Abstract
Introduction
We examined differences in hypoglycaemia risk between insulin glargine 300 U/mL (Gla-300) and insulin glargine 100 U/mL (Gla-100) in individuals with type 2 diabetes (T2DM) using the low blood glucose index (LBGI).
Methods
Daily profiles of self-monitored plasma glucose (SMPG) from the EDITION 2, EDITION 3 and SENIOR treat-to-target trials of Gla-300 versus Gla-100 were used to compute the LBGI, which is an established metric of hypoglycaemia risk. The analysis also examined documented (blood glucose readings < 3.0 mmol/L [54 mg/dL]) symptomatic hypoglycaemia (DSH).
Results
Overall LBGI in EDITION 2 and SENIOR and night-time LBGI in all three trials were significantly (p < 0.05) lower with Gla-300 versus Gla-100. The largest differences between Gla-300 and Gla-100 were observed during the night. In all three trials, individual LBGI results correlated with the observed number of DSH episodes per participant (EDITION 2 [r = 0.35, p < 0.001]; EDITION 3 [r = 0.26, p < 0.001]; SENIOR [r = 0.30, p < 0.001]). Participants at moderate risk of experiencing hypoglycaemia (defined as LBGI > 1.1) reported 4- to 8-fold more frequent DSH events than those at minimal risk (LBGI ≤ 1.1) (p ≤ 0.009).
Conclusions
The LBGI identified individuals with T2DM at risk for hypoglycaemia using SMPG data and correlated with the number of DSH events. Using the LBGI metric, a lower risk of hypoglycaemia with Gla-300 than Gla-100 was observed in all three trials. The finding that differences in LBGI are greater at night is consistent with previously published differences in the pharmacokinetic profiles of Gla-300 and Gla-100, which provides the physiological foundation for the presented results.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-020-00808-y) contains supplementary material, which is available to authorized users.
Keywords: Basal insulin, Glycaemic variability, Hypoglycaemia, Insulin glargine, Type 2 diabetes
Key Summary Points
| Optimal management of diabetes requires not only the achievement of glycaemic targets, but also the analysis of blood glucose fluctuations potentially leading to hyper- and hypoglycaemia. |
| Most glycaemic variability metrics are inherently biased towards hyperglycaemia, but the well-established low blood glucose index (LBGI) focusses entirely on low blood glucose excursions. |
| In this study, we used daily self-monitored plasma glucose (SMPG) profiles to assess the LBGI and to predict hypoglycaemia risk in the EDITION 2, EDITION 3 and SENIOR trials, each of which investigated the use of insulin glargine 300 U/mL (Gla-300) versus insulin glargine 100 U/mL (Gla-100) in type 2 diabetes. |
| In all three studies, the LBGI correlated well with the reported number of documented symptomatic hypoglycaemia events and could be used to identify those at risk of hypoglycaemia. |
| Using the LBGI as a metric of hypoglycaemia risk, a lower risk was observed for Gla-300 versus Gla-100 in all three trials, corroborating previous analyses of pharmacokinetic and pharmacodynamic profiles. |
Introduction
Daily fluctuations in blood glucose (BG) levels, captured under the term glycaemic variability (GV), are a well-recognised problem in the day-to-day management of type 1 and type 2 diabetes (T1DM and T2DM) [1], and are considered a risk factor for severe hypoglycaemia [2, 3], microvascular complications [4], cognitive impairment, lower quality of life and negative emotions [5, 6]. While HbA1c has been the gold standard for assessing average glycaemic control, it is a measure of exposure to BG over months and therefore does not account for the more rapid changes in glycaemic control and associated risks for hypoglycaemia that can occur every day [1]. New evidence, and a better understanding of the importance of GV, is shifting attention to GV and other measures that reflect the dynamics of BG fluctuations beyond HbA1c.
