Abstract
Objective:
To describe the epidemiology, morbidity, and mortality of new or progressive multiple organ dysfunction syndrome (NPMODS) in children with severe sepsis.
Design:
Secondary analysis of a prospective, cross-sectional, point prevalence study.
Setting:
International, multi-center pediatric intensive care units.
Patients:
Pediatric patients with severe sepsis identified on five separate days over a 1 year period.
Interventions:
None
Measurements and Main Results:
Of 567 patients from 128 PICUs in 26 countries were enrolled, 384 (68%) developed MODS within seven days of severe sepsis recognition. Three hundred twenty-seven had MODS on day of sepsis recognition. Ninety-one of these patients developed progressive MODS, while an additional 57 subsequently developed new MODS, yielding a total proportion with severe sepsis-associated NPMODS of 26%. Hospital mortality in patients with progressive MODS was 51% compared to patients with new MODS (28%) and those with single-organ dysfunction without MODS (10%), p<0.001. Survivors of NPMODS also had a higher incidence of moderate to severe disability defined as a Pediatric Overall Performance Category (POPC) of ≥ 3 and an increase of ≥ 1 from baseline: 22% vs. 29% vs. 11% for progressive, new, and no MODS, respectively, p<0.001.
Conclusion:
Development of NPMODS is common (26%) in severe sepsis and was associated with a higher risk of morbidity and mortality than severe sepsis without NPMODS. Our data supports the use of NPMODS as an important outcome in trials of pediatric severe sepsis, though efforts are needed to validate that reducing NPMODS leads to improvements in more definitive morbidity and mortality endpoints.
Keywords: severe sepsis, multiple organ dysfunction syndrome, children, epidemiology
INTRODUCTION
Pediatric sepsis remains a leading cause of childhood mortality across the world.1–3 For the subset of patients treated in pediatric intensive care units (PICUs), mortality ranges between 10–25%.2,4–8 Within this group of critically ill patients, multiple organ dysfunction syndrome (MODS) and new or progressive MODS (NPMODS) have long been recognized as significant risk factors for adverse outcomes in pediatric sepsis.6,9–13 The recent Sepsis Prevalence, Outcomes, and Therapy (SPROUT) point prevalence study conducted in 128 PICUs across 26 countries reported 25% all-cause hospital mortality for pediatric severe sepsis.7,8 In this cohort, 58% of patients presented with MODS at the time of severe sepsis recognition, and 40% either died during the hospitalization or developed new or progressive MODS (NPMODS) during the 7 day observation period.7,8 Prior studies have also demonstrated unfavorable functional outcomes for pediatric severe sepsis survivors with MODS. In a retrospective analysis of the pediatric activated protein C for severe sepsis trial (RESOLVE), percentage of survivors with poor functional outcome increased from 6.5% to 28.6% as number of organ system dysfunction increased from ≤ 2 to ≥ 5.13
However, limited data exist about which specific individual organ dysfunctions contribute to the morbidity and mortality risk and which therapeutic interventions are utilized for patients with sepsis-associated MODS or NPMODS. There are also few data comparing outcomes in pediatric severe sepsis patients who present with MODS to those with initial single-organ dysfunction who go on to develop NPMODS. Moreover, while the use of NPMODS as a primary outcome in clinical trials has been increasingly utilized,14,15 there remains a need to establish the degree of association between NPMODS and more definitive clinical endpoints, such as mortality.
We sought to determine the prevalence and evolution of MODS in pediatric patients treated for severe sepsis in a PICU setting through a secondary analysis of the SPROUT dataset.7,8 We hypothesized that the pattern and timing of sepsis-associated organ dysfunction and development of NPMODS, are associated with mortality, morbidity, and disability outcomes. These data may help inform future trials of pediatric severe sepsis considering NPMODS as both a surrogate marker of mortality as well as a stand alone primary outcome measure.
METHODS
SPROUT was a prospective, cross-sectional point prevalence study performed at 128 PICUs in 26 countries on five separate days between June 2013 to June 2014.7 Site participation was voluntary, with no funding provided to the participating sites. Ethics approval was obtained at all sites with a waiver of informed consent granted at all but three sites at which written consent was required for data collection. Complete details of the SPROUT study methodology have been previously published.7
Study population
All patients >42 weeks corrected gestational age and <18 years-old being treated in a participating ICU at 9:00am local time on each study day were screened for severe sepsis according to the 2005 International Pediatric Sepsis Consensus Conference criteria.16 For the purposes of screening, only clinical data available within the 24 hours preceding the 9:00 am study day time were used, yielding a study cohort with active severe sepsis. Patients who had surgery involving cardiopulmonary bypass in the preceding five days were excluded from the SPROUT study.
