Significance
There are >20 different endogenous opioid peptides derived from the three precursors proopiomelanocortin, proenkephalin, and prodynorphin; a long-standing question is the biological utility of having this variety of peptides. We addressed this question by systematically evaluating ligand binding and signaling properties of the peptides at each of the three opioid receptor types. Contrary to the prevailing notion, we show that all of the peptides bind and activate the three opioid receptors and that shorter β-endorphin peptides exhibit agonistic activity. Finally, we demonstrate that some endogenous peptides favor particular signaling pathways at the three receptors leading to biased signaling. These findings highlight the complexity of signaling where multiple opioid receptors are expressed and/or many opioid peptides are released.
Keywords: GPCRs, opioid receptors, biased agonism, opioid peptides
Abstract
Opioids, such as morphine and fentanyl, are widely used for the treatment of severe pain; however, prolonged treatment with these drugs leads to the development of tolerance and can lead to opioid use disorder. The “Opioid Epidemic” has generated a drive for a deeper understanding of the fundamental signaling mechanisms of opioid receptors. It is generally thought that the three types of opioid receptors (μ, δ, κ) are activated by endogenous peptides derived from three different precursors: Proopiomelanocortin, proenkephalin, and prodynorphin. Posttranslational processing of these precursors generates >20 peptides with opioid receptor activity, leading to a long-standing question of the significance of this repertoire of peptides. Here, we address some aspects of this question using a technical tour de force approach to systematically evaluate ligand binding and signaling properties ([35S]GTPγS binding and β-arrestin recruitment) of 22 peptides at each of the three opioid receptors. We show that nearly all tested peptides are able to activate the three opioid receptors, and many of them exhibit agonist-directed receptor signaling (functional selectivity). Our data also challenge the dogma that shorter forms of β-endorphin do not exhibit receptor activity; we show that they exhibit robust signaling in cultured cells and in an acute brain slice preparation. Collectively, this information lays the groundwork for improved understanding of the endogenous opioid system that will help in developing more effective treatments for pain and addiction.
The endogenous opioid system comprises the three opioid receptors, mu (μOR), delta (δOR), and kappa (κOR) and peptides acting at these receptors (1). The peptides are generated from the proteolytic cleavage of the precursor proteins prodynorphin, proenkephalin, and proopiomelanocortin, leading to the generation of >20 peptides (1, 2); endogenous opioid peptides contain an “opioid motif” (Tyr-Gly-Gly-Phe-Met/Leu) at their amino terminals (3). Although it is reported that endogenous opioid peptides can bind to μOR, δOR, or κOR (4), dynorphins (Dyn) are generally described as endogenous κOR agonists, endorphins as μOR, and enkephalins as δOR agonists (5, 6). A long-standing question in the field has been the physiological significance of the presence of so many endogenous opioid peptides in the central nervous system.
For several years it was thought that receptor activation by an agonist resulted in G protein-mediated signaling through all downstream cascades. More recent studies have demonstrated that G protein-coupled receptors (GPCRs) can generate, in addition to G protein-dependent signaling, G protein-independent signaling (7–10). The latter occurs following β-arrestin recruitment to the phosphorylated receptor, where it functions as a scaffold-enabling signaling through different molecules (11, 12). This discovery led to the concept of ligand-directed receptor signaling, also referred to as functional selectivity or biased agonism (13). Moreover, growing evidence shows that different orthosteric agonists can stabilize distinct receptor conformations and activate distinct downstream signaling pathways (9, 10, 14); this raises the possibility that endogenous opioid peptides could vary in this manner as well (13).
For opioid receptors, studies showed that mice lacking β-arrestin2 exhibited enhanced and prolonged morphine-mediated antinociception, and a reduction in side-effects, such as development of tolerance and acute constipation (15, 16). This led to studies examining whether μOR agonists exhibit biased signaling (17–20), and to the identification of agonists that preferentially activate G protein-mediated pathways (21–27). However, very few studies have examined whether endogenous opioid peptides exhibit signaling bias. One study examined functional selectivity of a small panel of endogenous peptides specifically at μOR and found that α-neoendorphin (α-neoend) and Met-enk RF exhibit distinct bias profiles (28). It is possible that endogenous opioid peptides not only produce biased signaling at a single opioid receptor but also have differential bias among the three different opioid receptors. To systematically evaluate this possibility, we examined the binding and signaling (G protein activity and β-arrestin2 recruitment) at μOR, δOR, and κOR of a panel of 22 peptides. To minimize factors that might obscure signal bias, such as cellular background, assay conditions, and kinetics of signaling (29), we carried out binding and signaling assays ([35S]GTPγS and β-arrestin recruitment) using one cellular background (U2OS cells expressing either μβgalOR, δβgalOR, or κβgalOR), and performed experiments under identical conditions in the presence of a mixture of peptidase inhibitors. We observed that the majority of the peptides bind to and signal at all three opioid receptors. In addition, some peptides (e.g., Dyn B13) exhibit biased signaling at only one receptor (μOR), while others (e.g., Met-enk RF) exhibit differential bias at each of the three opioid receptors (G protein bias at μOR, β-arrestin bias at δOR, and no bias at κOR). Together, these results reveal the unique functional selectivity profiles of the different endogenous opioid peptides at individual opioid receptors.
Results
Putative Endogenous Ligands of μOR, δOR, and κOR Bind and Signal at the Three Opioid Receptors.
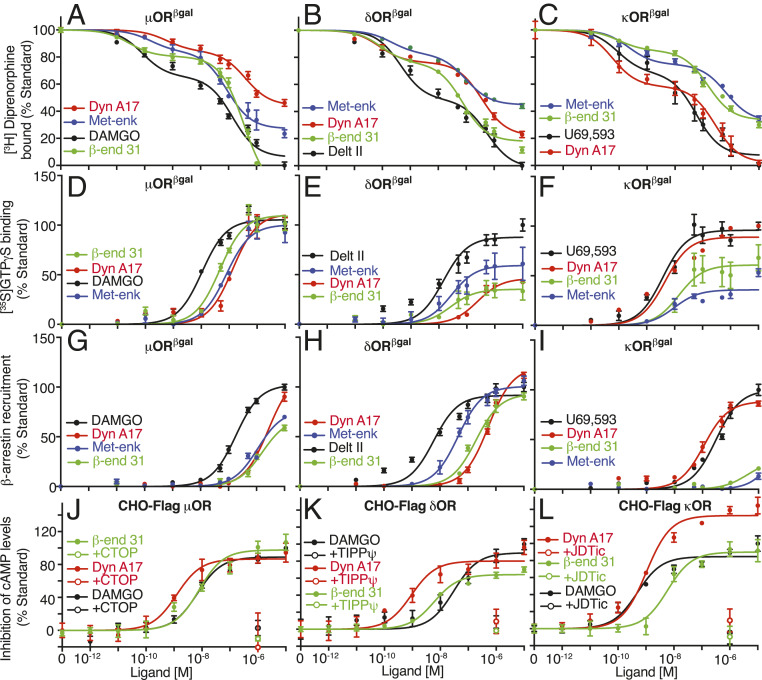
A general notion in the field is that dynorphins are endogenous κOR agonists, while endorphins are μOR and enkephalins δOR agonists (5, 6). To test if these peptides have receptor promiscuity, we examined binding of β-endorphin31 (β-end 31; putative μOR ligand), Met-enk (putative δOR ligand), and Dyn A17 (putative κOR ligand) to the three opioid receptors. All three peptides displace [3H]diprenorphine binding from the three opioid receptors, although they exhibit differences in affinity at individual receptors (Fig. 1 A–C and SI Appendix, Table S1). Next, we examined the signaling profiles of these peptides using G protein activity and β-arrestin recruitment assays. The three peptides induced a dose-dependent increase in [35S]GTPγS binding, and were full agonists at μOR and partial agonists at δOR and κOR (except for Dyn A17, which was a full agonist at κOR) when compared to reference standards (Fig. 1 D–F and SI Appendix, Figs. S1 and S2 and Tables S2–S4). Interestingly, in the β-arrestin recruitment assay these peptides were full agonists at δOR, while only Dyn A17 was a full agonist at μOR and κOR (Fig. 1 G–I and SI Appendix, Figs. S1 and S2 and Tables S2–S4). We confirmed that β-end 31 and Dyn A17 signal at μOR, δOR, or κOR by measuring inhibition of cAMP levels in an additional model, Chinese hamster ovary (CHO) cells expressing Flag-epitope tagged receptors. The peptides dose-dependently inhibited cAMP levels at all three opioid receptors and this was blocked by receptor-selective antagonists (Fig. 1 J–L and SI Appendix, Table S5). Together, our results indicate that these peptide ligands are less selective than proposed, binding to and signaling via all three opioid receptors.
