Abstract
Background:
Individuals with chronic inflammatory diseases (CIDs) are at elevated risk for cardiovascular diseases, but data are limited regarding risk for heart failure (HF).
Objectives:
We compared the risks of incident HF among a variety of CIDs, and to determine whether risks varied by severity of inflammation within each CID.
Methods:
We analyzed an electronic health records database from a large urban medical system and compared individuals with CIDs vs. frequency-matched controls without CIDs, all of whom were in regular outpatient care. We determined rates of incident HF using the Kaplan-Meier method, and subsequently used multivariable-adjusted proportional hazards models to compare HF risks for each CID. Exploratory analyses determined HF risks by proxy measures of CID severity.
Results:
Of 37,636 patients (n=18,278 with CIDs, n=19,358 controls without CIDs), there were 960 incident HF cases over a median 3.6 years. We observed elevated risks for incident HF among patients with systemic sclerosis [hazard ratio (HR) 7.26, 95% confidence interval (CI) 5.72–9.21, p<0.01], systemic lupus erythematosus (SLE) (HR 3.15, 95% CI 2.41–4.11, p<0.01), rheumatoid arthritis (HR 1.39, 95% CI 1.13–1.71, p<0.01), and human immunodeficiency virus (HIV) (HR 1.28, 95% CI 0.99–1.66, p=0.06). There was no association of psoriasis or inflammatory bowel disease with incident HF, although patients with these CIDs with higher C-reactive protein levels had higher HF risks than controls.
Conclusions:
Systemic sclerosis and SLE were associated with the highest risks of HF, followed by rheumatoid arthritis and HIV. Measures of inflammation were associated with HF risk across different CIDs.
Keywords: chronic inflammatory diseases, autoimmune disorders, inflammation, heart failure, electronic cohort
Introduction
Individuals with chronic inflammatory diseases (CIDs) have elevated risks for atherosclerotic cardiovascular disease (ASCVD) (1–11). The American College of Cardiology (ACC) and American Heart Association (AHA) recognize CIDs – including psoriasis, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and human immunodeficiency virus (HIV) – as ASCVD risk-enhancing factors (12), but fewer data exist regarding heart failure (HF) in CIDs. Small studies have demonstrated elevated HF risks in persons with specific CIDs but used heterogeneous definitions of HF and generally considered CIDs in isolation (8,13–20).
We investigated risks for incident HF in several CIDs and non-CID controls. In addition to the CIDs listed above, we included systemic sclerosis (SSc) given known associations with pulmonary hypertension (21) and inflammatory bowel disease (IBD) given data suggesting heightened ASCVD risk (and perhaps HF risk) in IBDs (22–24). We hypothesized that people with CIDs have elevated risks for HF but that these risks vary by CID type and severity.
Methods
We created an electronic health record-based cohort of persons with CIDs and non-CID controls receiving regular outpatient care using the Northwestern Medicine Enterprise Data Warehouse (NMEDW), which stores clinical observations on >6.6 million people in a large urban medical system since 2000. For this study, we defined regular outpatient care as ≥1 CID specialty and/or primary care outpatient visit at least once every two years between baseline (the first in-person outpatient encounter) and censoring dates (death, HF, or most recent in-person outpatient encounter at least one year after baseline date). Highlighting the external validity of the NMEDW, our previous investigations of HIV-associated CVDs in the NMEDW yielded findings comparable to those of multicenter US HIV cohorts (25–29). The cohort creation and research protocol was approved by the institutional review board at Northwestern University (Chicago, IL, USA).
We first identified adults 18 years and older with CIDs during the period of observation from 1/1/2000–1/1/2019 using the following validated criteria: two or more International Classification of Diseases-9 (ICD-9) or ICD-10 diagnosis codes within a two year period for SSc (30), IBD (31–34), and psoriasis (35,36). SLE required three diagnosis codes in three separate months as described previously (37,38). RA required two diagnosis codes and a prescription for a disease-modifying anti-rheumatic drug as defined previously (39–41). HIV was defined as previously (42) based on serology, plasma HIV RNA (viral load), and/or at least three instances in which both HIV viral load and CD4 T lymphocyte cell count (CD4) were ordered on the same date. Non-CID controls were frequency-matched with CID patients on age, sex, insurance status, baseline year, and baseline presence/absence of hypertension and/or diabetes.
Data regarding demographics and insurance were obtained from most recent clinical visits. Baseline hypertension was defined using administrative codes (ICD-9 401 – 405 and ICD-10 I10 - I15) on any date prior to one year after the baseline date (43,44). Measured blood pressure values were not used to define hypertension given the heterogeneity of visit types and potential for systematic differences in measurement across exposure groups (45,46). Baseline diabetes was defined using validated ICD-9 or ICD-10 administrative codes (ICD-9 250 and ICD-10 E10 - E11, E13) and either hemoglobin A1c > 6.5% or prescription of antidiabetic medications on any date prior to one year after the baseline date (47). Coronary heart disease (CHD) was identified by validated code definitions for myocardial infarction, angina, prior percutaneous coronary intervention, or other ischemic heart disease which have high levels of agreement with expert chart review (48–51). Chronic kidney disease (CKD) was defined by ICD-10 codes (N18 – N19). Smoking and alcohol have high rates of missingness in NMEDW and were therefore not included as covariates. Baseline total cholesterol and low-density lipoprotein-cholesterol were obtained from measurements taken closest to baseline, within one year of baseline. Body-mass index (BMI) was calculated from the closest-to-baseline concurrent height and weight measurements. Deaths were confirmed by electronic health record chart review and linked social security death index.
