Summary
Antitoxins are becoming recognized as proteins that regulate more than their own synthesis; for example, we found previously that antitoxin MqsA of the Escherichia coli toxin/antitoxin (TA) pair MqsR/MqsA directly represses the gene encoding the stationary-phase sigma factor RpoS. Here, we investigated the physiological role of antitoxin DinJ of the YafQ/DinJ TA pair and found DinJ also affects the general stress response by decreasing RpoS levels. Corroborating the reduced RpoS levels upon producing DinJ, the RpoS-regulated phenotypes of catalase activity, cell adhesins and cyclic diguanylate decreased while swimming increased. Using a transcriptome search and DNA-binding assays, we determined that the mechanism by which DinJ reduces RpoS is by repressing cspE at the LexA palindrome; cold-shock protein CspE enhances translation of rpoS mRNA. Inactivation of CspE abolishes the ability of DinJ to influence RpoS. Hence, DinJ influences the general stress response indirectly by regulating cspE.
Introduction
The role of toxin–antitoxin (TA) pairs in bacterial physiology is becoming more clear as antitoxin MqsA actively participates in the general stress response of Escherichia coli by regulating more than its own promoter (Wang et al., 2011). MqsA helps mediate the general stress response via its repression of rpoS, which encodes the stationary-phase sigma factor RpoS; RpoS is the master regulator of the stress response (Pesavento et al., 2008) and controls 500 genes in E. coli (Hengge, 2008). By repressing rpoS, MqsA reduces the concentration of the internal messenger cyclic diguanylate (c-di-GMP), thereby increasing motility and decreasing biofilm formation and catalase activity (Wang et al., 2011). Upon oxidative stress, MqsA is rapidly degraded by Lon protease resulting in induction of rpoS, which in turn increases c-di-GMP, inhibits motility and increases cell adhesion and biofilm formation. Therefore, TA systems have an important impact on cell physiology by influencing such developmental cascades as the switch from planktonic cells to biofilm cells (Wang and Wood, 2011).
Thirty-seven chromosomal TA systems have been characterized in E. coli so far (Tan et al., 2011) and the YafQ/DinJ TA system was initially characterized by Motiejūnaite and colleagues (2005). YafQ/DinJ are grouped in the RelE/RelB TA family (Gotfredsen and Gerdes, 1998), and the growth inhibition of toxin YafQ is counteracted by DinJ (Motiejūnaite et al., 2005). YafQ is an endoribonuclease that associates with the ribosome through the 50S subunit and blocks translation elongation through mRNA cleavage at 5′-AAA-G/A-3′ sequences (Prysak et al., 2009). The gene that encodes the antitoxin, dinJ (damage inducible gene) was predicted to be regulated by the LexA repressor (Lewis et al., 1994); LexA acts as a transcriptional repressor of SOS-regulated genes but is inactivated in response to DNA damage resulting in their induction (Butala et al., 2009). DinJ is not well-characterized; for example, there is no structural and little physiological information about it, however DinJ is more stable in the absence of the Lon and ClpXP proteases (Prysak et al., 2009).
Palindromes are often present at TA promoters and are sites of antitoxin binding to confer auto-regulation (Gerdes et al., 2005). YafQ and DinJ form a stable complex (Motiejūnaite et al., 2007), which binds the dinJ-yafQ palindrome (5′-CTGAATAAATATACAG-3′, −16 to −33 from the translation start site) (Prysak et al., 2009) which overlaps the consensus LexA binding site (5′-TACTG(TA)5CAGTA-3′) (Fernández De Henestrosa et al., 2000), suggesting that this module is regulated by DNA damage (Prysak et al., 2009). Although this is the only TA pair whose palindrome shares homology with the LexA binding site, the physiological role of this TA system in the general stress response has not been characterized.
The cold-shock proteins (Csp) of the CspA protein family consist of nine homologous proteins (CspA to CspI) that help the cell acclimate to low temperature conditions (Bae et al., 1999). However, CspE functions both at physiological temperatures and during cold shock (Phadtare et al., 2006). CspE is a single stranded nucleic acidbinding protein (Phadtare and Inouye, 1999) that plays a role in chromosome condensation by binding to distant DNA regions containing contiguous deoxythymine residues and dimerizing, thereby condensing the intervening DNA (Johnston et al., 2006). In addition, production of CspE increases RpoS through rpoS message stabilization (Phadtare et al., 2002; Phadtare et al., 2006). Therefore, CspE is involved in the regulation of RpoS, the global stress response regulator, as part of the complex stress response network of E. coli (Phadtare and Inouye, 2001; Phadtare et al., 2002).
In the present study, given that the palindrome recognized by DinJ is related to LexA and that antitoxin MqsA influences RpoS (Wang et al., 2011), we investigated whether antitoxin DinJ also influences the stress response. By using a genetic background devoid of the major E. coli TA pairs (the Δ6 strain of Wang et al., 2011), which lacks the MqsR/MqsA, MazF/MazE, RelE/RelB, ChpB, YoeB/YefM and YafQ/DinJ TA systems (Table 1), interpretation of the results was simplified. We find that the antitoxin DinJ reduces RpoS at the level of translation by repressing cspE, which encodes cold-shock protein CspE that facilitates the translation of rpoS mRNA. Hence, DinJ influences the general stress response indirectly by regulating cspE.
Table 1.
Escherichia coli bacterial strains and plasmids used in this study.
| Strains | Genotype | Source |
|---|---|---|
| MG1655 | F− λ− ilvG rfb-50 rph-1 | Blattner et al. (1997) |
| Δ6 | MG1655 ΔmazEF ΔrelBEF ΔchpB ΔyefM-yoeB ΔdinJ-yafQ ΔmqsRA Ω KmR | Wang et al. (2011) |
| Δ6 ΔKmR | Δ6 ΔKmR | Wang et al. (2011) |
| BW25113 | lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | Datsenko and Wanner (2000) |
| BW25113 rpoS | BW25113 ΔrpoS Ω KmR | Baba et al. (2006) |
| BW25113 cspE | BW25113 ΔcspE Ω KmR | Baba et al. (2006) |
| DH5α | luxS supE44 ΔlacU169(Δ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Ren et al. (2004c) |
| Plasmids | ||
| pCA24N | CmR; lacIq, pCA24N | Kitagawa et al. (2005) |
| pCA24N-dinJ | CmR; lacIq, pCA24N PT5-lac::dinJ+ | Kitagawa et al. (2005) |
| pCA24N-mqsA | CmR; lacIq, pCA24N PT5-lac::mqsA+ | Kitagawa et al. (2005) |
| pCA24N-lon | CmR; lacIq, pCA24N PT5-lac::lon+ | Kitagawa et al. (2005) |
| pBS(Kan) | KmR; pBS(Kan) | Canada et al. (2002) |
| pBS(Kan)-dinJ | KmR; pBS(Kan)Plac::dinJ+ | this study |
Note that the Δ6 strains used in this study lack an insertion sequence in the promoter of flhD that gives the parent strain MG1655 artificially high motility. CmR and KmR are chloramphenicol and kanamycin resistance respectively.
