Abstract
Objective:
Excitotoxic injury involving N-methyl-D-aspartate (NMDA) receptor hyperactivity contributes to epilepsy-related memory dysfunction (ERMD). Current treatment strategies for ERMD have limited efficacy and fail to target the underlying pathophysiology. The present pilot study evaluated the efficacy of memantine, an NMDA receptor antagonist, for the treatment of ERMD in adults with focal-onset seizures.
Methods:
Subjects underwent cognitive testing at baseline, after a 13-week randomized, parallel-group, double-blinded phase (of memantine titrated to 10 mg bid or placebo), and following a 13-week open-label extension phase (of memantine titrated to 10 mg bid). The selective reminding test (SRT) continuous long-term retrieval (CLTR) score and 7/24 Spatial Recall Test learning score served as the primary outcome measures. Secondary measures included tests of attention span, fluency, visual construction, and response inhibition, as well as assessments of quality of life, depression, sleepiness, and side effects.
Results:
Seventeen subjects contributed data to the blinded phase (n = 8 memantine, n = 9 placebo). No significant differences were seen between groups on the primary or secondary outcome measures. Pooled data at the end of the open-label phase from 10 subjects (initially randomized to memantine n = 3 or placebo n = 7) demonstrated statistically significant improvement from baseline in CLTR score, memory-related quality of life, spatial span, and response inhibition. No significant changes were evident in depression, sleepiness, side effects, or seizure frequency throughout the trial.
Keywords: Seizures, Memory disorders, Cognition, Receptors, N-methyl-D-aspartate
1. Introduction
Cognitive deficits are common in the setting of epilepsy, affecting patients who are newly diagnosed as well as those with chronic seizures [1,2]. Deficits may affect multiple domains, including attention, executive function, visuospatial skills, language, and memory [1]. While patients with left temporal lobe seizures often have particular deficits in verbal memory [3], memory difficulties are also evident in other forms of focal-onset epilepsy, both pre-and postsurgical resection [4]. These memory deficits may be the most distressing aspect of epilepsy for patients.
Unfortunately, treatment options for epilepsy-related memory dysfunction (ERMD) are limited, and there are no approved medications for this purpose. Memantine, a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, has been proposed as a novel treatment for ERMD. It is believed that excitotoxicity, mediated by glutamate acting on hippocampal NMDA receptors, causes hippocampal sclerosis [5]. This process leads to further seizures and memory dysfunction. Blocking the pathological activation of NMDA receptors has shown promising results with respect to memory function in animal models of epilepsy. In rats, MK-801 injection prior to electrical stimulation-induced seizures reduced seizure frequency and intensity and mitigated impairments in spatial memory [6]. N-[1-(2-thienyl)cyclohexyl] piperidine (TCP), causing NMDA receptor blockade, improved spatial memory test performance in the setting of soman-induced seizures in guinea pigs [7]. When memantine was administered to pentylenetetrazol-kindled rats, spatial learning and memory improved [8]. In contrast, when memantine was administered to nonkindled rats, there was task impairment. These findings suggested that the benefit from memantine in this animal model occurred only in the setting of excitotoxicity.
Memantine is approved for the treatment of moderate to severe Alzheimer’s disease, with studies suggesting significant cognitive improvements [9,10]. In Tariot et al. [9] and Reisberg et al. [10], improvements were noted in the Severe Impairment Battery, which measures performance on a number of low-level tasks that may be impaired by severe dementia, including tests of attention, orientation, language, memory, visuospatial ability, and construction. These studies also noted improvements in completion of activities of daily living, ratings of neuropsychiatric symptoms, clinicians’ ratings of change, and behavior. The time course of benefit is less clear, however, with some studies demonstrating sustained improvement and slowed decline [9] and others showing more transient benefits over the first several weeks of treatment [10].
It is unknown if an NMDA antagonist such as memantine would be of benefit in humans with focal-onset epilepsy and ERMD. One prior randomized, controlled, double-blinded study reported greater improvements in memory performance for memantine-treated subjects with various types of epilepsy compared with placebo, supporting the need for further study [11]. The present randomized, double-blind, parallel-group, placebo-controlled study tested the hypothesis that treatment with memantine would improve memory test performance compared with placebo in subjects with focal-onset epilepsy. We also evaluated the hypothesis that the benefit of memantine would be specific to memory, predicting no improvement in verbal and nonverbal span and fluency, visual construction, and response inhibition. Such findings would lend support to the hypothesis that blockade of NMDA receptor hyperactivity in the hippocampus would lead to improved performance on cognitive tasks that depend specifically on the integrity of that hippocampus, provide evidence for the use of memantine in the treatment of ERMD, and more generally substantiate the hypothesis that NMDA receptor hyperactivity is an appropriate target for intervention.
2. Methods
2.1. Design
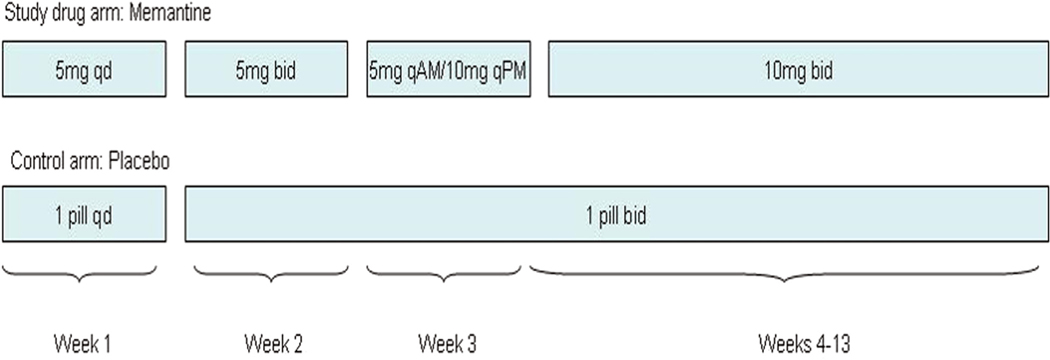
This study was conducted in two phases. In Phase 1, subjects were assigned by stratified block randomization to either a memantine treatment or placebo group, with randomization performed by the research pharmacy using an online tool (http://www.randomization.com). The blocks were stratified by sex and seizure frequency (< 1 focal-onset seizure per month or ≥ 1 focal-onset seizure per month). Investigators, who were conducting enrollment, testing, and data analysis procedures, and subjects were blinded to group assignment, with randomization records held by the pharmacy. The treatment group was placed on memantine, slowly titrated to 10 mg bid over Weeks 1–4. This dosage was maintained during Weeks 4–13 (Fig. 1). The placebo tablets, which were also taken for 13 weeks, were indistinguishable in appearance and number.
Fig. 1.
Titration of medication. Identical memantine titration schedules were employed in both Phase 1 and Phase 2.