Incident HF was the primary outcome in this study and defined using validated inpatient or outpatient diagnosis codes (52–54). HF events coded within the first year after baseline were considered baseline and not incident diagnoses to minimize the potential for misclassification of prevalent but not-yet-recorded HF as incident HF. Right heart failure (RHF) was defined among people with HF if they had ICD-9 diagnosis codes for cor pulmonale (416.9, 415.0) or ICD-10 codes for RHF (I50.81) or biventricular failure (I50.82). We excluded patients with baseline HF and patients with missing baseline demographic data. Follow-up time was defined as the time between baseline date and the earliest of incident HF, death, and most recent face-to-face encounter through January 1st, 2019.
For statistical analyses, we first constructed cumulative HF incidence curves for the CID subtypes stratified by age and sex, then used Cox proportional hazards models to analyze associations of CIDs with incident HF. Non-CID controls were the reference group. Models were constructed with specific CIDs as the exposures, with multivariable adjustment for age, sex, race, insurance status, hypertension, and diabetes in Model 1, additional adjustment for CKD in Model 2, and adjustment additionally for baseline and interim CHD in Model 3. Sensitivity analyses requiring two administrative code-based HF diagnosis codes were also performed to determine whether a less sensitive but more specific definition of HF yielded differing results.
For secondary analyses, we used peak C-reactive protein (CRP) as a proxy for CID-inflammation severity and determined risks for HF by CRP tertiles (3,55). For people with HIV (PWH), we used nadir CD4 as a marker of immune progression/disease severity given known associations of lower CD4 with HF among PWH (18,44). These analyses were limited to patients who had CRP levels (for all CIDs except HIV) or CD4 count (for HIV) measured.
Two-sided p-values < 0.05 were considered significant. All analyses were performed using R (The R Foundation for Statistical Computing) version 3.5.1.
Results
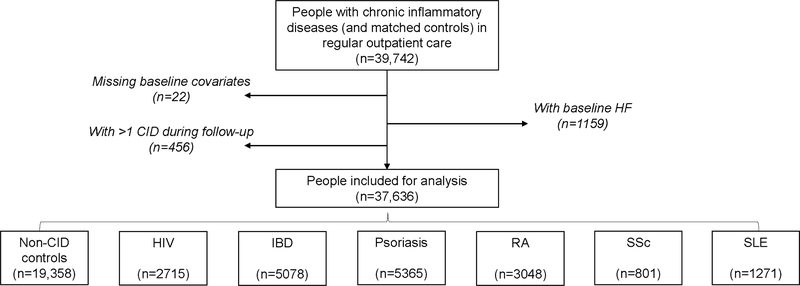
Of 39,742 people with CIDs and frequency-matched non-CID controls in regular outpatient care, we excluded 1159 with HF at baseline, 22 with missing covariates, and 456 with >1 CID (Figure 1). Demographics and clinical covariates were consistent with known distributions for CIDs (Table 1) (56). Patients with RA and SSc were generally older and PWH younger; >80% of patients with RA, SSc, and SLE were women whereas the opposite was true for PWH. The baseline prevalence in all CID groups of CHD was <5% and of diabetes was <10%. Hypertension and CKD were particularly common among people with SLE.
Figure 1. Cohort creation flow diagram.
Persons with chronic inflammatory diseases and controls matched 1:1 based on demographics, baseline year, hypertension, and diabetes were analyzed after excluding persons with baseline heart failure and those with missing baseline co-variates. HF = Heart Failure; HIV = human immunodeficiency virus; IBD = inflammatory bowel disease; Pso = psoriasis; RA = rheumatoid arthritis; SSc = systemic sclerosis; SLE = systemic lupus erythematosus.
Table 1:
Baseline Characteristics of each chronic inflammatory disease (CID) group and the control group
| CID Group | |||||||
|---|---|---|---|---|---|---|---|
| None (n=19358) | HIV (n=2715) | IBD (n=5078) | Pso (n=5365) | RA (n=3048) | SSc (n=801) | SLE (n=1271) | |
| Age, years | 48.60 (16.65) | 42.53 (11.40) | 44.55 (16.49) | 49.72 (15.95) | 55.92 (14.60) | 52.73 (13.37) | 43.23 (14.76) |
| Sex, F, n (%) | 11100 (57.3) | 415 (15.3) | 2890 (56.9) | 2774 (51.7) | 2475 (81.2) | 672 (83.9) | 1142 (89.9) |
| Race, n (%) | |||||||
| White | 12776 (66.0) | 1207 (44.5) | 4063 (80.0) | 4081 (76.1) | 1855 (60.9) | 521 (65.0) | 569 (44.8) |
| Black | 2165 (11.2) | 885 (32.6) | 292 (5.8) | 165 (3.1) | 426 (14.0) | 71 (8.9) | 339 (26.7) |
| Hispanic | 1370 (7.1) | 253 (9.3) | 204 (4.0) | 313 (5.8) | 342 (11.2) | 73 (9.1) | 163 (12.8) |
| Asian | 857 (4.4) | 48 (1.8) | 95 (1.9) | 192 (3.6) | 107 (3.5) | 24 (3.0) | 72 (5.7) |
| Other | 2190 (11.3) | 322 (11.9) | 424 (8.3) | 614 (11.4) | 318 (10.4) | 112 (14.0) | 128 (10.1) |
| Insurance, n(%) | |||||||
| Medicaid | 1002 (5.2) | 222 (8.2) | 168 (3.3) | 205 (3.8) | 133 (4.4) | 40 (5.0) | 115 (9.0) |
| Medicare | 4775 (24.7) | 369 (13.6) | 989 (19.5) | 1243 (23.2) | 1183 (38.8) | 231 (28.8) | 324 (25.5) |
| Private | 9760 (50.4) | 978 (36.0) | 2993 (58.9) | 3097 (57.7) | 1333 (43.7) | 357 (44.6) | 631 (49.6) |
| Self-pay | 3821 (19.7) | 1146 (42.2) | 928 (18.3) | 820 (15.3) | 399 (13.1) | 173 (21.6) | 201 (15.8) |
| BMI, kg/m3 | 27.76 (6.44) | 26.55 (5.71) | 26.31 (6.08) | 29.27 (6.93) | 28.63 (7.13) | 25.90 (6.14) | 28.14 (7.80) |
| HTN, n(%) | 3983 (20.6) | 296 (10.9) | 662 (13.0) | 1217 (22.7) | 770 (25.3) | 121 (15.1) | 362 (28.5) |
| DM, n(%) | 1441 (7.4) | 147 (5.4) | 232 (4.6) | 452 (8.4) | 291 (9.5) | 29 (3.6) | 92 (7.2) |
| CHD baseline, n(%) | 772 (4.0) | 67 (2.5) | 124 (2.4) | 191 (3.6) | 130 (4.3) | 36 (4.5) | 59 (4.6) |
| CKD, baseline, n(%) | 486 (2.5) | 146 (5.4) | 116 (2.3) | 128 (2.4) | 111 (3.6) | 35 (4.4) | 232 (18.3) |
| SBP, mmHg | 123.50 (17.05) | 125.80 (17.20) | 121.24 (16.14) | 126.53 (16.97) | 125.75 (17.26) | 118.75 (17.71) | 121.46 (16.81) |
| TC, mg/dL | 187.36 (40.10) | 173.47 (41.86) | 176.50 (43.40) | 185.80 (39.71) | 182.81 (39.10) | 175.26 (42.12) | 173.20 (42.49) |
| LDL-c, mg/dL | 109.42 (36.53) | 101.46 (33.36) | 100.39 (36.21) | 106.95 (34.29) | 100.78 (31.90) | 100.22 (31.08) | 97.47 (36.44) |
Notes: HIV = human immunodeficiency virus; IBD = inflammatory bowel disease; Pso = psoriasis; RA = rheumatoid arthritis; SSc = systemic sclerosis; SLE = systemic lupus erythematosus. BMI = body mass index; CHD = coronary heart disease; CKD = chronic kidney disease; DM = diabetes mellitus; F = female; HTN = hypertension; LDL = low-density lipoprotein-cholesterol; SBP = systolic blood pressure; TC = total cholesterol.
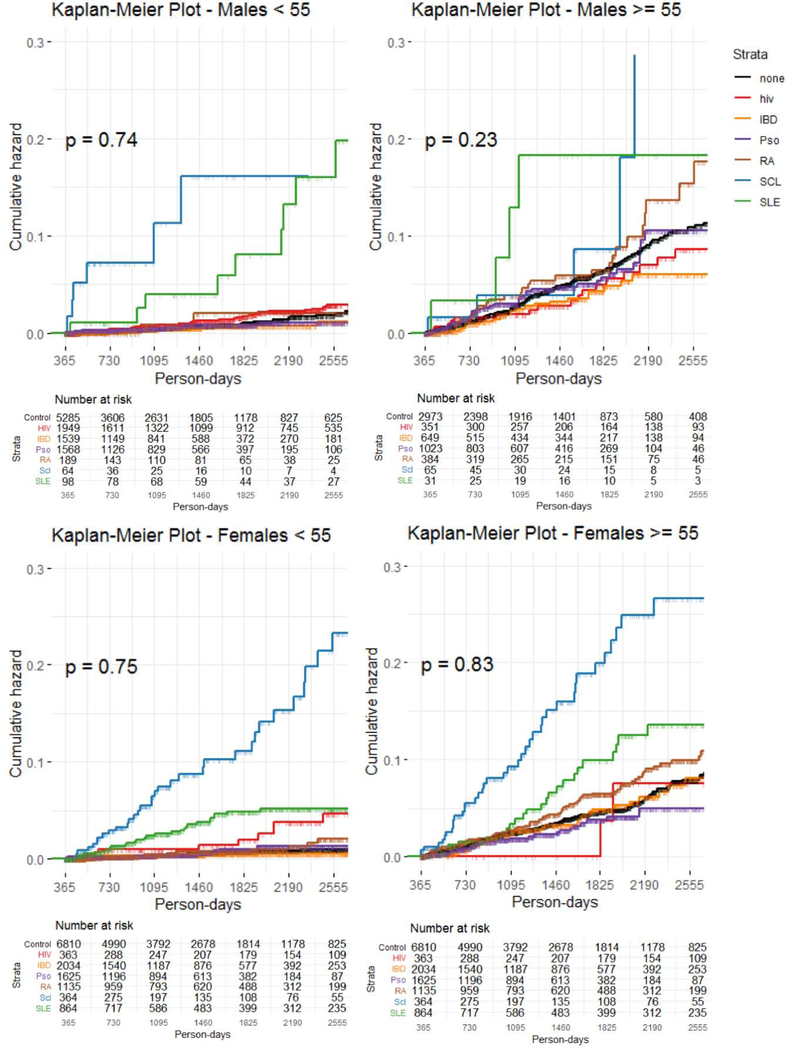
There were 1008 incident HF events over a median follow-up of 3.6 years. Proportions of each CID group with HF during follow-up are shown in Table 2. Descriptive cumulative incidence curves for incident heart failure in each CID group, stratified by age and sex, are shown in Figure 2 (A–D). Patients with SSc had the highest incidence of HF in every subgroup, followed by SLE, RA, and HIV.
