Graphical abstract
Abbreviations: ADME, administration distribution, metabolism and elimination; ADR, adverse drug reaction; ASO, antisense oligonucleotide; BCAA, branched-chain amino acid; CoQ10, Coenzyme Q10; CYP, cytochrome P450; DMD, Duchenne muscular dystrophy; EAA, essential amino acid; EGCG, Epigallocatechin-3-gallate; EMA, European Medicines Agency; EMS, Eosinophilia-Myalgia Syndrome; FDA, Food and Drug Administration; GTE, green tea extract; NAC, N-acetyl cysteine; NO, nitric oxide; NSAID, nonsteroidal anti-inflammatory drug; PD, pharmacodynamics; PK, pharmacokinetics; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species
Keywords: Duchenne muscular dystrophy, Dietary supplements, Adverse drug reactions, Safety, COVID-19, SARS-CoV-2
Abstract
At the moment, little treatment options are available for Duchenne muscular dystrophy (DMD). The absence of the dystrophin protein leads to a complex cascade of pathogenic events in myofibres, including chronic inflammation and oxidative stress as well as altered metabolism. The attention towards dietary supplements in DMD is rapidly increasing, with the aim to counteract pathology-related alteration in nutrient intake, the consequences of catabolic distress or to enhance the immunological response of patients as nowadays for the COVID-19 pandemic emergency. By definition, supplements do not exert therapeutic actions, although a great confusion may arise in daily life by the improper distinction between supplements and therapeutic compounds. For most supplements, little research has been done and little evidence is available concerning their effects in DMD as well as their preventing actions against infections. Often these are not prescribed by clinicians and patients/caregivers do not discuss the use with their clinical team. Then, little is known about the real extent of supplement use in DMD patients. It is mistakenly assumed that, since compounds are of natural origin, if a supplement is not effective, it will also do no harm. However, supplements can have serious side effects and also have harmful interactions, in terms of reducing efficacy or leading to toxicity, with other therapies. It is therefore pivotal to shed light on this unclear scenario for the sake of patients. This review discusses the supplements mostly used by DMD patients, focusing on their potential toxicity, due to a variety of mechanisms including pharmacodynamic or pharmacokinetic interactions and contaminations, as well as on reports of adverse events. This overview underlines the need for caution in uncontrolled use of dietary supplements in fragile populations such as DMD patients. A culture of appropriate use has to be implemented between clinicians and patients’ groups.
1. Introduction
Duchenne muscular dystrophy (DMD) is a severe, progressive muscle wasting disorders affecting around 1 in 5 000 newborn boys. First symptoms arise around two to three years of age, after which a progressive loss of muscle tissue leads to wheelchair dependency, usually in the early teens. Hereafter respiratory and cardiac failure occur, which are often the cause of death before the age of thirty [1]. It is caused by mutations in the DMD gene, located on Xp21, which encodes for the dystrophin protein, leading to its absence [2,3]. A less severe form is Becker muscular dystrophy (BMD), which a short yet partly functional dystrophin protein is expressed [2,3]. Dystrophin provides stability to the muscle fibres by linking the cytoskeletal actin to the extracellular matrix. In its absence, muscle cells get damaged easily, which leads to chronic inflammation, continuous cycles of degeneration and regeneration and eventually replacement of muscle tissue by adipose and fibrotic tissue [4]. Corticosteroids are at the moment the main pharmacological treatment for DMD. Furthermore, treatment of, among others, respiratory and cardiac failure belongs to the standard care for DMD [[5], [6], [7]].
Recently three therapies that target the primary effect, have received marketing authorization. In Europe, the European Medicines Agency (EMA) has approved ataluren, applicable to patients carrying premature stop codons [8], and in the United States, the Food and Drug Administration (FDA) has licensed eteplirsen and golodirsen, relevant for patients amenable for exon 51 and 53 skipping, respectively [9,10]. The research in the field is intense and in the last few years, EMA and FDA granted the orphan designation to several drugs with various mechanism of actions like, among the other, monoamine oxidase inhibitors (rasagiline), ion transporters blockers (rimeporide) and histone deacetylase inhibitors (givinostat) and at different level of clinical investigation. Lists of designated orphan drugs and trials ongoing in DMD and BMD are available and accessible online (https://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm; https://ec.europa.eu/health/documents/community-register/html/reg_od_act.htm?sort=a; https://clinicaltrials.gov/).
Other potential primary therapies, like gene therapy using microdystrophins and exon skipping of other exons are investigated [11]. These therapies have so far shown moderate improvements and most of them are not applicable to all patients, targeting the secondary effects of the lack of dystrophin could be an alternative approach. Furthermore, it could serve as an additional treatment to enhance the effects of primary medicines [11]. With the aim to facilitate the research process, EMA and FDA released guidelines for the development of medicinal products for the treatment of Duchenne or Becker muscular dystrophy [12] (https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-duchenne-becker-muscular-dystrophy; https://www.fda.gov/media/92233/download).
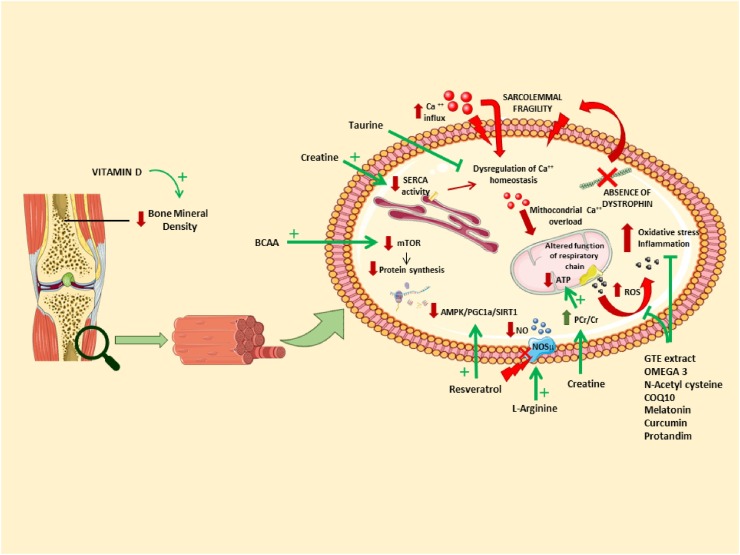
Disturbances of the metabolic system are one of the secondary consequences of the absence of dystrophin [13]. Changes in insulin signalling and mitochondrial function have been observed in animal models and patients [[14], [15], [16], [17], [18]]. DMD patients show alterations in body composition and energy expenditure [[19], [20], [21]]. In glucocorticoid-naïve boys at young age up to 50 % of patients is overweight [22,23,24]. Effects are exacerbated using corticosteroids, which is now the main treatment for DMD. These can lead to weight gain, cushingoid features, hyperglycaemia and growth restrictions [25]. Older patients, however, are at risk of underweight and malnutrition, amongst others due to increasing difficulties with eating [22,26,27]. Therefore, the importance of nutritional management becomes more and more recognized [28,29]. Knowledge is, however, lacking what are the best recommendations for DMD patients of different ages. The current guidelines only give general recommendations in the field of nutrition [5].
One of the aspects of nutrition is the use of dietary supplements. At the moment, only the use of vitamin D if the serum level of 25hydroxyvitamin D is below 30 ng/mL, and calcium if intake is low, is recommended [5]. It is advised to follow the dietary reference intakes for the general population [30]. It is known that many patients use other nutritional supplements without prescription, but information on the magnitude and the exact supplements used is lacking. This is also influenced by geographical and cultural differences, which increases the uncertainty in this field for the DMD community. Similarly, extraordinary emergencies, like those related by COVID-19 by the outbreak of SARS-CoV-2 virus, may reinforce the idea that implementation of diet with vitamins and other supplements can enhance the immune response, thus protecting fragile patients such as DMD as well as BMD patients. This can lead to further fragmentation of the situations worldwide, that are in turn poorly controlled by health specialists.
The aim of this review is to briefly review the long list of dietary supplements commonly used by DMD patients and easily available without medical prescription. Other than briefly mention the presumed mechanism of their claimed beneficial action, the present work mainly focuses to underline that their use needs to be carefully balanced with the limited information about proper dosing and different pathological phases to observe the efficacy and the high risk of toxicity related to the uncontrolled use. Also, a better distinction has to be made between “dietary supplements” and “natural active principles” as the rationale for their use is markedly different along with doses, benefit/safety ratio and experimental settings for validation.
2. Complex therapeutic regimens and risk of adverse drug reactions (ADR)
Complex and chronic pathologies in the paediatric population, such as DMD, are at high risk of adverse drug reactions (ADR), due to the combination of factors. These include the little information available about drug safety in children, the altered pharmacokinetic (PK) and pharmacodynamic (PD) process due to both body development and pathology progression, and mostly for the possible interactions between drugs. In fact, according to the pathology phase, DMD patients are exposed to multiple therapies: standards of care prescribe the early start of treatment with steroids, with various other medications added on the top, e.g. to prevent and control cardiovascular complication (e.g. ACE inhibitors, beta-blockers) or other pathology processes, or new drugs when patients take part in clinical trials [[5], [6], [7],31]. Polytherapy should also take into account supplements, such as the above-mentioned vitamin D to delay steroid-induced osteoporosis.
A careful post-marketing monitoring of ADR is crucial for assessing the risk/ benefit ratio of drugs and complex therapeutic protocols in the real world conditions [32].
2.1. Mechanisms of drug-to-drug and drug-to-supplement interactions
Drug-to-drug (D-D) and drug-to-supplement (D-S) interactions can occur at both the PD and the PK level. Biological effects of any sort of bioactive compounds, of either natural, chemical or biosynthetic source, are due to high-affinity interaction with molecular targets with intensity of effect and risk/benefit ratio being also related to dosing. Two compounds acting on the same target, or on its cellular path, may lead to synergistic or antagonistic PD interaction with the result of enhancing effects, disclosing potential ADR or reducing therapeutic efficacy. Importantly, the inability to reach the therapeutic effect, due to inadequate target engagement is by itself an ADR [33,34].
Clinically relevant D-D and D-S interactions can also occur during each of the four distinct phases (ADME) of PK (Fig. 1 ), Consequently, this may profoundly affect the reciprocal level of a drug or its metabolite in the body, including main target or off-sites, and consequently therapeutic action and toxicity. Interactions at the level of the enzymes of phase I and phase II metabolic reactions are key ones. Many compounds can act as enzyme inducers or inhibitors which would result in either reduced or enhanced exposure of another therapeutic, respectively. Similarly, synergistic or antagonistic interaction can occur at any other level, from drug absorption to distribution (i.e. competition for plasma protein binding) and elimination, also via specific interaction with transporters and other targets. According to these considerations novel approaches for predicting ADME interactions, i.e. by in silico studies, are ongoing [35,36].
Fig. 1.
Phases of ADME and primary mechanisms involved in drug-to-supplement interactions.
3. General aspects in safety control by law of dietary supplements
Dietary supplements include one or more ingredients labelled to enrich diet, including vitamins, minerals, herbs, amino acids, whey protein, essential fatty acids and other components, formulated for oral use as pills, tablets, syrups, etc.
In USA and EU-countries, dietary supplements are regulated as food, and no prove of safety nor formal approval is required before their commercialization; however, due to the intense and global interest toward these products, there is an increasing attention by regulators with respect to safety issues [37,38]. For instance, for a new dietary ingredient, the manufacturer must provide information about the reasonable evidence for safe human use of the product.
Legally, dietary supplements cannot be claimed to exert any action towards pathology, either for diagnosis, prevention or treatment. Rather, the health claim for a dietary supplement is for correcting nutritional deficiencies that increase the risk of a health-related condition. In parallel, nutrient content claims and structure/function claims are related to the amount of the nutrient and how this has an impact on physiological functions. As described by the Office of Dietary Supplements of NIH (https://ods.od.nih.gov/), although structure/function claims do not require FDA approval, the label should clearly indicate that the product has not been approved by FDA nor intends to have any drug-related action [[37], [38], [39]].
Regulators, such as FDA and European Commission (through the European Food Safety Authority; EFSA, https://www.efsa.europa.eu/en/topics/topic/food-supplements) are active in post-marketing safety surveillance and in providing specific guidance to evaluate sources, and establishing limits for ingredients and contaminants. Consumers, health professionals and industry members can report unwanted reactions to dietary supplements and regulators can take actions to protect the public from unsafe products, including withdrawal due to proven or suspected harmful effects and toxicity. Reasons for recalls include contamination by microbiological species, pesticides, and heavy metals as well as abnormal presence of dietary ingredients with respect to what claimed in the label, including absence, reduction or excess, or the presence of other active principles [40]. In fact, many supplements contain ingredients that have strong biological effects, and such products may not be safe in all people, especially if an underlying pathology or an acute viral or bacterial infection requires concomitant use of other drugs, increasing the risk of potential harmful interactions (see Directive 2002/46/EC [41]).
It should be underlined that food and dietary supplements are also freely available on the web, which increases the risk of consumers being exposed to products of limited quality in terms of content of active principles and contaminants.
4. The reason for attention to supplements and nutraceuticals in DMD
A great interest has been raised toward dietary supplements in DMD [42]. One of the main reasons resides in the possibility to get adjuvant control of secondary pathogenic events, possibly related to altered metabolism, diet intake or dietary requirements, and based on their claimed mechanism of action. Then, supplements and nutraceuticals can potentially control inflammation and oxidative stress, provide a clue to overcome impaired protein synthesis/proteolysis related to acute necrosis or act as energizer or metabolic booster at skeletal muscle level [42]. The actions of some supplements in DMD have been supported by well-designed preclinical studies, while for many others the action is elusive, and more evidence is needed [28]. Based on what is stated in paragraph 3, any reference to a “therapeutic” action of food supplements without a clear link to dietary deficiency or metabolic requirement, would be misleading. Some of the nutritional supplements could be important in case of a clear nutritional deficit, as may occur in specific phases of the pathology. In this respect, if a dietary deficiency is corrected with a supplement, then an adjuvant therapeutic-like action can be expected. It is anticipated that this would, in any case, be the most reasonable use of any dietary supplement although not always easy to proof preclinically in animal models [37]. The dark side of nutritional supplementation is related to the wrong assumption that these compounds, because of natural origin and normally present in diet, are safe and pivotal to maintain the wellbeing state and potentially help to prevent other pathological conditions, as those related to infections as in case of pandemic COVID-19. This holistic view is totally misleading, since the basis for the action of these exogenous compounds, especially at the high doses chronically used as supplements, follows the same mechanisms that underlie efficacy and safety issues of regular drugs. As anticipated supplements may contain natural active principles that have drug-like effects, like steroids present in a Chinese supplement with claimed beneficial effects in DMD boys [43], or statins present in red yeast rice [44]. In this case, an active principle of natural origin can have therapeutic action, i.e. impact on pathology-related events, and act as a disease modifier. However, the active principle should be considered per se as a drug, with no specific reference to dietary requirement and follow the same rule and experimental validation needed for pharmaceutical compounds [34]. For most natural compounds the boundary is very narrow, with doses and underlying rationale making the big differences. Consumers and patients need to be properly informed.
Other than being wrong, the assumption of natural-being-safe is dangerous, because caregivers can decide in an autonomous way and without medical prescription or suggestion to implement a supplement in the diet of a DMD boy with the idea that this is without danger and can only help. To increase the level of complexity, legislation can differ in various countries worldwide, with some preparations being considered somewhere “dietary supplements” and “pharmaceutical products” elsewhere. In a global world, as that of rare disease, this can increase confusion and misuse [37]. Unfortunately, this attitude exposes patients to high risk of ADR, may delay or counteract the effect of other therapeutics, and also adds economic burden to families, due to both the high costs of this class of compounds and the poor control of pathology progression.
5. Effects of the most used dietary supplements in DMD: side effects, mechanisms of toxicity and interactions
A wide range of dietary supplements is used by DMD patients. The present review is not aimed at entering into the specific rationale of their use; however, in Fig. 2 their main site of action is indicated, while concepts about the mechanism of action and an overview of the preclinical and clinical studies performed in DMD is given in Table 1 . As general considerations, a variety of both preclinical and clinical experimental approaches have been used and many contradictory results have been obtained over the years. Variables including age, stage of the disease, dosage, route of administration, time of exposure and readouts, have to be considered when reviewing efficacy data of these compounds or when designing preclinical and clinical studies. Importantly, the scientific community increasingly recognizes the need of properly conducting preclinical studies in this field, also for de-risking the possible clinical assessment of supplement need in DMD patients [45], similarly to the standardization in drug preclinical studies [46]. When testing dietary supplements different nutritional requirements between species should also be considered and accurately balanced with the more limited information about doses/levels needed to correct an alteration, so to reach the proper amount in target tissues and have a biological impact, further complicating the general picture. Based on the above consideration, we will presently focus on the main mechanisms that can account for possible safety concerns or harmful interactions for the most used dietary supplements in DMD (Table 1) as well as describing specific cases of reported toxicity by pharmacovigilance monitoring in the wide population (Table 2 ).
Fig. 2.
Targets of dietary supplements. The absence of dystrophin affects both mainly, but not exclusively, skeletal muscle. Many signalling pathways in myofibers are disturbed (in red). Nutraceuticals and dietary supplements can act on different parts of the pathology (in green). This figure was modified from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License; http://smart.servier.com/. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Table 1.
