Abstract
Background:
The aims of this study were: investigate the frequency of HIV-1 RNA levels discordance between the cerebrospinal fluid (CSF) and plasma and of CSF viral escape (CVE) in patients HIV-1 subtype C on antiretroviral therapy; evaluate the CSF white blood cell (WBC) performance characteristics in predicting CSF discordance in HIV+ group; and the frequency of cognitive impairment in individuals with CSF HIV discordance or escape.
Methods:
HIV-1 RNA levels were assessed in plasma and CSF samples from 68 HIV+ participants without opportunistic infection.
Results:
CSF discordance was found in 7.4% and CVE in 10%, with comparable frequencies between HIV-1B and C. Twenty samples (29%) showed increased CSF WBC counts. This group had higher CSF and plasma HIV-1 RNA levels than the group with normal WBC counts (p < 0.0001 and 0.006, respectively). The odds of CSF discordance were 18 times higher for a person with CSF WBC count of >5cells/mm3 than the group with normal CSF WBC count. CSF WBC counts (cut-off of 15 cells/mm3) showed high-performance characteristics as a predictive biomarker of CSF discordance (AUC the ROC curve 0.98). The frequency of cognitive impairment for CSF escape or discordance, was 83%, and 80%. The odds of cognitive impairment in these groups were 19 and 15 times higher, than those for a HIV(−) person.
Conclusion:
Viral discordance or escape in the CNS occurs at a comparable frequency for HIV-1C and HIV-1B. The CSF WBC count was effective as a predictive biomarker of CSF and plasma discordance.
Keywords: HIV-1, cerebrospinal fluid (CSF), central nervous system (CNS), white blood cell, scape, discordance
CSF inflammation begins shortly after HIV infection and persists throughout the early stages of immunosuppression (Hollander et al. 1994). The CSF white blood cell (WBC) count is a sensible biomarker of intrathecal inflammation (Hollander et al. 1994; Peluso et al. 2012; Manesh et al. 2019; Monteiro de Almeida et al. 2006). The CSF HIV RNA level was found to be significantly associated with the CSF WBC count (Buffet et al. 1991), supporting the hypothesis that a substantial part of the virus in the CSF of people with HIV (PWH) is locally produced by mononuclear cells (Martin et al. 1998) or that CSF WBC traffic into CSF when CSF HIV RNA is elevated.
Previous studies have reported a subset of individuals (~5% to 21%) presenting with CSF viral escape (CVE), regardless of sustained HIV suppression in the blood during antiretroviral therapy (ART) (Clifford 2010; Eden et al. 2010; Lescure et al. 2013). Despite effective suppression of viremia with ART, HIV can still replicate in the CNS, with the development of quasispecies in patients with acute or subacute neurological manifestations (Haggert and Stevenson 1991; Zarate et al. 2007; Harrington et al. 2009). CSF pleocytosis was presumed to reflect virological control failure in the CNS (Eden et al. 2010; Cusini et al. 2013; Ferretti et al. 2015; Pérez-Valero et al. 2019).
The majority of research studies on CSF and plasma HIV-1 RNA discordance (hereinafter CSF discordance) and CVE have been carried out in settings where HIV-1B predominates (Peluso et al. 2012; Canestri et al. 2010; van Lelyveld et al. 2010; Katlama et al. 2010; Bogoch et al. 2011; Bingham et al. 2011; Del Palacio et al.2012), and little is known about the CSF discordance and CVE of the non-B HIV subtypes. HIV-1 subtype C has been proposed to be less neuropathogenic than subtype B (Satishchandra et al. 2000), on the basis of an in vitro defective transactivator of transcription (Tat) chemokine dimotif in the position (C30C31) that might influence cellular trafficking and CNS inflammation (Ranga et al. 2004). The frequency of CVE in PWH infected with subtype C is largely unknown. Therefore, this cross-sectional study was performed to investigate the impact of the defective Tat chemokine dimotif on the frequency of CSF discordance and CVE among PWH infected with HIV-1C and HIV-1B.
The aims of this study were to investigate the frequency of CSF discordance and CVE in participants chronically infected with HIV-1C and who are on ART. In addition, we evaluated the diagnostic characteristics of the CSF WBC count to ascertain its performance in predicting CSF discordance in PWH without opportunistic CNS infections. We also investigated the frequency of cognitive impairment in participants with CSF discordance or CVE.
RESEARCH DESIGN AND METHOD
Samples
The HIV-positive (HIV+) participants were recruited at Hospital de Clínicas, Universidade Federal do Paraná, HC-UFPR, Brazil. This study was approved by the UCSD Institutional Review Board (IRB), the HC-UFPR IRB, and the Brazil National IRB (CONEP). Individuals with opportunistic CNS infections were not included in this study. All volunteers provided paired blood and CSF samples, and underwent serological testing to confirm the HIV status before enrollment (BRASIL, 2018).
A total of 68 paired CSF and blood samples from the HIV+ participants were tested. The CSF samples were collected by lumbar puncture (LP), which was performed using an atraumatic spinal needle and aseptic technique. For participants with a clinically resistant infection, the infecting HIV subtype was genotyped with pol sequences, whereas env sequences were used for all other participants. Genotyping revealed that 27 individuals were infected with HIV-1B and 40 with non-B HIV-1 subtypes (C, n = 26; BF, 10; BC, 1; CF, 1; and F, 2). The HIV-1 subtype could not be genotyped in one participant.
The HIV-negative (HIV–) participants (n = 48) were recruited from the HC-UFPR blood bank, described previously (de Almeida et al. 2013).
Methods
The total CSF WBC count (cells/mm3) and cell-type differentials were quantified using fresh non-centrifuged CSF samples immediately after the LP. CSF pleocytosis was defined as a WBC count of >5 cells/mm3.
Quantification of plasma and CSF HIV RNA levels
The VERSANT® HIV-1 RNA 3.0 bDNA Kit (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) was used for CSF or plasma samples. It was used 1 mL of CSF or plasma. The assays were performed immediately after sample collection. Samples with an HIV RNA level of <50 copies/mL were considered under the detection limit.
