Abstract
Calcium phosphate is the inorganic mineral of hard tissues such as bone and teeth. Due to their similarities to the natural bone, calcium phosphates are highly biocompatible and biodegradable materials that have found numerous applications in dental and orthopedic implants and bone tissue engineering. In the form of nanoparticles, calcium phosphate nanoparticles (CaP’s) can also be used as effective delivery vehicles to transfer therapeutic agents such as nucleic acids, drugs, proteins and enzymes into tumor cells. In addition, facile preparation and functionalization of CaP’s, together with their inherent properties such as pH-dependent solubility provide advantages in delivery and release of these bioactive agents using CaP’s as nanocarriers. In this review, the challenges and achievements in the intracellular delivery of these agents to tumor cells are discussed. Also, the most important issues in the design and potential applications of CaP-based biominerals are addressed with more focus on their biodegradability in tumor microenvironment.
Keywords: Calcium phosphate, nanoparticles, degradable, cancer, drug delivery, intracellular delivery, gene delivery
1. Introduction
Developing a wide range of therapeutic and diagnostic agents by recent advancements in nanotechnology has drawn an increasing attention toward their intracellular delivery. Most front-line cancer drugs are water insoluble, untargeted and have dose-limiting side effects [1]. In addition, several groups of these therapeutic agents (e.g., DNA or siRNA) are both charged and large molecules and it is difficult for them to cross the cell membrane to elicit their desired effects. Therefore, an appropriate delivery system is required to assist their trafficking and cellular uptake. Although, recombinant viral vectors demonstrated high efficacy for treatment of human diseases, their major drawbacks and eventual clinical failure have led to quick shift toward synthetic non-viral carriers [2, 3].
To overcome limitations of drug delivery techniques, several formulations of nanoparticles have been proposed, including biodegradable polymers [4], gold [5], iron oxide [6, 7], liposomes [8], silica [9], dendrimers [10] and calcium phosphate based mineral systems [11, 12]. An ideal drug carrier should be able to incorporate potential bioactive agents either physically or chemically and protect them in the bloodstream. Furthermore, the carrier complex should de-assemble gradually and provide sustained drug release over prolonged period of time to increase therapeutic efficiency [13]. In addition, it should provide a feasible mechanism to specifically bind to target cells or tissues, in order to reduce their off-target effects and enhance the on-site drug concentration. Among different synthetic vectors calcium phosphate nanoparticles (CaP’s) have shown promising results toward the abovementioned criteria.
Synthetic CaP’s in the form of carbonate apatite are similar to inorganic mineral of natural bone, which are highly biocompatible and biodegradable materials, extensively used in orthopedic implants and bone tissue engineering [14]. Furthermore, CaP’s hold outstanding bioactivity and tailorable biodegradability that distinguish them from the other biominerals and make them an excellent option for drug delivery in nanomedicine applications, orthopedics and dentistry [15–18] or as adjuvants in different vaccines [19–22]. Moreover, their innate properties such as pH-dependent solubility along with facile and straightforward preparation and functionalization make them useful in diverse therapeutic applications, including cancer therapy [23]. Biodegradable nanoparticles are generally preferred for cancer therapy since their predictable body clearance pathways and mechanisms makes them safer candidates for clinical applications [24].
Finally, site-specific cellular entry plays an important role in the bioactivity and bioavaiability of the delivered biomolecules. Studies have shown that physical and chemical characteristics of CaP nanoparticles including size, charge, morphology, composition and surface chemistry are the critical parameters that determine the route of internalization of nanomedicines [25–28]. Other essential factors such as dose of bio-agents and their functionalities depend on the cellular entry pathway and the final location inside the cells [29]. The cell has a high number of organelles thus, it is important that the delivery system releases its payload in the specific site in order to induce the desired effect. Insights into how the cell passes its components to the specific cellular compartments could improve drug design for better management of the tumors.
This paper summarizes recent achievements in preparation and cancer therapy applications of CaP nanoparticles and reviews opportunities and difficulties associated with various available strategies to overcome their cellular barriers. In addition, their potential applications as carriers of bio-agents (e.g., cancer drugs and nucleic acids) and their required properties are discussed. Moreover, the critical roles of their intrinsic characteristics such as pH-dependent solubility for facilitated delivery and release of bioactive agents are addressed in details. Compared with previous publications reviewing CaP design and applications in nanomedicine (e.g., [30–32]), our review provides a new materials science perspective, focusing more on biodegradability of different crystallographic phases of CaP’s designed as cancer therapeutics. This is a major safety concern hindering clinical translation of different formulations of nanomedicines developed for cancer therapy.
2. CaP Biominerals for Intracellular Delivery to Cancer Cells
2.1. Tuning CaP’s Physiochemical Properties for Cancer Therapy
Size and Morphology:
Binding to specific cell membrane receptors, trafficking inside the cells, intracellular flow and pharmacokinetics, extravasation and clearance mechanisms depend on nanoparticles size and morphology [25, 27, 33–35]. Among various techniques for preparation of cancer therapeutic CaP’s [36], synthesis in the presence of nucleic acids (e.g., DNA, siRNA and oligonucleotides) has been reported as a facile and straightforward method [22, 37, 38]. This method usually results in particles with larger sizes (~100–200 nm). However, recent approaches such as laser ablation enabled synthesis of CaP-based particles as small as 3 nm [39]. Larger nanoparticles usually have a short blood circulation time due to rapid accumulation in reticuloendothelial system (RES), while ultrasmall nanoparticles smaller than 8–10 nm have a fast renal clearance from the blood. Therefore, synthesis of CaP nanoparticles should be optimized to tune their sizes within the range of 10–80 nm, as also recommended for other types of nanoparticles, in order to achieve longest blood circulation time and highest amount of uptake by tumors [24].
As one of the pioneering studies, more than three decades ago, Graham and Van Der Eb discovered that entrapment of biomolecules such as nucleic acids into the CaP’s can be used for tuning nanoparticles physiochemical properties such as size and morphology [40]. Their approach involved mixing of a calcium chloride solution, containing these biomolecules (e.g., DNA), with phosphate-buffered saline (PBS) for in situ co-precipitation of CaP nano- and microparticles (Figure 1). Although this approach had notable advantages such as low cost and facile preparation method, the rapid growth of precipitates resulted in large particle sizes and therefore low transfection efficiency [41].
Figure 1.
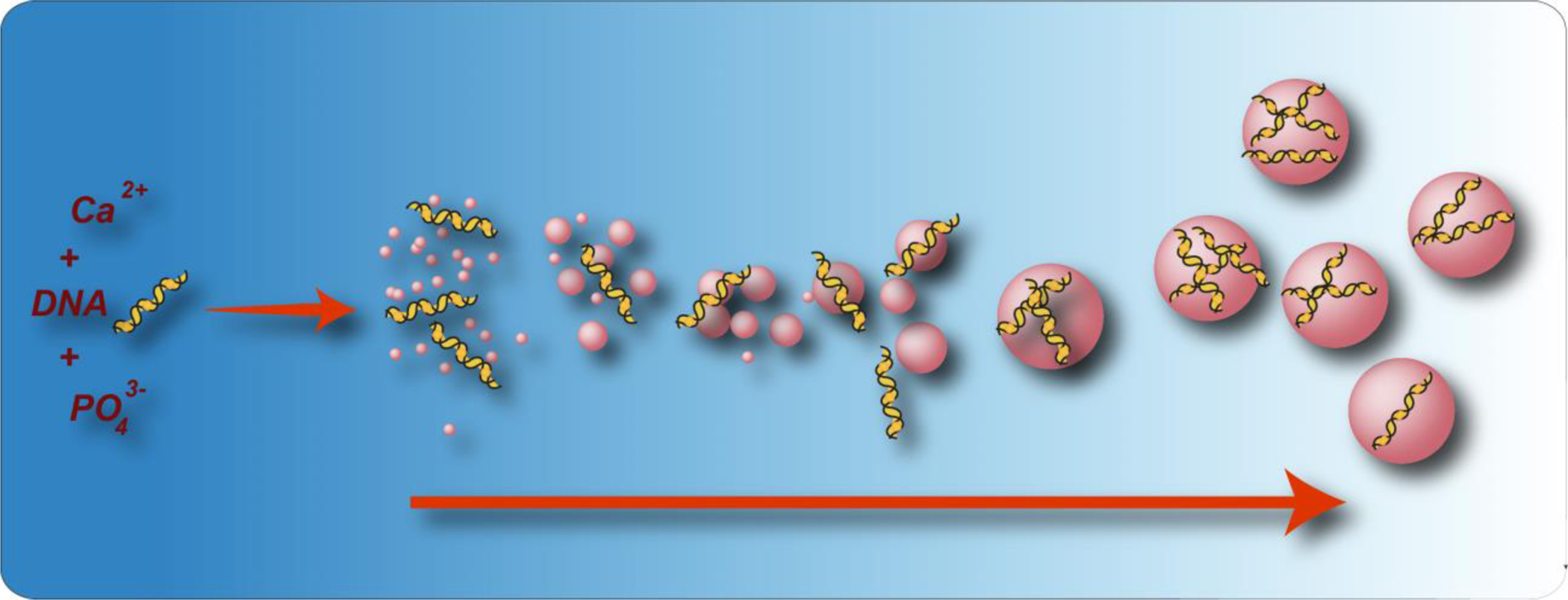
(a) Schematic showing co-precipitation of calcium phosphate nanoparticles in the presence of DNA molecules. (Reprinted with permission from ref. [37]. Copyright 2019 American Chemical Society)
Later studies showed that controlling synthesis parameters such as temperature, concentration of calcium and phosphate ions, pH, and reaction time are critical to achieve smaller size precipitates [42–44]. Since, the reaction between calcium and phosphate ions in the precursor solutions is spontaneous, adjusting their concentrations and selection of their precursors play important roles in producing a consistent particle size and morphology [45, 46]. After initial burst of nucleation, there is a high affinity for rapid aggregation of particles, due to the van der Waal’s and secondary surface interaction between particles. Particle aggregation is a common phenomenon in colloidal suspensions and is much stronger in the nano-sized range, due to the intrinsic surface reactivity of the nanoparticles. It is reported that using dilute solutions of initial precursors of calcium and phosphate ions can minimize these aggregations by controlling the kinetics of precipitation reaction [47]. However, lower concentration of calcium ions can also reduce the CaP precipitation rates and therefore optimized conditions should be found for each type of mineralization solution and biomolecule. For example, it is known that addition of magnesium to mineralization solution can help to obtain CaP nanoparticles with smaller sizes by inhibiting their crystal growth [48]. This lower growth rate is attributed to distortion of atomic structure of the CaP particles, since some Ca2+ ions are substituted with smaller Mg2+ cations.
Incorporation of block copolymers with a polycarboxylate section, such as poly (ethylene glycol)-block-poly (aspartic acid) (PEG-PAA) can also suppress the crystal growth of CaP nanoparticles. This approach involves mixing of a HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer containing phosphate and PEG-PAA with a Tris buffer containing Ca2+ and biomolecules (e.g., DNA). It has been known that PAA gets adsorbed on CaP crystals during the growth and compensates the increased interfacial energy. Utilizing this strategy resulted in formation of monodispersed nanoparticles with a core-shell structure. For example, when DNA was added to the Ca2+-containing Tris buffer, the core of the resulted nanoparticles consisted of DNA-incorporated CaP crystals that were surrounded by hydrophilic palisades of PEG shell [49, 50]. It was shown that small size and stable nanoparticles could form only above certain value of PEG-PAA concentrations and the particles size could be tuned by modifying the PEG-PAA concentration. In another study, poly (ethylene glycol)-block-poly (methacrylic acid) or PEG-PMA was used to stabilize CaP crystals. The resulted nanoparticles were in the range of several hundreds of nanometers with a considerable colloidal stability, due to the steric hindrance effect of PEG palisade. The PMA segment of the nanocrrier underwent a conformational change at pH 4–6 and formed a more hydrophobic coating as compared to physiological pH. This pH-dependent conformational transition enhanced the interaction of nanoparticles with the membrane of endosomes and facilitated endosomal escape [51]. Pre-mixing of the precursor solutions containing phosphate ions with PEG and then addition of Ca2+-containing solutions was also reported as an efficient method to generate stable CaP nanoparticles, due to chelating effect of the PEG molecules with phosphates [52].
As it is shown in Table 1, most of the recent CaP-based in vivo cancer therapy studies have used spherical calcium phosphate nanoparticles or shells for delivery of the therapeutic agents to cancer tissues. This is mainly because of mild mineralization conditions used for preparation of the CaP’s, which usually results in formation of spherical and round morphologies that are more stable thermodynamically. Rod-shape morphologies have also been prepared by relatively higher temperature synthesis methods. For example, a recent study by Sun et al. [53] discussed synthesis and efficacy of hydroxyapatite nanorods for targeting mitochondria of the cancer cells to inhibit growth of lung cancer xenografts in mice. However, spherical nanoparticles are generally preferred for therapeutic delivery applications, since they provide highest possible specific surface area for loading drugs or nucleic acid molecules. Other CaP morphologies such as nano-clusters [54] and porous structures [55, 56] have also been investigated as high-loading capacity candidates for cancer therapy applications, but the relatively larger size of these particles and multiple steps involved in their synthesis are considered as the major drawback hindering their potential applications in clinical trials.
Table 1.
An overview of the recent in vivo cancer therapeutic studies using calcium phosphate-based nanoparticles. Nanoparticles physiochemical properties (e.g., crystallographic phase, hydrodynamic size, shape, surface coating and targeting molecules), chemical procedures used for their surface functionalization, cancer models and therapeutic approaches are listed for each study. Observation of endocytosis in each study is shown with YES or NO. Endocytosis in tumor cells has been reported for CaP nanoparticles with different sizes, crystallographic phases, shapes and surface coatings. (abbreviations sorted alphabetically: 2DG: 2-deoxy-D-glucose; α-TOS: alphα-tocopheryl succinate; ABX-EGF scFv: EGFR- specific single chain fragment antibody; AHA: alendronate-hyaluronan graft polymer; BSA: bovine serum albumin; CaP: Calcium phosphate; DOX: Doxorubicin; Gd: Gadolinium MRI contrast agent; HAp: Hydroxyapatite; MIT: Mitoxantrone; mPEG-SH: methoxy-polyethylene glycol with thiol groups; NA: not applicable; NPs: Nanoparticles; NS: not characterized or specified in the reported study.; PAA: Poly acrylic acid; PDT: Photo dynamic therapy; PEG: Polyethylene glycol; PEGS: Pegylated poly(glycerol sebacate); PTT: Photothermal therapy; RGD: Arginyl glycyl aspartic acid; TPGS: Polyethylene glycol 1000 vitamin E succinate; TSG-6: (TNF)-stimulated gene 6; uPA: Urokinase plasminogen activator analogues peptide; VER: Verapamil.
| CaP phase | Shape | Size (nm) | Coating | Targeting molecule | Functionalization method | Application/Endocytosis reported? | Ref. |
|---|---|---|---|---|---|---|---|
| Amorphous CaP | Spherical | 40–50 | Lipid | Folic acid and/or ABX-EGF scFv | Two-step lipid coating and then covalent bonding of targeting molecules | siRNA delivery to breast tumor/ (NO) | [23] |
| CaP (NS) (degradable) | Spherical | 100–150 | Cupper sulfide/Pluronic® F-68 | NS | Electrostatic adsorption for F-68 capping | Mitochondrial Ca2+ homeostasis and PTT of breast tumor/ (YES) | [58] |
| CaP (NS) | Spherical | 150 | Hyaluronic acid | Hyaluronic acid | Electrostatic adsorption of cross-linked hyaluronic acid | siRNA delivery to mouse melanoma tumor/ (YES) | [59] |
| CaP (NS) | Spherical | 20 | PEG-RGD | RGD peptide | Formation of CaP’s inside drug-loaded PEG-RGD micelles | Delivery of Drug-resistance inhibitor (VER) and a chemotherapeutic agent (MIT) to hepatocellular carcinoma (liver cancer)/ (YES) | [60] |
| CaP (NS) | Spherical | 44–47 | Lipid/PEG | Bispecific antibody (BsAb) | Non-covalent adsorption of BsAb to lipid-coated CaP’s | Inhibit growth of the triple negative breast tumor by siRNA delivery and PTT in triple negative breast tumor/ (YES) | [61] |
| CaP (NS) | Spherical | 125 | Lipid | Galactose derivative | Two-step lipid coating and non-covalent bonding of galactose derivatives | siRNA delivery to hepatocellular carcinoma (liver cancer)/ (YES) | [62] |
| CaP (NS) | Spherical | 100 | PAA/ PEG/ gold nanorods | NA | Assembly of gold nanorods to the PAA-coated CaP’s and then coating with mPEG-SH | DOX delivery and PTT of liver cancer/ (YES) | [63] |
| CaP (NS) | Spherical | 170 | AHA | Hyaluronic acid | Electrostatic adsorption of hyaluronic acid to CaP | siRNA delivery to lung cancer/ (YES) | [64] |
| CaP (NS) | Spherical | 68 | folic acid/ PEG | Folic acid | Two-step lipid coating and then covalent bonding of folic acid | Prevention of breast tumor metastasis by vitamin E (α-TOS) delivery/ (YES) | [65] |
| Calcium phosphonate | Spherical | 42 | Lipid/PEG coated | miRNA-155 | Microemulsion to form miRNA loaded lipid-coated CaP’s and then coating with PEG-lipid in organic phase | miRNA delivery to tumor associated macrophages in murine sarcoma cancer/ (YES) | [66] |
| CaP (NS) | Spherical | 194 | Chitosan | NA | Co-precipitation of siRNA-loaded CaP’s in the presence of chitosan | siRNA delivery to Hela cervical cancer/ (YES) | [67] |
| HAp | Spherical nano-clusters | 30–40 | Polyglutamic acid | NA | Polyglutamic acid for self-assembly of ultrasmall HAp NPs | Synergistic DOX delivery and increased calcium influx for therapy of gastric adenocarcinoma/ (YES) | [54] |
| CaP (NS) | CaP shell to encapsulate siRNA | 113 | PEG | uPA | Formation of siRNA-loaded CaP’s inside PEG-uPA micells | siRNA delivery to triple negative breast cancer/ (YES) | [68] |
| HAp | Granular and nanorods | 17 and 61×29 | NA | NA | No surface functionalization was used | To evaluate effects of size and shape of HAp (no drug)in melanoma/ (YES) | [69] |
| HAp | Spherical | 38 | A54, TPGS and Gd | A54 peptide | CaP formation in the presence of A54-TPGS and Gd | MRI-guided DOX delivery to hepatic cancer/ (YES) | [70] |
| CaP (NS) | Spherical | 207 | PEI-cholesterol | VEGF siRNA | siRNA-loaded CaP’s were coated with a layer of PEI-cholestrol | siRNA delivery and inhibition of breast tumor angiogenesis/ (NO) | [71] |
| Amorphous CaP (degradable) | CaP coated upconversion NPs | 78 | PAA | NA | Silica-coated upconversion NPs were coated with CaP and PAA | DOX delivery and PDT of cervical cancer/ (YES) | [72] |
| HAp | nanorod | 10×50 | NA | NA | No surface functionalization was used | Mitochondria-targeting to inhibit lung cancer growth/ (YES) | [53] |
| HAp | almost spherical and porous | 217 | PEG/ Folic acid | Folic acid | Folic acid was covalently conjugated to polyethylene glycol-coated HAp NPs | Drug (epirubicin) delivery to murine colorectal cancer/ (YES) | [55] |
| HAp | mesoporous and spherical | NS | NA | NA | Electrostatic adsorption of 2DG, DOX and then radiolabeling for SPECT imaging | 2DG and DOX delivery to breast tumor and SPECT imaging using radiolabeling/ (NO) | [56] |
| CaP (NS) | Spherical | 140 | BSA | NA | CaP mineralization in the presence of BSA as template | TSG-6 delivery for treatment of liver fibrosis/ (NO) | [73] |
| HAp | core/shell PEGS/ HAp micelle | 20–30 | PEGS | NA | HAp NPs were formed in PEGS micelles | pH responsive DOX delivery to breast tumor/ (NO) | [74] |
| HAp | Nanorods loaded into a titanium implant | 46×13 | NA | NA | No surface functionalization was used | mitochondrial-dependent apoptosis and stimulating immune response in rabbit models/ (NO) | [75] |
| HAp and oligo-HAp (degradable) | silica/HAp hybrid | 99 | Hyaluronan (HA) and oligo-hyaluronan (oHA) | Hyaluronan (HA) and oligo-hyaluronan (oHA) | HA or oHA were covalently conjugated to the surface of silica/HAp | DOX delivery to breast cancer/ (YES) | [76] |
| CaP (NS) | spherical | 140 | MMP-2 cleavable peptide and PEG-lipid | NA | Peptide and PEG-lipid coating created by microemulsion | siRNA delivery to liver cancer/ (YES) | [77] |
Despite the extensive use of CaP-based vectors for gene and drug delivery during the last 30 years, there are still concerns regarding inconsistencies in transfection or drug delivery efficacy of the synthesized CaP’s in different studies. Recent studies have indicated that such inconsistencies can be minimized by tuning the stoichiometry (Ca/P ratio) and crystallographic phase of the CaP particles, addition of cations such as strontium or zinc, using optimized mode of mixing, and selecting proper stabilizing macromolecules (i.e., nucleic acids, oligonucleotides and synthetic polymers) with specific molecular structures [22, 37, 47, 57]. Therefore, accurate controlling of these elaborated synthesis parameters should be considered for reproduction of the nano-sized CaP particles with efficient condensation and bounding of the desired macromolecules.
Biodegradability and Crystallographic Phase:
Degradability of the cancer therapeutic CaP’s in acidic tumor microenvironment or endolysosomal organelles is the other major characteristic that should be considered and designed properly for their clinical translational applications. In fact, most of the nanoparticles studied for nanomedicine are not degradable in body and this has been considered as one of the major safety concerns delaying their translation to human applications. There are a wide range of calcium phosphates with different Ca:P ratios and crystallographic phases (Table 2), and among these different phases, amorphous calcium phosphates and hydroxyapatite are the most common types of CaP’s used for cancer therapy. Degradation of the CaP’s is pH-dependent and changes with their crystallographic structures, and Ca/P ratio [78–80]. Generally, degradability of different CaP phases in acidic buffers is sorted as follows [32] (“≫” denotes much greater solubility.):
Table 2.
