Abstract
Introduction:
Children with ventricular pre-excitation are at risk for sudden death.
Methods:
This retrospective pediatric study identified patients >8 years of age who had undergone electrophysiology study (EPS). Our primary objective was to determine the performance characteristics of non-invasive risk stratification. Subjects were separated into two groups. Group 1 was asymptomatic or had non-specific symptoms (palpitations, chest pain, light-headedness) without documented supraventricular tachycardia (SVT). Group 2 had syncope, documented SVT, or a life-threatening event. As a secondary aim, we tested whether patients with severe symptoms had a shorter time from date of diagnosis to date of invasive risk stratification.
Results:
Among 93 patients with an average age of 14.2 years, 25 patients had documented SVT, 6 had syncope and 1 had a life-threatening event. The sensitivity of non-invasive risk stratification was 7%. The specificity was 91%. The positive predictive valve was 14% and the negative predictive value was 84%. Even patients with severe symptoms commonly underwent non-invasive risk stratification prior to EPS, albeit at a lower rate (Group 1, 98%; Group 2 84%, p=0.02). The median time to EPS was 4.2 months (Group 1) and 4.5 months (Group 2, p=0.63).
Conclusion:
Non-invasive risk stratification was a poor predictor of invasive risk stratification. Cardiologists should counsel families about the limitations of non-invasive risk stratification and consider starting with invasive risk stratification and possible ablation. Counterintuitively, severe symptoms were not associated with a shorter time to electrophysiology study.
Keywords: Pediatric, ventricular pre-excitation, sudden death, risk stratification, Wolff-Parkinson-White syndrome
INTRODUCTION
In pediatrics, ventricular pre-excitation (VPE) is the most common cardiac electrophysiology diagnosis associated with sudden death. A 2012 expert consensus statement provided guidance to minimize the risk of sudden death.[1] The statement recommended an algorithm for risk stratification that depended on non-invasive risk stratification using exercise stress tests. In some centers, ambulatory monitors (Holters) have also been used for risk stratification.
Since the publication of the consensus statement, studies have called into question the efficacy of non-invasive risk stratification to classify a patient with VPE as lower-risk for sudden death. Reports of patients with lower-risk pathways who subsequently suffered life-threatening events continue to be published.[2,3] Although sudden death outcomes in asymptomatic patients with VPE are rare, they are not insignificant. For example, a 2015 meta-analysis reported malignant atrial fibrillation in up to 9% of patients and ventricular fibrillation in up to 2%.[4]
While the concern that non-invasive risk stratification imperfectly predicts risk of sudden death is not new, contemporary data are particularly important because the central question of whether the 2012 HRS consensus statement requires revision is important for general cardiologists and electrophysiologists. There have been mixed data to date.[5] Kiger et al suggested that non-invasive risk stratification was insufficient, noting that two patients with lower-risk characteristics died or required resuscitation in their study of 295 patients (~1%).[2] In a contrasting study, Wackel et al reported that low-risk exercise stress testing successfully predicted low-risk invasive testing, although they limit these findings to invasive testing without isoproterenol.[6] Mah et al found that intermittent VPE did not rule out potentially high-risk pathways. Etheridge et al demonstrated that life threatening events (LTE) were the sentinel symptom in 65% of cases with a LTE.[7] In addition, the 2015 review and meta-analysis found that there was a significant risk for arrhythmia in patients with VPE who did not undergo ablation, with most of the ventricular fibrillation documented in children.[4]
Whether non-invasive risk stratification in VPE is useful is particularly important to general cardiologists who decide whether patients should be referred to an electrophysiologist. In addition, obtaining non-invasive testing prior to referral may delay invasive risk stratification or add unnecessary costs and hassle for the family. In our medical catchment area, as in many others, it is common for pediatric cardiologists to obtain non-invasive risk stratification prior to referring the patient for electrophysiology study. While some tests occur quickly, it takes some patients weeks or months to be scheduled for an exercise stress test, depending on both hospital and family factors. We hypothesized that the process of obtaining these non-invasive studies might delay the time to electrophysiology study, without substantially informing risk stratification for sudden death.