The definitions of the following terms are based on the quantification of CSF HIV RNA in paired CSF and plasma samples. (1) Discordance between the CSF and plasma HIV RNA levels was defined as follows: (a) CSF HIV RNA level greater than 1 Log10 of the plasma viral load (Canestri et al. 2010). (b) Additionally, a separate group of CSF samples with HIV RNA levels higher than the plasma levels (regardless of the magnitude of the difference) was studied. (2) CVE was defined as any HIV RNA in the CSF above the limit of detection of the assay used, despite undetectable plasma levels by the same assay. It reflects the loss of control of brain HIV infection in a patient on effective ART (Peluso et al. 2012). In three participants for whom serial CSF and plasma samples were collected, CVE was classified into the following subtypes, on the basis of longitudinal analysis of the CSF and plasma HIV-1 RNA levels: (1) CSF blip, a single occurrence of CVE while suppressed in the plasma; (2) CSF slow suppression, a CVE with preceding lack of suppression in the plasma; and (3) persistent CVE, ≥2 consecutive CVEs while suppressed in the plasma (Joseph et al. 2016; Mukerji et al. 2017).
Neuropsychological assessments
All participants underwent a neuropsychological assessment, which covered seven ability domains with 18 individual tests. Global deficit scores (GDS), a measure of cognitive impairment, was calculated. Neuropsychological assessments was described in detail previously (de Almeida et al. 2013).
Data analyses
The results are presented as the median and interquartile range (IQR) or the percentage (%), as appropriate. Comparisons between groups were made using the chi-square test, Fisher’s exact test, or Mann-Whitney nonparametric test (unadjusted analysis), where appropriate. Comparisons between HIV RNA (Log10) in matched CSF and plasma samples was done with Wilcoxon signed rank test. Differences were considered statistically significant at the 5% alpha level. The 95% confidence intervals (95% CIs) for impaired proportions were calculated using the normal distribution with no continuity correction. For the correlation analysis, the correlation coefficients (rs) were estimated using Spearman’s rank-order method.
The performance characteristics of the increased CSF WBC count (index test) in predicting CSF discordance were evaluated. The CSF and plasma HIV RNA levels were used as the reference. The following performance characteristics of the CSF WBC count were calculated: sensitivity; specificity; accuracy (efficiency); positive and negative predictive values (PPV, NPV); Youden index (Galen and Gambino, 1975); positive and negative clinical utility index (CUI+, CUI−). The CUI values were classified as follows: excellent, ≥0.81; good, ≥0.64; fair, ≥0.49; poor, ≤0.49; and very poor, ≤0.36 (Mitchell 2008; Mitchell 2011). The positive and negative likelihood ratio (LR+, LR−) were calculated, where an LR+ value of ≥10.0 indicates that a positive test almost confirmed the disease, a value of ~6.0 indicates that the disease was present, and a value of ~1.0 indicates that the test was not able to show whether the disease was present or not. An LR+ value of ≤0.1 indicates that the disease was practically absent (McGee 2002; Akobeng 2007A). The receiver operating characteristic (ROC) curve was used to evaluate the ability of the CSF WBC count to accurately classify impaired and normal participants and to establish the best cut-off of the CSF WBC count increase (Akobeng 2007B).
RESULTS
The demographic, clinical, and laboratory characteristics of the groups studied (viz., HIV-1 as a whole and categorized by subtypes B or C, and CSF WBC cell counts) are presented in Table 1; the groups with CSF discordance or CVE and virological suppression in the CSF and plasma (aviremia) are summarized in Table 2.
Table 1.
Demographics, AIDS clinical characteristics and treatment in HIV+ participants as a whole, categorized by HIV-1 subtypes B and C participants, and CSF WBC > 5cell/mm3 and on normal range
| HIV + (n= 68) | HIV1-B (n=27) | HIV1-C (n=26) | p | WBC> 5cell/mm3 (n=20) | WBC≤ 5cell/mm3 (n=48) | p | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, years | 43 (35; 48) | 44(36.5; 50) | 43 (34.5; 47.5) | 0.450 | 42 (34; 46) | 43.5(35; 49) | 0.310 |
| Education, years | 8 (5; 11) | 8(5; 12) | 7 (5; 11.5) | 0.550 | 9.5 (6; 13) | 7 (5; 11) | 0.140 |
| Sex - male, n (%) | 33 (49.0%) | 14 (51.9%) | 11 (42.3%) | 0.590 | 12(60.0%) | 21(44.0%) | 0.290 |
| Disease and Treatment | |||||||
| Duration of infection (mths) | 89 (31; 135) | 91.03 (61.63; 144) | 81.37(27.82; 132) | 0.450 | 51 (15; 131) | 93(46; 136) | 0.172 |
| AIDS | 55 (80.9%) | 22 (81.5%) | 19 (73.1%) | 0.526 | 13(65.0%) | 42 (87.5%) | 0.045 |
| GDS | 0.65(0.30;105) | 0.95 (0.275; 1.725) | 0.50 (0.225; 0.875) | 0.126 | 0.58(0.25;0.97) | 0.72(0.3;1.10) | 0.567 |
