Abstract
Objective
The aim of this review and meta-analysis was to assess the effects and safety of modified Si-Miao pill (mSMP) in treatment of rheumatoid arthritis.
Design
A systematic literature search was carried out in eight databases from their available dates of inception to April 2020. After screening, fifteen randomized, controlled trials (RCTs) comparing the effects and safety of mSMP in combination with western medicine (including disease-modifying antirheumatic drugs (DMARDs) and nonsteroidal anti-inflammatory drugs (NSAIDs)) in treating rheumatoid arthritis patients were included after screening.
Results
In comparison with DMARDs, or coadministration of DMARDs and NSAIDs, mSMP in combination with western medicine significantly lowered erythrocyte sedimentation rate (mean difference (MD) = -10.61, 95% confidence interval (CI) [−12.19, −9.03]), C-reactive protein (MD = −6.50, 95% CI [−8.43, −4.56]), rheumatoid factors (MD = −17.31, 95% CI [−24.34, −10.27]), swollen joint count (MD = −1.63, 95% CI [−2.29, −0.97]), tender joint count (MD = −1.98, 95% CI [−2.34, −1.62]), and morning stiffness time (MD = −24.37, 95% CI [−29.41, 19.33]) and ameliorated the condition of patients (odds ratio (OR) = 3.69, 95% CI [2.64, 5.14]). Additionally, mSMP in combination with western medicine seemed safer (OR = 0.49, 95% CI [0.30, 0.81]).
Conclusion
The results of the meta-analysis study have shown that mSMP in combination with western medicine therapies appears to be more effective and safer than western medicine alone in the treatment of rheumatoid arthritis including reducing inflammatory markers and adverse events and improving symptoms. Howbeit, more high-grade, large-scale RCTs of mSMP in various countries and regions are still needed.
1. Introduction
Rheumatoid arthritis (RA), one of the most common autoimmune diseases, is characterized by symmetrical inflammatory polyarthritis and progressive joint destruction of unknown etiology. It affects approximately 1% of the population at any age, burdening the social economy for its high disability [1, 2]. At present, immunosuppression and anti-inflammatory effects are the main mechanisms of drugs to treat RA. Disease-modifying antirheumatic drugs (DMARDs), including conventional synthetic DMARDs, biological DMARDs, and targeted DMARDs, are thought to be first-line drugs internationally [3]. Nonsteroidal anti-inflammatory drugs (NSAIDs) aim to relieve the pain and inflammation rapidly. However, the various side effects of DMARDs and NSAIDs, such as leukopenia and gastrointestinal, are common and cannot be ignored [4]. Epidemiological studies have found out that patients with RA treated with anti-TNF antibody therapy were at an increased risk of serious infections and a dose-dependent increased risk of malignancies [5]. In addition, huge medical care cost of biological and targeted DMARDs makes it infeasible in many countries, and only 8.3% of patients have received biological DMARDs in China [6]. Furthermore, quite a few people are not sensitive to the current DMARDs. In order to treat RA more effectively and safely with less cost, it is necessary to explore new pharmacologic treatment for RA.
Traditional Chinese medicine (TCM), as a multicomponent and multitarget approach, has been proven to be effective in the treatment of RA in terms of reducing toxicity and increasing the efficacy of mechanisms [7, 8]. Although the use of TCM is common in Southeast Asia, it is rare in other regions. Si-Miao pill (SMP) is primarily composed of Phellodendri Chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, and Coicis Semen. It has been widely used in the treatment of various arthralgia diseases, especially rheumatoid arthritis in China. According to modern pharmacology, the Si-Miao pill and numerous monomers extracted from it are also invalidated efficaciously in treating RA in vitro and in vivo [9]. The modified Si-Miao pill (mSMP) is derived from the Si-Miao pill, adjusting the composition according to different syndrome types for more effectiveness [10–15]. While mSMP has been applied in RA for a long time, a systematically evidence-based study is vacant. We assessed the effects and safety of mSMP combined with western medicine (DMARDs and NSAIDs) in the treatment of RA systematically by the meta-analysis of randomized controlled trials (RCTs).
2. Methods
This study was designed according to the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systemic Review and Meta-analyses (PRISMA) guidelines to ensure accuracy and reliability [16]. The review protocol was registered in the PROSPERO database before the start of the review process (CRD42019133738). The PRISMA checklist was presented in Appendix 1 in Supplementary Materials.