Our study had two objectives. The primary aim was to determine performance characteristics of non-invasive risk stratification. The secondary aim was to test whether severe symptoms are associated with a shorter time to invasive risk stratification.
METHODS
We performed a retrospective review of invasive EPS studies performed between 2012 and 2017 at a single tertiary pediatric center. We excluded patients under 8 years old because age and size considerations change non-invasive screening choices, especially for exercise stress tests. We excluded patients with significant congenital heart disease. Hemodynamically insignificant septal defects, ductus arteriosus, mitral valve prolapse and isolated left superior vena were not considered significant. Patient age at diagnosis was defined by the earliest available pre-excited electrocardiogram (ECG). If patients underwent more than one EPS for recurrent pre-excitation, the latency period was defined as the difference between the date of the first pre-excited ECG and the date of the first EPS.
Non-invasive Risk Stratification
Data on non-invasive risk stratification were collected from Holters, exercise stress tests (EST) and resting 12 lead ECGs. All patients were assumed to be higher-risk unless non-invasive testing demonstrated lower-risk characteristics. Patients were classified lower-risk by non-invasive testing if they had either abrupt beat-to-beat loss of pre-excitation on Holter/EST or at least one ECG demonstrating absence of pre-excitation prior to EPS.
Invasive Risk Stratification
All invasive data were determined during intracardiac EPS under general anesthesia. Higher-risk classification by EPS was determined using previously established criteria: an accessory pathway effective refractory period (APERP) ≤ 250 msec, accessory pathway block rate during steady-rate atrial pacing (APB) ≤ 250 msec, shortest pre-excited R-R interval during atrial fibrillation (SPERRI) ≤ 250 msec[8], multiple pathways[9], or inducible atrio-ventricular re-entrant tachycardia.[10] If isoproterenol was used, APERP, APB, and SPERRI were tabulated using the same thresholds. Our base cases analysis considered data both on and off isoproterenol for the classification of higher-risk patients. We performed two sensitivity analyses. First, we tabulated risk characteristics for invasive cases only in the baseline setting, without isoproterenol. Second, we tabulated risk characteristics if the presence of isolated, inducible orthodromic reciprocating tachycardia were not considered a higher-risk feature and the data were otherwise tabulated as above, including isoproterenol data.
Definition of Groups
Patients were grouped based on symptom severity. Patients with no cardiac symptoms or patients with non-specific symptoms (palpitations, chest pain or lightheadedness) without documented SVT, were assigned to Group 1. Patients with a history of syncope, documented SVT, or with a LTE by previously published criteria[3] were assigned to Group 2. Study data were collected and managed using the REDCap (Research Electronic Data Capture) system hosted at Northwestern University Clinical and Translational Sciences Institute.[11] Analysis was performed with Stata IC 15 (College Station, TX).
Statistical Analysis
Descriptive statistics summarized key variables for patients. Fisher exact analysis was used for categorical variables. Our time variable for survival analysis was the time between the date of the first pre-excited ECG and the date of the EPS. A log-rank test for equality of survivor functions was used to evaluate latency dichotomized between Group 1 and Group 2 and a Cox regression model was used for multivariate analysis of time-survival data. All analyses assumed a two-sided type I error rate of 0.05. Because the overall goal was to detect candidates that have a low risk of a sudden death event, positive and negative predictive values evaluated non-invasive tests in terms of ability to accurately detect a lower-risk pathway by EPS. Thus, “true positive” was a lower-risk result on both non-invasive and invasive testing. “True negative” was a higher-risk result on both non-invasive and invasive testing. “False positive” was a lower-risk result on non-invasive testing, but a higher-risk result on invasive testing. “False negative” was a higher-risk result on non-invasive testing and lower-risk result on invasive testing. Sensitivity, specificity, positive predictive value and negative predictive value were then assigned using standard conventions.