Fig. 1.
Representative opioid peptides bind and signal at μOR, δOR, and κOR. (A–C) Displacement of [3H]diprenorphine binding by β-end 31, Met-enk, and Dyn A17 in membranes (20 μg) from cells expressing either μβgalOR (A), δβgalOR (B), or κβgalOR (C). (D–F) [35S]GTPγS binding in membranes (20 μg) from cells expressing either μβgalOR (D), δβgalOR (E), or κβgalOR (F). (G–I) β-Arrestin recruitment in cells expressing either μβgalOR (G), δβgalOR (H), or κβgalOR (I). (J–L) Inhibition of cAMP levels in CHO cells expressing Flag epitope-tagged μOR (J), δOR (K), or κOR (L). Antagonists (10 μM) to μOR, CTOP (J), to δOR, TIPPψ (K), and to κOR, JDTic (L) block β-end 31-, Met-enk–, and Dyn A17- (1 μM) mediated inhibition in cAMP levels. DAMGO (μOR), Delt II (δOR), and U69,593 (κOR) were used as standards. Data are mean ± SE from three to six independent experiments.
Shorter Forms of β-Endorphin Exhibit Agonistic Activity.
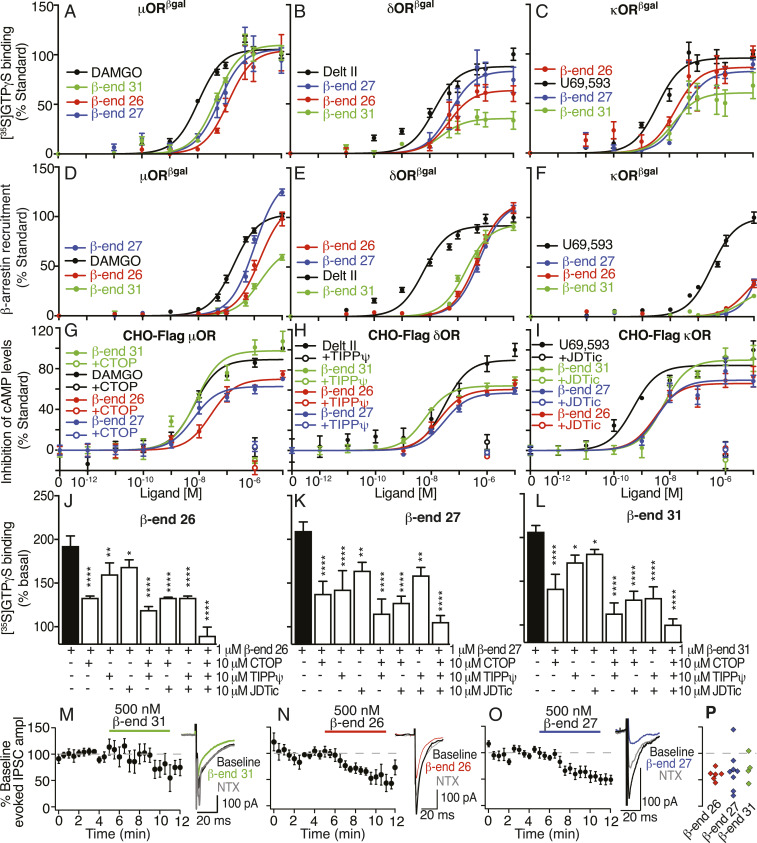
A dogma in the field has been that shorter forms of β-end 31(β-end 26 and β-end 27) exhibit reduced (or no) activity at opioid receptors (30–32). In particular, it has been proposed that β-end 27 functions as an opioid receptor antagonist because it attenuates β-end 31 or etorphine-mediated analgesia (32, 33). To directly test this hypothesis, we examined the pharmacological properties of β-end 31and shorter peptides at the three opioid receptors. Like β-end 31, both β-end 26 and β-end 27 exhibited a biphasic profile of [3H]diprenorphine displacement at the three opioid receptors and all three peptides caused a dose-dependent increase in [35S]GTPγS binding with nanomolar potencies (Fig. 2 A–C and SI Appendix, Fig. S1 and Tables S1–S4). Thus, all three peptides are full agonists at μOR and only β-end 26 and β-end 27 are full agonists at κOR (Fig. 2 A–C and SI Appendix, Fig. S2 and Tables S2–S4). Interestingly, in the β-arrestin recruitment assay, while β-end 31 was a partial agonist at μOR, both β-end 26 and β-end 27 were full agonists, with the efficacy of β-end 27 being significantly higher than that of the standard DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin) (Fig. 2 D–F and SI Appendix, Figs. S2 and S3 and Tables S2–S4). We confirmed the agonistic properties of β-end peptides at μOR, δOR, or κOR using a second cell line expressing N-terminally tagged receptors (CHO cells expressing Flag-epitope tagged receptors). These peptides dose-dependently inhibited cAMP levels at each of the three opioid receptors; this was blocked by receptor-selective antagonists (Fig. 2 G–I and SI Appendix, Table S5).
Fig. 2.
β-Endorphin peptides signal at μOR, δOR, and κOR. (A–C) [35S]GTPγS binding in membranes (20 μg) from cells expressing either μβgalOR (A), δβgalOR (B), or κβgalOR (C). (D–F) β-Arrestin recruitment in cells expressing either μβgalOR (D), δβgalOR (E), or κβgalOR (F). (G–I) Inhibition of cAMP levels in CHO cells expressing Flag epitope-tagged μOR (G), δOR (H), or κOR (I). Antagonists to μOR, CTOP (G), to δOR, TIPPψ (H), and to κOR, JDTic (I) block β-end peptide-mediated inhibition in cAMP levels. DAMGO (μOR), Delt II (δOR), and U69,593 (κOR) were used as standards in A–I. (J–L) Increases in [35S]GTPγS binding by β-end peptides in striatal membranes (20 μg) were completely blocked by a combination of antagonists to μOR (CTOP), δOR (TIPPψ), and to κOR (JDTic). (M–P) β-End peptides inhibit evoked synaptic GABAAR-mediated IPSC amplitude (ampl) onto VTA neurons in acute brain slices. Time courses for the effects of 500 nM β-end 31 (M, Left), β-end 26 (N, Left), or β-end 27 (O, Left) responses averaged across neurons are shown. Example IPSC traces show inhibition by 500 nM β-end 31 (M, Right), β-end 26 (N, Right), or β-end 27 (O, Right) and partial reversal by 1 μM naltrexone (NTX). (P) Summary showing the magnitude of the change in evoked IPSC amplitude induced by the endorphin peptides in each neuron tested. Data are mean ± SE from three to six independent experiments for A–L and mean ± SE for M–P (n = 4 to 8 neurons from three rats each).*P < 0.05; **P < 0.01; ****P < 0.0001, one-way ANOVA.
