Abstract
BACKGROUND:
Metastatic colorectal cancer (mCRC) outcomes continue to improve, but they vary significantly by race and ethnicity. We hypothesize that these disparities arise from unequal access to care.
MATERIALS AND METHODS:
The Harris Health System (HHS) is an integrated health delivery network that provides medical care to the underserved, predominantly minority population of Harris County, Texas. As the largest HHS facility and an affiliate of Baylor College of Medicine’s Dan L. Duncan Comprehensive Cancer Center, Ben Taub Hospital (BTH) delivers cancer care through multidisciplinary subspecialty that prioritize access to care, adherence to evidence-based clinical pathways, integration of supportive services, and mitigation of financial toxicity. We performed a retrospective analysis of minority patients diagnosed with and treated for mCRC at BTH between 1/2010 and 12/2012. Kaplan-Meier survival curves were compared to survival curves from randomized control trials reported during that time period.
RESULTS:
We identified 103 patients; 40% were black, 49% were Hispanic, and 12% were Asian or Middle Eastern. 35% reported a language other than English as their preferred language. 74% of patients with documented coverage status were uninsured. 84% of patients received standard chemotherapy with a clinician-reported response rate of 63%. Overall survival for BTH patients undergoing chemotherapy was superior to that of subjects enrolled in the CRYSTAL trial (median 24.0 vs 19.9 months, p = 0.014).
CONCLUSION:
HHS provides a health delivery infrastructure through which minority patients with socioeconomic challenges experience clinical outcomes comparable to highly selected patients enrolled in randomized control trials. Efforts to resolve CRC disparities should focus on improving access of at-risk populations to high-quality comprehensive cancer care.
Keywords: minority, disparities, metastatic, colorectal, cancer
MICROABSTRACT
Metastatic colorectal cancer (mCRC) outcomes continue to improve, but they vary significantly by race and ethnicity. Hypothesizing that these disparities arise from unequal access to care rather than intrinsic biology, we showed that survival of 103 consecutive mCRC patients treated at an academic safety net hospital that treats the underserved, predominantly minority population of Harris County, Texas was superior to that of subjects enrolled in the CRYSTAL trial. Our findings suggest that administering high-quality comprehensive cancer care to vulnerable populations can overcome disparities reported in the medical literature.
INTRODUCTION
Colorectal cancer (CRC) accounts for over 135,000 new cancer cases and 50,000 deaths each year in the United States, making it the third most common malignancy and second most common cause of cancer death[1]. CRC mortality rates continue to decline due to detection of premalignant and early-stage disease through CRC screening and higher cure rates of regional disease with multimodality therapy. Outcomes of metastatic CRC (mCRC) have also improved significantly over the last few decades, with recent multicenter, randomized control trials yielding median overall survival ranging from 20 months in unselected populations to 30 months in patients with KRAS wild type tumors treated with anti-EGFR therapy[2–10]. However, the outcomes observed in clinical trials, which include predominantly Caucasian patients with limited comorbidities, excellent performance status, and access to high-quality health care, have not been reproduced in all populations. Whereas prior to 1980, CRC mortality rates were lower among African Americans than non-Hispanic whites, they are now nearly 40% higher in African Americans with distant-stage disease accounting for approximately 60% of the overall white-black disparity[11–14].
Numerous studies have addressed the potential etiology of racial disparities in CRC[15–17]. Some argue that CRC is inherently more aggressive in certain populations. For example, when compared to non-Hispanic whites, African Americans are more likely to be younger and less healthy at diagnosis and to harbor tumors that are higher stage, higher grade, and localized to the proximal colon[18]. Other studies have shown that certain biological factors such as vitamin D deficiency, DNA methylation, and pharmacogenetics may disproportionately affect CRC outcomes in black patients[19–21]. Although racial differences in the clinical, pathological, and molecular features of CRC may exist, a growing body of literature suggests that inferior survival among minority CRC patients arises primarily from poor access to comprehensive cancer care rather than differences in tumor biology. Factors such as insufficient staffing of medical facilities, substandard diagnostic evaluations, delays in diagnosis and treatment, and lack of access to novel therapies compromise adherence to national treatment guidelines and, in turn, adversely affect cancer-related outcomes, particularly among African Americans with advanced stage disease[22–27]. Hypothesizing that, with access to high-quality comprehensive cancer care, minority and socioeconomically disadvantaged patients with mCRC can achieve outcomes comparable to those of the highly selected, predominantly Caucasian patients enrolled in randomized control trials, we conducted a retrospective analysis of minority patients treated for mCRC in an academic safety-net healthcare system.
MATERIALS AND METHODS
Study design
In this retrospective study, we recorded and analyzed baseline characteristics, treatment administration, tumor response, and overall survival among minority patients treated for mCRC in the Harris Health System (HHS) in Harris County, Texas. The Baylor College of Medicine (BCM) Institutional Review Board and Harris Health System Research and Sponsored Programs approved the research protocol and waived the requirement to obtain informed consent and HIPAA authorization.