Data collection
Data were collected from the medical record regarding patient demographics, comorbid conditions, and infectious etiology. Laboratory data and types of therapies provided were collected within a 48-hour window around the study day (9:00am on the day prior through 9:00am on the day after the study day). Pediatric Index of Mortality-3 (PIM3) score17 was calculated at PICU admission and the Pediatric Logistic Organ Dysfunction (PELOD) score18 was calculated on the study day. Patients were followed until hospital discharge (censored at 90 days if still hospitalized) to determine all-cause mortality, morbidity, and disability outcomes. The Pediatric Overall Performance Category (POPC) ordinal scale was used to measure new disability or change in baseline level of disability.19
The day of severe sepsis recognition was assigned as “day 1” and was identified by chart review as the first calendar day on which a patient met consensus criteria for severe sepsis.16 Presence of NPMODS was determined by daily screening for dysfunction of the cardiovascular, respiratory, hematologic, hepatic, renal, neurologic, and gastrointestinal organ systems for six additional days following day 1 of severe sepsis recognition to give a total study period of seven days. Although the consensus criteria used to screen patients for severe sepsis also include organ dysfunction criteria,16 patients had to meet the more stringent definitions published and used by Proulx, et al. to identify daily presence of specific organ dysfunctions in order to meet criteria for MODS and NPMODS.6 Although NPMODS as a trial outcome is commonly assessed for 28 days, SPROUT had censored this evaluation at seven days due to prior reports that the overwhelming majority of organ dysfunctions occurred within one week of onset of sepsis and critical illness in children.10
MODS was defined as two or more concurrent organ systems dysfunctions. Both new as well as progressive MODS were considered to be NPMODS. New MODS was defined as a patient with ≤1 organ dysfunction on day 1 of sepsis recognition who subsequently developed ≥2 concurrent organ dysfunctions. Progressive MODS was defined as a patient with existing MODS (≥2 organ dysfunctions) on day 1 of sepsis recognition who developed at least one other concurrent organ dysfunction.14,15 MODS or NPMODS was not considered present if a single organ system dysfunction resolved prior to development of a different single organ dysfunction on the next day. In the original SPROUT analysis, death was also considered as meeting criteria for NPMODS, even if the patient exhibited only single organ dysfunction pre-mortem.7 However, in the present analysis, only documented pre-mortem organ dysfunction based on clinical and laboratory criteria available prior to death were used to define NPMODS in order to analyze organ dysfunction as a risk factor antecedent to mortality. Chronic organ dysfunctions established prior to sepsis recognition were not considered to be NPMODS unless new organ dysfunction developed in line with published criteria.6
Statistical Analysis
Data were analyzed using STATA (Version 12.1; College Station, TX). Continuous data are presented as medians with interquartile range (IQR) and analyzed using the Wilcoxon rank sum or Kruskal-Wallis tests. Categorical data are presented as proportions and analyzed using the Fisher’s exact or chi-squared tests. Data across ordered groups were compared using a non-parametric test of trend of ranks. We used logistic regression to determine the association of either each individual organ system dysfunction or NPMODS with hospital mortality. We then used multivariable logistic regression to assess potential confounding effects of covariates on the association of NPMODS with hospital mortality. Covariates that were significantly different between patients with versus without NPMODS were tested as possible confounders of