Different crystallographic phases of calcium phosphates and their pH stability range and water solubility as representatives of biodegradability in weakly acidic microenvironment of the tumors or acidic pH of the endosomes and lysosomes. (Adapted and Reprinted with permission from refs. [32] and [31]. Copyright 2019 Royal Society of Chemistry)
| Compound | Chemical formula | Ca/P ratio | Solubility at 25 °C, −log(Ksp) | Solubility at 25 °C (g L−1) | pH stability range at 25 °C |
|---|---|---|---|---|---|
| Monocalcium phosphate monohydrate (MCPM) | Ca(H2PO4)2·H2O | 0.5 | 1.14 | ~ 18 | 0.0–2.0 |
| Monocalcium phosphate anhydrous (MCPA) | Ca(H2PO4)2 | 0.5 | 1.14 | ~ 17 | b |
| Dicalcium phosphate anhydrous (DCPA, monetite) | CaHPO4 | 1.0 | 6.90 | ~ 0.048 | b |
| Dicalcium phosphate dihydrate (DCPD, brushite) | CaHPO4·2H2O | 1.0 | 6.59 | ~ 0.088 | 2.0–6.0 |
| Octacalcium phosphate (OCP) | Ca8(HPO4)2(PO4)4·5H2O | 1.33 | 96.6 | ~ 0.0081 | 5.5–7.0 |
| Amorphous calcium phosphates (ACP) | CaxHy(PO4)z·nH2O, n = 3–4.5; 15–20% H2O | 1.2–2.2 | a | a | ~ 5–12 |
| Calcium-deficient hydroxyapatite (CDHA, is also called precipitated hydroxyapatite) | Ca10−x(HPO4)x(PO4)6−x(OH)2−x | 1.5–1.67 | −85.1 | ~ 0.0094 | 6.5–9.5 |
| Hydroxyapatite (HAP) | Ca10(PO4)6(OH)2 | 1.67 | 116.8 | ~ 0.0003 | 9.5–12 |
| Fluorapatite (FAP) | Ca10(PO4)6F2 | 1.67 | 120 | ~ 0.0002 | 7–12 |
| α-Tricalcium phosphate (α-TCP) | α-Ca3(PO4)2 | 1.5 | 25.5 | ~ 0.0025 | c |
| β-Tricalcium phosphate (β-TCP) | β-Ca3(PO4)2 | 1.5 | 28.9 | ~ 0.0005 | c |
| Tetracalcium phosphate (TTCP, hilgenstockite) | Ca4(PO4)2O | 2.0 | 38–44 | ~ 0.0007 | c |
Cannot be measured precisely, always metastable.
Stable at temperatures above 100 °C.
These compounds cannot be precipitated from aqueous solution.
Amorphous Calcium Phosphates ≫ α-tricalcium phosphates ≫ β-tricalcium phosphates > Calcium-deficient hydroxyapatite ≫ hydroxyapatite > fluorapatite
Amorphous calcium phosphates are expected to degrade faster, since there is no long-range crystallographic bonding in their atomic structure, which results in easier detachment of the atomic layers by consuming less thermodynamic energy. Liu et el. [72] designed nanoparticles that were coated with amorphous CaP coatings. Their results showed that this amorphous CaP layer could be degraded in weakly acidic microenvironment of the tumors, to enhance release of the chemotherapeutic drugs that were entrapped inside them. Also, a recent in vitro study showed similar results for apoptosis of breast cancer cells, using degradable and drug-loaded amorphous CaP nanoparticles [81]. CaP’s in the form of calcium-deficient hydroxyapatite and tricalcium phosphate have also shown promising degradability, especially at lower pH values, which makes them favorable candidates. Kang et al. [76] coated drug-loaded silica nanoparticles with calcium-deficient hydroxyapatite and showed that the hydroxyapatite layer could be degraded in low-pH environment of the tumors to release chemotherapy agents. Calcium-deficient hydroxyapatites formed by substitution of Ca2+ with other ions such as Fe2+ and Mg2+ have also been developed for cancer therapy [82]. For example, iron-doped magnetic hydroxyapatite nanoparticles were developed and used for treatment of liver [83] and colon [84] cancers using hyperthermia. Mg-doped hydroxyapatites have also been used to induce apoptosis in cancer cells, due to release of Ca2+ and Mg2+ ions as the result of nanoparticles degradation [85]. Additionally, calcium-deficient hydroxyapatite nanoparticles formed by doping hydroxyapatite with small drug molecules (e.g., 5-Fluorouracil) can also be used as effective cancer chemotherapy agents [86]. Also, it is possible to accelerate degradation of the CaP’s by applying external stimuli such as near infra-red laser irradiation. For example, Xu et al. [58] doped ultrasmall CaP’s into cupper sulfide (CuS) hollow nanoparticles and showed that irradiation of the core-shell particles with near infra-red laser can cause disintegration of the CaP’s, as a result of photothermal interactions between CuS and light. Their in vivo studies showed that released Ca2+ ions could enhance apoptosis rate of the cancer cells. Safety and biocompatibility of the degradation by-products are also important factors that should be carefully considered in future design of the biodegradable CaP’s for cancer therapy. More studies are required to investigate the performance and degradability of different phases of CaP’s such as amorphous calcium phosphates, tricalcium phosphates and calcium-deficient hydroxyapatites in low-pH environment of the tumors or endolysosomes. However, in situ characterization of the CaP’s inside the cells or tumors is a challenging procedure that needs sophisticated materials science expertise. Such characterizations become even more challenging, considering transition of different CaP phases to each other (e.g., transition of calcium-deficient hydroxyapatite to tricalcium phosphate [87]) at low pH environments of the tumors.
2.2. Surface Functionalizing and In vivo Cancer Targeting
Surface functionalization of the cancer therapeutic CaP’s is necessary to prolong their circulation in blood or adding targeting molecules (e.g., peptides and antibodies) to their surface. These two phenomena enable CaP’s enhanced permeation and retention (EPR) in tumors, leading to higher therapeutic efficacy with lower dosage of the administered nanoparticles. As it is shown in Table 1, various methods have been used for surface functionalization of the CaP’s, using covalent or non-covalent (electrostatic) conjugation of macromolecules (e.g., polymers, peptides and nucleic acids) to their surface. Overall, these methods can be categorized into four different strategies. The procedure descriptions, and major advantages and disadvantages of each procedure are summarized in Table 3. Selection of the surface functionalization procedure depends on type of targeted tumors and approaches used for their treatment.
Table 3.
Common chemical methods used for surface modification of CaP-based nanoparticles with different types of macromolecules such as nucleic acids (e.g., siRNA and DNA), cancer targeting agents (e.g., peptides and antibodies), and polymers (e.g., PEG, PEI and hyaluronic acid), designed for in vivo cancer therapy studies. (PEI:Polyethylenimine)
| Method Description | Common Macromolecules | Advantages | Disadvantages | Examples |
|---|---|---|---|---|
| Macromolecules used to form micelles for CaP mineralization | Liposomes, hyaluronic acid and peptides conjugated to polymers (e.g., PEG) |
|
|
[60, 68, 74, 77] |
| Macromolecules used as homogenously dispersed templates for controlled growth of mineralized CaP’s (no micelles) | Nucleic acids and proteins (e.g., BSA) |
|
|
[67, 70] |
| Surface modification of mineralized CaP’s by electrostatic adsorption of macromolecules | Cancer targeting molecules and nucleic acids |
|
|
[61, 62] |
| Covalent bonding of macromolecules to the surface of mineralized CaP’s | Cancer targeting molecules, Polymers (e.g., PEG or PEI derivatives) and hyaluronic acid |
|
|
[23, 55, 65] |
Surface functionalization usually increases the hydrodynamic size of the CaP’s, due to addition of large molecules to their surface. As mentioned before, CaP’s with larger hydrodynamic sizes usually have shorter blood circulation time and therefore less uptake by tumors. However, it is known that addition of polyethylene glycol (PEG) to the surface of the CaP’s can help increasing the blood circulation time, due to steric hinderance and less protein adsorption of the PEG molecules. Some recent in vivo studies based on using PEG or PEG-derivative molecules for surface modification of the cancer therapeutic CaP’s are listed in Table 1.
Surface functionalization with different types of cationic or anionic molecules can also be used for tuning the surface charge of the CaP’s, which is a crucial characteristic determining adhesion of CaP’s to cancer cells. In addition, electrostatic repulsive forces between charged CaP’s, helps to prevent their aggregation and therefore increases the chances of their uptake by tumors. Klesing et al. [28] functionalized CaP’s with different surface charges by adding a mixture of anionic carboxymethyl cellulose (CMC) polymer and photoactive porphyrin dye to the CaP’s. The negative charge of these particles was then inverted by adding a layer of cationic poly (ethyleneimine) (PEI). Then, they assessed the effect of CaP’s surface charge on their ability to pass through the cell membrane. Their results showed that CaP’s coated with positively-charged PEI molecules had higher uptake and therefore, more therapeutic efficacy.
Targeted delivery of CaP nanoparticles to tumors is also critical in order to increase their therapeutic efficacy and diminish their undesirable off-target effects. Covalent or non-covalent binding of peptides [70] or antibodies [88] to the surface of CaP’s have been reported as effective methods to improve their specific targeting. Type of the targeting molecules that should be conjugated to CaP’s depends on cancer cells and receptors available on their membranes. An extensive list of these targeting molecules and methods for loading them to the surface of CaP’s are provided in Tables 1 and 3 and some of them will be discussed below.
Folic acid is one of the common molecules used for targeting folate-receptors on cancer cells such as breast and colon cancers and has been used in several recent CaP-based in vivo studies [23, 55, 65]. Folic acid is a small molecule and its conjugation to CaP’s does not increase their hydrodynamic size significantly. Therefore, it is a suitable targeting molecule that can be used for specific delivery of the therapeutic molecules to tumors using CaP nanoparticles. Also, CaP’s can be functionalized with conjugates of folic acid and PEG (i.e., FA-PEG) for increasing their specific delivery to tumors, as a result of synergistic targeting effect of folic acid and steric hinderance of the PEG molecules [65]. Hyaluronic acid (a sugar-based polymer) has also been used for targeted delivery of CaP’s to tumors with CD44 receptors. Negative charge of hyaluronic acid facilitate its electrostatic adsorption to calcium ions (Ca2+) on the surface of CaP’s through a simple mixing reaction. In two recent studies, CaP’s were functionalized by electrostatic adsorption of hyaluronic acid and were used for targeted siRNA delivery to melanoma and lung cancer [59, 64]. A modified procedure has also been developed by Kang et al. [76] for covalent bonding of hyaluronic acid to CaP’s for targeted drug delivery to breast tumors.
Conjugation of antibodies to CaP’s can also be used for their targeted delivery to cancers. For example, Tang et al. [23] conjugated EGFR-specific single chain fragment antibody (ABX-EGF scFv) to lipid-coated CaP’s and used the nanoparticles for specific siRNA delivery to breast tumor in mice. In a separate study, a bispecific antibody (BsAb) was loaded to the surface of lipid-coated CaP’s by non-covalent adsorption and the resulted nanoparticles were used for in vivo siRNA delivery and inhibition of breast tumors [61]. While the extensive list of available antibodies offer different opportunities for targeting various cancers, their major drawback in nanomedicine is their large sizes, which also increases the hydrodynamic size of the nanoparticles inevitably. These larger size nanoparticles have shorter blood circulation half-life and therefore show less uptake by tumors. Unlike antibodies, cancer targeting peptides with smaller sizes can be designed and prepared for specific delivery of the nanoparticles to tumors. For example, Zhang et al. [70] mineralized CaP’s in the presence of a synthetic peptide (A54) and used them for targeted drug delivery to hepatic cancers. Recent studies showed that conjugation of PEG molecules to peptides such as arginyl glycyl aspartic acid (RGD) and urokinase plasminogen activator analogues peptide (uPA) can help increasing the blood circulation half-life and tumor uptake of the CaP’s [60, 68].
2.3. Endocytosis of CaP’s in Cancer Cells
Cell membrane, a flexible lipid bilayer, separates the intracellular milieu from the environment and plays a vital role in internalization of therapeutic molecules such as nucleic acids, proteins and drugs. Since most cancer therapeutic agents have large sizes and are hydrophobic, they cannot cross the cell membrane easily and require nanoscale carriers that can facilitate their intracellular delivery. Among different types of nanoparticles, CaP’s have been extensively used for this purpose, mainly due to their biocompatibility, degradability and facile synthesis methods. However, efficient delivery of these agents to different types of cancer cells requires a thorough understanding of their nanostructures, crystallographic phases, biofunctionalities and the mechanisms by which they get internalized inside each specific type of target cells.
Generally, the uptake pathways of synthetic nanoparticles can be divided into two major categories: endocytic uptake pathway and nonendocytic delivery [89–91]. Endocytosis is defined as the vesicular uptake of the nanoparticles by invagination and pinging of the part of cell membrane [92, 93]. Endocytic routes are divided into several subgroups (Figure 2) including phagocytosis, macropinocytosis, clathrin-mediated endocytosis, caveolae-mediated endocytosis, and nonclathrin- and noncaveolae-mediated endocytosis [94]. Clathrin mechanism is a multi-step endocytosis process, which is activated by binding of ligands on the surface of the nanoparticles to their specific receptors on cell membrane. Also, caveolae endocytosis is characterized as a small invagination of the cell membrane to internalize the nanoparticles [95]. Pinocytosis, on the other hand, refers to the hydrophobic or electrostatic interaction of nanoparticles with the plasma membrane and their internalization following a mechanism similar to uptake of fluid-phases. It should be noted that endocytic mechanisms are not simply limited to internalizing the nanoparticles and include intracellular transport of different types of macromolecules and nutrients. In fact, they are involved in more complex levels of cellular master plan that control and regulate the spatial and temporal dynamics of cell signaling circuitry [96]. Therefore, cancer therapeutic efficacy of the nanoparticles is closely linked to their uptake mechanisms and subsequent biological responses of the targeted cells.
Figure 2.
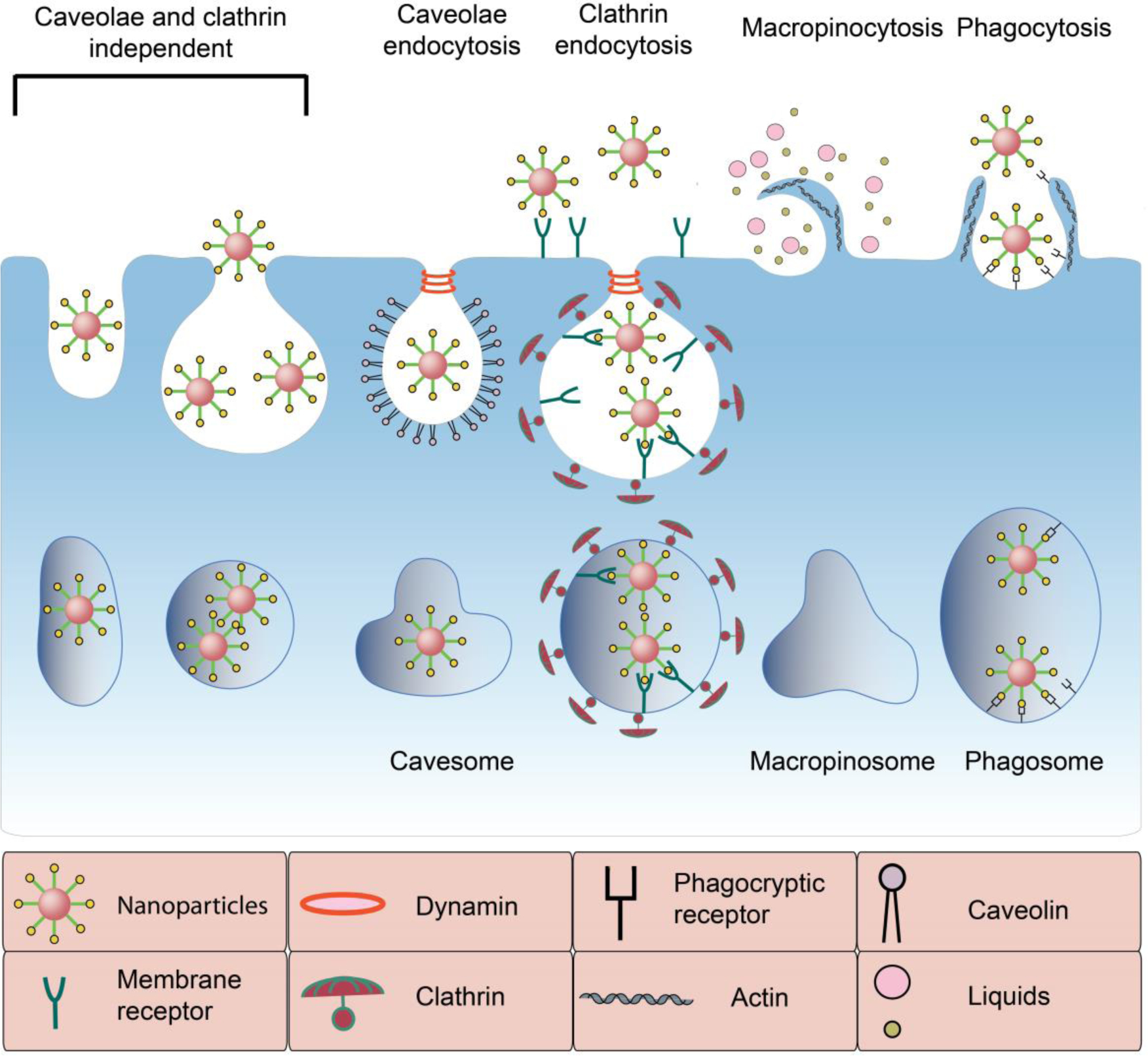
Schematic illustrating different CaP endocytic pathways. Nanoparticles are taken up into cancer cells via different routes including clathrin-dependent or clathrin-independent endocytosis (e.g., macropinocytosis, phagositosis and caveolae-mediated endocytosis). This process is highly complex and over 50 different proteins are participating in it. Intracellular trafficking and subsequent biological response depend on these pathways.
Endocytosis has been reported as the main mechanism for cellular uptake of the CaP’s in different cancer therapeutic studies (Table 1). Through endocytosis pathway macromolecules assembled on the surface of the CaP’s (e.g., coating molecules, targeting peptides or antibodies) interact with the receptors on the plasma membrane of the cancer cells. Various receptors on the cell membrane can be targeted by selection or engineering of functional groups or ligands on the surface of the CaP’s, as listed in Table 1 (also see Section 2.2). High-resolution visualization techniques such as electronic microscopies, have shown that multiple simultaneous mechanisms are often involved in internalization of nanoparticle-based endocytic cargos [97, 98]. For example, clathrin and caveolae-mediated pathways have been identified as the main routes for polymeric [99–101] and lipid-based [99] nano-delivery systems. Most of the cancer therapeutic CaP’s are also coated and functionalized with polymers and lipids and therefore similar mechanisms are usually involved in their endocytosis.
Almost 20 years ago, it was first thought that intracellular trafficking of CaP’s has been only toward the clathrin-mediated endocytosis [102]. However, a decade later, studies by Olton et al. indicated that cellular uptake mechanism of CaP’s might be facilitated by both clathrin- and caveolae-dependent endocytosis [26]. It is currently known that the exact endocytosis mechanisms of the CaP’s depends on their surface coating molecules, size, morphology and type of the targeted cells. For example, Sun et al. [53] used in vitro studies to delineate endocytosis mechanism of the CaP’s (10 nm × 50 nm hydroxyapatite nanorods, without any specific surface coating) in a lung cancer cell line. They inhibited caveolae-mediated pathway using a biochemical agent (methyl β-cyclodextrin) and found that uptake of the CaP’s was blocked, showing their uptake was through the caveolae-mediated mechanism. Conversely, when they blocked the clathrin-mediated pathway using a different inhibiting agent (chlorpromazine), the uptake of the CaP’s did not change, showing that clathrin was not involved in uptake of their rod-shape nanoparticles. Similar assays were used by Zhou et al. [59] to evaluate endocytosis mechanism of spherical-shape hyaluronic acid-coated CaP’s (hydrodynamic size ~ 150 nm) in melanoma. Their results indicated that these CaP’s entered cancer cells through a combination of micropinocytosis, and caveolae- or clathrin-mediated pathways. Qiu et al. [64], however, evaluated endocytosis of almost similar CaP’s (hyaluronic acid-coated, size ~ 170 nm) in a lung cancer cell line and showed that only caveolae- or clathrin-mediated pathways were actively involved in internalization of their nanoparticles, suggesting dependency of the endocytosis mechanism on type of the cells. Later, it was shown that caveolae-mediated mechanism was the main pathway for internalization of smaller size CaP’s (~ 20 nm) that were coated with PEG and a liver cancer targeting peptide (Arginyl glycyl aspartic acid or RGD) [60]. However, a separate study using HeLa (cervical cancer) cells reported clathrin pathway as the major endocytosis mechanism for 140 nm CaP’s that were coated with a co-polymer of PEG and poly(benzoxaborole) [103]. Overall, these examples together suggest that endocytosis mechanism of the CaP’s is dependent on type of the cancer cells, and nanoparticles physiochemical properties such as size, coating molecules, and morphology. Therefore, accurate investigations are necessary to delineate and tune the exact endocytosis pathway for each specific type of CaP’s and targeted cells.
2.4. The Endosomal Escape Phenomenon
After endocytosis, CaP’s tend to be localized inside the vesicles (i.e., early endosomes) with no access to the cytosol. These early endosomes either mature to the late endosomes and fuse with lysosome for degradation or recycle their contents outside the cell membrane (i.e., exocytosis) [104]. This uptake mechanism usually facilitates targeting of the endolysosomal compartments by the bio-agents loaded on CaP’s [105]. However, entrapment inside these vesicles and subsequent degradation of the therapeutic bio-agents by lysosomes is undesirable when targeting of the other cellular organelles such as nucleus or cytoplasm is required. Therefore, escaping from endosome microenvironment is an essential requirement for trafficking of nucleic acids, proteins, drugs and other molecules that function outside the endosome compartment.
Several surface coating strategies have been employed to facilitate CaP’s endosomal escape. One of the most common approaches is based on incorporating fusiogenic lipids such as dioleoylphosphatidylethanolamine (DOPE) on the surface of CaP’s. DOPE has the ability to undergo structural change from a stable lipid bilayer at physiological pH 7 to an inverted hexagonal structure at low pH environments. This conformational transition causes DOPE to fuse with endosomal membrane and destabilizes it [89]. Another well-known method for escaping from endosomal network is by coating CaP’s with polymers that show significant buffering capacity at pH values ranging from 5.2 to 7. For example, polycations such as polyethyleneimine (PEI) mediate this escape through a process called proton-sponge effect [106]. At low endosomal pH, these polymers become more protonated, leading to influx of chloride ions and protons. This process follows by influx of water molecules that eventually induces osmotic swelling and rupture of the endosome membrane, leading to release of its content within the cytoplasm (Figure 3) [107].
Figure 3.