Mechanisms of action and outcome readouts for efficacy in preclinical and/or clinical studies, for dietary supplements most commonly used in DMD.
| Compound | Claimed mechanism of action | Preclinical studies in mdx mice: assessed endpoints | Evidences from clinical trials in DMD patients |
|---|---|---|---|
| Branched-chain amino acids | Increase protein synthesis | Modulation of vascular function | ↓ Protein degradation [50] |
| Myofibre phenotype | No improvements [51] | ||
| Motor function [206] | |||
| L-arginine | Enhance utrophin expression | Utrophin expression [62,63] | In combination with metformin (AMPK activator) [60]: |
| Necrosis [63] | ↑ Activity of mitochondrial electron transport chain | ||
| Respiratory function [63] | ↓ Oxidative stress | ||
| ↑ Clinical scores | |||
| Glutamine | Antioxidant activity | Oxidative stress | ↑ Insulin secretion [83] |
| Protein breakdown [207] | ↓ Protein degradation [82] | ||
| No effect on muscle function or strength [80,81] | |||
| Creatine | Enhance metabolism | Necrosis [86,87] | ↑ (PCr)/inorganic phosphate (Pi) ratio [90] |
| ↑ Ca2+-ATPase (SERCA2) content | Energy metabolism [86,88] | ↑ Muscle strength [90,919293] | |
| Muscle force [87,88,89] | ↓ Markers of bone breakdown [92] | ||
| ↑ Fat free mass [92] | |||
| Taurine | Antioxidant and anti-inflammatory activity; restoration of Ca2+ homeostasis | Muscle damage [103] | |
| Oxidative stress [103] | |||
| Muscle function [89] | |||
| Membrane conductivity [89] | |||
| Cardiac function [104] | |||
| Muscle strength (in combination with prednisolone) [105] | |||
| Green tea extract | Antioxidant and anti-inflammatory activity | Muscle damage and CK plasma levels [110,111] | Clinical trial ended but data not available |
| Necrosis [111,112113] | |||
| Regeneration [110] | |||
| Oxidative stress [111,112113] [208], | |||
| Muscle force [111,113] | |||
| Protandim | Antioxidant and anti-inflammatory activity | Oxidative stress markers [114] | |
| Omega-3 fatty acids | Anti-inflammatory activity | Necrosis [133] | ↓ Insulin resistance |
| Inflammation [133,134] | Trend towards a decrease of muscle loss [135] | ||
| Vitamin D | Restored insufficient 25-hydroxyvitamin D serum levels in DMD boys | Muscle force | In combination with calcium-rich diet [210]: |
| Muscle damage [209] | ↑ Bone mineral density | ||
| ↑ Bone mineral content | |||
| Resveratrol | Anti-inflammatory activity | Muscle damage (CK/LDH plasma levels) [103] | |
| Oxidative stress [103,150,151] | |||
| Inflammation [211] | |||
| Autophagy/mitophagy [150,212] | |||
| Muscle force [103] | |||
| Utrophin expression [211] | |||
| Curcumin | Anti-inflammatory activity | Muscle strength [152,153] | |
| Muscle damage [152,153] | |||
| Inflammation markers [152,153] | |||
| NF-kappaB activity [152,153] | |||
| N-Acetyl cysteine | Antioxidant activity | Muscle damage (CK plasma levels) [168,169] | |
| Necrosis [168,169] | |||
| Inflammation [169,170] | |||
| Oxidative stress [169,170] | |||
| βdystroglycan and utrophin expression [172] | |||
| Coenzyme Q10 | Antioxidant activity | ↑ Muscle strength [179] | |
| No effect on echocardiographic parameters [182] | |||
| Melatonin | Antioxidant activity | Muscle damage (CK plasma levels) | ↓ Oxidative stress [187] |
| Oxidative stress | ↓ Plasma pro-inflammatory cytokines [187] | ||
| Muscle force [186] | ↓ Hyperoxidative status in erythrocytes [213] |
Table 2.
Adverse events reports. Selection of adverse drug reactions reported for the different compounds.
| Product | Patient | Country | Year | Adverse reaction | Possible interaction with other drugs/ supplements |
|---|---|---|---|---|---|
| Mix of BCAA | Multiple reports | UK | 2015−2018 | Vomiting; dyspnoea; diarrhoea; dehydration | – |
| Female; 70 years | Canada | 2012 | Asthenia; cold sweat; diarrhoea; feeling abnormal; vomiting | – | |
| Female; 23 years | Canada | 2015 | Abdominal pain; cheilitis; diarrhoea; disorientation; dyspnoea; feeling abnormal; gait disturbance; gengival pain; hypersensitivity; ocular hyperaemia; palpitations; paraesthesia; peripheral swelling; swelling face; throat tightness | – | |
| Male | Canada | 2016 | Blood glucose decreased | insulin | |
| Male; 29 years | Canada | 2015 | Abdominal pain; anxiety; blood pressure increased; chest pain; dehydration; diarrhoea; disorientation; dyskinesia; gait disturbance; heart rate increased; hyperhidrosis; hyperventilation; hypokinesia; muscle spasms; pain in extremity; respiratory rate increased; seizure; tongue discoloration; toxicity to various agents; yellow skin | ibuprofen; nadolol | |
| Male; 19 years | Canada | 2013 | Rhabdomyolysis | energy drink + creatine | |
| Female; 63 years | Canada | 2017 | Blood pressure fluctuation | lisinopril; ASA; metoprolol; N-acetylcysteine | |
| Leucine | Female; 80 years | USA | 2010 | Vitamin B6 increased; pain in extremity; neuropathy peripheral; hypoaesthesia | – |
| Mix of arginine, others amino acids and caffeine | Female; 31 years | Canada | 2009 | Asthenia; burning sensation; Chest discomfort; Daydreaming; Disability; Dizziness; Feeling of body temperature change; Fine motor skill dysfunction; Heart rate increased; Movement disorder; Musculoskeletal stiffness; Palpitations | – |
| L-arginine | Male; 64 years | Canada | 2012 | Diarrhoea; myocardial infarction; nausea | carvedilol; atorvastatin; furosemide; warfarin |
| Male; 12 years | Canada | 2015 | Chromaturia; flank pain; haematuria | clonidine | |
| Male; 69 years | USA | 2014 | Palpitations; heart rate increased | – | |
| Female; 86 years | USA | 2016 | Neutrophil and monocyte percentage increase; lymphocyte percentage decrease; hyponatraemia; hypertension; hyperkalaemia; heart rate irregular; haematocrit decreased; fatigue; dizziness; discomfort; blood urea increased; blood sodium, calcium and chloride decreased; blood albumin decreased; atrioventricular block complete; alanine aminotransferase increased | – | |
| Multiple reports | UK | 2006−2015 | Supraventricular arrhythmias; malaise; neutropenia | – | |
| Multiple reports | Europe | 2019 | Cardiac and gastrointestinal disorders, hypotension | – | |
| L-glutamine | Female; 48 years | Canada | 2002 | Ear disorder; headache; hypoacusis; pruritus; rectal haemorrhage; vomiting | ginseng |
| Female 40 years | USA | 2014 | Psychomotor hyperactivity; panic attack; nervousness; heart rate increased; disturbance in attention; discomfort; confusional state; cerebrovascular accident | – | |
| Female; 27 years | USA | 2014 | Vomiting; nausea; jaundice; hepatic enzyme increased; hepatic enzyme increased; chills; body temperature increased; blood bilirubin increased; abdominal pain | – | |
| Female; 65 years | USA | 2015 | Syncope; nausea; dizziness; blood pressure decreased | – | |
| Not specified | USA | 2017 | Vomiting; vision blurred; paraesthesia; neurological symptom; nausea; insomnia; hypoaesthesia; dysarthria; dizziness; depression; confusional state; balance disorder; anxiety; amnesia | – | |
| Creatine | Male; 16 years | Canada | 2008 | Blood creatine increased; chest pain; renal failure; thrombosis | – |
| Male; 34 years | Canada | 2006 | Hyperhidrosis; nausea; vomiting | – | |
| Male; 28 years | USA | 2014 | Muscle spasms; blood creatine phosphokinase increased | – | |
| Male; 25 years | USA | 2014 | Acute renal failure; iatrogenic injury; blood thyroid stimulating hormone decreased; blood creatine increased | – | |
| Taurine | Male; 14 years) | Canada | 2007 | Arrhythmia; dry mouth; rash; skin discolouration; speech disorder; tongue oedema; tremor | – |
| Male; 41 years | USA | 2016 | Renal pain; micturition urgency; back pain | – | |
| Not specified | USA | 2017 | Vomiting; pyrexia; nausea; hospitalisation; hepatic pain; headache; abdominal pain upper | – | |
| Multiple reports | UK | 2014−2018 | Diarrhoea; vomiting; headache; dyspnoea; flushing | – | |
| Green tea | Female; 63 years | Canada | 2008 | Constipation ; dizziness; fatigue; phlebitis superficial | turmeric; aspirin |
| Male; 63 years | Canada | 2015 | Haemorrhagic stroke | – | |
| Female; 17 years | Canada | 2016 | Abdominal distension; atelectasis; blood creatinine increased; chest pain; hepatic failure; hepatitis acute; hepatomegaly; malaise; nausea; pain; pleural effusion; renal failure; renal tubular necrosis; transaminases increased; urine output decreased; vomiting; weight increased | ethinyl estradiol; norgestimate; ibuprofen | |
| Female; 23 years | Canada | 2014 | Liver function test abnormal; hepatitis acute; hepatic enzyme increased; blood bilirubin increased; blood alkaline phosphatase increased; aspartate aminotransferase increased | – | |
| Female; 66 years | USA | 2014 | Hepatitis; fatigue; dyspnoea; diarrhoea; chromaturia | – | |
| Male; 70 years | USA | 2014 | Heart rate irregular | – | |
| Omega 3-6-9 Fish Oil | Female; 46 years | Canada | 2005 | Arrhythmia; dizziness; lethargy; palpitations; pruritus; rash papular; urticaria | – |
| Female; 65 years | Canada | 2010 | Rash macular; rash pruritic | – | |
| Female; 57 years | Canada | 2010 | Pruritus; rash; skin disorder | – | |
| Male; 38 years | Canada | 2012 | Blood mercury abnormal | – | |
| Female; 58 years | Canada | 2012 | Abdominal pain; arthralgia; asthenia; chest discomfort; cough; fall; flushing; gallbladder disorder; hair texture abnormal; headache; joint swelling; lymphadenopathy; muscle spasms; nausea; neck pain; nephrolithiasis; neuropathy peripheral; oesophageal pain; pain in extremity; peripheral swelling; skull fracture; spider vein; | salbutamol; imatinib; allopurinol | |
| Male; 88 years | Canada | 2018 | Bedridden; drug ineffective; drug interaction; fall; hypoacusis; movement disorder; nocturia; vision blurred | calcium; vitamin D 400 I.U.; mirabegron | |
| Male; 58 years | USA | 2014 | Thrombosis; respiratory tract congestion; liver function test abnormal; fatigue; dyspnoea | – | |
| Not specified | USA | 2014 | Diarrhoea; abdominal pain upper | – | |
| Female; 57 years | USA | 2015 | Rash pruritic; rash erythematous | – | |
| Female; 64 years | USA | 2015 | Low density lipoprotein increased; high density lipoprotein increased | – | |
| Multiple reports | UK | 2003−2018 | Thrombocytopenia; nausea; diarrhoea; arthralgia; pain in extremity; rash | – | |
| Multiple reports | Europe | 2019 | Abnormal clotting factors, trombocytopenia, gastrointestinal disorders, musculoskeletal stiffness, myalgia, blood arsenic increased, blood and urine mercury abnormal | – | |
| Vitamin D | Female; 57 years | USA | 2014 | Palpitations; hypotension; heart rate increased; headache | – |
| Female; 53 years | USA | 2014 | Nephrolithiasis | calcium carbonate | |
| 2 months | USA | 2015 | Vitamin D increased; failure to thrive; blood calcium increased | – | |
| Female; 78 years | Canada | 2010 | Angioedema; lip blister; lip swelling; oral candidiasis; pharyngeal oedema; rash generalized | atenolol; prednisone; ranitidine | |
| Female | Canada | 2016 | Transient ischaemic attack | calcium; ibuprofen | |
| Male; 4 years | Canada | 2017 | Creatinine urine increased; nephrolithiasis; red blood cells urine positive | – | |
| Multiple reports | UK | 2000−2018 | Nausea; vomiting; diarrhoea; abdominal pain upper; hypercalcemia; dehydration; myalgia; pruritus | – | |
| Male; 79 years | New Zeeland | 2004 | Oedema peripheral | colecalciferol oral (suspect); prednisone oral (concomitant) | |
| Female; 64 years | New Zeeland | 2014 | Angina pectoris; arthralgia; chest pain; dysgeusia; headache | alendronic acid; colecalciferol oral (suspect) | |
| Multiple reports | Europe | 2019 | Gastrointestinal disorders; osteomalacia | – | |
| Resveratrol | Not specified | USA | 2016 | Transient ischaemic attack; blood pressure increased | – |
| Not specified | USA | 2016 | Atrial fibrillation | – | |
| Not specified | USA | 2017 | Sensation of pressure; paraesthesia; heart rate decreased; blood pressure increased | Omega Q | |
| Not specified | USA | 2018 | Palpitations; heart rate irregular; heart rate increased; atrial fibrillation | – | |
| Curcumin | Male 66 years | Canada | 2014 | Urine arsenic increased | – |
| Multiple reports | UK | 2015−2019 | Gastrointestinal disorders, headaches | – | |
| Female, 75 years | USA | 2014 | Vomiting, pain, nausea, headache, dizziness, deafness | – | |
| Not specified | USA | 2015 | Sinus congestion, nausea, headache | – | |
| Female, 57 years | USA | 2015 | Vomiting, ultrasound kidney abnormal, renal failure, nausea, hypophosphataemia, hypokalaemia, haematocrit decreased, blood creatinine increased. | – | |
| N-acetyl cysteine | Female; 70 years | USA | 2016 | Speech disorder; migraine without aura; headache; convulsion; confusional state; blood pressure increased; aphasia; amnesia | – |
| Male; 80 years | Canada | 2002 | Asthenia; chills; diarrhoea; dizziness; hypotension; myocardial infarction; nausea; rash; renal failure; serum sickness; skin exfoliation; vomiting | – | |
| Female; 16 years | New Zealand | 2016 | Angioedema; bronchospasm; rash; vomiting | acetylcysteine injection (suspect); paracetamol oral (concomitant) | |
| Multiple reports | Europe | 2019 | Gastrointestinal disorders, chest pain, asthma, respiration abnormal | – | |
| CoQ10 | Male; 70 years | Canada | 2005 | Drug interaction; prothrombin level increased | fluticasone; atorvastatin; warfarin |
| Male; 80 years | USA | 2014 | Pruritus; paraesthesia; hypertension; feeling hot; burning sensation | – | |
| Female; 58 years | USA | 2015 | Flatulence; diarrhoea; abdominal distension | – | |
| Female; 64 years | USA | 2015 | Pain; myopathy; muscular weakness; hepatic enzyme increased; gait disturbance; fatigue; dysstasia; cataract; asthenia; arthralgia | red yeast rice | |
| Male; 72 years | USA | 2015 | Palpitations; blood pressure systolic increased | – | |
| Melatonin | Female; 74 years | USA | 2014 | Insomnia; heart rate increased; dyspnoea; blood pressure increased | – |
| Female; 64 years | USA | 2014 | Wheezing; hypertension; dyspnoea; cough; chest discomfort | – | |
| Male; 61 years | USA | 2005 | Blood glucose increased | – | |
| Female; 59 years | USA | 2008 | Thrombocytopenia; platelet count decreased; hypersensitivity; ecchymosis; contusion | – | |
| Multiple reports | UK | 2014−2018 | Thrombocytopenia; tachycardia; muscle twitching; nausea | – | |
| Female; 69 years | Canada | 2012 | Muscle twitching | – | |
| Female; 63 years | Canada | 2012 | Blood pressure increased; confusional state; dizziness; dyskinesia; dysphagia; dyspnoea; heart rate increased; nausea; pain in jaw; palpitations; tremor | – | |
| Female; 18 years | Canada | 2014 | Headache; nervous system disorder; palpitations; tachycardia; vomiting | – | |
| Multiple reports | Europe | 2019 | Gastrointestinal disorders, agitation, confusional state, amnesia, insomnia, panic disorder | – |
New Zealand: https://medsafe.govt.nz/Projects/B1/ADRSearch.asp.
5.1. Branched-chain amino acids
The anabolic effect of branched-chain amino acids (BCAAs; leucine, isoleucine, and valine) on animal and human muscles has been well described in several studies [47,48] and is primarily due to BCAAs’ capability to affect pathways involved in protein synthesis (mTOR, S6K1). BCAAs are considered essential amino acids (EAAs) for humans, and, like the other six EAAs, they need to be necessarily introduced through the diet. However, recently, doubts were raised about the real ability of BCAAs to stimulate muscle anabolism, occurring when the rate of muscle protein synthesis exceeds the muscle protein breakdown and depends on the presence of an adequate amount of intracellular EAAs. The intake of BCAAs alone cannot prevent, in the post-absorptive state, the protein breakdown required for the production of the remaining six EAAs, making it impossible to create an anabolic state [49].
In addition, contradictory outcomes have been described in clinical studies [50,51], reinforcing the need for proper preclinical assessment in animal models in order to obtain more consistent and reliable results that may be useful for translational research on BCAA effects.