Table 2:
Frequency and median time to incident heart failure (HF) and death for each CID group and the control group
| CID group | N | Incident HF (%) | HF Incidence Rate (event/1000 person-years) | Median time to HF (days) | Death (%) | Median time to death (days) |
|---|---|---|---|---|---|---|
| Control | 19,358 | 436 (2.25%) | 0.598 | 1272 | 22 (0.11%) | 1086.5 |
| HIV | 2,715 | 79 (2.91%) | 0.488 | 1827 | 16 (0.59%) | 2347 |
| IBD | 5,078 | 73 (1.44%) | 0.749 | 1092 | 6 (0.12%) | 838 |
| Pso | 5,365 | 91 (1.7%) | 0.940 | 891 | 2 (0.04%) | 776.5 |
| RA | 3,048 | 124 (4.07%) | 0.713 | 1263 | 6 (0.2%) | 1019 |
| SSc | 801 | 87 (10.86%) | 0.822 | 1077 | 2 (0.25%) | 1043.5 |
| SLE | 1,271 | 70 (5.51%) | 0.735 | 1211.5 | 7 (0.55%) | 1596 |
Abbreviations as in Table 1.
Figure 2. Heart failure cumulative incidence among chronic inflammatory disease groups.
Kaplan-Meier cumulative incidence curves for incident heart failure by chronic inflammatory disease group over median follow-up period of 3.6 years. Curves presented for demographic subsets. CID = chronic inflammatory disease; other abbreviations as in Figure 1.
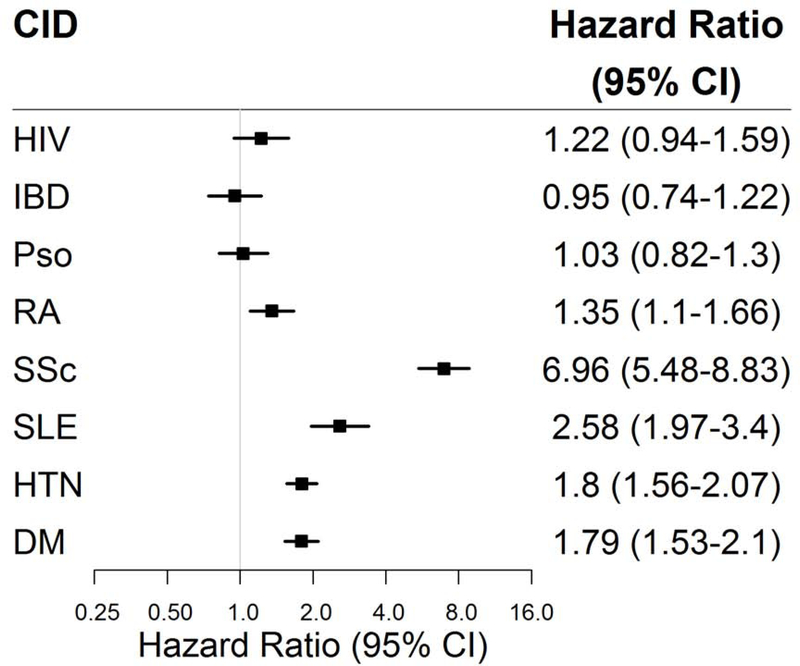
After adjustment for age, sex, race, insurance status, and baseline diabetes and hypertension (Model 1, Central Illustration), we observed significantly elevated risks for incident HF among patients with SSc [hazard ratio (HR) 7.25, 95% CI 5.71–9.21, p<0.001], SLE (HR 3.15, 95% CI 2.41–4.11, p<0.001), and RA (HR 1.39, 95% CI 1.13–1.71, p<0.001). PWH had borderline significantly elevated HF risk (HR 1.28, 95% CI 0.99–1.66, p=0.06). By comparison, hazard ratios for HF in people with hypertension or diabetes at baseline were 1.93 and 1.91, respectively (p<0.05 for both). There was no significant association of psoriasis or IBD with HF. Findings were largely unchanged after additional adjustment for CKD (Model 2, Central Illustration) and CHD (Online Figure 1), and in sensitivity analyses with different administrative code-based HF definitions (Supplemental File 1).
Central Illustration. Heart failure risk among chronic inflammatory disease groups.
Adjusted cox proportional hazard ratios (square markers) and 95% confidence intervals (error bars) for incident heart failure for each chronic inflammatory disease group are presented in forest plots after adjustment for (A) age, sex, race, insurance, hypertension, diabetes (Model 1) and (B) additional adjustment for chronic kidney disease (Model 2). CID = chronic inflammatory disease; other abbreviations as in Figure 1.
We further phenotyped people who developed HF based on left ventricular size and ejection fraction (EF), presence of RHF, medication use, and peak B-type natriuretic peptide (BNP) (Table 3). The predominant HF phenotype in SSc and RA was heart failure with preserved ejection fraction (HFpEF) and RHF was present in a far higher proportion of SSc patients with RHF than other CIDs. Patients with SLE had equal numbers of heart failure with reduced ejection fraction (HFrEF) and HFpEF. For psoriasis and RA, although there were not heightened risks of HF compared to controls, HFpEF was the predominant phenotype among those who developed HF. Although PWH with HF had, on average, the most severe phenotypes (lowest LVEF, most LV dilation, and highest BNPs), they were by far the least likely to be taking angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers and among the least likely to be taking beta blockers.