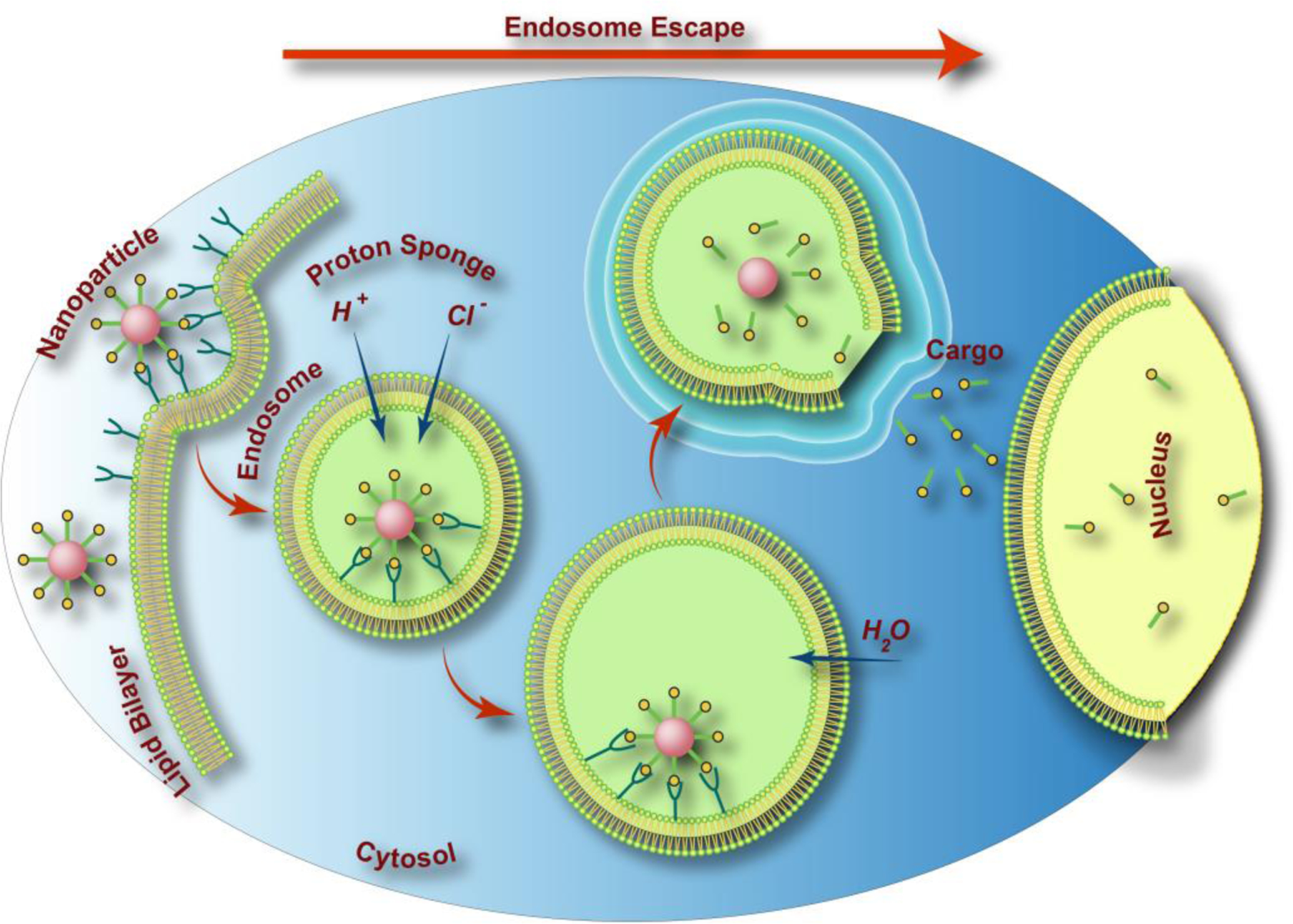
Schematic drawing of CaP’s endosomal escape in cancer cells through the proton-sponge effect. The maturation of endosome to lysosome creates the acidic environment that along with various enzymes degrades the content of lysosome (e.g., CaP nanoparticles). H+ ions diffuse into the endosomes by proton pumps and subsequently for maintaining the charge neutrality Cl− ions enter to this vesicle. The influx of these ions along with influx of water causes the endosomes to swell and rupture, releasing its trapped payload inside the cytosol, a process generally known as endosomal escape.
It is worth mentioning, that the use of PEI coating is usually associated with toxicity that bounds their application. To overcome this issue, Lee et al. [108] proposed new hybrid nanocomposite particles consisting of PEI-PEG (polyethylene glycol) copolymer grafted to nanoparticles. This system showed safe and non-toxic delivery functionality, by inducing a proton-sponge effect and taking the advantage of electrostatic and steric interaction of the polymer palisade. Similar studies showed that coating of CaP nanoparticles with PEI significantly increased their transfection efficiency and protein expression [109]. PEI coating provides electro-steric stabilization and necessary positive charge to the CaP’s and increases their uptake through the electrostatic interaction with negatively charged plasma membrane. However, an optimum concentration of low molecular weight PEI or its copolymers (e.g., PEI-PEG) should be used to avoid the undesirable cytotoxicity [110, 111]. After escaping from endosome environment, the biomolecules loaded on CaP’s should be localized at the target organelles to induce their proposed functions. For instance, nucleic acids such as DNA should reach to nucleus for expression [57], while others such as siRNA can function within cytoplasm [64]. Likewise, cancer therapeutic drugs and proteins have different sites of action and their proper intracellular trafficking is required for effective treatment [16, 112, 113]. Delivery of nucleic acids (i.e., DNA and siRNA) and chemotherapeutic drugs by CaP’s will be discussed more in Sections 3.1 and 3.2.
In addition to the mechanisms discussed above, CaP’s that are fully or partially degradable in acidic pH of the endosomes are also ideal candidates to enable endosomal escape as a result of releasing calcium ions and other lysosomolytical molecules. However, this mechanism is highly dependent on solubility of the CaP’s at lower pH values, which is correlated to their crystallographic structure (Table 2). Different in vitro studies have been used to simulate endosomal degradation (partial or full degradation) of CaP’s and subsequent release of their cargos. For example, Bisht et al. [114] studied the pH-dependent release profile of plasmid DNA (pSVβgal) from hydroxyapatite CaP’s that were degradable at low pH values. They used agarose gel electrophoresis to quantify pSVβgal release at two different buffers with pH values of 5 and 8 during various incubation intervals (0, 1, 2, 4 and 24h). No pSVβgal was released from CaP’s at pH=5 and at zero hour. However, consistentpSVβgal release was observed at later incubation times (2, 4 and 24h). There was no indication of pSVβgalrelease from these particles even after 24h, when the pH of the incubating buffer was increased to 8. This indicates pH-dependency of the pSVβgal release as a result of CaP degradation.
CaP’s (Ca:P ~ 0.98) encapsulating different organic fluorophores were also developed and used to verify the effect of endosomal pH on dissolution rate of these nanoparticles. Figure 4 shows the diffusion coefficients and hydrodynamic radii of these nanoparticles at pH values of 4 and 7. The hydrodynamic sizes of the CaP’s incubated at pH 4 was almost similar to the values observed for a solution containing free Cy3 fluorophore molecules, indicating that CaP nanoparticles were fully dissolved at such a low pH. In addition, the diffusion coefficient shifted to significantly higher values when the pH was decreased from 7 to 4, further confirming CaP’s dissolution and subsequent release of their encapsulated contents within low pH environments, similar to that of endolysosomes [115]. In vitro evaluation using bovine aortic endothelial cells also indicated that cells were effectively stained with these nanoparticles, which further validated the dissolution of nanoparticles in the endosome. Several other studies used similar methods and reported full or partial dissolution of CaP-based nanoparticles (e.g., amorphous calcium phosphates [81, 116], hydroxyapatite [70, 117] or calcium-deficient hydroxyapatite [118]) at lower pH values for controlled release of different drugs (e.g., doxorubicin [70, 119] and cisplatin [117]) or proteins (e.g., bovine serum albumin or BSA, and green fluorescent protein or GFP [116].
Figure 4.
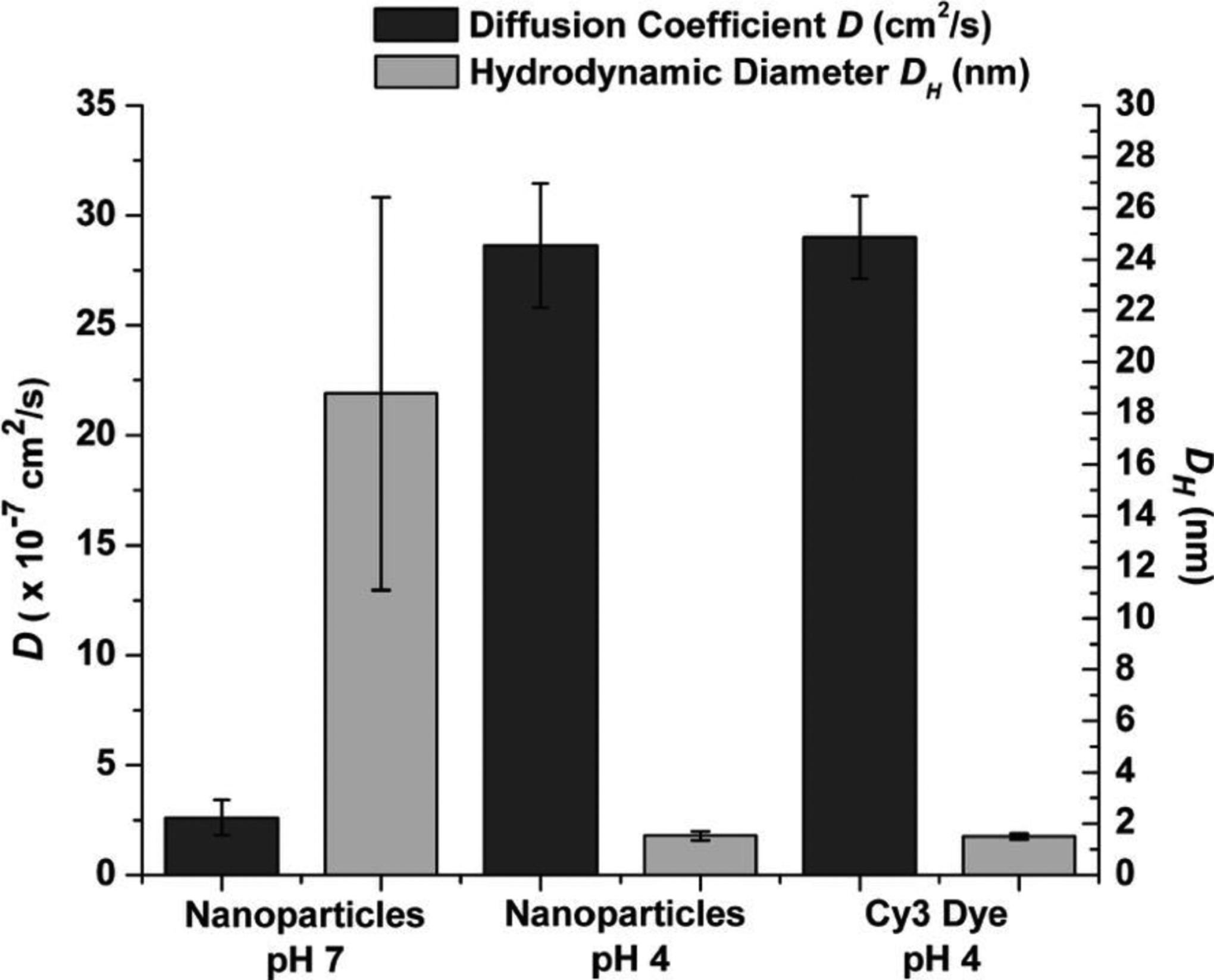
Hydrodynamic size (gray) and diffusion coefficient D (black) of nanoparticles at pH 4 and pH 7 and free Cy3 dye at pH 4. Larger hydrodynamic size at pH 7 indicates the nanoparticles specific size distribution. In addition, diffusion coefficients and hydrodynamic size of nanoparticles and free Cy3 dye at pH 4 are in the same range, suggesting dissolution of the nanoparticles at pH 4. *p<0.0001 for pH 4 vs. pH 7. (Adapted with permission from ref. [115]. Copyright 2008 American Chemical Society)
Overall, among different types of CaP’s, hydroxyapatite-based nanoparticles are preferred candidates for intracellular delivery of the therapeutics through endosomal escape pathway. This is mainly because of their less degradability at physiological pH, compared to their relatively higher degradability at acidic pH of the endosomes (See Section 2.1 for details). Hydroxyapatite is one of the most stable phases of CaP’s at physiological pH. This characteristic facilitates its synthesis and drug-loading, as a wide range of drug molecules are only stable at physiological pH. Also, less degradability at physiological pH helps to prolong shelf-life of the hydroxyapatite nanoparticles before their in vivo administration. These properties enable hydroxyapatite nanoparticles to protect therapeutic agents at physiological pH conditions and release them in the targeted intracellular organelle.
2.5. Releasing Calcium Ions: An Implication for Cancer Therapy
The normal concentration of calcium and phosphate ions in the cells and blood are in the range of millimolar [121]. It has been shown that any change in concentration of calcium ions can trigger a variety of physiological responses and eventually lead to apoptosis [122–124]. Accordingly, several studies indicated the potential toxicity and inhibition of proliferation in various cell lines, due to the different characteristics of CaP’s [125–127]. CaP nanoparticles have high surface area to volume ratio, which differs them from the bulk material that they are derived from. Due to this property their cellular toxicity is different from their bulk counterparts.
Motskin et al. [120] used human macrophage cell lines to evaluate and compare in vitro cytotoxicity of different hydroxyapatite nanoparticles with spray dried calcium phosphate microparticles. They showed that preparation methods significantly influence the nanoparticles physiochemical properties (Figure 5) and therefore their toxicity level. Nanoparticles generated by sol-gel method showed the greatest toxicity. In addition, microparticles that were prepared through spray drying showed significantly lower cytotoxicity, due to their less macrophage uptake. As it can be seen in Figure 6, the toxicity level of these different types of nanoparticles was associated with the amount of uptake, in which the higher the amount of internalized particles, the greater the cytotoxicity. It is reported that this toxicity enhancement is due to release of calcium ions from these internalized particles and subsequent interference with the intracellular calcium ions homeostasis [58].
Figure 5.
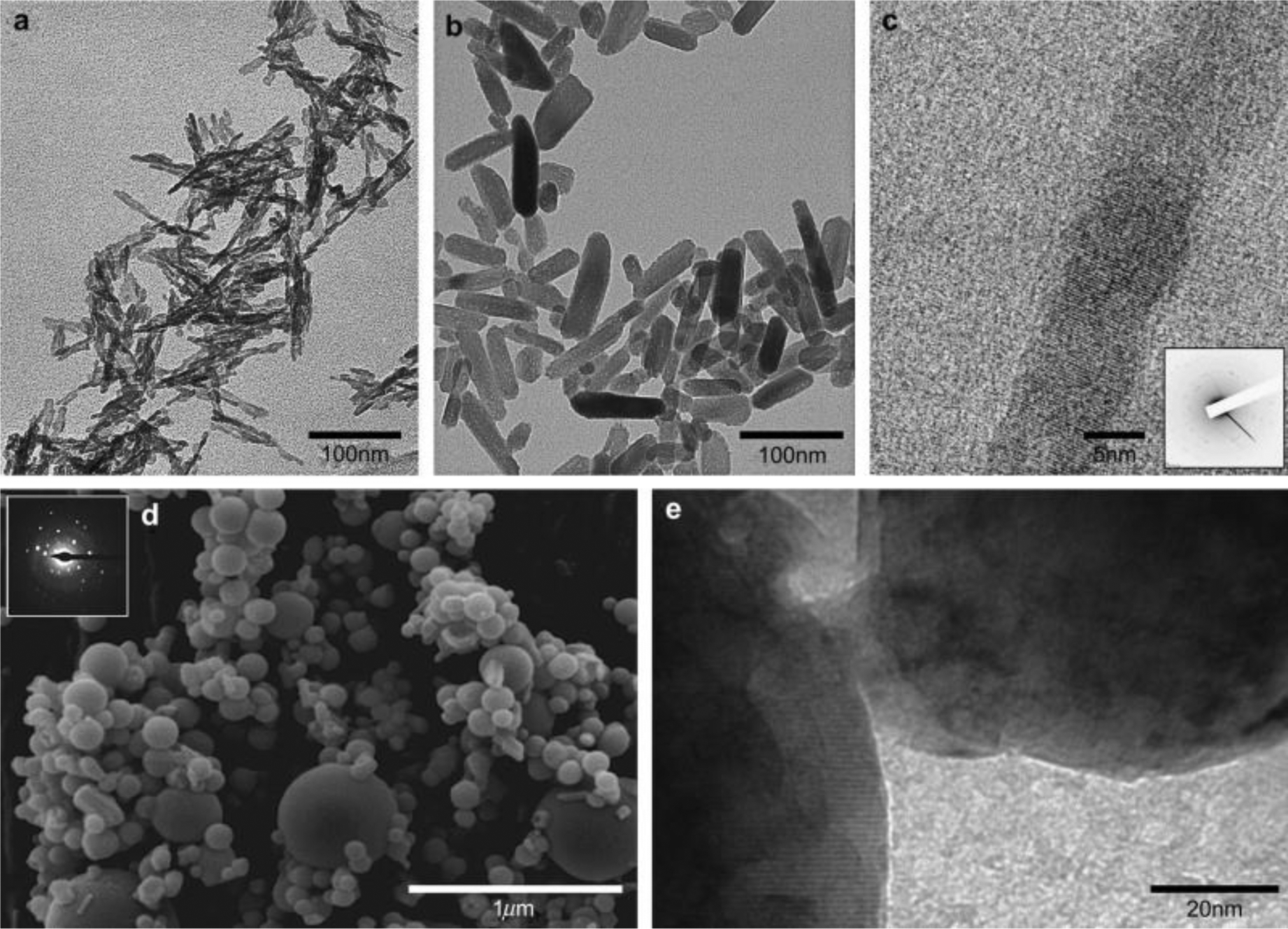
(a) TEM micrograph of as precipitated hydroxyapatite (HA) calcium phosphate-based nanoparticles; (b) The gel form of HA; (c) High resolution TEM of the gel and selected area electron diffraction (SAED) pattern of a single nanoparticle; (d) TEM micrograph and SAED diffraction pattern (inset) of commercial particles that were used for comparison; (e) High resolution TEM image of the commercial particles, presenting the smooth surface of spherical particles. (Reproduced with permission from ref. [120]. Copyright 2009 Elsevier)
Figure 6.
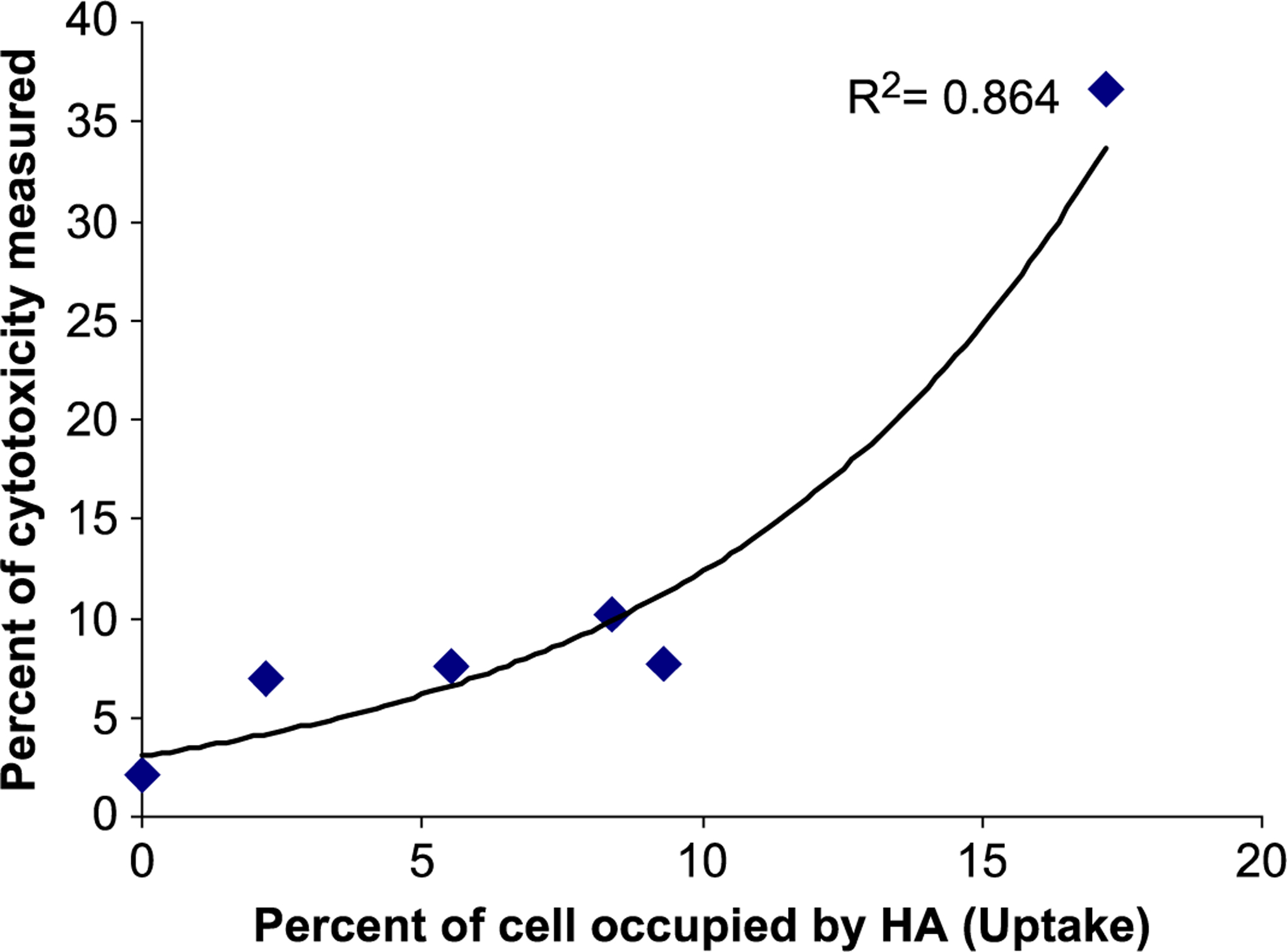
Correlation between uptake of CaP-based nanoparticles (HA) and associated cytotoxicity. P<0.005, Correlation co-efficient −0.929, R2 =0.864. (Reproduced with permission from ref. [120]. Copyright 2009 Elsevier)
In a separate study, Neumann et al. [128] used standard calcium phosphate transfection method (addition of a mixture of DNA and Ca2+ solutions to the cells [129, 130]) and two types of synthesized CaP/DNA nanoparticles to compare variation of the intracellular calcium level and related toxicity in T24 cells (human bladder carcinoma) as a result of transfection. Their observations indicated that when standard calcium method was used, intracellular calcium level rapidly raised to more than one hundred times higher than the normal level, whereas the CaP/DNA nanoparticles only resulted in minor changes (Figure 7). Such a low calcium disturbance was associated with the moderately small nanoparticle-mediated calcium uptake and efficient calcium extrusion mechanism which helped to balance the intracellular calcium concentration. In contrast, the irregular calcium spiking in response to standard calcium phosphate transfection was due to rapid dissolution of large particles after endocytosis. The uptake of these particles dramatically increased the intracellular calcium concentration and this excessive amount of calcium ions could not be pumped out of cells instantly. Instead, they were gradually extruded into the cell culture medium, up to 48 h after transfecting the cells. In addition, it was shown that transfection using standard calcium phosphate method could increase the overall concentration of calcium ions in the cells, whereas calcium variation because of transfecting with CaP/DNA nanoparticles was only limited to subcellular sections.
Figure 7.
