Abstract
Context
Pretreatment with α-adrenergic receptor blockers is recommended to prevent hemodynamic instability during resection of a pheochromocytoma or sympathetic paraganglioma (PPGL).
Objective
To determine which type of α-adrenergic receptor blocker provides the best efficacy.
Design
Randomized controlled open-label trial (PRESCRIPT; ClinicalTrials.gov NCT01379898)
Setting
Multicenter study including 9 centers in The Netherlands.
Patients
134 patients with nonmetastatic PPGL.
Intervention
Phenoxybenzamine or doxazosin starting 2 to 3 weeks before surgery using a blood pressure targeted titration schedule. Intraoperative hemodynamic management was standardized.
Main Outcome Measures
Primary efficacy endpoint was the cumulative intraoperative time outside the blood pressure target range (ie, SBP >160 mmHg or MAP <60 mmHg) expressed as a percentage of total surgical procedure time. Secondary efficacy endpoint was the value on a hemodynamic instability score.
Results
Median cumulative time outside blood pressure targets was 11.1% (interquartile range [IQR]: 4.3–20.6] in the phenoxybenzamine group compared to 12.2% (5.3–20.2)] in the doxazosin group (P = .75, r = 0.03). The hemodynamic instability score was 38.0 (28.8–58.0) and 50.0 (35.3–63.8) in the phenoxybenzamine and doxazosin group, respectively (P = .02, r = 0.20). The 30-day cardiovascular complication rate was 8.8% and 6.9% in the phenoxybenzamine and doxazosin group, respectively (P = .68). There was no mortality after 30 days.
Conclusions
The duration of blood pressure outside the target range during resection of a PPGL was not different after preoperative treatment with either phenoxybenzamine or doxazosin. Phenoxybenzamine was more effective in preventing intraoperative hemodynamic instability, but it could not be established whether this was associated with a better clinical outcome.
Keywords: pheochromocytoma, sympathetic paraganglioma, -adrenergic receptor blocker, hemodynamic instability
Pheochromocytoma and sympathetic paraganglioma (PPGL) are neuro-endocrine tumors originating from chromaffin cells in the adrenal medulla and extra-adrenal sympathetic paraganglia, respectively (1). Overproduction of catecholamines is a key feature of PPGL and responsible for an increased cardiovascular risk (2–4). Curative surgical resection is the treatment of choice except in cases of metastatic disease (5).
Resection of a PPGL is associated with a high risk of hemodynamic instability and subsequent cardiovascular complications due to uncontrolled release of catecholamines in response to various anesthesiologic and surgical stimuli (6–8). To minimize intraoperative hemodynamic instability, pretreatment with an α-adrenergic receptor blocker is recommended to antagonize the α-receptor mediated vasoconstrictive effects of catecholamines (5,9). Two frequently prescribed drugs for this purpose are phenoxybenzamine, a nonselective and noncompetitive α 1- and α 2-adrenergic receptor blocker, and doxazosin, a selective and competitive α 1-adrenergic receptor blocker. Studies evaluating pretreatment with either phenoxybenzamine or doxazosin have shown conflicting results with respect to intraoperative blood pressure control. Whereas some studies suggested phenoxybenzamine to be superior to doxazosin, other investigators found the opposite or did not find any difference (10–14). Without exception, however, these studies were nonrandomized and retrospective in design and predominantly small-sized. Apart from blood pressure levels, hemodynamic instability is also reflected by the amount of vasoactive medication and intravenous fluids required to correct an abnormal blood pressure (15–17).
The present randomized multicenter study was initiated to compare the efficacy of pretreatment with either phenoxybenzamine or doxazosin on the intraoperative hemodynamic stability during PPGL resection.
Materials and Methods
Pheochromocytoma Randomized Study Comparing Adrenoreceptor Inhibiting Agents for Preoperative Treatment (PRESCRIPT) trial was an investigator-initiated multicenter, randomized controlled, open-label trial conducted between January 2012 and December 2017 at 9 sites in The Netherlands. The trial protocol was approved by the institutional review board of the University Medical Center Groningen, University of Groningen, The Netherlands, in compliance with the Dutch Medical Research Involving Human Subjects Act and the Declaration of Helsinki. All patients provided written informed consent. The PRESCRIPT trial has been registered under ClinicalTrials.gov number NCT01379898. The Consolidated Standards of Reporting Trials statement was followed for presentation of the current study (18).
Participants
Adult patients aged 18 years or older with a recently diagnosed PPGL and an indication for surgical resection were considered eligible. Inclusion criteria were a diagnosis of nonmetastatic PPGL with elevated plasma or urinary (nor)metanephrine concentrations, a minimum tumor diameter of 1 cm on computed tomography or magnetic resonance imaging, and visualization on functional imaging (eg, I123-MIBG scintigraphy or [18F]DOPA-PET). Exclusion criteria were metastatic PPGL, severe hemodynamic instability necessitating presurgical admission to the intensive care unit, or pregnancy.