the association of NPMODS with mortality. To test for confounding, each covariate was added to a bivariable model that included NPMODS as the independent variable and hospital mortality as the dependent variable. Only those covariates that changed the base model odds ratio (OR) by 5% or greater were considered to be true confounders for inclusion in the final multivariable model.20 Unadjusted and adjusted ORs are presented with 95% confidence intervals (95% CI). Statistical significance was defined as a p-value <0.05 when comparing patient characteristics and therapies. Comparison across the eight clinical outcomes was adjusted using Bonferroni correction with statistical significance defined as a p-value <0.006 (0.05/8).
RESULTS
Of the 569 PICU patients with severe sepsis enrolled in the SPROUT study, two declined to consent for data collection, leaving 567 patients for the current analysis. Respiratory and cardiovascular dysfunction were the most frequent organ dysfunctions during the week following sepsis recognition (82% and 65%, respectively) and were most likely to be present at the time of severe sepsis recognition (87% and 79%, respectively) (Table 1). Respiratory, neurologic, or gastrointestinal dysfunction present on the day of sepsis recognition or developing at any point within 7 days of severe sepsis onset conferred the highest risk of death with OR (95% CI) of 4.8 (2.3–10.1), 4.6 (2.8–7.4), and 4.0 (2.2–7.3), respectively (Table 1).
Table 1:
Specific organ system dysfunctions and risk of hospital death
| Organ System | Present within 7 days of sepsis recognition | Present at time of sepsis recognition | ||
|---|---|---|---|---|
| N (%)a | OR (95% CI) of death | N (%)b | OR (95% CI) of death | |
| Cardiovascular | 366 (65) | 2.6 (1.7, 4.1) | 288 (79) | 1.36 (0.93–2.0) |
| Respiratory | 467 (82) | 4.8 (2.3, 10.1) | 408 (87) | 2.1 (1.3, 3.8) |
| Renal | 70 (12) | 2.9 (1.7, 4.8) | 26 (37) | 2.2 (0.98, 5.0) |
| Hepatic | 52 (9) | 3.0 (1.7, 5.4) | 22 (42) | 2.4 (0.98, 5.6) |
| Hematologic | 123 (22) | 1.3 (0.86, 2.1) | 73 (59) | 1.4 (0.8, 2.5) |
| Neurologic | 87 (15) | 4.6 (2.8, 7.4) | 61 (70) | 3.8 (2.2, 6.6) |
| GI | 48 (8) | 4.0 (2.2, 7.3) | 31 (65) | 3.4 (1.6, 7.0) |
Proportion of the study population with organ dysfunction present at any time within 7 days of sepsis recognition.
Proportion of each organ dysfunction that was present at time sepsis of recognition.
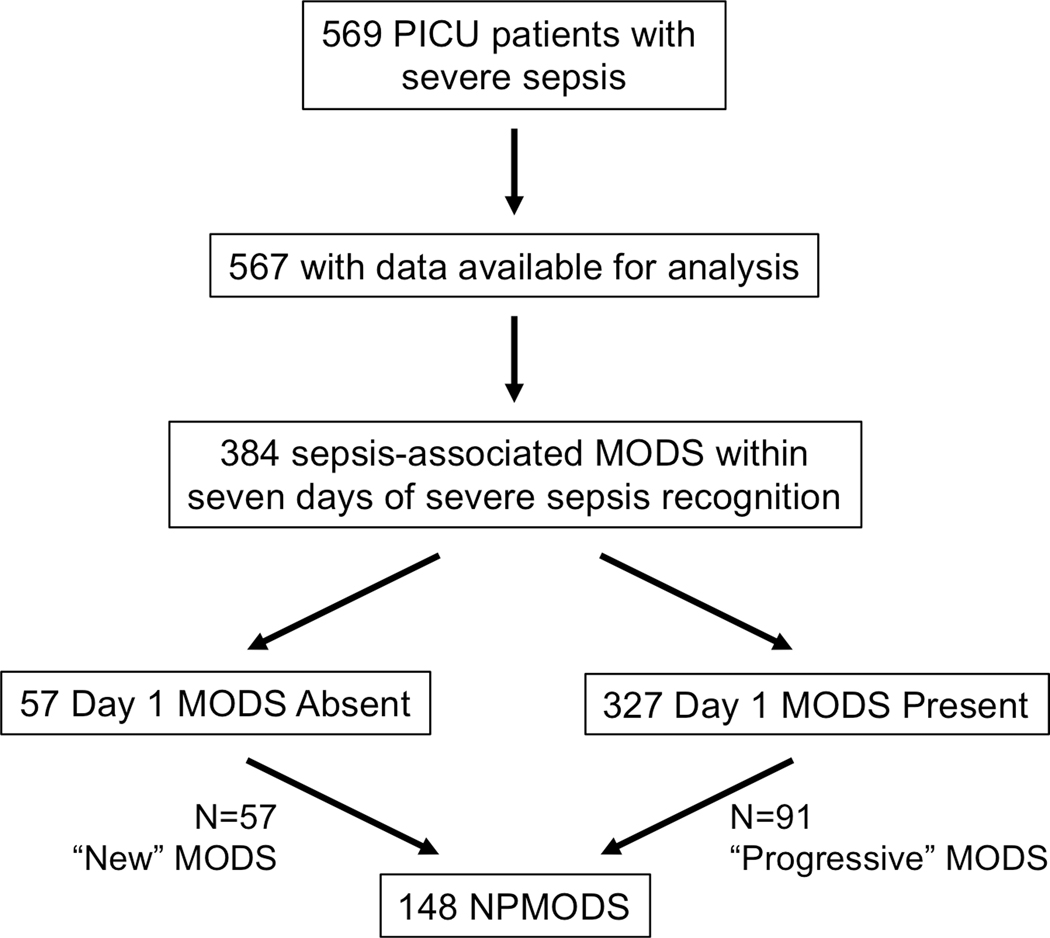
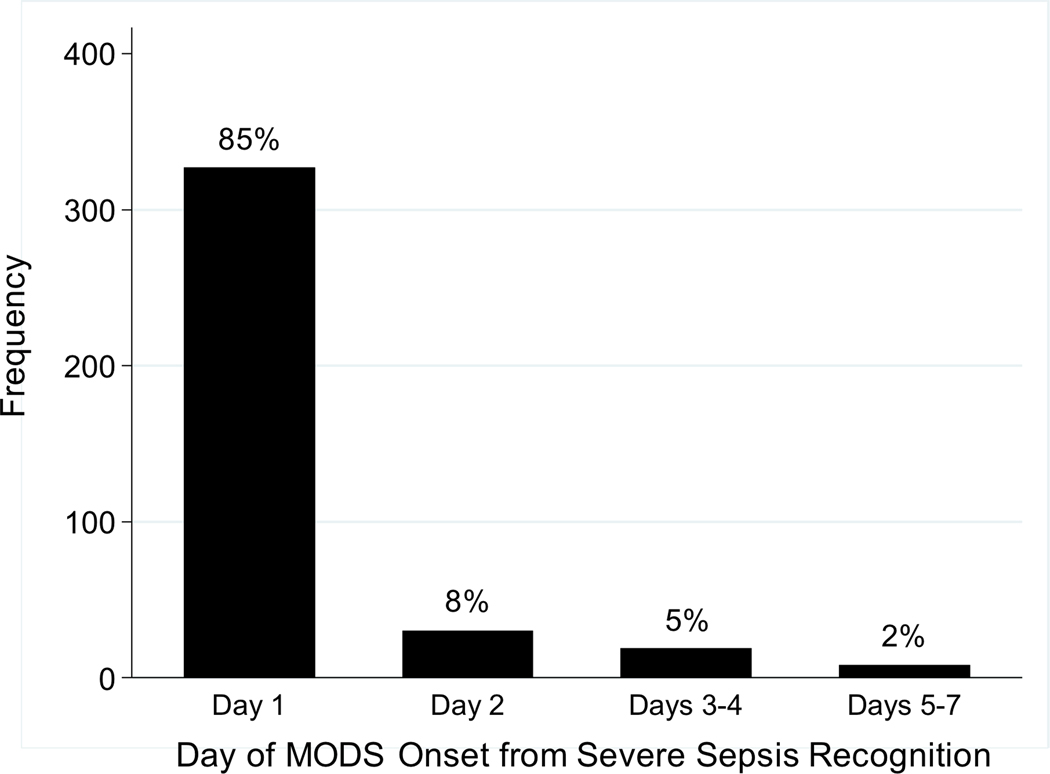
Overall, 384 (68%) patients developed sepsis-associated MODS within one week of severe sepsis recognition and 148 (26%) developed NPMODS (Figure 1). The onset of MODS was most common on day 1 (85% of all patients with MODS; Figure 2), of whom 91 went on to develop progressive MODS. Of the 240 patients who did not present with MODS, 57 went on to develop new MODS. The total proportion with severe sepsis-associated NPMODS was thus 148 (26%) of all identified severe sepsis patients. The proportion of patients who developed NPMODS did not differ based on whether MODS was present or absent on day 1 (p=0.28).
Figure 1.
Flow diagram of severe sepsis patients presenting with or without MODS and subsequently developing NPMODS or having no progression of MODS.
Figure 2.
Timing of MODS onset within the first seven days following severe sepsis recognition. Day 1 is the day of sepsis recognition.
The characteristics of patients with and without day 1 MODS and subsequent NPMODS are shown in Table 2. Age, sex, race, and source of PICU admission were not associated with MODS or NPMODS. As expected, patients with MODS on day 1 had higher PIM3 scores than patients without MODS on day 1, with the subset who also developed progressive MODS having the highest PIM3 scores at PICU admission. Patients with MODS on day 1 who developed NPMODS were also more likely to have malignancy or solid organ/bone marrow transplant as comorbid conditions.