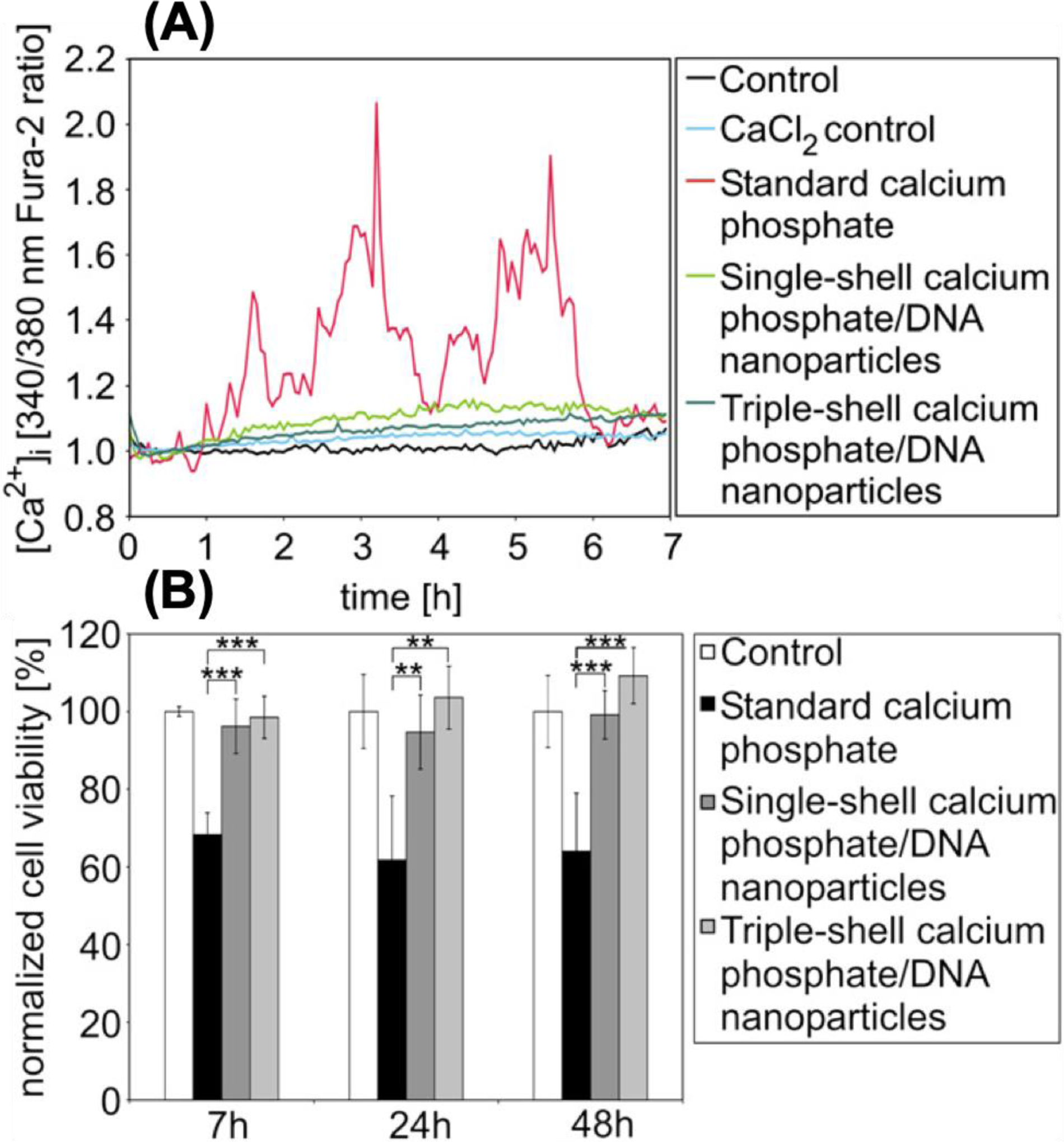
Variation of intracellular calcium level (A) and cell viability results (B) obtained from transfected T24 cells at different incubation times. Cells were transfected with single-shell and triple-shell CaP nanoparticles and results were compared with standard calcium phosphate and control samples (PBS). Fura-2 (shown in A) is a sensitive indicator dye for measuring intracellular calcium. It gets excited at 340 and 380 nm and the ratio of the emissions at these wavelengths is a representative of intracellular calcium [131]. For cell viability tests, mean values of absorption were defined as 100% for control cells during the MTT assay for cell viability quantifications. Graph bars shown in (B) represent four independent experiments. (**: p <0.01, ***: p <0.001). (Reprinted with permission from ref. [128]. Copyright 2009 Elsevier)
It is worth noting that different cells have various calcium sensitivities and each type of CaP nanoparticle has its own specific degradation and Ca2+ release kinetics [132]. Recently, Wu et al. [69] showed that intracellular Ca2+ release depends on CaP’s size, morphology, crystallinity, specific surface area (SSA) and synthesis method. First, they generated five types of hydroxyapatite nanoparticles with varying characteristics (Figures 8.A and B) using different synthesis methods (i.e., different combination of air drying, hydrothermal treatment and calcination). Then, using in vitro and in vivo studies, they evaluated efficacy of these different CaP’s for melanoma treatment, based on their Ca2+ release (Figure 8.C). Their in vitro studies showed that, granular nanoparticles with smaller size, higher SSA, and lower crystallinity (HA-A) were the most effective nanoparticles in inhibiting the viability of A375 melanoma cells (~ 34.90% viability at day 3). This was mainly because of higher degradation rate and Ca2+ release from these relatively amorphous nanoparticles, as discussed in Section 2.1. Also, while all samples were able to induce apoptosis, HA-A and HA-B showed highest apoptosis rates (20.10% for HA-A and 19.41% for HA-B, at day 3). Interestingly, none of these nanoparticles caused any apoptosis in normal human epidermal fibroblasts (HSF) after incubation in similar conditions, suggesting the tumor specificity of their therapeutic effects. Their mice studies showed that HA-A and HA-C were more effective in limiting the growth of melanoma tumor xenografts, compared with other samples, and HA-A therapeutic efficacy was more than HA-C (49.1% vs. 34.0 at day 23).
Figure 8.
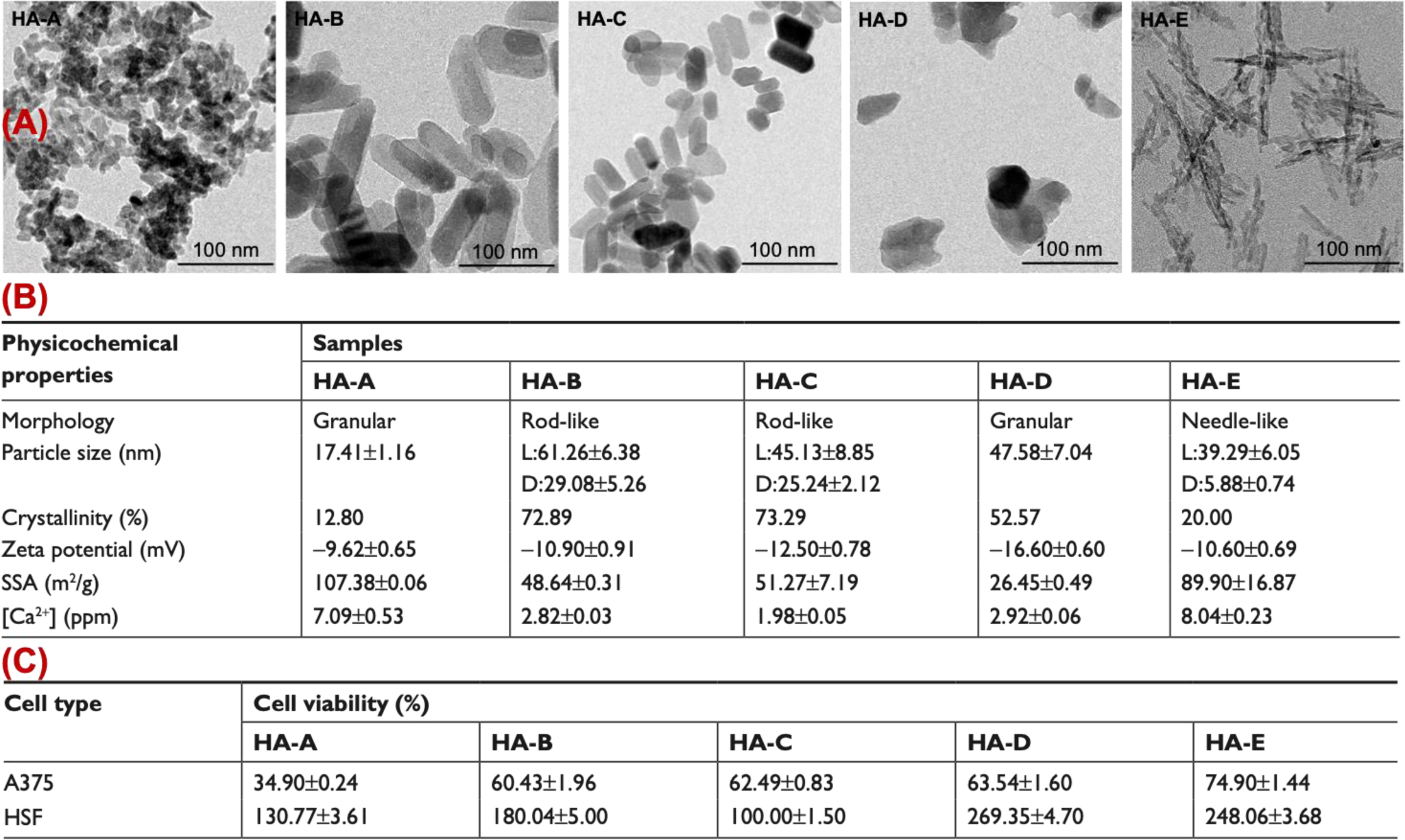
Effect of CaP’s morphology, size, crystallinity, zeta potential, and specific surface area (SSA) on viability of melanoma (A375) and normal human epidermal fibroblasts (HSF) cells. (A) Transmission electronic microscopy images of five different types of hydroxyapatites shown as HA-A, HA-B, HA-C, HA-D and HA-E. (B) Physiochemical properties and (C) cell viability efficacy of these five batches. (Adopted with permission from ref. [69]. Copyright 2019 DovePress)
Several other studies have reported tumor-specificity of this toxicity effect. For example, Sun et al. [53], synthesized hydroxyapatite nanorods (width ~10 nm and length ~ 50 nm, without any surface functionalization or drug loading) and incubated them with human lung cancer (A459) and normal bronchial epithelial cells (16HBE, control samples). Their results showed that these nanorods could selectively cause apoptosis in cancer cells, without any noticeable effect on epithelial cells. This was because degradation of nanorods consistently increased the amount of Ca2+ in cancer cells, while the Ca2+ elevation in normal control cells was transient. Their mice studies resulted in 40% tumor growth inhibition, confirming the in vitro results. Drug-loaded CaP’s can also be designed for simultaneous release of Ca2+ and chemotherapeutic drugs, for enhanced cancer therapy. Xiaoyu et al. [54] generated Doxorubicin-loaded CaP’s (spherical, 30–40 nm, clusters of hydroxyapatite nanoparticles with low crystallinity) that could degrade after cell internalization, releasing the drug and increasing the intracellular Ca2+ simultaneously for more effective therapy of gastric adenocarcinoma.
Intracellular Ca2+ release can also be enhanced using external stimuli, such as laser irradiation. Recently, Xu et al. [58] synthesized spherical nanoparticles that were coated with a CuS shell (final size ~ 100–150 nm). CuS shell was designed to generate heat in response to near infrared light (808 nm) for enhancing degradation of the CaP’s. Their in vitro and in vivo studies showed that intracellular degradation of the CaP nanoparticles (so called Ca2+ nanogenerators) inside the endosomes and release of excessive Ca2+ ions in cytoplasm could disrupt the mitochondrial Ca2+ homeostasis resulting in apoptosis of the breast cancer cells (Figure 9). This is a promising fundamental study that can possibly open up new prospects for light-triggered cancer therapy using degradable CaP nanoparticles.
Figure 9.
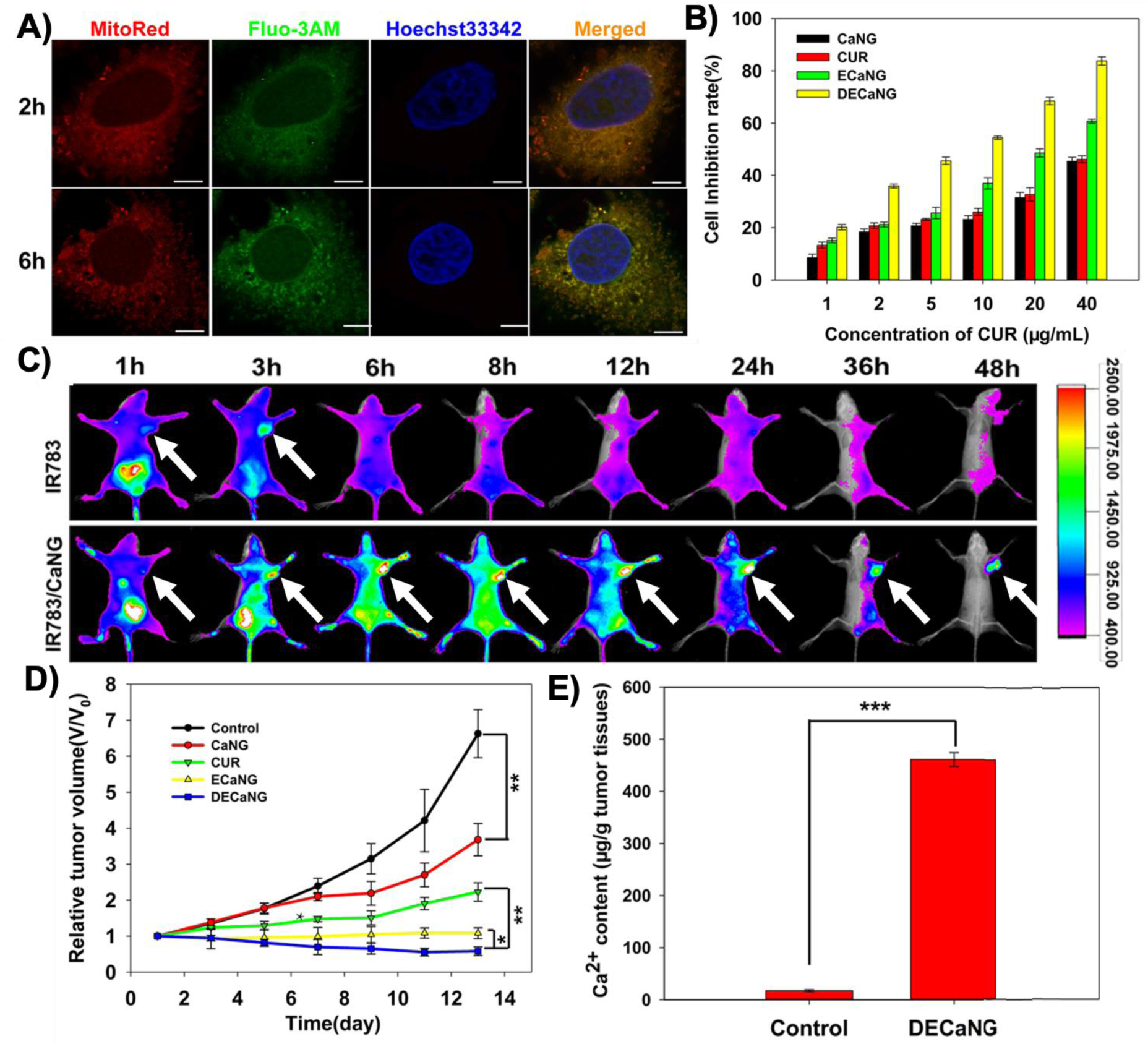
(A) Confocal fluorescent images of the MCF-7 breast cancer cells showing distribution of Ca2+ (stained with Fluo-3AM) released from degraded CaP nanoparticles (so called calcium nanogenerators or CaNG) in mitochondria (stained with MitoRed) at different incubation times (2 and 6 h). Hoechst33342 was used for staining the nucleus. (Scale bars: 7.5 μm) (B) Degradation of the CaP nanoparticles disrupted the mitochondrial Ca2+ homeostasis and resulted in enhanced apoptosis (growth inhibition) of the cancer cells. (C) In vivo fluorescent imaging at different post-injection times indicated these nanoparticles could accumulate in subcutaneous tumors (shown by white arrows), when they were tagged with IR783 fluorescent molecules, compared with free IR783 molecules. (D) Mitochondrial disruption of the Ca2+ homeostasis helped to inhibit tumor growth and enhanced the therapeutic effects, especially when combined with photothermal therapy. (E) Significantly higher amount of Ca2+ in tumor tissues obtained from the mice treated with DECaNG compared with control mice only injected with saline. CaNG: the synthetic CaP nanoparticles; CUR: curcumin, an agent to enhance release of Ca2+ from endosomes to cytoplasm; ECaNG: enhanced Ca2+ nanogenerators or CUR-loaded CaNGs;DECaNG: dual enhanced Ca2+ nanogenerator, CaNG loaded with both CUR and a fluorescent molecule for simultaneous Ca2+ release and photothermal therapy effects. (Adopted with permission from ref. [58]. Copyright 2018 American Chemical Society)
3. Delivery of Therapeutics to Tumors Using Degradable CaP Biominerals
3.1. Nucleic Acids
The ability to apply gene therapy (e.g., introducing a therapeutic DNA into the nucleus of cells) holds promise for treatment of wide variety of acquired and inherited diseases, including tumors. Numerous nano-delivery systems have been developed to introduce desired genes into target cancer cells. A gene delivery vector must demonstrate biocompatibility, stability, diminished or no toxicity and sustained-released kinetics [133–137]. Among different types of nonviral vectors, calcium phosphate-based gene delivery systems have shown promising in vitro and in vivo results which makes them one of the best candidates for clinical translation [15, 138, 139]. As mentioned earlier, co-precipitation of nucleic acids (e.g., DNA and siRNA) and CaP is a well-established and straightforward method for preparing a composite delivery vehicle. The reason for good adherence of nucleic acids to calcium phosphate is thought to be due to the electrostatic affinity of positively charged calcium ions in CaP to the negatively charged, phosphate groups in backbone of nucleic acids [22, 140]. Furthermore, addition of positively charged moieties such as amino groups to the surface of the CaP nanoparticles has been reported as an effective method for higher nucleic acid loading and improving transfection [141, 142]. Also, synthesis of the nucleic acid-loaded CaP’s can be easily modified to incorporate both nucleic acid and drugs molecules into CaP’s for simultaneous transfection and cancer chemotherapy [143, 144].
Despite safety and efficiency of CaP/nucleic acid nanocarriers, this technique suffers from limited reproducibility and difficulty in controlling transfection parameters, which is related to the nanoparticles physical and structural properties, such as size, morphology, crystallinity and composition [34, 47, 145]. Furthermore, studies have shown that transfection efficiency not only changes with these physiochemical properties, but also depends on the ability of nano-delivery systems to effectively encapsulate and protect the nucleic acid molecules from enzymatic attacks either inside the bloodstream or cells. Different organic and inorganic additives have been incorporated into CaP’s to preserve their small size and protect them from enzymatic attacks. For instance, it was shown that addition of magnesium ions to CaP mineralization solution suppresses the crystal growth by creating distortions in the crystal structure of the CaP’s, which results in improving their transfection efficiency [48]. In addition studies have shown that incorporation of polymers such as block-copolymers of poly(ethylene glycol) (PEG) and poly(aspartic acid) (PAA) enhances the transfection efficiency [50]. It is known that PEG is an amphiphilic and biocompatible polymer that confers the colloidal stability of CaP nanoparticles by steric effect [117]. This polymer is also well known for its stealth property when applied in vivo [146, 147].
Welzel et al. [148, 149] reported a simple in situ precipitation method to prepare well-defined CaP/DNA nanoparticles with CaP core and a DNA shell. This method showed better control over size tuning, resulting in relatively higher transfection efficiency and stability of the CaP’s in biological media. Despite these advantages, it was found that transfection efficiency of such core-shell system could not be further amplified, possibly due to the degradation of DNA molecules inside the lysosome compartment (before they reach the nucleus). Therefore, it is required to protect the DNA from such degradation and facilitate its transport into the nucleus. To achieve this goal, it was suggested to add an external layer of CaP (double-shell in Figure 10) to the surface of nanoparticles [130]. Since these precipitates were susceptible to agglomeration, another layer of DNA (triple-shell in Figure 10) was also added to impart the electrostatic colloidal stability. Alternative molecules such as PEI [150, 151] or its derivatives (e.g., PEI-cholesterol [152]) were also used as the third layer for electrostatic stabilization of these nanoparticles, but additional considerations are required to monitor toxicity and degradation pathway of the PEI layer. The transfection efficiency of these nanocarriers has been tested in different in vitro studies using various cell lines, such as human cervical carcinoma (Hela), T-HUVEC and leukocyte tyrosine kinase (LTK). The in vitro results indicated that the transfection efficiency of triple-shell nanoparticles is significantly higher than single-shell approach in all tested cell lines, confirming that such a simple additional step can protect DNA molecules from endolysosomal enzymatic attacks.
Figure 10.
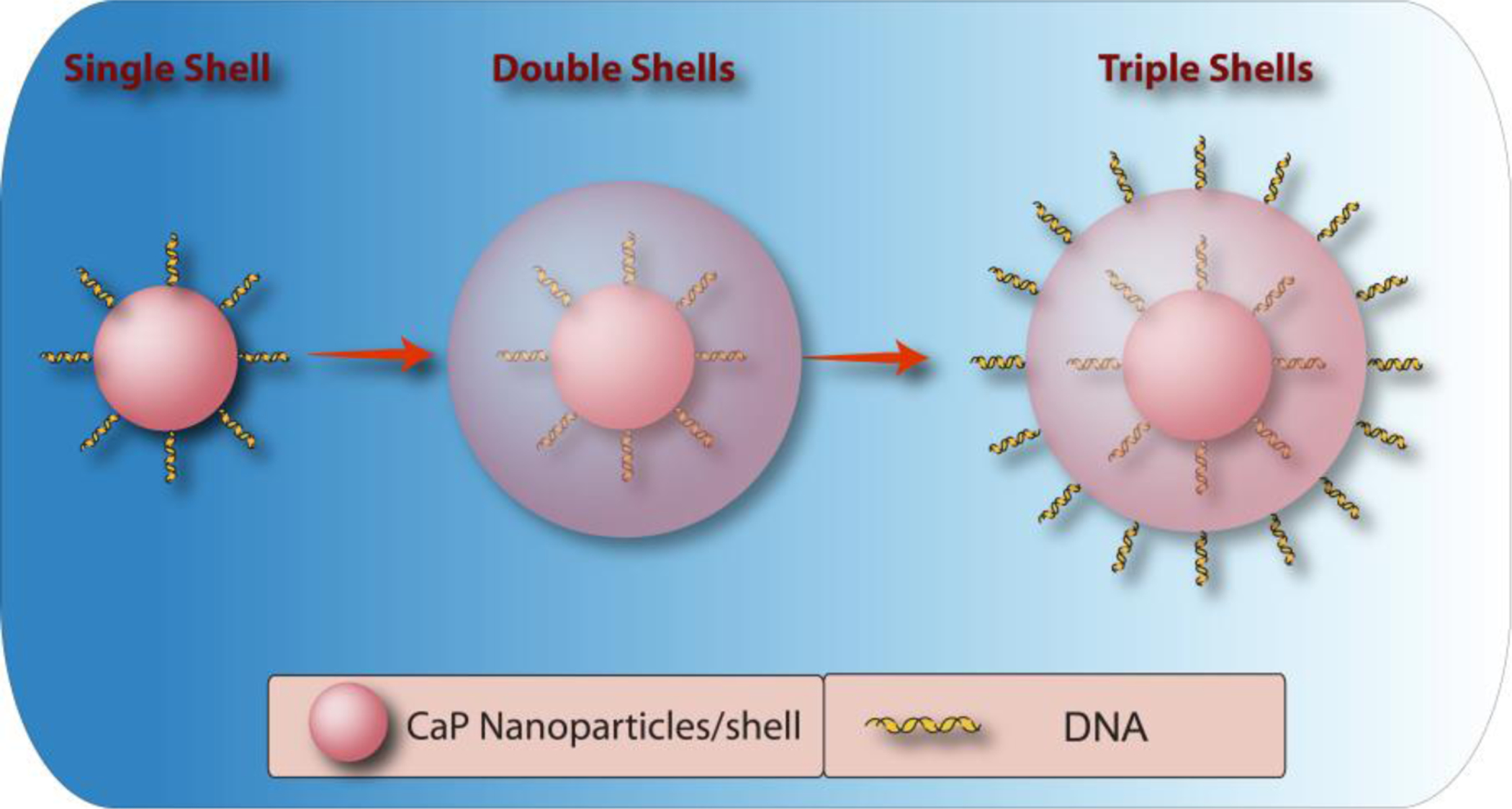
Schematic illustration of triple-shell CaP nanoparticles incorporating DNA. First DNA coated CaP nanoparticles are synthesized, followed by mineralization of an additional CaP shell. Finally the CaP coated nanoparticles are coated with another DNA layer to prevent their aggregation in biological media.