Upon completion of Phase 1, subjects entered a 13-week open-label extension period (Phase 2). This was offered midway through the study, based on subject request. Hence, a smaller subset of participants completed Phase 2. All participants in Phase 2 were placed on memantine, with the same titration schedule as in Phase 1, slowly increasing to 10 mg bid over Weeks 14–17. The subjects then remained on memantine at 10 mg bid, until the conclusion of the study at Week 26. The subjects assigned to the memantine group in Phase 1 completed the titration procedure along with the subjects originally assigned to placebo, thus having their dosage reduced and then increased. Subjects were asked to return unused medication at the final study visit to monitor compliance.
2.2. Subjects
Subjects were outpatients recruited from tertiary epilepsy centers in the Boston and Atlanta areas from 2008 to 2013, with final follow-up in 2014. Participants were diagnosed with focal-onset epilepsy, either symptomatic or idiopathic, based on clinical history, imaging studies, and ictal and/or interictal electroencephalography (EEG). All subjects had self-reported memory dysfunction. Additional inclusion criteria were age 18–65 years, normal intelligence quotient (IQ) (≥ 80) as estimated by the Wechsler Test of Adult Reading (WTAR), fluency in English, ability to give consent, capability of living independently and completing activities of daily living, and a stable frequency of seizures.
The anticonvulsant drugs had to remain unchanged during the 26-week trial or the subject would be withdrawn from the study.
Exclusion criteria included nonepileptic seizures, pregnancy, breastfeeding, renal tubular acidosis, urinary tract infections, and severe renal impairment (creatinine clearance ≤ 29 mL/min). Patients with conditions that may affect memory by other mechanisms were also excluded, such as progressive neurologic illness, current alcohol or drug abuse, Alzheimer’s disease, nutritional deficiencies, infections, metabolic/electrolyte disorders, and narcotic, anticholinergic or older generation antihistamines (i.e. diphenhydramine) taken within three days of testing. Seizures must not have occurred within three days of testing. If clinical seizures occurred during testing, data from that session were excluded. If blood pressure was persistently elevated from baseline when checked at Weeks 5, 13, 18, and 26, defined as a systolic or diastolic blood pressure increase ≥10 points with use of the study drug, the subject would be excluded from further participation. Status epilepticus, a generalized tonic–clonic seizure (if there was no prior history of convulsions), or a doubling of seizure frequency during the trial would also cause the subject to be withdrawn.
2.3. Cognitive testing
Subjects completed three testing sessions: prior to treatment (base-line), Week 13 (end of the Phase 1 blinded period), and Week 26 (end of the Phase 2 open-label extension period). Cognitive testing included measures of anterograde verbal memory (Buschke selective reminding test [SRT]) [12,13], anterograde visual learning and memory (7/24 Spatial Recall Test) [14], auditory-verbal working memory and immediate span (Digit Span) [15], visuospatial working memory and span (Spatial Span) [15], visuospatial construction (Block Design) [16,17], verbal fluency [18], design fluency [18], and executive function (Stroop Color– Word Interference) [19]. Subjects also completed questionnaires to assess impact on medication side effects (Adverse Events Profile [AEP]) [20], quality of life (quality of life in epilepsy inventory [QOLIE-89]) [21], sleep quality (Epworth sleepiness scale [ESS]) [22], and mood (neurological disorders depression inventory for epilepsy [NDDI-E]) [23].
The SRT continuous long-term retrieval (CLTR) score and 7/24 Spatial Recall Test learning score were the primary outcome measures. In the SRT, subjects recall a list of 12 words over 6 repeated trials. They are “selectively reminded” of the missed words prior to each immediate recall trial. The CLTR score represents the words recalled on all subsequent trials, reflecting long-term storage (LTS), with possible scores ranging from 0 to 80 (maximum — women: 72; men < 60 years of age: 77; men ≥ 60 years of age: 80, when adjusted for sex and age). In the 7/24 Spatial Recall Test, subjects reproduce spatial arrangements of disks within a grid using short-term memory. The learning score is the sum of all correctly placed disks over five trials, with possible scores ranging from 0 to 35. Alternate forms were used for both tests to minimize practice effects, with the order of test form administration counterbalanced across subjects (ABA vs. BAB). Details regarding each test are provided online in Supplement Table 1.
All study procedures took place at Emory University, Newton–Wellesley Hospital, and Massachusetts General Hospital. The study was approved by their respective institutional review boards, and over-sight was provided by an independent medical monitor. Signed informed consent was obtained from all subjects before participation.
2.4. Statistical analysis
2.4.1. Phase 1
A “change score” was calculated for each neuropsychological measure, subtracting the pretreatment (Session 1, baseline) score from the post-double-blind (Session 2, Week 13) score. To evaluate the hypothesis that treatment with memantine would lead to improved performance on memory tasks, the SRT-CLTR and 7/24 Spatial Recall Test learning change scores were compared between the placebo and memantine treatment groups. The prediction was that the mean change scores would differ between the two groups, with greater positive change scores reflecting improvement in performance in the memantine treatment group.
To assess the hypothesis that improvement was selective for memory, change scores on the digit span, spatial span, block design, verbal fluency, design fluency, and Stroop tasks were compared between the placebo and memantine treatment groups. We predicted no significant differences between the mean change scores in the two groups.
The change scores on the memory subscale from the QOLIE-89 were evaluated to test the hypothesis that treatment with memantine would result in subjective memory improvement. The expectation was that the mean change scores would differ between the placebo and memantine treatment groups, with greater positive change scores reflecting improvement of subjective memory in the memantine treatment group.
2.4.2. Phase 2
The prediction was that subjects taking placebo would demonstrate improvement in the SRT-CLTR and 7/24 Spatial Recall Test learning scores between the first (baseline) and third (post-open-label, Week 26) testing sessions. The expectation was that subjects randomized to memantine would also demonstrate improved memory test scores between pretreatment and post-open-label testing sessions, reflecting sustained benefit. Because of the small sample size, the two subject groups were pooled.
If a subject had missing data for a given cognitive measure, his/her data were excluded only for that specific task. A missing response to a single item on the AEP was replaced by the subject’s mean response for the remaining items on the questionnaire (n = 2).
For each comparison, if the distributions were normal based upon skew, kurtosis, histogram plots, and Shapiro–Wilk tests, we would per-form t-tests. Should the distributions appear non-normal, we would proceed with nonparametric Wilcoxon rank sum and Wilcoxon signed-rank tests for analyses of Phases 1 and 2, respectively. A two-tailed p-value of < 0.05 was considered as the threshold for statistical significance.
