Abstract
Although combination antiretroviral therapy (cART) has simplified over the past decade, polypharmacy is increasing for older people living with HIV (PLWH) due to the emergence of multiple health comorbidities. This study examined predictors of, and relationships between, objective (Medication Management Test-Revised (MMT-R)) and self-reported medication management ability in older (≥50 years) PLWH (n=146) compared to HIV uninfected (HIV-) individuals (n=60). PLWH scored worse on the MMT-R and had a higher pill burden compared to HIV- individuals. MMT-R failure was predicted by HIV status, race, reading level, and worse executive functioning, as well as history of Hepatitis C and detectable viral load in PLWH. Self-reported ability to manage medications did not relate to MMT-R score. Older PLWH may not self-describe concerns regarding their ability to manage complex medication regimens. Our results emphasize the need for objective measurements of medication management ability.
Keywords: Polypharmacy, Neuropsychology, HIV, Medication therapy management, Executive function
Introduction
Since the implementation of combination antiretroviral therapy (cART), the life expectancy of people living with HIV (PLWH) has increased and is similar to the HIV uninfected (HIV-) general population (Sabin 2013). As of 2015, almost half of all PLWH in the United States were ≥50 years old and this number will continue to rise. Older PLWH (≥50 years) represent a unique clinical challenge as this population is at increased risk for developing serious age-related comorbidities (e.g. cardiovascular disease, diabetes, neurodegenerative diseases) that require medications (Greene et al., 2013; Greene et al., 2014). Consequently, although cART regimens have become less complex, polypharmacy has become a significant concern among older PLWH (Gimeno-Gracia et al., 2016).
Previous research has suggested several predictors of performance on objective measures of medication management in PLWH. Specifically, demographic factors such as lower education, higher rate of unemployment, and clinical factors such as depression and hepatitis C co-infection (Hep C) (Heaton et al., 2004; Patton et al., 2012) correspond to suboptimal performance on tests of medication management. Medication management performance is also strongly associated with cognitive ability. Poorer executive functioning, motor/psychomotor function, and memory have repeatedly been identified as strong predictors of poorer medication management abilities (Albert et al., 1999; Heaton et al., 2004; Patton et al., 2012). Prospective memory, that is, remembering to complete future tasks, is particularly relevant to medication management (Thames et al., 2013; Woods et al., 2008).
Identifying significant predictors of poor medication management ability in PLWH represents an important step towards the development of interventions to support optimal treatment outcomes. Older PLWH represent a particularly important area of focus as they may be at an additional risk both for age-related comorbidities and functional impairment in managing medications due to interactions between age-related and HIV-related neurocognitive impairment. To date, relationships between objective and self-reported medication management in older PLWH compared to HIV-uninfected individuals are not well-understood. This gap was addressed in the present study. We compared 146 older PLWH and 60 older HIV- uninfected individuals on objective and subjective measures of medication management. Factors associated with self-reported medication management were examined in PLWH.
Methods
Participants
Older (≥50 years old) PLWH (n = 146) who were chronically-infected (> 1 year), and on a stable cART regimen for ≥ 12 months were recruited from the Washington University School of Medicine (WUSM) Infectious Disease Clinic and the WUSM AIDS Clinical Trial Unit (ACTU) (87% undetectable viral load (≤ 50 copies/mL)). Older (≥50 years old) HIV- controls (n = 60) were also recruited from the general community through WUSM. Exclusion criteria included < 8 years of education, inability to read or write in English, inability to provide written informed consent, history of head injury with loss of consciousness >30 minutes, self-reported major psychiatric disorders (e.g. severe depression, schizophrenia, Bipolar disorder), central nervous system opportunistic infections, or current use of illicit drugs other than marijuana at time of study (assessed by urine drug screen). Serostatus of all HIV- controls was confirmed using a buccal oral swab test. The study was approved by the Washington University Institutional Review Board, and all participants were financially compensated for participation.
Demographic information (age, sex, race, education) and employment status (currently employed, retired, on disability, or unemployed) was recorded.