Results
DinJ reduces RpoS
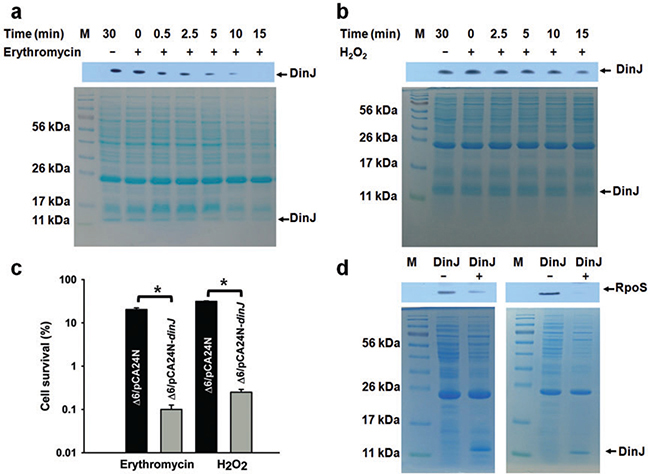
Since antitoxin MqsA was shown to repress rpoS (Wang et al., 2011), we investigated the possible role of antitoxin DinJ on rpoS transcription and RpoS protein levels to see if this antitoxin also impacted the general stress response. We utilized strain Δ6 that is deleted for six sets of TA systems; hence, DinJ could be studied in a background without the best-studied TA systems. Since the effects of antitoxins on gene regulation should only be important during stress when proteases degrade the antitoxin and derepress genes (Wang et al., 2011; Wang and Wood, 2011), we investigated several stresses to identify a condition where DinJ was labile. Using heat, acid, oxidative and antibiotic stresses (erythromycin, gentamicin, mitomycin C, ampicillin, tetracycline and nalidixic acid), we determined that DinJ was degraded in the presence of erythromycin (75 μg ml−1 for 10 min) with a half life of less than 2 min (Fig. 1A). In contrast, DinJ was stable in the presence of the other stresses with half lives greater than 15 min (hydrogen peroxide result shown in Fig. 1B). In addition, production of DinJ decreased cell viability by 220 ± 10-fold with erythromycin (Fig. 1C). Erythromycin, a macrolide antibiotic produced by Streptomyces erythreus (Weber et al., 1985), inhibits protein synthesis by binding to the 50S ribosomal subunit and inducing the dissociation of peptidyl-tRNAs from the ribosomes after the initiation of mRNA translation (Tenson et al., 2003). Hence, this condition was chosen to determine the impact of DinJ on cell physiology. In addition, DinJ also reduced cell viability under oxidative stress (20 mM H2O2 for 10 min) by 125 ± 20-fold (Fig. 1C). Therefore, DinJ reduces the ability of the cells to respond to erythromycin and oxidative stress, and DinJ is degraded during erythromycin stress.
Fig. 1.
DinJ is degraded during erythromycin stress and affects cell viability and RpoS levels. Lanes 3–8 in the upper panel (Western) show DinJ levels as detected by a His-tag antibody at different time points with 75 μg ml−1 erythromycin (A) and 20 mM H2O2 (B) stress for Δ6/pCA24N-dinJ. For both (A) and (B), the corresponding whole cell lysates were visualized by SDS-PAGE (lower panels). dinJ was induced from pCA24N-dinJ via 1 mM IPTG. Two independent cultures were used for each strain and one representative data set is shown here.
C. Percentage of cells which survive erythromycin (75 μg ml−1) stress for 10 min or oxidative stress induced by 20 mM H2O2 for 10 min. Error bars indicate standard error of mean (n = 2). Significant changes are marked with an asterisk for P < 0.05.
D. Lanes 2 and 3 show RpoS levels as detected by an anti-RpoS antibody for Δ6/pCA24N-dinJ (DinJ+) and Δ6/pCA24N (DinJ-). Two independent cultures were used for each strain and both experiments are shown.
Using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) to investigate whether DinJ affects rpoS, we applied erythromycin stress and found rpoS was repressed slightly (2.6 ± 0.2-fold). More significantly, production of DinJ dramatically decreased RpoS levels (Fig. 1D). Therefore, DinJ changes primarily RpoS levels in the cell, and the reduction in viability seen upon adding erythromycin is likely the result of reducing RpoS levels.
DinJ reduces cyclic diguanylate thereby increasing swimming and decreasing cell adhesins and catalase activity
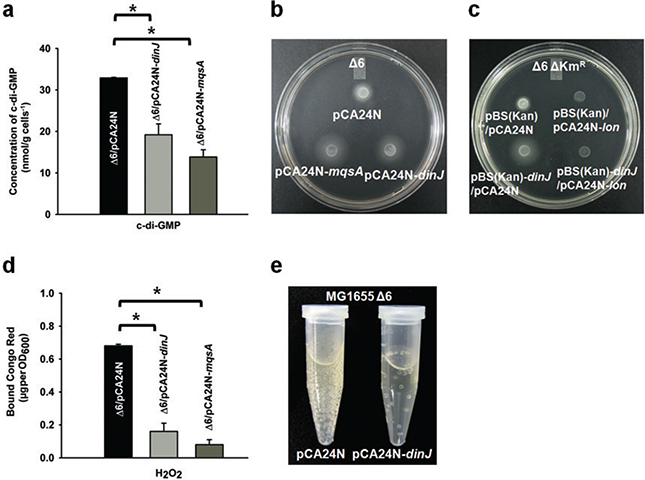
To confirm that DinJ reduces RpoS, we investigated the phenotypes related to RpoS activity. Since RpoS is a positive regulator of diguanylate cyclases (Landini, 2009), it was expected that c-di-GMP concentrations should decrease in the presence of DinJ. As expected, upon overexpressing dinJ from a plasmid in stationary-phase cells, the intracellular c-di-GMP concentration decreased by 1.7 ± 0.2-fold (Fig. 2A). The positive control MqsA (Wang et al., 2011) also reduced c-di-GMP by 2.4 ± 0.3-fold (Fig. 2A). Therefore, DinJ reduces c-di-GMP concentrations.
Fig. 2.
DinJ reduces cyclic diguanylate thereby increasing swimming and decreasing cell adhesins and catalase activity.
A. Cyclic diguanylate concentrations in stationary-phase cultures (starving cells) of Δ6/pCA24N-dinJ, Δ6/pCA24N-mqsA and Δ6/pCA24N after 15 h at 37°C. Error bars indicate standard error of mean (n = 2). Significant changes are marked with an asterisk for P < 0.05.