3. Results
3.1. Subjects
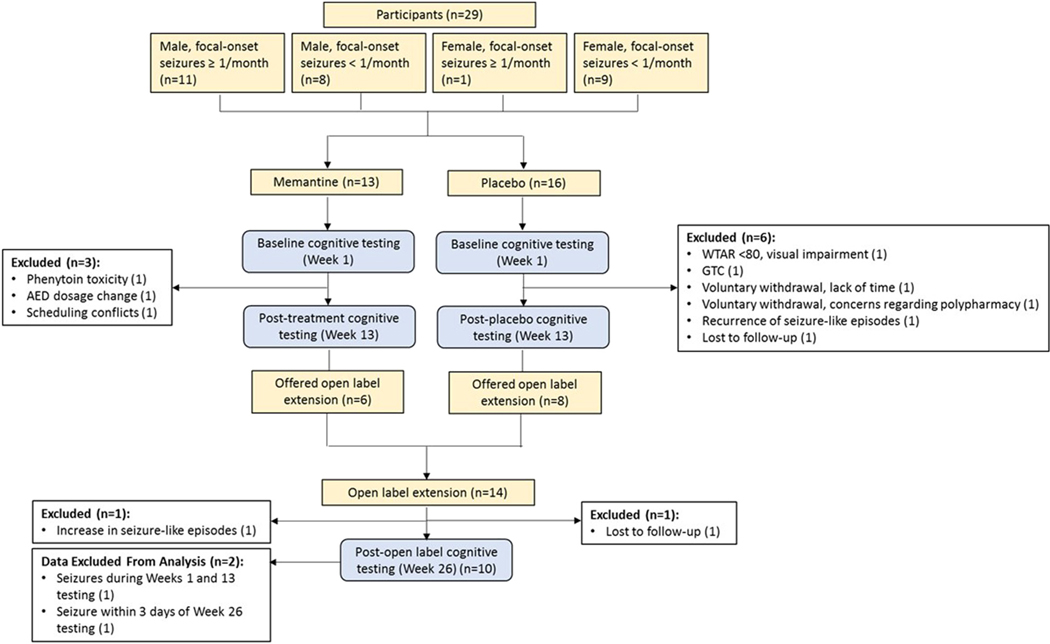
A total of 29 subjects entered the study, with 13 randomized to memantine treatment and 16 randomized to the placebo group (Fig. 2). Twenty subjects completed the blinded Phase 1 (10 subjects in each group); reasons for noncompletion or exclusion are noted in Fig. 2. Fourteen subjects entered the open-label phase. Given the small number of participants, Phase 2 data were pooled. A total of 10 pooled subjects yielded data included in the Phase 2 analysis (n = 3 initially randomized to memantine and n = 7 initially randomized to placebo), with reasons for noncompletion noted in Fig. 2.
Fig. 2.
Study flow diagram. AED = antiepileptic drug, GTC = generalized tonic–clonic seizure, WTAR = Wechsler Test of Adult Reading.
One subject in the memantine treatment group had a focal-onset seizure with impairment of awareness during both baseline and post-double-blind testing sessions, and the data were removed from the analysis.
Demographics of the participants who completed the double-blind trial are reported in Table 1. Wilcoxon rank sum tests were used to assess potential differences in baseline performance between subjects in the memantine and control groups that contributed data to the Phase 1 portion of the trial. While raw scores suggested better performance in the memantine group on the SRT, the 7/24 Spatial Recall Test, spatial span, block design, the Stroop task, and design fluency, as well as better memory-related quality of life (Table 2), these differences were not statistically significant.
Table 1.
Demographic data.
| MEMANTINE | PLACEBO | |
|---|---|---|
| Mean age (range), years | 46.5 (22–62) | 48.2 (31–62) |
| Male/female, n | 6/2 | 6/3 |
| Right/leG handedness, n | 7/1 | 8/1 |
| Race/ethnicity, n | White, 7 Black or African American, 1 |
White, 9 |
| Mean education (range), years | 15.5 (11–19) | 15.8 (12–20.5) |
| Age at seizure onset (range), years | 32.9 (11–61) | 28.9 (3.5–61)* |
| Mean duration of epilepsy (range), years | 13.8 (0.8–41) | 20.0 (0.92–42.7)* |
| Seizure localization, n | L temporal, 5 L hemisphere, localization unknown/not specified, 1 Focal, localization unknown/not specified, 2 |
L temporal, 5 R fronto-central, 1 L hemisphere, localization unknown/not specified, 1 Focal fronto-temporal, lateralization unknown/not specified, 1 Focal, localization unknown/not specified, 1 |
| Anticonvulsant medications, n Carbamazepine Lamotrigine Levetiracetam Oxcarbazepine Phenobarbital Valproic acid |
2 5 3 1 1 1 |
0 6 5 1 0 1 |
| Number of anticonvulsants, n One Two Three |
5 1 2 |
6 2 1 |
L = left, R = right.
n = 1 in the placebo group with age of onset and duration of epilepsy unknown.
Demographic information is shown only for subjects with data included in the Phase 1 analysis.
None of the group differences were significant, based on p N 0.05 for Fisher’s exact (sex, handedness, race, seizure lateralization, and mono-vs. polytherapy) and Wilcoxon rank sum (age at testing, education, age at seizure onset, and duration of epilepsy) tests.
Table 2.
Cognitive test scores at baseline (Session 1) and end of the double-blind phase (Session 2, Week 13).
| MEMANTINE | PLACEBO | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEST | BASELINE MEAN (SD) | END OF DOUBLE-BLIND MEAN (SD) | MEAN CHANGE (SD) | BASELINE MEDIAN (IQR) | END OF DOUBLE-BLIND MEDIAN (IQR) | MEDIAN CHANGE (IQR) | BASELINE MEAN (SD) | END OF DOUBLE-BLIND MEAN (SD) | MEAN CHANGE (SD) | BASELINE MEDIAN (IQR) | END OF DOUBLE-BLIND MEDIAN (IQR) | MEDIAN CHANGE (IQR) |
| 7–24 Spatial Recall Testn | ||||||||||||
| Learning | 31.86 (3.34) | 32.86 (2.55) | 1.00 (2.00) | 34.00 (7.00) | 33.00 (3.00) | −1.00 (3.00) | 27.44 (5.46) | 29.22 (3.56) | 1.78 (4.99) | 28.00 (10.00) | 30.00 (5.00) | 1.00 (8.00) |
| Immediate Recall | 6.29 (1.50) | 6.43 (0.79) | 0.14 (1.77) | 7.00 (1.00) | 7.00 (1.00) | 0.00 (1.00) | 6.00 (1.58) | 6.11 (1.36) | 0.11 (1.45) | 6.00 (1.00) | 7.00 (3.00) | 0.00 (2.00) |
| Delayed Recall | 5.71 (1.60) | 6.29 (1.25) | 0.57 (1.62) | 6.00 (3.00) | 7.00 (2.00) | 0.00 (1.00) | 5.56 (1.74) | 6.22 (0.83) | 0.67 (1.41) | 6.00 (3.00) | 6.00 (2.00) | 0.00 (1.00) |
| Selective Reminding Test | ||||||||||||
| LTSa | 40.63 (9.81) | 47.63 (12.13) | 7.00 (7.86) | 40.50 (19.00) | 52.00 (13.00) | 9.00 (13.00) | 37.11 (17.48) | 44.56 (14.44) | 7.44 (12.11) | 35.00 (28.00) | 47.00 (26.00) | 9.00 (14.00) |
| CLTRb | 32.75 (10.33) | 37.13 (12.90) | 4.38 (7.60) | 35.50 (21.00) | 39.00 (10.00) | 6.00 (13.00) | 24.67 (12.55) | 32.78 (14.47) | 8.11 (9.51) | 21.00 (17.00) | 29.00 (24.00) | 7.00 (16.00) |
| Delayed Recall | 6.25 (3.77) | 6.75 (4.10) | 0.50 (3.25) | 8.00 (7.00) | 8.00 (7.00) | 1.00 (5.00) | 5.67 (3.91) | 6.56 (4.04) | 0.89 (3.98) | 6.00 (7.00) | 6.00 (8.00) | 1.00 (3.00) |
| Digit Span | 18.50 (3.67) | 19.25 (3.99) | 0.75 (3.62) | 18.50 (6.00) | 20.50 (6.00) | 0.50 (6.00) | 19.33 (5.75) | 19.00 (4.27) | −0.33 (4.15) | 19.00 (11.00) | 18.00 (7.00) | −1.00 (8.00) |
| Spatial Spanc | 16.25 (4.20) | 17.13 (3.98) | 0.88 (2.23) | 16.50 (7.00) | 16.50 (6.00) | 2.00 (2.00) | 14.67 (1.50) | 17.11 (2.37) | 2.44 (2.88) | 15.00 (2.00) | 17.00 (4.00) | 3.00 (6.00) |
| Block Designn | 59.57 (4.28) | 61.43 (5.74) | 1.86 (3.93) | 59.00 (6.00) | 60.50 (13.00) | 0.00 (3.00) | 54.22 (7.98) | 57.22 (5.91) | 3.00 (5.20) | 55.00 (13.00) | 56.00 (9.00) | 2.00 (7.00) |
| Stroop Color Word Interference Test | 119.78 (29.33) | 110.82 (27.74) | −8.97 (10.67) | 112.19 (20.61) | 99.17 (23.80) | −8.88 (16.15) | 120.93 (29.34) | 110.36 (26.21) | −10.57 (24.66) | 126.00 (42.80) | 109.00 (45.44) | −6.00 (11.16) |