Measures of Medication Management
Medication Management Test-Revised
The Columbia Medication Management Test-Revised (MMT-R) (Albert et al., 1999; Heaton et al., 2004) was developed to provide objective measures of medication inference and pill dispensing skills among PLWH. The medication inference tasks requires participants to examine and answer questions about a simulated medication regimen and over-the-counter medication insert. The pill dispensing section requires participants to manipulate a pill organizer designed for a one-week supply of medications. Scores on the MMT-R range from 0-10, with higher values indicating better performance. A score <5 reflects impairment (Patton et al., 2012).
Self-report Measure
A self-report measure assessed pill burden (total number of pills taken daily from medications and vitamins/supplements verified through medical records) and methods utilized to facilitate adherence (e.g., phone reminders). Participants were asked if they had run out of medications in the past two months or if they experienced more difficulty managing medications now compared to the past. A positive response to at least one of these two questions represented perceived “difficulty” with medication management (Thames et al., 2011b).
Neuropsychological Assessment
All participants were administered neuropsychological tests representing five cognitive domains, including executive function, motor/psychomotor speed, learning, retention, and language. Raw scores were transformed into demographically-corrected domain Z scores. The test battery has been previously used to assess cognitive impairment in PLWH (Paul et al., 2018).
Prospective-Retrospective Memory Questionnaire
Significant relationships between prospective memory and medication management ability have been identified in PLWH and HIV- controls (Woods et al., 2008; Woods et al., 2014). The Prospective-Retrospective Memory Questionnaire (Smith et al., 2000) is a 16-item questionnaire that asks the participant to rate how often different prospective and retrospective memory failures occur. Ratings are scored using a five-point Likert scale from 1 (never) to 5 (often). Scores for the prospective memory subscale were analyzed in the current study.
Wide-Range Achievement Test 3 Reading Subscale
The Reading test of the Wide-Range Achievement Test 3 (WRAT3) was included as a measure of reading level. Raw scores were transformed to age-adjusted standard scores using the appropriate norms (Wilkinson et al., 2006).
Substance Use
Participants self-reported (yes/no) substance use (marijuana, cocaine, opiates, methamphetamine, barbiturates, benzodiazepines, phencyclidine and alcohol) within the past six months at study enrollment. Nicotine use at the time of enrollment was also documented using self-report.
Beck Depression Inventory II (BDI-II)
Current symptoms of depression were assessed using the Beck Depression Inventory II (BDI-II) (Beck et al., 1996).
Clinical Variables
Current CD4 T-cell count, nadir CD4 T-cell count, undetectable viral load (≤ 50 copies/mL) status, estimated duration of infection, total time (in months) on cART, and Hep C were collected from medical records for all PLWH.
Analyses
Older PLWH and HIV- controls were first compared on the MMT-R as a continuous variable using an analysis of covariance (ANCOVA) test. The proportion of individuals that failed the MMT-R was compared between the two groups with a Chi-square analysis. Next, a hierarchical binary logistic regression analysis was conducted to examine predictors of impairment on the MMT-R. HIV status (first block); demographic variables, substance use, BDI-II score, cognitive performance for each domain, and PRMQ prospective memory subscale scores (second block) were included as predictors. For significant predictors, a second hierarchical binary logistic regression analysis examined interactions between significant predictors and HIV status. Associations between MMT-R failure and HIV variables (e.g. CD4 count, percent undetectable viral load) were also examined using independent sample t-tests or Chi-square analyses, where appropriate. This approach was repeated to examine predictors of subjective ratings of medication management, with MMT-R score also included as a potential predictor.
Finally, the total number of daily pills was compared between PLWH with undetectable viral load (≤ 50 copies/mL) (n= 127) versus detectable viral load (n=19).
Results
Sample demographic characteristics are provided in Table 1. The two groups were similar with regards to age, race, and years of education. However, the PLWH group included a greater proportion of males, individuals with lower reading performances, higher BDI-II scores, and a higher rate of unemployment. PLWH also had a significantly higher daily pill burden compared to HIV- controls (all p’s < 0.05). However, it should be noted that both the HIV- and PLWH groups reported an average daily pill burden of ≥5 pills, meeting the definition of polypharmacy.
Table 1.