B. Swimming motility after 12 h of growth at 37°C for Δ6 cells producing DinJ and MqsA via pCA24N-dinJ and pCA24N-mqsA. Two independent cultures were used, and a representative image is shown here.
C. Swimming motility after 12 h of growth at 37°C for Δ6 ΔKmR cells producing DinJ from pBS(Kan)-dinJ and Lon from pCA24N-lon via 1 mM IPTG. Chloramphenicol (30 μg ml−1) and kanamycin (50 μg ml−1) were used for maintaining the pCA24N-based and pBS(Kan)-based plasmids. Controls where lon was induced in the absence of DinJ were also included to show the direct effect of each gene on motility. Two independent cultures were used, and a representative image is shown here.
D. Cellulose/curli formation of Δ6/pCA24N-dinJ, Δ6/pCA24N-mqsA and Δ6/pCA24N after 180 min at 30°C with oxidative stress (2 mM H2O2). Error bars indicate standard error of mean (n = 2). Significant changes are marked with an asterisk for P < 0.05.
E. Images of Δ6/pCA24N-dinJ versus Δ6/pCA24N cultures (turbidity of 1) 10 min after adding 40 mM H2O2. Two independent cultures were used for each strain, and one representative data set is shown. Bubbles are oxygen produced by the decomposition of hydrogen peroxide by catalase: 2 H2O2 to 2 H2O + O2. DinJ was produced from pCA24N-dinJ via 0.5 mM IPTG for the c-di-GMP assay and via 1 mM IPTG for the stress assays.
Since c-di-GMP levels are reduced in the presence of antitoxin DinJ, motility should increase due to the lower RpoS levels since RpoS inhibits flhD, the master regulator of motility (Pesavento et al., 2008). As expected, production of DinJ in Δ6 increased motility by 1.7 ± 0.1-fold (Fig. 2B). The positive control MqsA also increased motility by 2.5 ± 0.1-fold (Fig. 2B). Moreover, producing Lon and DinJ simultaneously abolished the ability of DinJ to increase motility (Fig. 2C). Therefore, Lon degrades DinJ as it does for several antitoxins (Christensen et al., 2001; Wilbaux et al., 2007; Jorgensen et al., 2009; Kim et al., 2010; Wang et al., 2011), and DinJ increases swimming motility.
Given that RpoS levels are reduced, both curli and cellulose production should likewise be decreased since RpoS is a positive regulator of csgD (Pesavento et al., 2008). Using Congo red, a dye that binds to both cellulose and curli (Ma and Wood, 2009), we found that producing DinJ decreased curli/cellulose production 5.4 ± 1.2-fold in the presence of erythromycin stress (7.5 μg ml−1 for 3 h) when compared with an empty plasmid. Similar to the results under erythromycin stress, producing DinJ in the presence of oxidative stress decreased curli/cellulose production by 4 ± 1-fold (2 mM H2O2 for 180 min at 30°C) (Fig. 2D). We used oxidative stress along with erythromycin since RpoS is crucial for resistance to oxidative stress (Sammartano et al., 1986; Hengge-Aronis, 2002) and since it regulates the antioxidant activities of catalase and superoxide dismutase (Lacour and Landini, 2004). The positive control MqsA also decreased curli/cellulose by 8 ± 2-fold (Fig. 2D). Therefore, DinJ decreases adhesin formation.
Given that RpoS levels are reduced, catalase activity should be decreased when DinJ is overproduced since RpoS is a positive regulator of catalase activity. To confirm this, we checked catalase activity by both a colorimetric assay with lactoperoxidase and dicarboxidine (Macvanin and Hughes, 2010) and a bubble formation assay (Wang et al., 2011). As expected, overproduction of DinJ in MG1655 Δ6 decreased the catalase activity by a 13 ± 3-fold compared with the empty plasmid control (only trace catalase activity was seen in the DinJ-producing strain). Catalase converts H2O2 to H2O and O2; hence, the reduced ability of the cells to decompose H2O2 when DinJ reduces RpoS was demonstrated by a dramatic reduction in oxygen bubbles upon addition of H2O2 to Δ6 (Fig. 2E). Therefore, DinJ decreases catalase activity.
DinJ represses cspE
To determine the mechanism by which DinJ reduces RpoS levels, we investigated which genes were differentially expressed during production of DinJ using a whole-transcriptome analysis. Exposure to erythromycin (75 μg ml−1 for 10 min) significantly altered the expression of 925 genes as compared with the untreated control based on a cut-off ratio of 2.5 (Table 2). Of these, 822 genes were induced, while 103 genes were repressed.
Table 2.
Partial list of differentially expressed genes for Δ6/pCA24N-dinJ versus Δ6/pCA24N.
| Group and gene name | b number | Fold change | Description of encoded protein |
|---|---|---|---|
| Stress response | |||
| osmB | b1283 | −3.0 | Osmotically and stress inducible lipoprotein |
| cspE | b0623 | −3.5 | DNA-binding transcriptional repressor, cold-shock protein |
| umuC | b1184 | 3.7 | SOS mutagenesis and repair, DNA polymerase V, subunit D |
| umuD | b1183 | 4.0 | DNA polymerase V subunit, error-prone repair |
| ybeS | b0646 | 3.0 | Protein folding, heat shock protein binding |
| ygeG | b2851 | 3.0 | Response to stress |
| Cell wall, membrane and motility/chemotaxis | |||
| csgB | b1041 | 2.5 | Curlin nucleator protein, homology with major curlin, CsgA |
| eaeH | b0297 | 2.5 | Cell adhesion |
| fliC | b1923 | −6.1 | Flagellar filament structural protein (flagellin) |
| fliK | b1943 | 3.2 | Flagellar hook-length control protein |
| yaiC | b0385 | 2.8 | Cellulose, biofilm, motility regulator, diguanylate cyclase; CsgD-regulated |
| yehD | b2111 | 2.6 | Predicted fimbrial-like adhesin protein |
| yfjP | b2632 | 3.0 | Required for swarming phenotype |
| DNA replication, recombination and repair | |||
| oraA | b2698 | 3.0 | Blocks RecA filament extension; inhibitor of RecAATPase, reduced resistance to DNA damage |
| recN | b2616 | 4.6 | DNA Recombination and repair |
| rusA | b0550 | 2.6 | DNA repair Endonuclease |
| yeeS | b2002 | 2.6 | CP4-44 prophage; predicted DNA repair protein |
| Transport | |||
| fruB | b2169 | 9.8 | Fructosephosphotransferase enzyme III |
| yddL | b1472 | 4.0 | Transport, OmpCFN porin family, N-terminal fragment |
| Metabolism | |||
| fruK | b2168 | 4.0 | Fructose-1-phosphate kinase; 1-phosphofructokinase |
| ybdK | b0581 | 4.3 | Glutathione biosynthesis, cysteine ligase activity |
Cells at 37°C had DinJ produced for 2 h via 1 mM IPTG then were adjusted to a turbidity of 1.0 and were exposed to erythromycin stress for 10 min in LB low-salt medium. The cut-off ratio was 2.5-fold.