Analysis of GV, introduced 20 years ago [7] and recently reviewed [8], has been a valuable tool for assessing risk for hypo- and hyperglycaemia in diabetes. While metrics such as the mean amplitude of glucose excursions (MAGE) and the standard deviation of blood glucose over time have been explored, most such measures have an inherent bias towards hyperglycaemia [8]. This is because BG values typically have an asymmetrical distribution; therefore, deviations towards hyperglycaemia (e.g. BG > 10 mmol/L [180 mg/dL]) have a wider range of values and tend to be numerically “heavier” and are therefore given more weight than variations towards hypoglycaemia (e.g. blood glucose < 3.9 mmol/L [70 mg/dL]) in statistical calculations [8]. To address this issue, the design of the low blood glucose index (LBGI), a GV-based metric of the risk for hypoglycaemia, ignores hyperglycaemia [3, 8–10] and places more emphasis on the hypoglycaemic range. The LBGI increases with the frequency and extent of hypoglycaemic excursions and has been used as a predictor of severe events; baseline LBGI has been reported to be a good predictor of hypoglycaemia in people with type 1 diabetes who switched to continuous subcutaneous insulin infusion [11], and the LBGI has been shown to be predictive of symptomatic severe hypoglycaemia in people with diabetes [2, 3].
Insulin glargine 100 U/mL (Gla-100), a once-daily long-acting basal insulin, has been approved in the US and Europe since 2000. It has good efficacy and safety profiles, with less hypoglycaemia compared with neutral protamine Hagedorn (NPH); however, hypoglycaemia is still observed in patients on Gla-100 [12–14]. To address this problem, a second-generation basal insulin analogue, insulin glargine 300 U/mL (Gla-300), was developed to provide a more stable and prolonged pharmacokinetic and pharmacodynamic (PK/PD) profile than Gla-100, the efficacy and safety of which was assessed versus that of Gla-100 in the EDITION 1–3 trials of different populations with T2DM [15–17]. Both Gla-300 and Gla-100 exhibit comparable effectiveness in achieving glycaemic control (as measured, for example, by reductions in HbA1c and self-monitored plasma glucose [SMPG]) in people with T2DM, although Gla-300 is associated with a lower risk of hypoglycaemia than Gla-100 [15–17]. When comparing PK/PD profiles, Gla-100 is associated with higher insulin concentrations and activity, particularly overnight [18]. In EDITION 2 (previously treated with basal insulin and oral antihyperglycaemic drugs [OADs]), the improved PK/PD profile of Gla-300 versus Gla-100 was associated with a 37% (p = 0.031) relative reduction in the annualised rate of nocturnal confirmed (≤ 3.9 mmol/L [≤ 70 mg/dL]) or severe hypoglycaemia [17]. Similarly, in EDITION 3 (insulin naïve), the relative risk of experiencing confirmed (≤ 3.9 mmol/L [≤ 70 mg/dL]) or severe hypoglycaemia at night with Gla-300 versus Gla-100 was 0.86 (95% confidence interval [CI] 0.69–1.07) [15]. Subsequently, in the SENIOR study of older individuals with T2DM aged ≥ 65 years, the hypoglycaemia benefit of Gla-300 versus Gla-100 was most marked in the oldest subpopulation of people aged ≥ 75 years.
In this reanalysis of the data from the EDITION 2, EDITION 3 and SENIOR comparative studies of Gla-300 and Gla-100 [15, 17, 19], we calculated the LBGI to examine if there were differences in this GV-based metric of hypoglycaemia risk between these two basal insulins. We also explored whether the LBGI may help to identify people with T2DM who are at an increased risk of hypoglycaemia.
Methods
Trials Included in the Analysis
The EDITION 2, EDITION 3 and SENIOR trials compared Gla-300 with Gla-100 in different populations of individuals with T2DM (Table S1 in the Electronic supplementary material, ESM) [15, 17, 19]. All three trials had a treat-to-target design with a fasting SMPG target of 4.4–5.6 mmol/L (79–100 mg/dL) in both EDITION trials and a less stringent target of 5.0–7.2 mmol/L (90–130 mg/dL) for the older population in SENIOR [15, 17, 19]. Once-daily subcutaneous injections of Gla-300 or Gla-100 were self-administered at approximately the same time every evening (between prior to the evening meal and bedtime). The analysis included SMPG daily profiles and data on documented symptomatic hypoglycaemia (DSH). SMPG profiles included plasma glucose readings taken before and 2 hours after each meal, at bedtime and at 3:00 a.m. in EDITION 2 and EDITION 3 studies and before each meal, at bedtime and at 3:00 a.m. in the SENIOR study. DSH was defined as a symptomatic event confirmed by a blood glucose reading below 3 mmol/L (54 mg/dL).