Randomization and procedures
Patients were randomized to pretreatment with either phenoxybenzamine or doxazosin extended-release in a 1:1 ratio using randomly permuted blocks with alternating block sizes of 2 and 4 stratified by center with interactive Web-based randomization software. Before the start of pretreatment, blood samples were drawn after 30 min of supine rest and stored at –80°C until determination of plasma free (nor)metanephrine and catecholamines concentrations using high-pressure liquid chromatography tandem mass spectrometry with online solid-phase extraction in a central reference laboratory (19). Treatment was started 2 to 3 weeks before surgery using blood pressure guided dose titration with a maximum dosage of 70 mg phenoxybenzamine twice daily or 24 mg doxazosin twice daily (Fig. 1), in accordance with the maximum dosages previously reported for this indication (10). It was at the discretion of the treating physician whether the drug treatment would take place in the outpatient or inpatient clinic. During the whole pretreatment period, blood pressure and heart rate were measured twice daily with a certified automated electronic blood pressure monitor just before ingestion of the study drugs. Each measurement consisted of a single recording after 5 min of supine rest and subsequently after 3 min in upright posture. Blood pressure and heart rate measurements were either performed at home by the patients themselves after careful instructions or at the hospital by medical personnel. Target values were a blood pressure <130/80 mmHg in the supine position and a systolic blood pressure between 90 and 110 mmHg in the upright position (20). Nifedipine extended-release 30 to 90 mg once daily was added when these targets were not reached despite a maximum dosage of either study drug. Heart rate target values were <80 bpm and <100 bpm in the supine and upright position, respectively. Metoprolol extended-release 50 to 200 mg once daily was added in case these targets were not achieved. In addition, patients were advised to consume a diet containing at least 15 g of sodium chloride per day (5). During the last 24 hours before surgery, 2 liters of 0.9% saline was administered intravenously. Resection of the PPGL was postponed if the supine blood pressure was >160/100 mmHg on the day before surgery. In each participating center, patients were treated by a dedicated team of endocrinologists, surgeons, and anesthesiologists.
Figure 1.
Flow-chart of the trial procedure.
Abbreviations: BP, blood pressure; HR, heart rate; ER, extended-release; i.v., intravenous.
Blood pressure and heart rate during surgery were monitored by continuous intra-arterial measurement. Hemodynamic management was performed using a standardized operating procedure describing in detail the anesthesiologic procedures including the indications for pharmacological interventions and the preferred vasoactive medication. All supplementary material and figures are located in a digital research materials repository (21). Intraoperative hemodynamic targets were systolic blood pressure <160 mmHg, mean arterial pressure >60 mmHg, and heart rate <100 bpm. Administration of vasoactive medication was only allowed when hemodynamic variables were outside these targets. After surgery, patients were monitored at the postanesthesia or intensive care unit. Postoperative pharmacological interventions to correct hemodynamic deviations were applied according to the standard operating procedure. We extracted all data on blood pressure, heart rate, intravenous volume therapy, and vasoactive medication from the electronic patient data monitoring system starting at induction of anesthesia and ending at discharge from the postanesthesia care unit or intensive care unit. Both duration and amplitude of hemodynamic variables outside the target range were assessed and cumulative dosages of vasoactive medication were calculated.
Outcome measures
The primary endpoint of our study was the cumulative intraoperative time outside the blood pressure target range, expressed as a percentage of the time interval between induction of anesthesia (ie, first administration of propofol) and suturing of the incision. As a secondary efficacy endpoint, we used the Hemodynamic Instability score (HI-score), a validated semiquantitative score reflecting the degree of hemodynamic instability (17). In short, the HI-score consists of 3 intraoperative components: hemodynamic variables (ie, blood pressure and heart rate), cumulative dosage of vasoactive medication, and fluid therapy. For each of these 3 components, incremental points are attributed according to the magnitude of deviation from predefined thresholds as well as infusion rates of vasoactive drugs and fluids. Thus, a higher HI-score represents a higher degree of overall hemodynamic instability. For the present study, we modified the original HI-score by including the dosages of vasodilating drugs and β-adrenergic receptor blockers (21).
Other secondary efficacy endpoints were (i) the frequency, duration, and magnitude of a systolic blood pressure >160 mmHg; mean arterial pressure <60 mmHg; and heart rate >100 bpm; (ii) number and cumulative dosages of intraoperatively administered vasoactive drugs; and (iii) duration of postoperative administration of vasopressive drugs. Safety endpoints were cardiovascular complications and mortality from the first administration of study medication until 30 days after surgery. In addition, the frequency of postoperative glucose levels ≤3.5 mmol/L and length of hospital stay were assessed. Preoperative adverse events were assessed and graded according to the Common Terminology Criteria for Adverse Events (22).
Statistical analysis
The sample size was calculated at a total of 134 subjects to demonstrate a relative reduction of 20% in intraoperative time outside the predefined blood pressure targets, assuming a frequency of 8 ± 4%, between patients pretreated with phenoxybenzamine or doxazosin with a power of at least 80% and a 2-sided alpha of .05. Patients who never received the allocated treatment were excluded from all analyses. We performed all efficacy and exploratory analyses in a modified intention-to-treat population, meaning that we excluded subjects in whom pathological examination of the resected tumor was inconsistent with a PPGL since these patients were not at risk for catecholamine-induced hemodynamic instability. The safety analysis was performed in all patients who received the allocated treatment, including the cases in which another pathological diagnosis than PPGL was established (21). Continuous variables are presented as mean ± SD or median (IQR) where appropriate. Categorical variables are presented as absolute number or percentages. Continuous variables were compared using a t test or Mann–Whitney U test. Nonparametrical effect sizes were calculated using Rosenthal’s formula (23). Categorical data were analyzed using Chi-square or Fisher’s exact test. Two-sided P-values <.05 were considered significant. All statistical analyses were carried out with SPSS version 23 (IBM Corporation, Armonk, NY, US).
Exploratory analyses
Exploratory analyses were carried out to assess the relationship between efficacy endpoints and cardiovascular complications. In addition, determinants of hemodynamic instability were explored for identification of potential risk factors. The relationship between achievement of preoperative blood pressure targets and intraoperative hemodynamic instability was assessed in a multivariable regression model. Further details are provided in the supplemental material (21).