Likewise, CaP nanoparticles can be stabilized with an outer layer of small-interfering RNA (siRNA). Similarly, triple-shell design resulted in highest gene silencing efficiency, due to the protection of siRNA molecules from intracellular degradation [153]. In addition, multi-shell nanoparticles with a PEI layer demonstrated higher siRNA transfection efficiency in three different cancer cell lines (T24, NIH3T3 and Hela), when compared with nanoparticles without PEI [110]. Recent studies showed that other hydrophilic polymers (e.g., alendronate-hyaluronan graft polymer [64]), natural cationic molecules (e.g., lipids [23, 61]) and amino acids (i.e., arginine molecules [154]) can also help stabilizing single core CaP nanoparticles and adsorb siRNA molecules for effective in vitro and in vivocancer therapy, based on endosomal escape of the nanoparticles and releasing siRNA into cytoplasm after degradation. Addition of targeting molecules (e.g., folic acid for breast cancer[23], galactose moieties for hepatocellular carcinoma [62], hyaluronic acid for melanoma [59], and arginylglycylaspartic acid (RGD) for bladder cancer [155]) has also been used for specific in vitro and in vivo siRNA delivery using CaP’s.
CaP-based nanostructure coatings, fabricated from biomimetic solutions (e.g., simulated body fluid (SBF)), can also be used for enhanced transfection. This technique was first proposed by Kokubo et al. more than three decades ago [156]. Although hydroxyapatite was the primary type of CaP’s used for this approach, other types of CaP’s with different crystallographic phases, morphologies and structures have also been prepared by adjusting the synthesis parameters such as pH, ion concentration and temperature. Results indicated that physiochemical properties of these coatings have significant influence on transfection efficacy in different cell lines. For example, Shen et al. demonstrated the co-precipitation of DNA with SBF solutions of different ion content and concentrations onto the cell-culture surfaces [157]. The CaP coating layers had different nanostructures and Ca:P ratios and were able to support cell attachment and proliferation. In addition, the dissolution of CaP layer provided high concentration of DNA in the vicinity of the cultured cells. The mineral stability and subsequent DNA release rate was correlated to different structures and compositions of the precipitates. Since then, many studies have been conducted to evaluate dissolution of these minerals and their effects on gene transfection efficiency in various cell types [158, 159].
Different techniques are still under progress to improve the transfection efficiency of CaP nanoparticles. For example, lipid-coated CaP nanoparticles incorporating siRNA molecules were developed using a water-in-oil microemulsion method (Figure 11) [23, 160]. In this system, the core of liposome-polycation-DNA (LPD) was replaced by acid-sensitive CaP [160]. The resulted formulation was called liposome/calcium/phosphate (LCP). The dissolution of CaP core in the acidic environment of endosomes increased the osmotic pressure. This increased pressure resulted in swelling and disruption of endosomes, followed by releasing the encapsulated siRNA into cytoplasm. Modification of these LCP nanoparticles with PEG resulted in about 70 and 50% gene silencing efficacy in cultured (in vitro) and xenografted (mice) H460 tumor cells, respectively.
Figure 11.
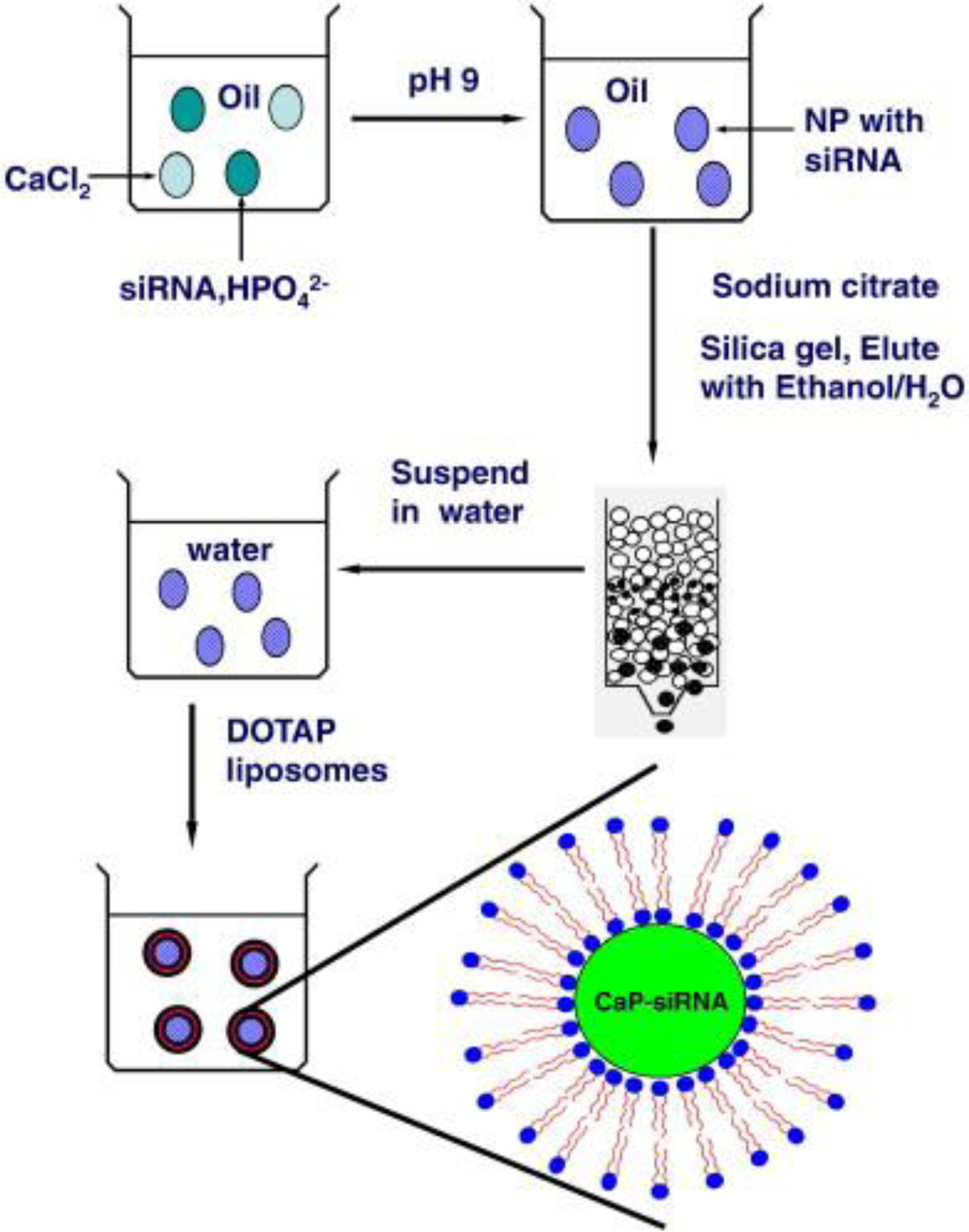
Schematic showing a typical method used for preparation of siRNA-loaded lipid/calcium/phosphate nanoparticles (LCP NPs). (Adopted with permission from ref. [160]. Copyright 2010 Elsevier)
For in vivo nucleic acid delivery applications, the CaP design criteria are even more demanding. For example, it is required to prepare the CaP nanoparticles that are able to efficiently incorporate adequate amount of nucleic acids and protect them in bloodstream. Zhang et al. [161], designed a novel hybrid micelle of siRNA and CaP’s, to address these two important requirements. In this system, siRNA and PEG were conjugated viaan environment-sensitive disulfide linkage. This complex was further modified with CaP’s to form hybrid micelles of PEG-SS-siRNA/CaP. The evaluation of physiochemical properties of these hybrid CaP’s showed that they had a spherical core-shell morphology with a particle size ranging from 90 to 120 nm (Figure 12). The disulfide bond could break-down in the acidic pH of the endosomes and selectively cleaved and detached the PEG protecting layer from the nanoparticles. This cleavage resulted in destabilization and dissolution of CaP’s and subsequent release of the siRNA into cytosol. The amphiphilic PEG layer also helped to improve colloidal stability and stealth property of the nanoparticles. Cellular uptake of these hybrid micelles was evaluated in vitro using Huh-7 cells. The results revealed significantly higher cellular uptake and subsequent gene knockdown efficiency, indicating promising characteristics of this PEGylated CaP-based nano-delivery system for in vivo applications.
Figure 12. (A).
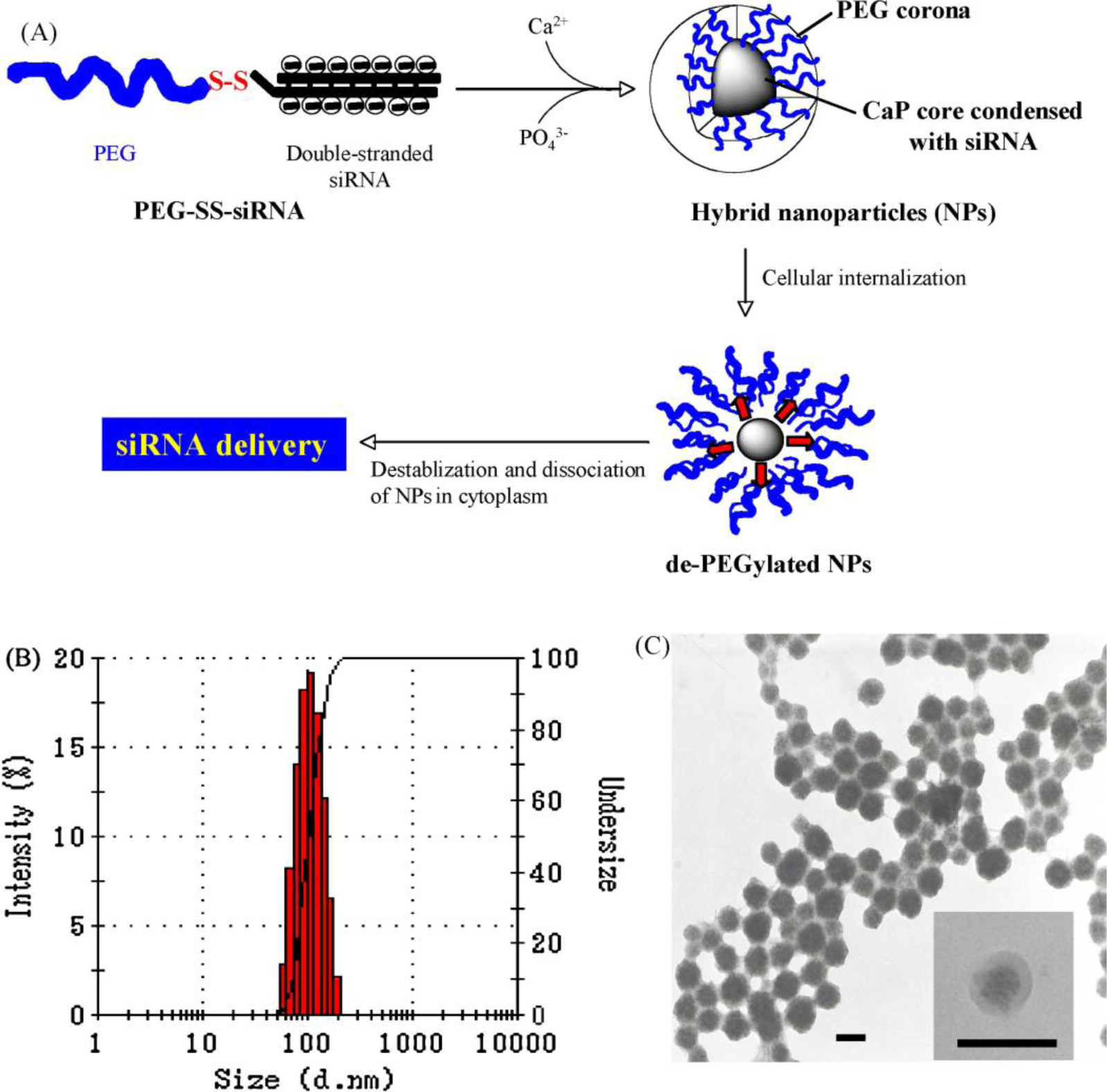
Schematic showing the chemical design of the PEG–SS–siRNA/CaP nanoparticle; (B) Hydrodynamic size distribution and (C) TEM micrographs of the synthesized PEG–SS–siRNA/CaP nanoparticle; (TEM scale bar: 100 nm). (Reprinted with permission from ref. [161]. Copyright 2009 WILEY)
A recent study by Huang et al. [62] showed efficacy of lipid/calcium/phosphate nanoparticles (LCP NPs) for preventing the tumor growth by releasing VEGF (vascular endothelial growth factor) siRNA to orthotopic hepatocellular carcinoma (HCC) tumors in murine liver. LCP NPs were loaded with siRNA and then eight different types of galactoside derivatives were conjugated to them for targeting asialoglycoprotein receptors (ASGPR) on the HCC cells. In vitro and in vivo tests demonstrated that β-D-galactoside derivative had the highest HCC targeting efficiency. Tail vein injected LCP NPs were accumulated in HCC tumor and degraded in acidic environment of the lysosomes, releasing VEGF siRNA to the cytosol. siRNA downregulated VEGF expression in tumor cells and prevented tumor growth by an anti-angiogensis mechanism (Figure 13).
Figure 13.
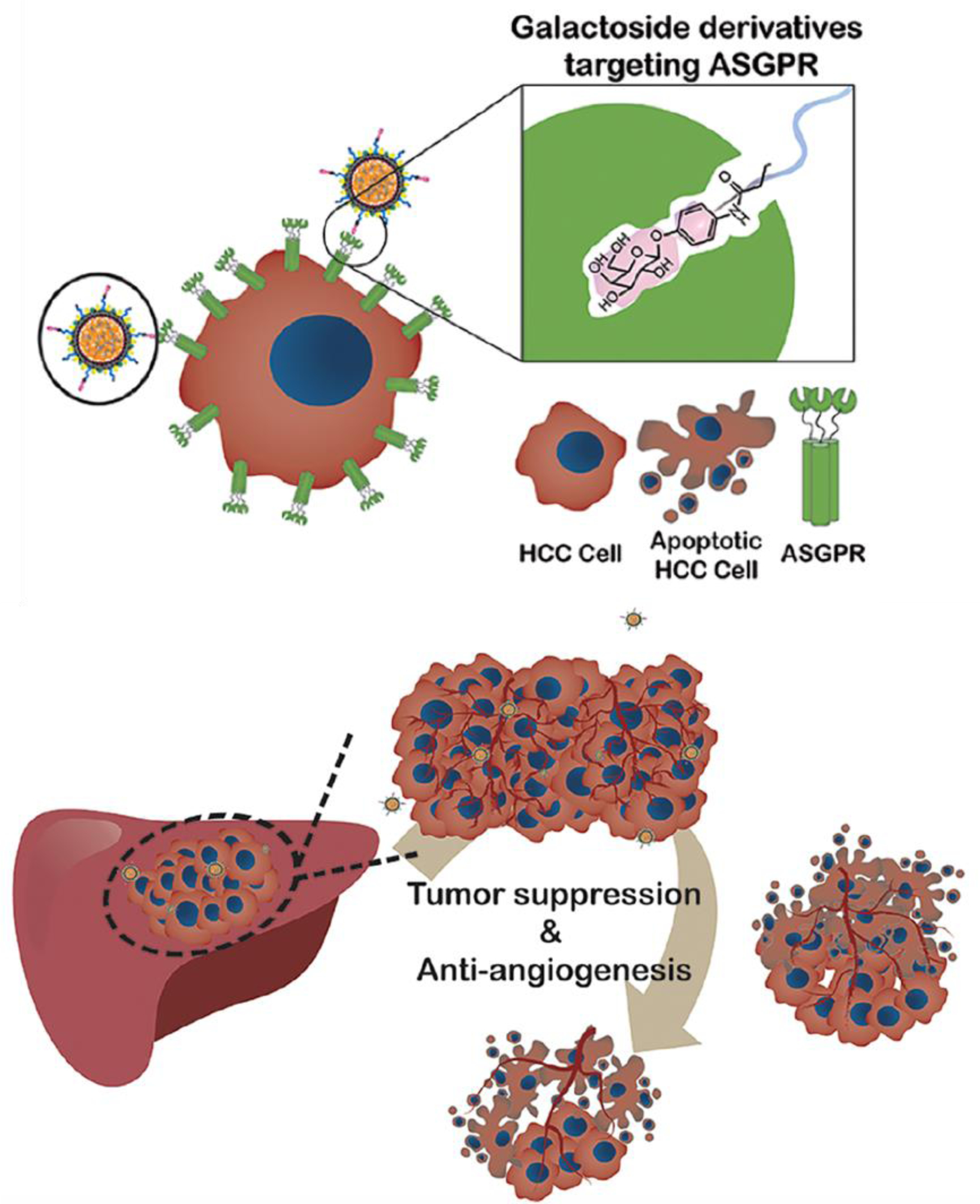
(A) Targeted delivery of VEGF (vascular endothelial growth factor) siRNA to murine orthotopic hepatocellular carcinoma (HCC) tumors in liver, using lipid/calcium/phosphate nanoparticles (LCP NPs) as carriers. β-D-galactoside was conjugated to the LCP NPs for targeting the asialoglycoprotein receptors (ASGPR) on the membrane of the HCC cells. LCP NPs were degraded in low pH environment of the HCC lysosomes and released VEGF siRNA that downregulated expression of the VEGF in HCC cells and inhibited tumor growth by anti-angiogenic effect. (Adapted with permission from ref [62]. Copyright 2018 American Chemical Society)
3.2. Drugs
The ability of the cancer chemotherapy drugs to induce designated therapeutic functions depends on their successful delivery to the tumor tissues, followed by their effective internalization into the target organelles such as nucleus or cytoplasm. However, most cancer drugs are fragile, impermeable and have undesirable biodistributions [162–164]. Therefore, they require a carrier system (e.g., nanoparticles) that can protect them from the harsh extracellular environments and release them in a sustained manner inside targeted cancer tissue [165, 166]. CaP’s have been a subject of increasing attention for cancer drug delivery due to their unique physiochemical characteristics such as controlled synthesis, pH-dependent degradability and high capacity to encapsulate different types of drugs.
Several strategies have been reported for loading cancer drugs into CaP’s, depending on size and water-solubility level of the desired drug and the targeted cancers (Table 1). One of the commonly used approaches is based on loading drugs into different types of micelles that are designed to act as templates for mineralization of CaP nano-shells. For example, Wang et al. [60], used a multi-step procedure to synthesize CaP’s for in vivodrug delivery to hepatocellular carcinoma. First, they linked hydrophilic polyethylene glycol (PEG) to hydrophobic phosphatidylserine (PS) to generate micelles by self-assembly of these molecules. RGD peptide (Arginyl glycyl aspartic acid) was also conjugated to these molecules (PS-PEG-RGD) for cancer targeting. As it is shown in Figure 14, Mitoxantrone (MIT, cancer drug) was trapped inside these micelles during their formation (Step 1). Then, CaP shells were formed around these micelles after addition of Ca2+ and phosphate ion precursors (Step 2). Finally, a solution containing Verapamil (VER, a drug-resistance inhibitor drug) was added to MIT-loaded CaP’s and the mixture was sonicated to help VER adsorption to the surface of nanoparticles (Step 3).
Figure 14.
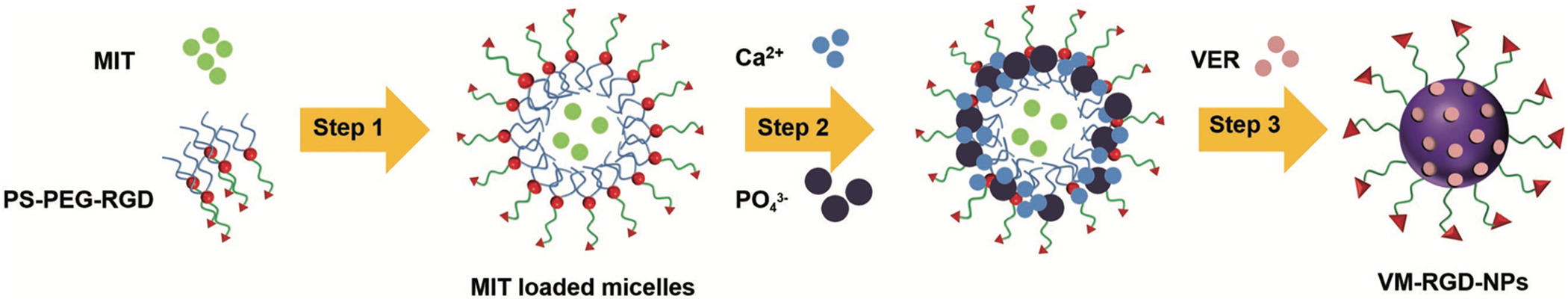
Two common mechanisms for designing drug-loaded CaP nanoparticles. Drugs can be entrapped inside micelles that act as templates for mineralization of CaP’s. Also, physical adsorption of drugs to CaP’s has been commonly used for synthesizing drug-loaded CaP’s. Schematic shows entrapment of Mitoxantrone (MIT) in PS-PEG-RGD micelles (Step 1). CaP shell was formed on the micelles to seal this drug (Step 2). A second drug (Verapamil or VER) was also added to the surface of CaP’s by mixing and physical adsorption (Step 3). RGD is a cancer targeting peptide. (Reprinted with permission from ref. [60]. Copyright 2018 WILEY-VCH Verlag GmbH & Co.)