LBGI was computed using the SMPG daily profile data and a formula that has been used extensively in the past 20 years to gauge patients’ risk for hypoglycaemia [2, 3, 8, 10, 11, 20]. Briefly, the LBGI formula puts progressively increasing weights on lower SMPG values, thereby emphasising glucose variability in the hypoglycaemic range. Thus, by design, LBGI is particularly sensitive to hypoglycaemia and has been shown to be a robust predictor of symptomatic hypoglycaemic episodes [2, 3]. Further details on the computation of LBGI are provided in the ESM.
Statistical Analyses
LBGI was analysed as the response variable in a linear mixed effects model with covariates including treatment (Gla-300 and Gla-100), treatment period (titration and maintenance), and treatment by treatment period interaction as fixed effects and subject as a random effect (Table 1). Mean LBGI at each collection time point was also summarised for each treatment and study (Fig. 1). The mean number and glucose nadir of DSH were summarised by patients with/without moderate hypoglycaemia risk (with moderate risk defined as LBGI > 1.1) for two treatments combined and for each study (Fig. 2). A P value from a two-sample t-test assuming unequal variance was provided to test the mean difference between these two risk groups. To further explore the association between LBGI and DSH while accounting for baseline factors, a negative binomial regression model was fitted with number of DSH events as the dependent variable and baseline age, baseline duration of diabetes, baseline HbA1c and LBGI-defined risk category as covariates for each study. SAS 9.4 software was used for statistical analyses.
Table 1.
Hypoglycaemia risk comparison of participants with T2DM treated with Gla-300 versus Gla-100
| Risk index | EDITION 2 | EDITION 3 | SENIOR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gla-300 | Gla-100 | p value | Gla-300 | Gla-100 | p value | Gla-300 | Gla-100 | p value | |
| LBGI | |||||||||
| Titration | 0.33 | 0.51 | < 0.0001 | 0.24 | 0.30 | 0.0587 | 0.28 | 0.34 | 0.1167 |
| Maintenance | 0.41 | 0.50 | 0.043 | 0.38 | 0.41 | 0.4205 | 0.34 | 0.44 | 0.0125 |
| Overall p value | 0.0003 | 0.1012 | 0.0084 | ||||||
| Nocturnal LBGI | |||||||||
| Titration | 0.72 | 1.29 | < 0.0001 | 0.50 | 0.59 | 0.1838 | 0.39 | 0.51 | 0.0604 |
| Maintenance | 0.98 | 1.24 | 0.0387 | 0.73 | 0.92 | 0.0176 | 0.52 | 0.67 | 0.0270 |
| Overall p value | < 0.0001 | 0.0167 | 0.0082 | ||||||
The data points represent mean values unless otherwise indicated
Gla-100 insulin glargine 100 U/mL, Gla-300 insulin glargine 300 U/mL, LBGI low blood glucose index
p value represents treatment differences between Gla-300 and Gla-100 within the titration, maintenance or overall (titration and maintenance) trial periods
Fig. 1.