RESULTS
Patients
We identified 93 pediatric patients with VPE who underwent EPS. The median age at the time of first diagnostic ECG was 14.2 years (SD 2.6). The median age at EPS was 15.1 years (Table 1). Severe symptoms were present in 32 patients (Group 2). Syncope was present in 6 (19%), documented SVT was present in 25 (78%). An emergency room ECG was the most common method of documenting pre-EPS SVT (Table 2). One patient had a history of a LTE. He had syncope and presented with pre-excited atrial fibrillation (clinical SPERRI 140 msec), requiring emergent cardioversion for hemodynamic instability.
Table 1.
Patient characteristics
| Number of patients | 93 |
| Median age at first electrocardiogram | 14.2 years (IQR 12.3 – 16.0) |
| Median age at electrophysiology study | 15.1 years (IQR 13.7 – 16.9) |
| Mean weight at electrophysiology study | 61.3 kg (SD 20.9) |
| Female | 38 (41%) |
| Group 1 | 61 (65%) |
| Group 2* | 32 (35%) |
| Documented SVT | 26 (81% of Group 2 patients) |
| Syncope | 7 (22%) |
| LTE (pre-excited atrial fibrillation) | 1 (3%) |
One patient in Group 2 had more than 1 serious symptom. LTE = life-threatening event; SVT = Supraventricular tachycardia; IQR= Interquartile range; SD = Standard deviation
Table 2.
Modality for Documentation of Supraventricular Tachycardia Prior to EPS
| Number of patients | 26 |
| Emergency Room Visit | 11 (42%) |
| Event Monitor | 9 (35%) |
| Transesophageal Pacing Study | 5 (19%) |
| Holter | 1 (4%) |
EPS = Electrophysiology Study
Twenty-five patients were asymptomatic (27%) and 36 patients reported chest pain, dizziness, shortness of breath, or a combination of these symptoms (39%).
Non-invasive risk stratification
In our cohort, 87 of 93 patients underwent non-invasive risk stratification with either a Holter or an EST prior to EPS (98% in Group 1; 84% in Group 2, p=0.02). Non-invasive testing classified only eight patients as lower-risk (8.6%); the remaining 79 patients were classified as higher-risk. Among the six patients who did not receive non-invasive risk stratification, three had SVT in the emergency department, two had SVT on an event monitor, and one had a LTE.
Correlation between Invasive and Non-Invasive Risk Stratification
During EPS, 79 patients had higher-risk pathways (85%). Non-invasive testing poorly predicted lower-risk characteristics during EPS (Figure 1). Among the eight patients with lower-risk non-invasive testing, seven (88%) had higher-risk pathways during EPS. The sensitivity of non-invasive risk stratification was 7%. The specificity of non-invasive risk stratification was 91%. The positive predictive valve was 14%. The negative predictive value was 84%.
Figure 1.

Test characteristics for non-invasive risk stratification
“Invasive (±) Isoproterenol”: Test characteristics of non-invasive testing compared against invasive testing with and without isoproterenol; “Invasive (−) Isoproterenol”: Test characteristics of non-invasive testing compared against invasive testing, without using data on isoproterenol. “Invasive (±) isoproterenol, (−) ORT”: Test characteristics of noninvasive testing compared against invasive testing with and without isoproterenol, but not considering ORT as a stand-alone criterion for higher-risk pathways. ORT: Orthodromic reciprocating tachycardia. “Positive predictive value” refers to the ability of non-invasive testing to successfully label a pathway as “lower-risk” that is also deemed lower-risk on invasive testing.
In our first sensitivity analysis, we evaluated invasive pathway characteristics at baseline (without isoproterenol infusion data). Without isoproterenol, the number of patients with higher-risk pathways by EPS decreased to 68 (73%) patients. Using these data, the non-invasive risk stratification had a sensitivity of 12%, a specificity of 93%, a positive predictive value of 38% and a negative predictive value of 74%. In our second sensitivity analysis, we excluded orthodromic reciprocating tachycardia during EPS as a stand-alone criterion to assign a patient to a higher-risk category. Non-invasive testing predicted this invasive outcome with a sensitivity of 10%, specificity of 94%, positive predictive value of 75%, and negative predictive value of 36%. The distribution of cases among all sensitivity analyses are shown in Figure 2.