| B/C, n | 27/26 | 27 | 26 | - | 6/8 | 21/18 | 0.544 |
| Current CD4 cells/mm3 | 369 (201; 534) | 457 (255; 614) | 359.5(176.5; 472.5) | 0.200 | 360 (241; 543) | 369 (193; 527) | 0.540 |
| Nadir CD4 cells/mm3 | 90 (33; 266) | 82 (26; 253.5) | 159 (16.5; 359.5) | 0.290 | 107 (11; 368) | 85 (43; 240) | 0.620 |
| CART, n(%) | 55 (80.9%) | 24 (88.9%) | 18 (69.2%) | 0.099 | 12 (60.0%) | 43 (89.6%) | 0.014 |
| CPE | 8 (6; 9) | 8 (6; 9) | 6 (5.5; 9) | 0.339 | 8.0 (6.5; 9.5) | 8.0 (5.5; 9.0) | 0.279 |
| Adherence, n(%) | 51/54 (94.4%) | 21/23(91.3%) | 18/18(100%) | 0.495 | 11/12 (91.7%) | 40/42 (95.2%) | 1.00 |
| Plasma HIV RNA (Log10) | 1.7 (1.7; 3.5) | 1.7(1.7; 1.97) | 2.8 (1.7; 3.8) | 0.012 | 3.14 (1.7; 4.1) | 1.7 (1.7; 2.3) | 0.006 |
| Plasma HIV RNA < 50 copies/mL | 38 (55.9%) | 20 (74.1%) | 9 (34.6%) | 0.006 | 6 (30.0%) | 32 (66.7%) | 0.008 |
| CSF | |||||||
| HIV RNA (Log10) | 1.7 (1.7; 2.8) | 1.7 (1.7; 2.2) | 2.2 (1.7; 2.9) | 0.084 | 2.96 (2.3; 3.6) | 1.7 (1.7; 1.9) | <0.0001 |
| HIV RNA <50 copies/mL, n(%) | 35 (51.5%) | 16 (59.3%) | 10 (38.5%) | 0.173 | 4 (20.0%) | 31(64.5%) | 0.0012 |
| HIV RNA CSF >plasma, n(%) [1] | 12 (17.7%) | 5 (18.5%) | 5 (19.2%) | 1.00 | 8 (40.0%) | 4 (8.3%) | 0.004 |
| CSF escape [2] | 7 (10.3%) | 5(14.8%) | 1(3.9%) | 0.351 | 3(15.0%) | 4 (8.3%) | 0.411 |
| Discordance [3] | 5(7.4%) | 2(7.4%) | 3(11.5%) | 0.670 | 5(25.0%) | 0 (0.0%) | - |
| WBC cells/mm3 | 2.1 (0.6; 7.2) | 1.6 (0.30; 4.85) | 2.65 (0.60; 11) | 0.160 | 13 (9.5;31.5) | 0.9 (0.5;2.4) | <0.0001 |
| Glucose mg/dL | 57 (53; 62) | 63 (54; 66) | 56(51.5; 59) | 0.007 | 59 (48; 61) | 56 (54; 63) | 0.396 |
| Total protein mg/dL | 40 (32; 46) | 42(35; 47.5) | 40(28.5;47) | 0.551 | 42.5 (35.5; 56.5) | 36.5 (28.5; 45.5) | 0.011 |
| Total protein >45 mg/dL, n(%) | 20 (29.4%) | 10 (37.0%) | 8 (30.8%) | 0.773 | 8 (40.0%) | 12 (25.0%) | 0.251 |
| Albumin mg/L | 223.5 (164; 288.5) | 248.0(189; 309) | 218(138.5; 300) | 0.328 | 226.5 (188.5; 360) | 222.5 (145.5; 284.5) | 0.104 |
| Q. Albumin | 0.0064 (0.0049; 0.0097) | 0.0082(0.0061; 0.0108) | 0.0060(0.0044; 0.0097) | 0.52 [4] | 0.0079 (0.0063; 0.0116) | 0.0061 (0.0045; 0.0092) | 0.014 |
| Lactic acid mmol/L | 1.6 (1.5; 1.8) | 1.65(1.35; 1.9) | 1.7(1.6; 1.8) | 0.640 | 1.7 (1.5; 1.9) | 1.6 (1.5; 1.8) | 0.580 |
| RBC cells/mm3 | 0.5 (0; 7.5) | 1.0(0; 24) | 0.8(0; 36.5) | 0.900 | 1.3 (0.2; 6.8) | 0.3 (0; 8.0) | 0.387 |
Data are median (IQR) or number of cases (%).
any value of CSF or blood HIV RNA.
CSF escape is defined as any HIV RNA levels in the CSF above the limit of detection of the assay used (usually 50 copies/mL) when the plasma HIV RNA levels is undetectable by the same assay.
CSF discordance is defined as CSF viral load (VL) greater than 1log10 of the plasma HIV RNA levels (independent of the number).
adjusted for plasma HIV VL suppression, nadir CD4.
CART, combination antiretroviral therapy
CNS Penetration-Effectiveness (CPE) rank (Letendre et al. 2010)
Table 2.
Demographics, AIDS clinical characteristics and treatment in the groups with HIV central nervous system escape, discordance, and aviremic in CSF and plasma
| A-Escape n=7 [1] | B-Discordance n= 5 [2, 3] | C-CSF/Pl Aviremic n=31 | D-HIV RNA Pl > CSF, n=23 | AxC p | BxC p | AxB p | CxD p | |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age, years | 39(36; 49) | 39(27; 51) | 42(35; 49.5) | 46(37; 48) | 0.598 | 0.552 | 1.0 | 0.807 |
| Education, years | 6 (4.5; 14.5) | 7(4; 13) | 8 (5.5; 11.5) | 6(5; 11) | 0.429 | 0.631 | 0.876 | 0.412 |
| Sex - male, n (%) | 2 (28.6%) | 3 (60.0%) | 18 (58.1%) | 9 (39.1%) | 0.222 | 1.0 | 0.558 | 0.271 |
| Disease and Treatment | ||||||||
| Duration of infection (mths) | 66.77(19.50; 145.4) | 5.57(3.17; 127.6) | 91 (43.28; 136) | 94(47.38; 143.2) | 0.429 | 0.032 | 0.268 | 1.0 |
| GDS | 1.353 (0.543; 2.710) | 1.11(0.32; 3.47) | 0.5(0.23; 1.0) | 0.65(0.28; 1.10) | 0.076 | 0.109 | 0.931 | 0.726 |
| GDS≥0.5, n (%) | 5/6 (83.3%) | 4/5 (80.0%) | 16/29 (55.2%) | 11/18 (61.1%) | 0.366 | 0.379 | 1.0 | 1.0 |
| B/C, n | 5/1 | 2/3 | 15/8 | 6/13 | 0.633 | 0.353 | 0.242 | 0.062 |
| Current CD4 cells/mm3 | 457(265; 747) | 239(98; 692) | 372(215; 626) | 347(159; 443) | 0.925 | 0.522 | 0.639 | 0.349 |
| Nadir CD4 cells/mm3 | 51(6; 293) | 6(1; 692) | 54(16.5; 193) | 266(85; 368) | 0.749 | 0.082 | 0.530 | 0.001 |
| CART, n(%) | 7(100.0%) | 4(80.0%) | 31(100.0%) | 14 (61.9%) | - | - | - | - |
| CPE | 8(6.5; 10) | 9 (6; 10) | 8(6; 9) | 6(5; 9) | 0.674 | 0.224 | 0.527 | 0.907 |
| Adherence, n(%) | 7 (100.0%) | 4/4(100.0%) | 26/28(92.9%) | 14/14(100.0) | - | - | - | - |
| Time current regimen (mths) | 1.87(0.26; 64 ) [3] | 32(0; 64) | 24.64(9.92; 44.53) | 24.87(4.89; 47.21) | 0.265 | 0.817 | 0.800 | 0.736 |
| Pl HIV RNA ( Log10) | 1.7 | 1.7(1.7; 3.76) | 1.7 | 3.77(2.81; 4.68) | 1.0 | 0.149 | 0.268 | <0.0001 |