5.1.1. Side effects, mechanism of toxicity and interactions
The most common side effects revealed during the aforementioned trials were gastrointestinal: decreased appetite, anorexia, nausea, and general stomach discomfort [51].
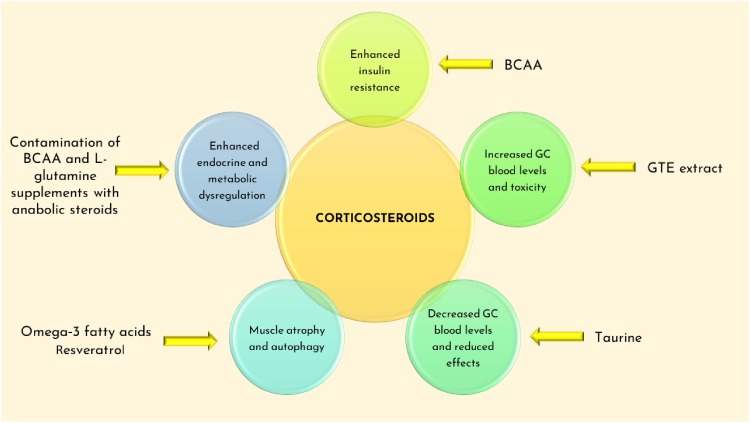
The potential anabolic effect of BCAAs is a costly process that requires a proper availability of energetic substrates: in fact, leucine administration promotes glucose uptake in skeletal muscle and enhances insulin response [52]. Consequently, it must be taken into account that BCAAs have a complex interaction with glucose metabolism. Supplementation might lower blood glucose levels (see Table 2), and this may have additive effects in DMD patients treated with antidiabetic drugs [53]. On the other hand, elevated BCAA can be prognostic of insulin resistance [54]; also, this aspect can be harmful in steroid treated DMD patients that already experience insulin resistance (v. Fig. 3 ). Another hypothesis to consider is the expression of BCAA transporters in skeletal muscle of dystrophic patients. In fact, muscle mass reduction could re-address the distribution of BCAAs towards other tissues, with changes in the amino acid flow. So far, no data seem to be available concerning BCAA levels in DMD patients.
Fig. 3.
Main effects of supplements interactions with glucocorticoids (GC) used to treat DMD (deflazacort and α-methylprednisolone). BCAA might enhance the insulin resistance caused by the chronic use of GC. Induction (GTE extract) or inhibition (Taurine) of CYP3A4 and P-gp (Gte extract) could influence the GC blood levels, with potential toxic effects or reduced bioavailability. Omega-3 fatty acids, resveratrol and GC might activate common pathways involved in muscle atrophy. Supplements contaminations with anabolic steroids could interfere with GC mechanisms of action and alter endocrine balance. Mechanisms are described in the text.
The work of Carunchio et al. in 2010 [55] also suggested that excessive use of BCAAs (with a BCAA-enriched diet, 2.5 % valine, isoleucine and leucine in 1:1:1 ratio) in sport athletes caused a major increase in the risk of amyotrophic lateral sclerosis (ALS) due to enhanced hyperexcitability of cortical neurons and excitotoxicity (see commentary by Manuel & Heckman, 2011 [56]). A risk to alter calcium homeostasis by BCAA has also been proposed, which would be dangerous in DMD subjects.
BCAAs are also important for the ammonia detoxification in skeletal muscle, promoting the synthesis of glutamate, which acts as a substrate for glutamine production. On the other hand, an excess of BCAAs supplementation and, consequently, of glutamine synthesis, may increase glutamine breakdown and the production of ammonia in the intestine and kidneys. This condition might lead to the development of hepatic encephalopathy. For these reasons, attention should be given to the dose of BCAAs administrated [57].
5.1.2. Contaminants
A study of 2004 [58] revealed the presence of dehydroepiandrosterone (DHEA) and 4androstenedione not indicated on the label of BCAA supplements. The abuse of anabolic steroids might cause relevant side effects and alters the normal hormonal production in the body [59], especially in DMD patients already under steroid therapy (v. Fig. 3).
5.2. L-arginine and l-citrulline
Another proposed approach to counteract muscle wasting in DMD is enhancing utrophin expression, a protein sharing 80 % homology with dystrophin. Utrophin has a similar function in the maintenance of the dystrophin-glycoprotein complex (DGC) integrity and myofibre structure. A widespread strategy to improve utrophin expression was to enhance the activity of the muscular isoform of nitric oxide synthase (NOS-μ) and NO synthesis by means of NO-donors administration, like l-arginine (or its precursor, l-citrulline [60,61]). NO is also critical to support mitochondrial function and biogenesis [62,63]. Interestingly, a recent study also showed that the levels of l-arginine and its precursor l-citrulline were significantly lower in mdx muscles with respect to wild type ones [64]. A recent study showed that l-homoarginine (one of the direct products of l-Arg metabolism in human) serum levels were significantly lower in Becker muscular dystrophy patients [61]. This evidence, if confirmed in DMD patients, might give more support to the requirement of l-arginine and l-citrulline supplementation through diet.
Although no other clinical trials with l-arginine administration alone or in combination with different drugs in DMD boys are currently published, a wide range of evidence in healthy and unhealthy subjects have been collected [65,66] (Table 1). l-arginine was administered alone or in combination with multiple active ingredients, in various dosage forms and with varying duration of treatment. This marked heterogeneity in experimental procedures makes it difficult to establish the upper level of intake of l-arginine and the accurate evaluation of its safety and real effect.
5.2.1. Side effects, mechanism of toxicity and interactions
The absence of serious adverse effects related to l-arginine or l-citrulline administration in human trials confirms the safety profile of these amino acids. However, the most common side effects reported were nausea and mild diarrhoea [67,68]. This is probably due to the increase of local NO synthesis by means of stimulation of intestinal NO synthase (NOS-1) activity, promoted by l-arginine ingestion. As it is well known, NO is strictly related to intestinal function at different levels, including motility, water retention and electrolyte transport. The excess of local NO synthesis might generate an imbalance in the equilibrium between intestinal absorption and secretion, promoting abnormal water loss and, consequently, nutrient depletion [69].
L-arginine contributes to the regulation of hemodynamic. In fact, animal and human studies demonstrated a hypotensive effect of l-arginine administration, not only related to the increase of NO synthesis but also to the enhanced endothelial cell function and the decreased resistance of peripheral blood vessels [[70], [71], [72]]. l-arginine also induced hyperkalaemia in animals and humans [73]. A recent study suggested that l-arginine and other dibasic amino acids increased plasma potassium levels, promoting the metal ion efflux from liver and pancreas to the extracellular aqueous environment [74].
L-arginine supplementation downregulates the expression, in liver and ileum, of cationic transporter-1 (CAT-1) that is also able to transport other basic and essential amino acids, like lysine and histidine, with a consequent reduction of EAAs plasma levels. This condition should be avoided in DMD patients. Furthermore, it was demonstrated that l-arginine affected the expression of enzymes involved in its metabolism, as such as arginase I, reducing its own bioavailability [75].
Considering these effects on vascular homeostasis and electrolytes balance, uncontrolled administration of l-arginine, particularly in unhealthy subjects, should be avoided. While an improvement of peripheral blood flow may contrast functional ischemia in DMD patients [76], l-arginine might cause excessive low blood pressure in DMD patients under treatment with antihypertensive drugs and could have a synergistic effect with agents that increase potassium levels, such as beta- and alpha-blockers. In accordance with this assertion, three cases of palpitations, irregular heartbeat, tachycardia and syncope were reported in healthy subjects after taking arginine supplementation [77] (see Table 2).
5.2.2. Contaminants
No relevant contaminations have been reported for l-arginine. In 2014, an investigation by the FDA discovered N-acetyl- l-leucine instead of l-citrulline in lots of these supplements with subsequent evidence of hyperammonaemia and related health hazards in patients [68].
5.3. Glutamine
Dystrophic muscles show a low glutamine concentration and DMD boys exhibit a significant decrease of glutamine bioavailability in the post-absorptive state, with the result that this amino acid should be considered “conditionally essential” for DMD patients [78]. Skeletal muscle plays a crucial role in the stock, metabolism and release of glutamine, maintaining approximately 80 % of the entire body amino acid content [79]. According to this point, it is conceivable that the muscle-wasting condition would account for glutamine deficiency, paving the way for a proper rationale to proceed with glutamine supplementation in several muscle pathologies, including DMD.
Nonetheless, clinical trials in DMD patients revealed a lack of functional benefits of glutamine supplementation compared to placebo [80,81], despite the encouraging results obtained on the reduction of whole-body protein degradation [82].
5.3.1. Side effects, mechanism of toxicity and interactions
A vast number of studies in human subjects that investigated the safety profile of glutamine have been published. No evidence of adverse effects appeared, supporting the harmless of glutamine supplementation [67]. The two most common adverse effects reported after l-glutamine consumption are nausea and vomiting (see Table 2).
Interestingly, a recent study investigated the effect of glutamine on glucose metabolism in DMD boys [83]. Glutamine, in fact, is involved in gluconeogenesis as a source of carbon skeletons via the tricarboxylic acid cycle (TCA) cycle. After 5 h of glutamine infusion, a rapid and transient increase of insulin plasma levels, and a prompt return to normal values when blood glutamine concentration was maintained at basal level were observed. For this reason, DMD patients under insulin therapy should avoid glutamine co-administration.
5.3.2. Contaminants
Also, in glutamine supplements contaminations with anabolic steroids (4-estren3,17-dion, 4-androsten3,17-dion) were found (v. Fig. 3) [84].
5.4. Creatine
In the last decades, creatine (Cr) has become the most popular dietary supplementation among athletes, both professionals and amateurs. The reason for this widespread popularity of Cr is attributable to the benefits on muscle performance globally observed in the sports field, not only at competitive level. Cr elicits various effects on muscle metabolism, managing energy resources in skeletal muscle cells. Cr and its phosphorylated form phosphocreatine (PCr) are responsible for the ATP shuttling between mitochondria and cytosol. In this way, during physical activity, myofibres have access to a dynamic reserve of energetic substrates, which remains locked within the cell, also thanks to the negative charge of PCr. Moreover, Cr is involved in the increase of muscle fibre size that might be related to an improvement of protein synthesis or a decrease of protein breakdown [85].
Muscles of DMD patients and mdx mice show a marked depletion of Cr and PCr concentration [86]. The administration of creatine in animal models demonstrated an enhancement of muscle strength and oxidative capacity of skeletal muscle cells with a reduction of myofibres necrosis [[86], [87], [88], [89]]. These beneficial effects arose from an essential property of Cr to reduce the chronic Ca2+-overload, a major feature in DMD myofibres. Importantly, it must be taken into account that the effects of Cr supplementation might be strictly related to the disease phase [[90], [91], [92], [93]]. Despite these promising results, long-term studies would be necessary to assess benefits and potential side effects of Cr, particularly analysing interaction with corticosteroids.
5.4.1. Side effects, mechanism of toxicity and interactions
Aside from anecdotal cases of gastrointestinal distress and muscle cramps, a side effect that potentially represents a serious hazard for DMD patients is the weight gain observed after Cr supplementation, becoming more significant with prolonged use of the compound [94]. The increase of body mass is mainly due to water retention and this effect might represent a relevant issue for patients with reduced mobility, and cardiac and hemodynamic sufferance, like DMD boys.
In literature, high variability in responsiveness to creatine supplementation was described. This variance primarily depends on the muscular content of Cr, that is higher in fast-twitch type II fibres. In accordance with this evidence, muscles consisting mainly of type fibres II are more susceptible to Cr supplementation. As is well known, in dystrophic muscles the fibre type mainly affected by pathology is the fast one and a progressive replacement of type II myofibres with fibrotic tissue is observed. Due to these reasons, a lack of responsiveness to Cr supplementation in DMD patients might be expected [95].
There are serious concerns about the renal safety of Cr. In detail, literature review and adverse events reports (see Table 2) mentioned cases of renal dysfunction in subjects with kidney disease history but also in healthy people taking Cr, even at the recommended dose [96,97]. Furthermore, there are no studies investigating the effect of Cr long-term administration in children/adolescent. Similarly, no clear evidence is available about the interaction of Cr with drugs potentially harmful for the kidney or mainly excreted via kidney, and possibly used on demand in DMD patients, including antibiotics, antivirals, hydroxychloroquine and other drugs of potential help in viral infections as COVID-19 [[98], [99], [100]].
5.4.2. Contaminants
It must be considered that Cr supplements most commonly traded in supermarkets, drugstores and also on internet, is a synthetic product derived from the reaction between sarcosine and cyanamide. Possible contaminants produced during this process are creatinine, arsenic, dicyandiamide and dihydrotrianzines; all compounds potentially hazardous for human health [85]. For this reason, careful attention should be paid to the source of the product, rather relying on brands prescribed by clinicians, which ensure the traceability of ingredients and which are subject to inspections that guarantee the respect of the good laboratory practices.
5.5. Taurine
One of the supplements with the most multitasking potential is certainly taurine, an amino acid found free in mammalian tissues and particularly abundant in excitable ones. In the human body, taurine exerts various actions, ranging from the antioxidant and the anti-inflammatory effect to the regulation of ion channels functions. This plethora of actions explains the potential use of taurine in the adjuvant therapy of some disorders that affect the muscular system, like myotonia, sarcopenia and disuse muscle atrophy [101]. This is particularly true in conditions characterized by altered tissue amount of taurine as it occurs at different extent in the various phases of dystrophic pathology [89,102]. Preclinical studies in mdx mice showed amelioration of functional parameters and markers of necrosis, oxidative stress and inflammation, being more effective than creatine [89,101,103,104]. Accordingly, taurine appears to be most effective in early phases of muscle and cardiac pathology, and synergic effect effects with glucocorticoids have been described [105]. This profile may envisage the potential usefulness in chronic use.
5.5.1. Side effects, mechanism of toxicity and interactions
Taurine supplementation might interfere with hemodynamic: a study in male rats demonstrated that acute injection of taurine induced hypotension and vasodilatation whereas a long-term supplementation in drinking water had hypertensive effect [106]. However, a possible interaction with drugs for blood pressure should be considered.
Taurine also modulates the induction of cytochrome P450 (CYP) 3A [107]. For this reason, a possible interaction with CYP3A4 inducers such as glucocorticoids might decrease blood levels of steroids and other drugs in DMD patients (v. Fig. 3). Furthermore, taurine levels in the body are also controlled by its renal transport, a sodium and chloride dependent mechanism. Then taurine may influence diuresis; in turn, altered kidney function may alter taurine equilibrium in the body [101,108]. Then, the previous considerations raised for creatine about interaction with drugs at kidney level, also apply to taurine.
Adolescent and children consumption of energy drinks with caffeine and taurine is raising globally. Some studies observed an increase in systolic blood pressure and heart rate after consumption. In Table 2 was also reported a case of a young subject who shown symptoms like diarrhoea, tremors and speech disorders after energy drinks consumption. Taurine also induced an increase of left ventricular stroke volume by suppressing sympathetic nervous stimulation and influencing calcium uptake in cardiac muscle cells and a long-term exposure may cause hypoglycaemia [109].
5.5.2. Contaminants
None described.
5.6. Green tea extract
Green tea extract (GTE), derived from Camellia sinensis leaves, contains flavonoids catechins (epigallocatechin-3-gallate (EGCG), epicatechin gallate, epicatechins, gallic acid) that have claimed antioxidant properties. For this reason, GTE supplementation was tested in mdx mice to counteract the oxidative stress in dystrophic muscle cells. Preclinical studies demonstrated that GTE improved muscle function and reduced necrosis in muscle tissues of treated animals. Even though encouraging results are available, there is still a lack of consistency among different studies [[110], [111], [112], [113]].
EGCG is the principal polyphenol antioxidant present in green tea and has main potency in preclinical tests. EGCG was also tested in DMD patients (https://www.clinicaltrials.gov/ct2/show/NCT01183767) but, currently, there are no results available.
GTE extract is also a component of Protandim® (LifeVantage Corp., San Diego, CA), an over-the-counter supplement with antioxidant activity [114]. Other herbal components of this supplement are, in detail, Bacopa monniera extract (45 % bacosides), Silybum marianum extract (70 %–80 % silymarin), Withania somnifera (Indian ginseng) powder, and curcumin (95 %) from Curcuma longa. Protandim was firstly tested on healthy human subjects and a reduction of oxidative stress markers was observed. 6-months administration of Protandim in mdx mice confirmed the positive results with reduction of oxidative damage and profibrotic factors. However, no relevant effects on muscle histology and functional parameters were described.
5.6.1. Side effects, mechanism of toxicity and interactions
The most frequent side effects are due to the presence of caffeine in GTE extracts, and are headache, nervousness, sleep problems, vomiting, diarrhoea, irritability, irregular heartbeat, tremor, heartburn, dizziness, ringing in the ears, convulsions, and confusion [115] (see also Table 2). Caffeine has also a diuretic effect [116] and this may exacerbate the urinary calcium loss in DMD patients.
Excessive GTE consumption induced iron deficiency anaemia (case report) [117]. High dose of GTE has hepatic adverse effects: in animal models, EGCG induced hepatotoxicity and several case reports showed hepatic adverse events in humans, associated with an increase of livers enzymes [118,119] (see Table 2).