Table 2:
Patient characteristics
| Variable | Day 1 MODS Absent | Day 1 MODS Present | P-Value | |||
|---|---|---|---|---|---|---|
| NPMODS Absent N=183 | NPMODS Present N=57 | NPMODS Absent N=236 | NPMODS Present N=91 | All Groupsd | NPMODS absent vs. presente | |
| Age (years) | 5 (1–11) | 3 (0.3–11) | 2 (0.6–10) | 4 (0.5–12) | 0.09 | 0.56 |
| Sex (male) | 88 (48) | 30 (53) | 137 (58) | 47 (52) | 0.24 | 0.73 |
| Race, n (%) | 0.23 | 0.11 | ||||
| White | 84 (46) | 24 (42) | 104 (44) | 40 (44) | ||
| Black | 20 (11) | 5 (9) | 41 (17) | 14 (15) | ||
| Asian | 23 (13) | 13 (23) | 26 (11) | 16 (18) | ||
| Other/Unknown | 56 (31) | 15 (26) | 65 (28) | 21 (23) | ||
| Source PICU Admit, n (%) | 0.25 | 0.18 | ||||
| ED | 56 (31) | 19 (33) | 73 (31) | 19 (21) | ||
| Hospital ward | 48 (26) | 18 (32) | 58 (24) | 34 (38) | ||
| Operating room | 16 (9) | 5 (9) | 26 (11) | 3 (3) | ||
| Other hospital | 55 (30) | 15 (26) | 67 (28) | 29 (32) | ||
| Other | 8 (4) | 0 | 12 (5) | 6 (7) | ||
| Previously healthy, n (%) | 33 (18) | 17 (30) | 54 (23) | 24 (26) | 0.19 | 0.08 |
| Comorbid conditionsa, n (%) | ||||||
| Respiratory | 68 (37) | 12 (21) | 66 (28) | 26 (29) | 0.07 | 0.15 |
| Gastrointestinal | 54 (30) | 15 (26) | 48 (20) | 24 (26) | 0.17 | 0.63 |
| Cardiovascular | 37 (20) | 13 (23) | 69 (29) | 17 (19) | 0.10 | 0.22 |
| Genetic | 41 (22) | 10 (18) | 48 (20) | 16 (18) | 0.78 | 0.34 |
| Hematologic/Immunologic | 38 (21) | 5 (9) | 39 (17) | 32 (35) | <0.001 | 0.08 |
| Neuromuscular | 47 (26) | 8 (14) | 31 (13) | 11 (12) | 0.004 | 0.11 |
| Malignancy | 25 (14) | 6 (11) | 27 (11) | 22 (24) | 0.03 | 0.05 |
| Prematurity | 27 (15) | 9 (16) | 33 (14) | 7 (8) | 0.33 | 0.28 |
| Metabolic | 21 (11) | 4 (7) | 27 (11) | 10 (11) | 0.084 | 0.50 |
| Renal | 14 (8) | 5 (9) | 23 (10) | 13 (14) | 0.38 | 0.24 |
| Organ transplant/BMT | 16 (9) | 4 (7) | 14 (6) | 20 (22) | 0.001 | 0.001 |
| PIM-3b | 2.4 (1.4–4.8) | 2.3 (1.6–6.7) | 4.5 (1.8–10.7) | 7.7 (4.1–15.0) | <0.001 | <0.001 |
| PELODc | 10 (1–11) | 11 (3–20) | 11 (2–13) | 11 (3–21) | <0.001 | <0.001 |
Data presented as median (interquartile range) unless noted.
ED, emergency department; BMT, bone marrow transplant; PIM, pediatric index of mortality; PELOD, pediatric logistic organ dysfunction; MODS, multiple organ dysfunction syndrome
Categories do not add up to 100% because some patients had multiple comorbid conditions.
PIM-3 was measured at time of PICU admission.
PELOD score was calculated from data within a 48-hour time window around the study day (9:00 am on the day before to 9:00 am on the day after the study day).
Statistical comparison across all four groups using Kruskal-Wallis test for continuous variables and Fisher’s exact or chi-squared tests for categorical variables
Statistical comparison between patients with NPMODS absent versus present (irrespective of day 1 MODS presence) using Wilcoxon rank sum for continuous variables and Fisher’s exact or chi-squared tests for categorical variables
Microbiology results are shown in Supplemental Digital Content—Table 1. Patients with MODS on day 1 were more likely to have bacteremia or hospital acquired infections, but neither were associated with NPMODS. Fungal infections were more common in patients with versus without NPMODS (19% versus 11%, p=0.02).
Sepsis-related therapies utilized within the 48-hour data collection window are shown in Table 3. All therapies were used more frequently in patients with MODS than those without MODS, with the highest utilization rates in patients with day 1 MODS and progressive MODS.
Table 3:
Therapies used within the 48-hour data collection window
| Day 1 MODS Absent | Day 1 MODS Present | P-value | ||||
|---|---|---|---|---|---|---|
| Therapy | NPMODS Absent N=183 | NPMODS Present N=57 | NPMODS Absent N=236 | NPMODS Present N=91 | All Groupse | NPMODS absent vs. presentf |
| Invasive mechanical ventilation | 87 (48) | 47 (82) | 209 (89) | 78 (86) | <0.001 | 0.001 |
| Vasoactive infusiona | 44 (24) | 33 (58) | 171 (72) | 66 (73) | <0.001 | 0.001 |
| Albumin | 26 (14) | 17 (30) | 62 (26) | 30 (33) | 0.001 | 0.008 |
| Blood productsb | 45 (25) | 27 (47) | 104 (44) | 56 (62) | 0.001 | <0.001 |
| Corticosteroids | 58 (32) | 26 (46) | 114 (48) | 44 (48) | 0.004 | 0.19 |
| Insulinc | 8 (4) | 8 (14) | 25 (11) | 16 (18) | 0.002 | 0.004 |
| RRTd | 7 (4) | 7 (12) | 34 (14) | 33 (36) | <0.001 | <0.001 |
Data presented as n (%).
G/GM-CSF, granulocyte/granulocyte-macrophage colony stimulating factor; IVIG, intravenous immunoglobulin; RRT, renal replacement therapy; ECMO, extracorporeal membrane oxygenation; PICC, peripherally inserted central catheter
Includes dopamine >5 mg/kg/min, dobutamine >5 mg/kg/min, or any dose of epinephrine, norepinephrine, vasopressin, phenylephrine, milrinone, levosimendan, or a vasodilator.
Includes packed red blood cells, platelets, fresh frozen plasma, cryoprecipitate, granulocytes, and whole blood.
Includes intravenous insulin by continuous infusion only.
Includes hemodialysis, all continuous renal replacement modalities, and peritoneal dialysis.
