Abstract
This review describes a presentation at a recent symposium entitled “SUs in the treatment of T2DM: a fresh look and new insights” on Wednesday September 18, 2019 at the 55th Annual Meeting of the European Association for the Study of Diabetes (EASD) in Barcelona, Spain. It examines the current role of sulfonylureas (SUs) in the management of type 2 diabetes mellitus (T2DM) and gives the author’s personal perspective of how this therapeutic class has performed in both local and international guidelines. The place of SUs within current guidelines is highlighted, and a critical appraisal of the reasons for the differences between guidelines given. Finally, comparison of evidence-based guidelines and consensus reports is discussed.
Keywords: Consensus report, Evidence-based guidelines, Sulfonylureas, Type 2 diabetes mellitus
Key Summary Points
| Key guidelines on the treatment of type 2 diabetes mellitus (T2DM) include those from the World Health Organization (WHO) and the International Diabetes Federation (IDF) and are evidence-based, whereas the popular joint report from the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) is a consensus |
| Numerous regional guidelines on diabetes treatment are currently available, including the scientifically rigorous and independent National Institute for Health and Care Excellence (NICE) guideline in the UK |
| This review provides a critical appraisal of differences between various guidelines, and compares evidence-based guidelines with consensus reports, on the role of sulfonylureas (SUs) in the management of T2DM |
| Most international and regional guidelines differentiate between different SUs |
| SUs remain widely recommended as safe and effective glucose-lowering agents, with low absolute rates of severe hypoglycaemia |
Introduction
The four main internationally recognised guidelines or consensus reports for the treatment of type 2 diabetes mellitus (T2DM) are from the European Association for the Study of Diabetes (EASD)/American Diabetes Association (ADA) [1], Diabetes Canada [2], the World Health Organization (WHO) [3] and the International Diabetes Federation (IDF) [4]. Of these, EASD/ADA is the most popular despite it being only a consensus report, and not meeting the Institute of Medicine requirements for trustworthy guidelines [5]. The current article describes the place of sulfonylureas (SUs) within current international guidelines for the management of T2DM, and critically examines the quality of the guideline development process and of the evidence to support those recommendations. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by the authors.
The Place of Sulfonylureas Within Guidelines
The EASD/ADA consensus report recommends metformin as initial therapy, with further management based on whether or not patients have established atherosclerotic cardiovascular disease (ASCVD) or chronic kidney disease (CKD) and/or heart failure (HF). In these patients, SUs are the last choice of drug, despite the fact that SUs were taken by up to 50% of patients in cardiovascular outcomes trials (CVOTs) and benefits were shown in these patients [6–8]. Furthermore, the EASD/ADA divides patients without ASCVD or CKD into the following groups: those with a compelling need to minimise hypoglycaemia, for whom insulin and insulin secretagogues (e.g. SUs) are last-choice therapy; those with a compelling need to minimise weight gain or promote weight loss, for whom SUs are last-choice therapy; and those where cost is a major issue, who are the only patients for whom they recommend use of SUs after metformin [1].
In contrast, the Canadian guidelines are well considered and evidence based, with no hierarchy for patients without cardiovascular disease (CVD) [2]. Physicians are guided to select the best drug to use for each patient; SUs are not recommended in elderly patients or those with CKD, but may be useful in those requiring rapid blood glucose lowering. Gliclazide is recommended as the first-choice SU because, compared with other SUs, it has a lower risk of hypoglycaemia, cardiovascular (CV) events and mortality [2].
In the development of their guidelines, the WHO reviewed results of seven systematic reviews conducted between 2007 and 2017 to establish the standard of care for second- and third-line therapies in a resource-limited setting, with a focus on SUs, thiazolidinediones (TZDs), dipeptidyl peptidase 4 (DPP4) inhibitors and sodium–glucose co-transporter 2 (SGLT2) inhibitors [9]. The WHO noted the following: DPP4 inhibitors were less effective than SUs in terms of lowering glycosylated haemoglobin (HbA1c), with a treatment difference of − 0.12%; TZDs and insulin are associated with weight gain, while SGLT2 and DPP4 inhibitors favour weight loss; there is less hypoglycaemia with TZDs, DPP4 inhibitors and SGLT2 inhibitors; and there are no differences in CVD and mortality risk between drug classes in the studies analysed [9]. In terms of hypoglycaemia, the odds of severe hypoglycaemia were higher with SUs, but the absolute risk for severe hypoglycaemia could not be determined from randomised controlled trials (RCTs) because there were too few events; the risk of hypoglycaemia (of varying severity) ranged from 0.2 to 1.8 events per 100 person-years. With regard to the cost-effectiveness of SUs, the cost of DPP4 inhibitors was 3.5–30 times higher, SGLT2 inhibitors were 4.5–26 times higher and TZDs were 2.6–6 times higher [3]. The WHO guidelines concluded that SUs are the first-line drugs of choice in patients who do not tolerate metformin, that SUs should be added to metformin in patients not at HbA1c target (strong recommendation; moderate quality evidence), and that SUs with a better safety record for hypoglycaemia (e.g. gliclazide) are preferred in patients for whom hypoglycaemia is a concern [9].