| plasma HIV RNA < 50 copies/mL | 7 (100.0%) | 3 (60.0%) | 31 (100.0%) | 0 (0%) | - | - | - | - |
| CSF | ||||||||
| HIV RNA (Log10) | 2.05(1.85; 3.11) | 3.112(2.85; 5.13) | 1.7 | 2.69(1.94; 3.14) | <0.0001 | 0.0004 | 0.030 | <0.0001 |
| HIV RNA <50 copies/mL, n(%) | 0 (0%) | 0 (0%) | 31(100%) | 4 (17.39%) | - | - | - | - |
| WBC cells/mm3 | 2.5 (1.1; 38.5) | 37 (20; 382) | 0.90 (0.30; 2.95) | 3.10(0.95; 10.35) | 0.113 | 0.001 | 0.106 | 0.010 |
| Glucose mg/dL | 54 (49; 59) | 44 (38; 61) | 60(55.5; 65.5) | 56(52; 59) | 0.010 | 0.007 | 0.268 | 0.011 |
| Total protein mg/dL | 42 (36; 123) | 91 (42; 339) | 40(30.5; 46.5) | 36(30.5; 45) | 0.397 | 0.003 | 0.073 | 0.529 |
| Total protein >45 mg/dL, n(%) | 2 (28.6%) | 4 (80.0%) | 10 (32.3%) | 4 (17.4%) | 1.0 | 0.064 | 0.242 | 0.347 |
| Albumin mg/L | 248 (211; 611) | 501 (311; 1770) | 236 (155; 292) | 195(144; 266.5) | 0.328 | 0.003 | 0.048 | 0.340 |
| Q. Albumin | 0.0082 (0.0067; 0.0224) | 0.0164 (0.0096; 0.0917) | 0.0062 (0.0045; 0.0101) | 0.0060 ( 0.0047; 0.0090) | 0.153 | 0.003 | 0.048 | 0.861 |
| Lactic acid mmol/L | 1.7 (1.55; 2.34) | 1.9 (1.7; 2.8) | 1.6(1.45; 1.85) | 1.7 (1.6; 1.8) | 0.412 | 0.027 | 0.268 | 0.489 |
| RBC cells/mm3 | 2 (0.15; 19) | 30 (2; 92) | 0.6 (0; 24) | 0.3 (0.0; 8.0) | 1.0 | 0.030 | 0.048 | 0.257 |
Data are median (IQR), number of cases (%)
CSF escape is defined as any VL in the CSF above the limit of detection of the assay used (usually 50 copies/mL) when the VL in the plasma is undetectable by the same assay. All cases were on CART.
CSF discordance is defined as CSF viral load (VL) greater than 1log10 of the plasma VL (independent of the number).
Data are median (min, max).
CART, combination antiretroviral therapy; Pl, Plasma
CSF, cerebrospinal fluid.
CNS Penetration-Effectiveness (CPE) rank (Letendre et al. 2010)
The individuals infected with either HIV-1B or HIV-1C were similar in age, gender, years of education. Participants with subtype B were significantly more likely to have undetectable plasma HIV RNA, participants with HIV-1C had lower CSF glucose levels than those with subtype B, although CSF glucose levels in both groups were between reference range. The relationship of the CSF HIV RNA level in paired CSF and plasma samples (escape, discordance, and CSF and plasma aviremia) are summarized in Table 2. Participants with discordance had significantly higher CSF WBC and higher protein, and albumin quotient than those who were undetectable in both compartments. In the HIV+ group, combination antiretroviral therapy (CART), mostly protease inhibitors, was prescribed to 55 participants (81%). The median (IQR) CNS penetration effectiveness rank of the antiretrovirals was 8 (6; 9), and adherence (AIDS Clinical Trials Group, adherence questionnaire) was maintained by 51 (93%) participants. In the HIV+ group as a whole, there were positive correlations between the CSF WBC count and the CSF HIV RNA level (Log10) [rs = 0.614 (95%CI: 0.434–0.747), p < 0.0001] and the plasma HIV RNA level (Log10) [rs = 0.386 (95%CI: 0.155–0.577), p = 0.001]. For the HIV-1B and HIV-1C groups, there was correlation between the CSF WBC count and CSF HIV RNA level (Log10) [rs = 0.586 (95%CI: 0.254–0.795), p = 0.001; and rs = 0 656 (95%CI: 0.350–0.836), p = 0.0003, respectively], but not between the CSF WBC count and the plasma HIV RNA level (Log10).
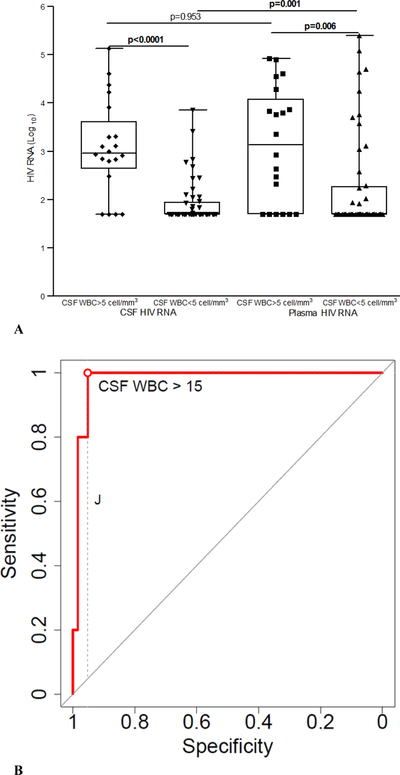
Twenty samples (29%) showed increased CSF WBC counts (>5 cells/mm3), with predominantly lymphocytes (85–100%), the median (IQR) WBC count was 13 (9.5; 31.5) cells/mm3 (Table 1). Participants with CSF WBC counts of >5 cells/mm3 had higher HIV RNA levels both in the CSF and plasma than the group with normal WBC counts (p < 0.0001 and 0.006, respectively). Participants with pleocytosis had significantly higher quotient of albumin and were less likely to have undetectable plasma and CSF HIV RNA than those with normal CSF WBC (Table 1; Figure 1A). Four participants (20%) have CSF pleocytosis with undetectable CSF HIV viral load, median (minimum; maximum) of CSF WBC in this group 14.65 (6.3; 27) cells/mm3. Whereas 31 (64.5%) participants in the group without CSF pleocytosis have undetectable CSF HIV viral load (p=0.0012) (Table 1; Figure 1A).
Figure 1.
A. Cerebrospinal fluid (CSF) and plasma HIV RNA levels (Log10) in the groups with cerebrospinal fluid (CSF) white blood cell (WBC) count increased (>5 cell/mm3) and on normal range (≤5 cell/mm3).
The line in the center of the box represents median; the superior and inferior borders of the box represent interquartiles (IQRs); the whiskers represent the least and greatest values; the number of cases in each group are indicated by the dots.
B. Receiver operating characteristic (ROC) curve to evaluate predictive characteristics of CSF WBC in characterizing subjects with CSF HIV RNA discordance.