| DKEFS Verbal Fluency | 96.00 (21.59) | 96.63 (13.75) | 0.63 (12.01) | 100.00 (41.00) | 98.00 (19.00) | −2.00 (15.00) | 96.44 (27.25) | 101.11 (23.68) | 4.67 (11.73) | 86.00 (39.00) | 98.00 (43.00) | 3.00 (15.00) |
| DKEFS Design Fluency | 30.38 (9.67) | 36.50 (6.99) | 6.13 (7.74) | 32.00 (16.00) | 36.50 (13.00) | 8.50 (13.00) | 30.11 (4.91) | 33.78 (3.15) | 3.67 (6.71) | 31.00 (9.00) | 34.00 (4.00) | 1.00 (13.00) |
| QOLIE Memory | 43.43 (14.21) | 52.15 (20.83) | 8.72 (20.07) | 45.14 (25.56) | 64.03 (39.86) | 7.92 (37.51) | 34.60 (23.78) | 45.25 (25.87) | 10.65 (14.34) | 26.67 (38.48) | 34.17 (43.07) | 10.00 (24.72) |
LTS = long-term storage; CLTR = continuous long-term retrieval; DKEFS = Delis–Kaplan Executive Functioning System; QOLIE = quality of life in epilepsy inventory. The table contains raw scores, with the CLTR and LTS scores including standard adjustments for age and sex.
Differences in baseline performance and change scores compared across memantine and placebo groups did not reach statistical significance.
Data were excluded from 3 subjects because of seizures during/within 3 days of testing. Hence, data were based on n = 8 memantine group and n = 9 placebo group.
Due to isolated missing measures (1 subject), n = 7 memantine and n = 9 placebo.
Marginally significant improvement in posttreatment/placebo (Session 2) compared with baseline (Session 1) performance at p = 0.05 in the memantine group.
Significant improvement in posttreatment/placebo (Session 2) compared with baseline (Session 1) performance at p = 0.033 in the placebo group.
Significant improvement in posttreatment/placebo (Session 2) compared with baseline (Session 1) performance at p = 0.042 in the placebo group. Such results are likely due to practice effects and should be interpreted with caution.
3.1.1. Phase 1
As results were of non-normal distributions, Wilcoxon rank sum tests were used to compare the change scores (from baseline to post-double-blind) for each cognitive measure across the memantine and placebo groups. None of the group differences in change score reached statistical significance (all p values > 0.05; Table 2). Wilcoxon signed-rank tests were used to assess differences between baseline and post-double-blind (Session 2, Week 13) performance in each group. In the memantine group, improvement in SRT-LTS was marginally significant at p = 0.05 (Session 1 mean 40.63, standard deviation (sd) 9.81; Session 2 mean 47.63, sd 12.13). Significant improvements were noted for SRT-CLTR (Session 1 mean 24.67, sd 12.55; Session 2 mean 32.78, sd 14.47; p = 0.033) and spatial span (Session 1 mean 14.67, sd 1.50; Session 2 mean 17.11, sd 2.37; p = 0.042) in the placebo group.
3.1.2. Phase 2
As results were of non-normal distributions, Wilcoxon signed-rank tests were used to compare the change in performance on each cognitive measure from baseline (Session 1) to post-open-label (Session 3, Week 26) in the pooled sample (Table 3). Statistically significant improvement was noted in the SRT-CLTR score (Session 1 mean 25.70, sd 11.04; Session 3 mean 40.30, sd 17.28; p = 0.013), a primary outcome measure of memory performance. All other comparisons of objective memory task performance were nonsignificant (p values N 0.05). Subjective memory-related quality of life scores significantly improved (Session 1 mean 36.36, sd 18.36; Session 3 mean 47.89, sd 22.14; p = 0.015).
Table 3.
Cognitive test scores at baseline (Session 1) and the post-open-label phase (Session 3, Week 26).
| POOLED DATA (n=10) | ||||||
|---|---|---|---|---|---|---|
| TEST | BASELINE MEAN (SD) | END OF OPEN LABEL MEAN (SD) | MEAN CHANGE (SD) | BASELINE MEDIAN (IQR) | END OF OPEN LABEL MEDIAN (IQR) | MEDIAN CHANGE (IQR) |
| 7–24 Spatial Recall Test | ||||||
| Learningt | 28.80 (5.12) | 32.20 (3.36) | 3.40 (5.36) | 28.00 (11.00) | 34.00 (7.00) | 5.00 (9.00) |
| Immediate Recall | 6.50 (0.71) | 6.50 (0.85) | 0.00 (1.16) | 7.00 (1.00) | 7.00 (1.00) | 0.00 (2.00) |
| Delayed Recall | 5.40 (1.96) | 6.60 (1.27) | 1.20 (2.44) | 6.00 (3.00) | 7.00 (0.00) | 0.00 (3.00) |
| Selective Reminding Test | ||||||
| LTS | 40.80 (13.03) | 50.10 (12.95) | 9.30 (12.73) | 49.00 (23.00) | 57.00 (23.00) | 7.00 (4.00) |
| CLTRa | 25.70 (11.04) | 40.30 (17.28) | 14.60 (14.59) | 34.00 (20.00) | 49.00 (41.00) | 8.00 (27.00) |
| Delayed Recall | 6.00 (3.59) | 7.10 (3.81) | 1.10 (3.11) | 9.00 (5.00) | 9.00 (6.00) | 1.00 (3.00) |
| Digit Span | 20.30 (4.62) | 20.60 (3.92) | 0.30 (2.95) | 19.00 (8.00) | 21.00 (7.00) | 1.00 (3.00) |
| Spatial Spanb | 13.60 (2.91) | 16.00 (2.83) | 2.40 (2.12) | 14.50 (6.00) | 17.00 (5.00) | 3.00 (2.00) |
| Block Design | 57.60 (6.04) | 58.40 (7.55) | 0.80 (6.36) | 57.50 (8.00) | 60.00 (12.00) | 0.50 (7.00) |
| Stroop Color Word Interference Testc | 125.99 (36.11) | 109.92 (27.67) | −16.07 (21.28) | 113.45 (60.50) | 104.90 (48.25) | −10.15 (13.98) |
| DKEFS Verbal Fluency | 100.50 (27.62) | 104.70 (28.68) | 4.20 (13.62) | 95.50 (44.00) | 107.50 (37.00) | 4.50 (23.00) |
| DKEFS Design Fluency | 29.20 (5.10) | 33.40 (9.01) | 4.20 (10.29) | 29.50 (10.00) | 34.00 (13.00) | 3.50 (11.00) |
| QOLIE Memoryd | 36.36 (18.36) | 47.89 (22.14) | 11.53 (10.54) | 27.78 (33.05) | 43.75 (42.99) | 9.87 (21.32) |
LTS = long-term storage; CLTR = continuous long-term retrieval; DKEFS = Delis–Kaplan Executive Functioning System; QOLIE = quality of life in epilepsy inventory.
