Abstract
The gut microbial diversity of Thai people was investigated between two large cohorts, adult and elderly subjects, from the middle region of Thailand; the cohorts were divided into different age groups of healthy adult (73) and elderly subjects (47). The diversities of the groups were characterized using a pyrosequencing technique with primers targeting the V6–V8 region of the 16S rRNA gene, and a significant decrease in the Firmicutes and Bacteroidetes ratio from 7.3 to 4.5 was observed with increased age. The microbiota of the adult and elderly groups had a significantly higher abundance of the phylum Actinobacteria, including the three species Bifidobacterium adolescentis, Bifidobacterium longum and Bifidobacterium pseudocatenulatum, and the phylum Bacteroidetes containing the four species Bacteroides uniformis, Bacteroides ovatus, Bacteroides caccae and Bacteroides thetaiotaomicron. Firmicutes showed no significant differences between the two groups. Eleven species belonging to Firmicutes, Bacteroidetes and Proteobacteria were shared by at least 90% of all subjects and defined as core gut microbiota of healthy Thai, among which a high abundance of Escherichia coli was particularly characterized in Thai elderly individuals. Multiple linear regression analysis of age, gender, BMI and diet consumption frequency showed the correlation of age with Bacteroides and Bifidobacterium. Rice consumption frequency showed a significant positive correlation with Bacteroides, while no correlation was found for other factors. Taken together, in the gut of Thai adults, Bifidobacterium decreased and Bacteroides increased with age, while rice consumption increased the abundance of Bacteroides. These link of age and food, especially rice carbohydrate, to gut microbiota and health could be ultimately proposed as the Thai feature.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02265-7) contains supplementary material, which is available to authorized users.
Keywords: Gut microbiota, Core gut microbiome, Age-related gut microbial changes, Food consumption frequency
Introduction
The human gastrointestinal tract harbors the largest and most complex bacterial ecosystem which plays a key role in human health and well-being (Biagi et al. 2010; Power et al. 2013; Brüssow 2013). The intestinal microbiota of healthy individuals serve numerous important functions for the host, including pathogen protection, nutrient processing, host metabolism and immune modulation (Sekirov et al. 2010; Vrieze et al. 2010; Clemente Jose et al. 2012). The development of the human microbiota is a dynamic process throughout the different stages of human life. During the first years of life, the intestinal tract is inhabited by microbes with unstable dynamics (Favier et al. 2002). The human gut microbiota composition at 2–3 years of age further changes with the introduction of solid foods, causing a more complex and stable community similar to the composition of the adult microbiota (Favier et al. 2002; Palmer et al. 2007; Koenig et al. 2011). Although this ecosystem displays high stability in healthy humans, its composition undergoes individual variations (Tiihonen et al. 2010; Ottman et al. 2012; Power et al. 2013). The balance of the gut microbiota composition can be affected by physiological changes in the gastrointestinal tract during the aging process, as well as by modifications in lifestyle, nutritional behavior and the functionality of the host immune system (Bartosch et al. 2004; Mueller et al. 2006; Biagi et al. 2010; Zhao et al. 2011; Yatsunenko et al. 2012).
Several microbiota studies using different methods have aimed to describe the features of the aged gut microbiota (Hayashi et al. 2003; Bartosch et al. 2004; Mueller et al. 2006; Mariat et al. 2009; Claesson et al. 2012). Most common studies have shown decreasing numbers of strict anaerobes (bifidobacteria, bacteroides and clostridia) and increasing numbers of facultative anaerobes (streptococci, staphylococci, enterococci and enterobacteria) in elderly subjects (Hayashi et al. 2003; Bartosch et al. 2004; Mueller et al. 2006; Mariat et al. 2009). However, the differences in lifestyle and dietary habits of individuals from different areas can also affect the gut microbiota, particularly the dominant gut microbiota belonging to the Firmicutes and Bacteroidetes phyla (Mueller et al. 2006; Claesson et al. 2012). Clostridium cluster XIVa, a member of the Firmicutes phylum, was decreased in elderly subjects from Japan (Hayashi et al. 2003), Italy (Mueller et al. 2006; Biagi et al. 2010) and Finland (Mäkivuokko et al. 2009), while it was increased in elderly subjects from Germany (Mueller et al. 2006). The species Faecalibacterium prausnitzii (Costridium cluster IV), known for its anti-inflammatory properties and ability to produce short-chain fatty acids (SCFAs), was markedly decreased in Italian elderly and centenarian subjects (Mueller et al. 2006; Biagi et al. 2010). A decrease in both Clostridium cluster XIVa and F. prausnitzii was reported to correlate with frailty, hospitalization, antibiotic treatment and nonsteroidal anti-inflammatory therapy (Bartosch et al. 2004; Van Tongeren et al. 2005; Zwielehner et al. 2009; Tiihonen et al. 2010).
Thailand is a geographically diverse country divided into four regional parts—central, northern, southern and northeastern. Each region has its own lifestyle and eating habits, which are quite different from those of other countries. Thai food is based on a balance of different spices combined within an individual dish, which is mostly eaten with steamed rice and fresh vegetables. Such cuisine is very different from Western-style diets, which are high in fat/sugar and low in fiber (Conlon and Bird 2015). La-ongkham et al. (2015) recently reported that healthy Thai children (aged 8–11 years) from northeastern Thailand who had a significantly higher consumption frequency of meat and a wide variety of carbohydrate sources (noodle, fermented rice noodle and sweet potato), including vegetables and fruit, had a significantly higher abundance of lactobacilli, Clostridium coccoides-Eubacterium rectale, Clostridium leptum, Prevotella and Bacteroides fragilis than those from the central region who had a significant preference for Western-style diets such as breakfast cereal and cow milk. It seemed that the high frequency consumption of varieties of carbohydrates, protein sources, fruits and vegetables by northeastern children promoted a high abundance of bacterial species in the phyla Firmicutes and Bacteroidetes. Therefore, it was interesting to gain more information on the microbial community of two more generations, adult and elderly subjects, in Thailand who have their own lifestyle and dietary habits. Characterization of the gut microbiota composition of healthy elderly subjects was compared with younger adults from the middle part of Thailand using a high-throughput pyrosequencing technique. The core gut microbiome of healthy Thai subjects and the correlations among gut microbiota, age and dietary habits were also considered.
Materials and methods
Fecal samples
Total fecal samples were obtained from 73 adult (30–40 years) and 47 elderly (≥65 years) subjects from central Thailand. All subjects were phenotypically well characterized for their physical and cognitive health status. They had not taken antibiotics for 1 month prior to sampling, they had regular excrement habits, no history of gastrointestinal disease such as gastritis, peptic ulcer, gastric cancer, colorectal cancer and inflammatory bowel disease and had not exhibited diarrhea for the previous month. A stool sampling kit consisting of a sample collection tube, cotton swabs and sterile tissue papers was given to each subject together with a questionnaire related to their food consumption behavior. Fresh fecal samples were collected in sterile tubes, kept on ice during transportation and stored immediately at − 20 °C in the laboratory for 15 ± 5 days prior to DNA extraction as described by La-ongkham et al. (2015).
Dietary assessment
Dietary intake was assessed with a self-administered, qualitative food frequency questionnaire (FFQ). The short-type FFQ used in this study contained 14 items related to food consumption in the month before sampling. The food types were classified into high-protein diets (beef, pork, chicken, fish, eggs and cow milk) and high-carbohydrate diets (rice and rice noodle, Thai traditional fermented rice noodle (khanomjeen), bread, whole grain, sweet potato and dietary fiber (vegetables and fruit).The consumption frequency evaluations were performed at nine frequency levels as previously described by La-ongkham et al. (2015).
DNA extraction
The total bacterial genomic DNA from each sample was extracted from 50 mg of each fecal sample as previously described by La-ongkham et al. (2015). The DNA was eluted with sterilized pure water and kept at − 20 °C until use.