The IDF Guidelines Task Force considered that there are many clinical practice guidelines around the world to manage T2D at the local, regional and international level, with significant differences in some topics that may confuse physicians regarding the management of their patients. With the objective to provide recommendations that will facilitate their decision-making processes in their daily real-word practice, the 2017 IDF guidelines appraised all 23 available national and international guidelines using the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument [4]. Of these, nine guidelines scoring more than 70% with AGREE II criteria were selected, as well as three popular guidelines (ADA, AACE and IDF 2014). With regard to monotherapy, the IDF recommends metformin as the preferred first-line choice, but other glucose-lowering drugs are recommended if metformin is not tolerated, preferably an SU (not glibenclamide), an alpha-glucosidase inhibitor (AGI) or a DPP4 inhibitor. For dual (second-line) therapy, the best choice of add-on therapy includes SUs (not glibenclamide), a DPP4 inhibitor, SGLT2 inhibitor or AGI. Glucagon-like peptide 1 receptor agonists (GLP-1RAs) can be used if weight loss is a priority and the drug is affordable [4].
In addition to internationally recognised guidelines, regional guidelines include the National Institute for Health and Care Excellence (NICE) [10] in the UK, the South Asian Federation of Endocrine Societies (SAFES) [11], the Royal Australian College of General Practitioners (RACGP)/Diabetes Australia [12] and the Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA) [13]. The NICE guidelines are regularly updated, with the last update being August 2019 [10]. They are scientifically rigorous, with an emphasis on safety, efficacy and cost-effectiveness, and consider all SUs to be safe and effective glucose-lowering agents suitable for use in first-, second- and third-line therapy. They recommend SU use preferentially over SGLT2 inhibitors and GLP-1RAs [10]. The next NICE update will take into account results of the CVOTs.
Of the other regional guidelines, the SAFES recommend gliclazide modified-release (MR) as the preferred SU because of its reduced mortality, better CV outcomes and renal protection compared with conventional SUs [11]. It is also preferred in elderly or overweight/obese patients, those at increased risk of hypoglycaemia or CVD, patients with previous CVD and during Ramadan [11]. Similarly, the RACGP/Diabetes Australia, SEMDSA, Dutch and Italian guidelines all favour SUs as second-line therapy, and differentiate gliclazide from other SUs, highlighting the lower CV risk, more than 50% fewer hypoglycaemia episodes, weight neutrality, proven microvascular benefits and lower costs with gliclazide [12–15].
Critical Appraisal of Reasons for Differences Between Guidelines
The reasons for differences between the guidelines vary. These include the paradox surrounding why guidelines may exist, as outlined in Table 1, the fact that some are consensus-based rather than evidence-based guidelines, as well as the scientific rigour, with which the evidence is applied. Differences between guidelines can also arise depending on their stated priorities, e.g. the target audience (general practitioners versus specialists), outcomes being measured (subjective non-measurable quality outcomes versus reliance on objective measurable outcomes), cost-effectiveness (considerations of the cost of implementing the guidelines’ suggested interventions versus having no considerations for cost-effectiveness), safety considerations (considering only short-term versus long-term safety data), ease of implementation (considering the complexities involved in implementing one treatment strategy over another), access to therapies (e.g. gliclazide is unavailable in the USA so is not highlighted in American guidelines) and finally author bias and conflicts of interest.
Table 1.