Next, we examined if β-end peptides exhibit agonistic activity in brain tissue. We found that they increased [35S]GTPγS binding to striatal membranes; this was completely blocked only with a combination of antagonists of μOR (CTOP), δOR (TIPPψ), and κOR (JDTic) (Fig. 2 J–L). These results suggest that β-end peptides can elicit signaling through all three opioid receptors in brain tissue. We also tested for synaptic effects with whole-cell electrophysiology in acute ventral tegmental area (VTA) slices. It is well established that μOR activation strongly inhibits GABA release onto VTA neurons (34–36). We found that β-end 26 and β-end 27, like β-end 31, inhibit electrically evoked GABA-mediated inhibitory postsynaptic currents in VTA neurons; this is partially reversed by the opioid antagonist naltrexone (Fig. 2 M–P). These results further support the notion that β-end 26 and β-end 27 exhibit agonistic activity at opioid receptors in vivo.
Dynorphin Peptides Bind to and Signal at μOR, δOR, and κOR.
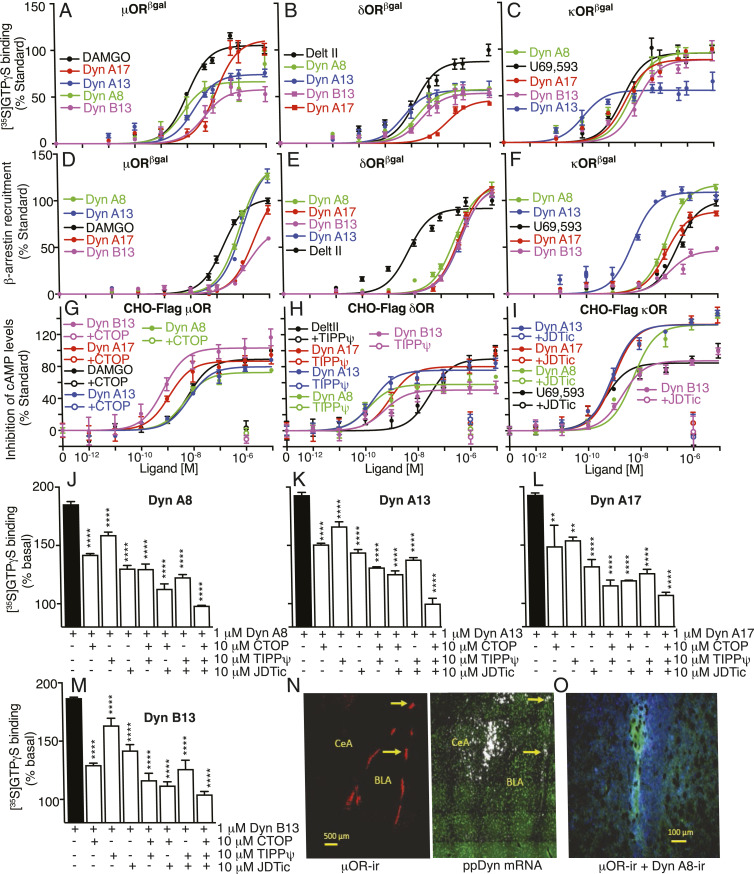
It is widely believed that prodynorphin-derived peptides (particularly Dyn A17) exert their physiologic effects exclusively via κOR (5, 6). We investigated binding and signaling by prodynorphin-derived peptides and found that all tested peptides bind to the three opioid receptors (Fig. 1 and SI Appendix, Table S1). Characterization of signaling revealed that the peptides induced a dose-dependent increase in G protein activity with nanomolar potency (Fig. 3 A–C and SI Appendix, Fig. S1 and Tables S2–S4) but with subtle differences in efficacy. For example, while at κOR the majority of tested dynorphin peptides had full agonistic activity, at δOR all peptides had partial activity, and at μOR only α-neoend and Dyn B13 had partial agonistic activity (SI Appendix, Fig. S2). The Dyn peptides dose-dependently recruited β-arrestin at each of the three opioid receptors (Fig. 3 D–F and SI Appendix, Figs. S2 and S3). Dyn A17, Dyn A13, and Dyn A8 had full agonist activity at all three receptors (SI Appendix, Fig. S2), α-neoend and β-neoend had partial activity at the three receptors, and Dyn B13 had full agonist activity only at δOR (SI Appendix, Fig. S2). Interestingly at μOR, Dyn A8 and Dyn A13 were more efficacious than the standard, DAMGO, while at δOR all Dyn peptides, except α-neoend and β-neoend, were as efficacious as the standard, Delt II (SI Appendix, Fig. S3). Next, we examined downstream signaling at μOR, δOR, or κOR by measuring inhibition of cAMP levels in CHO cells expressing Flag-epitope tagged receptors. We found that all tested Dyn peptides dose-dependently inhibited cAMP levels at each of the three opioid receptors; this was blocked by the respective receptor-selective antagonists (Fig. 3 G–I and SI Appendix, Table S5). Together, these results indicate that dynorphin peptides can bind to and signal via all three opioid receptors. Finally, we also found that tested Dyn peptides increased [35S]GTPγS binding to striatal membranes; this was completely blocked only when a combination of antagonists to μOR, δOR, and κOR were used (Fig. 3 J–M). These results indicate that Dyn peptides can signal through μOR, δOR, and κOR, not just in heterologous systems but also in the brain.
Fig. 3.
Dynorphin peptides signal at μOR, δOR, and κOR. (A–C) [35S]GTPγS binding in membranes (20 μg) from cells expressing either μβgalOR (A), δβgalOR (B), or κβgalOR (C). (D–F) β-Arrestin recruitment in cells expressing either μβgalOR (D), δβgalOR (E), or κβgalOR (F). (G–I) Inhibition of cAMP levels by Dyn A8, Dyn A13, Dyn A17, and Dyn B13 in CHO cells expressing Flag epitope-tagged μOR (G), δOR (H), or κOR (I). Antagonists to μOR, CTOP (G), to δOR, TIPPψ (H), and to κOR, JDTic (I) block Dyn A8-, Dyn A13-, Dyn A17-, or Dyn B13-mediated inhibition in cAMP levels. DAMGO (μOR), Delt II (δOR), and U69,593 (κOR) were used as standards. (J–M) Increases in [35S]GTPγS binding by Dyn peptides in striatal membranes (20 μg) are completely blocked by a combination of antagonists to μOR (CTOP), δOR (TIPPψ), and to κOR (JDTic). (N, Left) Low-magnification image of a coronal section through rat amygdala showing high levels of expression of μOR immunoreactivity (μOR-ir; red) in ITC clusters of the basolateral nucleus of amygdala (BLA). μOR-ir is barely visible in the CeA. (N, Right) In situ hybridization autoradiographic imaging of an adjacent section with immunohistochemical staining for the neuronal marker NeuN (green), showing silver grains (white) indicating the presence of ppDYN mRNA; ppDYN mRNA is apparent in many cells in the CeA, and in a few of the ITC cell clusters. Yellow arrows (N) indicate μOR-expressing areas in ITC clusters that also express ppDYN mRNA. (O) Higher-magnification image of an ITC cluster in amygdala showing immunocytochemically detected Dyn A8 expression (blue) in proximity to μOR labeling (green). Data (A–M) are mean ± SE from three to six independent experiments. Images (N and O) are representative images from sections obtained from four or more rats. **P < 0.01; ****P < 0.0001, one-way ANOVA.