Results
Participants
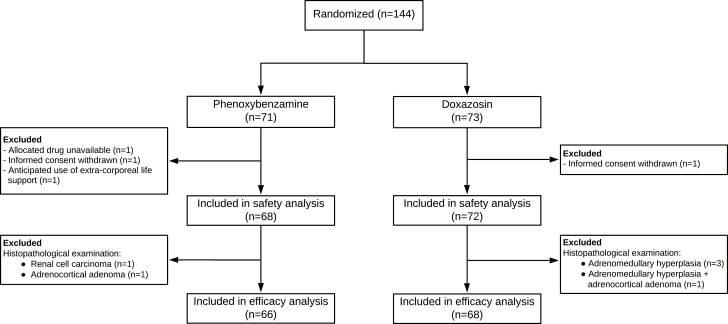
A total of 144 patients were enrolled in the trial. Four patients were excluded from all analyses because the allocated treatment was never initiated, leaving 140 patients who completed the study. Notably, in 6 patients the final pathology report did not reveal a PPGL (21). Thus, a total of 134 patients met the criteria for the modified intention-to-treat population (phenoxybenzamine group: n = 66, doxazosin group: n = 68). The safety analysis was performed using the data of all 140 patients who completed the study (21).
Baseline characteristics and preoperative blood pressure values are presented in Table 1. There were no differences between the 2 groups with respect to demographic characteristics, cardiovascular risk factors, American Society of Anesthesiologists physical score, plasma free (nor)metanephrine, or catecholamine secretion patterns. The median duration of pretreatment was 14 days in both groups, and patients received a median dosage of 120 (78–140) mg phenoxybenzamine or 40 (32–48) mg doxazosin on the day before surgery. A calcium channel blocker was administered to 42.4% of the patients in the phenoxybenzamine group compared to 39.7% in the doxazosin group (P = .86). A higher proportion of patients in the phenoxybenzamine group received metoprolol (89.4% vs. 66.2%, P < .01), which was also prescribed at higher dosages.
Table 1.
Patient characteristics
| Characteristic | Phenoxybenzamine (n = 66) | Doxazosin(n = 68) | P-value |
|---|---|---|---|
| Female, n (%) | 34 (51.5) | 36 (52.9) | >.99 |
| Age (years), mean ± SD | 54 ± 15 | 54 ± 15 | .87 |
| BMI (kg/m2), median (IQR) | 25.6 (23.6–29.0) | 25.1 (22.4–29.1) | .49 |
| Smoking | .72 | ||
| Never, n (%) | 28 (42.4) | 32 (47.1) | |
| Previous, n (%) | 18 (27.3) | 19 (27.9) | |
| Current, n (%) | 20 (30.3) | 17 (25.0) | |
| Prior cardiovascular event,an (%) | 17 (25.8) | 11 (16.7) | .29 |
| ASA class | .39 | ||
| I, n (%) | 11 (16.7) | 10 (14.7) | |
| II, n (%) | 34 (51.5) | 43 (63.2) | |
| III, n (%) | 20 (30.3) | 15 (22.1) | |
| IV, n (%) | 1 (1.5) | 0 (0.00) | |
| Germline mutation, n (%) | .75 | ||
| Yes, n (%) | 17 (25.8) | 17 (25.0) | |
| No, n (%) | 37 (56.1) | 37 (54.4) | |
| Not assessed, n (%) | 12 (18.2) | 14 (20.6) | |
| Tumor localization, n (%) | .27 | ||
| Unilateral pheochromocytoma, n (%) | 59 (89.4) | 65 (95.6) | |
| Bilateral pheochromocytoma, n (%) | 5 (7.6) | 1 (1.5) | |
| Sympathetic paraganglioma, n (%) | 2 (3.0) | 2 (2.9) | |
| Maximum tumor diameter (mm), median (IQR) | 38 (28–51) | 42 (29–61) | .62 |
| Biochemical profile | |||
| Plasma-free metanephrine (nmol/L), median (IQR) | 1.37 (0.29–5.64) | 1.04 (0.21–3.39) | .09 |
| Plasma-free normetanephrine (nmol/L), median (IQR) | 4.33 (1.63–10.11) | 3.41 (1.52–8.44) | .69 |
| Plasma epinephrine (nmol/L), median (IQR) | 0.48 (0.23–2.13) | 0.40 (0.19–1.41) | .26 |
| Plasma norepinephrine (nmol/L), median (IQR) | 4.47 (2.91–11.91) | 4.87 (3.03–17.69) | .29 |
| Duration of pretreatment (days), median (IQR) | 14 (13–20) | 14 (13–19) | .86 |
| Medication on day before surgery | |||
| Daily dosage study drug (mg), median (IQR) | 120 (78–140) | 40 (32–48) | — |
| Patients receiving any CCB, median (IQR) | 28 (42.4) | 27 (39.7%) | .86 |
| Daily dosage nifedipine (mg),b median (IQR) | 60 (30–90) | 60 (30–90) | .76 |
| Patients receiving any β-blocker, median (IQR) | 59 (89.4) | 45 (66.2) | <.01 |
| Daily dosage metoprolol (mg),c median (IQR) | 100 (50–150) | 50 (50–100) | <.01 |
| Hemodynamic variables at randomization | |||
| Supine | |||
| SBP (mmHg), median (IQR) | 144 (124–156) | 138 (122–152) | .44 |
| DBP (mmHg), median (IQR) | 82 (73–88) | 80 (72–87) | .38 |
| HR (bpm), median (IQR) | 76 (66–85) | 71 (63–78) | .02 |
| Upright | |||