CSF HIV RNA discordance was defined as CSF HIV RNA greater than 1log10 of the plasma HIV RNA (Canestri et al. 2010); optimal cut-off point 15 cells/mm3; area under the curve (AUC) = 0.981; 95% CI = 0.951−1. The AUC acts as a single measure, independent of prevalence, which summarizes the discriminative ability of a test across the full range of cut-offs, where the higher the AUC is, the better the test will be. The higher the AUC, the better the test. A perfect test would have an AUC of 1.0, while a completely ineffective test (where the curve falls on diagonal line) has an AUC of 0.5. Youden’s index (J) is the difference between the true positive rate and the false positive rate. According to its definition, “J” is the vertical distance between the ROC curve and the first bisector (or chance line), dashed line. Maximizing this index allows to find, from the ROC curve, an optimal cut-off point independently from the prevalence.
Group with CSF and plasma HIV RNA discordance
CSF discordance (Canestri et al. 2010) in the HIV+ group as a whole was found in 5 (7.35%) individuals (one of these participants was not on CART). The frequency of CSF discordance was comparable between the HIV-1 subtypes, with 2 (7.4%) individuals infected by HIV-1B and 3 (11%) infected by HIV-1C (p = 0.67) (Table 1). In the group with CSF WBC counts of >5 cells/mm3, discordance was found in 5 (25%) individuals, whereas in the group with CSF WBC counts of ≤5 cells/mm3, discordance was not found (Table 1). The odds of CSF HIV viral load discordance were 18.36 times higher for a person with a CSF WBC count of >5 cells/mm3 than for a person with a CSF WBC count within the normal range (≤5 cells/mm3) [odds ratio (OR) = 18.36 (95% CI: 2.05–164.34), p = 0.009]. Two of the participants with CSF discordance and increased CSF WBC counts (20 and 382 cells/mm3) were further studied by next-generation sequencing, with results showing HIV genetic compartmentalization in the CNS, both cases were HIV-1C, described previously (de Almeida et al. 2017; 2018A).
We also analyzed CSF discordance in the individuals with CSF viral loads higher than those of the plasma, independent of the HIV RNA values in both compartments. In the HIV group as a whole, there were 12 (17.65%) such individuals, with the number being comparable between those with HIV-1B and those with HIV-1C (n = 5, 18.52% and n = 5, 19.23%, respectively; p = 1.0). The CSF WBC count in the group with higher HIV RNA levels in CSF than in the plasma was 15.5 (3; 38.5), whereas that in the group with lower HIV RNA levels in CSF than in the plasma was 1.7 (0.6; 3.8) (p = 0.003). The Log10 CSF HIV RNA level was 3.0 (2.3; 4.1) in the group with higher HIV RNA in the CSF than in the plasma, whereas it was 1.7 (1.7; 2.3) in the group with lower HIV RNA in the CSF than in the plasma (p < 0.0001). The median (IQR) plasma HIV RNA levels of these two groups were 1.97 (1.7; 3.5) and 1.7 (1.7; 3.5), respectively (p = 0.84).
In the group with CSF WBC counts of >5 cells/mm3, the CSF viral load was higher than the plasma viral load in 8 (40%) individuals, whereas in the group with CSF WBC counts of ≤5 cells/mm3, the same was true for 4 (8.3%) individuals (p = 0.004) (Table 1). The odds of the HIV RNA level being higher in the CSF than in the plasma were 8.8 times higher for a PWH with a CSF WBC count of >5 cells/mm3 than for a person with a CSF WBC count within the normal range [OR = 8.76 (CI 95%: 2.21–34.8), p = 0.002].
Group with CSF HIV escape
CVE (Peluso et al., 2012) in the HIV group as a whole was found in 7 (10.29%) individuals (Table 1). The frequency of CVE was higher in the individuals with HIV-1B than in those with HIV-1C, although the numbers did not reach significance [n = 5 (14.81%) and n = 1 (3.85%), respectively, p = 0.61]. CVE was found in 3 (15%) individuals in the group with CSF WBC counts of >5 cells/mm3, and in 4 (8%) individuals in the group with CSF WBC counts of ≤5 cells/mm3 (p = 0.411) (Table 1). CNS penetration ARV regimens (Letendre et al. 2010) were comparable between groups described (Table 2)
Performance characteristics of the increased CSF WBC counts
The optimal cut-off point of CSF WBC count in predicting CSF discordance (Canestri et al. 2010) was >15 cells/mm3 (Figure 1B). However, CSF WBC counts with a cut-off of >3.8 cells/mm3 were efficient in predicting CSF discordance independent of the HIV RNA values in both compartments, with an AUC value of 0.78 (95% CI: 0.597−0.963). The performance characteristics of the increased CSF WBC count in predicting CSF discordance are indicated in Table 3 and Figure 1B.
Table 3.
Performance characteristics of cerebrospinal fluid white blood cell count for predicting CSF and plasma HIV RNA discordance diagnosis
| HIV RNA | CSF>plasma [1] | CSF discordance [2] |
|---|---|---|
| CSF WBC [3] | >3.8 cell/mm3 | >15.0 cell/mm3 |
| Sensitivity | 75.00 | 100.00 |
| Specificity | 77.00 | 95.00 |
| PPV | 41.00 | 62.50 |
| NPV | 94.00 | 100.00 |
| Efficiency (test score) | 77.00 | 96.00 |
| Youden Index | 0.52 | 0.95 |
| LR+ | 3.23 | 21.00 |
| LR− | 0.33 | 0.00 |
| CUI+ | 0.31 | 0.63 |
| CUI− | 0.72 | 0.95 |
| AUC | 0.78 | 0.98 |
PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood; LR−, negative likelihood; CUI+, Clinical utility index positive; CUI−, Clinical utility index negative; AUC, area under the receiver operating characteristic (ROC) curve.
HIV RNA higher than plasma, independent of the value of CSF or plasma HIV RNA.
CSF HIV RNA greater than 1log10 of the plasma viral load (VL) (Canestri et al. 2010)
Cut off points chosen by the ROC curve.
Longitudinal CSF and HIV RNA analysis
In three participants for whom serial CSF and plasma samples were collected, CVE was classified in the following forms: for two individuals with HIV-1C infection, as CSF slow suppression and CSF and plasma discordance (this case was not on CART) (de Almeida et al. 2017; 2018A); and for one individual with HIV-1B as persistent CVE.