Statistical comparison across all four groups using Fisher’s exact or chi-squared tests for categorical variables
Statistical comparison between patients with NPMODS absent versus present (irrespective of day 1 MODS presence) using Fisher’s exact or chi-squared tests for categorical variables
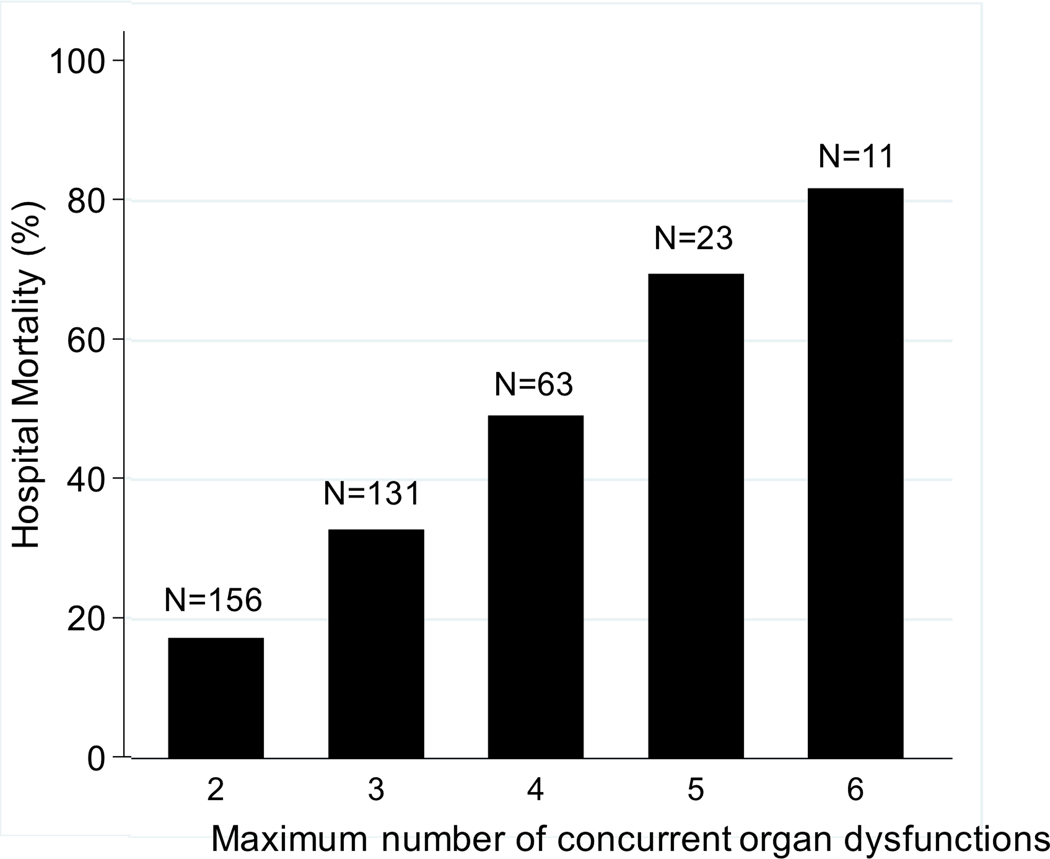
Patients with NPMODS had higher hospital mortality (62 of 148, 42%) than patients without NPMODS (83 of 419, 20%), p<0.001 (Table 4). Hospital mortality in patients with progressive MODS was 51% compared to patients with new MODS (28%) or those with single-organ dysfunction without MODS (10%), p<0.001. Mortality also increased in a step-wise fashion with increasing maximum number of organ dysfunctions (p<0.001; Figure 3). However, mortality did not differ by day of MODS onset (p=0.74; Supplemental Digital Content-Figure 1). In a multivariable regression model, NPMODS remained independently associated with hospital mortality after controlling for age, solid organ/bone marrow transplant comorbidity, illness severity (PIM3), bacteremia, and presence of a fungal infection (adjusted OR 2.85, 95% CI 1.84, 4.40, p<0.001).
Table 4:
Patient Outcomes
| Outcome Measure | Day 1 MODS Absent | Day 1 MODS Present | P-valuec | |||
|---|---|---|---|---|---|---|
| NPMODS Absent N=183 | NPMODS Present N=57 | NPMODS Absent N=236 | NPMODS Present N=91 | All Groupsd | NPMODS absent vs. presente | |
| PICU mortality | 18 (10) | 16 (28) | 62 (26) | 43 (47) | <0.001 | <0.001 |
| Hospital mortality | 19 (10) | 16 (28) | 64 (27) | 46 (51) | <0.001 | <0.001 |
| PICU LOS, median (IQR) | 10 (4–23) | 15 (10–33) | 18 (9–43) | 19 (10–39) | <0.001 | 0.01 |
| Hospital LOS, median (IQR) | 20 (10–38) | 30 (17–53) | 34 (15–71) | 30 (17–62) | 0.001 | 0.10 |
| Vasoactive-free days, median (IQR) | 28 (26–28) | 23 (9–27) | 21 (6–25) | 17 (0–23) | <0.001 | <0.001 |
| Ventilator-free days, median (IQR) | 26 (16–28) | 10 (0–22) | 12 (0–22) | 6 (0–17) | <0.001 | <0.001 |
| At least mild disabilitya | 29 (18) | 21 (51) | 53 (31) | 13 (29) | <0.001 | 0.005 |
| At least moderate disabilityb | 18 (11) | 12 (29) | 33 (19) | 10 (22) | 0.02 | 0.02 |
Data presented as n (%) unless noted. P-values represent comparisons using Kruskal-Wallis for continuous and Fisher’s exact or chi-squared tests for categorical variables.
IQR, interquartile range; PICU, pediatric intensive care unit; LOS, length of stay; NPMODS, new or progressive multiple organ dysfunction syndrome
Any increase in Pediatric Overall Performance Category (POPC) from baseline to hospital discharge in the 422 hospital survivors.
Discharge Pediatric Overall Performance Category ≥3 and an increase of ≥1 from baseline in the 312 hospital survivors.
Statistical significance defined as p<0.006 after Bonferroni correction for multiple comparisons.
Statistical comparison across all four groups using Kruskal-Wallis test for continuous variables and Fisher’s exact or chi-squared tests for categorical variables
Statistical comparison between patients with NPMODS absent versus present (irrespective of day 1 MODS presence) using Wilcoxon rank sum for continuous variables and Fisher’s exact or chi-squared tests for categorical variables
Figure 3.
Hospital mortality and total number of patients by maximum number of concurrent organ system dysfunctions.
Morbidity outcome measures (Table 4) were also significantly different across patient categories after correction for multiple comparisons. In particular, median PICU length of stay in patients who never developed MODS was nearly half that experienced by patients who presented with MODS and then developed NPMODS (10 vs. 19 days, p<0.001). Median ventilator-free days was four-times lower between these same two groups (26 vs. 6 days, p<0.001). Patients with NPMODS also suffered higher rates of disability as measured by POPC score at hospital discharge.
DISCUSSION
In this secondary analysis of a large international cohort of pediatric patients in which 8.2% of the 6,925 patients screened were treated for severe sepsis in a PICU, we found that two-thirds of PICU patients developed MODS and one-quarter developed NPMODS within one week of meeting criteria for severe sepsis. The risk of NPMODS did not differ between patients with or without MODS at the time of sepsis recognition, and NPMODS increased the odds of death in patients with both single and multiple organ dysfunction at presentation. Our results suggest that NPMODS could be considered as a distinct sepsis phenotype, separate from single or multiple organ dysfunction, and carries the highest risk of poor outcomes. By considering NPMODS as a distinct phenotype rather than as a marker of increased sepsis severity, investigators could then identify features that predict development of NPMODS and then prospectively test interventions applied prior to rather than after development of NPMODS as a way to impact overall mortality. Our data confirm that NPMODS is an important primary outcome for use in clinical trials and has a strong independent association with death and functional disability. Future efforts validating that a reduction in NPMODS improves more definitive morbidity and mortality endpoints are necessary.