| SBP (mmHg), median (IQR) | 136 (122–151) | 138 (124–151) | .94 |
| DBP (mmHg), median (IQR) | 84 (78–94) | 85 (77–93) | .94 |
| HR (bpm), median (IQR) | 87 (73–98) | 82 (76–94) | .03 |
| Hemodynamic variables day before surgery, median (IQR) | |||
| Supine | |||
| SBP (mmHg), median (IQR) | 132 (116–143) | 124 (115–138) | .07 |
| DBP (mmHg), median (IQR) | 74 (67–84) | 69 (63–80) | .02 |
| HR (bpm), median (IQR) | 73 (64–83) | 71 (65–80) | .62 |
| Upright | |||
| SBP (mmHg), median (IQR) | 120 (107–133) | 120 (104–130) | .55 |
| DBP (mmHg), median (IQR) | 71 (65–81) | 71 (64–82) | .86 |
| HR (bpm), median (IQR) | 90 (83–106) | 86 (74–98) | .03 |
| Preoperative targets achieved, n (%) | <.01 | ||
| Supine BP <130/80 + upright SBP 90–110, n (%) | 16 (24.6) | 13 (19.7) | |
| Supine BP <130/80, n (%) | 13 (20.0) | 28 (42.4) | |
| Upright SBP 90–110, n (%) | 1 (1.5) | 5 (7.6) | |
| None, n (%) | 35 (53.8) | 20 (30.3) | |
| Surgical approach | .69 | ||
| Laparoscopy, n (%) | 48 (72.7) | 44 (64.7) | |
| Laparotomy, n (%) | 9 (13.6) | 12 (17.6) | |
| Posterior retroperitoneoscopic, n (%) | 9 (13.6) | 12 (17.6) | |
| Type of anesthesia | .86 | ||
| Total intravenous, n (%) | 40 (60.6) | 43 (63.2) | |
| Balanced inhalation, n (%) | 26 (39.4) | 25 (36.8) | |
| Epidural anesthesia, n (%) | 7 (10.6) | 9 (13.6) | .79 |
| Anesthesia duration (min),d median (IQR) | 140 (112–164) | 145 (110–164) | .91 |
| Surgical duration (min),e median (IQR) | 95 (71–127) | 99 (72–120) | .76 |
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; BP, blood pressure; CCB, calcium channel blocker; IQR, interquartile range; SBP, systolic blood pressure; DBP, diastolic blood pressure.
aHistory of coronary artery disease, heart failure, stroke, peripheral artery disease, or aortic aneurysm.
bNifedipine was prescribed in 87% of patients receiving any CCB. Median (IQR) shown of only these cases. In the remaining cases, amlodipine, barnidipine, or verapamil was prescribed.
cMetoprolol was prescribed in 88% of patients receiving any β-blocker. Median (IQR) shown of only these cases. In the remaining cases, propranolol, atenolol, or bisoprolol was prescribed.
dTime from induction of anesthesia until suturing of the incision.
eTime from incision until suturing of the incision.
Efficacy outcomes
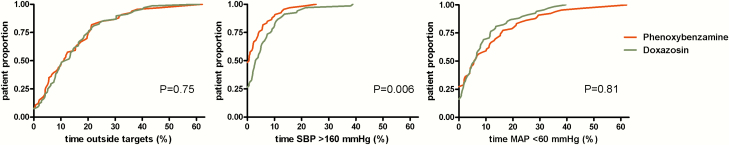
The primary endpoint (ie, the median cumulative time outside the blood pressure target range during surgery) was 11.1% (4.3–20.6) in the phenoxybenzamine group compared to 12.2% (5.3–20.2) in the doxazosin group (P = .75, r = 0.03; Fig. 2). The median total HI-score was lower in the phenoxybenzamine group compared to the doxazosin group (38.0 [28.8–58.0] vs. 50.0 [35.3–63.8], P = .02, r = 0.20). Peak systolic blood pressure, cumulative time and frequency of systolic blood pressure >160 mmHg, and the amount of vasodilating drugs were all lower in the phenoxybenzamine group (Table 2). Frequency and duration of a mean arterial pressure <60 mmHg or heart rate >100 bpm were not different between groups (Table 2). There were no differences between phenoxybenzamine and doxazosin with respect to the occurrence of postoperative hypotension defined as a mean arterial blood pressure < 60 mmHg or the use of vasoconstrictive/inotropic drugs (40.0% vs. 38.8%, P > .99), the proportion of patients requiring vasopressors (33.3% and 32.4%, P > .99), or the duration of vasopressor treatment (402 [161–1185] vs. 490 [163–1167] min, P = .98).
Figure 2.
Cumulative distribution of the percentage of total intraoperative time with blood pressure outside the target values (ie, systolic blood pressure >160 mmHg and MAP <60 mmHg). The x axis represents the cumulative time outside of the respective blood pressure targets. The y axis represents the cumulative proportion of patients.
Table 2.