Why guidelines exist: the guidelines paradox
| Optimistic view | vs | Pessimistic view |
|---|---|---|
| Our guidelines are based on strong evidence | vs | If you have good evidence, you do not need a guideline (because the evidence speaks for itself) |
| Our guidelines help doctors to offer the best modern treatments to their patients | vs | Guidelines help societies maintain their status, and to compete with other organizations for funding |
| We issue guidelines as a service to the profession and humanity | vs | Guidelines are issued for a self-serving purpose |
| Our guidelines are popular because of their scientific quality | vs | Guidelines are popular when they have marketing appeal |
Adapted and modified from a presentation made by Eam [38] with permission from the author
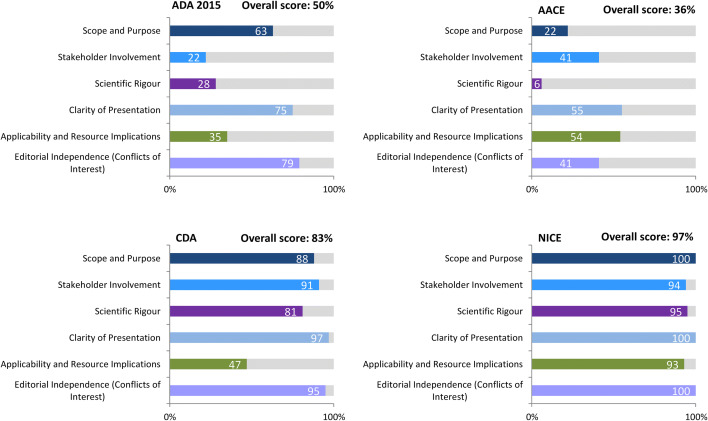
The AGREE II instrument enables comparison of the quality of guidelines, assessing the methodological rigour and transparency with which a guideline is developed [16]. Twenty-three items are organised within six domains, each of which captures a unique dimension of guideline quality (i.e. scope and purpose, stakeholder involvement, scientific rigour of development, clarity of presentation, applicability and editorial independence), with an additional two global rating items. Each item is rated on a 7-point scale and domain scores calculated by averaging the scores given by multiple appraisers [16].
Appraisal by the IDF of all of the available diabetes guidelines in 2017 using AGREE II revealed large differences in quality [4]. For example, the scientific rigour score was 6% for the AACE guidelines, 28% for the ADA 2015 guidelines, 81% for the Canadian guidelines and 95% for the NICE guidelines. The NICE guidelines also scored 100% for scope and purpose, clarity of presentation and editorial independence (i.e. conflicts of interest). Overall scores were 36% for AACE, 50% for ADA 2015, 83% for the Canadian guidelines and 97% for NICE, suggesting a conflict between the quality of guidelines and the popularity of consensus reports (Fig. 1) [4].
Fig. 1.
Summary of appraisal of four key guidelines by the AACE, ADA, NICE and the CDA conducted by the International Diabetes Federation using AGREE II [4]. AACE American Association of Clinical Endocrinologists, ADA American Diabetes Association, CDA Canadian Diabetes Association, NICE National Institute for Health and Care Excellence
Evidence-Based Guidelines Versus Consensus Reports
A consensus has been light-heartedly described by Margaret Thatcher, a former UK prime minister, as “the process of abandoning all beliefs, principles, values and policies in search of something in which no one believes, but to which no one objects” and, according to the Israeli diplomat and politician Abba Eben, “a consensus means that everyone agrees to say collectively what no one believes individually”.
Despite being elevated to the status of guidelines by medical practitioners, the EASD/ADA publication is a consensus report of its ten authors, and makes no evidence-graded recommendations [1]. In fact, the authors state “though evidence based, the recommendations presented herein are the opinions of the authors”. There is also some inconsistency with how drugs within a class are handled in the consensus report [1]. The EASD/ADA report handles newer drug classes (e.g. GLP-1RAs and SGLT2 inhibitors) differently to older ones, by attempting to grade the evidence within the newer drug classes for CVD benefit (“liraglutide > semaglutide > exenatide extended release, and empagliflozin > canagliflozin > dapagliflozin”) and weight loss benefit (“semaglutide > liraglutide > dulaglutide > exenatide > lixisenatide”). The same is not applied to older “off-patent” drug classes even where clear differences exist within the class, such as the cardiovascular benefits of pioglitazone versus rosiglitazone [17], or the hypoglycaemic risk [18, 19], renal safety [20, 21] and cardiovascular safety with gliclazide versus glimepiride.