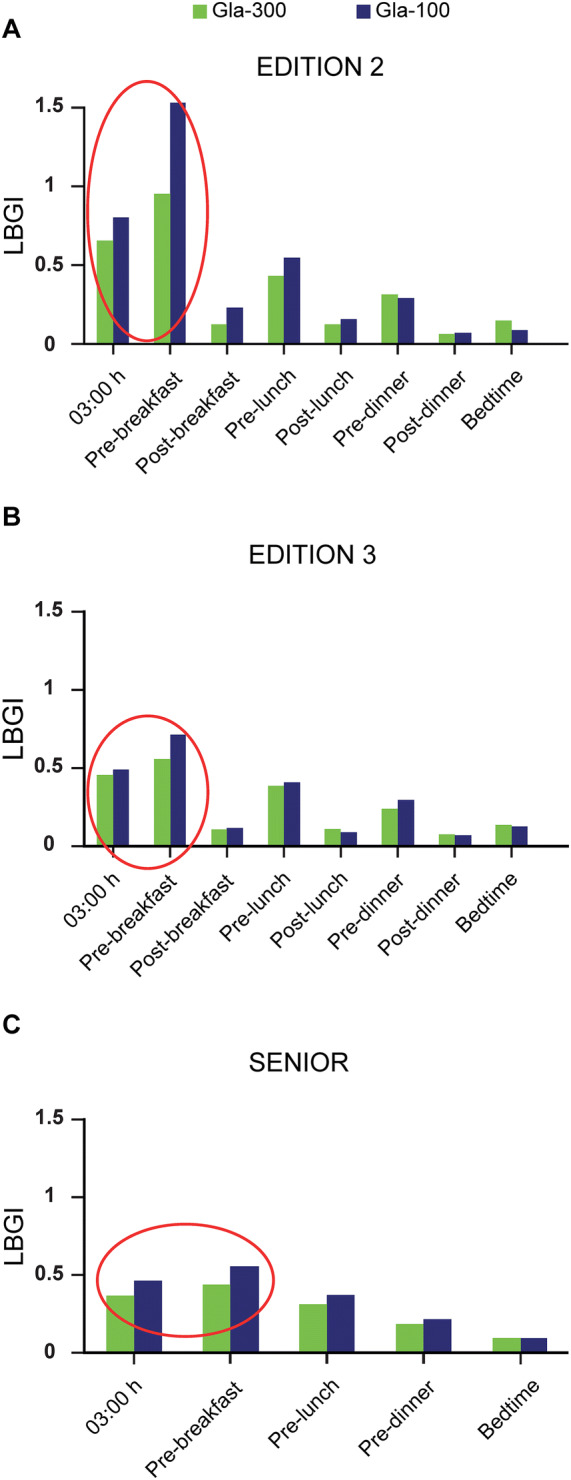
Daily profiles of LBGI calculated using data at each time point for Gla-300 versus Gla-100 in the a EDITION 2 b EDITION 3 and c SENIOR trials. Measurements were only performed at the five time points shown in SENIOR. Gla-100 insulin glargine 100 U/mL, Gla-300 insulin glargine 300 U/mL, LBGI low blood glucose index
Fig. 2.
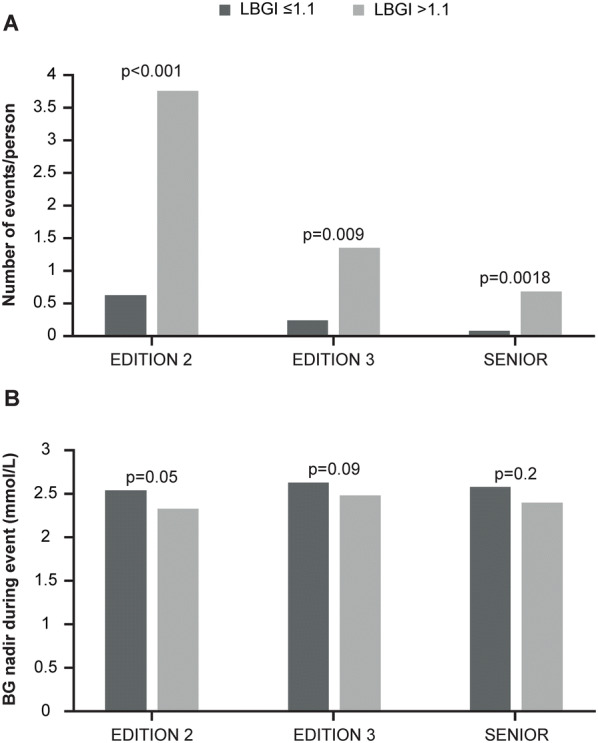
The a number and b glucose nadir of documented symptomatic hypoglycaemia events stratified by LBGI ≤ 1.1 and > 1.1. BG blood glucose, LBGI low blood glucose index
Compliance with Ethics Guidelines
This analysis did not involve primary data collection by the authors, and all reanalysed data from EDITION 2, EDITION 3 and SENIOR were anonymised, so separate ethical approval was not required. Appropriate local or national ethics committees approved the protocols for the multicentre EDITION 2, EDITION 3 and SENIOR trials, which were conducted according to Good Clinical Practice and the Declaration of Helsinki.