GTE has an inhibitory effect on drug-metabolizing enzymes: CYP1A2, 2C9, 2D6, and 3A4 [120]. The concomitant administration of GTE and drugs metabolized by the aforementioned cytochromes should be avoided, including steroids (v. Fig. 3), antibiotics and anti-viral drugs.
In addition, EGCG is a competitive inhibitor of the proton-coupled folate transporter (PCFT) that mediates the intestinal absorption of folic acid. Green tea intake could, therefore, interfere with the transport of folate across the small intestine and decrease the bioavailability of this essential vitamin [121]. The lack of folate absorption alters cysteine metabolism, inducing hyperhomocysteinemia (HHcy) [122]. HHcy is involved in skeletal muscle malfunction and could exacerbate the muscle degeneration in DMD.
The number of GTE-cardiovascular drug interactions reported in humans increased in the last years and now include simvastatin, rosuvastatin, nadolol, sildenafil, tacrolimus and warfarin. However, the list might be longer, as suggested by the results of animal experiments with diltiazem, verapamil and nicardipine. GTE may, on one hand, increase the exposure to some drugs (simvastatin, tacrolimus), and, on the other hand, reduce the exposure to others (rosuvastatin, nadolol) [123]. Green tea consumption might also impair the correct gastrointestinal absorption of antibiotics (i.e. amoxicillin [124]) and also inhibits P-glycoprotein (P-gp) activity, a transporter that promotes the cellular efflux of a large variety of drugs including steroids and antiretroviral, increasing their potential toxicity (v. Fig. 3) [125,126]. Importantly, glucuronidation is the most important reaction of phase II metabolism of catechins, catalysed by UDP-glucuronosyltransferase (UGT) isoenzymes (1A3, 1A8, 1A9) [127]. DMD patients treated with drugs or other phenol-based nutraceuticals metabolized by UGT may, therefore, incur in unwanted interactions with GTE [128]. For instance, UGT1A9 is also the major enzyme that metabolizes ataluren, a novel drug recently approved for DMD to promote ribosomal read-through for patients with premature stop codon mutations [31,129]. Its metabolism could be delayed or impaired by GTE, with potential toxic effects.
Focusing on Protandim, most common adverse effects are related to allergic reactions to one or more herbal components of the product. (https://www.tandfonline.com/doi/full/10.3109/21678421.2015.1088707).
5.6.2. Contaminants
Thirty-two per cent of Chinese tea exceeded the national maximum permissible concentration for Pb [130]. Sources of green tea contamination are: pesticides (HCH and DDTs), environmental contaminants (Polycyclic aromatic hydrocarbons (PAHs)), mycotoxins and microorganisms (Aspergillum, Penicillium, Fusarium), toxic elements (mercury (Hg), lead (Pb), arsenic (As), cadmium (Cd), aluminium (Al), chromium (Cr), and nickel (Ni)), radioactive contaminants (in plantations near areas like Chernobyl and Fukushima), and plant growth regulators [131]. Pb is more bioavailable to tea plants growing in highly acidic soils: Japanese tea had the highest Pb contamination whereas Indian teas had the highest percentage of Cd leaching [132].
On 2012, LifeVantage Corporation announces voluntary recall for lots of Protandim due to contamination with small metal fragments in final products. (https://www.in.gov/isdh/files/LifeVantage_Corporation_Recall.pdf)
5.7. Omega-3 fatty acids
Inflammation is a hallmark of dystrophic muscles: for this reason, omega-3 fatty acids (ω3FAs) that are claimed to have anti-inflammatory effects, were tested in dystrophic animals. Supplementation with the omega-3 fatty acid eicosapentaenoic acid (EPA) in mdx mice induced a decrease of inflammation, creatine kinase and improved muscle regeneration [133,134].
Omega-3 long-chain polyunsaturated fatty acids (Ω3LCPUFA; 2.9 g/day) intake for 6 months reduced insulin resistance in boys with DMD and showed trends towards slowing the progression of muscle loss and decreasing the fat mass [135].
5.7.1. Side effects, mechanism of toxicity and interactions
A few side effects have been reported in subjects using ω3FA supplements. Some of these include fishy breath, upset stomach, diarrhoea, and nausea [136].
PUFA are metabolized and often bio-activated by at least four families of CYP, leading to possible PK interaction with a large number of drugs metabolized by CYP enzymes [137].
In addition, PUFA can lead to several PD interactions with drugs acting as anticoagulants and modulating blood homeostasis. An increment of the International Normalized Ratio (INR) in a patient on therapy with warfarin has been reported. It is important to recall that heparins are used empirically in COVID-19 and risk of bleeding in association with omega 3 should be considered. The patient referred to take a high dose of fish oil (2000 mg/d) daily. PUFA, in fact, might interfere with platelet aggregation, with an additive anticoagulant effect [138]. Accordingly, PUFA may have interactions with NSAIDs.
A recent study [139] demonstrated that omega‐3 fatty acids are capable of increasing the activity of the ubiquitin‐proteasome system (UPS). UPS is a protein network responsible for inducing muscle atrophy and, if not inhibited, promotes protein breakdown. Long-term administration of GC inhibits IGF‐1/PI‐3 K/Akt/mTOR pathway that normally suppresses the UPS activity. For this reason, the co-administration of omega-3- fatty acids and GC might enhance the pro-atrophic effects of steroids in skeletal muscle.
The excessive consumption of fish oil may also cause hypervitaminosis (vitamin D and A) [136].
5.7.2. Contaminants
ω3FA sources (fish and fish oils) are often exposed to environmental contaminants/toxins like mercury, polychlorinated biphenyls and dioxins [140]. A case report of 2012 shown an abnormal mercury blood level in a 38 years old man in Canada (see Table 2). Fish oil is also susceptible to oxidation and this process contributes to the rancid conversion of the compound, producing the typical “fishy” smell and aftertaste. This issue suggests an inadequate purification of the product by the manufacturer [140].
Fish oils taken as dietary supplements may contribute significantly to daily intakes of organochlorine contaminants due to the presence of pesticides in marine environments [141]. Most common non-animal sources of ω3FA are flaxseed, walnut, echium and algal oil. Other potential plant sources are soybean and canola that were genetically modified to contain higher quantity of ω3FA. However, as no indication about the use and safety of these oils are spread from the food agencies, the consumption of OGM products should be careful [142].
5.8. Vitamin D
Insufficient 25hydroxyvitamin D (25(OH)D) serum levels are seen in DMD boys. This is mainly due to the chronic corticosteroid use by DMD boys. In addition, reduced sun exposure due to little outdoor activities can play a role. Lowered levels of 25(OH)D contribute to reducing bone mineral density with an increasing probability of fractures of long bones and vertebrae. Vitamin D supplementation is a widespread strategy adopted in DMD boys to counteract corticosteroid-induced osteoporosis and is recommended by the standards of care [5,143]. Considering that vitamin D insufficiency may have detrimental role in the immune system, its supplementation in DMD patients should not be interrupted during viral pandemic emergencies, such as COVID-19 [144]. However, caution should be taken according to what described in 5.8.1 and for contaminants (see below).
5.8.1. Side effects, mechanism of toxicity and interactions
Vitamin D is generally considered safe when taken in appropriate amounts but can cause side effects when taken chronically in high doses. Side effects of vitamin D include weakness, fatigue, dizziness, nausea, vomiting, headache, anorexia, and others. Vitamin D assumption can further increase hypercalcemia as frequently reported i.e. in the UK (Table 2) and consequently, calcium supplementation needs to monitored and balanced [145,146]. Excess vitamin D is also associated with symptoms related to hypercalcemia, such as hypertension, anorexia, nausea, and possible kidney damage [30].
25-hydroxylation of vitamin D3 is the first step to produce the most common form of circulating vitamin D and occurs in the liver by means of a reaction catalysed by several CYP enzymes, such as CYP27A1 and CYP3A4 [147]. Literature reports occasional cases of drug-drug interactions with drugs metabolized by the same CYP (e.g., anticonvulsants), with an accelerated degradation of vitamin D and consequent osteomalacia [148].
Vitamin D intake is associated with improved absorption of essential inorganic elements (calcium (Ca), magnesium (Mg), copper (Cu), zinc (Zn), iron (Fe), and selenium(Se)) but has also been linked to enhanced uptake of toxic elements such as aluminium (Al), cadmium (ca), cobalt (Co), and lead (Pb) as well as radioactive isotopes including caesium (Cs) and radioactive strontium (Sr). In children, elevated 25(OH)D3 levels are associated with an increase in blood lead levels [149]. Bioaccumulation of toxic metals, in turn, counteracts the renal synthesis of vitamin D active form.
5.8.2. Contaminants
In 2017, FDA released a warning letter about a potential contamination of batches of liquid Vitamin D products with bacteria Burkholderia cepacia (https://www.fda.gov/news-events/press-announcements/fda-warns-potential-contamination-multiple-brands-drugs-dietary-supplements). This bacterium causes severe respiratory infections, particularly resistant to antibiotics treatment. Accordingly, contamination with B. capacia could be dangerous for subjects with respiratory distress and for children with immature immune system, like DMD boys.
5.9. Resveratrol and curcumin
Resveratrol supplementation has been associated with a variety of health benefits in skeletal muscle, such as increased oxidative capacity, decreased protein degradation, and decreased activation and inflammation. For this reason, this compound was tested in dystrophic animals and preclinical studies suggested that resveratrol supplementation may exert amelioration of muscle function and maturation of skeletal muscle cells by reducing oxidative stress [103,150,151].
The mechanism of NF-κB inhibition is proper of another compound already tested on mdx mice: curcumin. This curcuminoid, produced by Curcuma longa, was administered i.p. in mdx mice for 10 days [152]. Treated mice showed an amelioration in muscle function and a mitigation of dystrophic symptoms. Nevertheless, a previous study [153], demonstrated that NF-kappaB activity of mdx mice of three different ages (10 days; 4 and 8 weeks) was resistant to inhibition by oral curcumin supplementation, suggesting that the route of administration might heavily influence the bioavailability of the compound.
5.9.1. Side effects, mechanism of toxicity and interactions
In vitro studies, [154] showed that resveratrol is an irreversible inhibitor for CYP3A4 and a non-competitive reversible inhibitor for CYP2E1. CYP3A4 activity is important for the metabolism of calcium channel antagonists (e.g. felodipine, nicardipine), immunosuppressants (e.g. cyclosporine, tacrolimus), antihistamines (terfenadine), sildenafil, antiretrovirals; therefore, a possible interaction with drugs should be considered [[155], [156], [157]]. In addition, resveratrol is metabolized by liver and intestinal UGTs and may cause, like catechins, unwanted interactions with drugs metabolized by the same enzymes [127].
Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition [158]. Since glucocorticoids inhibit mTOR activity [159], possible additive effects should be taken into account [160] (v. Fig. 3).
Curcumin is generally considered as safe [161]. Studies in human subjects have shown only moderate adverse effects, like bloating, diarrhoea, headache and nausea [162,163].
5.9.2. Contaminants
None reported. However, careful attention of production chain for commercial resveratrol is advised, like pesticides, heavy metals and other contaminants can be present in grapes, soy, Polygonon cuspidatum and other resveratrol sources [164].
Analyses on curcumin supplements revealed contamination with metanil yellow (C18H14N3NaO3S), a toxic colorant used to adulterate Curcuma longa powder [165]. Furthermore, other compounds commonly used as adulterants are pigments containing lead, with serious concerns about the safety of these products [166,167].
5.10. N-Acetylcysteine
Because muscles in dystrophic individuals may produce more reactive oxygen species (ROS) and are thought to be more susceptible to oxidative damage, antioxidant supplementation may serve as an adjunct therapy that can be easily incorporated into treatment plans. N-acetylcysteine (NAC) is a potent antioxidant and has been studied as a potential DMD treatment in mdx mice [[168], [169], [170]]. In detail, NAC is a precursor of the amino acid cysteine that can itself function directly as an antioxidant, and indirectly through glutathione synthesis. Cysteine is also a precursor of taurine synthesis and the increase in muscle taurine content contributes to the amelioration of strength and function of mdx muscle, both in vivo and ex vivo [171,172]. Importantly, although it has no direct anti-viral actions, NAC has been claimed to have protective effects against pulmonary damages during viral infections in both pre-clinical and clinical studies due to its antioxidant and mucolytic activities [[173], [174], [175]]. Then safety issues of NAC as supplement deserve attention, also for the focus it may have in the media, as occurring during the COVID-19 emergency.
5.10.1. Side effects, mechanism of toxicity and interactions
A clinical trial in cancer patients revealed mostly gastrointestinal side effects, including nausea, vomiting, bloating and diarrhoea [176] (Table 2).
Paradoxically, long-term administration of NAC in humans increases oxidative stress after an acute muscle injury induced by eccentric exercise, with higher blood levels of markers of inflammation [177].
A preclinical study in mdx mice showed that NAC significantly impaired body weight gain in young growing mice (both mdx and wild type), and reduced liver weight in C57 mice and muscle weight in mdx mice [178].
5.10.2. Contaminants
None described.
5.11. Coenzyme Q10
Coenzyme Q10 (CoQ10) is a naturally occurring compound that plays a fundamental role in cell bioenergetics as a cofactor in the mitochondrial electron transport chain. CoQ10 is also a potent antioxidant and may counteract the excessive ROS production in dystrophic myofibres. The compound was tested in different clinical trials in DMD patients with encouraging results: CoQ10 supplementation ameliorated the impaired myocardial function and physical performance [[179], [180], [181]], although no significant improvement in echocardiographic parameters was revealed [182]. Interestingly, idebenone, a synthetic analogue of CoQ10 sharing its antioxidant profile, is at an advanced stage of development for treating cardiorespiratory dysfunction of DMD patients [[182], [183], [184]].
5.11.1. Side effects, mechanism of toxicity and interactions
There are no serious adverse events reported. One patient developed a headache of moderate intensity associated with high blood levels of CoQ10. The event resolved with a decrease of the dose [179].
CoQ10 supplementation may result in a reduction of diastolic blood pressure and might increase the effect of medication with antihypertensive activity [185].
5.11.2. Contaminants
None described. Sources (animal tissues and extracts and whole grains) have to be ensured for quality and purity.
5.12. Melatonin
Several studies corroborate the view that melatonin might protect many tissues, including skeletal muscle, from oxidative stress, due to the antioxidant properties of this compound and its metabolites. In mdx mice, muscle of treated animals showed an amelioration of the contractile function of dystrophic myofibres, accompanied by a decrease of CK plasma levels [186].
In DMD patients, melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress, with a reduction of inflammation and muscle wasting [187].
Recently, interest has been raised around melatonin for its anti-inflammatory, immunomodulatory and sedative actions, that may foresee the use of melatonin as adjuvant to contrast the exaggerated immune response finally leading to acute lung injury and acute respiratory distress syndrome in critical care patients with COVID-19 [188]. These may further increase the use of melatonin as supplements by DMD patients as adjuvant in seasonal viral pandemics.
5.12.1. Side effects, mechanism of toxicity and interactions
Oral melatonin reduces blood coagulation activity with a dose-response relationship [189]. It has been reported that the evening administration of melatonin in hypertensive patients taking the calcium antagonist nifedipine, interfered with the mechanism of cardiovascular regulation, inducing an increase in blood pressure [190]. Melatonin seems to interfere with calcium signalling via calmodulin. This suggests caution should be taken in uncontrolled use of melatonin in hypertensive patients as well as possible interference with antihypertensive drugs.
Lastly, it should be underlined that the actual safety of melatonin was not properly assessed, in paediatric population. Doubts were raised over the potential harmful effect of melatonin supplementation on endocrine and reproductive systems in children and adolescents [191]. Further enquiries are needed to evaluate the effects of melatonin in young subjects.
5.12.2. Contaminations
A recent study demonstrated that a quarter of melatonin products sold in one city in Canada contained serotonin, some at potentially significant doses [192,193]. An increment of serotonin levels might cause symptoms like tachycardia, palpitations and hypertension as observed in some cases reported in Table 2. Reports indicated that the eosinophilia-myalgia syndrome (EMS) was triggered by the consumption of a contaminated batch of the dietary supplement l-tryptophan (l-Trp). Because melatonin is structurally like to l -Trp (both compounds contain the indole ring functionality), a study investigated the nature of the contaminants in commercial preparations of melatonin. Several contaminants in commercial preparations of melatonin were found (formaldehyde-melatonin condensation product and two structural isomers of hydroxymelatonin). Of interest, an EMS-related contaminant in l-Trp referred to as "peak C" has been proposed as a 5hydroxytryptophan isomer. These findings should raise serious questions about the consequences of long-term ingestion of melatonin preparations containing such impurities [194].
6. Potential interaction between dietary supplements and advanced therapies: an unexplored world
Increasing efforts in DMD are devoted toward therapies able to counteract the absence of dystrophin by means of innovative approaches, such as antisense oligonucleotides (ASO) for skipping mutated exons and restoring reading frame, gene editing such as CRISPR/Cas9, transfer of microdystrophin gene and cell therapies. Two ASOs, eteplirsen and golodirsen, have been recently approved by FDA and others are currently in clinical trials, as well as gene and cell therapies. Little or no data are present about the possible interaction of those innovative therapies with classical drugs and even more with dietary supplements. Below an attempt to underline the main points of interaction susceptibility of these innovative approaches.