Consistent with the qRT-PCR results, rpoS was not changed upon production of DinJ (1.1-fold). Critically, some stress-related genes were significantly repressed by DinJ including the gene for the DNA-binding transcriptional repressor cspE (3.5-fold) and cspC (2.3-fold). Given that CspE is a positive regulator for RpoS and that the promoter of cspE contains a LexA/DinJ-like palindrome, we focused on it as a possible negative regulator of RpoS. To confirm that DinJ represses the transcription of cspE, we quantified the transcript levels of cspE using qRT-PCR for Δ6/pCA24N-dinJ versus Δ6/pCA24N with erythromycin stress and found that DinJ repressed cspE dramatically (114 ± 35-fold). Therefore, DinJ either directly or indirectly represses cspE, which results in a reduction in RpoS.
DinJ binds the cspE promoter
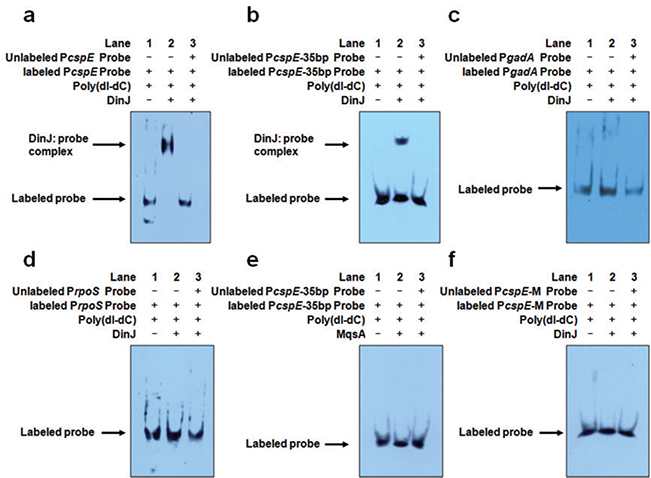
To determine if antitoxin DinJ directly controls the transcription of cspE by binding, an electrophoretic mobility shift assay (EMSA) was used for the 35 bp (from −87 to −53) and 176 bp fragments of PcspE (from −123 to +53), both of which contain the consensus LexA binding sequence (5′-TACTG(TA)5CAGTA-3′) (Fernández De Henestrosa et al., 2000) as shown in Fig. S1. EMSA revealed that DinJ binds both PcspE fragments (Fig. 3A and B). In contrast, for the negative control, at the same concentrations, there were no shifts for DinJ for the promoter of the negative control gadA (Fig. 3C) and also no shift for the promoter of rpoS (Fig. 3D, promoter sequence shown in Fig. S2). Also, antitoxin MqsA failed to bind PcspE (Fig. 3E). To confirm that DinJ binds PcspE, we mutated the LexA binding sequence from CTGG to GACC and found that DinJ no longer binds PcspE (Fig. 3F). Therefore, DinJ represses cspE by directly binding it while it does not regulate rpoS directly.
Fig. 3.
DinJ binds PcspE at the LexA palindrome but does not bind PrpoS.
A. The 176 bp DNA fragment of the cspE promoter (PcspE) was incubated with DinJ. Lanes 1–3: 6 ng of biotin-labelled PcspE; Lane 2: addition of 1.2 μg DinJ; and Lane 3: addition of 1.2 μg DinJ and 600 ng of unlabelled PcspE.
B. The 35 bp DNA fragment of cspE promoter (PcspE-35bp) was incubated with DinJ. Lanes 1–3: 30 pg of biotin-labelled PcspE; Lane 2: addition of 1.2 μg DinJ; and Lane 3: addition of 1.2 μg DinJ and 12 ng of unlabelled PcspE.
C. The 185 bp DNA fragment of gadA promoter (PgadA) was incubated with DinJ as a negative control. Lanes 1–3: 6 ng of biotin-labelled PgadA; Lane 2: addition of 1.2 μg DinJ; and Lane 3: addition of 1.2 μg DinJ and 600 ng of unlabelled PgadA.
D. The 185 bp DNA fragment of rpoS promoter (PrpoS) was incubated with DinJ. Lanes 1 to 3: 6 ng of biotin-labelled PrpoS; Lane 2: addition of 1.2 μg DinJ; and Lane 3: addition of 1.2 μg DinJ and 600 ng unlabelled PrpoS.
E. The 35 bp DNA fragment of cspE promoter (PcspE-35bp) was incubated with MqsA as a negative control. Lanes 1–3: 30 pg of biotin-labelled PcspE; Lane 2: addition of 1.2 μg MqsA; and Lane 3: addition of 1.2 μg MqsA and 12 ng of unlabelled PcspE. F. The mutated 35 bp cspE promoter (PcspE-M) lacking the LexA palindrome was incubated with DinJ. Lanes 1–3: 30 pg of biotin-labelled mutated PcspE; Lane 2: addition of 1.2 μg DinJ; and Lane 3: addition of 1.2 μg DinJ and 12 ng of unlabelled mutated PcspE. The unspecific competitor poly(dI-dC) was used for all samples.
DinJ depends on CspE to control RpoS levels and RpoS-controlled cell viability, swimming motility, cell adhesins, and catalase activity
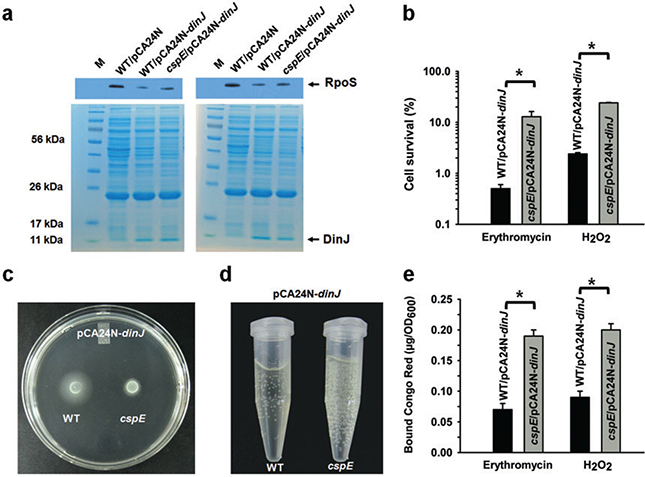
To confirm that DinJ controls indirectly the above phenotypes related to RpoS (resistance to erythromycin and hydrogen peroxide, cyclic diguanylate levels, swimming, cell adhesins and catalase activity) via its repression of cspE, we investigated whether DinJ was effective in the absence of CspE; i.e. we reasoned that deleting cspE would prevent DinJ from reducing RpoS levels and affecting these phenotypes. Using BW25113cspE/pCA24N-dinJ versus BW25113/pCA24N-dinJ, as expected, producing DinJ reduced RpoS but only in the presence of CspE (Fig. 4A). Thus, CspE is required for DinJ to regulate RpoS protein levels.