2.1. Search Strategy
Only Chinese and English articles were concerned. We conducted a systematic literature search including eight Chinese and foreign databases to ascertain trials including CNKI Databases, Wan Fang Database, Chinese Biomedical Literature database (CBM), PubMed, EMBASE, the Cochrane Library, Clinical Trails, and Web of Science. All of the databases were searched from their available dates of inception to April 2020. Search strategies were combined as follows. For the English databases, free text terms ((rheumatoid arthritis) OR (rheumatism)) AND ((si miao) OR (four subtleties)) were applied. For the Chinese databases, the terms were (si miao AND (lei feng shi guan jie yan OR lei feng shi guan jie yan (RA in Chinese)) NOT si miao yong and NOT si miao xiao bi).
2.2. Selection Criteria
We adopted the following criteria: (1) studies used mSMP in combination treatment with western medicine (including DMARDs and NSAIDs); (2) all partaken patients who were diagnosed with RA according to the authoritative diagnostic criterion of RA such as the 1987 or 2010 American College of Rheumatology (ACR)/European League Against Rheumatism Criteria; and (3) RCTs.
2.3. Exclusion Criteria
Exclusion criteria were as follows: (1) studies using mSMP alone, si miao yong an, and si miao xiao bi decoction were excluded; (2) case reports, reviews, and animal experiments were excluded; (3) diagnosis criteria are unclear; (4) studies without regulatory outcomes or studies in which the evaluation of curative effect is not standard were excluded; (5) articles that have no available full text were excluded.
2.4. Types of Outcome Measures
The primary outcomes were the main indicators correlating with disease activity including sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factors (RF), swollen joint count, and tender joint count. The secondary outcomes were some clinical symptoms reflecting disease activity consisting of effective rate, morning stiffness times, and adverse events (AEs). The decrease of ESR, CRP, RF, morning stiffness times, swollen joint count, and tender joint count and the increase of effective rate could reflect the therapeutic effects (TEs). AEs included abnormal liver function, hyperleukocytosis, acratia, erythema or itch of the skin, dental ulcer, and gastrointestinal discomfort.
2.5. Data Extraction and Management
The data were extracted by two independent reviewers (HW and YAH). Any discrepancy was resolved by consensus or judged by the corresponding author (SHT and ZC). All relevant data including characteristics of trails, the first author, year of publication, baseline characteristics of patients, number of patients, duration of the study, intervention methods, and outcome measures were extracted and entered into a template table. We assessed the included studies' quality on the basis of Cochrane Collaboration's risk of bias tool. There were three scores for each item, low risk, unclear, and high risk, according to following criteria: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) incomplete outcome data, (5) selective reporting, and (6) other biases.
2.6. Statistical Synthesis and Analysis
Review Manager 5.3 and Stata 12.0 were used to calculate the differences of effects and safety of mSMP in treating RA between the mSMP/experimental groups (mSMP treatment in combination with DMARDs that were combined or were not combined with NSAIDs) and the control groups (DMARDs that were combined or were not combined with NSAIDs). We applied the odds ratio (OR) and 95% confidence interval (CI) to appraise dichotomous data and mean difference (MD) to continuous data. Heterogeneity was evaluated according to the chi-square test and the Higgins I2 test. The fixed-effects model was applied when statistical heterogeneity was low that was I2 ≤ 50% or Chi2 test P < 0.10; otherwise, a random-effects model was employed. The subgroup analysis was performed to eliminate heterogeneity, and sensitivity analysis was adopted to probe the source of heterogeneity. If the data provided by the included studies were not appropriate for performing a meta-analysis, the study data were presented in narrative form. Publication bias was detected by Egger's regression asymmetry test.
3. Results
3.1. Study Selection and the Basic Documents
The flow chart of the study selection was given in Figure 1. In the primary screening, we retrieved 176 articles. After removing duplicates, titles and abstracts of 104 studies were screened. 27 records were excluded because they were a summary of experience or reviews (n = 27), animals or cell experiments (n = 15), or irrelevant diseases or medicines (n = 24). After the full-text reading of the resulting 38 studies, 20 records were excluded, seven of which were due to self-controlled studies and thirteen of which were owning to inconsistent interventions and four of which were due to no available raw data. Eventually, 15 RCTs were enrolled in the meta-analysis, including 1349 participants in total [17–31]. The characteristics of the studies were exhibited in Table 1. They were all carried out in China and published between 2007 and 2019 in Chinese. All of them were conducted as single-center trials. There was no statistically significant difference in gender and age between treatment groups and the control groups in the literature, and the course of the disease as well. The duration of intervention in the included RCTs ranged from 30 to 90 days. The dosage of mSMP was twice a day and the dosage of DMARDs or NSAIDs was the same between experiment (mSMP) and control groups. DMARDs in these RCTs included Methopterin (MTX), Leflunomide (LEF), Salazosulfapyridine (SASP), and Hydroxychloroquine (HCQ), while SAIDs included Diclofenac sodium, meloxicam, Voltaren, and Loxoprofen. Fourteen RCTs [17–23, 25–31] had definite TCM syndrome of the patients according to the criteria of diagnosis and therapeutic effect of diseases and syndromes in TCM, of which eleven [17, 19, 21–23, 25–29, 31] were dampness-heat, and three [18, 20, 30] were wind and dampness-heat. The formulations and compositions of mSMP were listed in Table 2. The decoction was used in thirteen articles [18–30] and the pill was used in two articles [17, 31]. The dosage of Phellodendri Chinensis cortex is from 10 to 15 g and that of Coicis Semen is from 15 to 30 g; the dosage of achyranthis bidentatae radix is from 15 to 30 g and that of atractylodes rhizome is from 10 to 15 g. Four articles [17, 28, 29, 31]adopted the original formula of SMP while other studies added herbs based on SMP.