6.1. Antisense oligonucleotides
ASOs approved for DMD are phosphorodiamidate morpholino oligomers (PMO), very stable molecules in biological systems, administered in weekly regimens and mostly eliminated as such by renal excretion. Then approved ASOs do not undergo main metabolism via hepatic microsomes nor are they substrates of main transporters in the body. So, it is generally believed that the level of interaction with drug, food and dietary supplements is low. Both eteplirsen and golodirsen have little if any activity as inhibitor or inducer of cytochromes [195,196], golodirsen being a weak inducer of CYP1A2. This cytochrome is involved in the metabolism of various commonly used drugs and also involved in the biotransformation of PUFA; then interaction cannot be excluded.
Although the available information does not support major harmful interactions, these compounds have potential toxicity at kidney and liver level, suggesting that these tissues could be a possible site of harmful interactions. Moreover, since they are not very well absorbed at cellular level and they are excreted very fast via the kidney, supplements increasing renal clearance could interfere with their efficacy. Other ASOs under development may have different profiles and specific interaction can occur. Patients in clinical trials should then avoid using dietary supplements without medical control.
6.2. Cell therapy
Cell therapy represents another innovative approach for many diseases, including DMD. Various muscle progenitor cells have attracted interest, with some of them, such as mesangioblasts, tested in clinical settings, due to their ability to cross vessel wall in order to reach the site of the damage [197,198]. Although very little is known about possible interaction with drugs or with dietary supplements, it is intuitive to predict they may occur at various stages of cell therapy. In fact, muscle precursors are highly proliferating cells which are known to critically depend, for proliferation, fusion and adhesion, on nutrients, such as glucose and amino acids, via high expression of specific transporters such as CD98hc [199,200]. Also, extravasation may need a concerted action of cytokines and molecules involved in oxidative stress signalling and endothelial permeability, which may also be modulated by either drug or supplements. Another point of interaction can be related to a massive immune response that may be induced by the administration of high amounts of cells, which can also be influenced by supplements such as BCAA [201]. Then, although not easy to decipher, interaction with complex outcome may occur between cell therapies and dietary supplements.
6.3. Gene therapy
The idea behind gene therapy is to treat the pathology by substituting the defective gene(s) or at least one copy of it. For what it concerns muscular dystrophy, considering the size of dystrophin protein (usually not fitting in an adeno-associated virus (AAV)-vector), the transfer of microdystrophin is under clinical assessment [202]. One of the main points of interaction relates to the need to control the immune reaction to viral vectors and the new protein, then immunosuppressive drugs can be required. Accordingly, supplements able to modulate PD or PK profile of immunosuppressive drugs should be used with caution.
Another promising approach for DMD is nowadays offered by genome editing using the CRISPR/CAS9 technique. For this approach, important concern can be raised for off-target actions and gene mismatch [203,204], which can modify key organ functions for drug and supplement mechanism and clearance.
Basically, each of these new therapies presents a peculiar mechanism of action, also because they use different cell machinery and they act at different levels (DNA/RNA/protein). For this reason, it will be pivotal to fully understand and not rule out possible interactions with supplements.
7. Discussion
During the last years, the interest in natural, dietary supplements is increasing in the DMD field as adjuvant approaches in controlling both dietary requirement and pathological events. The recent emergency due to the outbreak of SARS-CoV-2 further increased the interest toward dietary supplements, among the population worldwide. A recent report of New York Times on March 23, 2020, underlined the increase of sales of supplements with claimed action on cold and flu with respect to the same period of 2019, with specific peaks for so called immune boosters, among which vitamin C, zinc, and others, for the hope to prevent or better manage the Sars-CoV-2 infection by reinforcing natural defences (https://www.nytimes.com/2020/03/23/well/live/coronavirus-supplements-herbs-vitamins-colds-flu.html). Similarly, a Google search of “dietary supplements” and “COVID-19” on March 31st, 2020 led to more than 12 million of results, underlying the great interest on this topic from different stakeholders.
It is therefore understandable, and even more justified by the fragility of the population, that such interest also arises from DMD community. This for the hope to improve standard of care as well as to get protection in case of an unexpected health emergency, as dramatically arose for COVID-19 recently emerged from World Duchenne Organization (https://www.worldduchenne.org/news/live-covid-19-coronavirus-and-duchenne-becker-muscular-dystrophy/).
Many caregivers/patients may mistakenly assume these supplements are safe. They think that if a supplement will not be beneficial then it will also not be harmful, so it does not hurt to try. This can be amplified by media and internet, also in relation to economic interests. Consequently, the risks of dietary supplements are seriously underestimated and often their use is not discussed with experts (either medical specialists, practitioners or pharmacists), nor related to any proven nutritional deficits. In fact, most of the dietary supplements discussed herein and even those with claimed effect on the immune system, such as vitamin C, are present in food, especially fresh fruits and vegetables. Then, a proper diet, healthy and varied, is the first aid to supply necessary essential elements, amino acids and vitamins. Supplements can, on the other hand, exert unwanted effects related to the use of excessive doses or interact with other medicines patients are using, either inhibiting or enhancing their efficiency and cause serious health risks. There is often no solid proof of dietary supplements effectiveness, while many side effects have been reported, including the complication or the ineffectiveness of outcome of other therapies (see Table 2). Then, prolonged use of supplements may cause several adverse effects, that often lead to emergency department visits and, in severe cases, hospitalization. In United States alone, approximately 23 000 emergency department visits and 2154 hospitalizations are connected every year to side effects related to dietary supplement [205].
For DMD and also for BMD patients these interactions can be of particular relevance as they can occur with drugs used for standard care or with innovative therapies during clinical trials. A similar situation also applies in emergency cases, as in the current pandemic by Sars-CoV-2. In fact the treatment of symptomatic COVID-19 patients is still a largely empirical approach with a wide variety of drugs according to the phase of the disease and ranging from antiviral and antibiotics, repurposed old drugs, such as chloroquine and hydroxychloroquine, to immunosuppressants, monoclonal antibodies and low-molecular weight-heparins. All these class of therapeutics have either a low therapeutic index or can per se at high risk of ADR events which may increase with uncontrolled D-D and D-S associations. In addition, more than 1200 clinical trials are currently ongoing on COVID-19 worldwide (clinicaltrial.gov) which reinforce the need for caution in uncontrolled use of supplements.
Another issue is that the quality check of these supplements is not always as rigid as it should be and they can be contaminated, certainly when they are retrieved from less controlled sources, like via the internet.
Awareness of the risks of uncontrolled use should be raised amongst caregivers and patients. They should be encouraged to always first discuss with their clinicians and pharmacists before starting supplement use and only retrieve them from certified sources, like their pharmacy.
Supplement use can also be a confounder in clinical studies and thereby influence the results. Unexplored interactions may reduce the efficacy of innovative therapies in clinical trials or cause unexpected toxicity. A database about the use of food supplements in DMD and BMD population, also in relation to geographical distribution, would be important, also with the aim to carry on properly conducted clinical studies. In parallel, it is important to consider that there is an increasing awareness to focus on dietary requirement in DMD patients. Then a reinforcement of active surveillance may help to detect ADR by dietary supplements, while patients’ registries can be of support for better understanding supplements use in relation to geographic and cultural aspects. Accordingly, it is pivotal to reinforce translational research in the field of supplements, considering the different nutritional requirements in different species used for preclinical studies [28,29].
8. Conclusions
This review underlines that supplements may represent an important support in nutritional deficit occurring in DMD patients, although dosage and efficacy need to be better established with controlled studies [28]. Safety is a main concern, based on the concept of “primum non nocere” (first, do no harm). Caution has to be taken in properly discussing with experts before deciding to start using supplements. Also, supplements from unknown sources or acquired via the web or outside pharmacy or other authorized retailers should always be avoided. In this respect, the active role by regulators and health professionals helps to provide correct information, in order to avoid direct use of products that escape the normal paths of distribution or do not provide the possibility to check quality control or surveillance [37]. The uncontrolled use of dietary supplements may, in fact, represent an important confounding factor during the development of new therapies or during studies aimed at repurposing drugs in DMD. The pandemic emergency of COVID-19 provided further evidence of this critical issue, as seen by the greater demand for dietary supplements. Patients and caregivers aim to be protected, especially for the evident difficulties to put in place effective social distance for proper assistance of fragile patients. However, also in these conditions, the best prevention resides in efforts in maintaining proper food, hygiene and individual protection devices for patients and/or caregivers. Although specifically focused on DMD, the considerations raised in the present review can also apply to BMD and to other neuromuscular and non-neuromuscular rare patients, that share similar fragility, complexity and uncertainties.
Funding
The work has been supported by DPP-NL grant to ADL on pre-clinical assessment of dietary supplement effects in dystrophinopathies and raised in the frame of a task force on dietary issues and nutrition by World Duchenne Organization (https://www.worldduchenneday.org/category/nutrition-in-dmd/). The fellowship of DPP-NL to OC for a project on artificial muscle as a new platform for testing the efficacy of novel compounds is also gratefully acknowledged. The contribution of Italian PRIN 2017 grant 2017FJSM9S_005 to ADL is also acknowledged.
Declaration of Competing Interest
The authors disclose non-conflict of interest.
Acknowledgements
None.
References
- 1.Mercuri E., Muntoni F. Muscular dystrophies. Lancet (London, England) 2013;381(9869):845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Koenig M., Monaco A.P., Kunkel L.M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- 4.Blake D.J., Weir A., Newey S.E., Davies K.E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82(2):291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 5.Birnkrant D.J., Bushby K., Bann C.M., Apkon S.D., Blackwell A., Brumbaugh D., Case L.E., Clemens P.R., Hadjiyannakis S., Pandya S., Street N., Tomezsko J., Wagner K.R., Ward L.M., Weber D.R., Group DMDCCW Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251–267. doi: 10.1016/S1474-4422(18)30024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnkrant D.J., Bushby K., Bann C.M., Alman B.A., Apkon S.D., Blackwell A., Case L.E., Cripe L., Hadjiyannakis S., Olson A.K., Sheehan D.W., Bolen J., Weber D.R., Ward L.M., Group DMDCCW Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347–361. doi: 10.1016/S1474-4422(18)30025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnkrant D.J., Bushby K., Bann C.M., Apkon S.D., Blackwell A., Colvin M.K., Cripe L., Herron A.R., Kennedy A., Kinnett K., Naprawa J., Noritz G., Poysky J., Street N., Trout C.J., Weber D.R., Ward L.M., Group DMDCCW Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018;17(5):445–455. doi: 10.1016/S1474-4422(18)30026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas M., Vlcek V., Balabanov P., Salmonson T., Bakchine S., Markey G., Weise M., Schlosser-Weber G., Brohmann H., Yerro C.P., Mendizabal M.R., Stoyanova-Beninska V., Hillege H.L. European Medicines Agency review of ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscular Disorders: NMD. 2015;25(1):5–13. doi: 10.1016/j.nmd.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Aartsma-Rus A., Krieg A.M. FDA approves eteplirsen for duchenne muscular dystrophy: the next chapter in the eteplirsen Saga. Nucleic Acid Ther. 2017;27(1):1–3. doi: 10.1089/nat.2016.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank D.E., Schnell F.J., Akana C. Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy [published online ahead of print, 2020 Mar 5] Neurology. 2020 doi: 10.1212/WNL.0000000000009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhaart I.E.C., Aartsma-Rus A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019;15(7):373–386. doi: 10.1038/s41582-019-0203-3. [DOI] [PubMed] [Google Scholar]
- 12.Straub V., Balabanov P., Bushby K. Stakeholder cooperation to overcome challenges in orphan medicine development: the example of Duchenne muscular dystrophy. Lancet Neurol. 2016;15(8):882–890. doi: 10.1016/S1474-4422(16)30035-7. [DOI] [PubMed] [Google Scholar]
- 13.Heydemann A. Skeletal muscle metabolism in Duchenne and Becker muscular dystrophy-implications for therapies. Nutrients. 2018;10(6) doi: 10.3390/nu10060796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Cruz M., Sanchez R., Escobar R.E., Cruz-Guzman Odel R., Lopez-Alarcon M., Bernabe Garcia M., Coral-Vazquez R., Matute G., Velazquez Wong A.C. Evidence of insulin resistance and other metabolic alterations in boys with duchenne or becker muscular dystrophy. Int. J. Endocrinol. 2015 doi: 10.1155/2015/867273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuznetsov A.V., Winkler K., Wiedemann F.R., von Bossanyi P., Dietzmann K., Kunz W.S. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol. Cell. Biochem. 1998;183(1–2):87–96. doi: 10.1023/a:1006868130002. [DOI] [PubMed] [Google Scholar]
- 16.Camerino G.M., Cannone M., Giustino A. Gene expression in mdx mouse muscle in relation to age and exercise: aberrant mechanical-metabolic coupling and implications for pre-clinical studies in Duchenne muscular dystrophy. Hum. Mol. Genet. 2014;23(21):5720–5732. doi: 10.1093/hmg/ddu287. [DOI] [PubMed] [Google Scholar]
- 17.Gamberi T., Fiaschi T., Valocchia E. Proteome analysis in dystrophic mdx mouse muscle reveals a drastic alteration of key metabolic and contractile proteins after chronic exercise and the potential modulation by anti-oxidant compounds. J. Proteomics. 2018;170:43–58. doi: 10.1016/j.jprot.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Rybalka E., Timpani C.A., Cooke M.B., Williams A.D., Hayes A. Defects in mitochondrial ATP synthesis in dystrophin-deficient mdx skeletal muscles may be caused by complex I insufficiency. PLoS One. 2014;9(12):e115763. doi: 10.1371/journal.pone.0115763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogan S.E. Body composition and resting energy expenditure of individuals with Duchenne and Becker muscular dystrophy. Can. J. Diet. Pract. Res. 2008;69(4):208–212. doi: 10.3148/69.4.2008.208. [DOI] [PubMed] [Google Scholar]