Our findings on the timing of organ dysfunction relative to the time of sepsis recognition confirm what has been described in adult sepsis studies. First, the majority of renal and hepatic dysfunction occurred on days following the day of sepsis recognition parallels adult studies21,22 and suggests that these organs are most likely to exhibit delayed dysfunction following hypoxic-ischemic injury from intial circulatory dysfunction and hypoxemia. Moreover, while cardiovascular dysfunction currently defines pediatric septic shock, the presence of respiratory, neurologic, and gastrointestinal dysfunction were associated with the highest risk of mortality. These findings are consistent with the recent changes to the adult definitions of septic shock as a high-risk condition that includes severe or multiple organ dysfunction with both circulatory and systemic cellular/metabolic dysfunction.23
While we observed that NPMODS was significantly associated with mortality and others have used NPMODS as an endpoint in clinical trials, clear data linking the existing construct of NPMODS to death in pediatric sepsis has been lacking. Moreover, we also found that NPMODS is most likely to occur soon after sepsis onset. Just as Proulx, et al. reported in 1994,10 nearly all of our NPMODS patients developed added organ dysfunction within the first 72 hours following sepsis recognition. These results support prior studies that the majority of NPMODS ocurrs within the first four days following sepsis onset, suggesting that future surveillance and data collection may reduce resource utilization by limiting the duration of monitoring to a period less than the commonly used, though unsubstantiated, window of 28 days. However, one must balance the duration of monitoring, with the possibility that a reduction in early NPMODS could unmask an unintended later incidence of new organ dysfuncton.
In addition to supporting therapeutic efforts to reduce or prevent NPMODS, we found that NPMODS was also associated with morbidity, including functional disability, particularly in those patients without MODS on the day of sepsis recognition. This association with morbidity would allow identification of those patients at increased risk of non-lethal disability and most in need of post-ICU follow-up services to maximize post-illness recovery after discharge.
Given a high overall occurrence and strong correlation with both mortality and morbidity, NPMODS appears to be useful both clinically and scientifically. For clinical trial design, NPMODS provides a more common clnical endpoint than death, yet appears adequate as a surrogate for more definitive mortality and morbidity outcomes. The higher incidence of NPMODS should facilitate more feasible clinical trials with superior statistical power. NPMODS meets many of the criteria proposed by Prentice24 and expanded by Fleming and DeMets25 to be useful as a surrogate marker for mortality. These criteria require that a valid surrogate endpoint yield unambiguous information about potential treatment effects and that treatment effects on the surrogate marker predict the replaced clinical outcome. Additionally, the true impact of reducing NPMODS on sepsis-associated mortality, while seemingly connected, remains unsubstantiated.
The limitations of this report include those inherent to a cross-sectional point-prevalence design rather than a longitudinal observational design. This limits clinical data availability, including labs obtained or therapies performed outside of the 48 hour window around the arbitrarily selected study day. Additionally, because study day one was anchored to the day of sepsis recognition and not to day of ICU admission and since we did not collect the number of days between ICU admission and subsequent sepsis onset, we are unable to determine if length of ICU stay, duration of mechanical ventilation, or other factors prior to sepsis recognition were associated with risk of NPMODS.
Despite these limitations, our findings suggest that NPMODS could be considered a distinct sepsis phenotype rather than a marker of sepsis severity. In this way, NPMODS can be used not only as a predictor of outcome but also as an endpoint to identify prospectively which patients are at specific risk for eventual development of NPMODS or a specific organ dysfunction. In turn, defining specific characteristics of pre-existing or presenting conditions that predict eventual development of NPMODS may allow clinicians to best weigh risks and benefits of therapies targeting specific pathophysiologic processes associated with this particular sepsis phenotype.
A framework for evaluating the effectiveness and utility of defining NPMODS as a specific sepsis phenotype has been proposed by Angus, et al.26 These authors propose that usefulness of a definition will depend on their intended use for (1) clinical care, (2) research, (3) surveillance, and (4) quality improvement and audit and that the domains to be considered in these four areas include reliability, validity, measurement burden, and timeliness with concurrent care. Our findings suggest that NPMODS may add utility to all four areas. They also support future studies to assess the proposed domains of validity, reliability, and measurement burden of NPMODS as an outcome measure for clinical trials and to assess the ease with which both the bedside clinician and the research investigator can identify NPMODS in real time. Additionally, the validity and utility of identifying NPMODS as a distinct sepsis phenotype rather than an indicator of sepsis severity must be explored as well.
CONCLUSION
In summary, development of NPMODS is common (26%) in severe sepsis and carries a significantly higher risk of mortality independent of MODS at time of presentation for critically ill children with severe sepsis. Mortality was not associated with day of MODS onset, but did increase step-wise with increasing maximal number of concurrent organ dysfunctions. NPMODS provides a useful addition to more definitive morbidity and mortality outcomes for clinical trials in pediatric severe sepsis and may prove to be clinically relevant in future sepsis definitions. In order to validate more completely the utility of NPMODS, future studies must now demonstrate that a reduction in NPMODS leads to improvements in traditional morbidity and mortality endpoints.
Supplementary Material
Supplemental Digital Content-Figure 1. Hospital mortality by day of MODS onset within the first seven days following severe sepsis recognition. Day 1 is the day of sepsis recognition.
ACKNOWLEDGEMENTS
We would like to acknowledge the contributions of all the participating SPROUT investigators.
Funded by: Financial support was provided by the Endowed Chair, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine and the Center for Pediatric Clinical Effectiveness at The Children’s Hospital of Philadelphia. Dr. Weiss was also supported by NIGMS K23GM110496. Financial support for data collection in all UK centers was provided by the UK National Institute of Health (NIHR) Clinical Research Network and in Southampton by the Southampton NIHR Wellcome Trust Clinical Research Facility.