In order for an opioid receptor to be the target of a Dyn peptide in a brain circuit, neurons expressing the receptor should be in the vicinity of Dyn peptide release sites. To investigate this, we examined localization of μOR immunoreactivity with either prepro-dynorphin (ppDYN) mRNA detection or Dyn A8 immunoreactivity in the amygdala since studies report that neurons in the network of intercalated cells (ITC) surrounding the basolateral and lateral amygdala express a high density of μOR (37), that μOR activation modulates basolateral amygdala inputs to the central amygdala (CeA) (38), and that prodynorphin is expressed in the CeA (39). Consistent with the literature, we detected high levels of μOR immunoreactivity in ITC clusters (Fig. 3N), and using in situ hybridization we detected ppDYN mRNA in some ITC clusters that also contain μOR (Fig. 3N). We also found dense labeling for ppDYN mRNA in the CeA (Fig. 3N) and Dyn A8 immunoreactivity around individual μOR-expressing ITC cell groups (Fig. 3O). Together, these results are consistent with the idea that the small bioactive peptides processed from prodynorphin are present in close proximity to the μOR-expressing ITC cells.
Opioid Peptides Exhibit Biased Signaling.
A comparison of the signaling profiles of β-end and prodynorphin peptides at the three opioid receptors (SI Appendix, Tables S2–S4) suggests that they could exhibit biased signaling. In order to systematically investigate the extent of biased signaling by endogenous opioid peptides, we expanded the panel of peptides examined for G protein activity and β-arrestin recruitment to the 20 peptides that contain the opioid “address” sequence (Tyr-Gly-Gly-Phe-Met/Leu) and two additional peptides with a slightly modified sequence: Acetylated β-end 26 and Des-Tyr-γ-end. Ligand binding analysis revealed that tested opioid peptides exhibit a biphasic profile of displacement (SI Appendix, Table S1). In G protein activity assays, each of the 20 peptides caused dose-dependent activation at all three receptors (SI Appendix, Fig. S1 and Tables S2–S4). In addition, β-arrestin recruitment assays showed that the 20 peptides caused dose-dependent signaling via all three receptors (SI Appendix, Fig. S3 and Tables S2–S4). Consistent with prior reports indicating that acetylation of β-end and lack of Tyr residue in γ-end reduces the activity of these peptides (40, 41), the least amount of signaling was observed with acetylated β-end 26 and Des-Tyr-γ-end (SI Appendix, Figs. S1 and S3).
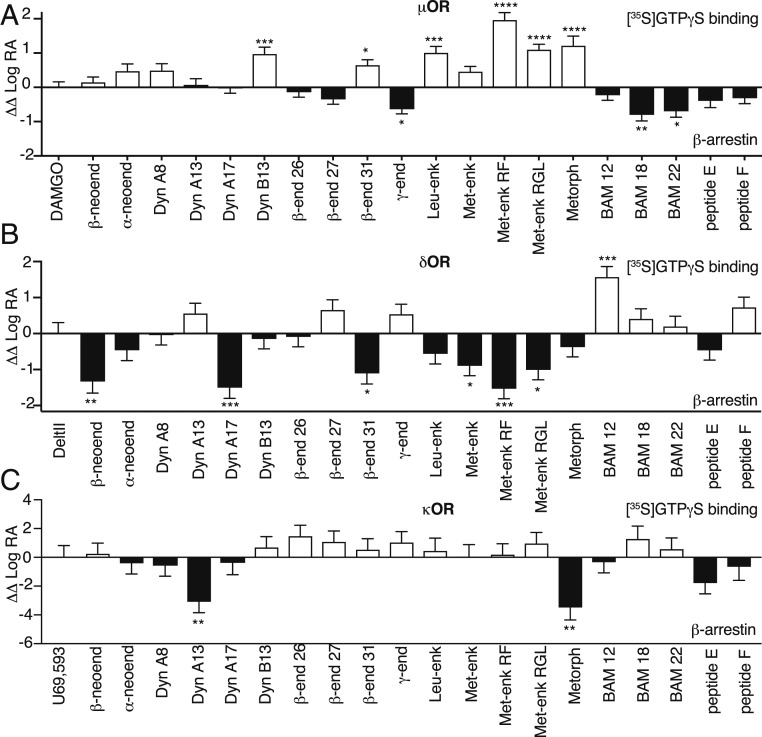
In order to calculate a bias factor that would allow comparison between signaling by opioid peptides and the standards DAMGO (μOR), Delt II (δOR), or U69,593 (κOR), we used the data obtained from [35S]GTPγS binding and β-arrestin recruitment assays and the quantification method described by Kenakin et al. (42); the latter was previously used to examine biased agonism for a small number of endogenous opioid peptides at μOR (28). We followed the step-wise protocol described by Nagi and Pineyro (43). This analysis revealed that at μOR, Met-enk RF exhibited highest G protein bias and BAM 18 exhibited highest β-arrestin bias (Fig. 4A and SI Appendix, Tables S6–S8). At δOR, BAM 12 exhibited significant G protein bias while β-neoend, Dyn A17, and Met-enk RF exhibited significant β-arrestin bias (Fig. 4B and SI Appendix, Tables S6–S8). At κOR, none of the peptides exhibited significant G protein bias, and only Dyn A13 and metorphamide exhibited significant β-arrestin bias (Fig. 4C and SI Appendix, Tables S6–S8). Interestingly, some of the peptides appear to exhibit receptor-specific biased signaling. For example, Met-enk RF and Met-enk RGL exhibited significant G protein bias at μOR, β-arrestin bias at δOR, and no bias at κOR (Fig. 4), while metorphamide exhibited significant G protein bias at μOR, no bias at δOR, and β-arrestin bias at κOR (Fig. 4). Together, these results indicate that some opioid peptides have substantially different bias factors across all three opioid receptors.
Fig. 4.
Bias plots for endogenous opioid peptides at μOR, δOR, and κOR. Bias analysis for signaling by endogenous opioid peptides at μOR (A), δOR (B), or κOR (C) was performed as described in Materials and Methods. Data are mean ± SE from three to six independent experiments. One-way ANOVA; *P > 0.05; **P > 0.01; ***P > 0.001; ****P > 0.0001.
Discussion
One important finding in this study is that shorter β-end peptides, β-end 26 and β-end 27, function as opioid receptor agonists. This is in contrast to previous reports that these shorter peptides exhibit limited or no activity at opioid receptors; β-end 27 was found to dose-dependently attenuate β-end 31 or etorphine-mediated analgesia (32, 33). One possibility is that the reported antagonistic effect of β-end 27 and lack of activity for β-end 26 in vivo is due to the peptides being cleaved to a form that retains affinity but not activity at opioid receptors; most of the studies with these peptides were carried out in the absence of protease inhibitors. However, studies show that peptide activity is greatly increased by the application of protease inhibitors (44, 45). Consistent with this, studies carried out to directly test the activity of β-end 27 using [35S]GTPγS binding to C6 membranes expressing μOR showed that this peptide exhibited agonistic activity and this response was not affected by protease blockade (46). Furthermore, supporting the idea that β-end 27 exhibits agonistic activity another study found β-end 27 to be ∼10 times more hypotensive than β-end 31 on mean arterial pressure (31). Systemically investigating the activity of β-end peptides in more controlled systems could help clarify their physiological roles.
