been submitted to the NCBI GEO database (accession no. GSE128317). All whole-genome sequencing data have been submitted to the NCBI SRA (accession no. PRJNA526508).