Pyrotag sequencing
The V6-V8 region of the bacterial 16S rRNA gene was amplified using PCR with a eubacterial universal primer set, Q-968F-# (5′-CWSWSWWSHT WACGCGARGAACCTTACC -3′) and Q-1390R-# (5′-CWSWSWWSHT TGACGGGCGGTGWGTAC-3′), where # indicates a series of barcode sequence tags underlined in the sequence (Nakayama 2010). Each genomic DNA sample of approximately 10–100 ng was used as the template in 50 µl of PCR reaction. For each sample, the PCR mixture contained 5 µl of 10 × Ex Taq buffer, 4 µl of 2.5 mM each dNTP, 1 µl of 10 pmol of each primer, 0.25 µl of 5 U/µl TaKaRa Ex Taq HS (Takara Bio, Japan) and dH2O added to obtain a final volume of 50 µl. The PCR conditions were as follows: initial denaturation at 98 °C for 2 min 30 s, 20 cycles of 98 °C for 15 s, 54 °C for 30 s, and 72 °C for 30 s with a final extension at 72 °C for 5 min. The PCR amplicons were purified using a QIAquick 96 PCR Purification Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The DNA concentration of the purified PCR products was determined with a NanoDrop ND-1000 spectrophotometer (Nanodrop Technologies, USA). Approximately 100 ng of each purified amplicon from each sample was pooled and purified using ethanol precipitation prior to pyrosequencing (Nakayama et al. 2013). Serial dilution of each amplicon fragment obtained was performed and amplified on a special bead (one fragment per bead), loaded and fixed onto a GS FLX Titanium Pico Titer Plate with dividers separating the reaction chambers according to the pyrosequencing process of the manufacturer’s protocol (454 Life Sciences, Roche, the Netherlands). Then, each amplicon mixture was applied using emulsion PCR (emPCR) with a GS FLX Titanium LV emPCR kit (Lib-L) v2 for nucleotide sequencing.
Processing of 454 pyrosequencing data
The obtained 454 batch data were sorted using the QIIME 1.7.0 software package to acquire each sample batch. The parameters used in this script were set according to Nakayama et al. (2013) as follows: 1 (minimum sequence length) = 408, e (maximum number of errors in barcode) = 0, reverse primer mismatches = 3, a (maximum number of doubtful bases) = 3 and L (maximum sequence length) = 500. In this step, any low-quality or ambiguous reads were determined based on the characteristics of each sequence and removed. The total reads were further filtered to remove noisy and chimeric sequences, and then operational taxonomic unit (OTU) clustering was performed with a 97% threshold identity via USEARCH v5.2.236 in QIIME. The phylogenetic composition was determined using QIIME against the Greengenes 13_5 database. Chao1 and Shannon index values were derived from QIIME. Weighted UniFrac PCoA and subsequent permutational multivariate analysis of variance analysis (PERMANOVA) were also performed in the QIIME platform.
Taxonomic analysis
Each OTU sequence was classified into bacterial population data from the phylum to the genus level (hierarchical level) by the RDP Classifier (https://rdp.cme.msu.edu/classifier/classifier.jsp), where the confidence threshold for classification was set at 80%. Species-level identification was performed on RDP Seqmatch (https://rdp.cme.msu.edu/seqmatch/seqmatch.jsp), which displayed the 20 closest 16S rDNA sequences of cultured strains. The SeqmatchQ400 algorithm designed by Nakayama et al. (2013) was used to convert the RDP Seqmatch results to species composition. If more than two different species showed the same highest score, the one with the highest count in the top 20 lists was selected. The criterion used to identify species was a cut off at ≥ 0.84 for the S_ab score, which statistically corresponds to approximately 97% sequence identity (Woese 1987). The relative abundance of each taxon was determined by dividing the assigned read counts by the total read counts, and the result was expressed as a percentage.
Statistical analyses
Statistical analyses were performed using the statistical software package SPSS version 17 for comparing means between groups. Stata/SE 12.0 was used for multiple linear regression analysis, and the “vegan” package in R version 3.5.2 was used for “PCA”, “Adonis” and “envfit” analyses. The normality of the data was checked using the Kolmogorov–Smirnov test. Continuous data are presented as the mean ± standard deviation (mean ± SD). Differences between groups were compared using one-way ANOVA for continuous data or the Mann–Whitney U test when data were not normally distributed. All statistical assessments were two-tailed, and p < 0.05 was considered statistically significant. Correlations between variables were determined by applying Spearman’s rank correlation (r) in SPSS 17.0. Principal component analysis (PCA) of genus composition data was performed using the “rda” command in the vegan package in R. PERMANOVA was performed by using the “adonis2” command, and envfit analysis was performed by using the “envfit” command in the “vegan” package in R.
Results
Characterization of Thai subjects and their dietary habits
The characteristics of the 120 Thai subjects consisting of 73 adult and 47 elderly subjects with ages of 30–40 years and > 65 years, respectively, are shown in Table 1. The elderly subjects were significantly heavier (p = 0.020) and had a greater body mass index (BMI, p = 0.001) than the adult subjects. However, the BMI of 22.39–24.30 kg/m2 on average for the two age groups was classified as nonobese.
Table 1.
Characteristics of 120 Thai adult and elderly age subjects
| Characteristic | All subjects (n = 120) | Adult (30–40 years) (n = 73) | Elderly (65–79 years) (n = 47) | p valueb |
|---|---|---|---|---|
| Agea (years) | 48.28 ± 17.43 | 34.60 ± 3.19 | 69.53 ± 3.44 | 0.001 |
| Female (no.) | 83 | 64 | 29 | |
| Male (no.) | 37 | 19 | 18 | |
| Weighta (kg) | 59.26 ± 10.32 | 57.52 ± 11.06 | 61.97 ± 8.46 | 0.020 |
| Heighta (m) | 1.60 ± 0.08 | 1.60 ± 0.07 | 1.60 ± 0.08 | 0.782 |
| BMIa (kg/m2) | 23.14 ± 3.22 | 22.39 ± 3.33 | 24.30 ± 2.68 | 0.001 |
aData shown as mean ± SD
bAverages of group differences between adult and elderly groups compared for significance at p < 0.05 using the Mann–Whitney U test (SPSS 17.0 statistical software)
Table 2 lists the six most popular carbohydrate sources related to food consumption by the Thai subjects. Both groups consumed rice as their staple food with no significant difference in intake frequency, while the adult subjects ate noodles and khanomjeen more frequently than the elderly subjects. On the other hand, the elderly subjects consumed significantly more vegetables (p = 0.019) and fruit (p = 0.001) than the adult subjects.
Table 2.