Demographics of the participants
| Variables | HIV- | PLWH | p-value |
|---|---|---|---|
| Age (years) (SD) | 59.7 (8.7) | 57.8 (6.9) | 0.09 |
| Education (years) (SD) | 14.1 (2.3) | 13.5 (2.6) | 0.14 |
| Sex, n (% Male) | 28 (47%) | 117 (80%) | <.001 |
| Race, n (% AAa) | 41 (68%) | 84 (58%) | 0.15 |
| % Employed | 53% | 31% | 0.002 |
| BDI-IIb | 5.6 (5.8) | 8.5 (7.1) | 0.01 |
| Clinical values | |||
| Current CD4, Median (IQRc) | N/A | 534 (375, 746) | N/A |
| Nadir CD4, Median (IQR) | N/A | 105 (20, 284) | N/A |
| Undetectable viral load N (%) | N/A | 127 (87%) | N/A |
| Hepatitis C co-infection N (%) | N/A | 17 (12%) | N/A |
| Duration of infection (months), Median (IQR) | N/A | 220 (144, 301) | N/A |
| Months on cART, Median (IQR) | N/A | 187 (90, 240) | N/A |
| Total daily pills (SD) | 5.1 (5.7) | 8.9 (5.4) | <0.001 |
| MMT-Rd score (SD) | 7.0 (2.5) | 5.4 (3.1) | <0.001 |
| Self-reported difficulty managing medications (% yes) | 17% | 21% | 0.50 |
AA=African American;
BDI-II=Beck Depression Inventory II;
IQR=interquartile range;
=MMT-R= Medication Management Test-Revised, SD= standard deviation of the participants
Performance on the MMT-R
Older PLWH (M=5.4, SD=3.1) performed significantly worse on the MMT-R compared to HIV- controls (M=7.0, SD=2.5) (higher scores reflect better performance, p <0.001; Cohen’s d = 0.6) (see Figure 1). The proportion of individuals failing the MMT-R was also significantly different between groups, with older PLWH exhibiting a significantly higher impairment rate (42%) compared to HIV- individuals (17%) (p<0.001;).
Figure 1.
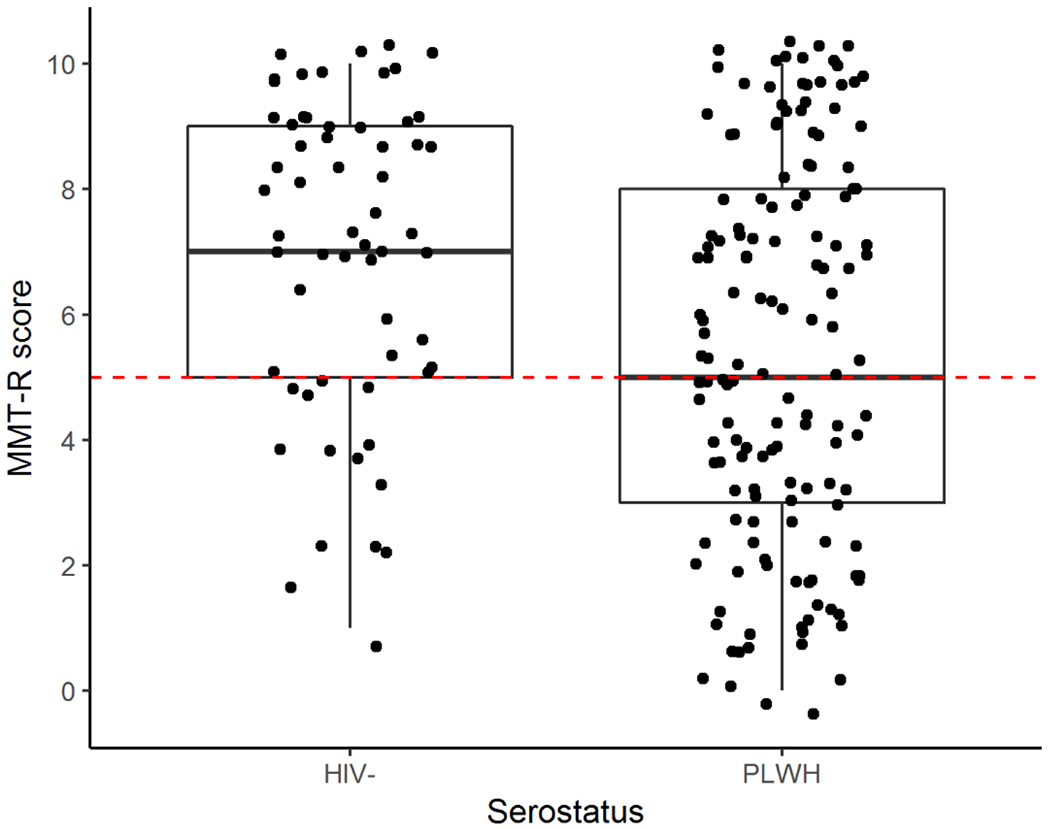
Average MMT-R scores were lower in PLWH than in HIV- controls. The dotted line indicates the cutoff (<5) for classifying failure on the MMT-R.