Figure 2.

Internal Agreement of Three Methods to Determine “Higher-Risk” Pathway Characteristics
A: Invasive (±) Isoproterenol; B: Invasive (−) Isoproterenol; C: Invasive (±) Isoproterenol, (−) ORT.
Shortest Pre-Excited RR Intervals during Atrial Fibrillation (SPERRI)
SPERRI was documented in 47/93 cases (50%). SPERRI was tested on isoproterenol in only five cases and the SPERRI transitioned from lower-risk (> 250 msec) to higher-risk (≤ 250 msec) on isoproterenol in two of the five. Among the 44 cases where SPERRI was > 250 msec, 33/44 were higher-risk based on APERP or APB criteria and 11 were concluded to be lower-risk. One patient had lower-risk SPERRI at baseline, higher-risk SPERRI on isoproterenol and had multiple pathways.
Transesophageal Pacing
Six patients underwent transesophageal pacing (TEP) studies with baseline and isoproterenol prior to invasive EPS (age range: 8 to 16 years old at TEP). In five patients, the TEP was performed due to patient family preference; in one patient TEP was performed at the recommendation of the primary electrophysiologist. All six also had Holter monitors prior to EPS (1/6 with abrupt loss of pre-excitation and 5/6 with persistent pre-excitation throughout). TEP showed higher-risk characteristics in 5 patients. The one patient with lower-risk characteristics on both non-invasive testing and TEP had an APERP and APB of 450 by invasive testing. He had orthodromic reciprocating tachycardia on TEP and EPS. Ablation was performed in all six patients.
Time to Electrophysiology Study
The median latency period between diagnosis and EPS for patients without severe symptoms (Group 1) was 4.2 months (IQR 2.6 – 16.7). The median latency period for patients who had syncope, documented SVT or a LTE (Group 2) was 4.5 months (IQR 2.7 – 14.5). In a log-rank test for equality of survivor functions, there was no difference in the latency period between the two groups (Figure 3, p=0.63). In addition, the presence or absence of higher-risk non-invasive risk stratification did not predict latency (in univariate analysis, p=0.88; in multivariable Cox regression, controlling for symptom severity, p=0.84 [95% CI 0.42–2.05]).
Figure 3.
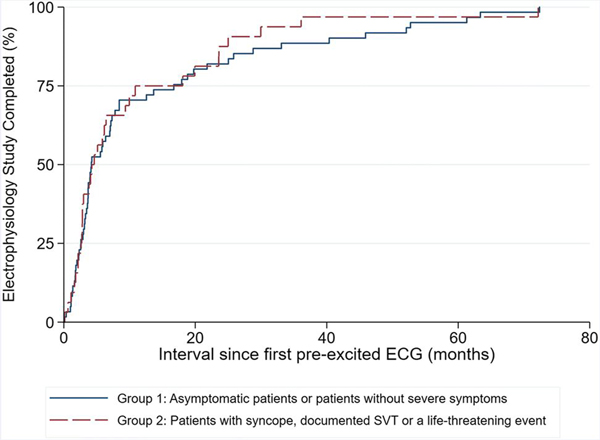
Time between initial diagnosis and electrophysiology study, stratified by the symptom severity.
A Kaplan-Meier survival curve documents no significant difference by log rank test (p=0.63), when dividing our population into two groups: Group 1: No cardiac symptoms or patients with palpitations, chest pain or non-specific cardiac symptoms without documented SVT. Group 2: Syncope, documented SVT or a life-threatening event.