Dietary consumption frequency of 120 Thai adult and elderly subjects
| Food item | Average intake frequencya (times/day) | |||
|---|---|---|---|---|
| All subjects (n = 120) | Adult (n = 73) | Elderly (n = 47) | p valueb | |
| High-carbohydrate diet | ||||
| Rice | 2.33 ± 0.80 | 2.26 ± 0.81 | 2.43 ± 0.80 | 0.196 |
| Noodle | 0.38 ± 0.44 | 0.42 ± 0.45 | 0.31 ± 0.42 | 0.008 |
| Bread | 0.38 ± 0.38 | 0.30 ± 0.25 | 0.51 ± 0.51 | 0.137 |
| Fermented rice noodle (Khanomjeen) | 0.20 ± 0.21 | 0.24 ± 0.23 | 0.13 ± 0.17 | 0.002 |
| Whole grain | 0.30 ± 0.43 | 0.24 ± 0.20 | 0.42 ± 0.64 | 0.350 |
| Potato/sweet potato | 0.14 ± 0.19 | 0.12 ± 0.15 | 0.17 ± 0.23 | 0.219 |
| High-protein diet | ||||
| Pork | 0.71 ± 0.60 | 0.86 ± 0.64 | 0.50 ± 0.48 | 0.001 |
| Chicken | 0.54 ± 0.60 | 0.79 ± 0.67 | 0.26 ± 0.35 | 0.001 |
| Fish | 0.62 ± 0.55 | 0.60 ± 0.55 | 0.71 ± 0.54 | 0.010 |
| Beef | 0.14 ± 0.29 | 0.19 ± 0.33 | 0.07 ± 0.21 | 0.001 |
| Egg | 0.58 ± 0.41 | 0.61 ± 0.41 | 0.52 ± 0.41 | 0.120 |
| Cow milk | 0.29 ± 0.46 | 0.36 ± 0.50 | 0.18 ± 0.36 | 0.020 |
| Dietary fiber | ||||
| Vegetables | 1.34 ± 0.86 | 1.22 ± 0.86 | 1.52 ± 0.84 | 0.019 |
| Fruit | 1.15 ± 0.81 | 0.92 ± 0.66 | 1.50 ± 0.90 | 0.001 |
aData shown as mean ± SD
bAverages of group differences between adult and elderly groups compared for significance at p < 0.05 using the Mann–Whitney U test (SPSS 17.0 statistical software)
For the high-protein diets, pork was the most frequently consumed item by the adult subjects (0.86 times/day), which consumed 1.7 times (p = 0.001) more pork than the elderly subjects. In addition, the consumption frequencies of chicken meat, beef and cow milk by the adult subjects were also significantly higher than those by the elderly subjects (p = 0.001, p = 0.001 and p = 0.020, respectively). On the other hand, fish meat was more frequently consumed by the elderly subjects (0.71 times/day), which consumed significantly more fish than the adult subjects (p = 0.010). However, no significant difference was found in egg consumption by either age group.
Sequencing coverage and estimation of bacterial diversity
The V6-V8 region of the 16S rRNA gene was amplified from the stool samples of the 120 Thai subjects and subjected to pyrotag sequencing. The obtained 454-sequence data were composed of 473,255 high-quality, filtered reads, corresponding to 3944 ± 948 reads per subject. All reads were clustered into 1599 OTUs at 97% sequence identity, and their representative sequences were used in taxonomic analysis; the reads were classified into 8 phyla, 15 classes, 41 families, 98 genera and 239 species.
The ecological diversity of the Thai gut microbiota was estimated for species richness using Chao1 (Chao 1984) and for both richness and evenness using the Shannon diversity index from the OTU data for each sample. Although the total Chao1 richness in the adult subjects was greater than that in the elderly subjects, corresponding to averages of 425 ± 124 and 413 ± 93 for the adult and elderly groups, respectively, the difference was not significant (p = 0.189). In addition, the Shannon index values of 6.0 ± 0.8 and 5.8 ± 0.8 for the adult and elderly groups did not significantly differ between the groups, respectively (p = 0.125).
Fecal bacterial community between Thai adult and elderly subjects
The gut microbiota of all 120 Thai subjects were classified into eight known phyla, namely Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Verrucomicrobia, Fusobacteria, Synergistetes and Lentisphaerae. More than 96% of the total sequences detected in all samples belonged to the four most abundant bacterial phyla, Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria, as shown in Fig. 1. Firmicutes was the most abundant phylum detected in the adult and elderly subjects, accounting for averages of 67.96 and 63.84% of the total sequences, respectively. The two phyla Bacteroidetes and Actinobacteria showed significantly different abundances between the two age groups. The phylum Bacteroidetes was significantly more abundant in the elderly subjects (15.69%) than in the adult subjects (11.13%, p = 0.019), whereas the abundance of the phylum Actinobacteria in the adult subjects (9.81%) was significantly higher by approximately 2.3 times than that in the elderly group (3.91%, p = 0.001). The phylum Proteobacteria was also present in both age groups at abundances ranging from 9% to 12.57% of total sequences, but there was no significant difference between the two age groups. However, the Firmicutes/Bacteroidetes ratio of the adult and elderly groups was 7.3 and 4.5, respectively, which were significantly different (p = 0.01).
Fig.1.
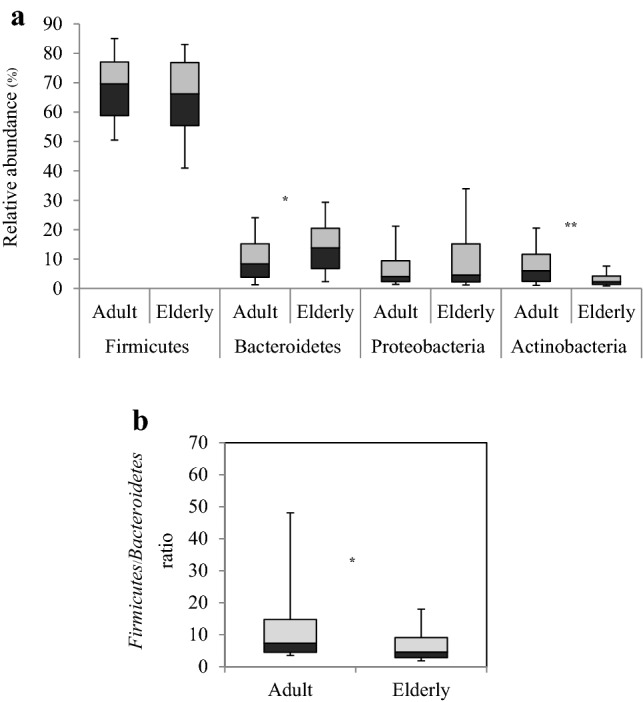
Box plots showing the relative abundance of the most dominant bacterial phyla and the Firmicutes/Bacteroidetes ratio in adult and elderly groups. a The four dominant bacterial phyla. Bacteroidetes was significantly higher in elderly subjects (p = 0.019), and Actinobacteria was significantly higher in adult subjects (p = 0.001). b The Firmicutes/Bacteroidetes ratio. The F/B ratio of the adult and elderly groups was significantly different (p = 0.01). Comparisons between groups were performed using the Mann–Whitney U test. The darker box represents the ratio of the 25th to 50th percentile. The lighter box represents the ratio of the 50th to 75th percentile. The horizontal line separating the two boxes is the 50th percentile. *p < 0.05, *p < 0.01
In total, 41 and 39 families were detected in the adult and elderly groups, respectively. The two families Lachnospiraceae and Ruminococcaceae, belonging to the Firmicutes phylum, were the most prevalent families detected in all subjects. These two families occupied more than 50% of the total sequences in both age groups. However, their abundances showed no significant difference between the two age groups. Only two families, Bacteroidaceae belonging to the phylum Bacteroidetes and Bifidobacteriaceae belonging to the phylum Actinobacteria, were significantly different between both age groups.The elderly subjects had a significantly higher relative abundance of Bacteroidaceae by approximately 2.2 times than the adult subjects (p = 0.001), whereas the abundance of Bifidobacteriaceae in the adult subjects was significantly higher than that in the elderly subjects by approximately 3.3 times (p = 0.001).
The numbers of genera observed in the adult and elderly groups were 97 and 95, respectively. Among these genera, Faecalibacterium was the most abundant genus in both the adult and elderly groups and had an abundance of 15.87 and 12.79% of the total sequences, respectively; however, the abundance of Faecalibacterium showed no significant difference between the two age groups. On the other hand, two genera, Bacteroides and Parabacteroides, belonging to the phylum Bacteroidetes were significantly more abundant in the elderly subjects than in the adult subjects by approximately 2.2 times (p = 0.001) and 1.3 times (p = 0.02), respectively. In contrast, in the adult group, the abundances of Bifidobacterium belonging to the phylum Actinobacteria and Dorea belonging to the phylum Firmicutes were significantly higher by approximately 3.3 times (p = 0.001) and 1.3 times (p = 0.01), respectively.