Abbreviations:
- NPMODS
new or progressive multiple organ dysfunction syndrome
- POPC
Pediatric Overall Performance Category
- PICU
pediatric intensive care unit
- SPROUT
Sepsis Prevalence, Outcomes, and Therapy
- PIM3
Pediatric Index of Mortality-3
- PELOD
Pediatric Logistic Organ Dysfunction
Footnotes
SPROUT STUDY INVESTIGATORS
North America: Canada: P. Fontela (Montreal Children’s Hospital-McGill), M. Tucci, M. Dumistrascu (Sainte Justine Hospital), P. Skippen, G. Krahn (BC Children’s Hospital); Puerto Rico: E. Bezares (Hospital Cardiovascular de Puerto Rico y el Caribe), G. Puig, A. Puig-Ramos (San Jorge Children’s Hospital), R. Garcia, M. Villar (University Pediatric Hospital); United States: M. Bigham, T. Polanski, S. Latifi, D. Giebner, H. Anthony (Akron Children’s Hospital), J. Hume, A. Galster, L. Linnerud (Amplatz Children’s Hospital), R. Sanders, G. Hefley (Arkansas Children’s Hospital), K. Madden (Boston Children’s Hospital), A. Thompson, S. Shein (Children’s Hospital of Pittsburgh), S. Gertz (Children’s Hospital-Hackensack), Y. Han, T. Williams, A. Hughes-Schalk (Children’s Mercy Hospital), H. Chandler (Children’s Healthcare of Atlanta), A. Orioles, E. Zielinski, A. Doucette (Children’s Hospital in Minnesota), A. Orioles, E. Zielinski, A. Doucette (Children’s Hospital St. Paul), C. Zebuhr, T. Wilson (Children’s Hospital Colorado), C. Dimitriades, J. Ascani, S. Layburn, S. Valley (Children’s Hospital New Orleans), B. Markowitz, J. Terry, R. Morzov (Children’s Hospital of Los Angeles), A. Mcinnes (Children’s Hospital of Monmouth), J. McArthur, K. Woods, K. Murkowski (Children’s Hospital of Wisconsin), M. Spaeder, M. Sharron (Children’s National Medical Center), D. Wheeler, E. Beckman, E. Frank, K. Howard (Cincinnati Children’s Medical Center), C. Carroll (Connecticut Children’s), S. Nett, D. Jarvis (Dartmouth Hitchcock), V. Patel (Dayton Children’s Hospital), R. Higgerson, L. Christie (Dell Children’s Medical Center), K. Typpo, J. Deschenes (Diamond Children’s Hospital), A. Kirby (Doernbecher Children’s Hospital), T. Uhl, K. Rehder, I. Cheifetz, S. Wrenn (Duke Children’s Hospital), K. Kypuros (El Paso Children’s Hospital), K. Ackerman (Golisano Children’s Hospital), F. Maffei, G. Bloomquist (Janet Weis/Geisinger), N. Rizkalla (Johns Hopkins), D. Kimura, S. Shah, C. Tigges (Le Bonheur Children’s Hospital), F. Su, C. Barlow (Lucile Packard Children’s Hospital), K. Michelson, K. Wolfe, D. Goodman, L. Campbell, L. Sorce (Lurie Children’s Hospital of Chicago), K. Bysani, T. Monjure (Medical City Children’s-Dallas), M. Evans (Medical University of South Carolina), B. Totapally, M. Chegondi, C. Rodriguez (Miami Children’s Hospital), J. Frazier, L. Steele (Nationwide Children’s Hospital), S. Viteri, A. Costarino (Nemours/ Alfred I. duPont Children’s Hospital), N. Thomas, D. Spear (Penn State Hershey Medical Center), E. Hirshberg, J. Lilley (Primary Children’s Medical Center), C. Rowan, C. Rider (Riley Hospital for Children), J. Kane (Rush Children’s Hospital), J. Zimmerman, C. Greeley (Seattle Children’s Hospital), J. Lin, R. Jacobs (St. Louis Children’s Hospital), M. Parker, K. Culver (Stony Brook University), L. Loftis, N. Jaimon, M. Goldsworthy (Texas Children’s Hospital), J. Fitzgerald, S. Weiss, V. Nadkarni, J. Bush, M. Diliberto (The Children’s Hospital of Philadelphia), C. Alen, M. Gessouroun (Oklahoma University Medical Center), A. Sapru, T. Lang, M. Alkhouli (University of California San Francisco), S. Kamath, D. Friel, J. Daufeldt (University of Iowa), D. Hsing, C. Carlo, S. Pon (Weill Cornell Medical Center), J. Scimeme, A. Shaheen (Wolfson Children’s Hospital), A. Hassinger, H. Qiao (Women and Children’s Hospital of Buffalo), J. Giuliano, J. Tala (Yale Children’s Hospital). South America: Argentina: D. Vinciguerra, A. Fernandez (Hospital Durand); Colombia: R. Carrero (Clínica Infantil Colsubsidio), P. Hoyos (Hospital de San Jose), J. Jaramillo, A. Posada (Hospital General de Medellín), L. Izquiierdo (Hospital Military Central), B.E. Piñeres Olave, J. Donado (Pablo Tobón Uribe); Chile: R. Dalmazzo, S. Rendich (Clínica Las Condes), L. Palma, M. Lapadula (Clínica Santa María), C. Acuna (Hospital Luis Calvo Mackenna), P. Cruces (Hospital Padre Hurtado) Europe: Belgium: S. Clement De Clety, M. Dujardin, C. Berghe, S. Renard (St. Luc University Hospital); Czech Republic: J. Zurek (Masaryk University); Germany: H. Steinherr (Klinikum Augsburg); Greece: K. Mougkou (Aghia Sophia Children’s Hospital), E. Critselis, K. Mougkou (P. & A. Kyriakou Children’s Hospital); Italy: M. Di Nardo, S. Picardo, F. Tortora (Bambino Gesu Area Rossa); E. Rossetti (Bambino Gesu Children’s Hospital); T. Fragasso, P. Cogo, R. Netto (Bambino Gesu Pediatrico); Lithuania: A. Dagys, V. Gurskis, R. Kevalas (Lithuanian University of Health Sciences); Netherlands: C. Neeleman, J. Lemson, C. Luijten (Radboud University Medical Centre); Poland: K. Wojciech, I. Pagowska-Klimek (Polish Mother Memorial Hospital), M. Szczepanska, J. Karpe (Szyszko Śląskiego University); Portugal: P. Nunes, H. Almeida (Hospital Prof Dr. Fernando Fonseca), J. Rios, M. Vieira (Centrol Hospitalar Lisboa Norte); Spain: J. P. Garcia Iniguez, P. Revilla (Childreńs Hospital Miguel Servet), J. Urbano, J. Lopez-Herce, A. Bustinza (Hospital General Universitario Gregorio Marañón), A. Cuesta, S. Hofheinz (Hospital 12 de Octubre), A. Rodriguez-Nunez (Hospital Clínico Universitario), S. Sanagustin, E. Gonzalez (Hospital de la Sant Creu Sant Pau), M. Riaza, R. Piaya (Hospital Universitario Madrid), P. Soler (Hospital Carlos Haya Materno Infantil), E. Esteban (Hospital Sant Joan de Déu), J. Laraudogoitia, C. Monge (Hospital Universitario Donostia), V. Herrera, J. Granados (Hospital Universitario Salamanca), C. Gonzalez (Hospital Virgen de la Arrixaca); Turkey: T. Koroglu, E. Ozcelik (Dokuz Eylul University); United Kingdom: P. Baines (Alder Hey Children’s