In managing T2DM, the EASD/ADA consensus report categorises and highlights patients with a “compelling need to minimise weight gain or promote weight loss” [1]. The report makes no mention of what these “compelling” indications might be, and there currently exists no evidence linking the weight loss from these drugs to any meaningful or measurable clinical outcomes. Weight loss with SGLT2 inhibitors of approximately 1.5–2.9 kg has been demonstrated in four network meta-analyses of data from major studies [22–25]. Slight reductions in body weight with GLP-1RAs versus placebo have also been documented in a number of trials (Table 2) [6–8, 26–28]. These slight reductions in body weight have been confirmed in a recent comprehensive network meta-analysis of diabetes medications [19]. Currently, there is no evidence linking the magnitude weight loss from SGLT2 inhibitors or GLP-1RAs with outcomes such as glycaemic control, cardiovascular benefits or mortality benefits. Evidence for direct benefits on other clinically relevant outcomes is also lacking. However, prescribing these newer agents for undefined “compelling” indications to “minimise weight gain or promote weight loss” after metformin results in a significant cost burden to the healthcare system. For example, on the basis of NADAC (National Average Drug Acquisition Cost), the cost of semaglutide at maximum dose in the US was 523-fold higher than glimepiride for a 30-day supply in September 2019 (USD 993.73 vs USD 1.90) [29]. The question of how long patients should continue this therapy for the weight benefit, and comparisons with the effectiveness of other approved weight loss interventions is not addressed in the EASD/ADA document. The evidence driving this prominent recommendation in the ADA/EASD algorithm deserves more critical appraisal.
Table 2.
Body weight loss with glucagon-like peptide 1 receptor agonists versus placebo
| Trial name | GLP-1RA | Body weight loss vs placebo (95% CI), kg | Median follow-up duration, years | References |
|---|---|---|---|---|
| LEADER | Liraglutide | 2.3 (− 2.54, − 1.99) | 3.8 | [6] |
| SUSTAIN-6 | Semaglutide 0.5 mg | 2.9 | 2.1 | [21] |
| Semaglutide 1.0 mg | 4.3 | |||
| HARMONY | Albiglutide | 1.8 (1.7, 2.0) | 1.3 | [7] |
| EXSCEL | Exenatide | 1.27 (− 1.4, − 1.13) | 3.2 | [20] |
| ELIXA | Lixisenatide | 0.7 (− 0.9, − 0.5) | 2.1 | [22] |
| REWIND | Dulaglutide | 1.46 (1.25, 1.67) | 5.4 | [5] |
CI confidence interval, GLP-1RA glucagon-like peptide 1 receptor agonist
The EASD/ADA algorithm also distinguishes patients without ASCVD/CKD who have a compelling need to minimise hypoglycaemia [1]. Avoidance of severe hypoglycaemia is a common reason for drug choice in all guidelines (and common sense). This is especially important in patients at highest risk who may suffer catastrophic consequences, including the frail elderly, operators of heavy machinery, drivers of public transport or heavy duty vehicles, airline pilots, and patients who are unaware of hypoglycaemia, live alone, have impaired cognition or mobility, or have a high risk of fall and fracture [13]. For these individuals, sensible physicians avoid SUs and insulin whenever possible or set higher HbA1c targets. In contrast, in the CAROLINA study, patients were given glimepiride 1 mg and protocol-titrated every 4 weeks to a dose of 4 mg daily [30, 31]. This was despite the fact that 35% of patients had established CVD, 34% were more than 70 years old, 18% had an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 (i.e. a contraindication to using more than 1 mg of glimepiride [20]) and 41% had baseline HbA1c below 7.0% [30, 31]. These are not patients in whom sensible physicians would prescribe glimepiride 4 mg. Despite this, the incidence of severe hypoglycaemia with glimepiride was 2.2% and the incidence of hospitalisation due to hypoglycaemia was 0.9% in this population of high-risk patients [31]. This confirms the WHO conclusion that there is a small absolute risk for severe hypoglycaemia (with glimepiride in CAROLINA) and explains why, despite a large 85% reduction in the relative risk of severe hypoglycaemia, linagliptin was not associated with better CV outcomes [31]. It is not difficult to speculate then, that if these high-risk patients had been excluded, or if they had used gliclazide, which has an approximately threefold lower incidence of hypoglycaemia than glimepiride and “is more similar to metformin than other SUs” [18, 19, 32], these results might have been even better.