Results
Overall LBGI (based on all relevant SMPG data) in the EDITION 2 and SENIOR studies and night-time LBGI (based on the 3:00 a.m. and pre-breakfast SMPG values) in all three trials were significantly (p < 0.05) lower with Gla-300 than with Gla-100 (Table 1). These differences were more apparent during the titration phases (first 16 weeks after study initiation) than in the maintenance phase in EDITION 2. In EDITION 3, only the nocturnal LBGI difference between Gla-300 and Gla-100 in the maintenance phase reached statistical significance. For the SENIOR study, the difference in both overall and nocturnal LBGI values between Gla-300 and Gla-100 reached statistical significance in the maintenance but not the titration phases.
The largest differences in LBGI between Gla-300 and Gla-100 were observed during the night (Table 1). The finding that the LBGI differences between Gla-300 and Gla-100 were more prominent at night was confirmed by examining the daily LBGI profiles. These profiles indicated a lower risk for hypoglycaemia with Gla-300 versus Gla-100 in all three trials, with the differences being most evident at 3:00 a.m. and before breakfast (Fig. 1).
In all three trials examined, the LBGI calculated for each participant correlated with the observed number of DSH episodes per participant (EDITION 2 [r = 0.35, p < 0.001]; EDITION 3 [r = 0.26, p < 0.001]; SENIOR [r = 0.30, p < 0.001]). Overall, most participants were at a low risk for hypoglycaemia (average LBGI < 1.1). However, the average LBGI ventured into the moderate-risk zone (defined as LBGI > 1.1 [10]) for Gla-100 pre-breakfast in EDITION 2 (Fig. 1). Additionally, there were individuals with nocturnal LBGI > 1.1 in all studies. Participants who were at moderate risk of experiencing hypoglycaemia [10] reported significantly more frequent DSH than those at minimal risk (LBGI ≤ 1.1) (Fig. 2a). Regression analysis showed that the difference in the number of DSH events between LBGI-defined risk categories was statistically significant (p < 0.01) for all three trials after adjustment for baseline age, baseline duration of diabetes and baseline HbA1c. There was a trend for those at moderate risk of hypoglycaemia (LBGI > 1.1) to have lower reported blood glucose nadirs for documented symptomatic hypoglycaemic events compared with those at minimal risk of hypoglycaemia (LBGI ≤ 1.1) (Fig. 2b). This difference achieved statistical significance in EDITION 2 (p = 0.05).
Discussion
The LBGI has previously been used to quantify the risk of hypoglycaemia based on SMPG data and to characterise glycaemic variability within the hypoglycaemic range with different treatments and populations [3, 7, 9, 10, 20–22]. Over the past 20 years, the LBGI has been shown to correlate with the occurrence of severe hypoglycaemia and has been reported to be higher in people with a history of severe hypoglycaemia [2, 3, 7]. Higher LBGI may indicate multiple mild hypoglycaemic events, a small number of severe hypoglycaemic events, or a combination of both [23].
People with diabetes who achieve mean glucose values within the target range could still be at risk of complications from hypoglycaemia and hyperglycaemia if they have high GV [24]. Therefore, for optimum glycaemic control, it is important to focus on GV in addition to HbA1c. LBGI, which by definition increases with the frequency and extent of hypoglycaemic excursions, was consistently lower for Gla-300 than Gla-100, particularly at night. This difference in LBGI between Gla-300 and Gla-100 was confirmed in all three clinical trials examined (the EDITION 2, EDITION 3 and SENIOR studies) [15, 17, 19]. Insulin degludec (IDeg) is another second-generation basal insulin analogue that has a less variable PK/PD profile than that of Gla-100 and has demonstrated similar glycaemic control to Gla-100 with less hypoglycaemia in phase 3 clinical trials. In future analyses, it would be of interest to use LBGI to directly compare Gla-300 with IDeg, as no difference in SMPG variability was seen in the recent BRIGHT trial [25], whereas PK/PD studies comparing the two second-generation basal insulin analogues have reported conflicting results [26, 27].
The daily LBGI profile was consistent with previously reported pharmacokinetic and pharmacodynamic differences between Gla-300 and Gla-100; at steady state, insulin concentration and glucose infusion rate profiles following the injection of Gla-300 were more constant over 24 h compared with those of Gla-100 [18]. It is notable that the periods when differences between Gla-300 and Gla-100 concentrations were most pronounced (e.g. 03:00 a.m. and pre-breakfast) coincide with the greatest differences in LBGI.