- 20.Saure C., Caminiti C., Weglinski J., de Castro Perez F., Monges S. Energy expenditure, body composition, and prevalence of metabolic disorders in patients with Duchenne muscular dystrophy. Diabetes Metab. Syndr. 2018;12(2):81–85. doi: 10.1016/j.dsx.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu-Fujiwara M., Komaki H., Nakagawa E., Mori-Yoshimura M., Oya Y., Fujisaki T., Tokita Y., Kubota N., Shimazaki R., Sato K., Ishikawa T., Goto K., Mochizuki H., Takanoha S., Ogata K., Kawai M., Konagaya M., Miyazaki T., Tatara K., Sugai K., Sasaki M. Decreased resting energy expenditure in patients with Duchenne muscular dystrophy. Brain Dev-Jpn. 2012;34(3):206–212. doi: 10.1016/j.braindev.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Martigne L., Salleron J., Mayer M., Cuisset J.M., Carpentier A., Neve V., Tiffreau V., Guimber D., Gottrand F. Natural evolution of weight status in Duchenne muscular dystrophy: a retrospective audit. Br. J. Nutr. 2011;105(10):1486–1491. doi: 10.1017/S0007114510005180. [DOI] [PubMed] [Google Scholar]
- 23.West N.A., Yang M.L., Weitzenkamp D.A., Andrews J., Meaney F.J., Oleszek J., Miller L.A., Matthews D., DiGuiseppi C. Patterns of growth in ambulatory males with Duchenne muscular dystrophy. J Pediatr-Us. 2013;163(6):1759–1763. doi: 10.1016/j.jpeds.2013.08.004. e1751. [DOI] [PubMed] [Google Scholar]
- 24.Bernabe-Garcia M., Rodriguez-Cruz M., Atilano S., Cruz-Guzman O.D.R., Almeida-Becerril T., Calder P.C., Gonzalez J. Body composition and body mass index in Duchenne muscular dystrophy: role of dietary intake. Muscle Nerve. 2019;59(3):295–302. doi: 10.1002/mus.26340. [DOI] [PubMed] [Google Scholar]
- 25.Davidson Z.E., Ryan M.M., Kornberg A.J., Sinclair K., Cairns A., Walker K.Z., Truby H. Observations of body mass index in Duchenne muscular dystrophy: a longitudinal study. Eur. J. Clin. Nutr. 2014;68(8):892–897. doi: 10.1038/ejcn.2014.93. [DOI] [PubMed] [Google Scholar]
- 26.Matthews E., Brassington R., Kuntzer T., Jichi F., Manzur A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016;(5) doi: 10.1002/14651858.CD003725.pub4. CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pane M., Vasta I., Messina S., Sorleti D., Aloysius A., Sciarra F., Mangiola F., Kinali M., Ricci E., Mercuri E. Feeding problems and weight gain in Duchenne muscular dystrophy. Eur. J. Paediatr. Neurol. 2006;10(5–6):231–236. doi: 10.1016/j.ejpn.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Verhaart I.E.C., van den Engel-Hoek L., Fiorotto M.L., Franken-Verbeek M., Vroom E. Nutrition in Duchenne muscular dystrophy 16-18 March 2018, Zaandam, the Netherlands. Neuromuscular Disorders: NMD. 2018;28(8):680–689. doi: 10.1016/j.nmd.2018.05.004. workshop p. [DOI] [PubMed] [Google Scholar]
- 29.Salera S., Menni F., Moggio M., Guez S., Sciacco M., Esposito S. Nutritional challenges in Duchenne muscular dystrophy. Nutrients. 2017;9(6) doi: 10.3390/nu9060594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D . Calcium. The national academies collection: reports funded by national institutes of health. In: Ross A.C., Taylor C.L., Yaktine A.L., Del Valle H.B., editors. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US), National Academy of Sciences; Washington (DC): 2011. [DOI] [Google Scholar]
- 31.Muntoni F., Desguerre I., Guglieri M., Osorio An, Kirschner J., Tulinius M., Buccella F., Elfring G., Werner C., Schilling T., Trifillis P., Zhang O., Delage A., Santos Cl, Mercuri E. Ataluren use in patients with nonsense mutation Duchenne muscular dystrophy: patient demographics and characteristics from the STRIDE Registry. J. Comp. Eff. Res. 2019;8(14):1187–1200. doi: 10.2217/cer-2019-0086. [DOI] [PubMed] [Google Scholar]
- 32.Patadia V.K., Coloma P., Schuemie M.J., Herings R., Gini R., Mazzaglia G., Picelli G., Fornari C., Pedersen L., van der Lei J., Sturkenboom M., Trifiro G., consortium E-A Using real-world healthcare data for pharmacovigilance signal detection - the experience of the EU-ADR project. Expert Rev. Clin. Pharmacol. 2015;8(1):95–102. doi: 10.1586/17512433.2015.992878. [DOI] [PubMed] [Google Scholar]
- 33.The European Parliament and the Council of the European Union Regulation (EU) no 1235/2010 of the European Parliament and of the Council. Off. J. Eur. Union. 2010;348:1–16. [Google Scholar]
- 34.The European Parliament and the Council of the European Union Regulation (EU) no 1027/2012 of the European Parliament and of the Council. Off. J. Eur. Union. 2012;316:38–40. [Google Scholar]
- 35.Petitet F., Barberan O., Dubus E., Ijjaali I., Donlan M., Ollivier S., Michel A. Development of an ADME and drug-drug interactions knowledge database for the acceleration of drug discovery and development. Expert Opin. Drug Discov. 2006;1(7):737–751. doi: 10.1517/17460441.1.7.737. [DOI] [PubMed] [Google Scholar]
- 36.Ryu J.Y., Kim H.U., Lee S.Y. Deep learning improves prediction of drug-drug and drug-food interactions. Proc. Natl. Acad. Sci. U. S. A. 2018;115(18):E4304–E4311. doi: 10.1073/pnas.1803294115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dwyer J.T., Coates P.M., Smith M.J. Dietary supplements: regulatory challenges and research resources. Nutrients. 2018;10(1) doi: 10.3390/nu10010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronis M.J.J., Pedersen K.B., Watt J. Adverse effects of nutraceuticals and dietary supplements. Annu. Rev. Pharmacol. Toxicol. 2018;58:583–601. doi: 10.1146/annurev-pharmtox-010617-052844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Cancer Society . 2015. FDA Regulation of Drugs Versus Dietary Supplements.https://www.cancer.org/treatment/treatments-and-side-effects/complementary-and-alternative-medicine/dietary-supplements/fda-regulations.html March. [Google Scholar]
- 40.Gupta R.C., Srivastava A., Lall R. Toxicity potential of nutraceuticals. Methods Mol. Biol. 2018;1800:367–394. doi: 10.1007/978-1-4939-7899-1_18. [DOI] [PubMed] [Google Scholar]
- 41.The European Parliament and the Council of the European Union Directive 2002/46/EC of the European parliament and of the council. Off. J. Eur. Union. 2002;183:51–57. [Google Scholar]
- 42.Nabukera S.K., Romitti P.A., Campbell K.A., Meaney F.J., Caspers K.M., Mathews K.D., Sherlock S.M., Puzhankara S., Cunniff C., Druschel C.M., Pandya S., Matthews D.J., Ciafaloni E., STARnet M.D. Use of complementary and alternative medicine by males with Duchenne or Becker muscular dystrophy. J. Child Neurol. 2012;27(6):734–740. doi: 10.1177/0883073811426501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samdup D.Z., Smith R.G., Il Song S. The use of complementary and alternative medicine in children with chronic medical conditions. Am. J. Phys. Med. Rehabil. 2006;85(10):842–846. doi: 10.1097/01.phm.0000233183.17059.b9. [DOI] [PubMed] [Google Scholar]
- 44.Woodman K.G., Coles C.A., Lamande S.R., White J.D. Nutraceuticals and their potential to treat duchenne muscular dystrophy: separating the credible from the conjecture. Nutrients. 2016;8(11) doi: 10.3390/nu8110713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Putten M., Aartsma-Rus A., Grounds M.D., Kornegay J.N., Mayhew A., Gillingwater T.H., Takeda S., Ruegg M.A., De Luca A., Nagaraju K., Willmann R. Update on standard operating procedures in preclinical research for DMD and SMA report of TREAT-NMD Alliance Workshop, Schiphol Airport, 26 April 2015, The Netherlands. J. Neuromuscul. Dis. 2018;5(1):29–34. doi: 10.3233/JND-170288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willmann R., Possekel S., Dubach-Powell J., Meier T., Ruegg M.A. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscular disorders: NMD. 2009;19(4):241–249. doi: 10.1016/j.nmd.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Blomstrand E., Eliasson J., Karlsson H.K., Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J. Nutr. 2006;136(Suppl. 1):269S–273S. doi: 10.1093/jn/136.1.269S. [DOI] [PubMed] [Google Scholar]
- 48.Moberg M., Apro W., Ekblom B., van Hall G., Holmberg H.C., Blomstrand E. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am. J. Physiol., Cell Physiol. 2016;310(11):C874–884. doi: 10.1152/ajpcell.00374.2015. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe R.R. Branched-chain amino acids and muscle protein synthesis in humans: myth or reality? J. Int. Soc. Sports Nutr. 2017;14:30. doi: 10.1186/s12970-017-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart P.M., Walser M., Drachman D.B. Branched-chain ketoacids reduce muscle protein degradation in Duchenne muscular dystrophy. Muscle Nerve. 1982;5(3):197–201. doi: 10.1002/mus.880050304. [DOI] [PubMed] [Google Scholar]
- 51.Mendell J.R., Griggs R.C., Moxley R.T., 3rd, Fenichel G.M., Brooke M.H., Miller J.P., Province M.A., Dodson W.E. Clinical investigation in Duchenne muscular dystrophy: IV. Double-blind controlled trial of leucine. Muscle Nerve. 1984;7(7):535–541. doi: 10.1002/mus.880070704. [DOI] [PubMed] [Google Scholar]
- 52.Gannon N.P., Schnuck J.K., Vaughan R.A. BCAA metabolism and insulin sensitivity - dysregulated by metabolic status? Mol. Nutr. Food Res. 2018;62(6):e1700756. doi: 10.1002/mnfr.201700756. [DOI] [PubMed] [Google Scholar]
- 53.Doi M., Yamaoka I., Nakayama M., Mochizuki S., Sugahara K., Yoshizawa F. Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J. Nutr. 2005;135(9):2103–2108. doi: 10.1093/jn/135.9.2103. [DOI] [PubMed] [Google Scholar]
- 54.Flores-Guerrero J.L., Oste M.C.J., Kieneker L.M., Gruppen E.G., Wolak-Dinsmore J., Otvos J.D., Connelly M.A., Bakker S.J.L., Dullaart R.P.F. Plasma branched-chain amino acids and risk of incident type 2 diabetes: results from the PREVEND prospective cohort study. J. Clin. Med. 2018;7(12) doi: 10.3390/jcm7120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carunchio I., Curcio L., Pieri M., Pica F., Caioli S., Viscomi M.T., Molinari M., Canu N., Bernardi G., Zona C. Increased levels of p70S6 phosphorylation in the G93A mouse model of Amyotrophic Lateral Sclerosis and in valine-exposed cortical neurons in culture. Exp. Neurol. 2010;226(1):218–230. doi: 10.1016/j.expneurol.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 56.Manuel M., Heckman C.J. Stronger is not always better: could a bodybuilding dietary supplement lead to ALS? Exp. Neurol. 2011;228(1):5–8. doi: 10.1016/j.expneurol.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holecek M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond). 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van der Merwe P.J., Grobbelaar E. Inadvertent doping through nutritional supplements is a reality. South Afr. J. Sport. Med. 2004;16(2):3–7. doi: 10.17159/2078-516X/2004/v16i2a180. [DOI] [PubMed] [Google Scholar]
- 59.Di Luigi L. Supplements and the endocrine system in athletes. Clin. Sports Med. 2008;27(1) doi: 10.1016/j.csm.2007.09.003. 131–ix. [DOI] [PubMed] [Google Scholar]
- 60.Hafner P., Bonati U., Erne B., Schmid M., Rubino D., Pohlman U., Peters T., Rutz E., Frank S., Neuhaus C., Deuster S., Gloor M., Bieri O., Fischmann A., Sinnreich M., Gueven N., Fischer D. Improved muscle function in duchenne muscular dystrophy through L-arginine and metformin: an investigator-initiated, open-label, single-center, proof-of-concept-study. PLoS One. 2016;11(1):e0147634. doi: 10.1371/journal.pone.0147634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanff E., Hafner P., Bollenbach A., Bonati U., Kayacelebi A.A., Fischer D., Tsikas D. Effects of single and combined metformin and L-citrulline supplementation on L-arginine-related pathways in Becker muscular dystrophy patients: possible biochemical and clinical implications. Amino Acids. 2018;50(10):1391–1406. doi: 10.1007/s00726-018-2614-7. [DOI] [PubMed] [Google Scholar]
- 62.Chaubourt E., Fossier P., Baux G., Leprince C., Israel M., De La Porte S. Nitric oxide and l-arginine cause an accumulation of utrophin at the sarcolemma: a possible compensation for dystrophin loss in Duchenne muscular dystrophy. Neurobiol. Dis. 1999;6(6):499–507. doi: 10.1006/nbdi.1999.0256. [DOI] [PubMed] [Google Scholar]
- 63.Voisin V., Sebrie C., Matecki S., Yu H., Gillet B., Ramonatxo M., Israel M., De la Porte S. L-arginine improves dystrophic phenotype in mdx mice. Neurobiol. Dis. 2005;20(1):123–130. doi: 10.1016/j.nbd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Mantuano P., Sanarica F., Conte E., Morgese M.G., Capogrosso R.F., Cozzoli A., Fonzino A., Quaranta A., Rolland J.F., De Bellis M., Camerino G.M., Trabace L., De Luca A. Effect of a long-term treatment with metformin in dystrophic mdx mice: a reconsideration of its potential clinical interest in Duchenne muscular dystrophy. Biochem. Pharmacol. 2018;154:89–103. doi: 10.1016/j.bcp.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 65.Alvares T.S., Meirelles C.M., Bhambhani Y.N., Paschoalin V.M., Gomes P.S. L-Arginine as a potential ergogenic aid in healthy subjects. Sports Med. (Auckland, NZ) 2011;41(3):233–248. doi: 10.2165/11538590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 66.Deveaux A., Pham I., West S.G., Andre E., Lantoine-Adam F., Bunouf P., Sadi S., Hermier D., Mathe V., Fouillet H., Huneau J.F., Benamouzig R., Mariotti F. L-arginine supplementation alleviates postprandial endothelial dysfunction when baseline fasting plasma arginine concentration is low: a randomized controlled trial in healthy overweight adults with cardiometabolic risk factors. J. Nutr. 2016;146(7):1330–1340. doi: 10.3945/jn.115.227959. [DOI] [PubMed] [Google Scholar]
- 67.Shao A., Hathcock J.N. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul. Toxicol. Pharmacol.: RTP. 2008;50(3):376–399. doi: 10.1016/j.yrtph.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Oketch-Rabah H.A., Roe A.L., Gurley B.J., Griffiths J.C., Giancaspro G.I. The importance of quality specifications in safety assessments of amino acids: the cases of l-tryptophan and l-citrulline. J. Nutr. 2016;146(12):2643S–2651S. doi: 10.3945/jn.115.227280. [DOI] [PubMed] [Google Scholar]
- 69.Grimble G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007;137(6 Suppl 2):1693S–1701S. doi: 10.1093/jn/137.6.1693S. [DOI] [PubMed] [Google Scholar]
- 70.Guridi J., Borgatello C., Scremin O.U. Arginine NO-dependent and NO-independent effects on hemodynamics. Eur. J. Pharmacol. 2014;729:138–143. doi: 10.1016/j.ejphar.2014.01.070. [DOI] [PubMed] [Google Scholar]
- 71.Nakaki T., Hishikawa K., Suzuki H., Saruta T., Kato R. L-arginine-induced hypotension. Lancet (London, England) 1990;336(8716):696. doi: 10.1016/0140-6736(90)92196-o. [DOI] [PubMed] [Google Scholar]
- 72.Vasdev S., Gill V. The antihypertensive effect of arginine. Int. J. Angiol. 2008;17(1):7–22. doi: 10.1055/s-0031-1278274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Massara F., Martelli S., Cagliero E., Camanni F., Molinatti G.M. The hypophosphatemic and hyperkalemic effect of arginine in man. J. Endocrinol. Invest. 1980;3(2):177–180. doi: 10.1007/bf03348247. [DOI] [PubMed] [Google Scholar]
- 74.Cremades A., Del Rio-Garcia J., Lambertos A., Lopez-Garcia C., Penafiel R. Tissue-specific regulation of potassium homeostasis by high doses of cationic amino acids. SpringerPlus. 2016;5:616. doi: 10.1186/s40064-016-2224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin S., Desai K. The effects of oral arginine on its metabolic pathways in Sprague-Dawley rats. Br. J. Nutr. 2020;123(2):135–148. doi: 10.1017/S0007114519002691. [DOI] [PubMed] [Google Scholar]
- 76.Thomas G.D. Functional muscle ischemia in Duchenne and Becker muscular dystrophy. Front. Physiol. 2013;4:381. doi: 10.3389/fphys.2013.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prosser J.M., Majlesi N., Chan G.M., Olsen D., Hoffman R.S., Nelson L.S. Adverse effects associated with arginine alpha-ketoglutarate containing supplements. Hum. Exp. Toxicol. 2009;28(5):259–262. doi: 10.1177/0960327109104498. [DOI] [PubMed] [Google Scholar]
- 78.Hankard R., Mauras N., Hammond D., Haymond M., Darmaun D. Is glutamine a’ conditionally essential’ amino acid in Duchenne muscular dystrophy? Clin. Nutr. (Edinburgh, Scotland) 1999;18(6):365–369. doi: 10.1016/s0261-5614(99)80017-x. [DOI] [PubMed] [Google Scholar]
- 79.Cruzat V., Macedo Rogero M., Noel Keane K., Curi R., Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10(11) doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Escolar D.M., Buyse G., Henricson E., Leshner R., Florence J., Mayhew J., Tesi-Rocha C., Gorni K., Pasquali L., Patel K.M., McCarter R., Huang J., Mayhew T., Bertorini T., Carlo J., Connolly A.M., Clemens P.R., Goemans N., Iannaccone S.T., Igarashi M., Nevo Y., Pestronk A., Subramony S.H., Vedanarayanan V.V., Wessel H., Group C. CINRG randomized controlled trial of creatine and glutamine in Duchenne muscular dystrophy. Ann. Neurol. 2005;58(1):151–155. doi: 10.1002/ana.20523. [DOI] [PubMed] [Google Scholar]
- 81.Mok E., Letellier G., Cuisset J.M., Denjean A., Gottrand F., Alberti C., Hankard R. Lack of functional benefit with glutamine versus placebo in Duchenne muscular dystrophy: a randomized crossover trial. PLoS One. 2009;4(5):e5448. doi: 10.1371/journal.pone.0005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mok E., Eleouet-Da Violante C., Daubrosse C., Gottrand F., Rigal O., Fontan J.E., Cuisset J.M., Guilhot J., Hankard R. Oral glutamine and amino acid supplementation inhibit whole-body protein degradation in children with Duchenne muscular dystrophy. Am. J. Clin. Nutr. 2006;83(4):823–828. doi: 10.1093/ajcn/83.4.823. [DOI] [PubMed] [Google Scholar]