Study setting
With an estimated population of over 4.5 million residents, Harris County, Texas – which encompasses the Houston metropolitan area – is the third most populous county and the most ethnically diverse urban region in the United States[28–30]. The HHS is an integrated health delivery network that utilizes federal and local tax revenue to provide comprehensive medical care to more than one million uninsured and underinsured Harris County residents, thereby serving as a safety-net healthcare system for the predominantly minority, socioeconomically disadvantaged population in the Houston area. As the largest HHS facility, Ben Taub Hospital (BTH) is 650-bed hospital staffed by BCM faculty, students, and postgraduate trainees. Comprehensive cancer care is delivered in multidisciplinary clinics by subspecialized clinicians who are full BCM faculty and members of the Dan L. Duncan Comprehensive Cancer Center. The BTH multidisciplinary gastrointestinal cancer program has instituted numerous quality measures through the years such as 1) expediting the referral process 2) establishing evidence-based clinical pathways for diagnosis and treatment, 3) granting patients access to all FDA-approved anti-neoplastic and supportive therapies, 4) optimizing use of the electronic medical record (EMR), 5) integrating supportive services (e.g. patient navigation, translation services, nutrition, and palliative care), and 6) mitigating patients’ financial toxicity. All newly referred patients are seen by a clinician within 1-2 weeks through a streamlined process facilitated by dedicated patient navigators, and cases requiring multidisciplinary input are discussed in a weekly gastrointestinal tumor board attended by representatives from medical oncology, surgical oncology, radiation oncology, gastroenterology, pathology, radiology, and case management. The multidisciplinary group regularly updates its consensus guidelines and clinical pathways for diagnostic evaluation, molecular testing, and clinical management. Patients have access to all intravenous and oral FDA-approved anti-neoplastic and supportive therapies, and a 30-bed infusion center is staffed by chemotherapy-trained nurses and pharmacists. All documentation, clinic orders, and chemotherapy orders are entered in the EPIC EMR to ensure optimal communication and coordination of care. Chemotherapy and supportive care protocols are devised and regularly updated by faculty and oncology pharmacists using BEACON software. Supportive services, including case management, counseling, nutrition, physical and occupational therapy, palliative care, and translation services, are available to all patients throughout their clinical course, and cancer navigators help to coordinate appointments. Financial toxicity is mitigated through the Harris Health Plan, a robust financial assistance program that facilitates the provision of high-quality, guideline-concordant care irrespective of a patient’s baseline socioeconomic status.
Study population
We included all minority patients ≥18 years old who were diagnosed with metastatic adenocarcinoma of the colon or rectum as their initial presentation of CRC between January 1, 2010 and December 31, 2012 and who were subsequently evaluated and treated at BTH. Minority patients were identified as those whose race was documented as African American, Hispanic, Asian, or Middle Eastern in the HHS EMR. Patients with non-metastatic (stage I-III) disease as their initial CRC presentation or patients with tumors of non-adenocarcinoma histology (e.g. neuroendocrine, squamous cell), non-colorectal location (e.g. small intestine, anus), or without pathological confirmation were excluded from the study.
Demographic and clinical factors
Patient data were extracted from the HHS EMR and stored on a BCM password-protected server. Patients’ race, age at diagnosis, sex, preferred language, and insurance status were extracted from the demographics section of the EMR. Clinical and pathological data, including baseline CEA, albumin, primary tumor location, histology, pathological grade, KRAS status, and mismatch repair status, were extracted from the laboratory, imaging, and pathology sections of the EMR. Of note, our study period preceded the standardization of extended RAS analysis. Patients’ oncology consultation and clinic notes were used to extract baseline body mass index (BMI), Charlson comorbidity index[31], treatment initiation date, treatment details (e.g. chemotherapeutic agents, radiation therapy, surgery), and response to therapy. Vital status and date of death were identified using the HHS Cancer Registry through January 31, 2017.
Outcomes
The primary outcome of this study was overall survival, defined as the time from pathological CRC diagnosis to date of death. Secondary outcomes included time to initiation of treatment, receipt of standard cancer-directed therapy in accordance with NCCN guidelines[32, 33], and chemotherapy response rates. The overall response rate was calculated as the proportion of patients experiencing complete or partial radiographic response as determined by the treating physician. Disease control rate was calculated as the proportion of patients with radiographic response or stable disease.
Statistical analysis
Baseline demographic, clinical, tumor, and treatment characteristics were reported as frequencies and percentages for categorical variables, means and standard deviations for continuous approximately normally distributed variables, and medians and interquartile ranges for ordinal or continuous non-normally distributed variables. These variables were reported for all patients but were not compared by race, as all patients in the study cohort were of minority status.
Using the algorithm developed by Guyot et al (available as an R script), we reconstructed the individual survival data and corresponding summary statistics from the Kaplan-Meier survival curves published in two randomized control trials, CRYSTAL (Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer) and PRIME (Panitumumab Randomized Trial In Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy)[34, 4, 8]. We selected the CRYSTAL and PRIME studies as historical controls because the antineoplastic agents included in these trials were similar, if not identical, to those offered to our patients during the study period. This method uses data generated from digitizing the published survival curve as well as the total sample size, number of events, and number remaining at risk at various time points to reconstruct a very accurate and highly reproducible approximation of the original individual-level data that the published curve summarizes. We then plotted Kaplan-Meier overall survival curves for our study cohort, stratified by KRAS status, and compared to the curves generated from CRYSTAL and PRIME using the log rank test, which very closely approximated the test result that would have accrued had the individual-level data been used. Analyses were performed in Stata Version 11 (Statacorp, College Station, TX), SAS Version 9.4 (SAS Institute, Cary, NC), and R Version 3.4.1. All reported p values are two-sided with p < 0.05 considered as statistically significant.