Overall, LBGI was not significantly different with Gla-300 versus Gla-100 in the EDITION 3 (insulin-naïve) study, but was significantly different in EDITION 2 (OAD + basal insulin) and SENIOR (older adults aged ≥ 65 years). Night-time LBGI was significantly different across all three trials. It is possible that the insulin-naïve population in EDITION 3, who had a shorter mean duration of T2DM versus participants in EDITION 2 and SENIOR, retained greater endogenous insulin and glucagon secretion and hence were better able to compensate for the more pronounced peaks and troughs of insulin activity with Gla-100 versus Gla-300. Given the longer mean duration of diabetes in participants in EDITION 2 (12.6 years) and SENIOR (15.3 years) versus EDITION 3 (mean 9.8 years), it is likely that the populations in SENIOR and EDITION 2 would have had more advanced T2DM versus those in EDITION 3 [15, 17, 19]. As the duration of T2DM increases, there is a progressive loss of endogenous glucoregulatory responses [28], which may increase the risk of individuals experiencing hypoglycaemia in response to peaks of exogenous insulin activity. This may explain why the risk of hypoglycaemia is increased with longer diabetes duration and longer duration of insulin treatment [29, 30]. In addition, the observation that final insulin doses were substantially higher in EDITION 2 (0.97 U/kg/day) versus EDITION 3 (0.67 U/kg/day) despite identical glucose targets [15, 17] supports the suggestion that participants in EDITION 2 had more demanding T2DM than the insulin-naïve group in EDITION 3, and were possibly less able to cope with the more pronounced peaks of insulin activity with Gla-100 versus Gla-300. It should be noted, however, that LBGI was still significantly different at night in the previously insulin-naïve population in EDITION 3, which corresponds to the period in which the PK/PD differences between Gla-300 and Gla-100 are most pronounced.
There is a growing awareness of the need to measure GV, and of the use of the GV-based LBGI as a predictive tool to identify people at higher risk of experiencing hypoglycaemia. In part, the use of continuous glucose monitoring (CGM) technology has highlighted the requirement for a more in-depth approach to assessing glucose control, with a focus that goes beyond HbA1c as the main parameter of interest [22]. While high GV in people with diabetes has been reported in clinical studies using CGM, discussions are ongoing regarding the most appropriate measures of GV to use [24, 31]. For example, the Advanced Technologies and Treatments for Diabetes (ATTD) consensus panel have recommended what they consider key metrics for reporting CGM data. These recommendations included LBGI to assess the risk of hypoglycaemia, but also other measures to assess the risk of hyperglycaemia, such as mean glucose, low glucose events (< 3.0 mmol/L [54 mg/dL]), percentage time in glucose target range (3.9–3.0 mmol/L [70–54 mg/dL]), percentage time in hyperglycaemia, events of elevated (> 10 mmol/L [> 180 mg/dL]) and very elevated (> 13.9 mmol/L [> 250 mg/dL]) glycaemia, and high blood glucose index (HBGI) [22]. Given that LBGI, HBGI and other GV measures can be calculated from SMPG data, the use of LBGI in our analysis is consistent with the international consensus in the field [22] and other recommendations [32].
Our analysis focusses entirely on the LBGI, rather than on other metrics of GV, which could be considered a limitation. Other limitations include the fact that only studies of Gla-300 versus Gla-100 were used in the analysis, and that CGM data were not available for comparison.
Conclusions
LBGI appears to be a good predictor for the risk of hypoglycaemia, and correlates well with the reported number of DSH episodes. People with T2DM at risk of experiencing hypoglycaemic episodes when treated with basal insulin may be identified by using SMPG data to calculate their LBGI, which may be a cost-effective alternative to CGM for hypoglycaemia risk prediction, particularly in larger populations. When LBGI was used as a metric of hypoglycaemic risk, a lower risk for hypoglycaemia with Gla-300 than with Gla-100 was reliably demonstrated in all three trials analysed. This finding was consistent with the differences in the pharmacokinetic profiles observed between Gla-300 and Gla-100, providing a physiological foundation for the presented results. While HbA1c measurements and targets are a central and familiar part of diabetes treatment, GV targets should also be incorporated into treatment programmes to reduce the risk of hypoglycaemia, hyperglycaemia and related diabetes complications [33].