The community structures of the gut microbiota of Thai individuals were compared between the adult and elderly groups by using weighted UniFrac PCoA analysis (Supplementary Fig. 1). Thereafter, a permutation analysis showed a marginally significant difference between the community structures between the adult and elderly groups (p = 0.119). We also performed principal component analysis (PCA) at the genus level and thereafter performed PERMANOVA. The community structure was significantly different between the elderly and adult groups (p = 0.001, R2 = 0.033), although the samples were not clustered within each group, as indicated by the low R2 value from PERMANOVA (Fig. 2a). However, the samples of the elderly group tended to be localized in the direction of the loading vector of the genus Escherichia/Shigella. The envfit analysis also indicated that age is associated with this PCA ordination, particularly the direction of the loading vector of the genus Escherichia/Shigella, whereas the other host factors, namely gender and BMI, were not statistically associated with the PCA ordination. The genus Escherichia/Shigella was shown to have a high abundance in the elderly subjects, although there was no statistically significant difference between these two age groups (Fig. 2b).
Fig. 2.
Gut microbiota of Thai adult and elderly subjects at the genus level. a Genus composition data of 120 samples were subjected to PCA and ordinated in the PC1-PC2 dimension. Elderly and adult samples were plotted as closed and open circles, respectively. The five genera with the largest loadings were positioned according to the vector of loading. The ellipse covers 95% of the samples belonging to the adult and elderly groups. The vectors of the host factor fitted to the PCA ordination are displayed by arrows. Asterisk beside the name of the factor represents statistical significance with p < 0.05. The samples of the elderly group tended to be localized in the direction of the loading vector of the genus Escherichia/Shigella (p = 0.001, R2 = 0.033). The envfit analysis also indicated that age is associated with this PCA ordination, particularly the direction of the loading vector of the genus Escherichia/Shigella, whereas the other host factors, namely gender and BMI, were not statistically associated with the PCA ordination. bBox plots showing the relative abundance of the genus Escherichia/Shigella in adult and elderly groups. The genus Escherichia/Shigella was shown to have a high abundance in the elderly subjects, although there was no statistically significant difference between these two age groups
Considering the potential species level, the top 20 most abundant species detected with more than 1% abundance in the adult and elderly subjects are listed in Table 3. Most species belonging to the phylum Firmicutes were detected in the adult and elderly subjects for up to 39.1 and 35.9% of total sequences, respectively. The abundances of those species in both subject groups, including Faecalibacterium prausnitzii, which was the most abundant from both age groups, were not significantly different. Three species of the phylum Proteobacteria, Escherichia coli, Klebsiella pneumoniae and Gemmiger formicilis, were detected in both the adult and elderly groups (7.8–11.5%). Their abundances were also not significantly different between the two age groups. The abundance of the phylum Bacteroidetes was higher in the elderly group (10.25%) than in the adult group (6.14%). Bacteroides uniformis, B. dorei and B. massiliensis dominated only in the elderly subjects, while the abundance of B. vulgatus was high in both the adult and elderly subjects. In contrast, only three species belonging to the phylum Actinobacteria, Bifidobacterium adolescentis, Bif. longum and Bif. pseudocatenulatum, were found to be higher in the adult group (4.95%), while those species were not found as the top 20 most abundant species in the elderly group. However, statistical analysis of these species from both groups showed that other species exhibited significant differences between the two age groups, as shown in Fig. 3, resulting in significantly higher abundances (about two-fold) of Dorea longicatena, Roseburia faecis and Blautia luti belonging to the phylum Firmicutes in the adult subjects compared to the elderly subjects. The abundances of Eubacterium eligens, Phascolarctobacterium faecium and Clostridium bolteae in the elderly subjects were significantly higher than those in the adult subjects by approximately twofold. Considering the phylum Bacteroidetes, Bacteroides uniformis, B. thetaiotaomicron, B. ovatus, B. caccae and Parabacteroides distasonis were detected in more than half of all elderly subjects at significantly higher abundance levels by approximately 1.3–4 times compared to the adult subjects. In contrast, Bifidobacterium adolescentis, Bif. longum and Bif. pseudocatenulatum belonging to the phylum Actinobacteria were found at significantly higher levels of approximately 2.3–6 times in the adult subjects than in the elderly subjects.
Table 3.
List of the top 20 most abundant species in adult and elderly subjects and species with abundances higher than 1%
| Adult | Elderly | ||||
|---|---|---|---|---|---|
| Phylum | Speciesa | Relative abundanceb (%) | Phylum | Speciesa | Relative abundanceb (%) |
| Firmicutes | Faecalibacterium prausnitzii | 16.66 | Firmicutes | Faecalibacterium prausnitzii | 13.55 |
| Firmicutes | Blautia wexlerae | 6.18 | Proteobacteria | Escherichia coli | 8.40 |
| Bacteroidetes | Prevotella copri | 4.37 | Firmicutes | Blautia wexlerae | 7.49 |
| Proteobacteria | Escherichia coli | 4.25 | Firmicutes | Eubacterium rectale | 4.70 |
| Firmicutes | Eubacterium rectale | 3.42 | Bacteroidetes | Bacteroides vulgatus | 4.13 |
| Actinobacteria | Bifidobacterium adolescentis | 2.40 | Bacteroidetes | Prevotella copri | 2.68 |
| Proteobacteria | Klebsiella pneumoniae | 2.01 | Proteobacteria | Gemmiger formicilis | 1.84 |
| Firmicutes | Ruminococcus torques | 1.92 | Firmicutes | Ruminococcus torques | 1.70 |
| Firmicutes | Megamonas funiformis | 1.82 | Verrucomicrobia | Akkermansia muciniphila | 1.53 |
| Bacteroidetes | Bacteroides vulgatus | 1.77 | Firmicutes | Eubacterium hadrum | 1.44 |
| Firmicutes | Clostridium bartlettii | 1.72 | Firmicutes | Roseburia inulinivorans | 1.37 |
| Proteobacteria | Gemmiger formicilis | 1.55 | Proteobacteria | Klebsiella pneumoniae | 1.26 |
| Firmicutes | Ruminococcus gnavus | 1.52 | Firmicutes | Megamonas funiformis | 1.23 |
| Actinobacteria | Bifidobacterium longum | 1.36 | Bacteroidetes | Bacteroides uniformis | 1.21 |
| Firmicutes | Eubacterium hadrum | 1.32 | Firmicutes | Ruminococcus bromii | 1.21 |
| Firmicutes | Roseburia inulinivorans | 1.19 | Bacteroidetes | Bacteroides dorei | 1.13 |
| Actinobacteria | Bifidobacterium pseudocatenulatum | 1.19 | Firmicutes | Ruminococcus obeum | 1.12 |
| Firmicutes | Ruminococcus obeum | 1.15 | Bacteroidetes | Bacteroides massiliensis | 1.10 |
| Firmicutes | Clostridium clostridioforme | 1.10 | Firmicutes | Clostridium clostridioforme | 1.04 |
| Firmicutes | Ruminococcus bromii | 1.09 | Firmicutes | Eubacterium eligens | 1.02 |
aSpecies was determined by RDP Seqmatch with a cut off at ≥0.84 for the S_ab score
bSpecies were ranked based on the average relative abundance for each age group
Fig.3.