Predictors of MMT-R failure
The hierarchical binary logistic regression analysis revealed several significant and trend-level predictors of failure on the MMT-R. The final model retained HIV status as a predictor (odds ratio [OR]=2.71, 95% confidence interval [CI] = 1.0-7.3; p=.048). Additionally, lower reading level (OR=1.07, CI= 1.0-1.1; p=.04), non-Caucasian race (OR=0.17, CI=0.06-0.5; p=.001), and poorer executive function (OR=2.3, CI=1.1-4.8; p=.02) emerged as significant predictors of failure on the MMT-R. A follow-up regression model that included HIV status and the significant predictors did not reveal any significant interaction effects (p-values >.05).
Associations between HIV variables and MMT-R performance in PLWH
With regards to HIV clinical variables, only undetectable viral load (≤50 copies/mL) (p=0.01) and Hep C (p=.0048) were significantly different between PLWH who failed or passed the MMT-R. Older PLWH who failed the MMT-R were more likely to have a detectable viral load (21% versus 7%) and Hep C co-infection (18% versus 7%) compared to older PLWH who passed the MMT-R.
Self-reported difficulty managing medications in older PLWH and HIV- controls
There was no significant difference in the proportion of HIV- controls (n=10; 17%) and PLWH (n=31; 21%) who self-reported difficulties in managing their own medications (p=0.50). When looking only at individuals who failed the MMT-R, this result did not change (p=0.86). Only two HIV- individuals (20%) and fourteen PWLH (23%) failed the MMT-R and self-reported difficulties in managing medications.
Predictors of self-reported difficulty managing medications
The hierarchical logistic regression analysis retained lower education (OR=0.81, CI=0.67-0.97; p=0.03), a higher number of daily pills (OR=1.1, CI=1.0-1.16; p=0.03), and worse self-reported prospective memory (OR=1.2, CI=1.1-1.2; p<0.001) as predictors of self-reported difficulty in managing medications. HIV status was not included in the final model, and none of the interaction terms including HIV status were significant (all p-values >0.05). Additionally, MMT-R performance was not retained in the model (p>0.05).
Associations between HIV variables and self-reported difficulty managing medications
There were no significant relationships between self-reported difficulty in managing medications and any of the HIV variables (all p-values >0.05), although an association between self-reported difficulties and detectable viral load was observed at a trend level (p=0.07).
Pill burden and undetectable viral load
PLWH with a detectable viral load (>50 copies/mL) reported a significantly higher daily pill burden (M=10.8, SD=5.1) compared to individuals with an undetectable viral load (M=8.1, SD=4.7) (p=.02; Cohen’s d=.55).
Discussion
The present study compared medication management ability on a commonly-used objective measure between older PLWH and HIV- individuals, predictors of MMT-R performance and interactions with HIV status. We also compared MMT-R performance to self-reported medication management ability. Failure rate on the MMT-R was significantly higher in older PLWH compared to similarly-aged HIV- individuals (42% versus 17%, respectively). The final regression model retained HIV status as a predictor of MMT-R performance and identified non-Caucasian race, lower reading level and worse executive function as significant predictors. A higher rate of detectable viral load was associated with failure on the MMT-R in PLWH. Importantly, MMT-R performance and self-reported difficulty managing medications were not significantly related in older PLWH or HIV- individuals.
The MMT was developed to serve as an objective measure of functional ability in PLWH. However, it is difficult to fully interpret performance and presence of functional deficits without inclusion of a healthy comparison group. Our study aimed to fill this gap and confirmed that older PLWH performed significantly worse on the MMT-R and are more likely to have difficulties managing their medications compared to age-matched HIV- individuals. Given that older PLWH also report a higher daily pill burden (polypharmacy) compared to older HIV- individuals, it is important for clinicians to recognize that PLWH may require additional attention and time to ensure that they can adequately manage their own medication regimen.