- 83.Letellier G., Mok E., Alberti C., De Luca A., Gottrand F., Cuisset J.M., Denjean A., Darmaun D., Hankard R. Effect of glutamine on glucose metabolism in children with Duchenne muscular dystrophy. Clin. Nutr. 2013;32(3):386–390. doi: 10.1016/j.clnu.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Martello S., Felli M., Chiarotti M. Survey of nutritional supplements for selected illegal anabolic steroids and ephedrine using LC-MS/MS and GC-MS methods, respectively. Food Addit. Contam. 2007;24(3):258–265. doi: 10.1080/02652030601013729. [DOI] [PubMed] [Google Scholar]
- 85.Persky A.M., Brazeau G.A. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol. Rev. 2001;53:161–176. [PubMed] [Google Scholar]
- 86.Passaquin A.C., Renard M., Kay L., Challet C., Mokhtarian A., Wallimann T., Ruegg U.T. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscular disorders: NMD. 2002;12(2):174–182. doi: 10.1016/s0960-8966(01)00273-5. [DOI] [PubMed] [Google Scholar]
- 87.Louis M., Raymackers J.M., Debaix H., Lebacq J., Francaux M. Effect of creatine supplementation on skeletal muscle of mdx mice. Muscle Nerve. 2004;29(5):687–692. doi: 10.1002/mus.20014. [DOI] [PubMed] [Google Scholar]
- 88.Payne E.T., Yasuda N., Bourgeois J.M., Devries M.C., Rodriguez M.C., Yousuf J., Tarnopolsky M.A. Nutritional therapy improves function and complements corticosteroid intervention in mdx mice. Muscle Nerve. 2006;33(1):66–77. doi: 10.1002/mus.20436. [DOI] [PubMed] [Google Scholar]
- 89.De Luca A., Pierno S., Liantonio A., Cetrone M., Camerino C., Fraysse B., Mirabella M., Servidei S., Ruegg U.T., Conte Camerino D. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J. Pharmacol. Exp. Ther. 2003;304(1):453–463. doi: 10.1124/jpet.102.041343. [DOI] [PubMed] [Google Scholar]
- 90.Banerjee B., Sharma U., Balasubramanian K., Kalaivani M., Kalra V., Jagannathan N.R. Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: a randomized, placebo-controlled 31P MRS study. Magn. Reson. Imaging. 2010;28(5):698–707. doi: 10.1016/j.mri.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 91.Felber S., Skladal D., Wyss M., Kremser C., Koller A., Sperl W. Oral creatine supplementation in Duchenne muscular dystrophy: a clinical and 31P magnetic resonance spectroscopy study. Neurol. Res. 2000;22(2):145–150. doi: 10.1080/01616412.2000.11741051. [DOI] [PubMed] [Google Scholar]
- 92.Tarnopolsky M.A., Mahoney D.J., Vajsar J., Rodriguez C., Doherty T.J., Roy B.D., Biggar D. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology. 2004;62(10):1771–1777. doi: 10.1212/01.wnl.0000125178.18862.9d. [DOI] [PubMed] [Google Scholar]
- 93.Walter M.C., Lochmuller H., Reilich P., Klopstock T., Huber R., Hartard M., Hennig M., Pongratz D., Muller-Felber W. Creatine monohydrate in muscular dystrophies: a double-blind, placebo-controlled clinical study. Neurology. 2000;54(9):1848–1850. doi: 10.1212/wnl.54.9.1848. [DOI] [PubMed] [Google Scholar]
- 94.Juhn M.S., Tarnopolsky M. Potential side effects of oral creatine supplementation: a critical review. Clin. J. Sport. Med. 1998;8(4):298–304. doi: 10.1097/00042752-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Candow D.G., Forbes S.C., Chilibeck P.D., Cornish S.M., Antonio J., Kreider R.B. Variables influencing the effectiveness of creatine supplementation as a therapeutic intervention for Sarcopenia. Front. Nutr. 2019;6:124. doi: 10.3389/fnut.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taner B., Aysim O., Abdulkadir U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus. 2011;4(1):23–24. doi: 10.1093/ndtplus/sfq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoshizumi W.M., Tsourounis C. Effects of creatine supplementation on renal function. J. Herb. Pharmacother. 2004;4(1):1–7. [PubMed] [Google Scholar]
- 98.Pazhayattil G.S., Shirali A.C. Drug-induced impairment of renal function. Int. J. Nephrol. Renovasc. Dis. 2014;7:457–468. doi: 10.2147/IJNRD.S39747. Published 2014 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu C.L., Chang C.C., Kor C.T. Hydroxychloroquine use and risk of CKD in patients with rheumatoid arthritis. Clin. J. Am. Soc. Nephrol. 2018;13(5):702–709. doi: 10.2215/CJN.11781017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith T., Bushek J., Prosser T. Vol. 12. 2020. (COVID-19 Drug Therapy – Potential Options). [Google Scholar]
- 101.De Luca A., Pierno S., Camerino D.C. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015;13:243. doi: 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Luca A., Pierno S., Liantonio A., Cetrone M., Camerino C., Simonetti S., Papadia F., Camerino D.C. Alteration of excitation-contraction coupling mechanism in extensor digitorum longus muscle fibres of dystrophic mdx mouse and potential efficacy of taurine. Br. J. Pharmacol. 2001;132(5):1047–1054. doi: 10.1038/sj.bjp.0703907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Capogrosso R.F., Cozzoli A., Mantuano P., Camerino G.M., Massari A.M., Sblendorio V.T., De Bellis M., Tamma R., Giustino A., Nico B., Montagnani M., De Luca A. Assessment of resveratrol, apocynin and taurine on mechanical-metabolic uncoupling and oxidative stress in a mouse model of duchenne muscular dystrophy: a comparison with the gold standard, alpha-methyl prednisolone. Pharmacol. Res. 2016;106:101–113. doi: 10.1016/j.phrs.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 104.Mele A., Mantuano P., De Bellis M., Rana F., Sanarica F., Conte E., Morgese M.G., Bove M., Rolland J.F., Capogrosso R.F., Pierno S., Camerino G.M., Trabace L., De Luca A. A long-term treatment with taurine prevents cardiac dysfunction in mdx mice. Transl. Res. 2019;204:82–99. doi: 10.1016/j.trsl.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Cozzoli A., Rolland J.F., Capogrosso R.F., Sblendorio V.T., Longo V., Simonetti S., Nico B., De Luca A. Evaluation of potential synergistic action of a combined treatment with alpha-methyl-prednisolone and taurine on the mdx mouse model of Duchenne muscular dystrophy. Neuropathol. Appl. Neurobiol. 2011;37(3):243–256. doi: 10.1111/j.1365-2990.2010.01106.x. [DOI] [PubMed] [Google Scholar]
- 106.El Idrissi A., Okeke E., Yan X., Sidime F., Neuwirth L.S. Taurine regulation of blood pressure and vasoactivity. Adv. Exp. Med. Biol. 2013;775:407–425. doi: 10.1007/978-1-4614-6130-2_31. [DOI] [PubMed] [Google Scholar]
- 107.Matsuda H., Kinoshita K., Sumida A., Takahashi K., Fukuen S., Fukuda T., Takahashi K., Yamamoto I., Azuma J. Taurine modulates induction of cytochrome P450 3A4 mRNA by rifampicin in the HepG2 cell line. Biochim. Biophys. Acta. 2002;1593(1):93–98. doi: 10.1016/s0167-4889(02)00345-2. [DOI] [PubMed] [Google Scholar]
- 108.Mozaffari M.S., Miyata N., Schaffer S.W. Effects of taurine and enalapril on kidney function of the hypertensive glucose-intolerant rat. Am. J. Hypertens. 2003;16(8):673–680. doi: 10.1016/s0895-7061(03)00915-4. [DOI] [PubMed] [Google Scholar]
- 109.Seifert S.M., Schaechter J.L., Hershorin E.R., Lipshultz S.E. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics. 2011;127(3):511–528. doi: 10.1542/peds.2009-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Evans N.P., Call J.A., Bassaganya-Riera J., Robertson J.L., Grange R.W. Green tea extract decreases muscle pathology and NF-kappaB immunostaining in regenerating muscle fibers of mdx mice. Clin. Nutr. 2010;29(3):391–398. doi: 10.1016/j.clnu.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakae Y., Dorchies O.M., Stoward P.J., Zimmermann B.F., Ritter C., Ruegg U.T. Quantitative evaluation of the beneficial effects in the mdx mouse of epigallocatechin gallate, an antioxidant polyphenol from green tea. Histochem. Cell Biol. 2012;137(6):811–827. doi: 10.1007/s00418-012-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buetler T.M., Renard M., Offord E.A., Schneider H., Ruegg U.T. Green tea extract decreases muscle necrosis in mdx mice and protects against reactive oxygen species. Am. J. Clin. Nutr. 2002;75(4):749–753. doi: 10.1093/ajcn/75.4.749. [DOI] [PubMed] [Google Scholar]
- 113.Dorchies O.M., Wagner S., Vuadens O., Waldhauser K., Buetler T.M., Kucera P., Ruegg U.T. Green tea extract and its major polyphenol (-)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am. J. Physiol., Cell Physiol. 2006;290(2):C616–625. doi: 10.1152/ajpcell.00425.2005. [DOI] [PubMed] [Google Scholar]
- 114.Qureshi M.M., McClure W.C., Arevalo N.L. The dietary supplement protandim decreases plasma osteopontin and improves markers of oxidative stress in muscular dystrophy mdx mice. J. Diet. Suppl. 2010;7(2):159–178. doi: 10.3109/19390211.2010.482041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doepker C., Franke K., Myers E., Goldberger J.J., Lieberman H.R., O’Brien C., Peck J., Tenenbein M., Weaver C., Wikoff D. Key findings and implications of a recent systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Nutrients. 2018;10(10) doi: 10.3390/nu10101536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marx B., Scuvee E., Scuvee-Moreau J., Seutin V., Jouret F. [Mechanisms of caffeine-induced diuresis] Medecine sciences: M/S. 2016;32(5):485–490. doi: 10.1051/medsci/20163205015. [DOI] [PubMed] [Google Scholar]
- 117.Fan F.S. Iron deficiency anemia due to excessive green tea drinking. Clin. Case Rep. 2016;4(11):1053–1056. doi: 10.1002/ccr3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.El-Bakry H.A., El-Sherif G., Rostom R.M. Therapeutic dose of green tea extract provokes liver damage and exacerbates paracetamol-induced hepatotoxicity in rats through oxidative stress and caspase 3-dependent apoptosis. Biomed. Pharmacother. 2017;96:798–811. doi: 10.1016/j.biopha.2017.10.055. [DOI] [PubMed] [Google Scholar]
- 119.Yu Z., Samavat H., Dostal A.M., Wang R., Torkelson C.J., Yang C.S., Butler L.M., Kensler T.W., Wu A.H., Kurzer M.S., Yuan J.M. Effect of green tea supplements on liver enzyme elevation: results from a randomized intervention study in the United States. Cancer Prev. Res. Phila. (Phila) 2017;10(10):571–579. doi: 10.1158/1940-6207.CAPR-17-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Satoh T., Fujisawa H., Nakamura A., Takahashi N., Watanabe K. Inhibitory effects of eight green tea catechins on cytochrome P450 1A2, 2C9, 2D6, and 3A4 activities. J. Pharm. Pharm. Sci. 2016;19(2):188–197. doi: 10.18433/J3MS5C. [DOI] [PubMed] [Google Scholar]
- 121.Kissei M., Itoh T., Narawa T. Effect of epigallocatechin gallate on drug transport mediated by the proton-coupled folate transporter. Drug Metab. Pharmacokinet. 2014;29(5):367–372. doi: 10.2133/dmpk.dmpk-14-rg-015. [DOI] [PubMed] [Google Scholar]
- 122.Veeranki S., Tyagi S.C. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int. J. Mol. Sci. 2013;14(7):15074–15091. doi: 10.3390/ijms140715074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Werba J.P., Misaka S., Giroli M.G., Shimomura K., Amato M., Simonelli N., Vigo L., Tremoli E. Update of green tea interactions with cardiovascular drugs and putative mechanisms. J. Food Drug Anal. 2018;26(2S):S72–S77. doi: 10.1016/j.jfda.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kiss T., Timár Z., Szabó A. Effect of green tea on the gastrointestinal absorption of amoxicillin in rats. BMC Pharmacol. Toxicol. 2019;20(1):54. doi: 10.1186/s40360-019-0332-8. Published 2019 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee L.S., Andrade A.S., Flexner C. Interactions between natural health products and antiretroviral drugs: pharmacokinetic and pharmacodynamic effects. Clin. Infect. Dis. 2006;43(8):1052–1059. doi: 10.1086/507894. [DOI] [PubMed] [Google Scholar]
- 126.Jodoin J., Demeule M., Beliveau R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta. 2002;1542(1–3):149–159. doi: 10.1016/s0167-4889(01)00175-6. [DOI] [PubMed] [Google Scholar]
- 127.Lu H., Meng X., Li C., Sang S., Patten C., Sheng S., Hong J., Bai N., Winnik B., Ho C.T., Yang C.S. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab. Dispos. 2003;31(4):452–461. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- 128.Wu B., Kulkarni K., Basu S., Zhang S., Hu M. First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics. J. Pharm. Sci. 2011;100(9):3655–3681. doi: 10.1002/jps.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kong R., Laskin O.L., Kaushik D., Jin F., Ma J., McIntosh J., Souza M., Almstead N. Ataluren pharmacokinetics in healthy japanese and caucasian subjects. Clin. Pharmacol. Drug Dev. 2019;8(2):172–178. doi: 10.1002/cpdd.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han W.Y., Zhao F.J., Shi Y.Z., Ma L.F., Ruan J.Y. Scale and causes of lead contamination in Chinese tea. Environ. Pollut. 2006;139(1):125–132. doi: 10.1016/j.envpol.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 131.Abd El-Aty A.M., Choi J.H., Rahman M.M., Kim S.W., Tosun A., Shim J.H. Residues and contaminants in tea and tea infusions: a review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014;31(11):1794–1804. doi: 10.1080/19440049.2014.958575. [DOI] [PubMed] [Google Scholar]
- 132.Brzezicha-Cirocka J., Grembecka M., Szefer P. Monitoring of essential and heavy metals in green tea from different geographical origins. Environ. Monit. Assess. 2016;188(3):183. doi: 10.1007/s10661-016-5157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Machado R.V., Mauricio A.F., Taniguti A.P., Ferretti R., Neto H.S., Marques M.J. Eicosapentaenoic acid decreases TNF-alpha and protects dystrophic muscles of mdx mice from degeneration. J. Neuroimmunol. 2011;232(1–2):145–150. doi: 10.1016/j.jneuroim.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 134.Carvalho S.C., Apolinario L.M., Matheus S.M., Santo Neto H., Marques M.J. EPA protects against muscle damage in the mdx mouse model of Duchenne muscular dystrophy by promoting a shift from the M1 to M2 macrophage phenotype. J. Neuroimmunol. 2013;264(1–2):41–47. doi: 10.1016/j.jneuroim.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 135.Rodriguez-Cruz M., Atilano-Miguel S., Barbosa-Cortes L., Bernabe-Garcia M., Almeida-Becerril T., Cardenas-Conejo A., Del Rocio Cruz-Guzman O., Maldonado-Hernandez J. Evidence of muscle loss delay and improvement of hyperinsulinemia and insulin resistance in Duchenne muscular dystrophy supplemented with omega-3 fatty acids: a randomized study. Clin. Nutr. 2019;38(5):2087–2097. doi: 10.1016/j.clnu.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 136.Riediger N.D., Othman R.A., Suh M., Moghadasian M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 2009;109(4):668–679. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 137.Westphal C., Konkel A., Schunck W.H. Cytochrome p450 enzymes in the bioactivation of polyunsaturated Fatty acids and their role in cardiovascular disease. Adv. Exp. Med. Biol. 2015;851:151–187. doi: 10.1007/978-3-319-16009-2_6. [DOI] [PubMed] [Google Scholar]
- 138.Buckley M.S., Goff A.D., Knapp W.E. Fish oil interaction with warfarin. Ann. Pharmacother. 2004;38(1):50–52. doi: 10.1345/aph.1D007. [DOI] [PubMed] [Google Scholar]
- 139.Fappi A., Neves J.C., Kawasaki K.A. Omega-3 multiple effects increasing glucocorticoid-induced muscle atrophy: autophagic, AMPK and UPS mechanisms. Physiol. Rep. 2019;7(1):e13966. doi: 10.14814/phy2.13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bays H.E. Safety considerations with omega-3 fatty acid therapy. Am. J. Cardiol. 2007;99(6A):35C–43C. doi: 10.1016/j.amjcard.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 141.Jacobs M.N., Santillo D., Johnston P.A., Wyatt C.L., French M.C. Organochlorine residues in fish oil dietary supplements: comparison with industrial grade oils. Chemosphere. 1998;37(9–12):1709–1721. doi: 10.1016/s0045-6535(98)00236-7. [DOI] [PubMed] [Google Scholar]
- 142.Lane K., Derbyshire E., Li W., Brennan C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: a review of the literature. Crit. Rev. Food Sci. Nutr. 2014;54(5):572–579. doi: 10.1080/10408398.2011.596292. [DOI] [PubMed] [Google Scholar]
- 143.Buckner J.L., Bowden S.A., Mahan J.D. Optimizing bone health in Duchenne muscular dystrophy. Int. J. Endocrinol. 2015;2015 doi: 10.1155/2015/928385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goldsmith J.R. Vitamin d as an immunomodulator: risks with deficiencies and benefits of supplementation. Healthcare (Basel) 2015;3(2):219–232. doi: 10.3390/healthcare3020219. Published 2015 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Koutkia P., Chen T.C., Holick M.F. Vitamin D intoxication associated with an over-the-counter supplement. N. Engl. J. Med. 2001;345(1):66–67. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 146.von Restorff C., Bischoff-Ferrari H.A., Theiler R. High-dose oral vitamin D3 supplementation in rheumatology patients with severe vitamin D3 deficiency. Bone. 2009;45(4):747–749. doi: 10.1016/j.bone.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 147.Jones G., Prosser D.E., Kaufmann M. Cytochrome P450-mediated metabolism of vitamin d. J. Lipid Res. 2014;55(1):13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hosseinpour F., Ellfolk M., Norlin M., Wikvall K. Phenobarbital suppresses vitamin D3 25-hydroxylase expression: a potential new mechanism for drug-induced osteomalacia. Biochem. Biophys. Res. Commun. 2007;357(3):603–607. doi: 10.1016/j.bbrc.2007.03.177. [DOI] [PubMed] [Google Scholar]