RESULTS
Study population
A query of the HHS Cancer Registry for minority patients diagnosed with mCRC between January 2010 and December 2012 yielded 131 patients. After review of patient-level EMR data, 28 patients were excluded from the study: six had no tissue diagnosis on record, six had stage I-III disease at the time of diagnosis, 15 had non-colorectal tumors or tumors of non-adenocarcinoma histology, and one was never evaluated or treated in the HHS. Ultimately, 103 patients were included in the study.
Baseline demographic, clinical, and tumor characteristics are summarized in Table 1. Our study cohort was predominantly male (64.1%), and the mean age at diagnosis was 54 years old (SD = 12). Forty-one patients (39.8%) were black, 50 (48.5%) were Hispanic, and 12 (11.7%) were of other races, including Asian and Middle Eastern. A language other than English was the preferred language of 35.0% of patients, and 74.4% of those with documented coverage status were uninsured. Median serum albumin was 3.2, and most patients (68.0%) had a Charlson comorbidity score of 0 (excluding cancer). Primary tumors were most frequently located in the left colon (53.4%), followed by the right colon (23.3%) and rectum (21.4%). Median serum CEA was 37. KRAS status was known in 74.8% of tumors, of which 36.4% harbored KRAS mutations. Mismatch repair status was known for 77.7% of tumors, of which 3.8% were mismatch repair deficient.
Table 1.
Baseline demographic, clinical, and tumor characteristics by minority race
| All (N = 103) | Black (N = 41) | Hispanic (N = 50) | Othera (N = 12) | |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis, mean (SD) | 53.7 (12.4) | 54.5 (11.7) | 51.7 (12.1) | 59.3 (14.7) |
| Sex | ||||
| Male | 66 (64.1) | 26 (63.4) | 32 (64.0) | 8 (66.7) |
| Female | 37 (35.9) | 15 (36.6) | 18 (36.0) | 4 (33.3) |
| Preferred language | ||||
| English | 67 (65.1) | 41 (100) | 16 (32.0) | 10 (83.3) |
| Not English | 36 (35.0) | 0 (0.0) | 34 (68.0) | 2 (16.7) |
| Insurance status | ||||
| Uninsuredb | 58 (56.3) | 22 (53.7) | 29 (58.0) | 7 (58.3) |
| Medicaid | 8 (7.8) | 4 (9.8) | 3 (6.0) | 1 (8.3) |
| Medicare | 7 (6.8) | 7 (17.1) | 0 (0.0) | 0 (0.0) |
| Private | 5 (4.9) | 0 (0.0) | 4 (8.0) | 1 (8.3) |
| Unknown | 25 (24.3) | 8 (19.5) | 14 (28.0) | 3 (25.0) |
| Clinical characteristicsc | ||||
| BMI, mean (SD) (N = 97) | 26.9 (6.1) | 29.1 (7.8) | 25.8 (4.3) | 24.3 (4.2) |
| Albumin, median (IQR) (N = 100) | 3.2 (1.0) | 3.2 (1.0) | 3.2 (1.0) | 3.3 (0.7) |
| Charlson comorbidity indexd | ||||
| 0 | 70 (68.0) | 25 (61.0) | 37 (74.0) | 8 (66.7) |
| 1-2 | 28 (27.2) | 14 (34.2) | 11 (22.0) | 3 (25.0) |
| ≥3 | 5 (4.9) | 2 (4.9) | 2 (4.0) | 1 (8.3) |
| Tumor characteristicsc | ||||
| Location of primary tumor | ||||
| Right colon | 24 (23.3) | 10 (24.4) | 13 (26.0) | 1 (8.3) |
| Left colon | 55 (53.4) | 23 (56.1) | 23 (46.0) | 9 (75.0) |
| Rectum | 22 (21.4) | 7 (17.1) | 13 (26.0) | 2 (16.7) |
| Throughoute | 2 (1.9) | 1 (2.4) | 1 (2.0) | 0 (0.0) |
| CEA, median (IQR) (N = 95) | 37 (302) | 43 (404) | 34 (230) | 15 (403) |
| Pathological grade | ||||
| Well differentiated | 19 (18.5) | 7 (17.1) | 8 (16.0) | 4 (33.3) |
| Moderately differentiated | 55 (53.4) | 22 (53.7) | 29 (58.0) | 4 (33.3) |
| Poorly differentiated | 14 (13.6) | 8 (19.5) | 5 (10.0) | 1 (8.3) |
| Unknown | 15 (14.6) | 4 (9.8) | 8 (16.0) | 3 (25.0) |
| KRAS statusf | ||||
| Wild type | 49 (47.6) | 18 (43.9) | 24 (48.0) | 7 (58.3) |
| Mutated | 28 (27.2) | 13 (31.7) | 14 (28.0) | 1 (8.3) |
| Unknown | 26 (25.2) | 10 (24.4) | 12 (24.0) | 4 (33.3) |
| Mismatch repair status | ||||
| Proficient | 77 (74.8) | 33 (80.5) | 36 (72.0) | 8 (66.7) |
| Deficient | 3 (2.9) | 0 (0.0) | 2 (4.0) | 1 (8.3) |
| Unknown | 23 (22.3) | 8 (19.5) | 12 (24.0) | 3 (25.0) |
Includes Asian, Middle Eastern
Covered by the Harris Health Plan, a financial assistance program for individuals with incomes under 300% of the federal poverty level
Collection of baseline clinical and tumor characteristics limited to within 90 days of tissue diagnosis date
Excluding cancer
Refers to patients with multiple synchronous primary tumors
Prior to expanded RAS testing
In comparison, the CRYSTAL trial randomized 1,217 patients with metastatic EGFR-positive, KRAS-unselected colorectal cancer to FOLFIRI alone or in combination with cetuximab. The study cohort was predominantly male (60.5%) with a median age of 61, and 96.5% of subjects had an ECOG performance status of 0-1 [4] Race and ethnicity were not reported in CRYSTAL. The PRIME study randomized 1,183 patients with mCRC to FOLFOX with or without panitumumab, and outcomes were assessed based on their KRAS mutational status. Like the CRYSTAL cohort, the PRIME population was predominantly male (63.3%) with a median age ranging from 61 to 63 years-old (depending on treatment group), and the vast majority of subjects (94.8%) had an ECOG performance status of 0-1. Most patients in PRIME were white (90.8%) and had a CEA greater than the upper limit of normal (79.2%)[8].