We also found that dynorphin peptides, classically thought of as κOR agonists, engage all three opioid receptors, with Dyn A17 showing a preference (∼25-fold) for κOR in [35S]GTPγS binding (SI Appendix, Tables S2–S4). Our observations agree with previous reports showing that dynorphin peptides bind with high affinity to all three opioid receptors in cells expressing individual receptors and in endogenous tissue (4, 47–52). Furthermore, studies using signaling assays, such as [35S]GTPγS binding, K+ current assays in Xenopus oocytes, or electrophysiological assays in hippocampal slices, also support the idea that dynorphin peptides exhibit agonist activity at opioid receptors other than κOR (46, 51–53). Finally, results from studies using the κOR antagonist, norBNI, that show incomplete blockade of Dyn A13-, Dyn A17-, or Dyn B13-mediated increases in [35S]GTPγS binding in striatal membranes (54) are consistent with our findings. Thus, dynorphin peptides are likely to exert their physiologic effects via activation of opioid receptors other than κOR in vivo. It is thus provocative that in the amygdala we found Dyn A8 immunoreactivity in close proximity to clusters of μOR-expressing neurons in the ITC network interfacing the basolateral and central amygdala, raising the possibility that Dyn A8 released here activates μORs on the ITC cell bodies or terminals in the central amygdala. Some ITCs or terminals within these cell clusters may express κOR as well. However, if receptor density is the key factor dictating the dominant target of a peptide, the density of μOR in the ITC cells is so high that Dyn A8 and other peptides derived from prodynorphin are most likely binding partners for μOR, whether or not κOR is also present.
Another finding in this study is that proenkephalin-derived opioid peptides also exhibit receptor promiscuity in that they engage all three opioid receptors, although to varying extent; the shorter enk peptides (Leu-enk, Met-enk, Met-enk RF, and Met-enk RGL) are less efficacious at recruiting β-arrestin to κOR compared to the other two opioid receptors. Studies used displacement binding assays to examine opioid receptor selectivity by proenkephalin-derived peptides. One study reported that Leu- and Met-enk bound preferentially to δOR; Met-enk RF and Met-enk RGL bound equally to μOR and δOR; BAM12, BAM22, metorphamide, and peptide E bound preferentially to μOR; and peptide F did not exhibit preference for any opioid receptor (5). Another study found that proenkephalin-derived peptides bound with nanomolar affinities to all three opioid receptors except for peptide F at κOR (>1 μM affinity) (4). The differences in binding profile of these peptides could be due to the membrane preparation used, assay buffer conditions, and whether or not protease inhibitors were used to prevent peptide degradation.
In ligand displacement studies we observed that some peptides exhibit complex binding profiles with a high-affinity (subnanomolar) and a low-affinity (high nanomolar) component. We also found that some peptides exhibited full displacement at one opioid receptor but partial (40 to 50% displacement) at other opioid receptors (SI Appendix, Table S1). This could be because of the different conformational states of the receptors and the differential ability of the endogenous peptides to displace [3H] diprenorphine bound to these conformations. It is also possible that stabilization of distinct conformations of the receptor by association with different proteins (including different pools of β-arrestin) contributes to our findings. Support for the latter comes from studies showing that association with β-arrestin can increase agonist affinity (55) and that β-arrestin bound to the receptor can exist in different conformations (56, 57). We interpret these data to indicate heterogeneity of receptor states in these preparations.
A previous study by Thompson et al. (28) examined biased signaling by a small panel of endogenous opioid peptides at μOR. Using assays for G protein activation, inhibition of intracellular cAMP levels, β-arrestin1/2 recruitment, phosphorylation of mitogen-activated protein kinase (ERK1/2), and μOR trafficking, they found that α-neoend, Met-enk, and Met-enk RF displayed differences in their biased signaling profile compared to DAMGO, while Leu-enk, β-end 31, Dyn A8, and Dyn B13 were similar to DAMGO. However, Thompson et al. reported that the endogenous opioid peptides tested lacked significant bias when comparing [35S]GTPγS binding and β-arrestin recruitment measurements. Here we found that β-end 31, Leu-enk, and Met-enk RF exhibit significant G protein bias compared to the standard (DAMGO) at μOR. The differences between our observations and the previous study (28) could be due to differences in cell line, the assay to measure β-arrestin recruitment, and the duration of agonist treatment (30 min for [35S]GTPγS binding and 5 min for β-arrestin recruitment) in the previous study. Given that binding of an agonist can stabilize distinct receptor conformations leading to preferential signaling through one pathway over another, and that this can be further influenced by the proteins in close proximity with the receptor, it is possible that the same agonist could give a different signaling profile for the same receptor expressed in different cell types (58, 59). Thompson et al. (28) compared μOR signaling with FlpIn CHO and AtT20 cell backgrounds, and examined the effect of different cellular context on the signaling profiles of three peptides (Met-enk, Met-enk RF, and α-neoend) that exhibited distinct signaling bias in their first report. They reported that despite changes in signaling profiles, peptides retained the bias (relative to the standard) across different cellular backgrounds, with some changes in magnitudes (28, 29). In this context, we note that some of the peptides reported to exhibit bias by Thompson et al. (28) (α-neoend, Dyn B13, Leu-enk) also exhibit similar bias in our study although the magnitudes are somewhat different (Fig. 4 and SI Appendix, Table S6). Together, these studies highlight the consistencies of ligand bias across systems and the appropriateness of heterologous systems to characterize functional selectivity.
In addition to the endogenous opioid system, a number of GPCRs, like the chemokine and somatostatin receptor systems, have multiple endogenous agonists. This raises the question about the physiological significance of the presence of many endogenous agonists for a receptor. In the case of chemokine receptors, studies show that endogenous agonists targeting the same receptor subtype exhibit a differential pattern of biased signaling that correlates with the different chemotactic profiles induced by the agonists (60, 61). In the case of somatostatin receptors, endogenous ligands to somatostatin receptor 2A induce unique patterns of receptor recycling (62). Together, these results suggest that multiple endogenous ligands for a receptor may enable fine-tuning of physiological responses.
To further elucidate the complexity and intricacy of signaling by endogenous opioid peptides, studies examining signaling at pathways downstream of G protein or β-arrestin, including receptor trafficking, are needed. We will also need to examine the temporal differences in signaling by endogenous opioid peptides and how this affects functional selectivity. In addition, it is possible that some endogenous opioid peptides are endogenous ligands for heteromers involving opioid receptors. For xamplee, BAM 22 exhibits unique signaling in cells coexpressing δOR and sensory neuron-specific receptor 4 (63). Thus, evaluation of signaling by endogenous opioid peptides at heteromers involving opioid receptors is also needed. Together, such studies will help us understand not only signaling by the endogenous opioid system but also the differences between these responses and those of small-molecule drugs used to treat pain or drugs with high abuse potential. Systematic investigations such as these are likely to help with the identification of unique signaling profiles for analgesic drug candidates with low abuse potential.
Materials and Methods
Materials.
DAMGO, deltorphin II, and U69,593, were from Bio-Techne Corporation. α-Neoend, β-neoend, Dyn A8, Dyn A13, Dyn A17, Dyn B1, acetylated β-end 26, β-end 26, β-end 27, β-end 31, γ-end, Des-Tyr-γ-end, Leu-enk, Met-enk, Met-enk RF, Met-enk RGL, metorphamide, BAM 12, BAM 18, BAM 22, peptide E, and peptide F were from Phoenix Pharmaceuticals. [3H]diprenorphine and [35S]GTPγS were from Perkin-Elmer. Paraformaldehyde (PFA) was from USB. Anti-μOR anti-serum was from Millipore, (Cat No. AB1774), mouse anti-NeuN primary antibody was from Chemicon. The HitHunter cAMP detection kit and the PathHunter Chemiluminescence detection kit were from DiscoverX (Eurofins Corporation).
Cell Lines.
μβgalOR, δβgalOR, and κβgalOR-expressing U2OS cells were from DiscoverX. These cells express μOR, δOR, or κOR C-terminally tagged with a ProLink/β-gal donor (PK) fragment and β-arrestin 2 tagged with a complementary β-gal activator (EA) fragment. Cells were grown in MEMα containing 10% (vol/vol) FBS, 2,000 μg to 2,000 Units/ml streptomycin-penicillin, 500 μg/mL geneticin, and 250 μg/mL hygromycin. CHO cells stably expressing either Flag-epitope tagged μOR, δOR, or κOR were grown in F12 media containing 10% (vol/vol) FBS, 2,000 μg to 2,000 Units/ml streptomycin-penicillin, 500 μg/mL geneticin.