Table 3:
Heart Failure Phenotypic Characteristics
| None (n = 436) | Hiv (n = 79) | IBD (n = 73) | Pso (n = 91) | RA (n = 124) | SSc (n = 87) | SLE (n = 70) | |
|---|---|---|---|---|---|---|---|
| Type (%) | |||||||
| Reduced EF (< 40) | 113 (25.9) | 33 (41.8) | 12 (16.4) | 18 (19.8) | 19 (15.3) | 10 (11.5) | 25 (35.7) |
| Borderline EF (40 to 50) | 78 (17.9) | 20 (25.3) | 13 (17.8) | 15 (16.5) | 24 (19.4) | 16 (18.4) | 17 (24.3) |
| Preserved EF (> 50) | 221 (50.7) | 25 (31.6) | 39 (53.4) | 50 (54.9) | 67 (54.0) | 59 (67.8) | 27 (38.6) |
| Missing EF | 24 (5.5) | 1 (1.3) | 9 (12.3) | 8 (8.8) | 14 (11.3) | 2 (2.3) | 1 (1.4) |
| Lowest LVEF (mean (SD)) | 47.71 (14.53) | 41.62 (14.45) | 50.23 (13.19) | 49.74 (14.38) | 50.06 (14.13) | 51.76 (11.61) | 43.22 (14.52) |
| Highest LVEDD (mean (SD)) | 4.92 (0.77) | 5.40 (0.77) | 4.83 (0.60) | 4.94 (0.73) | 4.86 (0.80) | 4.66 (0.64) | 5.11 (0.72) |
| Right Heart Failure (%)* | 35 (8.0) | 2 (2.5) | 2 (2.7) | 6 (6.6) | 12 (9.7) | 27 (31.0) | 3 (4.3) |
| Used Beta Blocker (%) | 229 (52.5) | 35 (44.3) | 44 (60.3) | 58 (63.7) | 75 (60.5) | 21 (24.1) | 38 (54.3) |
| Used ACE I or ARB (%) | 227 (52.1) | 28 (35.4) | 32 (43.8) | 55 (60.4) | 78 (62.9) | 55 (63.2) | 42 (60.0) |
| Peak BNP (mean (SD)) | 768.34 (1,053.04) | 1415.81 (1,562.77) | 569.13 (918.40) | 570.58 (912.93) | 801.48 (1,117.61) | 817.06 (1,053.16) | 1566.40 (1,600.47) |
Notes: EF – ejection fraction,
- Right Heart Failure classification based on having the following diagnosis codes: ICD10: I50.810Right heart failure, unspecified, I50.811-Acute right heart failure, I50.812-Chronic right heart failure, I50.813-Acute on chronic right heart failure ICD10: I27.81-Cor pulmonale (chronic), I26.09 (other PE with acute cor pulmonale) ICD9: 415.0 (acute cor pulmonale), 416.9 (cor pulmonale (chronic)
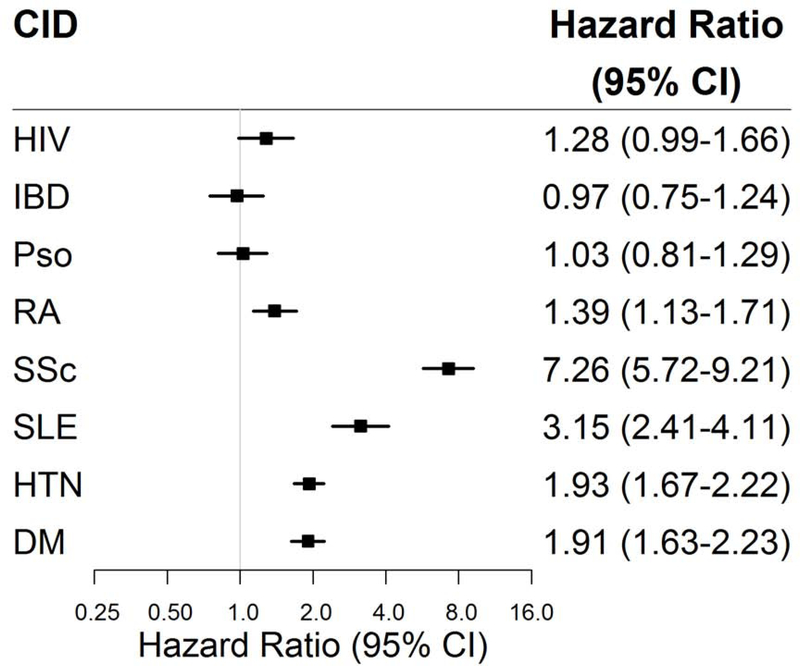
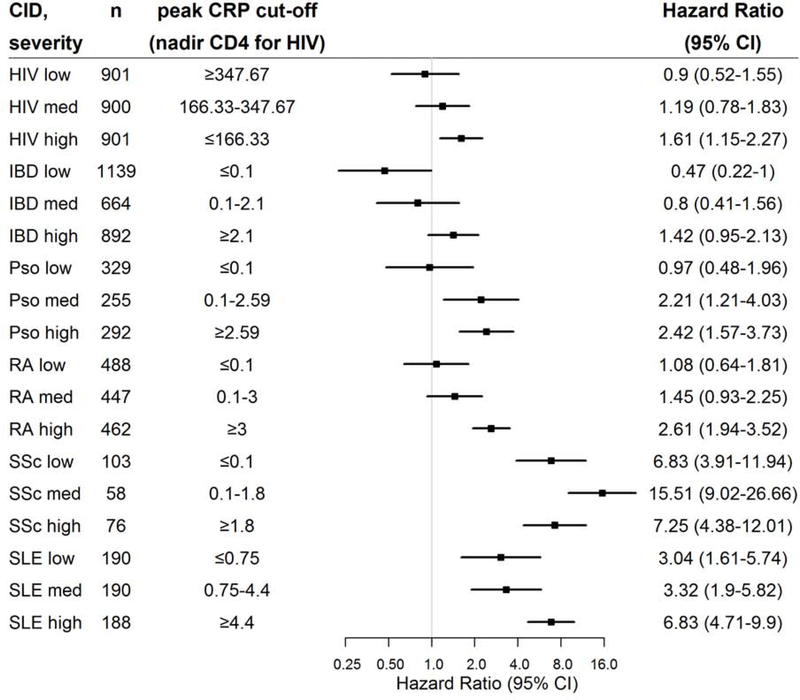
In exploratory analyses of patients with CRP measurements, there was a consistent, graded association within each CID whereby patients with higher CRP levels had higher HF risks. (Figure 3) Likewise, among PWH, those in the lowest tertile of nadir CD4 count had the highest HF risks.