Treatment and response to therapy
Treatment by individual chemotherapeutic agent and response to therapy are summarized in Table 2. Most patients (85.4%) received some type of cancer-directed therapy, with a median of 31 days between date of diagnosis and treatment initiation. Of those who were treated, 98.9% received chemotherapy. All patients treated with chemotherapy received a fluoropyrimidine (infusional 5-fluorouracil or capecitabine), 95.4% received oxaliplatin, 56.3% received irinotecan, and 60.9% receiving bevacizumab. Of the 47 patients with KRAS wild-type tumors who received chemotherapy, 29 (61.7%) received an anti-EGFR monoclonal antibody (cetuximab or panitumumab). Overall response to first-line chemotherapy was 62.8%, and disease control rate was 79.5%. Thirty-seven (42%) of the 88 treated patients underwent some type of surgery, of whom 34 (91.9%) underwent primary tumor resection and 12 (32.4%) underwent metastatectomy.
Table 2.
Treatment administration and response by minority race
| All (N = 88) | Black (N = 36) | Hispanic (N = 43) | Othera (N = 9) | |
|---|---|---|---|---|
| Treatment administration | ||||
| Days to treatment initiationb, median (IQR) | 31 (40) | 40 (34) | 24 (52) | 31 (15) |
| Chemotherapy | 87 (98.9) | 35 (97.2) | 43 (100) | 9 (100) |
| Fluoropyrimidinec | 87 (100) | 35 (100) | 43 (100) | 9 (100) |
| Oxaliplatin | 83 (95.4) | 34 (97.1) | 41 (95.4) | 8 (88.9) |
| Irinotecan | 49 (56.3) | 19 (54.3) | 25 (58.1) | 5 (55.6) |
| Bevacizumab | 53 (60.9) | 26 (74.3) | 21 (48.8) | 6 (66.7) |
| EGFR inhibitord (N = 47) | 29 (61.7) | 7 (41.2) | 17 (73.9) | 5 (71.4) |
| Regorafenib | 6 (6.9) | 5 (14.3) | 1 (2.3) | 0 (0.0) |
| Radiation therapy | 22 (25.0) | 9 (25.0) | 10 (23.3) | 3 (33.3) |
| Surgical therapy | 37 (42.0) | 17 (47.2) | 16 (37.2) | 4 (44.4) |
| Primary tumor resection | 34 (91.9) | 16 (94.1) | 15 (93.8) | 3 (75.0) |
| Metastatic resection | 12 (32.4) | 3 (17.7) | 6 (37.5) | 3 (75.0) |
| Other therapye | 6 (6.8) | 2 (5.6) | 2 (4.7) | 2 (22.2) |
| Response to chemotherapy | ||||
| First response | ||||
| Complete response | 3 (3.4) | 1 (2.9) | 1 (2.3) | 1 (11.1) |
| Partial response | 46 (52.9) | 17 (48.6) | 24 (55.8) | 5 (55.6) |
| Stable disease | 13 (14.9) | 9 (25.7) | 4 (9.3) | 0 (0.0) |
| Disease progression | 16 (18.4) | 6 (17.1) | 9 (20.9) | 1 (11.1) |
| No restaging | 9 (10.3) | 2 (5.7) | 5 (11.6) | 2 (22.2) |
| Disease control rate (%) | 79.5 | 81.8 | 76.3 | 85.7 |
| Response rate (%) | 62.8 | 54.6 | 65.8 | 85.7 |
| Best response | ||||
| Complete response | 6 (6.9) | 2 (5.7) | 3 (7.0) | 1 (11.1) |
| Partial response | 46 (52.9) | 18 (51.4) | 23 (53.5) | 5 (55.6) |
| Stable disease | 16 (18.4) | 8 (22.9) | 8 (18.6) | 0 (0.0) |
| Disease progression | 10 (11.5) | 5 (14.3) | 4 (9.3) | 1 (11.1) |
| No restaging | 9 (10.3) | 2 (5.7) | 5 (11.6) | 2 (22.2) |
| Disease control rate (%) | 87.2 | 84.9 | 89.5 | 85.7 |
| Response rate (%) | 66.7 | 60.6 | 68.4 | 85.7 |
Includes Asian, Middle Eastern
Defined as initiation of non-emergent chemotherapy, radiation therapy, or surgical resection
Infusional 5-fluorouracil or capecitabine
For KRAS wild type tumors
Includes radiofrequency ablation, microwave ablation, and portal vein embolization for liver metastases
Overall survival