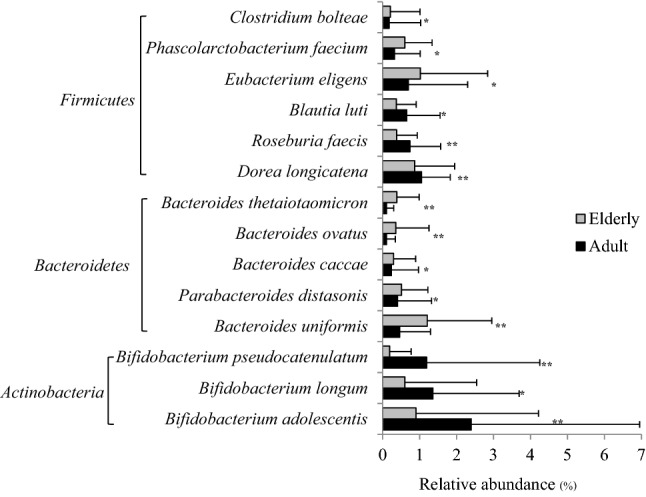
Comparison of gut microbiota at the species level between adult and elderly groups was performed using the Mann–Whitney U test. *p < 0.05, ** p < 0.01
Core gut microbiota of healthy Thai subjects
In this study, the 13 and 14 bacterial species found in 90% prevalence or more of all subjects in the adult and elderly groups were defined as core gut microbiota, as shown in Table 4. Eleven species (Blautia wexlerae, Blautia luti, Faecalibacterium prausnitzii, Escherichia coli, Ruminococcus obeum, Ruminococcus torques, Clostridium clostridioforme, Butyricicoccus pullicaecorum, Bacteroides vulgatus, Roseburia inulinivorans and Eubacterium hadrum) belonging to the three major phyla of Firmicutes, Bacteroidetes and Proteobacteria were commonly found in both age groups. Two additional species, Dorea longicatena and Clostridium bartlettii, and three species, Bacteroides uniformis, Streptococcus salivarius and Parabacteroides distasonis, showing a high prevalence only in the adult and elderly groups, respectively, could be used to differentiate these two age groups.
Table 4.
Core gut microbiota of healthy Thai adult and elderly subjects
| Adult | Elderly | ||||
|---|---|---|---|---|---|
| Speciesa | Prevalence (%) | Relative abundanceb (%) | Speciesa | Prevalence (%) | Relative abundancec (%) |
| Blautia wexlerae | 100.0 | 6.18 | Blautia wexlerae | 100.0 | 7.49 |
| Blautia luti | 98.6 | 0.65 | Faecalibacterium prausnitzii | 97.9 | 13.55 |
| Faecalibacterium prausnitzii | 97.3 | 16.66 | Bacteroides vulgatus | 97.9 | 4.13 |
| Escherichia coli | 97.3 | 4.25 | Ruminococcus torques | 97.9 | 1.70 |
| Ruminococcus obeum | 97.3 | 1.15 | Clostridium clostridioforme | 97.9 | 1.04 |
| Dorea longicatena | 95.9 | 1.06 | Escherichia coli | 95.7 | 8.40 |
| Ruminococcus torques | 93.2 | 1.92 | Roseburia inulinivorans | 95.7 | 1.37 |
| Clostridium clostridioforme | 93.2 | 1.10 | Bacteroides uniformis | 93.6 | 1.21 |
| Butyricicoccus pullicaecorum | 93.2 | 0.34 | Butyricicoccus pullicaecorum | 93.6 | 0.47 |
| Bacteroides vulgatus | 91.8 | 1.77 | Eubacterium hadrum | 91.5 | 1.44 |
| Clostridium bartlettii | 91.8 | 1.72 | Ruminococcus obeum | 91.5 | 1.12 |
| Roseburia inulinivorans | 91.8 | 1.19 | Streptococcus salivarius | 91.5 | 0.86 |
| Eubacterium hadrum | 90.4 | 1.32 | Parabacteroides distasonis | 91.5 | 0.51 |
| Blautia luti | 91.5 | 0.37 | |||
aSpecies was determined by RDP Seqmatch with a cut off at ≥0.84 for the S_ab score
bAverage of relative abundance (%) of all 73 adult subjects
cAverage of relative abundance (%) of all 47 elderly subjects
Correlation of gut microbiota, age and dietary consumption habit
The strength of association for the correlations of age, food consumption frequency and the relative abundance of dominant gut microbiota among the 120 Thai healthy subjects was evaluated using the correlation coefficient (r) value. Ranges of 0.5–1.0, 0.3–0.49 and 0.1–0.29 were defined for strong, moderate and weak correlations, respectively (AbuRuz et al. 2007). Only the association between age and microbial abundance is shown in Fig. 4. The genera Bacteroides and B. uniformis showed a moderate positive correlation with r values of 0.36 (p = 0.001) and 0.32 (p = 0.001), respectively, whereas Bifidobacterium sp., Bif. adolescentis and Bif. longum displayed a moderate negative correlation with r values of − 0.44 (p = 0.001), − 0.35 (p = 0.001) and − 0.32 (p = 0.001), respectively. To check the confounding of age, BMI, gender and diet consumption frequency for these correlations, multiple linear regression analysis were performed (Table 5). The results showed that except for Bif. adolescentis, the correlation between age and abundance of each bacterium remained significant (p < 0.05), while statistical significance was not observed between the abundance of each bacterium and BMI or gender. We further found a significant correlation of rice consumption with Bacteroides (p < 0.05 in Spearman correlation analysis). As a result, rice consumption frequency showed a significant positive correlation with both the genus Bacteroides and Bacteroides uniformis, while a significance in the correlation of age with these bacteria was maintained. No correlation was found for BMI and gender in these multiple linear regression analysis. Taken together, in the gut of Thai adults, Bifidobacterium decreased and Bacteroides increased with age, while rice consumption increased the abundance of Bacteroides.
Fig.4.
Spearman correlation analysis between age and gut microbiota of 120 Thai subjects was performed at the genus and species levels. r correlation coefficient. * p < 0.05, ** p < 0.01. a Correlation between age and the genus Bacteroides. b Correlation between age and Bacteroides uniformis. c Correlation between age and the genus Bifidobacterium. d Correlation between age and Bifidobacterium adolescentis. e Correlation between age and Bifidobacterium longum
Table 5.
Multiple linear regression analysis of age, BMI, gender, and rice consumption for Bacteroides and Bifidobacterium
| Genus or species | Factor | Coefficiency | Std. Err. | T | p | r |
|---|---|---|---|---|---|---|
| Total Bacteroides | Age | 0.164 | 0.041 | 3.99 | 0.000* | 0.355 |
| BMI | − 0.033 | 0.224 | − 0.15 | 0.883 | − 0.013 | |
| Gender | − 2.918 | 1.502 | − 1.94 | 0.055 | − 0.168 | |
| Rice | 1.796 | 0.852 | 2.11 | 0.037* | 0.180 | |
| Intercept | − 3.167 | 5.462 | − 0.58 | 0.563 | ||
| Bacteroides uniformis | Age | 0.020 | 0.007 | 2.92 | 0.004* | 0.269 |
| BMI | − 0.008 | 0.038 | − 0.22 | 0.827 | − 0.020 | |
| Gender | − 0.422 | 0.253 | − 1.67 | 0.099 | − 0.149 | |
| Rice | 0.300 | 0.144 | 2.09 | 0.039* | 0.184 | |
| Intercept | − 0.597 | 0.921 | − 0.65 | 0.518 | ||
| Total Bifidobacterium | Age | − 0.110 | 0.044 | − 2.49 | 0.014* | − 0.232 |
| BMI | − 0.410 | 0.241 | − 1.70 | 0.092 | − 0.160 | |
| Gender | 1.945 | 1.612 | 1.21 | 0.23 | 0.109 | |
| Rice | 0.158 | 0.915 | 0.17 | 0.863 | 0.015 | |
| Intercept | 18.193 | 5.863 | 3.10 | 0.002 | ||
| Bifidobacterium adolescentis | Age | − 0.042 | 0.023 | − 1.83 | 0.070 | − 0.175 |
| BMI | − 0.107 | 0.125 | − 0.85 | 0.395 | − 0.083 | |
| Gender | 0.338 | 0.838 | 0.40 | 0.688 | 0.038 | |
| Rice | − 0.167 | 0.475 | − 0.35 | 0.727 | − 0.032 | |
| Intercept | 6.593 | 3.048 | 2.16 | 0.033 | ||
| Bif. longum | Age | − 0.025 | 0.012 | − 2.03 | 0.044* | − 0.193 |
| BMI | − 0.035 | 0.066 | − 0.53 | 0.594 | − 0.051 | |
| Gender | 0.799 | 0.441 | 1.81 | 0.073 | 0.168 | |
| Rice | − 0.044 | 0.250 | − 0.18 | 0.859 | − 0.016 | |
| Intercept | 2.920 | 1.605 | 1.82 | 0.071 |
Discussion
Age could have a major effect on the human intestinal microbiota, which changes throughout the life of a human (Mueller et al. 2006; Biagi et al. 2010; Zhao et al. 2011; Yatsunenko et al. 2012). Age-related physiological changes in the gastrointestinal tract accompanied by nutritional behavior, lifestyle, frailty condition, antibiotic treatment and country-specific dietary habits have been investigated (Hayashi et al. 2003; Mueller et al. 2006; Biagi et al. 2010; Yatsunenko et al. 2012). Many studies have been conducted to characterize the main features of the gut microbiota in elderly individuals compared to younger adults. In this study, the gut microbiota communities of 47 healthy, elderly subjects and 73 adults were characterized. Considering the overall structure of their gut microbiota at the phylum level, the microbiota of the adult and elderly groups showed significant differences in Actinobacteria and Bacteroidetes, while there were no significant differences in Firmicutes between both age groups. The results were similar for elderly aged subjects (≥ 65 years) in Ireland, the microbiota of which were dominated by the phylum Bacteroidetes as well (Claesson et al. 2011). Recently, compositional changes in Japanese gut microbiota with age were observed (Odamaki et al. 2016). The relative abundances of Actinobacteria and Clostridia were significantly higher in the infant and adult clusters, respectively. The elderly cluster showed a significantly higher abundance of Betaproteobacteria, Deltaproteobacteria and Bacteroidetes, which was similar to our results.