Figure 3. Heart failure risk among chronic inflammatory disease (CID) groups stratified by severity of CID.
Adjusted cox proportional hazard ratios (square markers) and 95% confidence intervals (error bars) for incident heart failure (HF) for each CID group stratified by CID severity presented in a forest plot. CID severity defined by tertile of peak C-reactive protein level (and nadir CD4 for HIV). Adjusted for age, sex, race, insurance, hypertension, diabetes, and chronic kidney disease (Model 2). Abbreviations as in Figure 1. N for control = 19,358
Discussion
In this study, we quantified incident HF for patients with CIDs in a large urban medical system. We found that several CIDs were associated with elevated risks for HF, and the relative strength of association differed for different CIDs. SSc was associated with the highest risk for HF, followed by SLE, RA, and HIV. Higher inflammatory burden was associated with higher HF risk for all CIDs. Our findings are consistent with magnitudes of association observed in previous studies of single CIDs and HF (13,15–17,28), and provide further support to guidelines identifying psoriasis, RA, SLE, and HIV as risk-enhancing factors for CVD (12). SSc, SLE, RA, and HIV may warrant consideration for identification of ACC/AHA stage A heart failure (57).
Our findings underscore the importance of inflammation in HF and highlight the need for nuanced strategies to understanding, and potentially intervening upon, inflammatory triggers of HF. Inflammation is increasingly recognized as contributing to HF overall, and particularly HFpEF, through mechanisms such as microvascular dysfunction and endothelial function dysregulation leading to fibrosis and diastolic dysfunction (58). Our exploratory analyses show a clear, graded association between higher inflammatory burdens within each CID and higher risks for HF. The differences we observed in HF risk and phenotype across different CIDs reflect their complex and heterogeneous pathophysiologies. Therefore, while the role of systemic inflammation in HF is well-described (59,60) and higher systemic levels of chronic inflammation are associated with higher rates of incident HF and worse prognosis (61,62), reductionist approaches focusing on unified or specific inflammatory pathways may not adequately address diverse inflammatory pathophysiologies of HF. For instance, SSc may impart particularly elevated risk for HF due to coronary microvascular dysfunction and myocardial fibrosis (63). Additionally, there is an increased risk of Group I pulmonary hypertension leading to RHF in SSc. In our cohort, approximately a third of the HF patients had RHF in the SSc group, which was by far the highest proportion of RHF observed in any of the groups. In addition to the SSc group, we observed considerable heterogeneity in HF phenotypes and treatment across CID groups. For instance, although PWH had the most severe presentations of HF, on average (as indicated by lowest LVEF, highest LVEDD, and highest BNP), they were actually the least likely to be taking common HF medications. Further investigations into pathogenesis of, and effective treatments for, CID-associated HF are essential.
This study should be interpreted in the context of its limitations. This was an electronic health record-based case-cohort study in a single large urban medical system of people in clinical care. We sought to address major confounders by (1) frequency-matching controls (the key comparison population for each CID with respect to incident HF) on demographics and baseline hypertension and diabetes, and (2) adjusting for additional potential confounders in multivariable analyses. We also sought to account for confounding by clinical visit frequency and follow-up by applying a standard definition for cases and controls of being in regular outpatient care throughout the follow up period. Nevertheless, selection bias and residual confounding remain possible. Substance use was not consistently documented so we were not able to adjust for alcohol and tobacco use, a clear limitation if people with specific CIDs have significantly higher prevalence of smoking than controls even after matching on demographics and clinical CVD risk factors (64). Given the size of the cohort and the lack of standardized patient-reported outcomes, we were unable to determine CID severity, relying on CRP as the next best proxy measure. Although CRP was only available in two-fifths of total patients with CIDs, demographics and CVD risk factors were similar between those with CRP measured and those without, suggesting some degree of generalizability to our larger study population. Overall, we deemed these limitations, which we sought to address where possible, acceptable because they allowed us to analyze a large group of people with diverse CIDs in a single set of analyses, enabling meaningful description of relative HF risks.
Conclusions
People with SSc, SLE, and RA had significantly higher risks for incident HF compared with controls without CIDs, and this association was borderline significant for PWH. Higher levels of inflammatory biomarkers were associated with higher HF risks than observed among non-CID controls. Further studies are necessary to define the diverse pathophysiologies underlying HF in these different CIDs.
Supplementary Material
Adjusted cox proportional hazard ratios (square markers) and 95% confidence intervals (error bars) for heart failure for each group are presented in forest plots after adjustment for age, sex, race, insurance, hypertension, diabetes, chronic kidney disease, and coronary heart disease (Model 3). CID = chronic inflammatory disease; other abbreviations as in Figure 1.