Given the small number of subjects completing the open-label phase (n = 10, 3 of whom were originally assigned to memantine and 7 originally assigned to placebo), the data were pooled.
Significant change when post-open-label was compared with baseline:
p= 0.013,
p = 0.014,
p= 0.011,
p = 0.015.
A trend toward improvement was noted, 7/24 Spatial Recall Test learning score p = 0.07; SRT-LTS p = 0.06.
These improvements may be due to practice effects, the expectation of improvement, and/or multiple comparisons and should be interpreted with caution.
Assessment of the secondary cognitive outcome measures demonstrated significant improvement in spatial span (Session 1 mean 13.60, sd 2.91; Session 3 mean 16.00, sd 2.83; p = 0.014) and total Stroop score (Session 1 mean 125.99 s, sd 36.11; Session 3 mean 109.92 s, sd 27.67; p = 0.011, with shorter times of completion reflecting better performance). All other comparisons of secondary cognitive measures were nonsignificant (p values > 0.05).
Change scores from baseline to the final study visit (Week 26) were also compared between subjects originally assigned to the memantine group and those in the placebo group using Wilcoxon rank sum tests post hoc. No significant differences across groups were found for any outcome measure (p values > 0.05).
3.2. Additional post hoc analyses
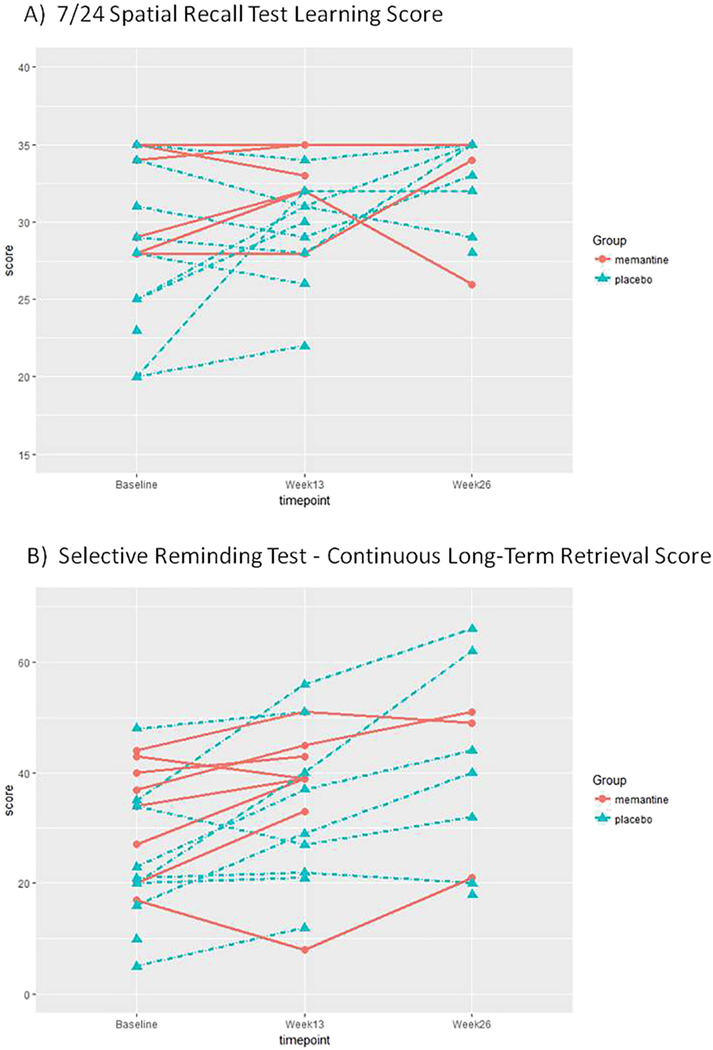
3.2.1. Individual analysis
Hair plots of individual subjects, organized by randomized group assignment, were created for the two primary outcome measures, the SRT-CLTR, and 7/24 Spatial Recall Test learning scores (Fig. 3).
Fig. 3.
Hair plots of individual subject performance. Hair plots of individual subject performance on the primary outcome measures, (A) 7/24 Spatial Recall Test learning score and (B) SRT-CLTR score, do not cluster into clear groups of “responders” or “nonresponders.”
3.2.2. Seizure frequency
A Wilcoxon signed-rank test was used to compare the change in average number of seizures per month from baseline (Session 1) to post-double-blind (Session 2, Week 13) in subjects who completed the two sessions and had available seizure data (n = 7 memantine group, n = 5 placebo group). No significant changes in seizure frequency were evident, with comparisons yielding p values > 0.05 (memantine group: Session 1 mean 0.88/month, sd 1.85 and Session 2 mean 0.55/month, sd 1.12; placebo group: Session 1 mean 9.43/month, sd 19.89 and Session 2 mean 4.7/month, sd 9.96). The apparent difference in baseline seizure frequency was not statistically significant (Wilcoxon rank sum test p > 0.05) and was driven by one subject in the placebo group with very frequent seizures. In a subset of subjects who completed the open-label extension phase and had available seizure data, change in seizure frequency was evaluated from baseline (Session 1) to post-open-label (Session 3, Week 26) using a Wilcoxon signed-rank test (n = 8, 3 of whom were originally in the memantine group and 5 were originally in the placebo group). No significant change was evident (Session 1 mean 6.17/month, sd 15.74 and Session 3 mean 0.56/month, sd 1.59, p > 0.05).