Hospital), A. Plunkett (Birmingham Children’s Hospital), P. Davis, S. George (Bristol Royal Hospital for Children), S. Tibby, J. Harris (Evelina Children’s Hospital), R. Agbeko, R. Lampitt (Great North Children’s Hospital–Newcastle), J. Brierley, M. Peters, A. Jones, T. Dominguez, T. Thiruchelvam, (Great Ormond Street), A. Deep, L. Ridley, W. Bowen (King’s College Hospital), R. Levin, I. Macleod (Royal Hospital for Sick Children), M. Gray, N. Hemat (St George’s Hospital), J. Alexander, S. Ali (University Hospital of North Staffordshire NHS Trust), J. Pappachan, J. McCorkell (University Hospital Southampton NHS Foundation Trust), P. Fortune, M. MacDonald, P. Hudnott (Royal Manchester Children’s Hospital). Asia: China: Q. Suyun (Beijing Children’s Hospital); India: S. Singhi, K. Nallasamy (Advanced Pediatrics), R. Lodha (All India Institute); Japan: N. Shime, Y. Tabata (Kyoto Prefectural), O. Saito, T. Ikeyama (Tokyo Metropolitan), T. Kawasaki (Shizuoka Children’s Hospital); Malaysia: L. Lum, A. Abidin, S. Kee (University Malaya Medical Center), S. Tang, R. Jalil (Kebangsaan Malaysia Medical Center); Singapore: Y. Guan, L. Yao (KK Women’s and Children’s Hospital), K. Lin, J. Ong (National University Hospital). Africa: South Africa: A. Salloo, L. Doedens, L. Mathivha (Chris Hani Baragwanath), G. Reubenson, S. Moaisi (Rahima Moosa), A. Pentz, R. Green (Steve Biko Academic Hospital). Australia: A. Schibler, A. Fernandez (Mater Children’s Hospital), S. Erickson (Princess Margaret Hospital), J. McEneiry, D. Long, T. Dorofaeff, M. Coulthard (Royal Children’s Hospital Brisbane), J. Millar, C. Delzoppo (Royal Children’s Melbourne), G. Williams, M. Morritt (Sydney Children’s Hospital), N. Watts, M. Morritt (Children’s Hospital Westmead). New Zealand: J. Beca, C. Sherring, T. Bushell (Starship Children’s Hospital)
REFERENCES
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2013;14:686–93. [DOI] [PubMed] [Google Scholar]
- 2.Jaramillo-Bustamante JC, Marin-Agudelo A, Fernandez-Laverde M, Bareno-Silva J. Epidemiology of sepsis in pediatric intensive care units: first Colombian multicenter study. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2012;13:501–8. [DOI] [PubMed] [Google Scholar]
- 3.Wolfler A, Silvani P, Musicco M, Antonelli M, Salvo I, Italian Pediatric Sepsis Study g. Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian Pediatric Intensive Care Units: a prospective national survey. Intensive care medicine 2008;34:1690–7. [DOI] [PubMed] [Google Scholar]
- 4.Ruth A, McCracken CE, Fortenberry JD, Hall M, Simon HK, Hebbar KB. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2014;15:828–38. [DOI] [PubMed] [Google Scholar]
- 5.Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 2007;369:836–43. [DOI] [PubMed] [Google Scholar]
- 6.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest 1996;109:1033–7. [DOI] [PubMed] [Google Scholar]
- 7.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global Epidemiology of Pediatric Severe Sepsis: the Sepsis PRevalence, OUtcomes, and Therapies Study. American journal of respiratory and critical care medicine 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erratum: Global Epidemiology of Pediatric Severe Sepsis: The Sepsis Prevalence, Outcomes, and Therapies Study. American journal of respiratory and critical care medicine 2016;193:223–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson JD, Pollack MM, Ruttimann UE, Glass NL, Yeh TS. Outcome of pediatric patients with multiple organ system failure. Critical care medicine 1986;14:271–4. [DOI] [PubMed] [Google Scholar]
- 10.Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA. Timing and predictors of death in pediatric patients with multiple organ system failure. Critical care medicine 1994;22:1025–31. [DOI] [PubMed] [Google Scholar]
- 11.Kutko MC, Calarco MP, Flaherty MB, et al. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2003;4:333–7. [DOI] [PubMed] [Google Scholar]
- 12.Leclerc F, Leteurtre S, Duhamel A, et al. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. American journal of respiratory and critical care medicine 2005;171:348–53. [DOI] [PubMed] [Google Scholar]
- 13.Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2013;14:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karam O, Tucci M, Ducruet T, et al. Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2011;12:512–8. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609–19. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2005;6:2–8. [DOI] [PubMed] [Google Scholar]
- 17.Straney L, Clements A, Parslow RC, et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2013;14:673–81. [DOI] [PubMed] [Google Scholar]
- 18.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet 2003;362:192–7. [DOI] [PubMed] [Google Scholar]
- 19.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Critical care medicine 2000;28:2616–20. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–36. [DOI] [PubMed] [Google Scholar]
- 21.Piccinni P, Cruz DN, Gramaticopolo S, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT). Minerva Anestesiol 2011;77:1072–83. [PubMed] [Google Scholar]
- 22.Vincent JL, Nelson DR, Williams MD. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Critical care medicine 2011;39:1050–5. [DOI] [PubMed] [Google Scholar]
- 23.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989;8:431–40. [DOI] [PubMed] [Google Scholar]
- 25.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med 1996;125:605–13. [DOI] [PubMed] [Google Scholar]
- 26.Angus DC, Seymour CW, Coopersmith CM, et al. A Framework for the Development and Interpretation of Different Sepsis Definitions and Clinical Criteria. Critical care medicine 2016;44:e113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content-Figure 1. Hospital mortality by day of MODS onset within the first seven days following severe sepsis recognition. Day 1 is the day of sepsis recognition.