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This analysis of data from EDITION 2, EDITION 3 and SENIOR was funded by Sanofi. The journal’s Rapid Service fee was also funded by Sanofi.
Medical Writing and Editorial Assistance
Editorial and writing assistance was provided by Sharon Eastwood (DPhil) of Fishawack Communications Ltd, and was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Presented at the 53rd Annual Meeting of the European Association for the Study of Diabetes, 11–15 September 2017, Lisbon, Portugal.
Disclosures
Boris Kovatchev has acted as a consultant and/or received honoraria from Sanofi. Professor Kovatchev has also received research support from Dexcom, Roche Diagnostics and Tandem Diabetes Care, and receives patent royalties managed by the University of Virginia from Johnson & Johnson, Dexcom, and Sanofi. Marc Breton has acted as a consultant for Roche Diagnostics, Ascensia Diabetes Care, and Sanofi, and has received research support from Ascensia Diabetes Care, Roche Diagnostics, Tandem Diabetes Care, Dexcom, Senseonics, and Novo Nordisk. In addition, Dr Breton receives patent royalties managed by the University of Virginia from Dexcom and Sanofi. Zhaoling Meng, Anna Cali and Riccardo Perfetti are/were Sanofi employees and shareholders. Riccardo Perfetti has changed affiliation since this study was conducted, to Applied Therapeutics, New York, USA.
Compliance with Ethics Guidelines
This analysis did not involve primary data collection by the authors, and all reanalysed data from EDITION 2, EDITION 3 and SENIOR were anonymised, so separate ethical approval was not required. Appropriate local or national ethics committees approved the protocols for the multicentre EDITION 2, EDITION 3 and SENIOR trials, which were conducted according to Good Clinical Practice and the Declaration of Helsinki.
Data Availability
All relevant data are contained within the manuscript. Proposals relating to the data should be directed to boris@virginia.edu. To gain access, data requestors will need to sign a data access agreement.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.12019059.
References
- 1.Satya Krishna SV, Kota SK, Modi KD. Glycemic variability: clinical implications. Indian J Endocrinol Metab. 2013;17:611–619. doi: 10.4103/2230-8210.113751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox DJ, Gonder-Frederick L, Ritterband L, Clarke W, Kovatchev BP. Prediction of severe hypoglycemia. Diabetes Care. 2007;30:1370–1373. doi: 10.2337/dc06-1386. [DOI] [PubMed] [Google Scholar]
- 3.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21:1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol. 2018;17:33. doi: 10.1186/s12933-018-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox D, Gonder-Frederick L, McCall A, Kovatchev B, Clarke W. The effects of glucose fluctuation on cognitive function and QOL: the functional costs of hypoglycaemia and hyperglycaemia among adults with type 1 or type 2 diabetes. Int J Clin Pract Suppl. 2002;20–26. [PubMed]
- 6.Cox DJ, McCall A, Kovatchev B, Sarwat S, Ilag LL, Tan MH. Effects of blood glucose rate of changes on perceived mood and cognitive symptoms in insulin-treated type 2 diabetes. Diabetes Care. 2007;30:2001–2002. doi: 10.2337/dc06-2480. [DOI] [PubMed] [Google Scholar]
- 7.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20:1655–1658. doi: 10.2337/diacare.20.11.1655. [DOI] [PubMed] [Google Scholar]
- 8.Kovatchev BP. Metrics for glycaemic control—from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol. 2017;13:425–436. doi: 10.1038/nrendo.2017.3. [DOI] [PubMed] [Google Scholar]
- 9.Kovatchev BP, Cox DJ, Gonder-Frederick L, Clarke WL. Methods for quantifying self-monitoring blood glucose profiles exemplified by an examination of blood glucose patterns in patients with type 1 and type 2 diabetes. Diabetes Technol Ther. 2002;4:295–303. doi: 10.1089/152091502760098438. [DOI] [PubMed] [Google Scholar]
- 10.Kovatchev BP, Cox DJ, Kumar A, Gonder-Frederick L, Clarke WL. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol Ther. 2003;5:817–828. doi: 10.1089/152091503322527021. [DOI] [PubMed] [Google Scholar]
- 11.Crenier L, Abou-Elias C, Corvilain B. Glucose variability assessed by low blood glucose index is predictive of hypoglycemic events in patients with type 1 diabetes switched to pump therapy. Diabetes Care. 2013;36:2148–2153. doi: 10.2337/dc12-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanofi. Prescribing information for Lantus®. Paris: Sanofi; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021081s072lbl.pdf. Accessed 6 Nov 2019.