3.2.3. Side effects
No significant difference was evident when AEP change scores from baseline (Session 1) to post-double-blind (Session 2, Week 13) were compared across groups using a Wilcoxon rank sum test (memantine group, n = 8: mean change − 3.64, sd 6.23; placebo group, n = 9: mean change −2.19, sd 4.10; p > 0.05). Similarly, no significant change in AEP score was evident when comparing baseline (Session 1) to the post-open-label extension phase (Session 3, Week 26) using a Wilcoxon signed-rank test in the pooled sample (n = 10; Session 1 mean 37.20, sd 7.74, Session 3 mean 34.70, sd 8.43; p > 0.05).
No significant changes in depression were found when comparing NDDI-E change scores from baseline (Session 1) to the post-double-blind phase (Session 2, Week 13) using a Wilcoxon rank sum test (memantine group, n = 8: mean change −1.75, sd 3.01; placebo group, n = 9: mean change −1.78, sd 2.77; p > 0.05). There were also no significant changes evident in the NDDI-E score when comparing baseline (Session 1) to the post-open-label extension phase (Session 3, Week 26) using a Wilcoxon signed-rank test in the pooled sample (n = 10; Session 1 mean 13.00, sd 5.64, Session 3 mean 12.10, sd 5.86; p > 0.05).
A small subset of subjects also completed the ESS, with no significant changes in sleep quality when comparing change scores from baseline (Session 1) to the post-double-blind phase (Session 2, Week 13) using a Wilcoxon rank sum test (memantine group, n = 3: mean change 2.00, sd 4.36; placebo group, n = 5: mean change 1.00, sd 1.23; p > 0.05). There were also no significant changes evident in the ESS when comparing baseline (Session 1) to the post-open-label extension phase (Session 3, Week 26) using a Wilcoxon signed-rank test in a pooled sample (n = 8, 3 of whom were originally in the memantine group and 5 were originally in the placebo group; Session 1 mean 6.50, sd 3.46, Session 3 mean 6.50, sd 4.18; p > 0.05).
4. Discussion
The present study was a randomized, double-blind, placebo-controlled trial of memantine for the treatment of ERMD, a drug currently Food and Drug Administration (FDA)-approved for the management of Alzheimer’s disease. Use of this drug was intended to target the excitotoxic pathways mediated by glutamate acting on NMDA receptors in the hippocampus that contribute to hippocampal sclerosis, further seizures, and memory dysfunction. Alteration of this excitotoxic path-way would be a novel, and potentially safe, approach to the treatment of memory loss.
Results demonstrated no overall effect of memantine on cognition when assessed at the end of the blinded period (Week 13). In the memantine group, improvement in SRT-LTS was only marginally statistically significant when baseline performance and posttreatment performance were compared. Significant improvements from baseline were noted for SRT-CLTR and spatial span scores but only in the placebo group. Pooled data at the end of the open-label phase (Week 26), however, showed significant improvement over baseline performance in verbal memory (SRT-CLTR), executive function (Stroop Color–Word Interference), spatial attention (spatial span), and memory-related quality of life. These improvements, however, may be due to practice effects, the expectation of improvement, and/or multiple comparisons and should be interpreted with caution. As subjects knew they were receiving active medication during Phase 2, they may have inflated quality-of-life ratings, believing that they were “supposed to” have improvement. While the present study design does not allow direct assessment of practice effects at Week 26, there are data to suggest that at test–retest intervals of 8.7–13.6 months in healthy subjects, there is poor to marginal reliability of the 10-item CLTR, with moderate practice effects of the CLTR and Stroop tasks [24].
In contrast, a recent randomized, controlled, double-blinded study in patients with epilepsy found robust benefits with the use of memantine when compared with placebo, as assessed by a global measure of cognition (Mini-Mental State Examination), memory testing (Wechsler Memory Scale), and quality of life [11]. In Marimuthu et al. [11], memory enhancement was evident in as little as 8 weeks with 5-mg daily dosing, and greater improvements were seen over time at 10 mg daily in their 16-week study.
The reason for a positive response with a lower dose and shorter duration of treatment in the Marimuthu et al. study [11], however, is unclear. Marimuthu et al. [11] included patients with various seizure types of unreported localization and etiology while the present study was restricted to focal-onset epilepsy. Localization in the current study was variable (Table 1), however, as other than exclusion of progressive neurological disease, additional etiological, imaging, or pathological data were not available. It is unknown whether certain subgroups of patients may benefit most. It is possible that subjects in Marimuthu et al. [11] were more impaired at baseline, leaving more room for improvement, although data are not available to allow direct comparison. Conversely, subjects in the current trial had a longer mean duration of epilepsy, which could correspond to greater hippocampal damage and less healthy tissue on which to act. While the cognitive test batteries differed across the studies, the SRT, our primary outcome measure, is particularly sensitive to changes in verbal memory in patients with epilepsy [13,25,26]. Perhaps most importantly, subjects in Marimuthu et al. [11] had a decline in seizure frequency during the trial. Based on its NMDA receptor-blocking properties, one might expect memantine to have an anticonvulsant effect, as demonstrated in the animal literature [27]. Whether anticonvulsant regimens remained stable in the Marimuthu et al. [11] study, however, was not specified. The degree to which seizure frequency improved and the extent to which this may account for their results are unknown. Seizure frequencies and medication regimens in the present study remained unchanged.
Both studies suggest safety and tolerability of memantine in patients with epilepsy. The present data demonstrated no significant changes in seizure frequency, medication side effects, depression, or sleep quality. One subject, originally randomized to memantine, withdrew during the open-label phase because of an increase in seizure-like episodes of uncertain etiology, after having tolerated the drug well during the blinded phase. No other withdrawals were due to side effects of the active treatment. These data are also important for patients with Alzheimer’s disease, for whom memantine is commonly prescribed, as they are at significantly higher risk of developing epilepsy than the general population.
Limitations of the present study include the small sample size that may have limited our ability to detect medication effects. Data do not suggest a robust effect of short-term treatment with memantine, but small changes may have been missed. Compliance was not well-documented, as pill bottles or remaining tablets were rarely returned. Future trials may consider the use of electronically monitored opening of pill bottles. In addition, raw baseline objective performance measures were slightly better in the memantine group on 10 out of 12 tasks, which could lead to ceiling effects or regression to the mean on some measures. The baseline differences across groups, however, did not reach statistical significance. The treatment and placebo groups were not systematically different with respect to the distribution of baseline CLTR scores from normative samples. Scores on the 7/24 Spatial Recall Test, however, suggested poorer performance in the placebo group compared to established norms. The poorer performance in controls may have further contributed to the relative lack of an effect in the treatment group, as the placebo group had a greater change in performance from baseline to Week 13 on the primary outcome measures, likely representing regression to the mean. While such scores would likely leave room for improvement in the treated group should the drug have been effective, lack of baseline impairment in the memantine group is a limitation of the study. Based upon hair plots of individual subjects (Fig. 3), however, it did not appear that participants clustered into clear groups of “responders” or “nonresponders” or that improvements were restricted to subjects with lower baseline scores. Future trials may consider using a specified degree of objective impairment as an inclusion criterion or stratified randomization based on degree of objective impairment. Finally, the study was of limited duration.