Ablation Outcomes
Ablation was attempted in 83 of 93 patients (89%). Ablation was acutely successful in 81/83 (98%). In the 10 patients for whom ablation was not attempted, two had inducible SVT, but were not ablated (1 had mechanical pathway termination during the case; 1 had a para-Hisian pathway and was not ablated). The remaining cases were adjudicated by the attending electrophysiologist to be too low-risk to ablate. None have had a subsequent LTE.
DISCUSSION
Non-invasive risk stratification poorly predicts invasive results
Since the publication of the 2012 expert consensus statement, the pediatric electrophysiology community has renewed its focus on risk stratification for sudden death in children with ventricular pre-excitation. The most important finding of our study was that lower-risk non-invasive testing does not reliably predict lower-risk pathway characteristics by invasive EPS in our study. For clinical purposes, the positive predictive value of 14% in our study is too low to help a patient decide about risk of sudden death. In other words, non-invasive risk stratification uses resources without changing the clinical risk calculation. These data, coupled with data from previously published studies, suggest that the expert consensus statement is ready for revision [2,3,6,12,13]
This point is important for general pediatric cardiologists as well as electrophysiologists. In our center, non-invasive risk stratification is often performed before referral to electrophysiology. While sudden death in this population is exceptionally rare, our data suggest that cardiologists may be falsely reassured by lower-risk non-invasive stratification. This is especially important because asymptomatic children may go on to have LTE, as documented in the international compendium by Etheridge et al.[3] Thus, our data support invasive EPS evaluation with potential ablation even in asymptomatic patients.
Results during isoproterenol testing
The addition of isoproterenol during EPS classified more patients in our study as “higher-risk”, but like many other studies, including a secondary analysis of the recent multicenter data from the Pediatric and Congenital Electrophysiology Society,[12] our data are too narrow to provide a definitive metric for testing on isoproterenol. A study by Kubuš et al showed similar findings, and a study by Moore et al also found that isoproterenol decreased accessory pathway characteristics (APERP, APB, SPERRI) during EPS.[13,14] The recent multicenter study by Etheridge et al includes matched clinical events prior to EPS. The authors conclude, “In order to prevent LTE, we may need to consider using isoproterenol and/or performing studies without general anesthesia to improve risk assessment, even at the risk of decreasing specificity.”[3] Our data support this.
Inducibility of orthodromic re-entrant tachycardia
There is disagreement about whether inducible ORT during electrophysiology study should be a sufficient criterion to categorize a pathway as “higher-risk”, if the other invasive risk characteristics are all lower-risk. Some groups have used this criterion;[7] others have not.[3] Our study suggests that eliminating this as a stand-alone criterion aligns the outcome of non-invasive testing (positive predictive value) more closely with invasive testing (Figure 1). However, even using this narrower endpoint, the test characteristics of non-invasive testing are poor. The best positive predictive value (75%) remains insufficient for clinical care. More importantly, our study used invasive EPS as the primary endpoint, not subsequent events. Thus the controversy remains: we cannot conclude whether ORT should be a stand-alone criterion.
Importantly, invasive EPS may not be an adequate metric for risk stratification. In a recent manuscript, Shwayder et al demonstrate that there is poor correlation between EP lab measurements of pathway characteristics under general anesthesia and the SPERRI when pre-excited atrial fibrillation is present clinically.[12] Non-invasive loss of pre-excitation is an insufficient measurement of risk when compared to invasive EPS. However, if invasive EPS is itself a poor risk prediction tool, there is even less reason to trust non-invasive risk stratification, especially as there are no data to suggest that results on a Holter or EST are more closely correlated to risk than invasive findings.
Trans-esophageal pacing
At our institution, TEP in this age group has typically been limited to unique cases or when there is a strong patient or provider preference. A study published by Koca et al, suggested that TEP is a useful tool in risk stratification, identifying patients with higher-risk pathways.[15] We report a limited number of patients with VPE who underwent TEP and all of the TEP patients ultimately underwent ablation.