Secondary efficacy endpoints
| Phenoxybenzamine (n = 66) | Doxazosin (n = 68) | P-value | |
|---|---|---|---|
| Systolic blood pressure >160 mmHg | |||
| Frequency, n (%) | 34 (51.5) | 49 (72.1) | .02 |
| Duration (%), mean (IQR) | 0.6 (0.0–4.6) | 3.1 (0.0–8.9) | <.01 |
| Maximum SBP (mmHg), mean (IQR) | 163 (146–188) | 181 (159–203) | <.01 |
| Vasodilating drugs | .02 | ||
| 0, n (%) | 29 (43.9) | 14 (20.6) | |
| 1, n (%) | 21 (31.8) | 23 (33.8) | |
| 2, n (%) | 10 (15.2) | 22 (32.4) | |
| 3, n (%) | 6 (9.1) | 8 (11.7) | |
| 4, n (%) | 0 (0) | 1 (1.5) | |
| Cumulative dosage MgSO4 (g), mean (IQR) | 0 (0–3) | 3 (0–4) | <.01 |
| Cumulative dosage phentolamine (mg), mean (IQR) | 0 (0–0.5) | 0 (0–4) | .16 |
| Mean arterial pressure <60 mmHg | |||
| Frequency, n (%) | 48 (72.7) | 56 (82.4) | .22 |
| Duration (%), mean (IQR) | 5.8 (0.0–16.0) | 6 (1–12) | .82 |
| Minimum MAP (mmHg), mean (IQR) | 53 (44–60) | 51 (46–57) | .36 |
| Vasoconstrictive/inotropic drugs | .46 | ||
| 0, n (%) | 17 (25.8) | 13 (19.1) | |
| 1, n (%) | 24 (36.4) | 27 (39.7) | |
| 2, n (%) | 23 (34.8) | 22 (32.4) | |
| 3, n (%) | 2 (3.0) | 6 (8.8) | |
| Infusion rate of fluids (mL/h), mean (IQR) | 632 (424–945) | 636 (484–896) | .81 |
| Cumulative dosage phenylephrine (µg), mean (IQR) | 0 (0–425) | 0 (0–300) | .98 |
| Cumulative dosage norepinephrine (µg), mean (IQR) | 55 (0–660) | 139 (0–603) | .52 |
| Heart rate >100 bpm | |||
| Frequency, n (%) | 26 (39.4) | 33 (48.5) | .30 |
| Duration (%), mean (IQR) | 0.0 (0.0–2.4) | 0.0 (0.0–3.2) | .47 |
| Maximum HR (bpm), mean (IQR) | 97 (85–115) | 100 (85–115) | .90 |
| Esmolol (mg), mean (IQR) | 0 (0–0) | 0 (0–0) | .61 |
Abbreviations: HR, heart rate; IQR, interquartile range; MAP, mean arterial pressure; SBP, systolic blood pressure.
Adverse events
There was no 30-day perioperative mortality in either treatment group. Perioperative complications are shown in Table 3. In each treatment group, there were 6 cardiovascular complications, occurring in 6 patients of the phenoxybenzamine group and 5 patients of the doxazosin group (8.8% vs 6.9%, P = 0.68). The number of subjects with postoperative hypoglycemia was not different (P = .19). During pretreatment, adverse events were reported by 80.9% and 92.4% of the phenoxybenzamine and doxazosin users, respectively (P = .08). All adverse events were graded as mild or moderate (ie, grade I or II) and are listed in the supplemental material (21). The total length of hospital stay was 14 (7–19) and 14 (8–18) days in the phenoxybenzamine and doxazosin group, respectively (P = .90).
Table 3.
Perioperative complications
| Number of events | ||
|---|---|---|
| Phenoxybenzamine (n = 68) | Doxazosin (n = 72) | |
| Cardiovascular events | ||
| Asystole | 0 | 1 |
| Atrial fibrillation/ flutter | 2 | 0 |
| Acute heart failure | 3 | 2 |
| Pulmonary embolism | 1 | 0 |
| Postoperative bleeding | 0 | 2 |
| Intestinal necrosis | 0 | 1 |
| Infection | ||
| Pneumonia | 4 | 5 |
| Urinary tract | 2 | 1 |
| Wound | 1 | 1 |
| Fever of unknown origin | 0 | 1 |
| Other | ||
| Excessive postoperative pain | 1 | 1 |
| Delirium | 1 | 0 |
| Intestinal perforation | 0 | 1 |
| Hypoglycemiaa | 8 | 4 |
aGlucose ≤3.5 mmol/L during the first 24 h postoperatively.
Exploratory analyses
The primary endpoint in patients with (n = 11) or without (n = 123) a cardiovascular complication was 11.8% (4.9–33.0) and 11.3% (5.0–20.0), respectively (P = .26). The associated HI-scores were 59.0 (43.8–73.0) and 42.5 (29.3–59.0), respectively (P = .03). In patients with (n = 104) or without (n = 30) preoperative use of a β-adrenergic receptor blocker, the primary endpoint was 11.4% (5.2–21.0) and 10.8% (2.5–17.4), respectively (P = .32). In addition, the associated HI-scores were 43.5 (32.3–59.0) and 49.0 (23.8–59.8) (P = .84), respectively.
Univariate analysis demonstrated that tumor size, total plasma-free metanephrines, and total plasma catecholamines were positively associated with the primary endpoint. Use of doxazosin, tumor size, total plasma-free metanephrines, and total plasma catecholamines were positively associated with the HI-score (21). These variables were subsequently tested in the multivariable linear regression model with the HI-score as a dependent variable. Total plasma-free metanephrines did not contribute significantly to the model and was removed. Achievement of different blood pressure targets was added. The final model demonstrated that the use of doxazosin, tumor size, and total plasma catecholamines were positively associated with the HI-score (21). The total model accounted for only a minority of the variance in HI-score (adjusted R2 = 0.16). Achievement of a supine blood pressure <130/80 mmHg, irrespective of the upright blood pressure, was negatively associated with the HI-score. Upright systolic blood pressure <90 mmHg was independently associated with an increased HI-score (21).
Discussion
In this first randomized controlled trial in patients scheduled for resection of a PPGL, we demonstrated that the cumulative time of blood pressure values outside the target range during PPGL surgery was not different after pretreatment with either phenoxybenzamine or doxazosin. Phenoxybenzamine was, however, more effective in preventing intraoperative systolic blood pressure above the target range and hemodynamic instability.