The EASD/ADA algorithm also highlights treatment choices where cost is a major issue, despite there being few places in the world where the cost of diabetes care is not a major problem. For example, in the USA, the cost of treating T2DM is increasing and with current costs of $237 billion per year [33]. The prominent inclusion of this category in the algorithm implies that the lower socioeconomic groups should get inferior treatment, which is rather disconcerting since guidelines should ensure cost-effective and equitable care for all. Cost-effectiveness is based on measurable clinical endpoints (positive and negative) and does not necessarily equate to being the cheapest option. In fact, head-to-head studies of gliclazide versus DPP4 inhibitors demonstrated similar efficacy, minimal weight gain with gliclazide and no cases of severe hypoglycaemia [34–36]. Also, at the time of publication of the ADA/EASD consensus, SGLT2 inhibitors and GLP-1RAs had not shown superiority when compared with conventional therapy with regard to major adverse CV event outcomes for patients without established ASCVD. A cost-effectiveness (taking into account all risks and benefits of) analysis for second-line therapies in patients without ASCVD undertaken in a first-world country clearly demonstrated that sulfonylureas remain the most cost-effective second-line therapy in patients inadequately controlled on metformin. In this analysis, the cost of gliclazide modified release was compared to all available DPP4 inhibitors, SGLT2 inhibitors, GLP-1 receptor agonists and insulins [37]. This analysis could find no measurable benefit that would have justified the higher cost of the other classes of drugs in patients without ASCVD.
Finally, the EASD/ADA do not provide guidelines for patients without compelling indications for particular drug classes in its algorithm [1], which is conceivably the majority of patients. Current evidence would suggest that in the absence of ASCVD, CKD or heart failure, a later-generation sulfonylurea (gliclazide > glimepiride) is still the most cost-effective second-line agent for these patients, even in a first world setting [12, 37]. This is probably the reason why, despite the negative narrative towards SUs over recent years, they remain the most widely prescribed second-line therapy [34], suggesting that practising physicians know something that the consensus experts do not.
Conclusions
SUs are still widely recommended and prescribed as safe and effective glucose-lowering drugs. The absolute rates of severe hypoglycaemia with later-generation SUs are low, as confirmed by the CAROLINA study and a recent meta-analysis [19]. Most international and regional guidelines prefer to differentiate among SUs, with gliclazide MR rated widely as having the lowest rates of hypoglycaemia and weight gain, and the best CV and renal safety. The EASD/ADA consensus report grades efficacy and safety for individual molecules in the newer drug classes but not for the older ones, lacks evidence for some of its major recommendations, and the algorithm does not assist primary care physicians with treating the majority of patients with T2DM.
Acknowledgements
Funding
Servier Medical Affairs, France, funded the development and publication of this article, including the journal’s Rapid Service Fee.
Authorship
The author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Medical Writing Assistance
The author would like to thank Andrea Bothwell, on behalf of Springer Healthcare Communications, who provided medical writing assistance with the first draft of this manuscript. This medical writing assistance was funded by Servier, France.
Prior Presentation
This article was based on the presentation given by the author at the symposium “SUs in the treatment of T2DM: a fresh look and new insights” during the 55th Annual Meeting of the European Association for the Study of Diabetes (EASD) in Barcelona Spain, 2019.
Disclosures
Aslam Amod has served on advisory panels, and participated in speaker bureaus and clinical trials for Aspen Pharmacare, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Novo Nordisk, Sanofi Aventis and Servier Laboratories.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.12030321.
References
- 1.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Canada Clinical Practice Guidelines Expert Committee. Lipscombe L, Booth G, et al. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes. 2018;42(Suppl 1):S88–S103. doi: 10.1016/j.jcjd.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Guidelines on second-and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. [PubMed]
- 4.International Diabetes Federation . Recommendations for managing type 2 diabetes in primary care. Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 5.Graham Robin, Mancher Michelle, Wolman Dianne Miller, Greenfield Sheldon, Steinberg Earl., editors. Clinical Practice Guidelines We Can Trust. Washington, D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- 6.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 9.Roglic G, Norris SL. Medicines for treatment intensification in type 2 diabetes and type of insulin in type 1 and type 2 diabetes in low-resource settings: synopsis of the World Health Organization guidelines on second- and third-line medicines and type of insulin for the control of blood glucose levels in nonpregnant adults with diabetes mellitus. Ann Intern Med. 2018;169(6):394–397. doi: 10.7326/M18-1149. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management (NG28). 2019. https://www.nice.org.uk/guidance/ng28. Accessed 14 Oct 2019.