Neuropsychological impairment
The frequency of global neuropsychological impairment based on a GDS of ≥0.5 in the escape, discordance, HIV RNA in plasma higher than in CSF, CSF and plasma aviremic, and HIV seronegative groups was 83%, 80%, 61%, 55%, and 21%, respectively (Figure 2). The HIV seropositive group performed significantly worse than the seronegative group in pairwise comparisons of the escape, discordance, or the CSF and plasma aviremia subgroups with those of the HIV− control group (p = 0.005, 0.014, 0.003 and 0.003, respectively). The three HIV seropositive subgroups were not different from each other when compared pairwise (p > 0.05). The odds of cognitive impairment for a participant with escape, discordance, HIV RNA in plasma higher than in CSF, and CSF and plasma aviremic were 19.00, 15.20, 5.97, and 4.68 times higher, respectively, than those for a person without HIV infection (p = 0.011, 0.020, 0.003 and 0.003, respectively).
Figure 2. Frequency of global neuropsychological impairment based on global deficit score (GDS) ≥ 0.5 in the escape, discordance, HIV viral load higher in plasma than cerebrospinal fluid (CSF), aviremic in CSF and plasma, and HIV seronegative control groups.
All HIV seropositive groups were significantly worse than the seronegative group, but not different from one another. Comparison of all four groups p=0.0004, pairwise comparisons of the groups escape, discordance, HIV RNA in plasma higher than in CSF and aviremic in CSF and plasma with the HIV− control group were p= 0.005, 0.014, 0.003, 0.003 respectively. The other comparisons were not significant (p>0.05). The odds of cognitive impairment for a participant with escape, discordance, HIV RNA in plasma higher than in CSF, and CSF and plasma aviremic were 19.0 (95 % CI: 2.0 to 181.6), 15.2 (95 % CI:1.6 to 151.5), 6.0 (95 % CI: 1.8 to 19.4) and 4.7 (95 % CI: 1.7 to 12.8) times higher, respectively, than those for a person without HIV infection (p = 0.011, 0.020, 0.003 and 0.003, respectively).
DISCUSSION
In this cross-sectional study of individuals chronically infected with HIV, the overall frequency of CSF discordance or CVE was low, similar to the frequencies described previously by other authors, which ranged from 4.5 to 21% (Eden et al. 2010; Peluso et al. 2012; Rawson et al. 2012; Nightingale et al. 2016; Anderson et al. 2017; Pérez-Valero et al. 2019). We demonstrated that CSF discordance or CVE occurred at comparable frequencies between HIV-1 subtypes C and B infections. To the best of our knowledge, this has not been described before, as previous studies included only HIV-1 subtype B (Spudich et al. 2006; Garvey et al. 2009; Canestri et al. 2010; Eden et al. 2010; Nightingale et al. 2016; Pérez-Valero et al. 2019). Our group had previously described two individuals infected by HIV-1C, who demonstrated CSF discordance and inflammatory reaction in the CSF with increase of the CSF WBC count, and an extensive biomarker panel study was carried out. The phylogenetic analyses of paired peripheral blood and CSF samples from both individuals revealed distinct CSF compartmentalization of the viruses suggesting that CSF escape and discordance are not simply due to trafficking of blood virus into CSF, but rather reflect and independent source of CSF virus from the CNS (de Almeida et al. 2017; 2018A). HIV genetic compartmentalization is defined when there is genetic differences (characterizing quasispecies) are seen between HIV in the compartments (Harrington et al. 2009; Schnell et al. 2010). These reports showed that independent and isolated HIV replication occurs in the CNS in individuals living with HIV-1C.
The findings of the present study will provide additional support for previously published studies that investigated differences between HIV-1B and HIV-1C; which found no difference in the frequency of HIV-associated neurocognitive disorders (de Almeida et al. 2013) or major depression (de Almeida et al. 2016A). Moreover, there was no molecular evidence to support the hypothesis of reduced intrathecal chemotaxis with HIV-1C relative to that with HIV-1B. β-chemokines, including monocyte chemoattractant protein 1 (MCP-1), and interleukins were elevated in the CSF in comparable proportions of the HIV-1B- and HIV-1C-infected participants (de Almeida et al. 2016). However, the same research group found subtype-dependent differences in amyloid pathway impairment (de Almeida et al. 2018B; 2018C; 2019).
Our study found comparable CSF WBC counts and frequencies of CSF pleocytosis between HIV-1B and HIV-1C infections, corroborating a previous study (Abdulle et al. 2008). These authors also found no subtype-dependent difference in CSF HIV RNA levels between HIV-1B and HIV-1C infections (Abdulle et al. 2008).
CSF and plasma HIV RNA were comparable in the group with CSF pleocytosis; although in the group with CSF WBC count between normal ranges HIV RNA was higher in plasma than CSF, suggesting the traffic of WBC as well as virus particles from blood to CSF. Participants with pleocytosis showed higher quotient of albumin indicating blood CSF barrier dysfunction, which can be interpreted as cause, or consequence or both of the increase of CSF WBC and HIV RNA levels. There is an extensive literature showing a positive correlation between the CSF WBC counts and CSF HIV RNA levels (Spector et al. 1993; Conrad et al. 1995; Pratt et al. 1996; Ellis et al. 1997; Price et al. 2001; Shacklett et al. 2004; Spudich et al. 2005; Nightingale et al. 2016; Anderson et al. 2017). It raises the question of whether the CSF WBC count contributes to the rising CSF HIV-1 RNA levels or whether it only represents a response to high CSF HIV-1 RNA levels (Spudich et al. 2005). Our results are in accordance with those of previous literature, but we went further by investigating CSF WBC counts and HIV RNA levels in individuals infected with HIV-1C. Increased CSF WBC count was found in 43% of participants showing CVE and in 100% of participants showing CSF discordance, similar to the results of another publication (Cusini et al. 2013). The CSF WBC count cut-off of 15 cells/mm3 showed very high performance characteristics as a predictive biomarker for CSF discordance. The LR+ value was high (i.e., 21). A LR+ value of ≥10.0 indicates that a positive test almost confirmed the diagnosis (McGee 2002; Akobeng 2007A).
There was a small number of participants with mild CSF pleocytosis and undetectable CSF HIV viral load; this can represent the persistence of latent HIV-1 in brain cellular reservoirs, as microglia or oligodendrocytes, despite the control of HIV in CSF (Wallet et al. 2019).
In this study patients with or without CSF HIV RNA discordance showed similar rates of neurocognitive impairment, similar to previously published (Pérez-Valero et al. 2019). Although, the odds of cognitive impairment for a PWH were higher, than those for a person without HIV infection. In the present study, neuropsychological impairment was present in 83% and 80% of the participants with CVE and CSF discordance, respectively. These results must be viewed with care, as the number of participants was low in each group. CVE has been described in association with and without symptoms (Eden et al. 2010; Ferretti et al. 2015), though symptomatic CVE comprises a range of symptoms rather than a single coherent entity. Asymptomatic CVE was reported in approximately 10% of a group of ART-treated patients with suppressed systemic viremia.