Treatment with an α-adrenergic receptor blocker prior to resection of a PPGL was first introduced in 1949 and has become part of routine clinical care since (24,25). All previous studies on the type of α-adrenergic receptor blocker were retrospective in design and suffered from several biases, such as the use of historical controls and the lack of a well-defined perioperative management protocol (10–14). In addition, these studies applied different blood pressure targets during surgery and raised conflicting results (10–14).
It should be noted that comparable intraoperative blood pressure levels can be achieved with the administration of a variable amount of vasoactive drugs and intravenous fluids by the anesthesiologist. The extent of these interventions has been acknowledged as a fundamental marker of hemodynamic instability (15–17). Therefore, we have recently developed and validated a clinical score for assessment of hemodynamic instability during surgery (17). Using this score as a secondary endpoint, we found a lesser degree of intraoperative hemodynamic instability after pretreatment with phenoxybenzamine. In particular, patients in the phenoxybenzamine group demonstrated a shorter duration of systolic blood pressure above 160 mmHg, a lower peak systolic blood pressure, and a concomitant lower requirement of vasodilating drugs. This might suggest that phenoxybenzamine offers a more effective inhibition of the α-adrenergic receptor than doxazosin, which could be explained by its noncompetitive antagonism compared to the competitive binding provided by doxazosin. Pretreatment with phenoxybenzamine did not result in more severe or a longer duration of postoperative hypotension, as previously suggested (26). We assume that this risk was minimized by the concomitant use of a high-sodium diet and the intravenous administration of saline the day before surgery (20). The higher rate of co-administration of β-adrenergic receptor blockers among patients allocated to phenoxybenzamine can be explained by the occurrence of reflex tachycardia as a result of inhibition of the presynaptic α 2-adrenergic receptor. Of note, neither the primary endpoint nor the hemodynamic instability score was affected by preoperative use of β-adrenergic receptor blockers.
The relevance of a more stable hemodynamic profile seems to be supported by the observation that patients who developed a postoperative cardiovascular complication had a higher hemodynamic instability score, despite the absence of a difference in primary endpoint. This observation is in agreement with other studies describing the adverse effects of hemodynamic instability on postoperative outcome (9,15,16,27–31). The rate of cardiovascular complications was not different between the treatment groups, but it should be noted that our study was not powered for this endpoint. Therefore, we were unable to demonstrate whether one of the study drugs resulted in a better clinical outcome. In view of the rarity of PPGL and the current complication rate, it would not be feasible to enroll the number of patients required to demonstrate a relevant difference in perioperative cardiovascular events (32). The absence of mortality in our study is in agreement with the literature (9,33,34). In the past decades, the perioperative mortality has decreased dramatically, most likely as a result of improvement of the medical management with use of α-adrenergic receptor blockers and major technical advances in both anesthesiology and surgery (7,20,35).
The importance of pretreatment with α-adrenergic receptor blockers has been questioned by some authors (36–38). These retrospective studies, however, suffered from a relevant selection bias, as both doctors’ and patients’ preferences were likely to have influenced the decision whether or not to initiate preoperative treatment with an α-adrenergic receptor blocker. In addition, these studies were confounded by the frequent use of antihypertensive agents other than α-adrenergic receptor blockers, the absence of a standardized management protocol before and during surgery, and the lack of detailed information on the nature and the extent of interventions required to control intraoperative hemodynamics. In view of the many limitations of these previous studies as well as the long-standing experience with preoperative administration of α-adrenergic receptor blockers, the use of these drugs generally remains recommended (5,39). The question as to whether pretreatment with an α-adrenergic receptor blocker could safely be omitted can only be answered in a randomized placebo-controlled trial.
The major strengths of the current study are its randomized controlled design, the use of a well-defined perioperative management protocol, the relatively large sample size of patients with a rare disease, and the comprehensive prospective data collection. Our study also has some limitations. Preoperative blood pressure targets were achieved in only a minority of the participants. In particular, a large majority did not reach the strict upright blood pressure target. It should be noted, however, that these blood pressure targets are mainly based on expert opinion and have never been evaluated prospectively before. Of potential interest, we showed that a preoperative supine blood pressure <130/80 mmHg is associated with less hemodynamic instability while an upright systolic blood pressure <90 mmHg is associated with more hemodynamic instability, as has been suggested previously (10). This finding could guide future recommendations concerning preoperative blood pressure targets. Furthermore, we did not include a placebo group, and study drugs were provided in an open-label fashion. Incorporation of a placebo arm was, however, considered to be unethical in view of current guidelines recommending pretreatment with an α-adrenergic receptor blocker (5,40). We have chosen for an open-label design because blinded administration of the study drugs would have required a double-dummy design with the ensuing risk of insufficient medication adherence due to the relatively large number of placebo and verum drugs that would need to be ingested by the participants. Limited availability of phenoxybenzamine in several countries likely affects the choice between phenoxybenzamine and doxazosin.
In conclusion, the duration of blood pressure being outside the target range during surgical resection of a PPGL was not different after preoperative treatment with either phenoxybenzamine or doxazosin. Phenoxybenzamine was more effective in preventing intraoperative hemodynamic instability, but it could not be established whether its use was associated with a better clinical outcome.