- 13.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet. 2008;371:1073–1084. doi: 10.1016/S0140-6736(08)60485-7. [DOI] [PubMed] [Google Scholar]
- 14.Riddle M. C., Rosenstock J., Gerich J. The Treat-to-Target Trial: Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 15.Bolli GB, Riddle MC, Bergenstal RM, Wardecki M, Goyeau H, Home PD, et al. Glycaemic control and hypoglycaemia with insulin glargine 300 U/mL versus insulin glargine 100 U/mL in insulin-naive people with type 2 diabetes: 12-month results from the EDITION 3 trial. Diabetes Metab. 2017;43:351–358. doi: 10.1016/j.diabet.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Riddle MC, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1) Diabetes Care. 2014;37:2755–2762. doi: 10.2337/dc14-0991. [DOI] [PubMed] [Google Scholar]
- 17.Yki-Jarvinen H, Bergenstal RM, Bolli GB, Ziemen M, Wardecki M, Muehlen-Bartmer I, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/mL versus insulin glargine 100 U/mL in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension. Diabetes Obes Metab. 2015;17:1142–1149. doi: 10.1111/dom.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units/mL provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units/mL. Diabetes Care. 2015;38:637–43. [DOI] [PubMed]
- 19.Ritzel R, Harris SB, Baron H, Florez H, Roussel R, Espinasse M, et al. A randomized controlled trial comparing efficacy and safety of insulin glargine 300 Units/mL versus 100 Units/mL in older people with type 2 diabetes: results from the SENIOR study. Diabetes Care. 2018;41:1672–1680. doi: 10.2337/dc18-0168. [DOI] [PubMed] [Google Scholar]
- 20.Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther. 2005;7:849–862. doi: 10.1089/dia.2005.7.849. [DOI] [PubMed] [Google Scholar]
- 21.Kovatchev B, Breton M, Clarke W. Analytical methods for the retrieval and interpretation of continuous glucose monitoring data in diabetes. Methods Enzymol. 2009;454:69–86. doi: 10.1016/S0076-6879(08)03803-2. [DOI] [PubMed] [Google Scholar]
- 22.Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabris Chiara, Patek Stephen D., Breton Marc D. Are Risk Indices Derived From CGM Interchangeable With SMBG-Based Indices? Journal of Diabetes Science and Technology. 2015;10(1):50–59. doi: 10.1177/1932296815599177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyser TA, Balo AK, Buckingham BA, Hirsch IB, Garcia A. Glycemic variability percentage: a novel method for assessing glycemic variability from continuous glucose monitor data. Diabetes Technol Ther. 2018;20:6–16. doi: 10.1089/dia.2017.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritzel R, Cheng A, Bosnyak Z, Boëlle-Le Corfec E, Cali AMG, Wang X, et al. Similar variability of fasting and 24-hr self-measured plasma glucose with Gla-300 vs IDeg-100 in insulin-naive adults with type 2 diabetes: the randomised BRIGHT trial. Diabetologia. 2018;61:S440.
- 26.Bailey TS, Pettus J, Roussel R, Schmider W, Maroccia M, Nassr N, et al. Morning administration of 0.4 U/kg/day insulin glargine 300 U/mL provides less fluctuating 24-h pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100 U/mL in type 1 diabetes. Diabetes Metab. 2018;44:15–21. doi: 10.1016/j.diabet.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Heise T, Norskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: lower day-to-day and within-day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab. 2017;19:1032–1039. doi: 10.1111/dom.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25:245–254. doi: 10.1111/j.1464-5491.2007.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 31.Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39:502–510. doi: 10.2337/dc15-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18(Suppl 2):S3–S13. doi: 10.1089/dia.2015.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39:273–282. doi: 10.4093/dmj.2015.39.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are contained within the manuscript. Proposals relating to the data should be directed to boris@virginia.edu. To gain access, data requestors will need to sign a data access agreement.