The present study was not free of limitations, such as the small number of samples with CVE and CSF discordance, mainly when categorized according to HIV-1 subtypes and the patterns of HIV RNA in paired CSF and plasma samples. However, the study was able to show the frequency of CVE or CSF discordance was comparable between the subtypes studied. The study was also limited by its cross-sectional design. A longitudinal study might be better in helping to gain an understanding of CVE and to classify the diverse forms of escape.
The positive points include the fact that this was the first study on CVE and CSF discordance to include participants infected with HIV-1C and not only HIV-1B, from the same geographical area. This was also the first study to evaluate the performance characteristics of the increased CSF WBC count in predicting CSF discordance. Consequently, this study will contribute to our understanding of the pathophysiology of HIV infection and the impact of HIV-1 genetic diversity in CNS HIV infection as well as the treatment. Moreover, to establish a biomarker that could predict CSF discordance as well as CVE is of great importance, especially in low- and middle-income countries where CSF HIV-1 viral loads are not routinely available.
In conclusion, viral discordance or escape in the CNS occurs at a comparable frequency with HIV-1C and HIV-1B infections. The CSF WBC count with a cut-off 15 cells/mm3 appears to be associated with high effectiveness as a predictive biomarker for CSF discordance. It should be useful for screening patients who will benefit from the CSF HIV RNA quantification. More studies are necessary, this cut-off needs to be tested in other populations and its performance should be confirmed. Our findings in conjunction with previous evidence of compartmentalization during CVE suggest that PWH with cognitive impairment may benefit from switching to an antiretroviral regimen with greater CNS penetration and with a resistance profile better matching CSF virus.
Acknowledgments
Source of Funding
This research was supported by NIH R21 MH76651 (principal investigators: R. Ellis, S. de Almeida) and HIV Neurobehavioral Research Center (HNRC; P30 MH062512).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
The authors state that there are no conflicts of interest regarding the publication of this article.
References
- Abdulle S, Hagberg L, Svennerholm B, Fuchs D, Gisslén M (2008) Cerebrospinal fluid viral load and intrathecal immune activation in individuals infected with different HIV-1 genetic subtypes. PloS One 3 (4):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akobeng AK (2007A) Understanding diagnostic tests 2: likelihood ratios, pre- and post-test probabilities and their use in clinical practice. Acta Pædiatr 96 (4):487–491. [DOI] [PubMed] [Google Scholar]
- Akobeng AK (2007B) Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Pædiatr 96 (5):644–647. [DOI] [PubMed] [Google Scholar]
- Anderson AM, Munoz-Moreno JA, McClernon D, et al. (2017) Prevalence and correlates of persistent HIV-1 RNA in cerebrospinal fluid during antiretroviral therapy. J Infect Dis 215(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham R, Ahmed N, Rangi P, et al. (2011) HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. Int J STD AIDS 22(10):608–609. [DOI] [PubMed] [Google Scholar]
- Bogoch II, Davis BT, Venna N (2011) Reversible dementia in a patient with central nervous system escape of human immunodeficiency virus. J Infect 63(3):236–239. [DOI] [PubMed] [Google Scholar]
- BRASIL. Ministério da Saúde. Secretaria de Vigilância em saúde. Departamento de vigilância, prevenção e controle das infecções sexualmente transmissíveis, do HIV/Aids e das Hepatites Virais Technical manual for the diagnosis of the HIV infection in Adults and Children Brasília, F, 2018 [Google Scholar]
- Buffet R, Agut H, Chieze F, et al. (1991) Virological markers in the cerebrospinal fluid from HIV-1-infected individuals. AIDS 5(12):1419–1424. [DOI] [PubMed] [Google Scholar]
- Canestri A, Lescure FX, Jaureguiberry S. et al. (2010) Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 50(5):773–778. [DOI] [PubMed] [Google Scholar]
- Clifford DB (2010) Viral escape in cerebrospinal fluid—an achilles heel of HIV therapy? J Infect Dis 202(12):1768–1769. [DOI] [PubMed] [Google Scholar]
- Conrad AJ, Schmid P, Syndulko K, et al. (1995) Quantifying HIV-1 RNA using the polymerase chain reaction on cerebrospinal fluid and serum of seropositive individuals with and without neurologic abnormalities. J Acquir Immune Defic Syndr 10(4):425–435. [DOI] [PubMed] [Google Scholar]
- Cusini A, Vernazza PL, Yerly S, et al. (2013) Higher CNS penetration-effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J Acquir Immune Defic Syndr 62(1):28–35. [DOI] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, de Pereira AP, et al. (2013) Neurocognitive impairment in HIV-1 subtype C- versus B-infected individuals in Southern Brazil. J Neurovirol 19(6): 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Barbosa FJ, Kamat R, et al. (2016A) Suicide risk and prevalence of major depressive disorder (MDD) among individuals infected with HIV-1 subtype C versus B in Southern Brazil. J Neurovirol 22(6):789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Rotta I, Jiang Y, et al. (2016 B) Biomarkers of Chemotaxis and Inflammation in Cerebrospinal Fluid and Serum in Individuals with HIV-1 Subtype C versus B. J Neurovirol 22(6):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Rotta I, Ribeiro CE, et al. (2017) Dynamic of CSF and serum biomarkers in HIV-1 subtype C encephalitis with CNS genetic compartmentalization: case study. J Neurovirol 23(3): 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Oliveira MF, Chaillon A, et al. (2018A) Transient and asymptomatic meningitis in human immunodeficiency virus-1 subtype C: a case study of genetic compartmentalization and biomarker dynamics. J Neurovirol 24(6):786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, Rotta I, et al. (2018B) Biomarkers of neuronal injury and amyloid metabolism in the cerebrospinal fluid of patients infected with HIV-1 subtypes B and C. J Neurovirol 24(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Tang B, Ribeiro CE, et al. (2018C) Neprilysin in the cerebrospinal fluid and serum of patients infected with HIV1-subtypes C and B. J Acquir Immune Defic Syndr 78(2):248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, Rotta I, et al. (2019) Blood amyloid-β protein isoforms are affected by HIV-1 in a subtype-dependent pattern. J Neurovirol [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Palacio, Tamarit M, Quereda C, et al. (2012) HIV Type 1 Viral Encephalitis After Development of Viral Resistance to Plasma Suppressive Antiretroviral Therapy. AIDS Res Hum Retrovir 28(1):83–86. [DOI] [PubMed] [Google Scholar]