Acknowledgments
We would like to thank all co-investigators who contributed to this study: A. N. A. van der Horst-Schrivers, N. A. M. Alagla, and W. J. Sluiter, Department of Endocrinology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; N. J. G. M. Veeger, Department of Epidemiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; M. I. van der Velde, Department of Anesthesiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; J. de Vries, S. Kruijff, and P. H. J. Hemmer, Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; I. P. Kema, Department of Laboratory Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; T. Keizer, B. H. W. Molmans, P. V. Nannan Panday, and A. M. T. Schmidt, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; S. A. A. Willems, Department of Anesthesiology, Radboud University Medical Center, Nijmegen, The Netherlands; J. F. Langenhuijsen, Department of Urology, Radboud University Medical Center, Nijmegen, The Netherlands; D. H. Thone-Passchier, L. A. Schwarte, and W. D. Lubbers, Department of Anesthesiology, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; H. J. Bonjer, C. Dickhoff, and H. H. Eker, Department of Surgery, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; P. van der Valk, Department of Pathology, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; P. de Graaf and P. G. H. M. Raijmakers, Department of Radiology and Nuclear Medicine, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; P. Thoral, Department of Intensive Care, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; E. W. C. M. van Dam, Department of internal Medicine, Endocrinology section, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; W. W. de Herder and J. Hofland, Department of Internal Medicine, Section of Endocrinology, Erasmus Medical Center, Rotterdam, The Netherlands; C. T. Favoccia and C. G. O. T. Bouman, Department of Anesthesiology, Erasmus Medical Center, Rotterdam, The Netherlands; G. D. Slooter, Department of Surgery, Máxima Medical Center, Eindhoven, The Netherlands; L. P. H. M. Le Mair, Department of Anesthesiology, Máxima Medical Center, Eindhoven, The Netherlands; P. C. M. Wouters-van Poppel, Department of Internal Medicine, Máxima Medical Center, Eindhoven, The Netherlands; J. Vuyk, Department of Anesthesiology, Leiden University Medical Center, Leiden, The Netherlands; M. W. Hollmann, Department of Anesthesiology, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands; E. J. M. Nieveen van Dijkum, Department of Surgery, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands; and R. G. Hoff, Department of Anesthesiology, University Medical Center Utrecht, Utrecht, The Netherlands.
Financial Support: This trial was supported by an unrestricted grant from the Ipsen pharmaceutical company. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or in writing of the report. The authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Clinical Trial Information: Clinical trial registration number NCT01379898 at ClinicalTrials.gov.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in the references.
References
- 1. DeLellis R, Heitz P, Eng C.. Pathology and Genetics: Tumours of Endocrine Organs (IARC WHO Classification of Tumours). Lyon, France: IARC Press; 2004. [Google Scholar]
- 2. Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab. 2013;98(3):1100–1106. [DOI] [PubMed] [Google Scholar]
- 3. Zelinka T, Petrák O, Turková H, Holaj R, Strauch B, Kršek M, Vránková AB, Musil Z, Dušková J, Kubinyi J, Michalský D, Novák K, Widimský J. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res. 2012;44(5):379–384. [DOI] [PubMed] [Google Scholar]
- 4. Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011;29(11):2049–2060. [DOI] [PubMed] [Google Scholar]
- 5. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 6. Thompson JE, Arrowood JG. Pheochromocytoma; surgical and anesthetic management. Anesthesiology. 1954;15(6):658–665. [DOI] [PubMed] [Google Scholar]
- 7. Ross EJ, Prichard BN, Kaufman L, Robertson AI, Harries BJ. Preoperative and operative management of patients with phaeochromocytoma. Br Med J. 1967;1(5534):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joris JL, Hamoir EE, Hartstein GM, Meurisse MR, Hubert BM, Charlier CJ, Lamy ML. Hemodynamic changes and catecholamine release during laparoscopic adrenalectomy for pheochromocytoma. Anesth Analg. 1999;88(1):16–21. [DOI] [PubMed] [Google Scholar]
- 9. Livingstone M, Duttchen K, Thompson J, Sunderani Z, Hawboldt G, Sarah Rose M, Pasieka J. Hemodynamic stability during pheochromocytoma resection: lessons learned over the last two decades. Ann Surg Oncol. 2015;22(13):4175–4180. [DOI] [PubMed] [Google Scholar]
- 10. Bruynzeel H, Feelders RA, Groenland TH, van den Meiracker AH, van Eijck CH, Lange JF, de Herder WW, Kazemier G. Risk factors for hemodynamic instability during surgery for pheochromocytoma. J Clin Endocrinol Metab. 2010;95(2):678–685. [DOI] [PubMed] [Google Scholar]
- 11. Kocak S, Aydintug S, Canakci N. Alpha blockade in preoperative preparation of patients with pheochromocytomas. Int Surg. 2002;87(3):191–194. [PubMed] [Google Scholar]
- 12. Prys-Roberts C, Farndon JR. Efficacy and safety of doxazosin for perioperative management of patients with pheochromocytoma. World J Surg. 2002;26(8):1037–1042. [DOI] [PubMed] [Google Scholar]
- 13. Zhu Y, He HC, Su TW, Wu YX, Wang WQ, Zhao JP, Shen Z, Zhang CY, Rui WB, Zhou WL, Sun FK, Ning G. Selective α1-adrenoceptor antagonist (controlled release tablets) in preoperative management of pheochromocytoma. Endocrine. 2010;38(2):254–259. [DOI] [PubMed] [Google Scholar]