- 149.Schwalfenberg G.K., Genuis S.J. Vitamin d, essential minerals, and toxic elements: exploring interactions between nutrients and toxicants in clinical medicine. TheScientificWorldJournal. 2015;2015 doi: 10.1155/2015/318595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sebori R., Kuno A., Hosoda R., Hayashi T., Horio Y. Resveratrol decreases oxidative stress by restoring mitophagy and improves the pathophysiology of dystrophin-deficient mdx mice. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/9179270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ljubicic V., Burt M., Lunde J.A., Jasmin B.J. Resveratrol induces expression of the slow, oxidative phenotype in mdx mouse muscle together with enhanced activity of the SIRT1-PGC-1alpha axis. Am. J. Physiol., Cell Physiol. 2014;307(1):C66–82. doi: 10.1152/ajpcell.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pan Y., Chen C., Shen Y. Curcumin alleviates dystrophic muscle pathology in mdx mice. Mol. Cells. 2008;25(4):531–537. [PubMed] [Google Scholar]
- 153.Durham W.J., Arbogast S., Gerken E., Li Y.P., Reid M.B. Progressive nuclear factor-kappaB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve. 2006;34(3):298–303. doi: 10.1002/mus.20579. [DOI] [PubMed] [Google Scholar]
- 154.Piver B., Berthou F., Dreano Y., Lucas D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol. Lett. 2001;125(1–3):83–91. doi: 10.1016/s0378-4274(01)00418-0. [DOI] [PubMed] [Google Scholar]
- 155.Chow H.H., Garland L.L., Hsu C.H., Vining D.R., Chew W.M., Miller J.A., Perloff M., Crowell J.A., Alberts D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. Phila. (Phila) 2010;3(9):1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Detampel P., Beck M., Krahenbuhl S., Huwyler J. Drug interaction potential of resveratrol. Drug Metab. Rev. 2012;44(3):253–265. doi: 10.3109/03602532.2012.700715. [DOI] [PubMed] [Google Scholar]
- 157.Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin. Drug Metab. Toxicol. 2007;3(4):583–598. doi: 10.1517/17425225.3.4.583. [DOI] [PubMed] [Google Scholar]
- 158.Park D., Jeong H., Lee M.N., Koh A., Kwon O., Yang Y.R., Noh J., Suh P.G., Park H., Ryu S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016;6:21772. doi: 10.1038/srep21772. https://doi.org/ARTN 2177210.1038/srep21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shimizu N., Yoshikawa N., Ito N., Maruyama T., Suzuki Y., Takeda S., Nakae J., Tagata Y., Nishitani S., Takehana K., Sano M., Fukuda K., Suematsu M., Morimoto C., Tanaka H. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011;13(2):170–182. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 160.Risson V., Mazelin L., Roceri M., Sanchez H., Moncollin V., Corneloup C., Richard-Bulteau H., Vignaud A., Baas D., Defour A., Freyssenet D., Tanti J.F., Le-Marchand-Brustel Y., Ferrier B., Conjard-Duplany A., Romanino K., Bauche S., Hantai D., Mueller M., Kozma S.C., Thomas G., Ruegg M.A., Ferry A., Pende M., Bigard X., Koulmann N., Schaeffer L., Gangloff Y.G. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J. Cell Biol. 2009;187(6):859–874. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sharma R.A., Euden S.A., Platton S.L. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 163.Carroll R.E., Benya R.V., Turgeon D.K. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia [published correction appears in Cancer Prev Res (Phila). 2012 Dec;5(12):1407. Dosage error in article text] Cancer Prev. Res. Phila. (Phila) 2011;4(3):354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen B.Y., Kuo C.H., Liu Y.C., Ye L.Y., Chen J.H., Shieh C.J. Ultrasonic-assisted extraction of the botanical dietary supplement resveratrol and other constituents of Polygonum cuspidatum. J. Nat. Prod. 2012;75(10):1810–1813. doi: 10.1021/np300392n. [DOI] [PubMed] [Google Scholar]
- 165.Dhakal S., Chao K., Schmidt W., Qin J., Kim M., Chan D. Evaluation of turmeric powder adulterated with metanil yellow using FT-Raman and FT-IR spectroscopy. Foods. 2016;5(2):36. doi: 10.3390/foods5020036. Published 2016 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gleason K., Shine J.P., Shobnam N. Contaminated turmeric is a potential source of lead exposure for children in rural Bangladesh. J. Environ. Public Health. 2014;2014 doi: 10.1155/2014/730636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Forsyth J.E., Nurunnahar S., Islam S.S. Turmeric means “yellow” in Bengali: lead chromate pigments added to turmeric threaten public health across Bangladesh. Environ. Res. 2019;179(Pt A):108722. doi: 10.1016/j.envres.2019.108722. [DOI] [PubMed] [Google Scholar]
- 168.Terrill J.R., Radley-Crabb H.G., Grounds M.D., Arthur P.G. N-Acetylcysteine treatment of dystrophic mdx mice results in protein thiol modifications and inhibition of exercise induced myofibre necrosis. Neuromuscular disorders: NMD. 2012;22(5):427–434. doi: 10.1016/j.nmd.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 169.de Senzi Moraes Pinto R., Ferretti R., Moraes L.H., Neto H.S., Marques M.J. Minatel E. N-acetylcysteine treatment reduces TNF-alpha levels and myonecrosis in diaphragm muscle of mdx mice. Clin. Nutr. 2013;32(3):472–475. doi: 10.1016/j.clnu.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 170.Whitehead N.P., Pham C., Gervasio O.L., Allen D.G. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J. Physiol. (Lond.) 2008;586(7):2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Terrill J.R., Pinniger G.J., Graves J.A., Grounds M.D., Arthur P.G. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. J. Physiol. (Lond.) 2016;594(11):3095–3110. doi: 10.1113/JP271418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moraes L.H., Bollineli R.C., Mizobuti D.S., Silveira Ldos R., Marques M.J., Minatel E. Effect of N-acetylcysteine plus deferoxamine on oxidative stress and inflammation in dystrophic muscle cells. Redox Rep.: Commun. Free Radic. Res. 2015;20(3):109–115. doi: 10.1179/1351000214Y.0000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Garozzo A., Tempera G., Ungheri D., Timpanaro R., Castro A. N-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infection. Int. J. Immunopathol. Pharmacol. 2007;20(2):349–354. doi: 10.1177/039463200702000215. [DOI] [PubMed] [Google Scholar]
- 174.McCarty M.F., DiNicolantonio J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus [published online ahead of print, 2020 Feb12] Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.2020.02.007. S0033-0620(20)30037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.De Flora S., Grassi C., Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur. Respir. J. 1997;10(7):1535–1541. doi: 10.1183/09031936.97.10071535. [DOI] [PubMed] [Google Scholar]
- 176.Pendyala L., Creaven P.J. Pharmacokinetic and pharmacodynamic studies of N-acetylcysteine, a potential chemopreventive agent during a phase I trial. Cancer Epidemiol. Biomark. Prev. 1995;4(3):245–251. [PubMed] [Google Scholar]
- 177.Childs A., Jacobs C., Kaminski T., Halliwell B., Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic. Biol. Med. 2001;31(6):745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 178.Pinniger G.J., Terrill J.R., Assan E.B., Grounds M.D., Arthur P.G. Pre-clinical evaluation of N-acetylcysteine reveals side effects in the mdx mouse model of Duchenne muscular dystrophy. J. Physiol. 2017;595(23):7093–7107. doi: 10.1113/JP274229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Spurney C.F., Rocha C.T., Henricson E., Florence J., Mayhew J., Gorni K., Pasquali L., Pestronk A., Martin G.R., Hu F., Nie L., Connolly A.M., Escolar D.M. Cooperative International Neuromuscular Research Group I. CINRG pilot trial of coenzyme Q10 in steroid-treated duchenne muscular dystrophy. Muscle Nerve. 2011;44(2):174–178. doi: 10.1002/mus.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Folkers K., Simonsen R. Two successful double-blind trials with coenzyme Q10 (vitamin Q10) on muscular dystrophies and neurogenic atrophies. Biochim. Biophys. Acta. 1995;1271(1):281–286. doi: 10.1016/0925-4439(95)00040-b. [DOI] [PubMed] [Google Scholar]
- 181.Folkers K., Wolaniuk J., Simonsen R., Morishita M., Vadhanavikit S. Biochemical rationale and the cardiac response of patients with muscle disease to therapy with coenzyme Q10. Proc. Natl. Acad. Sci. U. S. A. 1985;82(13):4513–4516. doi: 10.1073/pnas.82.13.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Salehi F., Zeinaloo A., Riasi H.R., Shamloo A.S. Effectiveness of Coenzyme Q10 on echocardiographic parameters of patients with Duchenne muscular dystrophy. Electron. Physician. 2017;9(3):3896–3904. doi: 10.19082/3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Phase III Study of Idebenone in Duchenne Muscular Dystrophy (DMD) - https://clinicaltrials.gov/ct2/show/NCT01027884.
- 184.Buyse G.M., Voit T., Schara U. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): a double-blind randomised placebo-controlled phase 3 trial. Lancet. 2015;385(9979):1748–1757. doi: 10.1016/S0140-6736(15)60025-3. [DOI] [PubMed] [Google Scholar]
- 185.Tabrizi R., Akbari M., Sharifi N., Lankarani K.B., Moosazadeh M., Kolahdooz F., Taghizadeh M., Asemi Z. The effects of coenzyme Q10 supplementation on blood pressures among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. High Blood Press. Cardiovasc. Prev. 2018;25(1):41–50. doi: 10.1007/s40292-018-0247-2. [DOI] [PubMed] [Google Scholar]
- 186.Hibaoui Y., Reutenauer-Patte J., Patthey-Vuadens O., Ruegg U.T., Dorchies O.M. Melatonin improves muscle function of the dystrophic mdx5Cv mouse, a model for Duchenne muscular dystrophy. J. Pineal Res. 2011;51(2):163–171. doi: 10.1111/j.1600-079X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 187.Chahbouni M., Escames G., Venegas C. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J. Pineal Res. 2010;48(3):282–289. doi: 10.1111/j.1600-079X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 188.Zhang R., Wang X., Ni L. COVID-19: Melatonin as a potential adjuvant treatment [published online ahead of print, 2020 Mar 23] Life Sci. 2020:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wirtz P.H., Spillmann M., Bartschi C., Ehlert U., von Kanel R. Oral melatonin reduces blood coagulation activity: a placebo-controlled study in healthy young men. J. Pineal Res. 2008;44(2):127–133. doi: 10.1111/j.1600-079X.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 190.Lusardi P., Piazza E., Fogari R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: a 24-hour study. Br. J. Clin. Pharmacol. 2000;49(5):423–427. doi: 10.1046/j.1365-2125.2000.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kennaway D.J. Potential safety issues in the use of the hormone melatonin in paediatrics. J. Paediatr. Child Health. 2015;51(6):584–589. doi: 10.1111/jpc.12840. [DOI] [PubMed] [Google Scholar]
- 192.Erland L.A., Saxena P.K. Melatonin natural health products and supplements: presence of serotonin and significant variability of melatonin content. J. Clin. Sleep Med. 2017;13(2):275–281. doi: 10.5664/jcsm.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grigg-Damberger M.M., Ianakieva D. Poor quality control of over-the-counter melatonin: what they say is often not what you get. J. Clin. Sleep Med. 2017;13(2):163–165. doi: 10.5664/jcsm.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Williamson B.L., Tomlinson A.J., Naylor S., Gleich G.J. Contaminants in commercial preparations of melatonin. Mayo Clin. Proc. 1997;72(11):1094–1095. doi: 10.1016/S0025-6196(11)63555-6. [DOI] [PubMed] [Google Scholar]
- 195.Andersson S., Antonsson M., Elebring M., Jansson-Lofmark R., Weidolf L. Drug metabolism and pharmacokinetic strategies for oligonucleotide- and mRNA-based drug development. Drug Discov. Today. 2018;23(10):1733–1745. doi: 10.1016/j.drudis.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 196.Sazani P., Magee T., Charleston J.S., Shanks C., Zhang J., Carver M., Saoud J., Kaye E. In vitro pharmacokinetic evaluation of eteplirsen, SRP-4045, and SRP-4053; three phosphorodiamidate morpholino oligomers (PMO) for the treatment of patients with duchenne muscular dystrophy (DMD) (P5.061) Neurology. 2015;84(14 Supplement) P5.061. [Google Scholar]
- 197.Cossu G., Previtali S.C., Napolitano S. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy [published correction appears in EMBO Mol Med. 2016 Dec 1;8(12):1470-1471] EMBO Mol. Med. 2015;7(12):1513–1528. doi: 10.15252/emmm.201505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tedesco F.S., Gerli M.F., Perani L. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci. Transl. Med. 2012;4(140) doi: 10.1126/sci. 140ra89. [DOI] [PubMed] [Google Scholar]
- 199.de la Ballina L.R., Cano-Crespo S., Gonzalez-Munoz E., Bial S., Estrach S., Cailleteau L., Tissot F., Daniel H., Zorzano A., Ginsberg M.H., Palacin M., Feral C.C. Amino acid transport associated to cluster of differentiation 98 heavy chain (CD98hc) is at the cross-road of oxidative stress and amino acid availability. J. Biol. Chem. 2016;291(18):9700–9711. doi: 10.1074/jbc.M115.704254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Malinovskaya N.A., Komleva Y.K., Salmin V.V., Morgun A.V., Shuvaev A.N., Panina Y.A., Boitsova E.B., Salmina A.B. Endothelial progenitor cells physiology and metabolic plasticity in brain angiogenesis and blood-brain barrier modeling. Front. Physiol. 2016;7:599. doi: 10.3389/fphys.2016.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang S., Zeng X., Ren M., Mao X., Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J. Anim. Sci. Biotechnol. 2017;8:10. doi: 10.1186/s40104-016-0139-z. Published 2017 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Duan D. Systemic AAV micro-dystrophin gene therapy for duchenne muscular dystrophy. Mol. Ther. 2018;26(10):2337–2356. doi: 10.1016/j.ymthe.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Koo T., Lu-Nguyen N.B., Malerba A. Functional rescue of dystrophin deficiency in mice caused by frameshift mutations using Campylobacter jejuni Cas9. Mol. Ther. 2018;26(6):1529–1538. doi: 10.1016/j.ymthe.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Young C.S., Pyle A.D., Spencer M.J. CRISPR for neuromuscular disorders: gene editing and beyond. Physiology. 2019;34(5):341–353. doi: 10.1152/physiol.00012.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Geller A.I., Shehab N., Weidle N.J., Lovegrove M.C., Wolpert B.J., Timbo B.B., Mozersky R.P., Budnitz D.S. Emergency department visits for adverse events related to dietary supplements. N. Engl. J. Med. 2015;373(16):1531–1540. doi: 10.1056/NEJMsa1504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Banfi S., D’Antona G., Ruocco C., Meregalli M., Belicchi M., Bella P., Erratico S., Donato E., Rossi F., Bifari F., Lonati C., Campaner S., Nisoli E., Torrente Y. Supplementation with a selective amino acid formula ameliorates muscular dystrophy in mdx mice. Sci. Rep. 2018;8(1):14659. doi: 10.1038/s41598-018-32613-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mok E., Constantin B., Favreau F., Neveux N., Magaud C., Delwail A., Hankard R. l-Glutamine administration reduces oxidized glutathione and MAP kinase signaling in dystrophic muscle of mdx mice. Pediatr. Res. 2008;63(3):268–273. doi: 10.1203/PDR.0b013e318163a259. [DOI] [PubMed] [Google Scholar]
- 208.Call J.A., Voelker K.A., Wolff A.V., McMillan R.P., Evans N.P., Hulver M.W., Talmadge R.J., Grange R.W. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J. Appl. Physiol. 2008;105(3):923–932. doi: 10.1152/japplphysiol.00028.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Debruin D.A., Andreacchio N., Hanson E.D., Timpani C.A., Rybalka E., Hayes A. The effect of vitamin d supplementation on skeletal muscle in the mdx mouse model of duchenne muscular dystrophy. Sports (Basel, Switzerland) 2019;7(5) doi: 10.3390/sports7050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bianchi M.L., Morandi L., Andreucci E., Vai S., Frasunkiewicz J., Cottafava R. Low bone density and bone metabolism alterations in Duchenne muscular dystrophy: response to calcium and vitamin D treatment. Osteoporos. Int. 2011;22(2):529–539. doi: 10.1007/s00198-010-1275-5. [DOI] [PubMed] [Google Scholar]
- 211.Gordon B.S., Delgado Diaz D.C., Kostek M.C. Resveratrol decreases inflammation and increases utrophin gene expression in the mdx mouse model of Duchenne muscular dystrophy. Clin. Nutr. 2013;32(1):104–111. doi: 10.1016/j.clnu.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 212.Kuno A., Hosoda R., Sebori R., Hayashi T., Sakuragi H., Tanabe M., Horio Y. Resveratrol ameliorates mitophagy disturbance and improves cardiac pathophysiology of dystrophin-deficient mdx mice. Sci. Rep. 2018;8(1):15555. doi: 10.1038/s41598-018-33930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chahbouni M., Escames G., Lopez L.C., Sevilla B., Doerrier C., Munoz-Hoyos A., Molina-Carballo A., Acuna-Castroviejo D. Melatonin treatment counteracts the hyperoxidative status in erythrocytes of patients suffering from Duchenne muscular dystrophy. Clin. Biochem. 2011;44(10–11):853–858. doi: 10.1016/j.clinbiochem.2011.04.001. [DOI] [PubMed] [Google Scholar]