These changes affected the F/B ratio, which significantly decreased with increasing age, from 7.3 to 4.5 between the adult and elderly groups (p = 0.01). This corresponded to the work done by Mariat et al. (2009), who reported a ratio of F/B for adult and elderly subjects in France that changed from 10.9 to 0.6. Such a shift (10.9–0.6) was greater than that in the current Thai study (7.3–4.5). However, Biagi et al. (2010) reported no significant differences among the F/B ratio of centenarians, elderly individuals and young adults. These results implied that the ratio of Firmicutes and Bacteroidetes, which were the most dominant phyla in the gut in each region, had its own signature (Biagi et al. 2010; Claesson et al. 2011; Nam et al. 2011).
The difference in the overall structure of the gut microbiota of adults and elderly individuals was also addressed by multivariate analyses, which showed significant differences in the permutation analysis, but there were no obvious clusters between these two groups in this study. Furthermore, an association of age with PCA coordination, particularly to the loading vector of the genus Esherichia/Shigella, was observed, although the abundance of this genus group did not significantly differ between the adult and elderly groups. These results indicated that the age-related change in gut microbiota depended on individuals and might be further affected by individual health status and dietary style.
The diversity values estimated for the species richness using Chao1 and for both richness and evenness using the Shannon diversity index of the gut microbiota in these two age groups were not significantly different, indicating that the microbial diversity in healthy, elderly subjects, including the number of species and species abundance, was still retained in the healthy gut microbiota when the age increased. This result was different from that reported by Weiskopf et al. (2009) and Biagi et al. (2012), who proposed that a loss in diversity in elderly individuals is associated with less resistance to pathogens and a natural decline in immune function, which agreed with Ottman et al. (2012). It has been proposed that the process of aging has a negative effect on the diversity of the gut microbiota, resulting in a decline in the state of the host’s health (Claesson et al. 2012). Although the diversity of both Thai adult and elderly subjects was not significantly different, the dominant species observed in the adult and elderly subjects were different. The adult group had a significantly higher abundance of Bifidobacterium pseudocatenulatum, Bif. longum, Bif. adolescentis, Blautia luti, Dorea longicatena and Roseburia faecis, while the elderly group had a higher abundance of Bacteroides vulgatus, B. uniformis, B. thetaiotaomicron, B. caccae, B. ovatus, Parabacteroides distasonis, Clostridium bolteae, Phascolarctobacteriun faecium and Eubacterium eligens. The decreasing abundance of bifidobacterial species diversity in elderly individuals is explained by their poor adhesion to the intestinal mucosa, although it is not clear if this is because of changes in the bacteria, or in the chemical composition and structure of colonic mucus (Ouwehand et al. 1999; He et al. 2001).
These different dominant species with different species abundance levels might consequently maintain the health of the host at each different age range. Some species of both Bifidobacterium and Bacteroides were reported to have beneficial functions in the host (Wexler 2007; Clemente et al. 2012). Bifidobacterium longum, Bif. infantis, Bif. breve and Bif. bifidum dominate in the infant flora, while Bif. adolescentis and Bif. catenulatum become more prevalent in the adult intestinal flora (Gavini et al. 2001; He et al. 2001; Hopkins and Macfarlane 2002; Léké et al. 2007; Palmer et al. 2007). In this study, the more abundant species found in Thai adult subjects compared to elderly subjects included the genus Bifidobacterium sp., Bif. adolescentis, Bif. longum and Bif. pseudocatenulatum. The multiple linear regression analysis of age, BMI and gender also showed the significant negative correlation between the genus Bifidobacterium and Bif. longum with age. Many Bacteroides species, such as B. distasonis, B. fragilis, B. ovatus B. caccae, B. uniformis and B. vulgatus, were present in healthy elderly subjects (Hopkins and Macfarlane 2002) as well as, healthy Japanese elderly subjects as determined by 16S rDNA libraries and T-RFLP (Hayashi et al. 2003). In this study, the multiple linear regression analysis of age, BMI and gender showed a significant positive correlation between the genus Bacteroides and B. uniformis with age. At the genus and species levels, many members of the Bacteroidetes phylum, especially in the genera Bacteroides and Parabacteroides, dominated in the elderly subjects. Bacteroides uniformis and Parabacteroides distasonis were classified as dominant core species in the elderly subjects. The abundances of B. thetaiotaomicron, B. ovatus, and B. caccae were significantly higher by approximately 1–3 times in the elderly subjects compared to the younger adult subjects. The findings of a higher abundance of the genus Bacteroides in Thai elderly subjects was similar to many previous reports and contributed to maintaining health. However, Mueller et al. (2006) investigated the bacterial community structures in four European populations (France, Germany, Italy and Sweden) and compared healthy young adult subjects and elderly subjects using the FISH technique. The author found that an age effect was only observed in the German and Italian groups, and this effect could be explained by country-specific dietary habits. German elderly subjects showed an increase in the Bacteroides-Prevotella group, while Italian elderly subjects showed a decrease in these bacteria when compared with younger adults. Therefore, the number of Bacteroides is not related to the aging process itself, but it may be influenced by frailty conditions, antibiotic treatment, hospitalization and country-specific dependence (Hopkins and Macfarlane 2002; Woodmansey et al. 2004; Mueller et al. 2006; Zwielehner et al. 2009).
Faecalibacterium prausnitzii, which has been suggested to serve as a biomarker for a healthy gut in both groups of Thai subjects, showed the highest abundance. F. prausnitzii, Bacteroides, Blautia, Dorea longicatena, Roseburia, Ruminococcus, Eubacterium, and Butyricicoccus pullicaecorum are commonly found in the human gut microbiota and are short chain fatty acid (SCFA)-producing bacteria (Duncan et al. 2002; Pryde et al. 2002; Taras et al. 2002; Hosseini et al. 2011; Nam et al. 2011; Park et al. 2012; Martínez et al. 2013; Geirnaert et al. 2014). The end products of bacterial metabolism, especially butyric acid, are vital for human health. F. prausnitzii can produce high amounts of butyrate and exhibit anti-inflammatory effects (Li et al. 2008; Sokol et al. 2008; Miquel et al. 2014). It was previously found that F. prausnitzii is commonly shared by healthy western individuals (Tap et al. 2009; Qin et al. 2010; Huse et al. 2012) and healthy young Chinese cohorts (Zhang et al. 2015). In this study, F. prausnitzii was the most abundant species (15.4% of total sequences), and the most prevalent OTUs (49 OTUs), detected in 98% of the 120 Thai individuals. This implied that the prevalence of such species across heterogeneous populations may indicate their essential nature for human health and would be a good sign of health for both age groups.