Fig. 4.
DinJ requires CspE to influence RpoS levels and RpoS-controlled viability, motility, cell adhesins and catalase activity.
A. Lanes 2, 3 and 4 show RpoS levels as detected by an anti-RpoS antibody for BW25113/pCA24N, BW25113/pCA24N-dinJ and BW25113 cspE/pCA24N-dinJ. Two independent cultures were used for each strain and both experiments are shown.
B. Percentage of cells (BW25113 cspE/pCA24N-dinJ versus BW25113/pCA24N-dinJ) which survive erythromycin (75 μg ml−1) stress for 10 min and oxidative stress induced by 20 mM H2O2 for 10 min. Error bars indicate standard error of mean (n = 2). Error bars indicate standard error of mean (n = 2). Significant changes are marked with an asterisk for P < 0.05.
C. Swimming motility after 12 h of growth at 37°C for BW25113 cspE/pCA24N-dinJ versus BW25113/pCA24N-dinJ. Two independent cultures were used, and a representative image is shown.
D. Images of BW25113 cspE/pCA24N-dinJ versus BW25113/pCA24N-dinJ cultures (turbidity of 1) 10 min after adding 40 mM H2O2. Bubbles are oxygen produced by the decomposition of hydrogen peroxide by catalase (2 H2O2 → 2 H2O + O2). Two independent cultures were used, and a representative image is shown.
E. Curli/cellulose production for strains BW25113 cspE/pCA24N-dinJ versus BW25113/pCA24N-dinJ after 3 h of incubation with 7.5 μg ml−1 erythromycin or 2 mM H2O2. All assays were performed at 30°C. Error bars indicate standard error of mean (n = 2). Significant changes are marked with an asterisk for P < 0.05.
Also as expected, we found that deleting cspE caused a 24 ± 2-fold increase in cell survival when DinJ was produced from pCA24N-dinJ under erythromycin stress compared with the wild-type strain (Fig. 4B). Similarly, deleting cspE also increased cell survival 10.2 ± 0.6-fold under oxidative stress (Fig. 4B). Hence, DinJ is not able to reduce RpoS levels without CspE so the cells survive better under stress without CspE. Also, deleting cspE abolished the increase in motility from DinJ (Fig. 4C). Furthermore, deleting cspE also prevented DinJ from reducing curli/cellulose under both erythromycin stress (2.6 ± 0.1-fold increase for cspE) and oxidative stress (2.1 ± 0.1-fold increase for cspE) (Fig. 4E). In addition, deleting cspE prevented DinJ from reducing catalase activity (3.73 ± 0.5-fold increase for cspE) and this is also illustrated by the larger bubble formation for the cspE strain (Fig. 4D). Together, these four sets of results convincingly show that CspE is crucial for DinJ to control phenotypes related to RpoS under the general stress response (cell survival with erythromycin and hydrogen peroxide, motility, production of adhesins and catalase activity).
Discussion
Previously, a single antitoxin, MqsA, was shown to regulate more than its own synthesis by regulating the general stress response through direct repression of rpoS via its mqsRA-like palindrome (Wang et al., 2011). As a result of this repression of rpoS, the concentration of the secondary messenger c-di-GMP is decreased, which results in increased motility, decreased production of adhesins, reduced biofilm formation and reduced catalase activity (Wang et al., 2011). Here, our results show that DinJ regulates cspE demonstrate that regulation of other genes may be a general feature of antitoxins. In addition, the results demonstrate that antitoxin DinJ influences RpoS activity via a novel, indirect mechanism: DinJ represses cspE which encodes the cold-shock protein CspE that induces translation of rpoS mRNA (Phadtare et al., 2002; Phadtare et al., 2006).
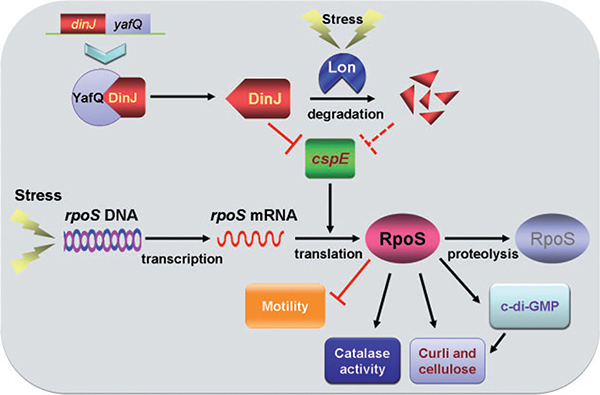
RpoS regulation is complex and includes regulation at the level of transcription, translation, protein stability and activity (Hengge, 2008). Positive regulators of rpoS translation include the cold-shock proteins CspC and CspE (Phadtare and Inouye, 2001), RNA binding protein Hfq (Soper et al., 2010), nucleoid protein HU (Balandina et al., 2001), and some small regulatory RNAs (DsrA, RprA and ArcZ) (Soper et al., 2010). Critically, our transcriptome study identified that DinJ represses cspE (Table 2) (these data were verified by qRT-PCR), and we showed that DinJ binds the promoter region of cspE at the LexA/CspE binding site (5′-CTGGATGCGCTTTCAG-3′) to repress cspE. Mutagenesis of the LexA/CspE binding site and the lack of binding of DinJ to the mutated promoter confirmed that DinJ represses cspE. Further evidence for DinJ repressing cspE was provided by the dependence on CspE for the effect of DinJ on RpoS-related phenotypes (resistance to erythromycin and hydrogen peroxide, swimming, cell adhesins and catalase activity). A schematic of our current understanding of how antitoxin DinJ mediates the general stress response is shown in Fig. 5.
Fig. 5.
Schematic of how DinJ impacts the general stress response. RpoS levels are reduced indirectly by DinJ through its control of cspE. Under erythromycin stress, DinJ is degraded by protease Lon, and cspE transcription is derepressed. The increased CspE levels lead to greater RpoS translation, which increases c-di-GMP concentrations, catalase activity and curli/cellulose production and decreases swimming. The lightning bolt indicates erythromycin stress, ‘→’ indicates induction and ‘⊥’ indicates repression.