Median overall survival was 22.7 months for the entire study population (excluding one patient who died on the date of diagnosis) and 24.0 months for those who received chemotherapy. As depicted in Figure 1a, the overall survival of BTH patients who received chemotherapy was superior to the KRAS-unselected cohort treated with FOLFIRI + cetuximab in CRYSTAL (24.0 months vs 19.9 months, p = 0.014). There was a trend towards improved survival among BTH patients with KRAS wild type tumors (62% of whom received anti-EGFR therapy) when compared to CRYSTAL subjects with KRAS wild type tumors who were treated with first-line FOLFIRI + cetuximab (29.3 months vs 23.8 months, p = 0.099) and PRIME subjects with KRAS wild type tumors who were treated with first-line FOLFOX + panitumumab (29.3 months vs 23.9 months, p = 0.128, see Figure 1b). Similarly, the median overall survival of BTH patients with KRAS mutated tumors who received chemotherapy was numerically higher than that of CRYSTAL subjects with KRAS mutated tumors who received first-line FOLFIRI alone (24.0 months vs 16.6 months) and PRIME subjects with KRAS mutated tumors treated with FOLFOX alone (24.0 months vs 19.1 months), but these differences did not reach statistical significance (p = 0.083 and p = 0.333, respectively, see Figure 1c).
Figure 1.
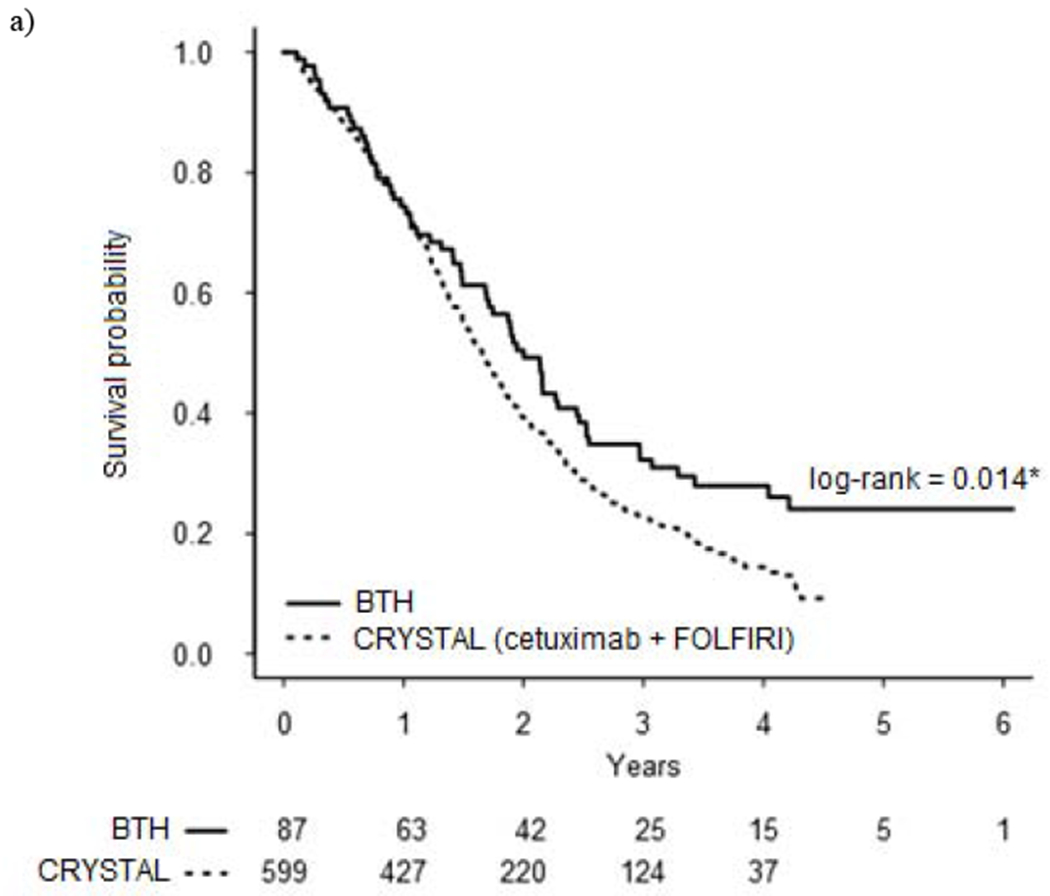


Kaplan-Meier overall survival curves for patients treated at BTH compared to CRYSTAL and PRIME trial cohorts
a) All patients
b) Patients with KRAS wild type metastatic colorectal cancer
c) Patients with KRAS mutated metastatic colorectal cancer
DISCUSSION
This retrospective study demonstrates that instituting definable and reproducible quality measures can address socioeconomic barriers that compromise cancer-related outcomes in underserved populations and, in doing so, yield favorable clinical outcomes. In a population comprised of underserved, minority patients presenting with extensive symptomatic disease, 85% of BTH patients with mCRC underwent cancer-directed therapy, 99% of whom received standard chemotherapy regimens endorsed by the NCCN. In comparison, a recent analysis of the National Cancer Database by Sineshaw and colleagues described lower rates of chemotherapy administration for mCRC in white and black patients alike (85.3% and 79.5%, respectively), and that differences in insurance coverage accounted for approximately half of the disparity in overall survival between white and black patients[35]. Median overall survival and response rates observed in the BTH population compared favorably to those reported in highly selected, predominantly Caucasian cohorts treated in multicenter randomized controlled trials. In fact, overall survival among BTH patients undergoing chemotherapy was statistically superior to the overall survival for the cohort enrolled in CRYSTAL. Analysis by KRAS status showed a trend towards improved survival in BTH patients with KRAS wild type and KRAS mutated tumors when compared to their CRYSTAL and PRIME counterparts, although the BTH sample size was small. Certainly, there was no evidence of inferior outcomes in the BTH cohort. Taken together, these data suggest that administering high-quality comprehensive cancer care to minority patients is feasible and that inferior outcomes reported among minority populations, in particular African Americans, likely arise from poor access to healthcare rather than intrinsic tumor biology.