Executive functioning was identified as a significant predictor of the objective measure of medication management (MMT-R). Our results complement previous studies that have identified cognitive performance as an important predictor of MMT-R performance in PLWH (Albert et al., 1999; Heaton et al., 2004; Patton et al., 2012; Thames et al., 2013). Conversely, self-reported prospective memory was a significant predictor for self-reported difficulty in managing medications. Prospective memory, or memory for future events has strong face validity in the context of medication management skills and has been reported to uniquely contribute to self-reported medication management ability in both PLWH (Woods et al., 2008) and older HIV- individuals (Woods et al., 2014). Interestingly, prospective memory has been reported to strongly correlate with, or classify as, a measure of executive function (Marsh and Hicks 1998; Martin et al., 2003) and both cognitive processes are heavily dependent on frontal networks (Burgess et al., 2003). Therefore, results from the present study indicate that while similar cognitive processes predict objective and self-reported medication management ability, self-rated cognitive ability did not associate with the objective measure of medication management and vice versa. These results indicate that older individuals are not likely to accurately describe their ability to manage their prescribed medication regimens. Similarly, performance on the objective measure of medication management (MMT-R) did not correspond to self-reported medication management ability. These results complement those in a study by Thames et al., 2011a who reported a similar lack of relationship between the MMT-R and self-reported medication management ability in a smaller group of slightly younger PLWH with more severe disease (n=8). Accuracy of self-reported functional ability is particularly important in PLWH, as ability to perform activities of daily living (often collected through self-report) is the distinguishing factor between a diagnosis of asymptomatic neurocognitive impairment and mild neurocognitive disorder under the HIV-associated neurocognitive disorder Frascati criteria (Antinori et al., 2007).
An important result of the present study was the significantly higher rate of unsuppressed viremia in older PLWH who failed the MMT-R. PLWH with a detectable viral load also reported a higher number of daily pills compared to PLWH with undetectable viral load. Anecdotally, the PLWH sample in the current study received care from a single clinic that stresses the importance of adherence to, and prioritization of, HIV medications. Future studies are needed to understand if individuals with higher pill burden select adherence to non-HIV medications versus their cART regimen. Additionally, a careful review and application of the American Geriatrics Society Beers criteria® (The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel, 2019) by clinicians may help reduce the number of potentially inappropriate medications older individuals are prescribed, thereby reducing daily pill burden.
It is important to note that although the MMT-R is widely used as a proxy measure of medication management ability, we did not observe participants’ real-world medication adherence. Therefore, any link made between the MMT-R or self-report measure and an individual’s actual compliance with their prescribed medications in this study is speculative. The literature is currently unclear as to whether the MMT-R accurately reflects real-world adherence (Albert et al., 1999; Patton et al., 2012). Additionally, the self-report measure has not yet been validated in an HIV population to our knowledge. Future studies are needed to assess the accuracy of the MMT-R or the self-report measures in assessing real-world medication adherence in older PLWH and HIV- individuals in order to draw more direct links between these measures, daily medication-taking practices, and viral suppression. Evaluating the MMT-R in a larger HIV- sample may also be beneficial in increasing the generalizability of our results to the healthy adult population as a whole. Finally, the current study is cross-sectional and subject to the inherent biases of this type of study design. Future work could focus on examining early and mid-life predictors of suboptimal medication management ability and adherence in older adults to inform interventions designed to improve adherence.
The ability to effectively manage medications has grown increasingly important as PLWH develop comorbidities requiring further medications in addition to their cART regimen. Clinicians or researchers who rely on patient self-report of medication management ability may fail to identify a significant proportion of PLWH who experience difficulty self-managing their medications. Screening older PLWH for cognitive impairment, particularly executive dysfunction, may help clinicians identify individuals who may have difficulty adhering to their medication regimen. Overall, results from the present study emphasize the need for objective measurements of medication management, particularly for older PLWH who experience a high pill burden.