4.1. Other approaches to ERMD treatment
Current treatment options for ERMD are limited, and if effective, memantine would provide a much needed alternative. Cognitive rehabilitation remains the primary approach but often yields only partial compensation. Benefits of verbal memory rehabilitation are particularly limited postleft temporal resection, in patients who are likely the most in need of treatment for verbal memory deficits [28]. Overall, cognitive therapy may be useful in selected patients to improve coping mechanisms, but it does not treat the memory loss or address the underlying pathologic process.
Studies of acetylcholinesterase inhibitors in the setting of ERMD have shown inconsistent results and questionable benefits. A pilot study by Fisher et al. [13] suggested promise for the use of donepezil, with improved verbal memory performance after three months of open-label treatment. A subsequent randomized, double-blind, placebo-controlled crossover trial of donepezil, however, showed no effect on memory [29] nor did a trial of galantamine demonstrate an effect on memory in patients with epilepsy [30]. Furthermore, although data are insufficient to establish causality, seizure exacerbation has been raised as a concern regarding the use of this drug class in patients with epilepsy [13,31].
Data regarding stimulant medications for the treatment of ERMD are similarly inconsistent. A small open-label, nonrandomized three-month pilot study of methylphenidate in patients with focal-onset epilepsy demonstrated improvements in multiple cognitive measures, including objective and subjective memory [32]. The effects of methylphenidate on memory were not confirmed, however, in a single-dose, double-blind, randomized, crossover trial [33]. The drug was found to improve attention and processing speed, but effects on memory were not significant. A case report also suggested the benefits of modafinil for the treatment of cognitive deficits due to anticonvulsant use, but no objective neuropsychological data were presented [34]. Seizure exacerbation has been raised as a concern with the use of stimulant medications, but increased seizure frequency has not been conclusively demonstrated [35].
Vinpocetine, a phosphodiesterase (PDE) type 1 inhibitor, maintains activation of proteins implicated in memory formation and synaptic plasticity. Data suggested improvements in Clinical Global Index in patients with various dementias [36], as well as decreased seizure frequency in patients with epilepsy [37,38], with the use of vinpocetine. Vinpocetine was shown to improve attention and memory in patients with cognitive deficits due to epilepsy or dementia, with greater benefit in patients with epilepsy [39]. The latter study was limited, however, by the unblinded design and absence of controls.
4.2. Future directions
Future studies will be required to address several issues. Marimuthu et al. [11] suggested the benefit of longer treatment duration, and it is possible that a longer duration of study is needed to attain a clear benefit in our subjects. Interestingly, the Alzheimer’s disease literature suggests that memantine may slow the progression of dementia, in addition to its more immediate beneficial effects. In a 24-week randomized, double-blind, placebo-controlled study in patients with moderate to severe Alzheimer’s disease, performance curves suggested the maintenance of cognitive function with memantine, compared with the decline of cognition seen with placebo. Treated subjects also exhibited less decline in independence with daily activities and overall clinical status [9]. In patients with epilepsy, it may be that the long-term use of memantine should also be assessed for its ability to prevent the chronic progression of ERMD [40].
Data suggest that if benefit from memantine is attained, it is likely a broad cognitive improvement, rather than a selective effect on memory. Hence, modulating NMDA receptor activity may affect distributed pathological networks, not restricted to the hippocampus. Other mechanisms may also be at play. Both epilepsy and Alzheimer’s disease involve abnormal amyloid deposition [41] and hyperphosphorylated tau pathology [42], and the possible benefit of memantine in these disorders may reflect a mechanism related to this shared pathology. Ultimately, trials of longer duration, with larger numbers of subjects, comprehensive neuropsychological test batteries, and analyses of underlying pathophysiology, will be necessary to determine whether, and how, memantine is effective for the treatment of epilepsy-related cognitive dysfunction.
Supplementary Material
Significance:
Results demonstrated no significant effect of memantine on cognition when assessed at the end of the blinded period. Pooled data at the end of the open-label phase showed significant improvement over baseline performance in measures of verbal memory, frontal-executive function, and memory-related quality of life. These improvements, however, may be due to practice effects and should be interpreted cautiously. Findings suggest a favorable safety profile of memantine in the setting of epilepsy.
AcknowledgThents
The authors wish to thank Barbara Dworetzky, M.D., for serving as an independent medical monitor; David Loring, Ph.D. and Binhuan Wang, Ph.D., for statistical guidance; Ashley Kopec, Ph.D., Samantha Donovan, and Kate Kielek for study coordination; and Forest Laboratories, Inc. for supply of the study drug. We also wish to thank the study participants for their time and effort. This study was supported by a grant from the American Academy of Neurology and American Brain Foundation (BAL). This work was also supported in part by Career Development Award number IK2 CX-001255–01 from the United States (U.S.) Department of Veterans Affairs Clinical Sciences R&D (CSRD) Service (BAL).
Footnotes
The trial was registered on clinicaltrials.gov under the following listing: Does Memantine Improve Verbal Memory Task Performance in Subjects With Partial Epilepsy and Memory Dysfunction? (NCT01054599).
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yebeh.2018.06.047.
Conflict of interest
None of the authors has any conflict of interest to disclose.