Since our results show clearly that DinJ is degraded under erythromycin stress (Fig. 1A) but not under heat, acid, oxidative, gentamicin, mitomycin C, ampicillin, tetracycline and nalidixic acid stress, we have determined the first conditions that lead to the degradation of DinJ and have shown that Lon is required for this degradation (Fig. 2C). We note however that we do not fully understand the relevance of erythromycin stress for DinJ degradation. Consistent with our results indicating DinJ is degraded by Lon, Prysak and colleagues (2009) also showed that Lon protease should be involved in the degradation of DinJ. This degradation of DinJ should lead to derepression of cspE transcription, which should result in an increase in RpoS. Hence, upon erythromycin stress, Lon is induced and degrades DinJ like other antitoxins, cspE is derepressed, RpoS levels increase, and the cell directs transcription towards stress-related genes, which includes increasing c-di-GMP concentrations and catalase activity.
In summary, our current results indicate that DinJ is involved in mediating the general stress response by indirect regulation of RpoS via its direct control of cspE. The results provide additional proof that the ubiquitous TA systems are far more than genomic debris. Furthermore, they provide insights into how antitoxins allow the cell to respond to stress (it is not well understood how external stress is mediated to the inside of the cell). Also, the results suggest new methods for controlling cell behaviour such as persistence and antibiotic resistance since if antitoxins could be made to more readily bind their targets, then the cell would be less able to respond to stress (i.e. antibiotics) and less able to become dormant through the action of toxins.
Experimental procedures
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids are listed in Table 1. Luria–Bertani (LB) (Sambrook et al., 1989) at 37°C was used for all the experiments unless noted. For construction of pBS(Kan)-dinJ, dinJ was PCR-amplified from E. coli MG1655 chromosomal DNA as a template using front primer dinJ-KpnI-F (primers are shown in Table S1), and rear primer dinJ-SacI-R. The PCR product was cloned into the multiple cloning site of pBS(Kan) (Canada et al., 2002) after double digestion with KpnI and SacI restriction enzymes. The dinJ gene in pBS(Kan) is under the control of a lac promoter. The pBS(Kan)-dinJ plasmid was confirmed by DNA sequencing with pBS(Kan)-seq primer. Cell growth was assayed using the turbidity at 600 nm for shake flasks. Kanamycin (50 μg ml−1) and chloramphenicol (30 μg ml−1) were used to maintain the pBS(Kan)-based and pCA24N-based plasmids (Kitagawa et al., 2005).
Survival assays
Overnight cultures were diluted to a turbidity of 0.05 and grown in LB low-salt medium (0.05% NaCl, 0.1% tryptone and 0.5% yeast extract) to a turbidity of 0.5, then 1 mM IPTG was used to induce dinJ for 2 h (low-salt medium was used to avoid osmotic stress). Cells were centrifuged and resuspended in LB to a turbidity of 1.0 and exposed to either erythromycin (75 μg ml−1) for 10 min or 20 mM H2O2 for 10 min (Wang et al., 2011). To investigate how DinJ affects cell survival, Δ6/pCA24N-dinJ and Δ6/pCA24N were used. To investigate how DinJ affects cell survival in the absence of CspE, BW25113 cspE/pCA24N-dinJ and BW25113/pCA24N-dinJ were used.
c-di-GMP assay
c-di-GMP was quantified using HPLC as described previously (Ueda and Wood, 2009). Δ6/pCA24N-dinJ, Δ6/pCA24N and Δ6/pCA24N-mqsA (positive control) were grown in 1 l LB medium for 2.5 h, then 0.5 mM IPTG was added to induce dinJ or mqsA for 15 h. A photodiode array detector (Waters, Milford, MA, USA) was used to detect nucleotides at 254 nm after the HPLC separation step. Synthetic c-di-GMP (BIOLOG Life Science Institute) was used as a standard and to verify the c-di-GMP peak via spiking. This experiment was performed with two independent cultures.
Swimming motility, curli/cellulose and catalase assays
Cell motility was examined on motility agar plates (1% tryptone, 0.25% NaCl and 0.3% agar) (Sperandio et al., 2002). Curli/cellulose production was quantified by the Congo red binding assay (Ma and Wood, 2009) performed at 30°C in the presence of erythromycin stress (7.5 μg ml−1 erythromycin for 180 min) or oxidative stress (2 mM H2O2 for 180 min). IPTG (1 mM) was added in both assays to induce dinJ via the pCA24N-based plasmids. Catalase activity was quantified by a colorimetric assay using dicarboxidine/lactoperoxidase to detect the remaining H2O2 (Macvanin and Hughes, 2010). Catalase activity was also tested by a bubble formation assay as described previously (Wang et al., 2011). Bubbles are oxygen produced by the decomposition of hydrogen peroxide by catalase (2 H2O2 → 2 H2O + O2).
RNA isolation and whole-transcriptome analysis
Whole-transcriptome analysis was performed using planktonic cells of Δ6/pCA24N-dinJ versus Δ6/pCA24N with 1 mM IPTG added at a turbidity of 0.5 for 2 h, then the cells were exposed to erythromycin (75 μg ml−1) for 10 min and harvested as quickly as possible to avoid mRNA degradation. Total RNA was isolated from cells as described previously (Ren et al., 2004a) with a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) using a bead beater (Biospec, Bartlesville, OK, USA) and RNAlater buffer (Applied Biosystems, Foster City, CA, USA) to stabilize the RNA. cDNA synthesis, fragmentation, hybridizations and data analysis were as described previously (González Barrios et al., 2006). The E. coli GeneChip Genome 2.0 array (Affymetrix, Santa Clara, CA, USA; P/N 900551) was used, and if the gene with the larger transcription rate did not have a consistent transcription rate based on the 11 probe pairs (P < 0.05), these genes were not used. A gene was considered differentially expressed when the P-value for comparing two chips was < 0.05 (to assure that the change in gene expression was statistically significant and that false positives arise less than 5%). Since the standard deviation for expression ratio for all the genes was 2.0, genes were considered differentially expressed if they had greater than 2.5-fold changes for condition (Ren et al., 2004b). Gene functions were obtained from the Ecogene database (http://www.ecogene.org/). The microarray raw data are deposited at the Gene Expression Omnibus of the National Center for Biotechnology Information (GSE30692).
qRT-PCR
After isolating RNA using RNAlater (Ambion), 50 ng of total RNA was used for qRT-PCR using the Power SYBR Green RNA-to-CT 1-Step Kit and the StepOne Real-Time PCR System (Applied Biosystems). Primers were designed using Primer3 Input Software (v0.4.0) and are listed in Table S2. The housekeeping gene rrsG was used to normalize the gene expression data. The annealed temperature was 60°C for all the genes in this study. To investigate the rpoS and cspE mRNA changes by DinJ under erythromycin stress conditions, overnight cultures of Δ6/pCA24N-dinJ and Δ6/pCA24N were inoculated into LB low-salt medium (0.05% NaCl) with an initial turbidity of 0.2 and grown to a turbidity of 0.5, then 1 mM IPTG was added for 2 h to induce dinJ until a turbidity ~ 3.0. After diluting to a turbidity ~ 1.0, cells were exposed to erythromycin (75 μg ml−1) for 10 min.