- 14. Weingarten TN, Cata JP, O’Hara JF, Prybilla DJ, Pike TL, Thompson GB, Grant CS, Warner DO, Bravo E, Sprung J. Comparison of two preoperative medical management strategies for laparoscopic resection of pheochromocytoma. Urology. 2010;76(2):508.e6–508.11. [DOI] [PubMed] [Google Scholar]
- 15. Yamazaki Y, Oba K, Matsui Y, Morimoto Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth. 2018;32(2):167–173. [DOI] [PubMed] [Google Scholar]
- 16. Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP, Thevathasan T, Pieretti A, Ferrone C, Hoeft A, Scheeren TWL, Thompson BT, Kurth T, Eikermann M. Effects of intraoperative fluid management on postoperative outcomes: a hospital registry study. Ann Surg. 2018;267(6):1084–1092. [DOI] [PubMed] [Google Scholar]
- 17. Buitenwerf E, Boekel MF, van der Velde MI, Voogd MF, Kerstens MN, Wietasch GJKG, Scheeren TWL. The haemodynamic instability score: Development and internal validation of a new rating method of intra-operative haemodynamic instability. Eur J Anaesthesiol. 2019;36(4):290–296. [DOI] [PubMed] [Google Scholar]
- 18. Schulz KF, Altman DG, Moher D; CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. [DOI] [PubMed] [Google Scholar]
- 19. de Jong WH, Graham KS, van der Molen JC, Links TP, Morris MR, Ross HA, de Vries EG, Kema IP. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clin Chem. 2007;53(9):1684–1693. [DOI] [PubMed] [Google Scholar]
- 20. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92(11):4069–4079. [DOI] [PubMed] [Google Scholar]
- 21. Buitenwerf E, Osinga TE, Timmers HJLM, Lenders JWM, Feelders RA,Eekhoff EMW, Haak HR, Corssmit EPM, Bisschop PHLT, Valk GD, GrooteVeldman R, Dullaart RPF, Links TP, Voogd MF, Wietasch JKG, Kerstens MN. Efficacy of phenoxybenzamine versus doxazosin on hemodynamic control during pheochromocytoma resection - a randomized controlled trial. Supplemental data. Figshare repository.https://figshare.com/articles/PRESCRIPT_SupplementalData_2019_pdf/9199682. doi: 10.6084/m9.figshare.9199682. Deposited August 1, 2019. [DOI]
- 22. National Cancer Institute. Common terminology criteria for adverse events.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Published 2017. Accessed September 9, 2018.
- 23. Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges L, eds. The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation;1994:231–244. [Google Scholar]
- 24. Grimson KS, Longino FH. Treatment of a patient with a pheochromocytoma; use of an adrenolytic drug before and during operation. J Am Med Assoc. 1949;140(16):1273. [DOI] [PubMed] [Google Scholar]
- 25. Manger WM. An overview of pheochromocytoma: history, current concepts, vagaries, and diagnostic challenges. Ann N Y Acad Sci. 2006;1073:1–20. [DOI] [PubMed] [Google Scholar]
- 26. Boutros AR, Bravo EL, Zanettin G, Straffon RA. Perioperative management of 63 patients with pheochromocytoma. Cleve Clin J Med. 1990;57(7):613–617. [DOI] [PubMed] [Google Scholar]
- 27. Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515. [DOI] [PubMed] [Google Scholar]
- 28. van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35–44. [DOI] [PubMed] [Google Scholar]
- 29. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515–523. [DOI] [PubMed] [Google Scholar]
- 30. Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123(1):79–91. [DOI] [PubMed] [Google Scholar]
- 31. Futier E, Lefrant JY, Guinot PG, Godet T, Lorne E, Cuvillon P, Bertran S, Leone M, Pastene B, Piriou V, Molliex S, Albanese J, Julia JM, Tavernier B, Imhoff E, Bazin JE, Constantin JM, Pereira B, Jaber S; INPRESS Study Group Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berends AMA, Buitenwerf E, de Krijger RR, Veeger NJGM, van der Horst-Schrivers ANA, Links TP, Kerstens MN. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: a nationwide study and systematic review. Eur J Intern Med. 2018;51:68–73. [DOI] [PubMed] [Google Scholar]
- 33. Kiernan CM, Du L, Chen X, Broome JT, Shi C, Peters MF, Solorzano CC. Predictors of hemodynamic instability during surgery for pheochromocytoma. Ann Surg Oncol. 2014;21(12):3865–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plouin PF, Duclos JM, Soppelsa F, Boublil G, Chatellier G. Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: analysis of 165 operations at a single center. J Clin Endocrinol Metab. 2001;86(4):1480–1486. [DOI] [PubMed] [Google Scholar]
- 35. Apgar V, Papper EM. Pheochromocytoma. Anesthetic management during surgical treatment. AMA Arch Surg. 1951;62(5):634–648. [PubMed] [Google Scholar]
- 36. Kong H, Li N, Li XY, Wang DX. The role of pre-operative α-blockade in patients with normotensive phaeochromocytoma or paraganglioma: A retrospective cohort study. Eur J Anaesthesiol. 2018;35(11):898–899. [DOI] [PubMed] [Google Scholar]
- 37. Groeben H, Nottebaum BJ, Alesina PF, Traut A, Neumann HP, Walz MK. Perioperative α-receptor blockade in phaeochromocytoma surgery: an observational case series. Br J Anaesth. 2017;118(2):182–189. [DOI] [PubMed] [Google Scholar]
- 38. Isaacs M, Lee P. Preoperative alpha-blockade in phaeochromocytoma and paraganglioma: is it always necessary? Clin Endocrinol (Oxf). 2017;86(3):309–314. [DOI] [PubMed] [Google Scholar]
- 39. Wolf KI, Santos JRU, Pacak K. WHY take the risk? we only live once: the dangers associated with neglecting a pre-operative alpha adrenoceptor blockade in pheochromocytoma patients. Endocr Pract. 2019;25(1):106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, Kimura N, Mannelli M, McNicol AM, Tischler AS; International Symposium on Pheochromocytoma Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab. 2007;3(2):92–102. [DOI] [PubMed] [Google Scholar]