Indeed, retrospective studies have shown that race does not impact administration of CRC-directed therapy or cancer-related outcomes in equal-access healthcare settings. In a retrospective analysis of over 2,000 patients treated for non-metastatic CRC in the Veterans Affairs (VA) system, Zullig et al. showed that Caucasians and African Americans received equally NCCN-compliant therapy[36]. Hofmann el al. reported no difference in surgical resection rates, administration of chemotherapy, or 5-year survival among 1,115 African Americans treated in Department of Defense (DOD) facilities when compared to Caucasians treated in the same equal-access healthcare system[37]. These observations extend beyond the Military Health System and Veterans Health Administration. For example, an analysis of 365 patients in the Emory University Hospital System identified age and diagnostic stage, but not race, as predictors of quality care and CRC mortality[38]. Working under the hypothesis that systems-based improvements can mitigate racial disparities in CRC outcomes, Laryea et al. performed a retrospective cohort study of 878 patients treated for CRC at an academic tertiary referral hospital that had implemented measures designed to enhance cultural competence in the workplace, strengthen patient navigation programs, and improve CRC screening rates among traditionally underrepresented groups. In contrast to the 40% disparity in CRC mortality between African Americans and Caucasians that preceded the implementation of these measures, the authors reported no difference in cancer-specific mortality or overall survival between these groups after implementation[39].
Our retrospective analysis of CRC-related outcomes among BTH patients reinforces these findings while presenting a unique perspective. Whereas each of the above analyses included only Caucasian and African American patients, we took a more comprehensive approach by including all minority populations, including those of Hispanic, Asian, and Middle Eastern descent. Including Hispanics is particularly important since, by 2060, Hispanics are expected to account for more than 30% of the entire US population[40]. Furthermore, in contrast to the population treated in a standard university hospital or VA/DOD facility, the BTH population is socially disadvantaged and largely uninsured, which necessitates a robust financial assistance program to ensure full access to anti-neoplastic and and supportive therapies. Finally, instead of including all stages of CRC, we chose to focus on metastatic disease in light of recent data suggesting that racial disparities in CRC treatment and survival outcomes are driven by distant-stage disease[14, 27]. We therefore identified a study cohort comprised of relatively young, underserved, minority patients with a high burden of disease who, despite significant clinical and socioeconomic challenges, routinely received guideline-compliant cancer care, achieved favorable responses to chemotherapy, and experienced overall survival rates comparable to those reported in multicenter randomized control trials. The relatively young age of our study population (median 54 years at diagnosis) is particularly timely given the rising incidence of CRC among individuals younger than 50, irrespective of race or ethnicity[41, 42]. Young-onset CRC appears to be inherently more aggressive and less chemo-responsive than older-onset CRC, and data from both the Texas Cancer Registry and National Cancer Institute have demonstrated inferior cancer-specific survival among young African Americans with CRC[43, 44].
This study has several limitations. First, the small size of our single-center cohort may limit the study’s applicability to the general population. Furthermore, the sub-specialization of BTH clinics and its robust patient assistance program may be more difficult to replicate in non-academic community settings with similar populations of uninsured or underinsured populations. Nevertheless, we have demonstrated the proof-of-principle that delivering comprehensive cancer care to an underserved segment of a major urban center is not only feasible but can yield favorable oncologic outcomes. Second, the retrospective nature of this study subjects its findings to selection bias, incomplete follow-up, variability in clinical decision-making, and inconsistencies in medical record documentation. On the other hand, one could argue that such heterogeneity provides a “real-world” perspective of CRC outcomes in a diverse and socioeconomically disadvantaged population treated with guideline-compliant CRC therapy.