File 1 – Results of Different Cox Proportional Hazard Models in Main and Sensitivity Analyses
Clinical Perspectives.
Competency in Medical Knowledge 1:
Systemic sclerosis, systemic lupus erythematosus, rheumatoid arthritis, and human immunodeficiency virus are associated with an increased risk of heart failure, in order of decreasing magnitude.
Competency in Medical Knowledge 2:
Among persons with chronic inflammatory diseases, those with higher inflammatory burden have higher risk for heart failure than those with lower inflammatory burden.
Translational Outlook 1:
Additional research is needed to better understand heart failure pathophysiology in chronic inflammatory diseases.
Translational Outlook 2:
The elevated heart failure risk we observed among persons with systemic sclerosis requires confirmation.
Acknowledgements:
The authors thank the Northwestern Medicine Enterprise Data Warehouse for logistical support.
Funding: American Heart Association Fellow-to-Faculty Award (16FTF31200010; PI: Feinstein) National Institutes of Health (P30AI117943 and UL1TR001422)
Abbreviations:
- ASCVD
atherosclerotic cardiovascular disease
- CHD
coronary heart disease
- CID
chronic inflammatory disease
- HF
heart failure
- HIV
human immunodeficiency virus
- IBD
inflammatory bowel disease
- PWH
persons with HIV
- SLE
systemic lupus erythematosus
- SSc
systemic sclerosis
- RA
rheumatoid arthritis
Footnotes
Disclosures/Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Teague H, Mehta NN. The Link Between Inflammatory Disorders and Coronary Heart Disease: a Look at Recent Studies and Novel Drugs in Development. Curr Atheroscler Rep 2016;18:3. [DOI] [PubMed] [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Grodzicky T et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001;44:2331–7. [DOI] [PubMed] [Google Scholar]
- 3.Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014;130:837–44. [DOI] [PubMed] [Google Scholar]
- 4.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol 2011;27:174–82. [DOI] [PubMed] [Google Scholar]
- 5.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- 6.Westerweel PE, Luyten RK, Koomans HA, Derksen RH, Verhaar MC. Premature atherosclerotic cardiovascular disease in systemic lupus erythematosus. Arthritis Rheum 2007;56:1384–96. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein MJ, Bahiru E, Achenbach C et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. The American journal of cardiology 2016;117:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy 2015;70:1300–8. [DOI] [PubMed] [Google Scholar]
- 9.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol 2009;145:700–3. [DOI] [PubMed] [Google Scholar]
- 10.Man A, Zhu Y, Zhang Y et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013;72:1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol 2011;106:741–7. [DOI] [PubMed] [Google Scholar]
- 12.Arnett DK, Blumenthal RS, Albert MA et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CH, Al-Kindi SG, Jandali B, Askari AD, Zacharias M, Oliveira GH. Incidence and risk of heart failure in systemic lupus erythematosus. Heart 2017;103:227–233. [DOI] [PubMed] [Google Scholar]
- 14.Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: a focus on heart failure. Heart Fail Clin 2014;10:339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantel A, Holmqvist M, Andersson DC, Lund LH, Askling J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. Journal of the American College of Cardiology 2017;69:1275–1285. [DOI] [PubMed] [Google Scholar]
- 16.Logstrup BB, Ellingsen T, Pedersen AB, Kjaersgaard A, Botker HE, Maeng M. Development of heart failure in patients with rheumatoid arthritis: A Danish population-based study. Eur J Clin Invest 2018;48:e12915. [DOI] [PubMed] [Google Scholar]
- 17.Khalid U, Ahlehoff O, Gislason GH et al. Psoriasis and risk of heart failure: a nationwide cohort study. Eur J Heart Fail 2014;16:743–8. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein MJ, Steverson AB, Ning H et al. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. J Am Heart Assoc 2018;7:e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aniwan S, Pardi DS, Tremaine WJ, Loftus EV Jr. Increased Risk of Acute Myocardial Infarction and Heart Failure in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2018;16:1607–1615 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverwood RJ, Forbes HJ, Abuabara K et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ 2018;361:k1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt SA, Jeppesen JL, Torp-Pedersen C et al. Cardiovascular Manifestations of Systemic Sclerosis: A Danish Nationwide Cohort Study. J Am Heart Assoc 2019;8:e013405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunu D-M, Timofte C-E, Ciocoiu M et al. Cardiovascular Manifestations of Inflammatory Bowel Disease: Pathogenesis, Diagnosis, and Preventive Strategies. Gastroenterol Res Pract 2019;2019:3012509–3012509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aniwan S, Pardi DS, Tremaine WJ, Loftus EV Jr. Increased Risk of Acute Myocardial Infarction and Heart Failure in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2018;16:1607–1615.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen SL, Ahlehoff O, Lindhardsen J et al. Inflammatory bowel disease is associated with an increased risk of hospitalization for heart failure: a Danish Nationwide Cohort study. Circ Heart Fail 2014;7:717–22. [DOI] [PubMed] [Google Scholar]
- 25.Feinstein MJ, Steverson AB, Ning H, Pawlowski AE, Schneider D, Ahmad FS, Sanders JM, Sinha A, Nance RM, Achenbach CJ, Delaney JAC, Heckbert SR, Shah SJ, Hanna DB, Hsue PY, Bloomfield GS, Longenecker CT, Crane HM, Lloyd-Jones DM. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. Journal of the American Heart Association 2018;7:e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinstein MJ, Hsue PY, Benjamin LA et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019;140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders JM, Steverson AB, Pawlowski AE et al. Atrial arrhythmia prevalence and characteristics for human immunodeficiency virus-infected persons and matched uninfected controls. PLoS One 2018;13:e0194754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freiberg MS, Chang CH, Skanderson M et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu JC, Li Y, Marcus GM et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. Journal of the American College of Cardiology 2013;61:2288–95. [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela A, Yaqub A, Fiorentino D, Krishnan E, Chung L. Validation of the ICD-9-CM code for systemic sclerosis using updated ACR/EULAR classification criteria. Scand J Rheumatol 2015;44:253–5. [DOI] [PubMed] [Google Scholar]