Radioligand Binding Studies.
Membranes were prepared from cells expressing either μβgalOR, δβgalOR, κβgalOR, or mouse striatum, as described previously (64). Displacement binding assays were carried out as described previously (65, 66) with membranes (20 μg) and [3H]diprenorphine (3 nM) in the absence and presence of different opioid peptides (10−12 to 10−5 M), except that the assay buffer consisted of 50 mM Tris⋅Cl buffer (pH 7.4) containing 100 mM NaCl, 10 mM MgCl2, 0.2 mM EGTA, and protease inhibitor mixture (Sigma-Aldrich; Buffer A) and incubation was carried out for 1 h at 30 °C. Nonspecific binding was determined in the presence of 10 μM cold diprenorphine. After subtracting nonspecific counts, values obtained in the absence of peptides were taken as 100%.
[35S]GTPγS Binding.
[35S]GTPγS binding assays were carried out as described previously (64). Briefly, membranes (20 μg) were incubated for 1 h at 30 °C in the absence or presence of DAMGO, Delt II, U69,593, or different opioid peptides (10−12 to 10−5 M final concentration) in Buffer A (described under radioligand binding studies) containing 30 μM GDP and 0.1 nM [35S]GTPγS. In experiments, examining the effect of receptor-specific antagonists, membranes were preincubated with the antagonists (10-μM final concentration) for 10 min prior to addition of standards or opioid peptides (1-μM final concentration). Nonspecific binding was determined in the presence of 10 μM cold GTPγS. After subtracting nonspecific counts, values obtained in the absence of peptides were taken as 100%.
β-Arrestin Recruitment Assay.
Cells expressing either μβgalOR, δβgalOR, or κβgalOR were plated in each well (5,000 cells) of a 96-well white clear-bottom plate in 100 μL of growth media. Next day, growth media was removed, cells were rinsed in Buffer A (described under Radioligand Binding Studies), and incubated in the absence or presence of either DAMGO, Delt II, U69,593, or different opioid peptides (10−12 to 10−5 M final concentration) in Buffer A for 1 h at 30 °C. β-Arrestin recruitment was measured using the PathHunter Chemiluminescence detection kit, as described in the manufacturer’s protocol.
cAMP Assay.
CHO cells (10,000 per well) expressing either Flag-epitope tagged μOR, δOR, or κOR were treated without or with either opioid peptides or the standards DAMGO, Delt II, or U69,593 (10−12 to 10−5 μM) in the absence or presence of 10-μM receptor antagonists (CTOP for μOR, TIPPψ for δOR, or JDTic for κOR) for 30 min at 37 °C in HBSS containing 10 mM Hepes, 20 μM forskolin, and 100 μM IBMX. cAMP levels were quantified using the HitHunter cAMP detection kit according to the manufacturer’s protocol. Dose–response curves obtained were normalized to that of the standards (DAMGO for μOR, deltorphin II for δOR, and U69,593 for κOR).
Bias Calculations.
Bias analyses were carried out using the method proposed by Kenakin et al. (28, 42) that is based on the Black and Leff operational method (67). For this we followed the step-wise protocol described by Nagi and Pineyro (43). Briefly, bias analysis was carried out as follows: 1) Dose–response curves obtained in [35S]GTPγS binding and β-arrestin recruitment assays were normalized to that of the standards (DAMGO for μOR, deltorphin II for δOR, and U69,593 for κOR). 2) Data points were fit to the four-parameter logistic equation in Prism 7.0 to obtain maximal response (span; Emax), EC50 values for all ligands and Hill coefficients for [35S]GTPγS binding and β-arrestin recruitment. Emax values obtained in step 2 were then analyzed by one-way ANOVA followed by Dunnett’s post hoc test to identify peptides that gave maximal responses in each signaling pathway and at each opioid receptor. This analysis helps identify opioid peptides that exhibit partial agonistic activity compared to the standards. 3) Data from step 2 was fit to the new operational model with TauKA ratios in Prism 7.0 by fitting values for KA (100 × EC50), “n” (Hill coefficient) and Emax. 4) Log(τ/KA) [also referred as Log (RA)] ratios for each ligand in different pathways were determined. 5) Subtract Log(τ/KA) ratio of the standard from those of the other ligands to obtain normalized coefficients ΔLog(τ/KA). 6) Obtain ΔΔLog(τ/KA) by subtracting ΔLog(τ/KA) ratios from different pathways. Organize the terms in the subtraction so as to obtain positive ΔΔLog(τ/KA) values. 7) Obtain the actual value of bias by calculating anti-Log ΔΔLog(τ/KA) values.
Animals.
Male Spague–Dawley rats (200 g) were used for electrophysiological, immunohistochemical, and in situ hybridization studies. All animal care studies and experimental procedures were approved in advance by the University of California, San Francisco, the Icahn School of Medicine at Mount Sinai, New York, as well as the Uniformed Services University of the Health Sciences Institutional Animal Care and Use Committees, and were conducted in accordance with the National Research Council Guide to the Care and Use of Laboratory Animals (68).
Slice Preparation and Electrophysiology.
Horizontal rat brain slices (200-μm thick) containing the VTA were prepared from adult rats as previously described (34). Electrophysiological measurements of the effect of β-end peptides (500 nM) on electrically evoked GABAAR inhibitory postsynaptic currents (IPSCs) were carried out as in Margolis et al. (34). The IPSC amplitude was calculated by comparing a 2-ms period around the peak to a 2-ms interval just before stimulation. In a subset of experiments, β-end effects were reversed with the opioid receptor antagonist naltrexone (1 μM).
Immunohistochemistry.
Male rats (∼200 g) were anesthetized with an intraperitoneal injection of ketamine (85 mg/kg) and xylazine (10 mg/kg) and perfused through the aorta with heparinized PBS followed by 4% PFA. The brains were dissected and placed in 4% PFA for 24 h at 4 °C and then cryoprotected by submersion in 20% sucrose at 4 °C for 3 d, frozen on dry ice, and stored at −70 °C until sectioned. Coronal sections (20 µm) through the amygdala (around −2.90 mm caudal to Bregma) (69) were cut using a cryostat (Leica CM1900), mounted on slides, fixed in 4% PFA for 5 min, washed in PBS then blocked in 10% normal goat serum (NGS) containing 0.3% Triton X-100 in PBS for 1 h. Sections were then incubated in guinea pig anti-μOR antiserum (1:1,000) (70) and rabbit anti-Dyn A(1–8) antiserum (1:500) (71) in carrier solution (5% NGS in 0.1% Triton X-100 in PBS) overnight at room temperature. After rinsing twice for 10 min in PBS, sections were incubated for 2 h in Alexa Fluor 488 labeled goat anti-guinea pig IgG (1:200) and Alexa Fluor 566 labeled goat anti-rabbit IgG (1:200). Finally, sections were rinsed twice for 10 min in PBS and cover-slipped with mounting medium. Images were obtained with a Leica DM RXA fluorescence microscope. Control sections with no primary antibody added showed no staining above a very low background staining. The guinea pig anti-μOR antibody AB1774 was raised against a synthetic peptide corresponding to the carboxy-terminus of the cloned rat μOR (70). The rabbit anti-Dyn A(1–8) antiserum, raised against Dyn A(1–8), shows less than 0.001% cross-reactivity with Dyn A(1–17) and no cross reactivity with α-neoend or Leu-enk (71).
In Situ Hybridization Autoradiography for ppDYN mRNA with NeuN Immunohistochemistry.