Third, there are important differences in baseline characteristics between our study cohort and the patients studied in CRYSTAL and PRIME. It has been well-established that most cancer patients are ineligible for clinical trial inclusion due to factors such as poor performance status, competing comorbidities, limited life expectancy, and inadequate social support, and that real-world patients experience inferior overall survival compared to participants in trials of poor-prognosis cancers such as mCRC[45, 46]. Whereas these findings would suggest inferior outcomes in our study cohort, we actually demonstrated comparable, if not superior, overall survival among BTH patients in comparison to the CRYSTAL and PRIME cohorts. We also defined overall survival as the time from diagnosis to death whereas in CRYSTAL and PRIME, overall survival was defined as the time from randomization to death. Given that the median time from diagnosis to initiation of treatment in our population was 31 days and that the time from randomization to initiation of therapy in randomized control trials typically ranges from 7-14 days, differences in defining overall survival are not likely to significantly alter the overarching results of our analysis. Finally, we compared all BTH patients with KRAS wild type tumors who received any chemotherapy with CRYSTAL and PRIME patients with KRAS wild type tumors who were randomized to upfront chemotherapy plus anti-EGFR therapy. Because only 62% of BTH patients with KRAS wild type tumors received anti-EGFR therapy, our analysis may have underestimated the survival difference between BTH and CRYSTAL/PRIME patients with KRAS wild type tumors who actually received anti-EGFR therapy.
In conclusion, this study provides a unique glimpse into a safety-net healthcare system that serves minority patients with significant clinical and socioeconomic challenges. We have demonstrated that, with high-quality comprehensive cancer care, minority patients with mCRC can achieve clinical outcomes comparable to the highly selected, predominantly Caucasian patients treated in multicenter randomized control trials. The Harris Health System’s prioritization of clinical pathways, access to care, EMR optimization, supportive service integration, and financial toxicity mitigation provides an exemplary model for reducing racial disparities in CRC outcomes. Future efforts should focus on designing, implementing, and evaluating similar healthcare delivery models to eliminate racial disparities in cancer care.
CLINICAL PRACTICE POINTS.
Numerous publications have reported inferior survival of metastatic colorectal cancer (mCRC) among racial/ethnic minorities. Whether these disparities arise from differences in intrinsic tumor biology or access to healthcare is not known. This study shows that the median overall survival of underinsured minority patients with mCRC who receive high-quality comprehensive cancer care is comparable, if not superior to, the median overall survival of mCRC previously reported in large, randomized control trials. These data suggest that disparities in mCRC survival stem from access to care rather than intrinsic biology and that future efforts should focus on designing, implementing, and evaluating similar healthcare delivery models to eliminate racial disparities in cancer care.
focused on clinical pathways for diagnosis and treatment, full access to anti-neoplastic and supportive therapies, optimization of EMR documentation and chemotherapy, integration of supportive services, and mitigation of financial toxicity, This study demonstrates that, by providing high-quality comprehensive cancer care focused on clinical pathways for diagnosis and treatment, full access to anti-neoplastic and supportive therapies, optimization of EMR documentation and chemotherapy, integration of supportive services, and mitigation of financial toxicity, minority patients with mCRC can achieve clinical outcomes comparable to the highly selected patients treated in randomized control trials.
Acknowledgements:
We thank Beverly Moy and Ryan D. Nipp for their mentorship and guidance, and Hui Zheng from the Harvard Catalyst for his statistical support. This work was supported by the Baylor College of Medicine Cancer Center Support Grant P30 CA125123.
Funding: This work was supported by the Cancer Center Support Grant [P30 CA125123].
Abbreviations:
- CRC
colorectal cancer
- HHS
Harris Health System
- BCM
Baylor College of Medicine
- BTH
Ben Taub Hospital
- EMR
electronic medical record
- BMI
body mass index
- NCCN
National Comprehensive Cancer Network
- DOD
Department of Defense
- CRYSTAL
Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer
- PRIME
Panitumumab Randomized Trial In Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures
Benjamin Musher receives salary support from LOKON pharma for serving as principal investigator for a Phase I trial in pancreatic cancer and receives consultation fees from Ipsen pharmaceuticals for Onivyde (FDA-approved for pancreatic cancer). Since both of these relationships pertain to pancreatic cancer rather than colorectal cancer, neither poses a conflict of interest in relation to the current study.