- 11.Kalra S, Aamir AH, Raza A, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian J Endocrinol Metab. 2015;19(5):577–596. doi: 10.4103/2230-8210.163171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royal Australian College of General Practitioners . General practice management of type 2 diabetes 2016–2018. East Melbourne: Vic: RACGP; 2016. [Google Scholar]
- 13.The Society for Endocrinology Metabolism and Diabetes of South Africa type 2 diabetes mellitus guidelines expert committee SEMDSA 2017 guidelines for the management of type 2 diabetes mellitus. J Endocrinol Metab Diabetes South Africa. 2017;22(Suppl 1):S1–S196. [Google Scholar]
- 14.Associazione Medici Diabetologi, Società Italiana di Diabetologia. Standard italiani per la cura del diabete mellito [in Italian]. Rome: Società Italiana di Diabetologia; 2018.
- 15.Rutten G, de Grauw WJC, Nijpels G, et al. NHG-Standaard Diabetes mellitus type 2 (derde herziening) Huisarts Wet. 2013;56(10):512–525. [Google Scholar]
- 16.AGREE Next Steps Consortium. Appraisal of guidelines for research and evaluation II: AGREE II instrument 2013. https://www.agreetrust.org/wp-content/uploads/2013/10/AGREE-II-Users-Manual-and-23-item-Instrument_2009_UPDATE_2013.pdf. Accessed 8 Oct 2019.
- 17.Johns E, McKay G, Fisher M. Glitazones (thiazolidinediones) Br J Cardiol. 2017;24:113–116. [Google Scholar]
- 18.Schernthaner G, Grimaldi A, Di Mario U, et al. GUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest. 2004;34(8):535–542. doi: 10.1111/j.1365-2362.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 19.Maloney A, Rosenstock J, Fonseca V. A model-based meta-analysis of 24 antihyperglycemic drugs for type 2 diabetes: comparison of treatment effects at therapeutic doses. Clin Pharmacol Ther. 2019;105(5):1213–1223. doi: 10.1002/cpt.1307. [DOI] [PubMed] [Google Scholar]
- 20.Guideline Development Group Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min) Nephrol Dial Transplant. 2015;30:ii1–ii142. doi: 10.1093/ndt/gfv100. [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Liu XY, Zhang N, Chen R, Zhao JG, Yu P. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complicat. 2015;29(8):1295–1303. doi: 10.1016/j.jdiacomp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740–751. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 24.Mearns ES, Sobieraj DM, White CM, et al. Comparative efficacy and safety of antidiabetic drug regimens added to metformin monotherapy in patients with type 2 diabetes: a network meta-analysis. PLoS One. 2015;10(4):e0125879. doi: 10.1371/journal.pone.0125879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18(8):783–794. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 26.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 29.Data Medicaid. Drug pricing and payment. https://data.medicaid.gov/Drug-Pricing-and-Payment/NADAC-as-of-2019-05-15/rt4v-78r4. Accessed 20 March 2020.
- 30.Marx N, Rosenstock J, Kahn SE, et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®) Diab Vasc Dis Res. 2015;12(3):164–174. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenstock J, Espeland M, Kahn SE, et al. The CAROLINA Trial—first results of the cardiovascular outcomes trial comparing linagliptin vs. glimepiride. American Diabetes Association (ADA) 79th Scientific Session: San Francisco; 2019.
- 32.Andersen SE, Christensen M. Hypoglycaemia when adding sulphonylurea to metformin: a systematic review and network meta-analysis. Br J Clin Pharmacol. 2016;82(5):1291–1302. doi: 10.1111/bcp.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riddle MC, Herman WH. The cost of diabetes care-an elephant in the room. Diabetes Care. 2018;41(5):929–932. doi: 10.2337/dci18-0012. [DOI] [PubMed] [Google Scholar]
- 34.Foley JE, Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naive patients with type 2 diabetes. Horm Metab Res. 2009;41(12):905–909. doi: 10.1055/s-0029-1234042. [DOI] [PubMed] [Google Scholar]
- 35.Filozof C, Gautier JF. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study. Diabet Med. 2010;27(3):318–326. doi: 10.1111/j.1464-5491.2010.02938.x. [DOI] [PubMed] [Google Scholar]
- 36.Hassanein M, Abdallah K, Schweizer A. A double-blind, randomized trial, including frequent patient-physician contacts and Ramadan-focused advice, assessing vildagliptin and gliclazide in patients with type 2 diabetes fasting during Ramadan: the STEADFAST study. Vasc Health Risk Manag. 2014;10:319–326. doi: 10.2147/VHRM.S64038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.New drugs for type 2 diabetes: second-line therapy—science report. Ottawa: CADTH therapeutic review. 2017;4 No. 1b. [PubMed]
- 38.Eam G. Society for Endocrinology, Metabolism and Diabetes of South Africa Congress. 2012; Cape Town.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.