Mixing CaP’s with solutions containing drug molecules such as Doxorubicin (DOX), α-tocopheryl succinate (α-TOS), and 2-deoxy-D-glucose (2DG) have also been reported as a feasible drug-loading approach in various CaP-based in vivo studies [54, 56, 63, 65, 72, 76, 167]. Physical adsorption of the drug molecules to the surface of CaP’s via electrostatic or hydrogen bonding has been the major drug-loading mechanism used in these studies. However, it should be noted that these weakly-bonded drug molecules might be detached from the surface of the CaP’s in blood stream and before their uptake by the tumors. Therefore, careful considerations are needed when this approach is used for loading drugs to CaP’s. Mineralization of CaP’s in the presence of drug molecules and entrapment of drug molecules into CaP’s has also been reported as an effective drug-loading method [70]. Drug release from these nanoparticles depends on degradation of the CaP’s within weakly acidic tumor microenvironment [168]. Also, controlling the amount of entrapped drug molecules and their sustained release need to be optimized for different types of drugs, considering their molecular size and therapeutic efficacy.
4. CaP’s for Multifunctional Cancer Therapy
CaP’s have been extensively studied for multimodal cancer therapeutic applications, by combining several therapeutic strategies in a relatively more complex nanoparticle design. For example, Sokolova et al. [169, 170] prepared multi-shell nanoparticles with CaP core and CpG oligonucleotide/CaP shells (Figure 15). These multi-shell nanoparticles were further functionalized with CpG and poly (I:C). Furthermore, a viral peptide of the influenza A virus hemagglutinin (HA) was incorporated inside these particles. Such functionalized CaP nanoparticles were able to protect the CpG molecules from initial degradation inside the cells and stimulated the antigen-specific CD4+ T-Cells strongly to produce immunostimulatory cytokines. In addition, tuning the composition of multi-shell nanoparticles enabled determining the required dose for in vivo targeting. The ability of these nanoparticles to encapsulate different antigens to stimulate antigen-presenting cells looks promising and paves the way for their future applications as effective cancer or antiviral vaccines.
Figure 15.
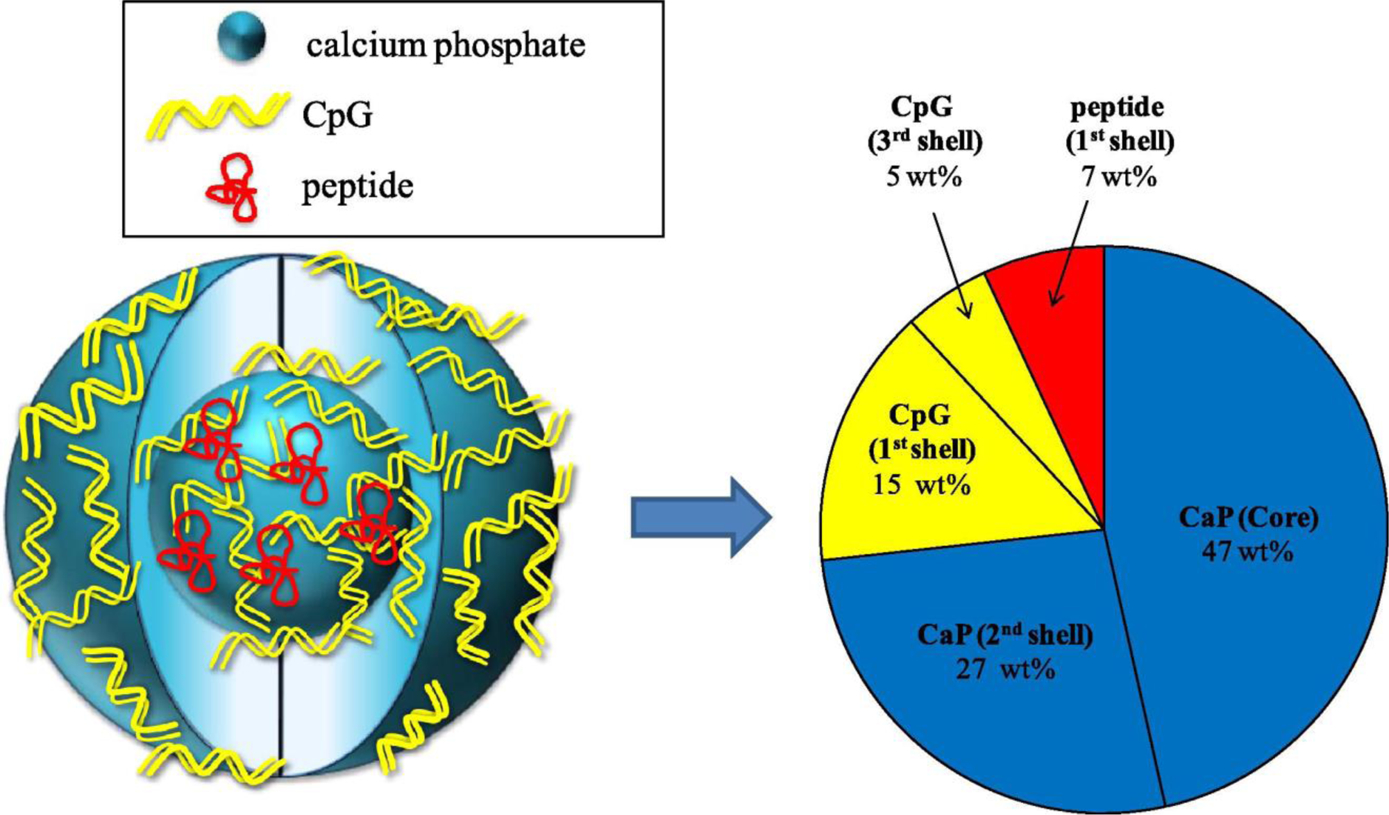
Composition distribution of a typical triple-shell calcium phosphate nanoparticles formulation that incorporates oligonucleotide (CpG) and a peptide. (Reprinted with permission from ref. [169]. Copyright 2011 Elsevier)
Another common strategy has been based on adding light-responsive moieties to the CaP’s for photo-induced cancer therapeutic applications such as photodynamic or photothermal therapy. These two therapeutic modalities have been used for treatment of various types of cancers, as well as curing the bacterial biofilms on the implant surfaces [171, 172]. These strategies are mostly based on ability of CaP nanoparticles to incorporate various light-responsive molecules such as photosensitive dyes. In photodynamic therapy, these non-toxic photosensitive dyes are used alongside a harmless visible light that is utilized to irradiate the tumor tissues. Interaction of these dyes with light leads to production of highly reactive oxygen species (i.e., singlet oxygen) that can destroy tumor cells. The photosensitizer dyes should be delivered into the cytoplasm of the tumor cells to be effective. However, these molecules are mostly insoluble in aqueous phases and have relatively small selectivity over malignant or healthy cells [173]. Therefore, incorporating them into nanoplatforms such as CaP’s is essential for their effective delivery to tumor cells.
A study by Schwiertz et al. [174] demonstrated the preparation of polymer-stabilized CaP nanoparticles functionalized with methylene blue or porphyrin as model photosensitizer dyes. Their results indicated that incorporation of these dyes into CaP nanoparticles can enhance their water solubility. Therefore, CaP’s could be used as carriers for their in vitro and in vivo administrations, instead of conventional administration methods such as using alcoholic solutions which impose side-effects. The phototoxicity of these nanoparticles with various concentrations of dyes was tested against different cell lines. The particles charge, incubation time, characteristics of the stabilizing polymer and type and concentration of photoactive dye altered the cell responses considerably. For instance, only moderate efficacy was observed for colon adenocarcinoma cells, whereas a relatively high phototoxicity was detected for synoviocytes.
On the other hand, photothermal therapy is based on heat generation due to interaction of the light-sensitive agents (e.g., dyes and gold nanoparticles) with laser irradiation. This heat can cause cancer cell apoptosis or ablation of the tumors at higher temperatures. A recent CaP-based study reported effective treatment of subcutaneously implanted liver tumors (HepG-2) in mice by combining photothermal therapy and chemotherapy (i.e., doxorubicin) [63]. In this study (Figure 16), hybrids of CaP nanoparticles and gold nanorods were loaded with doxorubicin and injected into mice via tail veins. A fraction of these nanoparticles were accumulated in the tumors and when irradiated with 808 nm laser, they released doxorubicin and generated heat as a result of surface plasmon effect of the gold nanorods. These synergistic therapeutic effects helped to treat the subcutaneous tumors and prolong the survival rate.
Figure 16.
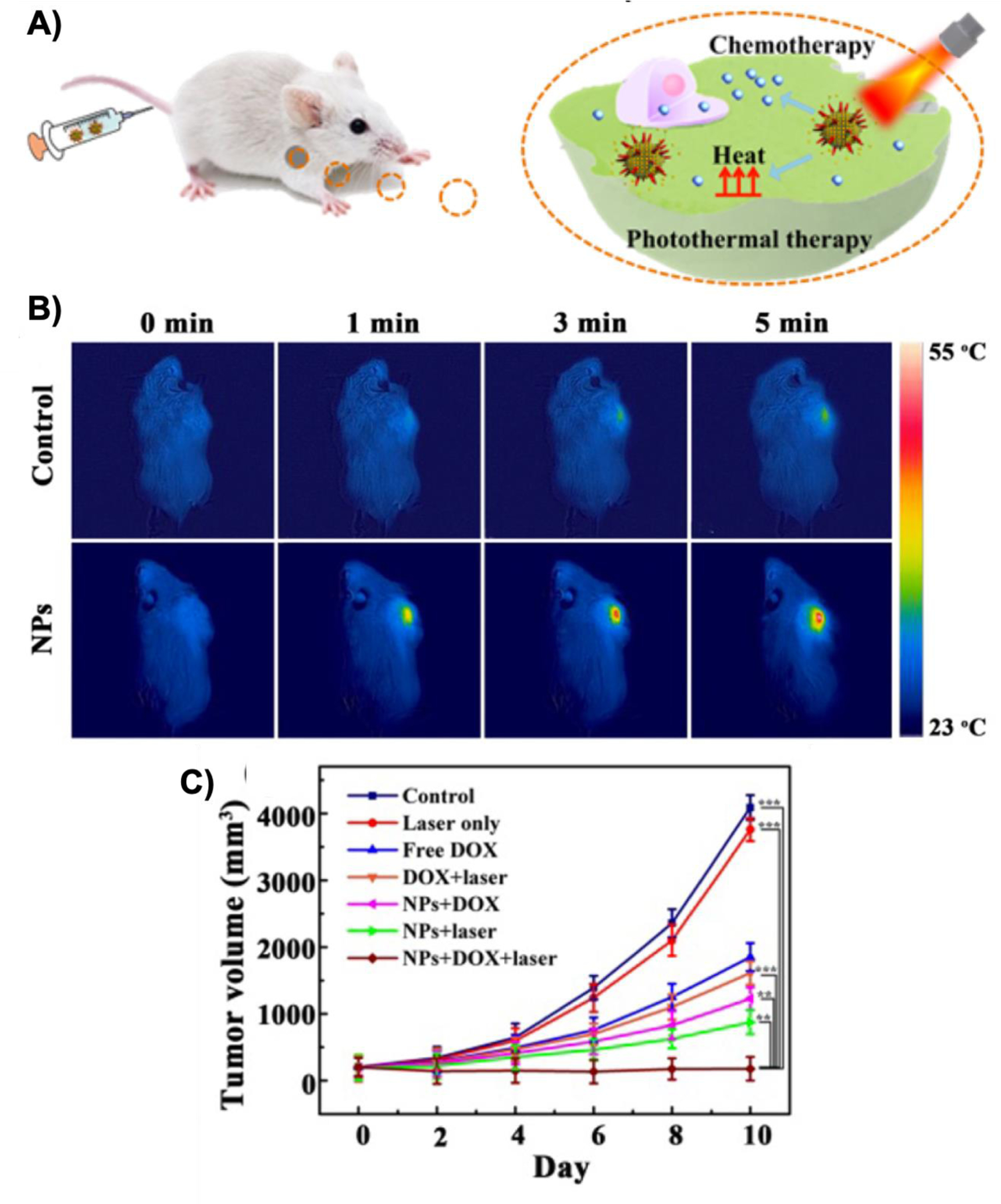
(A) Tumor treatment using CaP nanoparticles (NPs) as platforms for simultaneous photothermal therapy and chemotherapy. Hybrids of CaP nanoparticles and gold nanorods were loaded with Doxorubicin (DOX) and injected into mice via tail veins. Nanoparticles accumulated in the tumors released Doxorubicin (chemotherapy agent) and generated heat in response to laser irradiation. Temperature variation in tumors were monitored using thermal imaging (B) and enhanced the therapeutic effect of the nanoparticles as shown by “NPs+DOX+laser” in the tumor volume graph (C). (Adapted with permission from ref. [63]. Copyright 2017 American Chemical Society)
Tuning the surface chemistry is also helpful to enhance specific targeting of multifunctional CaP’s. For example, Barth et al. [175, 176] used calcium phosphosilicate nanoparticles (CPSNPs) to target breast cancer, pancreatic cancer and leukemia. These nanoparticles were functionalized with indocyanine green (ICG) near-infrared fluoroprobe, a diagnostic and photodynamic therapy agent, and then a novel bioconjugation technique was utilized for cell targeting. In vivo studies on murine leukemia model showed dramatic improvements in the efficiency of photodynamic therapy by using these functionalized nanoparticles. Another study by Ganesan et al. [177] demonstrated the preparation of CaP nanoparticles coated by phosphate-functionalized porphyrin (p-TPPP) as a stabilizer and fluorescence agent. In vitro studies by NIH 3T3 fibroblast cells confirmed a considerable amount of nanoparticles uptake after a few hours. The cells showed promising viability, indicating that prophyrin-loaded CaP nanoparticles could be used as biocompatible delivery vehicles to transfer fluorescing dyes to tumors.
Iron-doped CaP’s and hybrids of CaP’s with iron oxide nanoparticles have also been developed for magnetothermal therapy of the tumors. For example, Pernal et al. [178] dispersed iron oxide nanoparticles in hydroxyapatite nanoparticles as their carriers and evaluated the magnetothermal effects of the resulted nanocomposite for apoptosis of the human brain cancer cells (E297 and U87) and mouse osteosarcomas cells (K7M2) cells. Their in vitro analysis showed higher uptake and hyperthermia efficacy for hybrid hydroxyapatite/iron oxide nanoparticles, compared with iron oxide nanoparticles alone, suggesting hydroxyapatite facilitated uptake of the iron oxide nanoparticles. In a separate study, Adamiano et al. [179], coated superparamagnetic iron oxide nanoparticles with amorphous calcium phosphate and showed that hyperthermia effect of the resulted nanoparticles can reduce viability of the osteosarcoma and glioblastoma cancer cells in alternate magnetic fields of extremely low power. Similar results were shown by Mondal et al. [180] using hydroxyapatite-coated iron oxide nanoparticles for hyperthermia of the MG-63 osteosarcoma cells. Doping of the calcium phosphate nanoparticles with iron ions has also been used for magnetothermal hyperthermia [83, 84, 181, 182]. For example, Iafisco et al. [183] doped Doxorubicin-loaded hydroxyapatite nanorods with iron and showed more efficient Doxorubicin release from these superparamagnetic apatite nanoparticles under alternating magnetic fields. Additionally, in a recent study, Srinivasan et al. [182] synthesized hydroxyapatite nanoparticles in the presence of iron ions and showed that the resulted hydroxyapatite nanoparticles were partially stabilized with FeOOH and α-Fe2O3. Their results showed that, under an alternating magnetic field, these nanoparticles could increase the temperature from ~23 °C to 42 °C in 4 min. Magnetic calcium phosphates have also been used for other applications such as enzyme immobilization [184] or contrast enhancement in magnetic resonance imaging (MRI) [185].
5. Future Direction
Development of cancer drug delivery carriers using calcium phosphate biominerals, especially in the form of nanoparticles (CaP’s), has become of increasing interest. Unlike many other types of nanoparticles, CaP nanoparticles are biocompatible and biodegradable, which makes them suitable nanoplatforms with tunable body clearance pharmacokinetics. CaP degradation by-products are calcium complexes and phosphate groups which are naturally available in body. Also, they are on the same size scales as cellular ligands, receptors and channels and can be further modified or functionalized to attain specific physiochemical properties. Front-line cancer drugs are often untargeted and insoluble compounds that are easily recognized by reticuloendothelial system (RES) to get cleared from the blood by clearance organs (i.e., liver, spleen and kidney) in a short time. However, their solubility and circulation time can be modified when incorporated into the CaP nanoparticles.
In order to enhance the effectiveness of the drug-loaded CaP’s, it is essential to monitor their trafficking inside the target cells and other normal cells surrounding them. After endocytosis, the nanoparticles are typically dissolved in the acidic compartment of the cells that leads to increase of the intracellular calcium level. Any raise above the intracellular calcium level can cause the cell toxicity, due to imbalanced calcium exocytosis. Therefore, adjusting composition, morphology, size, surface chemistry and charge of the CaP nanoparticles play an important role in diminishing their toxicity. However, as discussed in this review, such a change in intracellular Ca2+ can be used for apoptosis of the cancer cells, when designed properly.
During the last several years, a large number of studies have been focused on developing of safe and effective CaP nanocarriers that are non-immunogenic and have potential to deliver a high concentration of drugs to the target cancer tissues. Despite all the outstanding progresses, there are still major scientific and technological issues that need to be overcome. For example, delivery of these nanoparticles to the target organs and tissues has two main challenges. The first is the kinetic barrier that requires nanostructural stability and long-time circulation of nanoparticles in bloodstream in the presences of a wide variety of enzymes, proteins and immune cells. The higher the ionic strength of the nanoparticles environment, the lower the electrostatic repulsion force between particles that makes them more prone to agglomeration. In addition, protein adsorption on the surface of nanoparticles and subsequent changes in surface characteristics (e.g., hydrophilicity and surface charge) is another major drawback that has to be addressed. Although, PEGylation technique is effective in extending the circulation time to some extent, it is still not able to effectively diminish the uptake of the nanocarriers by RES system. The second is the de-assembly or degradation of CaP’s at the desired site of action (i.e., weakly acidic tumor microenvironment or inside the endosomes and lysosomes). After successful uptake by tumors, it is required that nanoparticles undergo dissolution and release their cargo to induce the desired therapeutic effects extracellularly or intracellularly. Therefore, the ideal carrier should be able to encapsulate adequate amount of the bio-agents, transfer them stealthily via bloodstream and release them in a sustained manner at the targeted site.
Since calcium phosphate nanocarriers have distinctive pH-dependent physiochemical properties and are highly biocompatible, they are expected to open new prospects in the future of cancer nanomedicine. CaP’s degradability helps to predict their fate and clearance mechanisms during the clinical applications in future. Unknown body clearance mechanisms and pharmacokinetics of the nanoparticles have been the major drawback of nanomedicines till now and generation of degradable nanoparticles such as CaP’s can expedite the clinical translation of different nanomedicine approaches. Degradation of the CaP’s in tumor microenvironment and intracellular organelles is highly dependent on their crystallographic phase. Therefore, proper phase analysis of the CaP’s by using methods such as X-ray diffraction (XRD) is essential to tune their degradability. This has been a commonly neglected characteristic in most of the recent in vivo studies. Combination of CaP nanocarries with the already FDA approved drugs and imaging agents are predicted to face fewer regularity problems than the other nano-delivery vehicles. Expanding integrated understanding on the fundamentals of biology, chemistry and well-designed animal studies, along with appropriate stewardship, will enable having precise control at micro- and nano-scale levels to develope more effective and successful biodegradable and clinically safe CaP-based nanovectors for improved cancer therapy.
Degradability is a major challenge against clinical translation of nano-therapeutics.
Calcium phosphate nanoparticles (CaPs) are degradable at low pH.
Degradability makes CaPs suitable for clinically safer cancer therapeutic delivery.
Degradation mechanism of CaPs in cancer tissues is discussed.
Strategies for tuning CaPs degradation and therapeutic effects are summarized.
ACKNOWLEDGMENTS
This work was in part supported by National Institute of Health (NIH) K99/R00 Pathway to Independence award (grant No.# 1K99CA234208-01A1), NIH T32 CA196585 (Cancer-Translational Nanotechnology Training, Cancer-TNT) and NIH T32 CA009695 (Stanford Cancer Imaging Training, SCIT) Postdoctoral Fellowship Programs at Stanford University. HA also acknowledges supports by the Marie Sklodowska-Curie MINDED Project [grant agreement No 754490].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no conflict of interests.