A prodynorphin probe corresponding to nucleotides 365 to 732 of rat prodynorphin cDNA (accession no. NM_019374) was used for in situ hybridization; the latter was described in detail previously (72). Briefly, slide-mounted coronal sections including amygdala (from −1.8 to −3.6 mm caudal to Bregma) were hybridized with 35S-UTP-labeled riboprobe for ppDYN (approximately 2.04 × 106 dpm/100 µL) by incubating overnight at 55 °C, rinsed several times followed by incubation with RNase in buffer to remove free probe before processing for immunofluorescence immunohistochemistry to visualize the neuronal marker, NeuN, by incubation overnight with mouse anti-NeuN primary antibody (1:100; Chemicon). After rinsing, the sections were incubated with Alexa Flour 488 goat anti-mouse secondary antibody (1:200) for 2 h at room temperature, rinsed, followed by dehydration in ascending concentrations of ethanol, transferred to the dark room, and coated with Kodak NTB emulsion (Care Stream Health). After 18 d of exposure at 4 °C in the dark, the slides were developed and fixed using Kodak Dektol Developer and Kodak Fixer (Eastman Kodak). Slides were then cover-slipped using DAPI prolong (ThermoFisher Scientific). Images were obtained with a Leica DM RXA fluorescence microscope. Sets of low-magnification images across the amygdala were tiled to generate a composite image of the entire amygdala.
Data Analysis.
Data were analyzed using Prism 7.0 software. Displacement binding assay data were analyzed by comparing both One-site-Fit logIC50 and Two-sites-Fit logIC50 curves to determine for each dataset, which of the two equations fits best. Data for [35S]GTPγS binding and β-arrestin recruitment assays were analyzed as described above under bias calculations. Data from cAMP assays were analyzed using sigmoidal dose–response curves. Curves had R2 values >0.8 and <0.98. Statistical analysis was done using either one-way or two-way ANOVA. P < 0.05 was considered to be significant.
Data Availability Statement.
All relevant data, associated protocols and materials including their source are within the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Eckard Weber for the generous gift of the dynorphin A(1–8) antiserum; and Sheshat Mack for careful reading of the manuscript prior to submission. This work was supported by National Institute of Health Awards DA008863 and NS026880 (to L.A.D.); NIH Award DA03102 and US Department of Defense Award W81XWH-08-2-0575 (to B.M.C.); and R01AA026609 (to E.B.M.). S.S. was supported by a grant from Alfonso Martin Escudero Foundation.The opinions and assertions contained herein are the private opinions of the authors and are not to be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense or the Government of the United States.
Footnotes
The authors declare no competing interests.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000712117/-/DCSupplemental.
References
- 1.Stein C., Opioid receptors. Annu. Rev. Med. 67, 433–451 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Shenoy S. S., Lui F., Biochemistry, Endogenous Opioids (StatPearls, Treasure Island, FL, 2018). [PubMed] [Google Scholar]
- 3.Stein C., New concepts in opioid analgesia. Expert Opin. Investig. Drugs 27, 765–775 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Mansour A., Hoversten M. T., Taylor L. P., Watson S. J., Akil H., The cloned mu, delta and kappa receptors and their endogenous ligands: Evidence for two opioid peptide recognition cores. Brain Res. 700, 89–98 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Höllt V., Opioid peptide processing and receptor selectivity. Annu. Rev. Pharmacol. Toxicol. 26, 59–77 (1986). [DOI] [PubMed] [Google Scholar]
- 6.Schoffelmeer A. N., Warden G., Hogenboom F., Mulder A. H., Beta-endorphin: A highly selective endogenous opioid agonist for presynaptic mu opioid receptors. J. Pharmacol. Exp. Ther. 258, 237–242 (1991). [PubMed] [Google Scholar]
- 7.Kenakin T., Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol. Sci. 16, 232–238 (1995). [DOI] [PubMed] [Google Scholar]
- 8.Jarpe M. B., et al. , [D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P acts as a biased agonist toward neuropeptide and chemokine receptors. J. Biol. Chem. 273, 3097–3104 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Bohinc B. N., Gesty-Palmer D., β-arrestin-biased agonism at the parathyroid hormone receptor uncouples bone formation from bone resorption. Endocr. Metab. Immune Disord. Drug Targets 11, 112–119 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Galandrin S., Oligny-Longpré G., Bouvier M., The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol. Sci. 28, 423–430 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Luttrell L. M., Lefkowitz R. J., The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 115, 455–465 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Defea K., Beta-arrestins and heterotrimeric G-proteins: Collaborators and competitors in signal transduction. Br. J. Pharmacol. 153 (suppl. 1), S298–S309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson G. L., Canals M., Poole D. P., Biological redundancy of endogenous GPCR ligands in the gut and the potential for endogenous functional selectivity. Front. Pharmacol. 5, 262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rankovic Z., Brust T. F., Bohn L. M., Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorg. Med. Chem. Lett. 26, 241–250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raehal K. M., Walker J. K., Bohn L. M., Morphine side effects in beta-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 314, 1195–1201 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Bohn L. M., et al. , Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286, 2495–2498 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Rivero G., et al. , Endomorphin-2: A biased agonist at the μ-opioid receptor. Mol. Pharmacol. 82, 178–188 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPherson J., et al. , μ-Opioid receptors: Correlation of agonist efficacy for signalling with ability to activate internalization. Mol. Pharmacol. 78, 756–766 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raehal K. M., Schmid C. L., Groer C. E., Bohn L. M., Functional selectivity at the μ-opioid receptor: Implications for understanding opioid analgesia and tolerance. Pharmacol. Rev. 63, 1001–1019 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pradhan A. A., Smith M. L., Kieffer B. L., Evans C. J., Ligand-directed signalling within the opioid receptor family. Br. J. Pharmacol. 167, 960–969 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeWire S. M., et al. , A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 344, 708–717 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Tidgewell K., et al. , Herkinorin analogues with differential beta-arrestin-2 interactions. J. Med. Chem. 51, 2421–2431 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manglik A., et al. , Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruegel A. C., et al. , Synthetic and receptor signaling explorations of the mitragyna alkaloids: Mitragynine as an atypical molecular framework for opioid receptor modulators. J. Am. Chem. Soc. 138, 6754–6764 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rives M. L., Rossillo M., Liu-Chen L. Y., Javitch J. A., 6′-guanidinonaltrindole (6′-GNTI) is a G protein-biased κ-opioid receptor agonist that inhibits arrestin recruitment. J. Biol. Chem. 287, 27050–27054 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brust T. F., et al. , Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci. Signal. 9, ra117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maillet E. L., et al. , Noribogaine is a G-protein biased κ-opioid receptor agonist. Neuropharmacology 99, 675–688 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Thompson G. L., et al. , Biased agonism of endogenous opioid peptides at the μ-opioid receptor. Mol. Pharmacol. 88, 335–346 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Thompson G. L., et al. , Systematic analysis of factors influencing observations of biased agonism at the mu-opioid receptor. Biochem. Pharmacol. 113, 70–87 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Deakin J. F., Doströvsky J. O., Smyth D. G., Influence of N-terminal acetylation and C-terminal proteolysis on the analgesic activity of beta-endorphin. Biochem. J. 189, 501–506 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch M. D., Millington W. R., Endoproteolytic conversion of beta-endorphin-1-31 to beta-endorphin-1-27 potentiates its central cardioregulatory activity. Brain Res. 550, 61–68 (1991). [DOI] [PubMed] [Google Scholar]