Time to Electrophysiology Study
There was no statistically significant difference in the time from diagnosis of ventricular pre-excitation to EPS between patients with isolated VPE, without severe symptoms (Group 1) or patients with ventricular pre-excitation and syncope, documented SVT or LTE (Group 2). In fact, all Group 2 patients had a clear clinical indication for EPS, but 84% still received non-invasive risk stratification prior to referral and EPS. The similar latency in both groups highlights how deeply embedded non-invasive risk stratification is in pediatric cardiology practice patterns, at least in our single-center report. While there are likely some institutional and provider-dependent idiosyncrasies that we cannot discern with retrospective data collection, only six patients in this study were taken to EPS without either a Holter, EST, or other non-invasive risk stratification.
Limitations
These are retrospective results with the limitations of retrospective data sources, including idiosyncratic reasons for time-to-study latency in any given patient. In addition, we had more patients with higher-risk than lower-risk non-invasive risk stratification. This may reflect selection bias: patients with higher-risk non-invasive risk stratification may have been more likely to undergo invasive EPS. In one patient, SVT on a Holter was the determining factor that assigned the patient to Group 2, creating an artificial increase in the percentage of Group 2 patients who received non-invasive testing. Repeat analysis with this patient assigned to Group 1 did not change the statistical conclusions of the study.
Conclusion
Non-invasive risk stratification was a poor predictor of invasive risk stratification. In addition, children with ventricular pre-excitation and syncope, documented SVT or a life-threatening event did not have a shorter time to electrophysiology study than asymptomatic patients or those with less severe symptoms. Practitioners should counsel families about the limitations of non-invasive risk stratification and consider the option of invasive risk stratification and possible ablation in the setting of VPE.
Acknowledgments
The study was funded, in part, by the National Institutes of Health, National Heart, Lung and Blood Institute (grant number K23HL130554). REDCap access was provided by Northwestern University Clinical and Translational Sciences Institute (grant number NIH UL1TR001422).
Footnotes
All authors declare that they have no conflicts of interest to report.
COMPLIANCE WITH ETHICAL STANDARDS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- 1.Pediatric, Congenital Electrophysiology S, Heart Rhythm S, American College of Cardiology F, American Heart A, American Academy of P, Canadian Heart Rhythm S, Cohen MI, Triedman JK, Cannon BC, Davis AM, Drago F, Janousek J, Klein GJ, Law IH, Morady FJ, Paul T, Perry JC, Sanatani S, Tanel RE (2012) PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart rhythm 9 (6):1006–1024. doi: 10.1016/j.hrthm.2012.03.050 [DOI] [PubMed] [Google Scholar]
- 2.Kiger ME, McCanta AC, Tong S, Schaffer M, Runciman M, Collins KK (2016) Intermittent versus Persistent Wolff-Parkinson-White Syndrome in Children: Electrophysiologic Properties and Clinical Outcomes. Pacing Clin Electrophysiol 39 (1):14–20. doi: 10.1111/pace.12732 [DOI] [PubMed] [Google Scholar]