References
- [1].Adair JH, Parette MP, Altinoglu EI, Kester M, Nanoparticulate Alternatives for Drug Delivery, Acs Nano 4(9) (2010) 4967–4970. [DOI] [PubMed] [Google Scholar]
- [2].El-Aneed A, An overview of current delivery systems in cancer gene therapy., Journal of controlled release : official journal of the Controlled Release Society 94(1) (2004) 1–14. [DOI] [PubMed] [Google Scholar]
- [3].Somia N, Verma IM, Gene therapy: trials and tribulations., Nature reviews. Genetics 1(2) (2000) 91–99. [DOI] [PubMed] [Google Scholar]
- [4].Ulery BD, Nair LS, Laurencin CT, Biomedical applications of biodegradable polymers, J. Polym. Sci., Part B: Polym. Phys 49(12) (2011) 832–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Llevot A, Astruc D, Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer., Chem. Soc. Rev 41(1) (2012) 242–257. [DOI] [PubMed] [Google Scholar]
- [6].Mahmoudi M, Sahraian MA, Shokrgozar MA, Laurent S, Superparamagnetic Iron Oxide Nanoparticles: Promises for Diagnosis and Treatment of Multiple Sclerosis, Acs Chemical Neuroscience 2(3) (2011) 118–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mahmoudi M, Sant S, Wang B, Laurent S, Sen T, Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy., Advanced Drug Delivery Reviews 63(1–2) (2011) 24–46. [DOI] [PubMed] [Google Scholar]
- [8].Yang F, Jin C, Jiang YJ, Li J, Di Y, Ni QX, Fu DL, Liposome based delivery systems in pancreatic cancer treatment: From bench to bedside, Cancer Treatment Reviews 37(8) (2011) 633–642. [DOI] [PubMed] [Google Scholar]
- [9].Fernandez-Fernandez A, Manchanda R, McGoron AJ, Theranostic applications of nanomaterials in cancer: drug delivery, image-guided therapy, and multifunctional platforms., Appl. Biochem. Biotechnol 165(7–8) (2011) 1628–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].SZYMANSKI P, MARKOWICZ M, MIKICIUK-OLASIK E, NANOTECHNOLOGY IN PHARMACEUTICAL AND BIOMEDICAL APPLICATIONS: DENDRIMERS, NANO 06(06) (2011) 509. [Google Scholar]
- [11].Yih TC, Al-Fandi M, Engineered nanoparticles as precise drug delivery systems, J. Cell. Biochem 97(6) (2006) 1184–1190. [DOI] [PubMed] [Google Scholar]
- [12].Bohner M, Silicon-substituted calcium phosphates - a critical view., Biomaterials 30(32) (2009) 6403–6406. [DOI] [PubMed] [Google Scholar]
- [13].Tang Q-L, Zhu Y-J, Wu J, Chen F, Cao S-W, Calcium phosphate drug nanocarriers with ultrahigh and adjustable drug-loading capacity: One-step synthesis, in situ drug loading and prolonged drug release., NANOMEDICINE 7(4) (2011) 428–434. [DOI] [PubMed] [Google Scholar]
- [14].LeGeros RZ, Calcium phosphate-based osteoinductive materials., Chem. Rev 108(11) (2008) 4742–4753. [DOI] [PubMed] [Google Scholar]
- [15].Bose S, Tarafder S, Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review, Acta Biomaterialia (2011) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen Y, Li J, Kawazoe N, Chen G, Preparation of dexamethasone-loaded calcium phosphate nanoparticles for the osteogenic differentiation of human mesenchymal stem cells, Materials Chemistry B 5 (2017) 6801–6810. [DOI] [PubMed] [Google Scholar]
- [17].Cai X, Han B, Liu Y, Tian F, Liang F, Wang X, Chlorhexidine-Loaded Amorphous Calcium Phosphate Nanoparticles for Inhibiting Degradation and Inducing Mineralization of Type I Collagen, ACS Appl Mater Interfaces 9(15) (2017) 12949–12958. [DOI] [PubMed] [Google Scholar]
- [18].Iafisco M, Degli Esposti L, Ramirez-Rodriguez GB, Carella F, Gomez-Morales J, Ionescu AC, Brambilla E, Tampieri A, Delgado-Lopez JM, Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization, Sci Rep 8(1) (2018) 17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ahmadpour E, Sarvi S, Hashemi Soteh MB, Sharif M, Rahimi MT, Valadan R, Tehrani M, Khalilian A, Montazeri M, Fasihi-Ramandi M, Daryani A, Enhancing immune responses to a DNA vaccine encoding Toxoplasma gondii GRA14 by calcium phosphate nanoparticles as an adjuvant, Immunol Lett 185 (2017) 40–47. [DOI] [PubMed] [Google Scholar]
- [20].Rahimi MT, Sarvi S, Sharif M, Abediankenari S, Ahmadpour E, Valadan R, Ramandie MF, Hosseini SA, Daryani A, Immunological evaluation of a DNA cocktail vaccine with co-delivery of calcium phosphate nanoparticles (CaPNs) against the Toxoplasma gondii RH strain in BALB/c mice, Parasitol Res 116(2) (2017) 609–616. [DOI] [PubMed] [Google Scholar]
- [21].Morcl T, Hurst BL, Tarbet EB, Calcium phosphate nanoparticle (CaPNP) for dose-sparing of inactivated whole virus pandemic influenza A (H1N1) 2009 vaccine in mice, Vaccine 35(35 Pt B) (2017) 4569–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khalifehzadeh R, Arami H, CpG Molecular Structure Controls Mineralization of Calcium Phosphate Nanoparticles and their Immunostimulation Efficacy as Vaccine Adjuvants, Nanoscale (2020) In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tang J, Howard CB, Mahler SM, Thurecht KJ, Huang L, Xu ZP, Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands, Nanoscale 10(9) (2018) 4258–4266. [DOI] [PubMed] [Google Scholar]
- [24].Arami H, Khandhar A, Liggitt D, Krishnan KM, In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles, Chemical Society Reviews 44 (2015) 8576–8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mitragotri S, Lahann J, Physical approaches to biomaterial design., Nature Materials 8(1) (2009) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Olton DYE, Close JM, Sfeir CS, Kumta PN, Intracellular trafficking pathways involved in the gene transfer of nano-structured calcium phosphate-DNA particles, Biomaterials 32(30) (2011) 7662–7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu Liu, He Wang, Xiang Su, Zhang Zhang, An efficient calcium phosphate nanoparticle-based nonviral vector for gene delivery, International Journal of Nanomedicine (2011) 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Klesing J, Wiehe A, Gitter B, Grafe S, Epple M, Positively charged calcium phosphate/polymer nanoparticles for photodynamic therapy, Journal of Materials Science-Materials in Medicine 21(3) (2010) 887–892. [DOI] [PubMed] [Google Scholar]
- [29].Sahay G, Alakhova DY, Kabanov AV, Endocytosis of nanomedicines, J. Controlled Release 145(3)(2010) 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Degli Esposti L, Carella F, Adamiano A, Tampieri A, Iafisco M, Calcium phosphate-based nanosystems for advanced targeted nanomedicine, Drug Dev Ind Pharm 44(8) (2018) 1223–1238. [DOI] [PubMed] [Google Scholar]
- [31].Qi C, Musetti S, Fu L-H, Zhu Y-J, Huang L, Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications, Chemical Society Reviews 48 (2019) 2698–2737. [DOI] [PubMed] [Google Scholar]
- [32].Dorozhkin SV, Functionalized calcium orthophosphates (CaPO4) and their biomedical applications, J Mater Chem B 7(47) (2019) 7471–7489. [DOI] [PubMed] [Google Scholar]
- [33].Jiang W, Kim BYS, Rutka JT, Chan WCW, Nanoparticle-mediated cellular response is size-dependent, Nature Nanotechnology 3(3) (2008) 145–150. [DOI] [PubMed] [Google Scholar]
- [34].Zhang M, Li J, Xing G, He R, Li W, Song Y, Guo H, Variation in the internalization of differently sized nanoparticles induces different DNA-damaging effects on a macrophage cell line., Arch. Toxicol 85(12) (2011) 1575–1588. [DOI] [PubMed] [Google Scholar]
- [35].Chen HC, Sun B, Tran KK, Shen H, Effects of particle size on toll-like receptor 9-mediated cytokine profiles, Biomaterials 32(6) (2011) 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Arami H, Mohajerani M, Mazloumi M, Khalifehzadeh R, Lak A, Sadrnezhaad SK, Rapid formation of hydroxyapatite nanostrips via microwave irradiation, J. Alloys Compd 469 (2009) 391394. [Google Scholar]
- [37].Khalifehzadeh R, Arami H, DNA-Templated Strontium-Doped Calcium Phosphate Nanoparticles for Gene Delivery in Bone Cells, ACS Biomaterials Science and Engineering 5 (2019) 3201–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kakizawa Y, Furukawa S, Kataoka K, Block copolymer-coated calcium phosphate nanoparticles sensing intracellular environment for oligodeoxynucleotide and siRNA delivery, J. Controlled Release 97(2) (2004) 345–356. [DOI] [PubMed] [Google Scholar]
- [39].Rodio M, Coluccino L, Romeo E, Genovese A, Diaspro A, Garau G, Intartaglia R, Facile fabrication of bioactive ultra-small protein-hydroxyapatite nanoconjugates via liquid-phase laser ablation and their enhanced osteogenic differentiation activity, Journal of Materials Chemistry B 5 (2017) 279–288. [DOI] [PubMed] [Google Scholar]
- [40].Graham FL, Van Der Eb AJ, TRANSFORMATION OF RAT CELLS BY DNA OF HUMAN ADENOVIRUS-5, Virology 54(2) (1973) 536–539. [DOI] [PubMed] [Google Scholar]
- [41].Maitra A, Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy, Expert Review of Molecular Diagnostics 5(6) (2005) 893–905. [DOI] [PubMed] [Google Scholar]
- [42].Jordan M, Wurm F, Transfection of adherent and suspended cells by calcium phosphate, Methods 33(2) (2004) 136–143. [DOI] [PubMed] [Google Scholar]
- [43].Chowdhury E, Transfecting mammalian cells by DNA/calcium phosphate precipitates: Effect of temperature and pH on precipitation, Anal. Biochem 314(2) (2003) 316–318. [DOI] [PubMed] [Google Scholar]
- [44].Shubhra QTH, Oyane A, Araki H, Nakamura M, Tsurushima H, Calcium phosphate nanoparticles prepared from infusion fluids for stem cell transfection: process optimization and cytotoxicity analysis, Biomater Sci 5(5) (2017) 972–981. [DOI] [PubMed] [Google Scholar]
- [45].Das P, Jana NR, Length-Controlled Synthesis of Calcium Phosphate Nanorod and Nanowire and Application in Intracellular Protein Delivery, ACS Appl Mater Interfaces 8(13) (2016) 8710–20. [DOI] [PubMed] [Google Scholar]
- [46].Khan MA, Wu VM, Ghosh S, Uskokovic V ´, Gene delivery using calcium phosphate nanoparticles: Optimization of the transfection process and the effects of citrate and poly(L-lysine) as additives, Journal of Colloid and Interface Science 471 (2016) 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Olton D, Li J, Wilson ME, Rogers T, Close J, Huang L, Kumta PN, Sfeir C, Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: Influence of the synthesis parameters on transfection efficiency, Biomaterials 28(6) (2007) 1267–1279. [DOI] [PubMed] [Google Scholar]
- [48].Chowdhury EH, Kunou M, Nagaoka M, Kundu AK, Hoshiba T, Akaike T, High-efficiency gene delivery for expression in mammalian cells by nanoprecipitates of Ca-Mg phosphate, Gene 341 (2004) 77–82. [DOI] [PubMed] [Google Scholar]
- [49].Kakizawa Y, Kataoka K, Block Copolymer Self-Assembly into Monodispersive Nanoparticles with Hybrid Core of Antisense DNA and Calcium Phosphate, Langmuir 18(12) (2002) 4539–4543. [Google Scholar]
- [50].Kakizawa Y, Miyata K, Furukawa S, Kataoka K, Size-Controlled Formation of a Calcium Phosphate-Based Organic-Inorganic Hybrid Vector for Gene Delivery Using Poly(ethylene glycol)-block-poly(aspartic acid), Adv. Mater 16(8) (2004) 699–702. [Google Scholar]
- [51].Kakizawa Y, Furukawa S, Ishii A, Kataoka K, Organic-inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer, J. Controlled Release 111(3) (2006) 368–370. [DOI] [PubMed] [Google Scholar]
- [52].Huang X, Andina D, Ge J, Labarre A, Leroux JC, Castagner B, Characterization of Calcium Phosphate Nanoparticles Based on a PEGylated Chelator for Gene Delivery, ACS Appl Mater Interfaces 9(12) (2017) 10435–10445. [DOI] [PubMed] [Google Scholar]
- [53].Sun Y, Chen Y, Ma X, Yuan Y, Liu C, Kohn J, Qian J, Mitochondria-Targeted Hydroxyapatite Nanoparticles for Selective Growth Inhibition of Lung Cancer in Vitro and in Vivo, ACS Appl Mater Interfaces 8(39) (2016) 25680–25690. [DOI] [PubMed] [Google Scholar]
- [54].Xiaoyu M, Xiuling D, Chunyu Z, Yi S, Jiangchao Q, Yuan Y, Changsheng L, Polyglutamic acid-coordinated assembly of hydroxyapatite nanoparticles for synergistic tumor-specific therapy, Nanoscale 11(32) (2019) 15312–15325. [DOI] [PubMed] [Google Scholar]
- [55].Ansari L, Derakhshi M, Bagheri E, Shahtahmassebi N, Malaekeh-Nikouei B, Folate conjugation improved uptake and targeting of porous hydroxyapatite nanoparticles containing epirubicin to cancer cells, Pharm Dev Technol (2020) 1–9. [DOI] [PubMed] [Google Scholar]
- [56].Shamsi M, Majidi Zolbanin J, Mahmoudian B, Zolbanin NM, Maleki LA, Jafarabadi MA, Islamian JP, A study on drug delivery tracing with radiolabeled mesoporous hydroxyapatite nanoparticles conjugated with 2DG/DOX for breast tumor cells, Nucl Med Rev Cent East Eur 21(1) (2018) 32–36. [DOI] [PubMed] [Google Scholar]
- [57].Huang X, Li Z, Wu J, Hang Y, Wang H, Yuan L, Chen H, Small addition of Zn2+ in Ca2+@DNA results in elevated gene transfection by aminated PGMA-modified silicon nanowire arrays, Materials Chemistry B 7 (2019) 566–575. [DOI] [PubMed] [Google Scholar]
- [58].Xu L, Tong G, Song Q, Zhu C, Zhang H, Shi J, Zhang Z, Enhanced Intracellular Ca(2+) Nanogenerator for Tumor-Specific Synergistic Therapy via Disruption of Mitochondrial Ca(2+) Homeostasis and Photothermal Therapy, ACS Nano 12(7) (2018) 6806–6818. [DOI] [PubMed] [Google Scholar]
- [59].Zhou Z, Li H, Wang K, Guo Q, Li C, Jiang H, Hu Y, Oupicky D, Sun M, Bioreducible Cross-Linked Hyaluronic Acid/Calcium Phosphate Hybrid Nanoparticles for Specific Delivery of siRNA in Melanoma Tumor Therapy, ACS Appl Mater Interfaces 9(17) (2017) 14576–14589. [DOI] [PubMed] [Google Scholar]
- [60].Wang Q, Zhang X, Liao H, Sun Y, Ding L, Teng Y, Zhu W-H, Zhang Z, Duan Y, Multifunctional Shell-Core Nanoparticles for Treatment of Multidrug Resistance Hepatocellular Carcinoma, Advanced Functional Materials 28 (2018) 1706124. [Google Scholar]
- [61].Tang J, Li B, Howard CB, Mahler SM, Thurecht KJ, Wu Y, Huang L, Xu ZP, Multifunctional lipid-coated calcium phosphate nanoplatforms for complete inhibition of large triple negative breast cancer via targeted combined therapy, Biomaterials 216 (2019) 119232. [DOI] [PubMed] [Google Scholar]
- [62].Huang KW, Lai YT, Chern GJ, Huang SF, Tsai CL, Sung YC, Chiang CC, Hwang PB, Ho TL, Huang RL, Shiue TY, Chen Y, Wang SK, Galactose Derivative-Modified Nanoparticles for Efficient siRNA Delivery to Hepatocellular Carcinoma, Biomacromolecules 19(6) (2018) 23302339. [DOI] [PubMed] [Google Scholar]
- [63].Li S, Zhang L, Zhang H, Mu Z, Li L, Wang C, Rationally Designed Calcium Phosphate/Small Gold Nanorod Assemblies Using Poly(acrylic acid calcium salt) Nanospheres as Templates for Chemo-photothermal Combined Cancer Therapy, ACS Biomaterials Science and Engineering 3 (2017) 3215–3221. [DOI] [PubMed] [Google Scholar]
- [64].Qiu C, Wei W, Sun J, Zhang HT, Ding JS, Wang JC, Zhang Q, Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy, Nanoscale 8(26)(2016)13033–44. [DOI] [PubMed] [Google Scholar]
- [65].Wu Y, Liu J, Movahedi F, Gu W, Xu T, Xu ZP, Enhanced Prevention of Breast Tumor Metastasis by Nanoparticle-Delivered Vitamin E in Combination with Interferon-Gamma, Adv Healthc Mater (2020) e1901706. [DOI] [PubMed] [Google Scholar]
- [66].Zang X, Zhang X, Zhao X, Hu H, Qiao M, Deng Y, Chen D, Targeted Delivery of miRNA 155 to Tumor Associated Macrophages for Tumor Immunotherapy, Mol Pharm 16(4) (2019) 17141722. [DOI] [PubMed] [Google Scholar]
- [67].Li P, Yan Y, Zhang H, Wang R, Han H, Wang J, Treatment of cervical cancer by siRNA-loaded chitosan-coated calcium phosphate nanoparticles, Journal of Chinese Pharmaceutical Sciences 27(8) (2018) 517–529. [Google Scholar]
- [68].Dong Y, Liao H, Fu H, Yu J, Guo Q, Wang Q, Duan Y, pH-Sensitive Shell-Core Platform Block DNA Repair Pathway To Amplify Irreversible DNA Damage of Triple Negative Breast Cancer, ACS Appl Mater Interfaces 11(42) (2019) 38417–38428. [DOI] [PubMed] [Google Scholar]
- [69].Wu H, Li Z, Tang J, Yang X, Zhou Y, Guo B, Wang L, Zhu X, Tu C, Zhang X, The in vitro and in vivo anti-melanoma effects of hydroxyapatite nanoparticles: influences of material factors, Int J Nanomedicine 14 (2019) 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang N.-n., Yu R.-s., Xu M, Cheng X.-y., Chen C.-m., Xu X.-l., Lu C.-y., Lu K.-j., Chen M.-j., Zhu M.-l., Weng Q.-y., Hui J.-g., Zhang Q, Du Y-Z, Ji J.-s., Visual targeted therapy of hepatic cancer using homing peptide modified calcium phosphate nanoparticles loading doxorubicin guided by T1 weighted MRI, Nanomedicine: Nanotechnology, Biology, and Medicine 14 (2018) 2167–2178. [DOI] [PubMed] [Google Scholar]
- [71].Chen J, Sun X, Shao R, Xu Y, Gao J, Liang W, VEGF siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer, Int J Nanomedicine 12 (2017) 6075–6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Liu S, Li W, Dong S, Gai S, Dong Y, Yang D, Dai Y, He F, Yang P, Degradable Calcium Phosphate-Coated Upconversion Nanoparticles for Highly Efficient Chemo-Photodynamic Therapy, ACS Appl Mater Interfaces 11(51) (2019) 47659–47670. [DOI] [PubMed] [Google Scholar]
- [73].Wang M, Zhang M, Fu L, Lin J, Zhou X, Zhou P, Huang P, Hu H, Han Y, Liver-targeted delivery of TSG-6 by calcium phosphate nanoparticles for the management of liver fibrosis, Theranostics 10(1) (2020) 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wu Z, Ma X, Ma Y, Yang Z, Yuan Y, Liu C, Core/Shell PEGS/HA Hybrid Nanoparticle Via Micelle-Coordinated Mineralization for Tumor-Specific Therapy, ACS Appl Mater Interfaces 12(10) (2020) 12109–12119. [DOI] [PubMed] [Google Scholar]
- [75].Zhang K, Zhou Y, Xiao C, Zhao W, Wu H, Tang J, Li Z, Yu S, Li X, Min L, Yu Z, Wang G, Wang L, Zhang K, Yang X, Zhu X, Tu C, Zhang X, Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect, Sci Adv 5(8) (2019) eaax6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kang Y, Sun W, Li S, Li M, Fan J, Du J, Liang XJ, Peng X, Oligo Hyaluronan-Coated Silica/Hydroxyapatite Degradable Nanoparticles for Targeted Cancer Treatment, Adv Sci (Weinh) 6(13) (2019) 1900716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wu Z, Chen J, Sun Y, Zhao D, Shen M, Huang L, Xu Q, Duan Y, Li Y, Tumor Microenvironment-Response Calcium Phosphate Hybrid Nanoparticles Enhanced siRNAs Targeting Tumors In Vivo, Journal of Biomedical Nanotechnology 14 (2018) 1816–1825. [DOI] [PubMed] [Google Scholar]
- [78].Wang L, Nancollas GH, Calcium orthophosphates: crystallization and dissolution, Chem Rev 108(11) (2008) 4628–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang X, Cresswell M, Calcium Phosphate Materials for Controlled Release Systems, Inorganic Controlled Release Technology, Elsevier; 2016, pp. 161–187. [Google Scholar]
- [80].Dorozhkin SV, Dissolution mechanism of calcium apatites in acids: A review of literature, World J Methodol 2(1) (2012) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Liu J, Li L, Zhang R, Xu ZP, Development of CaP nanocomposites as Photothermal Actuators for Doxorubicin Delivery to Enhance Breast Cancer Treatment, Materials Science and Technology (2020) In press. [Google Scholar]
- [82].Tite T, Popa AC, Balescu LM, Bogdan IM, Pasuk I, Ferreira JMF, Stan GE, Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods, Materials (Basel) 11(11) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yang C-T, Li K-Y, Meng F-Q, Lin J-F, Young I-C, Ivkov R, Lin F-H, ROS-induced HepG2 cell death from hyperthermia using magnetic hydroxyapatite nanoparticles, Nanotechnology 29 (2018) 375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hou CH, Hou SM, Hsueh YS, Lin J, Wu HC, Lin FH, The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy, Biomaterials 30(23–24) (2009) 3956–60. [DOI] [PubMed] [Google Scholar]
- [85].Zhao Z, Espanol M, Guillem-Marti J, Kempf D, Diez-Escudero A, Ginebra MP, Ion-doping as a strategy to modulate hydroxyapatite nanoparticle internalization, Nanoscale 8(3) (2016) 1595–607. [DOI] [PubMed] [Google Scholar]
- [86].Tseng CL, Chen JC, Wu YC, Fang HW, Lin FH, Tang TP, Development of lattice-inserted 5-Fluorouracil-hydroxyapatite nanoparticles as a chemotherapeutic delivery system, J Biomater Appl 30(4) (2015) 388–97. [DOI] [PubMed] [Google Scholar]
- [87].Bakan F, A Systematic Study of the Effect of pH on the Initialization of Ca-deficient Hydroxyapatite to beta-TCP Nanoparticles, Materials (Basel) 12(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sokolova V, Shi Z, Huang S, Du Y, Kopp M, Frede A, Knuschke T, Buer J, Yang D, Wu J, Westendorf AM, Epple M, Delivery of the TLR ligand poly(I:C) to liver cells in vitro and in vivo by calcium phosphate nanoparticles leads to a pronounced immunostimulation, Acta Biomater 64 (2017) 401–410. [DOI] [PubMed] [Google Scholar]
- [89].Khalil IA, Uptake Pathways and Subsequent Intracellular Trafficking in Nonviral Gene Delivery, Pharmacological Reviews 58(1) (2006) 32–45. [DOI] [PubMed] [Google Scholar]
- [90].Chou LYT, Ming K, Chan WCW, Strategies for the intracellular delivery of nanoparticles, Chem. Soc. Rev 40(1) (2010) 233. [DOI] [PubMed] [Google Scholar]
- [91].Conner SD, Schmid SL, Regulated portals of entry into the cell., Nature 422(6927) (2003) 3744. [DOI] [PubMed] [Google Scholar]
- [92].Zuhorn IS, Kalicharan R, Hoekstra D, Lipoplex-mediated transfection of mammalian cells occurs through the cholesterol-dependent clathrin-mediated pathway of endocytosis., The Journal of biological chemistry 277(20) (2002) 18021–18028. [DOI] [PubMed] [Google Scholar]