- 32.Nicolas P., Li C. H., Beta-endorphin-(1-27) is a naturally occurring antagonist to etorphine-induced analgesia. Proc. Natl. Acad. Sci. U.S.A. 82, 3178–3181 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammonds R. G. Jr, Nicolas P., Li C. H., Beta-endorphin-(1-27) is an antagonist of beta-endorphin analgesia. Proc. Natl. Acad. Sci. U.S.A. 81, 1389–1390 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolis E. B., Fields H. L., Hjelmstad G. O., Mitchell J. M., Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J. Neurosci. 28, 12672–12681 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson S. W., North R. A., Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 12, 483–488 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonci A., Williams J. T., Increased probability of GABA release during withdrawal from morphine. J. Neurosci. 17, 796–803 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Likhtik E., Popa D., Apergis-Schoute J., Fidacaro G. A., Paré D., Amygdala intercalated neurons are required for expression of fear extinction. Nature 454, 642–645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaesse P., et al. , μ-Opioid receptor-mediated inhibition of intercalated neurons and effect on synaptic transmission to the central amygdala. J. Neurosci. 35, 7317–7325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchant N. J., Densmore V. S., Osborne P. B., Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: Evidence of two distinct endogenous opioid systems in the lateral division. J. Comp. Neurol. 504, 702–715 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Puett D., Hammonds R. G. Jr, Ling N., Contribution of the amino terminal tyrosine to the interaction of gamma-endorphin with opiate receptors. Peptides 3, 87–89 (1982). [DOI] [PubMed] [Google Scholar]
- 41.Akil H., Young E., Watson S. J., Coy D. H., Opiate binding properties of naturally occurring N- and C-terminus modified beta-endorphins. Peptides 2, 289–292 (1981). [DOI] [PubMed] [Google Scholar]
- 42.Kenakin T., Watson C., Muniz-Medina V., Christopoulos A., Novick S., A simple method for quantifying functional selectivity and agonist bias. ACS Chem. Neurosci. 3, 193–203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagi K., Pineyro G., Practical guide for calculating and representing biased signaling by GPCR ligands: A stepwise approach. Methods 92, 78–86 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Takahashi S., et al. , The enhancing effects of peptidase inhibitors on the antinociceptive action of [Met5]enkephalin-Arg6-Phe7 in rats. J. Pharmacol. Sci. 105, 117–121 (2007). [DOI] [PubMed] [Google Scholar]
- 45.McKnight A. T., Corbett A. D., Kosterlitz H. W., Increase in potencies of opioid peptides after peptidase inhibition. Eur. J. Pharmacol. 86, 393–402 (1983). [DOI] [PubMed] [Google Scholar]
- 46.Alt A., et al. , Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J. Pharmacol. Exp. Ther. 286, 282–288 (1998). [PubMed] [Google Scholar]
- 47.Quirion R., Pert C. B., Dynorphins: Similar relative potencies on mu, delta- and kappa-opiate receptors. Eur. J. Pharmacol. 76, 467–468 (1981). [DOI] [PubMed] [Google Scholar]
- 48.Hewlett W. A., Barchas J. D., Regional interactions of opioid peptides at mu and delta sites in rat brain. Peptides 4, 853–858 (1983). [DOI] [PubMed] [Google Scholar]
- 49.Young E. A., et al. , [3H]dynorphin A binding and kappa selectivity of prodynorphin peptides in rat, guinea-pig and monkey brain. Eur. J. Pharmacol. 121, 355–365 (1986). [DOI] [PubMed] [Google Scholar]
- 50.Garzón J., Sánchez-Blázquez P., Gerhart J., Loh H. H., Lee N. M., Dynorphin A: Inhibitory effect on other opiate ligand bindings in the mouse brain. Biochem. Pharmacol. 33, 2609–2614 (1984). [DOI] [PubMed] [Google Scholar]
- 51.Zhang S., et al. , Dynorphin A as a potential endogenous ligand for four members of the opioid receptor gene family. J. Pharmacol. Exp. Ther. 286, 136–141 (1998). [PubMed] [Google Scholar]
- 52.Merg F., et al. , Big dynorphin as a putative endogenous ligand for the kappa-opioid receptor. J. Neurochem. 97, 292–301 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Chavkin C., Henriksen S. J., Siggins G. R., Bloom F. E., Selective inactivation of opioid receptors in rat hippocampus demonstrates that dynorphin-A and -B may act on mu-receptors in the CA1 region. Brain Res. 331, 366–370 (1985). [DOI] [PubMed] [Google Scholar]
- 54.Zhou L., et al. , Characterization of kappa opioid receptor mediated, dynorphin-stimulated [35S]GTPγS binding in mouse striatum for the evaluation of selective KOR ligands in an endogenous setting. Neuropharmacology 99, 131–141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones B. W., Hinkle P. M., Arrestin binds to different phosphorylated regions of the thyrotropin-releasing hormone receptor with distinct functional consequences. Mol. Pharmacol. 74, 195–202 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carpenter B., Tate C. G., Active state structures of G protein-coupled receptors highlight the similarities and differences in the G protein and arrestin coupling interfaces. Curr. Opin. Struct. Biol. 45, 124–132 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Yin W., et al. , A complex structure of arrestin-2 bound to a G protein-coupled receptor. Cell Res. 29, 971–983 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kenakin T., Biased agonism. F1000 Biol. Rep. 1, 87 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmid C. L., Bohn L. M., Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol. Ther. 121, 285–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajagopal S., et al. , Biased agonism as a mechanism for differential signaling by chemokine receptors. J. Biol. Chem. 288, 35039–35048 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zweemer A. J., Toraskar J., Heitman L. H., IJzerman A. P., Bias in chemokine receptor signalling. Trends Immunol. 35, 243–252 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Zhao P., et al. , Agonist-biased trafficking of somatostatin receptor 2A in enteric neurons. J. Biol. Chem. 288, 25689–25700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breit A., Gagnidze K., Devi L. A., Lagacé M., Bouvier M., Simultaneous activation of the delta opioid receptor (deltaOR)/sensory neuron-specific receptor-4 (SNSR-4) hetero-oligomer by the mixed bivalent agonist bovine adrenal medulla peptide 22 activates SNSR-4 but inhibits deltaOR signaling. Mol. Pharmacol. 70, 686–696 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Gomes I., Filipovska J., Devi L. A., Opioid receptor oligomerization. Detection and functional characterization of interacting receptors. Methods Mol. Med. 84, 157–183 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Gomes I., et al. , A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. U.S.A. 101, 5135–5139 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomes I., Ijzerman A. P., Ye K., Maillet E. L., Devi L. A., G protein-coupled receptor heteromerization: A role in allosteric modulation of ligand binding. Mol. Pharmacol. 79, 1044–1052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Black J. W., Leff P., Operational models of pharmacological agonism. Proc. R. Soc. Lond. B Biol. Sci. 220, 141–162 (1983). [DOI] [PubMed] [Google Scholar]
- 68.National Research Council , Guide for the Care and Use of Laboratory Animals, (National Academies Press, Washington, DC, 7th Ed, 1996). [Google Scholar]
- 69.Paxinos G., Watson C., The Rat Brain in Sterotactic Coordinates (Academic Press, New York, 2001). [Google Scholar]
- 70.Rodriguez J. J., Mackie K., Pickel V. M., Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat caudate putamen nucleus. J. Neurosci. 21, 823–833 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weber E., Evans C. J., Barchas J. D., Predominance of the amino-terminal octapeptide fragment of dynorphin in rat brain regions. Nature 299, 77–79 (1982). [DOI] [PubMed] [Google Scholar]
- 72.Gouty S., Brown J. M., Rosenberger J., Cox B. M., MPTP treatment increases expression of pre-pro-nociceptin/orphanin FQ mRNA in a subset of substantia nigra reticulata neurons. Neuroscience 169, 269–278 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data, associated protocols and materials including their source are within the main text and SI Appendix.