Acknowledgements
Support for this work was provided by NIH-National Institute of Nursing Research (NINR) - R01-NR012907, R01-NR012657, and R01-NR014449. Research was conducted and supported by the Washington University Institute of Clinical and Translational Sciences (UL-TR000448 from the National Center for Advancing Translational Sciences).
Conflicts of interest and source of funding: Support for this work was provided by NIH-National Institute of Nursing Research (NINR) - R01-NR012907, R01-NR012657, and R01-NR014449. Research was conducted and supported by the Washington University Institute of Clinical and Translational Sciences (UL-TR000448 from the National Center for Advancing Translational Sciences). There are no conflicts of interest.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Albert SM, Weber CM, Todak G et al. AIDS Behav (1999) 3: 121 10.1023/A:1025483806464 [DOI] [Google Scholar]
- 2.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, …, Wojna VE Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69(18), 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 4.Burgess PW, Scott SK, Frith CD (2003). The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia, 47(8), 906–918. [DOI] [PubMed] [Google Scholar]
- 5.Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gómez FJ, Compaired-Turlán V, & Rabanaque-Hernández MJ (2016). Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clinical interventions in aging, 77, 1149–57. doi: 10.2147/CIA.S108072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene M, lustice AC, Lampiris HW, Valcour V (2013). Management of human immunodeficiency virus in advanced age. JAMA, 509(13), 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene M, Steinman MA, McNicholl IR, Valcour V (2014). Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older HIV-infected individuals. JAm Geriatr Soc., 62(3), 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaton R, Marcotte T, Mindt M, Sadek I, Moore D, Bentley H, … Grant I (2004). The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society, 10(3), 317–331. doi: 10.1017/S1355617704102130 [DOI] [PubMed] [Google Scholar]
- 9.Marsh RL, Hicks JL (1998). Event-based prospective memory and executive control of working memory. Journal of Experimental Psychology: Learning, Memory and Cognition. 24(2), 336–349. [DOI] [PubMed] [Google Scholar]
- 10.Martin M, Kliegel M, McDaniel MA (2003). The involvement of executive functions in prospective memory performance of adults. International Journal of Psychology, 38(A), 195–206. [Google Scholar]
- 11.Patton DE, Woods SP, Franklin D, Cattie JE, Heaton RK, Collier AC, Marra C, Clifford D, Gelman B, McArthur J, Morgello S, Simpson D, McCutchan JA, … Grant I (2012). Relationship of Medication Management Test-Revised (MMT-R) performance to neuropsychological functioning and antiretroviral adherence in adults with HIV. AIDS and behavior, 76(8), 2286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul RH, Cooley SA, Garcia-Egan PM, Ances BM. (2018). Cognitive performance and frailty in older HIV-positive adults. JAIDS, 79(3), 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabin CA. (2013). Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med., 11, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith GV, Della Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory. 2000;8:311–321. [DOI] [PubMed] [Google Scholar]
- 15.Thames AD, Becker BW, Marcot TD, … , Hinkin CH (2011a). Depression, cognition, and self-appraisal of functional abilities in HIV: An examination of subjection appraisal versus objective performance. The Clinical Neuropsychologist, 25, 2, 224–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thames AD, Kim MS, Becker BW, …, Hinkin CH (2011b) Medication and finance management among HIV-infected adults: The impact of age and cognition, Journal of Clinical and Experimental Neuropsychology, 33:2, 200–209, DOI: 10.1080/13803395.2010.499357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thames AD, Arentoft A, Rivera-Mindt M, Hinken CH (2013). Functional disability in medication management and driving among individuals with HIV: a 1-year follow-up study. Journal of Clinical and Experimental Neuropsychology, 35, 1, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGs Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society, 10.1111/igs.15767. [DOI] [PubMed]
- 19.Wilkinson GS, Robertson GJ. WRAT4: Wide Range Achievement Test professional manual. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 20.Woods SP, Moran LM, Carey CL, …, The HIVNeurobehavioral Research Center (HNRC) Group. (2008). Prospective memory in HIV infection: is “remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology, 23, 3, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods SP, Weinborn M, Maxwell BR, Gummery A, Mo K, Ng ARJ, Bucks RS (2014). Event-based prospective memory is independently associated with self-report of medication management in older adults. Aging and mental health, 18, 6, 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]