The core gut microbiota of the Thai adult and elderly subjects were dominated by the class Clostridia belonging to the phylum Firmicutes, including the phylum Bacteroidetes and the phylum Proteobacteria. These species were shared by at least 90% of all subjects among the Thai adult and elderly subjects, and these species are suggested as key dominant members of healthy Thai gut microbiota. However, it was noticed that only the Thai core gut microbiota consisting of E. coli occupied 4.3–8.4% of the total sequences. The PCA analysis at the genus level also indicated the association between age and the genus Escherichia/Shigella. Indeed, the microbiota of some subjects, notably elderly individuals, were highly colonized by these species and genera. Recently, Nakayama et al. (2015) also reported that healthy Thai children had a high abundance of E. coli compared to other Asian children. The high abundance of E. coli appears to be a common feature throughout all generations in Thailand, although the E. coli population has tendency to increase with aging worldwide. In fact, most E. coli species are harmless and are an important part of a healthy human intestinal tract, existing in a symbiotic state and providing resistance against pathogenic organisms. Several types of E. coli have many beneficial functions, such as the production of vitamin K2 (Kadner 1978; Bentley and Meganathan 1982) and a crucial role in food digestion (Alpert et al. 2009). In healthy individuals, the colonization of this species is tightly controlled by both host- and bacteria-derived mechanisms (Yang and Jobin 2014). However, some E. coli are pathogenic and cause diseases of intestinal and extraintestinal sites (Lindstedt et al. 2018). E. coli can be a sign of dysbiosis, such as Crohn’s disease (Darfeuille-Michaud et al. 2004), coeliac disease (De Palma et al. 2009) and inflammatory bowel disease (Becker et al. 2015). Therefore, the high abundance of E. coli in Thai might be either a risk for dysbiosis or good for health by providing specific and essential functions, similar to many commensal Clostridia, which make up a substantial part (10–40%) of the total bacteria in the gut microbiota (Lopetuso et al. 2013).
Diet-related changes in the gut ecosystem have been investigated along with their effects on gut microbiota changes (De Filippo et al. 2010; Wu et al. 2011; Claesson et al. 2012; Ruengsomwong et al. 2014; La-ongkham et al. 2015). In this study, we found a significant correlation of carbohydrates, especially rice consumption, with Bacteroides (p < 0.05) based on Spearman correlation analysis. For multiple linear regression analysis, rice consumption frequency showed a significant positive correlation with the genus Bacteroides and B. uniformis, while maintaining significance in the correlation of age with these bacteria. This corresponded to the work done by La-ongkham et al. (2015), who reported a high abundance of Prevotella and B. fragilis in children from the northeastern part who consumed high-frequency varieties of carbohydrates and dietary fiber. However, this result was opposite to gut microbial enterotypes with high abundances of Bacteroides from American adults and Thai non-vegetarian individuals who consumed more protein and animal fat (Wu et al. 2011; Ruengsomwong et al. 2014, 2016).
In conclusion, aging changes were associated with gut microbiota alteration: the abundance of Bacteroides increased and Bifidobacterium as well as Clostridium cluster XIVa decreased when age increased. Significantly high abundance of Bifidobacterium in adults and the ones of Bacteroides in elderlies including some species detected in both age groups sharing as core gut microbiome by at least 90% of the Thai individuals. It suggested the unique feature containing a balanced composition of many classes of bacteria in the Firmicutes, Bacteroidetes and Proteobacteria, which play important role functionally indispensable ecosystem in the maintenance of overall gut function. Although this study has a limitation due to the relatively small sample size, a further large-scale study to address the link of age and food to gut microbiota and health is warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to express their thanks to all the volunteers who were willing to provide fecal samples for this research. This work was supported by the Royal Golden Jubilee PhD. Scholarship Grant (RGJ-PhD) of the Thailand Research Fund (TRF) and Kasetsart University (PHD/0318/2552), partially supported by the Center for Advanced Studies for Agriculture and Food, Institute for Advanced Studies, Kasetsart University Under the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Ministry of Education, Thailand (CASAF PD011), by Grants-in-Aid for Scientific Research (B) No. 25304006 from the Japan Society for the Promotion of Science (JSPS) (to Jiro Nakayama) and performed under the Core-to-Core Program, which was financially supported by Japan Society for the Promotion of Science (JSPS), National Research Council of Thailand (NRCT), Vietnam Ministry of Science and Technology (MOST), the National University of Laos, Beuth University of Applied Sciences and Brawijaya University.
Compliance with ethical standards
Conflict of interest
The authors declare that have no conflict of interest in the publication.
Ethical statements
This study was approved by the Institute for the Development of Human Research Protection (IHRP) Department of Medical Sciences, Ministry of Public Health, Thailand with ethical approval number IHRP 311, and written informed consent was obtained from all participants.
Footnotes
Accession numbers Sequences obtained in this present study have been deposited in the DDBJ Sequence Read Archive (DRA) (BioProject accession number PRJDB5860, PSUB007333 and DRA accession number DRA005889).
References
- Alpert C, Scheel J, Engst W, Loh G, Blaut M. Adaptation of protein expression by Escherichia coli in the gastrointestinal tract of gnotobiotic mice. Environ Microbiol. 2009;11(4):751–761. doi: 10.1111/j.1462-2920.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- Bartosch S, Fite A, Macfarlane GT, McMurdo ME. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol. 2004;70(6):3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Neurath MF, Wirtz S. The intestinal microbiota in inflammatory bowel disease. ILAR J. 2015;56(2):192–204. doi: 10.1093/ilar/ilv030. [DOI] [PubMed] [Google Scholar]
- Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46(3):241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Candela M, Fairweather-Tait S, Franceschi C, Brigidi P. Ageing of the human metaorganism: the microbial counterpart. Age. 2012;34(1):247–267. doi: 10.1007/s11357-011-9217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H. Microbiota and healthy ageing: observational and nutritional intervention studies. Microb Biotechnol. 2013;6(4):326–334. doi: 10.1111/1751-7915.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. Nonparametric estimation of the number of classes in a population. Scand J Stat. 1984;11(4):265–270. [Google Scholar]
- Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, Weerd H, Flannery E. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- Clemente Jose C, Ursell Luke K, Parfrey Laura W, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon AM, Bird RA. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7(1):17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser A-L, Barnich N, Bringer M-A, Swidsinski A, Beaugerie L, Colombel J-F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127(2):412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102(8):1154–1160. doi: 10.1017/S0007114509371767. [DOI] [PubMed] [Google Scholar]
- Duncan SH, Hold GL, Barcenilla A, Stewart CS, Flint HJ. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int J Syst Evol Microbiol. 2002;52(5):1615–1620. doi: 10.1099/00207713-52-5-1615. [DOI] [PubMed] [Google Scholar]
- Favier CF, Vaughan EE, De Vos WM, Akkermans ADL. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68(1):219–226. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavini F, Cayuela C, Antoine J-M, Lecoq C, Lefebvre B, Membré J-M, Neut C. Differences in the distribution of bifidobacterial and enterobacterial species in human faecal microflora of three different (children, adults, elderly) age groups. Microb Ecol Health Dis. 2001;13(1):40–45. [Google Scholar]