Purification of DinJ
DinJ was produced in Δ6/pCA24N-dinJ via 1 mM IPTG at room temperature overnight. DinJ was purified using a Ni-NTA resin (Qiagen, Valencia, CA, USA) as described in the manufacture’s protocol. Purified DinJ was dialysed against buffer (25 mM Tris-HCl, pH 7.6) at 4°C overnight.
Electrophoretic mobility shift assays
To investigate binding of DinJ to promoter regions, EMSA was performed as described previously (Prysak et al., 2009) with some modification. Briefly, complementary oligonucleotides (35-mers) biotin labelled at the 3′ end (Table S1) were purchased from Integrated DNA technologies and were used to synthesize the wild-type LexA binding box and the corresponding mutated LexA binding box of the cspE promoter (from position −87 to −53) (Fig. S1). The combination of oligonucleotides was annealed at 65°C for 2 min and allowed to cool to room temperature. Promoter regions including the 176 bp fragment of PcspE (from position −123 to +53), the 185 bp fragment of PrpoS (from position −168 to +17), and the 185 bp fragment of PgadA (from position −205 to −21) were amplified using primers shown in Table S1, purified, and labelled with biotin using the biotin 3′ end DNA Labeling Kit (Pierce Biotechnology, Rockford, IL, USA). For the EMSA assay, biotin labelled target promoters were incubated with purified DinJ or MqsA (Brown and Page, 2010) either with or without unlabelled target DNA promoter for 120 min at room temperature in the reaction buffer [10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 5 mM CaCl2, 100 mM NaCl, 1 mM DTT, 5% glycerol and 0.1 mg ml−1 BSA]. Samples were run on a 6% DNA retardation gel (Invitrogen) at 100 V in 0.5× TBE for 75 min at 4°C. The bound protein/DNA mixtures were then transferred to a nylon membrane at 380 mA for 60 min then UV cross-linked at 302 nm. Chemiluminescence was performed with the LightShift Chemiluminescent EMSA Kit (Thermo Scientific).
Western blot analysis
To investigate the degradation of DinJ under stress and the effect of DinJ on RpoS, Western blots were performed as described previously (Wang et al., 2011). To ascertain DinJ levels, Δ6/pCA24N-dinJ and Δ6/pCA24N were grown to a turbidity of 0.1, then 0.5 mM ITPG was added to induce dinJ. When the turbidity reached 1, 200 μg ml−1 rifampin was added to inhibit transcription, and the cell pellets were exposed to various stress conditions including 75 μg ml−1 erythromycin (samples taken at 0, 0.5, 2.5, 5, 10, 15 min), 20 mM H2O2 (0, 2.5, 5, 10, 15 min), 2 μg ml−1 mitomycin C (0, 5, 15, 30 min), 100 μg ml−1 ampicillin (0, 5, 15, 30 min), 50 μg ml−1 gentamicin (0, 5, 15, 30 min), 15 μg ml−1 tetracycline (0, 5, 15, 30 min), 200 μg ml−1 nalidixic acid (0, 5, 15, 30 min), heat (50°C, 0, 5, 15, 30 min) and acid (pH 2.5, 0, 5, 15, 30 min). Samples were processed with 1 mM phenyl-ethylsulfonyl fluoride and protease inhibitor cocktail (Sigma-Aldrich) and sonicated twice on ice for 15 s. Soluble protein samples in supernatants were obtained by centrifuging the cell pellets at 17 000 g for 4 min. The protein concentration was assayed by the BCA assay. The same amount of protein (2 mg) was loaded into each well of a 12% SDS-PAGE gel, then transferred to a PVDF membrane, which was then blocked with 4% BSA in TBST (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. The Western blots were probed with a 1:2000 dilution of primary antibodies raised against a His tag (Cell Signaling Technology), and then with a 1:20 000 dilution of horseradish peroxidase-conjugated goat anti-mouse secondary antibodies (Millipore). To ascertain RpoS levels, strains were grown until a turbidity ~ 3.0 and a 1:2000 dilution of anti-RpoS monoclonal antibody (Neoclone) was used. To investigate how DinJ affects RpoS levels, Δ6/pCA24N-dinJ and Δ6/pCA24N were used. To investigate how DinJ affects RpoS levels in the absence of CspE, BW25113cspE/pCA24N-dinJ, BW25113/pCA24N-dinJ and BW25113/pCA24N were used.
Supplementary Material
Acknowledgements
This work was supported by the NIH (R01 GM089999). We are grateful for the Keio and ASKA strains provided by the Genome Analysis Project in Japan. T.W. is the T. Michael O’Connor Endowed Professor at Texas A & M University.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
References
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae W, Phadtare S, Severinov K, and Inouye M (1999) Characterization of Escherichia coli cspE, whose product negatively regulates transcription of cspA, the gene for the major cold shock protein. Mol Microbiol 31: 1429–1441. [DOI] [PubMed] [Google Scholar]
- Balandina A, Claret L, Hengge-Aronis R, and Rouviere-Yaniv J (2001) The Escherichia coli histone-like protein HU regulates rpoS translation. Mol Microbiol 39: 1069–1079. [DOI] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G III, Bloch CA, Perna NT, Burland V, Riley M, et al. (1997) The complete genome sequence of Escherichia coli K-12. Science 277: 1453–1462. [DOI] [PubMed] [Google Scholar]
- Brown BL, and Page R (2010) Preliminary crystallographic analysis of the Escherichia coli antitoxin MqsA (YgiT/b3021) in complex with mqsRA promoter DNA. Acta Crystallogr Sect F Struct Biol Cryst Commun 66: 1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butala M, Žgur-Bertok D, and Busby SJW (2009) The bacterial LexA transcriptional repressor. Cell Mol Life Sci 66: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada KA, Iwashita S, Shim H, and Wood TK (2002) Directed evolution of toluene ortho-monooxygenase for enhanced 1-naphthol synthesis and chlorinated ethene degradation. J Bacteriol 184: 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Mikkelsen M, Pedersen K, and Gerdes K (2001) RelE, a global inhibitor of translation, is activated during nutritional stress. Proc Natl Acad Sci USA 98: 14328–14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, and Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández De Henestrosa AR, Ogi T, Aoyagi S, Chafin D, Hayes JJ, Ohmori H, and Woodgate R (2000) Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol Microbiol 35: 1560–1572. [DOI] [PubMed] [Google Scholar]
- Gerdes K, Christensen SK, and Lobner-Olesen A (2005) Prokaryotic toxin–antitoxin stress response loci. Nat Rev Microbiol 3: 371–382. [DOI] [PubMed] [Google Scholar]
- González Barrios AF, Zuo R, Hashimoto Y, Yang L, Bentley WE, and Wood TK (2006) Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J Bacteriol 188: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotfredsen M, and Gerdes K (1998) The Escherichia coli relBE genes belong to a new toxin–antitoxin gene family. Mol Microbiol 29: 1065–1076. [DOI] [PubMed] [Google Scholar]