- Eden A, Fuchs D, Hagberg L, et al. (2010) HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 202(12):1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Hsia K, Spector SA, et al. (1997) Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol 42(5):679–688. [DOI] [PubMed] [Google Scholar]
- Ferretti F, Gisslen M, Cinque P, et al. (2015) Cerebrospinal fluid HIV escape from antiretroviral therapy. Curr HIV/AIDS Rep 12(2):280–288 [DOI] [PubMed] [Google Scholar]
- Galen RS, Gambino SR. Beyond normality, the predictive value and efficiency of medical diagnoses. New York: Wiley & Sons; 1975, 237 p. [Google Scholar]
- Garvey LJ, Everitt A, Winston A, et al. (2009) Detectable cerebrospinal fluid HIV RNA with associated neurological deficits, despite suppression of HIV replication in the plasma compartment. AIDS 23(11):1443–1444. [DOI] [PubMed] [Google Scholar]
- Haggert S, Stevenson M (1991) Predominance of distinct viral genotypes in brain and lymph node compartments of HIV-1-infected individuals. Viral Immunol 4(2):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington PR, Schnell G, Letendre SL, et al. (2009) Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS 23(8):907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander H, McGuire D, Burack JH (1994) Diagnostic lumbar puncture in HIV-infected patients: analysis of 138 cases. Am J Med 96(3):223–228. [DOI] [PubMed] [Google Scholar]
- Joseph J, Cinque P, Colosi D, et al. (2016) Highlights of the Global HIV-1 CSF Escape Consortium Meeting, 9 June 2016, Bethesda, MD, USA. J Virus Erad 2(4):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlama C, Valantin MA, Algarte-Genin M, et al. (2010) Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS 24(15):2365–2374. [DOI] [PubMed] [Google Scholar]
- Lescure FX, Moulignier A, Savatovsky J, et al. (2013) CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis 57(1):101–108. [DOI] [PubMed] [Google Scholar]
- Letendre S, Ellis R, Deutsch R, et al. , Correlates of time-to-loss-of-viral response in CSF and plasma in the CHARTER cohort. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; 2010, San Francisco, CA: 16–19 February (poster 430). [Google Scholar]
- Manesh A, Barnabas R, Mani S, et al. (2019) Symptomatic HIV CNS viral escape among patients on effective cART. Intern J Infect Dis 84: 39–43. [DOI] [PubMed] [Google Scholar]
- Martin C, Albert J, Hansson P, et al. (1998) Cerebrospinal fluid mononuclear cell counts influence CSF HIV-1 RNA levels. J Acquir Immundef Syndr Human Retrovirol 17(3):214–219. [DOI] [PubMed] [Google Scholar]
- McGee S (2002) Simplifying likelihood ratios. J Gen Intern Med 17(8):647–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ (2008) The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry 23(11):1191–1202. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ (2011) Sensitivity x PPV is a recognized test called the clinical utility index (CUI+). Eur J Epidemiol 26(3):251–252. [DOI] [PubMed] [Google Scholar]
- Monteiro de Almeida S, Letendre S, Zimmerman J, et al. (2006) Relationship of CSF leukocytosis to compartmentalized changes in MCP-1/CCL2 in the CSF of HIV-infected patients undergoing interruption of antiretroviral therapy. J Neuroimmunol 179(1–2):180–185. [DOI] [PubMed] [Google Scholar]
- Mukerji SS, Misra V, Lorenz D, et al. (2017) Temporal Patterns and Drug Resistance in CSF Viral Escape Among ART-Experienced HIV-1 Infected Adults. J Acquir Immune Defic Syndr 75(2):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S, Michael BD, Fisher M, et al. (2016) CSF/plasma HIV-1 RNA discordance even at low levels is associated with up-regulation of host inflammatory mediators in CSF. Cytokine 83:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S, Geretti AM, Beloukas A, et al. (2016) Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J Neurovirol 22(6):852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Ferretti F, Peterson J, et al. (2012) Cerebrospinal Fluid HIV Escape Associated with Progressive Neurologic Dysfunction in Patients on Antiretroviral Therapy with Well-Controlled Plasma Viral Load. AIDS 26(14):1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Valero I, Ellis R, Heaton R, et al. (2019) Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS 33(3):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt RD, Nichols S, McKinney N, et al. (1996) Virologic markers of human immunodeficiency virus type 1 in cerebrospinal fluid of infected children. J Infect Dis 174(2):288–293. [DOI] [PubMed] [Google Scholar]
- Price RW, Paxinos EE, Grant RM, et al. (2001) Cerebrospinal fluid response to structured treatment interruption after virological failure. AIDS 15(10):1251–1259. [DOI] [PubMed] [Google Scholar]
- Ranga U, Shankarappa R, Siddapa NB, et al. (2004) Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virology 78(5):2586–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson T, Muir D, Mackie NE, et al. (2012) Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J Infect 65(3):239–245. [DOI] [PubMed] [Google Scholar]
- Satishchandra P, Nalini A, Gourie-Devi M, et al. (2000) Profile of neurologic disorders associated with HIV/AIDS from Bangalore, South India (1989–96). Indian J Med Res 111:14–23. [PubMed] [Google Scholar]
- Shacklett BL, Cox CA, Wilkens DT, et al. (2004) Increased adhesion molecule and chemokinereceptor expression on CD8+ T cells trafficking to cerebrospinalfluid in HIV-1 infection. J Infect Dis 189(12):2202–2212. [DOI] [PubMed] [Google Scholar]
- Schnell G, Price RW, Swanstrom R, et al. (2010) Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J. virol 84(5):2395–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector SA, Hsia K, Pratt D, et al. (1993) Virologic markers of human immunodeficiency virus type 1 in cerebrospinal fluid. The HIV Neurobehavioral Research Center Group. J Infect Dis 168(1):68–74. [DOI] [PubMed] [Google Scholar]
- Spudich SS, Nilsson AC, Lollo ND, et al. (2005) Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis 5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S, Lollo N, Liegler T, et al. (2006) Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis 194(12):1686–1696. [DOI] [PubMed] [Google Scholar]
- van Lelyveld SF, Nijhuis M, Baatz F, et al. (2010) Therapy failure following selection of enfuvirtide resistant HIV-1 in cerebrospinal fluid. Clin Infect Dis 50:387–390. [DOI] [PubMed] [Google Scholar]
- Wallet C, De Rovere M, Van Assche J, et al. (2019) Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front Cell Infect Microbiol 9:362–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate S, Pond SLK, Shapshak P, et al. (2007) Comparative study of methods for detecting sequence compartmentalization in human immunodeficiency virus type 1. J Virol 81(12):6643–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]