The other authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2017. American Cancer Society, Atlanta: 2017. [Google Scholar]
- 2.Kemeny NE. Treatment of metastatic colon cancer: “the times they are A-changing”. J Clin Oncol. 2013;31(16):1913–6. doi: 10.1200/JCO.2013.49.4500. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14): 1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011. ;29(15):2011–9. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 5.Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011. ;22(7): 1535–46. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013. ;369(11): 1023–34. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 8.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25(7):1346–55. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10): 1065–75. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 10.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017;317(23):2392–401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SEER Cancer Statistics Factsheets: Colon and Rectum Cancer. National Cancer Institute, Bethesda, MD: http://seer.cancer.gov/statfacts/html/colorect.html 2017. [Google Scholar]
- 12.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002). Cancer Epidemiol Biomarkers Prev. 2006;15(4):792–7. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 13.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960-2005. Am J Public Health. 2010;100(10):1912–6. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30(4):401–5. doi: 10.1200/JCO.2011.37.5527. [DOI] [PubMed] [Google Scholar]
- 15.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006;24(14):2179–87. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 16.Tammana VS, Laiyemo AO. Colorectal cancer disparities: issues, controversies and solutions. World J Gastroenterol. 2014;20(4):869–76. doi: 10.3748/wjg.v20.i4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gastroenterol. 2009;15(30):3734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silber JH, Rosenbaum PR, Ross RN, Niknam BA, Ludwig JM, Wang W et al. Racial disparities in colon cancer survival: a matched cohort study. Ann Intern Med. 2014;161(12):845–54. doi: 10.7326/M14-0900. [DOI] [PubMed] [Google Scholar]
- 19.Fiscella K, Winters P, Tancredi D, Hendren S, Franks P. Racial disparity in death from colorectal cancer: does vitamin D deficiency contribute? Cancer. 2011;117(5): 1061–9. doi: 10.1002/cncr.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia YY, Ding YB, Liu XQ, Chen XM, Cheng SQ, Li LB et al. Racial/ethnic disparities in human DNA methylation. Biochim Biophys Acta. 2014;1846(1):258–62. doi: 10.1016/j.bbcan.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Sanoff HK, Sargent DJ, Green EM, McLeod HL, Goldberg RM. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol. 2009;27(25):4109–15. doi: 10.1200/JCO.2009.21.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas JS, Brawarsky P, Iyer A, Fitzmaurice GM, Neville BA, Earle C. Association of area sociodemographic characteristics and capacity for treatment with disparities in colorectal cancer care and mortality. Cancer. 2011;117(18):4267–76. doi: 10.1002/cncr.26034. [DOI] [PubMed] [Google Scholar]
- 23.Simpson DR, Martinez ME, Gupta S, Hattangadi-Gluth J, Mell LK, Heestand G et al. Racial disparity in consultation, treatment, and the impact on survival in metastatic colorectal cancer. J Natl Cancer Inst. 2013;105(23): 1814–20. doi: 10.1093/jnci/djt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laiyemo AO, Doubeni C, Pinsky PF, Doria-Rose VP, Bresalier R, Lamerato LE et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102(8):538–46. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halpern MT, Holden DJ. Disparities in timeliness of care for U.S. Medicare patients diagnosed with cancer. Curr Oncol. 2012;19(6):e404–13. doi: 10.3747/co.19.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chagpar R, Xing Y, Chiang YJ, Feig BW, Chang GJ, You YN et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30(9):972–9. doi: 10.1200/JCO.2011.39.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai Y, Wang C, Civan JM, Palazzo JP, Ye Z, Hyslop T et al. Effects of Cancer Stage and Treatment Differences on Racial Disparities in Survival From Colon Cancer: A United States Population-Based Study. Gastroenterology. 2016;150(5): 1135–46. doi: 10.1053/j.gastro.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Access Care. Harris Health System. https://www.harrishealth.org/en/patients/access-care/pages/default.aspx 2017.
- 29.Quick Facts: Harris County, Texas: United States Census Bureau; https://www.census.gov/quickfacts/table/PST045215/48201/accessible 2017. [Google Scholar]
- 30.Klineberg S The Kinder Houston Area Survey: Thirty-Six Years of Measuring Responses to a Changing America. Kinder Institute for Urban Research, Houston: 2017. [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 32.Colon Cancer. In: Clinical Practice Guidelines in Oncology (NCCN Guidelines). National Comprehensive Cancer Network; 2017. https://www.nccn.org/professionals/physician_gls/pdf/colon_blocks.pdf 2017. [Google Scholar]
- 33.Rectal Cancer. In: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). National Comprehensive Cancer Network; 2017. https://www.nccn.org/professionals/physician_gls/pdf/rectal_blocks.pdf 2017. [Google Scholar]
- 34.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sineshaw HM, Ng K, Flanders WD, Brawley OW, Jemal A. Factors That Contribute to Differences in Survival of Black vs White Patients With Colorectal Cancer. Gastroenterology. 2018;154(4):906–15 e7. doi: 10.1053/j.gastro.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31(28):3579–84. doi: 10.1200/JCO.2013.50.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmann LJ, Lee S, Waddell B, Davis KG. Effect of race on colon cancer treatment and outcomes in the Department of Defense healthcare system. Dis Colon Rectum. 2010;53(1):9–15. doi: 10.1007/DCR.0b013e3181bdcdb2. [DOI] [PubMed] [Google Scholar]
- 38.Berry J, Caplan L, Davis S, Minor P, Counts-Spriggs M, Glover R et al. A black-white comparison of the quality of stage-specific colon cancer treatment. Cancer. 2010;116(3):713–22. doi: 10.1002/cncr.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laryea JA, Siegel E, Klimberg S. Racial disparity in colorectal cancer: the role of equal treatment. Dis Colon Rectum. 2014;57(3):295–302. doi: 10.1097/DCR.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Census Bureau Projections Show a Slower Growing, Older, More Diverse Nation a Half Century from Now. United States Census Bureau, Suitland: 2012. https://www.census.gov/newsroom/releases/archives/population/cb12-243.html [Google Scholar]
- 41.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–8. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 42.Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015; 150(1): 17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang DY, Thrift AP, Zarrin-Khameh N, Wichmann A, Armstrong GN, Thompson PA et al. Rising Incidence of Colorectal Cancer Among Young Hispanics in Texas. J Clin Gastroenterol. 2017;51(1):34–42. doi: 10.1097/MCG.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holowatyj AN, Ruterbusch JJ, Rozek LS, Cote ML, Stoffel EM. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J Clin Oncol. 2016;34(18):2148–56. doi: 10.1200/JCO.2015.65.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015; 16:495. doi: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unger JM, Barlow WE, Martin DP, Ramsey SD, Leblanc M, Etzioni R et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3):dju002. doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]