- Hengge R (2008) The two-component network and the general stress sigma factor RpoS (σs) in Escherichia coli. Adv Exp Med Biol 631: 40–53. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R (2002) Signal transduction and regulatory mechanisms involved in control of the σs (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 66: 373–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Tavano C, Wickner S, and Trun N (2006) Specificity of DNA binding and dimerization by CspE from Escherichia coli. J Biol Chem 281: 40208–40215. [DOI] [PubMed] [Google Scholar]
- Jorgensen MG, Pandey DP, Jaskolska M, and Gerdes K (2009) HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea. J Bacteriol 191: 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Wang X, Zhang XS, Grigoriu S, Page R, Peti W, and Wood TK (2010) Escherichia coli toxin/antitoxin pair MqsR/MqsA regulate toxin CspD. Environ Microbiol 12: 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, and Mori H (2005) Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12: 291–299. [DOI] [PubMed] [Google Scholar]
- Lacour S, and Landini P (2004) σs-dependent gene expression at the onset of stationary phase in Escherichia coli: Function of σs-dependent genes and identification of their promoter sequences. J Bacteriol 186: 7186–7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landini P (2009) Cross-talk mechanisms in biofilm formation and responses to environmental and physiological stress in Escherichia coli. Res Microbiol 160: 259–266. [DOI] [PubMed] [Google Scholar]
- Lewis LK, Harlow GR, Gregg-Jolly LA, and Mount DW (1994) Identification of high-affinity binding-sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J Mol Biol 241: 507–523. [DOI] [PubMed] [Google Scholar]
- Ma Q, and Wood TK (2009) OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ Microbiol 11: 2735–2746. [DOI] [PubMed] [Google Scholar]
- Macvanin M, and Hughes D (2010) Assays of sensitivity of antibiotic-resistant bacteria to hydrogen peroxide and measurement of catalase activity. Methods Mol Biol 642: 95–103. [DOI] [PubMed] [Google Scholar]
- Motiejūnaite R, Armalyte J, Deputiene V, and Sužiedeliene E (2005) Escherichia coli dinJ-yafQ operon shows characteristic features of bacterial toxin–antitoxin modules. Biologija 4: 9–14. [Google Scholar]
- Motiejūnaite R, Armalyte J, Markuckas A, and Sužiedeliene E (2007) Escherichia coli dinJ-yafQ genes act as a toxin–antitoxin module. FEMS Microbiol Lett 268: 112–119. [DOI] [PubMed] [Google Scholar]
- Pesavento C, Becker G, Sommerfeldt N, Possling A, Tschowri N, Mehlis A, and Hengge R (2008) Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev 22: 2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadtare S, and Inouye M (1999) Sequence-selective interactions with RNA by CspB, CspC and CspE, members of the CspA family of Escherichia coli. Mol Microbiol 33: 1004–1014. [DOI] [PubMed] [Google Scholar]
- Phadtare S, and Inouye M (2001) Role of CspC and CspE in regulation of expression of RpoS and UspA, the stress response proteins in Escherichia coli. J Bacteriol 183: 1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadtare S, Inouye M, and Severinov K (2002) The nucleic acid melting activity of Escherichia coli CspE is critical for transcription antitermination and cold acclimation of cells. J Biol Chem 277: 7239–7245. [DOI] [PubMed] [Google Scholar]
- Phadtare S, Tadigotla V, Shin WH, Sengupta A, and Severinov K (2006) Analysis of Escherichia coli global gene expression profiles in response to overexpression and deletion of CspC and CspE. J Bacteriol 188: 2521–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prysak MH, Mozdzierz CJ, Cook AM, Zhu L, Zhang Y, Inouye M, and Woychik NA (2009) Bacterial toxin YafQ is an endoribonuclease that associates with the ribosome and blocks translation elongation through sequence-specific and frame-dependent mRNA cleavage. Mol Microbiol 71: 1071–1087. [DOI] [PubMed] [Google Scholar]
- Ren D, Bedzyk LA, Thomas SM, Ye RW, and Wood TK (2004a) Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol 64: 515–524. [DOI] [PubMed] [Google Scholar]
- Ren D, Bedzyk LA, Ye RW, Thomas SM, and Wood TK (2004b) Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli. Biotechnol Bioeng 88: 630–642. [DOI] [PubMed] [Google Scholar]
- Ren D, Bedzyk LA, Ye RW, Thomas SM, and Wood TK (2004c) Stationary-phase quorum-sensing signals affect autoinducer-2 and gene expression in Escherichia coli. Appl Environ Microbiol 70: 2038–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, and Maniatis T (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sammartano LJ, Tuveson RW, and Davenport R (1986) Control of sensitivity to inactivation by H2O2 and broadspectrum near-UV radiation by the Escherichia-coli katF locus. J Bacteriol 168: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper T, Mandin P, Majdalani N, Gottesman S, and Woodson SA (2010) Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci USA 107: 9602–9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V, Torres AG, and Kaper JB (2002) Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43: 809–821. [DOI] [PubMed] [Google Scholar]
- Tan Q, Awano N, and Inouye M (2011) YeeV is an Escherichia coli toxin that inhibits cell division by targeting the cytoskeleton proteins, FtsZ and MreB. Mol Microbiol 79: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenson T, Lovmar M, and Ehrenberg M (2003) The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J Mol Biol 330: 1005–1014. [DOI] [PubMed] [Google Scholar]
- Ueda A, and Wood TK (2009) Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885). PLoS Pathog 5: e1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, and Wood TK (2011) Toxin/Antitoxin systems influence biofilm and persister cell formation and the general stress response. Appl Environ Microbiol 77: 5577–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kim Y, Hong SH, Ma Q, Brown BL, Pu MM, et al. (2011) Antitoxin MqsA helps mediate the bacterial general stress response. Nat Chem Biol 7: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JM, Wierman CK, and Hutchinson CR (1985) Genetic analysis of erythromycin production in Streptomyces erythreus. J Bacteriol 164: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbaux M, Mine N, Guerout AM, Mazel D, and Van Melderen L (2007) Functional interactions between coexisting toxin–antitoxin systems of the ccd family in Escherichia coli O157:H7. J Bacteriol 189: 2712–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.