- [93].Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B, Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake., The Journal of biological chemistry 278(1) (2003) 585–590. [DOI] [PubMed] [Google Scholar]
- [94].Medina-Kauwe LK, Xie J, Hamm-Alvarez S, Intracellular trafficking of nonviral vectors, Gene Therapy 12(24) (2005) 1734–1751. [DOI] [PubMed] [Google Scholar]
- [95].Pelkmans L, Burli T, Zerial M, Helenius A, Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic, Cell 118(6) (2004) 767780. [DOI] [PubMed] [Google Scholar]
- [96].Scita G, Di Fiore PP, The endocytic matrix, Nature 463(7280) (2010) 464–473. [DOI] [PubMed] [Google Scholar]
- [97].Kumari S, Mg S, Mayor S, Endocytosis unplugged: multiple ways to enter the cell., Cell research 20(3) (2010) 256–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mayor S, Pagano RE, Pathways of clathrin-independent endocytosis, Nature Reviews Molecular Cell Biology 8(8) (2007) 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].REJMAN J, BRAGONZI A, CONESE M, Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes, Molecular Therapy 12(3) (2005) 468–474. [DOI] [PubMed] [Google Scholar]
- [100].Aa MAEM, Huth US, Hafele SY, Schubert R, Oosting RS, Mastrobattista E, Hennink WE, Peschka-Suss R, Koning GA, Crommelin DJA, Cellular Uptake of Cationic Polymer-DNA Complexes Via Caveolae Plays a Pivotal Role in Gene Transfection in COS-7 Cells, Pharm. Res 24(8)(2007)1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].von Gersdorff K, Sanders NN, Vandenbroucke R, De Smedt SC, Wagner E, Ogris M, The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type., Molecular therapy : the journal of the American Society of Gene Therapy 14(5) (2006) 745–753. [DOI] [PubMed] [Google Scholar]
- [102].Batard P, Jordan M, Wurm F, Transfer of high copy number plasmid into mammalian cells by calcium phosphate transfection, Gene 270 (2001) 61–68. [DOI] [PubMed] [Google Scholar]
- [103].Zhou Q, Wang Y, Xiang J, Piao Y, Zhou Z, Tang J, Liu X, Shen Y, Stabilized calcium phosphate hybrid nanocomposite using a benzoxaborole-containing polymer for pH-responsive siRNA delivery, Biomater Sci 6(12) (2018) 3178–3188. [DOI] [PubMed] [Google Scholar]
- [104].Gao W, Chan JM, Farokhzad OC, pH-Responsive Nanoparticles for Drug Delivery, Molecular Pharmaceutics 7(6) (2010) 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bareford LM, Swaan PW, Endocytic mechanisms for targeted drug delivery., Advanced Drug Delivery Reviews 59(8) (2007) 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M, Understanding biophysicochemical interactions at the nano-bio interface., Nature Materials 8(7) (2009) 543–557. [DOI] [PubMed] [Google Scholar]
- [107].Paulo CSO, Pires das Neves R, Ferreira LS, Nanoparticles for intracellular-targeted drug delivery., Nanotechnology 22(49) (2011) 494002. [DOI] [PubMed] [Google Scholar]
- [108].Lee H, Sung D, Veerapandian M, Yun K, Seo S-W, PEGylated polyethyleneimine grafted silica nanoparticles: enhanced cellular uptake and efficient siRNA delivery, Analytical and Bioanalytical Chemistry 400(2) (2011) 535–545. [DOI] [PubMed] [Google Scholar]
- [109].Hu J, Kovtun A, Tomaszewski A, Singer BB, Seitz B, Epple M, Steuhl K-P, Ergun S, Fuchsluger TA, A new tool for the transfection of corneal endothelial cells: Calcium phosphate nanoparticles., Acta Biomaterialia 22(10) (2011) A76–A77. [DOI] [PubMed] [Google Scholar]
- [110].Sokolova V, Neumann S, Kovtun A, Chernousova S, Heumann R, Epple M, An outer shell of positively charged poly(ethyleneimine) strongly increases the transfection efficiency of calcium phosphate/DNA nanoparticles, Journal of Materials Science 45(18) (2010) 4952–4957. [Google Scholar]
- [111].Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, Ellenbogen RG, Olson JM, Zhang MQ, PEI-PEG-Chitosan-Copolymer-Coated Iron Oxide Nanoparticles for Safe Gene Delivery: Synthesis, Complexation, and Transfection, Advanced Functional Materials 19(14) (2009) 2244–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Stepensky D, Quantitative aspects of intracellularly-targeted drug delivery., Pharm. Res 27(12) (2010) 2776–2780. [DOI] [PubMed] [Google Scholar]
- [113].Won Y-W, Lim KS, Kim Y-H, Intracellular organelle-targeted non-viral gene delivery systems., Journal of controlled release : official journal of the Controlled Release Society 152(1) (2011) 99–109. [DOI] [PubMed] [Google Scholar]
- [114].Bisht S, Bhakta G, Mitra S, Maitra A, pDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery., Int. J. Pharm 288(1) (2005) 157–168. [DOI] [PubMed] [Google Scholar]
- [115].Morgan TT, Muddana HS, Altinoglu EI, Rouse SM, Tabakovic A, Tabouillot T, Russin TJ, Shanmugavelandy SS, Butler PJ, Eklund PC, Yun JK, Kester M, Adair JH, Encapsulation of Organic Molecules in Calcium Phosphate Nanocomposite Particles for Intracellular Imaging and Drug Delivery, Nano Lett 8(12) (2008) 4108–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zeng B, Shi H, Liu Y, A versatile pH-responsive platform for intracellular protein delivery using calcium phosphate nanoparticlesj, Journal of Materials Chemistry B 3 (2015) 9115–9121. [DOI] [PubMed] [Google Scholar]
- [117].Ding Y, Zhai K, Pei P, Lin Y, Ma Y, Zhu H, Shao M, Yang X, Tao W, Encapsulation of cisplatin in a pegylated calcium phosphate nanoparticle (CPNP) for enhanced cytotoxicity to cancerous cells, J Colloid Interface Sci 493 (2017) 181–189. [DOI] [PubMed] [Google Scholar]
- [118].Victor SP, Paul W, Vineeth VM, Komeri R, Jayabalan M, Sharma CP, Neodymium doped hydroxyapatite theranostic nanoplatforms for colon specific drug delivery applications, Colloids Surf B Biointerfaces 145 (2016) 539–547. [DOI] [PubMed] [Google Scholar]
- [119].He Y, Zeng B, Liang S, Long M, Xu H, Synthesis of pH-Responsive Biodegradable Mesoporous Silica-Calcium Phosphate Hybrid Nanoparticles as a High Potential Drug Carrier, ACS Appl Mater Interfaces 9(51) (2017) 44402–44409. [DOI] [PubMed] [Google Scholar]
- [120].Motskin M, Wright DM, Muller K, Kyle N, Gard TG, Porter AE, Skepper JN, Hydroxyapatite nano and microparticles: correlation of particle properties with cytotoxicity and biostability., Biomaterials 30(19) (2009) 3307–3317. [DOI] [PubMed] [Google Scholar]
- [121].Oyane A, Kim HM, Furuya T, Kokubo T, Miyazaki T, Nakamura T, Preparation and assessment of revised simulated body fluids, Journal of Biomedical Materials Research Part A 65A(2) (2003)188–195. [DOI] [PubMed] [Google Scholar]
- [122].Cerella C, Diederich M, Ghibelli L, The Dual Role of Calcium as Messenger and Stressor in Cell Damage, Death, and Survival, International Journal of Cell Biology 2010 (2010) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ariano P, Zamburlin P, Gilardino A, Mortera R, Onida B, Tomatis M, Ghiazza M, Fubini B, Lovisolo D, Interaction of Spherical Silica Nanoparticles with Neuronal Cells: Size-Dependent Toxicity and Perturbation of Calcium Homeostasis, Small 7(6) (2011) 766–774. [DOI] [PubMed] [Google Scholar]
- [124].Mattson MP, Chan SL, Calcium orchestrates apoptosis, Nature Cell Biology 5(12) (2003) 1041–1043. [DOI] [PubMed] [Google Scholar]
- [125].Liu Z-S, Tang S-L, Ai Z-L, Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells., World journal of gastroenterology : WJG 9(9) (2003) 1968–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ewence AE, Bootman M, Roderick HL, Skepper JN, McCarthy G, Epple M, Neumann M, Shanahan CM, Proudfoot D, Calcium Phosphate Crystals Induce Cell Death in Human Vascular Smooth Muscle Cells A Potential Mechanism in Atherosclerotic Plaque Destabilization, Circulation Research 103(5) (2008) E28–E34. [DOI] [PubMed] [Google Scholar]
- [127].Dhawan A, Sharma V, Toxicity assessment of nanomaterials: methods and challenges., Analytical and Bioanalytical Chemistry 398(2) (2010) 589–605. [DOI] [PubMed] [Google Scholar]
- [128].Neumann S, Kovtun A, Dietzel ID, Epple M, Heumann R, The use of size-defined DNA-functionalized calcium phosphate nanoparticles to minimise intracellular calcium disturbance during transfection, Biomaterials 30(35) (2009) 6794–6802. [DOI] [PubMed] [Google Scholar]
- [129].Graham FL, van der Eb AJ, A new technique for the assay of infectivity of human adenovirus 5 DNA, Virology 52(2) (1973) 456–67. [DOI] [PubMed] [Google Scholar]
- [130].Sokolova VV, Radtke I, Heumann R, Epple M, Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles, Biomaterials 27(16) (2006) 3147–3153. [DOI] [PubMed] [Google Scholar]
- [131].Martinez M, Martinez NA, Silva WI, Measurement of the Intracellular Calcium Concentration with Fura-2 AM Using a Fluorescence Plate Reader Bio-protocol 7(14) (2017) e2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Pedraza CE, Bassett DC, McKee MD, Nelea V, Gbureck U, Barralet JE, The importance of particle size and DNA condensation salt for calcium phosphate nanoparticle transfection, Biomaterials 29(23) (2008) 3384–3392. [DOI] [PubMed] [Google Scholar]
- [133].Nimesh S, Gupta N, Chandra R, Strategies and advances in nanomedicine for targeted siRNA delivery, NANOMEDICINE 6(4) (2011) 729–746. [DOI] [PubMed] [Google Scholar]
- [134].Tan SJ, Kiatwuthinon P, Roh YH, Kahn JS, Luo D, Engineering Nanocarriers for siRNA Delivery, Small 7(7) (2011) 841–856. [DOI] [PubMed] [Google Scholar]
- [135].Chen S, Li F, Zhuo R-X, Cheng S-X, Efficient non-viral gene delivery mediated by nanostructured calcium carbonate in solution-based transfection and solid-phase transfection, Molecular Biosystems 7(10) (2011) 2841–2847. [DOI] [PubMed] [Google Scholar]
- [136].Klesing J, Chernousova S, Epple M, Freeze-dried cationic calcium phosphate nanorods as versatile carriers of nucleic acids (DNA, siRNA), J. Mater. Chem 22(1) (2011) 199. [Google Scholar]
- [137].Guo X, Huang L, Recent Advances in Nonviral Vectors for Gene Delivery, Acc. Chem. Res (2011)110826121905065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sokolova V, Epple M, Inorganic Nanoparticles as Carriers of Nucleic Acids into Cells, Angew. Chem. Int. Ed 47(8) (2008) 1382–1395. [DOI] [PubMed] [Google Scholar]
- [139].Zhang M, Kataoka K, Nano-structured composites based on calcium phosphate for cellular delivery of therapeutic and diagnostic agents, Nano Today 4(6) (2009) 508–517. [Google Scholar]
- [140].Chen W-Y, Lin M-S, Lin P-H, Tasi P-S, Chang Y, Yamamoto S, Studies of the interaction mechanism between single strand and double-strand DNA with hydroxyapatite by microcalorimetry and isotherm measurements, Colloids and Surfaces A: Physicochemical and Engineering Aspects 295(1–3) (2007) 274–283. [Google Scholar]
- [141].Mostaghaci B, Loretz B, Haberkorn R, Kickelbick G, Lehr C-M, One-Step Synthesis of Nanosized and Stable Amino-Functionalized Calcium Phosphate Particles for DNA Transfection, Chemistry of Materials 25 (2013) 3667–3674. [Google Scholar]
- [142].Mostaghaci B, Susewind J, Kickelbick G, Lehr CM, Loretz B, Transfection system of amino-functionalized calcium phosphate nanoparticles: in vitro efficacy, biodegradability, and immunogenicity study, ACS Appl Mater Interfaces 7(9) (2015) 5124–33. [DOI] [PubMed] [Google Scholar]
- [143].Bisso S, Mura S, Castagner B, Couvreur P, Leroux JC, Dual delivery of nucleic acids and PEGylated-bisphosphonates via calcium phosphate nanoparticles, Eur J Pharm Biopharm (2019). [DOI] [PubMed] [Google Scholar]
- [144].Tobin LA, Xie Y, Tsokos M, Chung SI, Merz AA, Arnold MA, Li G, Malech HL, Kwong KF, Pegylated siRNA-loaded calcium phosphate nanoparticle-driven amplification of cancer cell internalization in vivo, Biomaterials 34(12) (2013) 2980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Zhou C, Yu B, Yang X, Huo T, Lee LJ, Barth RF, Lee RJ, Lipid-coated nano-calcium-phosphate (LNCP) for gene delivery., Int. J. Pharm 392(1–2) (2010) 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Pittella F, Zhang M, Lee Y, Kim HJ, Tockary T, Osada K, Ishii T, Miyata K, Nishiyama N, Kataoka K, Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge-conversional polymer for efficient gene knockdown with negligible cytotoxicity, Biomaterials 32(11) (2011) 3106–3114. [DOI] [PubMed] [Google Scholar]
- [147].Chen FP, Liu CS, Wei J, Chen XA, Zhao Z, Gao YL, Preparation and characterization of injectable calcium phosphate cement paste modified by polyethylene glycol-6000, Mater. Chem. Phys 125(3) (2011) 818–824. [Google Scholar]
- [148].Welzel T, Radtke I, Meyer-Zaika W, Heumann R, Epple M, Transfection of cells with custom-made calcium phosphate nanoparticles coated with DNA, J. Mater. Chem 14(14) (2004) 2213. [Google Scholar]
- [149].Welzel T, Meyer-Zaika W, Epple M, Continuous preparation of functionalised calcium phosphate nanoparticles with adjustable crystallinity, Chem. Commun (10) (2004) 1204. [DOI] [PubMed] [Google Scholar]
- [150].Neuhaus B, Frede A, Westendorf AM, Epple M, Gene silencing of the pro-inflammatory cytokine TNF-a with siRNA delivered by calcium phosphate nanoparticles, quantified by different methods, Materials Chemistry B 3 (2015) 7186–7193. [DOI] [PubMed] [Google Scholar]
- [151].Frede A, Neuhaus B, Knuschke T, Wadwa M, Kollenda S, Klopfleisch R, Hansen W, Buer J, Bruder D, Epple M, Westendorf AM, Local delivery of siRNA-loaded calcium phosphate nanoparticles abates pulmonary inflammation, Nanomedicine 13(8) (2017) 2395–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhang J, Sun X, Shao R, Liang W, Gao J, Chen J, Polycation liposomes combined with calcium phosphate nanoparticles as a non-viral carrier for siRNA delivery, Journal of Drug Delivery Science and Technology 30 (2015) 1–6. [Google Scholar]
- [153].Sokolova V, Kovtun A, Prymak O, Meyer-Zaika W, Kubareva EA, Romanova EA, Oretskaya TS, Heumann R, Epple M, Functionalisation of calcium phosphate nanoparticles by oligonucleotides and their application for gene silencing, J. Mater. Chem 17(8) (2007) 721. [Google Scholar]
- [154].Bakan F, Kara G, Cokol Cakmak M, Cokol M, Denkbas EB, Synthesis and characterization of amino acid-functionalized calcium phosphate nanoparticles for siRNA delivery, Colloids Surf B Biointerfaces 158 (2017) 175–181. [DOI] [PubMed] [Google Scholar]
- [155].Wang Y-P, Liao Y-T, Liu C-H, Yu J, Alamri HR, Alothman ZA, Hossain MSA, Yamauchi Y, Wu KC-W, Trifunctional Fe3O4/CaP/Alginate Core-Shell-Corona Nanoparticles for Magnetically Guided, pH-Responsive, and Chemically Targeted Chemotherapy, ACS Biomaterials Science and Engineering 3 (2017) 2366–2374. [DOI] [PubMed] [Google Scholar]
- [156].Kokubo T, Formation of biologically active bone-like apatite on metals and polymers by a biomimetic process, Thermochim. Acta 280 (1996) 479–490. [Google Scholar]
- [157].Shen H, Tan J, Saltzman WM, Surface-mediated gene transfer from nanocomposites of controlled texture, Nature Materials 3(8) (2004) 569–574. [DOI] [PubMed] [Google Scholar]
- [158].Choi S, Murphy WL, Sustained plasmid DNA release from dissolving mineral coatings, Acta Biomaterialia 6(9) (2010) 3426–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Sun B, Tran KK, Shen H, Enabling customization of non-viral gene delivery systems for individual cell types by surface-induced mineralization., Biomaterials 30(31) (2009) 6386–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Li J, Chen Y-C, Tseng Y-C, Mozumdar S, Huang L, Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery, J. Controlled Release 142(3) (2010) 416421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Zhang M, Ishii A, Nishiyama N, Matsumoto S, Ishii T, Yamasaki Y, Kataoka K, PEGylated Calcium Phosphate Nanocomposites as Smart Environment-Sensitive Carriers for siRNA Delivery, Adv. Mater 21(34) (2009) 3520–3525. [Google Scholar]
- [162].Duncan R, The dawning era of polymer therapeutics., Nature Reviews Drug Discovery 2(5) (2003) 347–360. [DOI] [PubMed] [Google Scholar]
- [163].Sorensen DR, Leirdal M, Sioud M, Gene silencing by systemic delivery of synthetic siRNAs in adult mice., J. Mol. Biol 327(4) (2003) 761–766. [DOI] [PubMed] [Google Scholar]
- [164].Kakizawa Y, Kataoka K, Block copolymer micelles for delivery of gene and related compounds, Advanced Drug Delivery Reviews 54(2) (2002) 203–222. [DOI] [PubMed] [Google Scholar]
- [165].Gene therapy and tissue engineering in repair of the musculoskeletal system, 88(3) (2003) 467481. [DOI] [PubMed] [Google Scholar]
- [166].Luo D, Saltzman W, Synthetic DNA delivery systems, Nat. Biotechnol 18(1) (2000) 33–37. [DOI] [PubMed] [Google Scholar]
- [167].Wu Y, Gu W, Xu ZP, Enhanced combination cancer therapy using lipid-calcium carbonate/phosphate nanoparticles as a targeted delivery platform, Nanomedicine (Lond) 14(1) (2019) 77–92. [DOI] [PubMed] [Google Scholar]
- [168].Zhou ZF, Sun TW, Chen F, Zuo DQ, Wang HS, Hua YQ, Cai ZD, Tan J, Calcium phosphate-phosphorylated adenosine hybrid microspheres for anti-osteosarcoma drug delivery and osteogenic differentiation, Biomaterials 121 (2017) 1–14. [DOI] [PubMed] [Google Scholar]
- [169].Sokolova V, Knuschke T, Buer J, Westendorf AM, Epple M, Quantitative determination of the composition of multi-shell calcium phosphate-oligonucleotide nanoparticles and their application for the activation of dendritic cells., Acta Biomaterialia 7(11) (2011) 4029–4036. [DOI] [PubMed] [Google Scholar]
- [170].Sokolova V, Knuschke T, Kovtun A, Buer J, Epple M, Westendorf AM, The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation, Biomaterials 31(21) (2010) 56275633. [DOI] [PubMed] [Google Scholar]
- [171].Maisch T, Anti-microbial photodynamic therapy: useful in the future?, Lasers in Medical Science 22(2) (2007) 83–91. [DOI] [PubMed] [Google Scholar]
- [172].Castano AP, Mroz P, Hamblin MR, Photodynamic therapy and anti-tumour immunity, Nature Reviews Cancer 6(7) (2006) 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Konan YN, Gurny R, Allemann E, State of the art in the delivery of photosensitizers for photodynamic therapy., Journal of photochemistry and photobiology. B, Biology 66(2) (2002) 89–106. [DOI] [PubMed] [Google Scholar]
- [174].Schwiertz J, Wiehe A, GrAfe S, Gitter B, Epple M, Calcium phosphate nanoparticles as efficient carriers for photodynamic therapy against cells and bacteria, Biomaterials 30(19) (2009) 3324–3331. [DOI] [PubMed] [Google Scholar]
- [175].Barth BM, I Altinoglu E, Shanmugavelandy SS, Kaiser JM, Crespo-Gonzalez D, DiVittore NA, McGovern C, Goff TM, Keasey NR, Adair JH, Loughran TP Jr., Claxton DF, Kester M, Targeted Indocyanine-Green-Loaded Calcium Phosphosilicate Nanoparticles for In VivoPhotodynamic Therapy of Leukemia, Acs Nano 5(7) (2011) 5325–5337. [DOI] [PubMed] [Google Scholar]
- [176].Barth BM, Sharma R, Altinoglu EI, Morgan TT, Shanmugavelandy SS, Kaiser JM, McGovern C, Matters GL, Smith JP, Kester M, Adair JH, Bioconjugation of Calcium Phosphosilicate Composite Nanoparticles for Selective Targeting of Human Breast and Pancreatic Cancers In Vivo, Acs Nano 4(3) (2010) 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Ganesan K, Kovtun A, Neumann S, Heumann R, Epple M, Calcium phosphate nanoparticles: colloidally stabilized and made fluorescent by a phosphate-functionalized porphyrin, J. Mater. Chem 18(31) (2008) 3655. [Google Scholar]
- [178].Pernal S, Wu VM, Uskokovic V, Hydroxyapatite as a Vehicle for the Selective Effect of Superparamagnetic Iron Oxide Nanoparticles against Human Glioblastoma Cells, ACS Appl Mater Interfaces 9(45) (2017) 39283–39302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Adamiano A, Wu VM, Carella F, Lamura G, Canepa F, Tampieri A, Iafisco M, Uskokovic V, Magnetic calcium phosphates nanocomposites for the intracellular hyperthermia of cancers of bone and brain, Nanomedicine (Lond) 14(10) (2019) 1267–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Mondal S, Manivasagan P, Bharathiraja S, Santha Moorthy M, Nguyen VT, Kim HH, Nam SY, Lee KD, Oh J, Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment, Nanomaterials (Basel) 7(12) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Tampieri A, D’Alessandro T, Sandri M, Sprio S, Landi E, Bertinetti L, Panseri S, Pepponi G, Goettlicher J, Banobre-Lopez M, Rivas J, Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite, Acta Biomater 8(2) (2012) 843–51. [DOI] [PubMed] [Google Scholar]
- [182].Srinivasan B, Kolanthai E, Eluppai Asthagiri Kumaraswamy N, Jayapalan RR, Vavilapalli DS, Catalani LH, Ningombam GS, Khundrakpam NS, Singh NR, Kalkura SN, Thermally Modified Iron-Inserted Calcium Phosphate for Magnetic Hyperthermia in an Acceptable Alternating Magnetic Field, J Phys Chem B 123(26) (2019) 5506–5513. [DOI] [PubMed] [Google Scholar]
- [183].Iafisco M, Drouet C, Adamiano A, Pascaud P, Montesi M, Panseri S, Sardab S, Tampieri A, Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin, Journal of Materials Chemistry B 4 (2016) 57–70. [DOI] [PubMed] [Google Scholar]
- [184].Li D, Fang Z, Duan H, Liang L, Polydopamine-mediated synthesis of core-shell gold@calcium phosphate nanoparticles for enzyme immobilization, Biomater Sci 7(7) (2019) 2841–2849. [DOI] [PubMed] [Google Scholar]
- [185].Nakamura M, Oyane A, Kuroiwa K, Shimizu Y, Pyatenko A, Misawa M, Numano T, Kosuge H, Facile one-pot fabrication of calcium phosphate-based composite nanoparticles as delivery and MRI contrast agents for macrophages, Colloids Surf B Biointerfaces 162 (2018) 135–145. [DOI] [PubMed] [Google Scholar]