- 31.Ma C, Moran GW, Benchimol EI et al. Surgical Rates for Crohn’s Disease are Decreasing: A Population-Based Time Trend Analysis and Validation Study. Am J Gastroenterol 2017;112:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thirumurthi S, Chowdhury R, Richardson P, Abraham NS. Validation of ICD-9-CM diagnostic codes for inflammatory bowel disease among veterans. Dig Dis Sci 2010;55:2592–8. [DOI] [PubMed] [Google Scholar]
- 33.Hou JK, Tan M, Stidham RW et al. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn’s disease in the Veterans Affairs Health Care System. Dig Dis Sci 2014;59:2406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Chun J, Han KD et al. Increased end-stage renal disease risk in patients with inflammatory bowel disease: A nationwide population-based study. World J Gastroenterol 2018;24:4798–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lofvendahl S, Theander E, Svensson A, Carlsson KS, Englund M, Petersson IF. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden--a population-based register study. PLoS One 2014;9:e98024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asgari MM, Wu JJ, Gelfand JM et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996–2009. Pharmacoepidemiol Drug Saf 2013;22:842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walunas TL, Jackson KL, Chung AH et al. Disease Outcomes and Care Fragmentation Among Patients With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2017;69:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus 2010;19:741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jafri K, Taylor L, Nezamzadeh M et al. Management of hyperlipidemia among patients with rheumatoid arthritis in the primary care setting. BMC Musculoskelet Disord 2015;16:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SY, Servi A, Polinski JM et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther 2011;13:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum 2004;51:952–7. [DOI] [PubMed] [Google Scholar]
- 42.Felsen UR, Bellin EY, Cunningham CO, Zingman BS. Development of an electronic medical record-based algorithm to identify patients with unknown HIV status. AIDS care 2014;26:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pace R, Peters T, Rahme E, Dasgupta K. Validity of Health Administrative Database Definitions for Hypertension: A Systematic Review. Can J Cardiol 2017;33:1052–1059. [DOI] [PubMed] [Google Scholar]
- 44.Teixeira PL, Wei WQ, Cronin RM et al. Evaluating electronic health record data sources and algorithmic approaches to identify hypertensive individuals. J Am Med Inform Assoc 2017;24:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali S, Rouse A. Practice audits: reliability of sphygmomanometers and blood pressure recording bias. J Hum Hypertens 2002;16:359–61. [DOI] [PubMed] [Google Scholar]
- 46.Rouse A, Marshall T. The extent and implications of sphygmomanometer calibration error in primary care. J Hum Hypertens 2001;15:587–91. [DOI] [PubMed] [Google Scholar]
- 47.Wilke RA, Berg RL, Peissig P et al. Use of an electronic medical record for the identification of research subjects with diabetes mellitus. Clin Med Res 2007;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quan H, Li B, Saunders LD et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008;43:1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel AB, Quan H, Welsh RC et al. Validity and utility of ICD-10 administrative health data for identifying ST- and non-ST-elevation myocardial infarction based on physician chart review. CMAJ Open 2015;3:E413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014;9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fishman E, Barron J, Dinh J et al. Validation of a claims-based algorithm identifying eligible study subjects in the ADAPTABLE pragmatic clinical trial. Contemp Clin Trials Commun 2018;12:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang H, Turner M, Raju S et al. Identification of Acute Decompensated Heart Failure Hospitalizations Using Administrative Data. Am J Cardiol 2017;119:1791–1796. [DOI] [PubMed] [Google Scholar]
- 53.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One 2014;9:e104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmad FS, Chan C, Rosenman MB et al. Validity of Cardiovascular Data From Electronic Sources: The Multi-Ethnic Study of Atherosclerosis and HealthLNK. Circulation 2017;136:1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danesh J, Whincup P, Walker M et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ 2000;321:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev 2003;2:119–25. [DOI] [PubMed] [Google Scholar]
- 57.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 58.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 59.Blum A, Miller H. Pathophysiological role of cytokines in congestive heart failure. Annu Rev Med 2001;52:15–27. [DOI] [PubMed] [Google Scholar]
- 60.Damas JK, Gullestad L, Aukrust P. Cytokines as new treatment targets in chronic heart failure. Curr Control Trials Cardiovasc Med 2001;2:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalogeropoulos A, Georgiopoulou V, Psaty BM et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. Journal of the American College of Cardiology 2010;55:2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of Inflammation in Heart Failure. Curr Atheroscler Rep 2017;19:27. [DOI] [PubMed] [Google Scholar]
- 63.Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 2009;48 Suppl 3:iii45–8. [DOI] [PubMed] [Google Scholar]
- 64.Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K. Tobacco use among people living with HIV: analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob Health 2017;5:e578–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adjusted cox proportional hazard ratios (square markers) and 95% confidence intervals (error bars) for heart failure for each group are presented in forest plots after adjustment for age, sex, race, insurance, hypertension, diabetes, chronic kidney disease, and coronary heart disease (Model 3). CID = chronic inflammatory disease; other abbreviations as in Figure 1.
File 1 – Results of Different Cox Proportional Hazard Models in Main and Sensitivity Analyses