- 3.Etheridge SP, Escudero CA, Blaufox AD, Law IH, Dechert-Crooks BE, Stephenson EA, Dubin AM, Ceresnak SR, Motonaga KS, Skinner JR, Marcondes LD, Perry JC, Collins KK, Seslar SP, Cabrera M, Uzun O, Cannon BC, Aziz PF, Kubus P, Tanel RE, Valdes SO, Sami S, Kertesz NJ, Maldonado J, Erickson C, Moore JP, Asakai H, Mill L, Abcede M, Spector ZZ, Menon S, Shwayder M, Bradley DJ, Cohen MI, Sanatani S (2018) Life-Threatening Event Risk in Children With Wolff-Parkinson-White Syndrome: A Multicenter International Study. JACC Clinical electrophysiology 4 (4):433–444. doi: 10.1016/j.jacep.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 4.Al-Khatib SM, Arshad A, Balk EM, Das SR, Hsu JC, Joglar JA, Page RL (2016) Risk stratification for arrhythmic events in patients with asymptomatic pre-excitation: A systematic review for the 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart rhythm 13 (4):e222–237. doi: 10.1016/j.hrthm.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 5.Sharma AD, Yee R, Guiraudon G, Klein GJ (1987) Sensitivity and specificity of invasive and noninvasive testing for risk of sudden death in Wolff-Parkinson-White syndrome. Journal of the American College of Cardiology 10 (2):373–381. doi: 10.1016/s0735-1097(87)80021-9 [DOI] [PubMed] [Google Scholar]
- 6.Wackel P, Irving C, Webber S, Beerman L, Arora G (2012) Risk stratification in Wolff-Parkinson-White syndrome: the correlation between noninvasive and invasive testing in pediatric patients. Pacing Clin Electrophysiol 35 (12):1451–1457. doi: 10.1111/j.1540-8159.2012.03518.x [DOI] [PubMed] [Google Scholar]
- 7.Mah DY, Sherwin ED, Alexander ME, Cecchin F, Abrams DJ, Walsh EP, Triedman JK (2013) The electrophysiological characteristics of accessory pathways in pediatric patients with intermittent preexcitation. Pacing Clin Electrophysiol 36 (9):1117–1122. doi: 10.1111/pace.12144 [DOI] [PubMed] [Google Scholar]
- 8.Klein GJ, Gulamhusein SS (1983) Intermittent preexcitation in the Wolff-Parkinson-White syndrome. The American journal of cardiology 52 (3):292–296. doi: 10.1016/0002-9149(83)90125-x [DOI] [PubMed] [Google Scholar]
- 9.Brembilla-Perrot B, Chometon F, Groben L, Tatar C, Luporsi JD, Bertrand J, Huttin O, Beurrier D, Ammar S, Cedano J, Benzaghou N, Andronache M, Valizadeh R, Terrier De La Chaise A, Louis P, Selton O, Claudon O Marcon F (2008) Are the results of electrophysiological study different in patients with a pre-excitation syndrome, with and without syncope? Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 10 (2):175–180. doi: 10.1093/europace/eum300 [DOI] [PubMed] [Google Scholar]
- 10.Pappone C, Santinelli V, Rosanio S, Vicedomini G, Nardi S, Pappone A, Tortoriello V, Manguso F, Mazzone P, Gulletta S, Oreto G, Alfieri O (2003) Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. Journal of the American College of Cardiology 41 (2):239–244. doi: 10.1016/s0735-1097(02)02706-7 [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)-- a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 42 (2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shwayder MH, Escudero CA, Etheridge SP, Dechert BE, Law IH, Blaufox AD, Perry JC, Dubin AM, Sanatani S, Collins KK (2019) Difficulties with Invasive Risk Stratification Performed Under Anesthesia in Pediatric Wolff-Parkinson-White Syndrome. Heart rhythm. doi: 10.1016/j.hrthm.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 13.Kubus P, Vit P, Gebauer RA, Materna O, Janousek J (2014) Electrophysiologic profile and results of invasive risk stratification in asymptomatic children and adolescents with the Wolff-Parkinson-White electrocardiographic pattern. Circulation Arrhythmia and electrophysiology 7 (2):218–223. doi: 10.1161/circep.113.000930 [DOI] [PubMed] [Google Scholar]
- 14.Moore JP, Kannankeril PJ, Fish FA (2011) Isoproterenol administration during general anesthesia for the evaluation of children with ventricular preexcitation. Circulation Arrhythmia and electrophysiology 4 (1):73–78. doi: 10.1161/circep.110.958660 [DOI] [PubMed] [Google Scholar]
- 15.Koca S, Pac FA, Kavurt AV, Cay S, Mihcioglu A, Aras D, Topaloglu S (2017) Transesophageal and invasive electrophysiologic evaluation in children with Wolff-Parkinson-White pattern. Pacing Clin Electrophysiol 40 (7):808–814. doi: 10.1111/pace.13100 [DOI] [PubMed] [Google Scholar]