- Geirnaert A, Steyaert A, Eeckhaut V, Debruyne B, Arends JBA, Van Immerseel F, Boon N, Van de Wiele T. Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe. 2014;30:70–74. doi: 10.1016/j.anaerobe.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sakamoto M, Kitahara M, Benno Y. Molecular analysis of fecal microbiota in elderly individuals using 16S rDNA library and T-RFLP. Microbiol Immunol. 2003;47(8):557–570. doi: 10.1111/j.1348-0421.2003.tb03418.x. [DOI] [PubMed] [Google Scholar]
- He F, Ouwehand AC, Isolauri E, Hosoda M, Benno Y, Salminen S. Differences in composition and mucosal adhesion of bifidobacteria isolated from healthy adults and healthy seniors. Curr Microbiol. 2001;43(5):351–354. doi: 10.1007/s002840010315. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51(5):448–454. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]
- Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev. 2011;69(5):245–258. doi: 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- Huse SM, Ye Y, Zhou Y, Fodor AA. A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS ONE. 2012;7(6):e34242. doi: 10.1371/journal.pone.0034242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner RJ. Repression of synthesis of the vitamin B12 receptor in Escherichia coli. J Bacteriol. 1978;136(3):1050–1057. doi: 10.1128/jb.136.3.1050-1057.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La-ongkham O, Nakphaichit M, Leelavatcharamas V, Keawsompong S, Nitisinprasert S. Distinct gut microbiota of healthy children from two different geographic regions of Thailand. Arch Microbiol. 2015;197(4):561–573. doi: 10.1007/s00203-015-1089-0. [DOI] [PubMed] [Google Scholar]
- Léké A, Romond M, Mullié C. Insights in the human bifidobacterial flora through culture-dependent and independent techniques. Commun Curr Res Educ Top Trends Appl Microbiol. 2007;2:758–765. [Google Scholar]
- Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, Zhang Y, Shen J, Pang X, Zhang M, Wei H, Chen Y, Lu H, Zuo J, Su M, Qiu Y, Jia W, Xiao C, Smith LM, Yang S, Holmes E, Tang H, Zhao G, Nicholson JK, Li L, Zhao L. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci USA. 2008;105(6):2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt B-A, Finton MD, Porcellato D, Brandal LT. High frequency of hybrid Escherichia coli strains with combined intestinal pathogenic Escherichia coli (IPEC) and extraintestinal pathogenic Escherichia coli (ExPEC) virulence factors isolated from human faecal samples. BMC Infect Dis. 2018;18(1):544. doi: 10.1186/s12879-018-3449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5(1):23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2009;103(2):227–234. doi: 10.1017/S0007114509991553. [DOI] [PubMed] [Google Scholar]
- Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9(1):123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I, Muller CE, Walter J. Long-Term Temporal analysis of the human fecal microbiota revealed a stable core of dominant bacterial species. PLoS ONE. 2013;8(7):e69621. doi: 10.1371/journal.pone.0069621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel S, Martín R, Bridonneau C, Robert V, Sokol H, Bermúdez-Humarán LG, Thomas M, Langella P. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes. 2014;5(2):146–151. doi: 10.4161/gmic.27651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72(2):1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J. Pyrosequence-based 16S rRNA profiling of gastro-intestinal microbiota. Biosci Microflora. 2010;29(2):83–96. [Google Scholar]
- Nakayama J, Jiang J, Watanabe K, Chen K, Ninxin H, Matsuda K, Kurakawa T, Tsuji H, Sonomoto K, Lee Y-K. Up to species-level community analysis of human gut microbiota by 16S rRNA amplicon pyrosequencing. Biosci Microbiota Food Health. 2013;32(2):69–76. doi: 10.12938/bmfh.32.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Watanabe K, Jiang J, Matsuda K, Chao SH, Haryono P. Diversity in gut bacterial community of school-age children in Asia. Sci Rep. 2015;5:8397. doi: 10.1038/srep08397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam YD, Jung MJ, Roh SW, Kim MS, Bae JW. Comparative analysis of Korean human gut microbiota by barcoded pyrosequencing. PLoS ONE. 2011;6(7):e22109. doi: 10.1371/journal.pone.0022109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao J-z, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16(1):90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. 2012;2:104. doi: 10.3389/fcimb.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwehand AC, Isolauri E, Kirjavainen PV, Salminen SJ. Adhesion of four Bifidobacterium strains to human intestinal mucus from subjects in different age groups. FEMS Microbiol Lett. 1999;172(1):61–64. doi: 10.1111/j.1574-6968.1999.tb13450.x. [DOI] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-K, Kim M-S, Roh SW, Bae J-W. Blautia stercoris sp. Nov., isolated from human faeces. Int J Syst Evol Microbiol. 2012;62(4):776–779. doi: 10.1099/ijs.0.031625-0. [DOI] [PubMed] [Google Scholar]
- Power SE, O'Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr. 2013;111(3):387–402. doi: 10.1017/S0007114513002560. [DOI] [PubMed] [Google Scholar]
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217(2):133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J-M, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruengsomwong S, Korenori Y, Sakamoto N, Wannissorn B, Nakayama J, Nitisinprasert S. Senior Thai fecal microbiota comparison between vegetarians and non-vegetarians using PCR-DGGE and real-time PCR. J Microbiol Biotechnol. 2014;24(8):1026–1033. doi: 10.4014/jmb.1310.10043. [DOI] [PubMed] [Google Scholar]
- Ruengsomwong S, La-Ongkham O, Jiang J, Wannissorn B, Nakayama J, Nitisinprasert S. Microbial community of healthy Thai vegetarians and non-vegetarians, their core gut microbiota and pathogens risk. J Microbiol Biotechnol. 2016;26(10):1723–1735. doi: 10.4014/jmb.1603.03057. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux J-J, Blugeon S, Bridonneau C, Furet J-P, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet J-P, Ugarte E, Muñoz-Tamayo R, Paslier DLE, Nalin R, Dore J, Leclerc M. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11(10):2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- Taras D, Simmering R, Collins MD, Lawson PA, Blaut M (2002) Reclassification of Eubacterium formicigenerans Holdeman and Moore 1974 as Dorea formicigenerans gen. nov., comb. nov., and description of Dorea longicatena sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 52(2):423–428 [DOI] [PubMed]
- Tiihonen K, Ouwehand AC, Rautonen N. Human intestinal microbiota and healthy ageing. Ageing Res Rev. 2010;9(2):107–116. doi: 10.1016/j.arr.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Van Tongeren SP, Slaets JPJ, Harmsen HJM, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol. 2005;71(10):6438–6442. doi: 10.1128/AEM.71.10.6438-6442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze A, Holleman F, Zoetendal EG, de Vos WM, Hoekstra JBL, Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53(4):606–613. doi: 10.1007/s00125-010-1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22(11):1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- Wexler HM. Bacteroides: the good, the bad, and the Nitty–Gritty. Clin Microbiol Rev. 2007;20(4):593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese CR. Bacterial evolution. Microbiol Rev. 1987;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodmansey EJ, McMurdo MET, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol. 2004;70(10):6113–6122. doi: 10.1128/AEM.70.10.6113-6122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Jobin C. Microbial imbalance and intestinal pathologies: connections and contributions. Dis Model Mech. 2014;7(10):1131–1142. doi: 10.1242/dmm.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guo Z, Xue Z, Sun Z, Zhang M, Wang L, Wang G, Wang F, Xu J, Cao H, Xu H, Lv Q, Zhong Z, Chen Y, Qimuge S, Menghe B, Zheng Y, Zhao L, Chen W, Zhang H. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. ISME J. 2015;9(9):1979–1990. doi: 10.1038/ismej.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Qiao X, Zhu J, Zhang X, Jiang J, Hao Y, Ren F. Correlations of fecal bacterial communities with age and living region for the elderly living in Bama, Guangxi. China J Microbiol. 2011;49(2):186–192. doi: 10.1007/s12275-011-0405-x. [DOI] [PubMed] [Google Scholar]
- Zwielehner J, Liszt K, Handschur M, Lassl C, Lapin A, Haslberger AG. Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides, bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp Gerontol. 2009;44(6–7):440–446. doi: 10.1016/j.exger.2009.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