References
- [1].Taylor J, Kolamunnage-Dona R, Marson AG, Smith PEM, Aldenkamp AP, Baker GA, et al. Patients with epilepsy: cognitively compromised before the start of antiepileptic drug treatment? Epilepsia 2010;51(1):48–56. [DOI] [PubMed] [Google Scholar]
- [2].Jokeit H, Ebner A. Effects of chronic epilepsy on intellectual functions. Prog Brain Res 2002;135:455–63. [DOI] [PubMed] [Google Scholar]
- [3].Blum D. Decline in verbal memory associated with duration of epilepsy: an intracarotid amobarbital study. Epilepsy Behav 2001;2(5):448–53. [DOI] [PubMed] [Google Scholar]
- [4].Rausch R, Kraemer S, Pietras CJ, Le M, Vickrey BG, Passaro EA. Early and late cognitive changes following temporal lobe surgery for epilepsy. Neurology 2003;60(6):951–9. [DOI] [PubMed] [Google Scholar]
- [5].Meldrum BS. Excitotoxicity and selective neuronal loss in epilepsy. Brain Pathol 1993;3(4):405–12. [DOI] [PubMed] [Google Scholar]
- [6].Kelsey JE, Sanderson KL, Frye CA. Perforant path stimulation in rats produces seizures, loss of hippocampal neurons, and a deficit in spatial mapping which are reduced by prior MK-801. Behav Brain Res 2000;107(1–2):59–69. [DOI] [PubMed] [Google Scholar]
- [7].de Groot DM, Bierman EP, Bruijnzeel PL, Carpentier P, Kulig BM, Lallement G, et al. Beneficial effects of TCP on soman intoxication in guinea pigs: seizures, brain damage and learning behaviour. J Appl Toxicol 2001;21(Suppl. 1):S57–65. [DOI] [PubMed] [Google Scholar]
- [8].Jia L-J, Wang W-P, Li Z-P, Zhen J-L, An L-W, Duan R-S. Memantine attenuates the impairment of spatial learning and memory of pentylenetetrazol-kindled rats. Neurol Sci 2011;32(4):609–13. [DOI] [PubMed] [Google Scholar]
- [9].Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil. JAMA 2004;291(3):317. [DOI] [PubMed] [Google Scholar]
- [10].Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003;348(14):1333–41. [DOI] [PubMed] [Google Scholar]
- [11].Marimuthu P, Varadarajan S, Krishnan M, Shanmugam S, Kunjuraman G, Ravinder JR, et al. Evaluating the efficacy of memantine on improving cognitive functions in epileptic patients receiving antiepileptic drugs: a double-blind placebo-controlled clinical trial (Phase IIIb pilot study). Ann Indian Acad Neurol 2016;19(3):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learn Verbal Behav 1973;12:543–50. [Google Scholar]
- [13].Fisher RS, Bortz JJ, Blum DE, Duncan B, Burke H. A pilot study of donepezil for memory problems in epilepsy. Epilepsy Behav 2001;2(4):330–4. [DOI] [PubMed] [Google Scholar]
- [14].Barbizet J, Cany E. Clinical and psychometrical study of a patient with memory disturbances. Int J Neurol 1968;7(1):44–54. [PubMed] [Google Scholar]
- [15].Wechsler D. A standardized memory scale for clinical use. Aust J Psychol 1945;19: 87–95. [Google Scholar]
- [16].Kohs S. Intelligence measurement. New York, NY: Macmillan; 1823. [Google Scholar]
- [17].Wechsler D. WAIS [manual]. San Antonio, TX: Psychological Corporation; 1955. [Google Scholar]
- [18].Delis D, Kaplan E, Delis-Kaplan Kramer J. Executive Function Scale. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- [19].Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18(6): 643–62. [Google Scholar]
- [20].Baker GA, Middleton A, Jacoby A, et al. Initial development, reliability, and validity of a patient-based adverse event scale. Epilepsia 1994;35(Suppl. 7):80. [Google Scholar]
- [21].Devinsky O, Vickrey BG, Cramer J, Perrine K, Hermann B, Meador K, et al. Development of the quality of life in epilepsy inventory. Epilepsia 1995;36(11):1089–104. [DOI] [PubMed] [Google Scholar]
- [22].Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14(6):540–5. [DOI] [PubMed] [Google Scholar]
- [23].Gilliam FG, Barry JJ, Hermann BP, Meador KJ, Vahle V, Kanner AM. Rapid detection of major depression in epilepsy: a multicentre study. Lancet Neurol 2006;5(5): 399–405. [DOI] [PubMed] [Google Scholar]
- [24].Dikmen SS, Heaton RK, Grant I, Temkin NR. Test–retest reliability and practice effects of expanded Halstead–Reitan Neuropsychological Test Battery. J Int Neuropsychol Soc 1999;5(4):346–56. [PubMed] [Google Scholar]
- [25].Sabsevitz DS, Swanson SJ, Morris GL, Mueller WM, Seidenberg M. Memory outcome after left anterior temporal lobectomy in patients with expected and reversed Wada memory asymmetry scores. Epilepsia 2001;42(11):1408–15. [DOI] [PubMed] [Google Scholar]
- [26].Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia 2008;49(8):1377–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McLean MJ, Gupta RC, Dettbarn WD, Wamil AW. Prophylactic and therapeutic efficacy of memantine against seizures produced by soman in the rat. Toxicol Appl Pharmacol 1992;112(1):95–103. [DOI] [PubMed] [Google Scholar]
- [28].Helmstaedter C, Loer B, Wohlfahrt R, Hammen A, Saar J, Steinhoff BJ, et al. The effects of cognitive rehabilitation on memory outcome after temporal lobe epilepsy surgery. Epilepsy Behav 2008;12(3):402–9. [DOI] [PubMed] [Google Scholar]
- [29].Hamberger MJ, Palmese CA, Scarmeas N, Weintraub D, Choi H, Hirsch LJ. A randomized, double-blind, placebo-controlled trial of donepezil to improve memory in epilepsy. Epilepsia 2007;48(7):1283–91. [DOI] [PubMed] [Google Scholar]
- [30].Griffith HR, Martin R, Andrews S, LeBron Paige A, Ware J, Faught E, et al. The safety and tolerability of galantamine in patients with epilepsy and memory difficulties. Epilepsy Behav 2008;13(2):376–80. [DOI] [PubMed] [Google Scholar]
- [31].Mishra A, Goel RK. Adjuvant anticholinesterase therapy for the management of epilepsy-induced memory deficit: a critical pre-clinical study. Basic Clin Pharmacol Toxicol 2014;115(6):512–7. [DOI] [PubMed] [Google Scholar]
- [32].Moore JL, McAuley JW, Long L, Bornstein R. An evaluation of the effects of methylphenidate on outcomes in adult epilepsy patients. Epilepsy Behav 2002;3(1):92–5. [DOI] [PubMed] [Google Scholar]
- [33].Adams J, Alipio-Jocson V, Inoyama K, Bartlett V, Sandhu S, Oso J, et al. Methylpheni-date, cognition, and epilepsy: a double-blind, placebo-controlled, single-dose study. Neurology 2017;88(5):470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith BW. Modafinil for treatment of cognitive side effects of antiepileptic drugs in a patient with seizures and stroke. Epilepsy Behav 2003;4(3):352–3. [DOI] [PubMed] [Google Scholar]
- [35].Baptista-Neto L, Dodds A, Rao S, Whitney J, Torres A, Gonzalez-Heydrich J. An expert opinion on methylphenidate treatment for attention deficit hyperactivity disorder in pediatric patients with epilepsy. Expert Opin Investig Drugs 2008;17(1):77–84. [DOI] [PubMed] [Google Scholar]
- [36].Hindmarch I, Fuchs HH, Erzigkeit H. Efficacy and tolerance of vinpocetine in ambulant patients suffering from mild to moderate organic psychosyndromes. Int Clin Psychopharmacol 1991;6(1):31–43. [DOI] [PubMed] [Google Scholar]
- [37].Dutov AA, Tolpyshev BA, Petrov AP, Gladun VN. Use of cavinton in epilepsy. Zh Nevropatol Psikhiatr Im S S Korsakova 1986;86(6):850–5. [PubMed] [Google Scholar]
- [38].Dutov AA, Gal’tvanitsa GA, Volkova VA, Sukhanova ON, Lavrishcheva TG, Petrov AP. Cavinton in the prevention of the convulsive syndrome in children after birth injury. Zh Nevropatol Psikhiatr Im S S Korsakova 1991;91(8):21–2. [PubMed] [Google Scholar]
- [39].Ogunrin A. Effect of vinpocetine (Cognitol™) on cognitive performances of a Nigerian population. Ann Med Health Sci Res 2014;4(4):654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol 2003;54(4): 425–32. [DOI] [PubMed] [Google Scholar]
- [41].Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol 1994;87(5):504–10. [DOI] [PubMed] [Google Scholar]
- [42].Tai XY, Koepp M, Duncan JS, Fox N, Thompson P, Baxendale S, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain 2016;139(Pt 9):2441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.