Figure 1.

Study selection flow chart.
Table 1.
Clinical and demographic characteristics of patients with rheumatoid arthritis.
| Study (ref) | Number of participants experimental/control | Age (years) experimental/control | Intervention | Duration (days) | Outcomes | TCM syndrome | |
|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||
| Chen [23] | 35/35 | 50/50 | mSMP + MTX 7.5 + LEF 10 | MTX 7.5 + LEF 10 | 90 | Effective rate AEs | Dampness-heat |
| Hu [29] | 73/73 | 43.5 ± 5.0/43.0 ± 5.5 | mSMP + MTX 7.5 + Voltaren 75 | MTX 7.5 + Voltaren 75 | 90 | Effective rate ESR RF MST SJT AEs | Dampness-heat |
| Li [27] | 36/36 | 47.00 ± 2.35/48.00 ± 2.19 | mSMP + MTX 10 + Loxoprofen sodium 60 | MTX 10 + Loxoprofen sodium 60 | 90 | Effective rate ESR CRP RF MST SJT AEs | Dampness-heat |
| Li and Gao [19] | 30/30 | 52.16 ± 10.24/54.02 ± 14.76 | mSMP + MTX 10 + Voltaren 75 | MTX 10 + Voltaren 75 | 30 | Effective rate ESR CRP | Dampness-heat |
| Liu [22] | 32/31 | 44.5 ± 11.6/43.6 ± 13 | mSMP + MTX 5-10 + LEF 10 | MTX 5-10 + LEF 10 | 30 | Effective rate | Wind and dampness-heat |
| Liu and Yuan [26] | 90/88 | 42.18 ±4.76/42.18 ±4.76 | mSMP + MTX 15 + SASP 500 | MTX 15 + SASP 500 | 90 | Effective rate | Dampness-heat |
| Liu [22] | 35/30 | 46.89 ±9.15/45.40 ± 8.63 | mSMP + LEF 10 | LEF 10 | 30 | Effective rate ESR | Dampness-heat |
| Qian [18] | 44/44 | 44.7 ± 3.9/45.6 ± 3.7 | mSMP + MTX 7.5 + Meloxicam 7.5 | MTX 7.5 + Meloxicam 7.5 | 60 | Effective rate ESR | Wind and dampness-heat |
| Wang [24] | 45/44 | 50.1 ± 4.7/48.7 ± 4.6 | mSMP + MTX 10 | MTX 10 | 90 | Effective rate ESR RF AEs | Unclear |
| Wei [21] | 49/49 | 42.6 ± 5.7/41.3 ± 5.7 | mSMP + MTX 15 + Meloxicam 7.5 | MTX 15 + Meloxicam 7.5 | 42 | Effective rate ESR CRP RF MST | Dampness-heat |
| Yang [28] | 20/20 | 43/41 | mSMP + MTX 7.5 + Voltaren 75 | MTX 7.5 + Voltaren 75 | 90 | Effective rate ESR CRP RF MST SJT TJC AEs | Dampness-heat |
| Zeng [17] | 20/20 | 43/41 | mSMP + MTX 7.5 + HCQ 0.2 | MTX 7.5 + HCQ 0.2 | 90 | Effective rate ESR CRP RF SJT TJC AEs | Dampness-heat |
| Zhang [31] | 94/96 | 34.54 ± 12.51/35.33 ± 12.52 | mSMP + MTX 10 + Loxoprofen sodium 60 | MTX 10 + Loxoprofen sodium 60 | 90 | Effective rate ESR CRP RF SJT TJC AEs | Dampness-heat |
| Zhang [25] | 39/39 | 47.59 ± 10.18/47.95 ± 11.94 | mSMP + MTX 7.5-15 + Diclofenac sodium 75 | MTX 7.5-15 + Diclofenac sodium 75 | 90 | Effective rate ESR CRP RF SJT TJC AEs | Dampness-heat |
| Zhao [30] | 36/36 | 47.9 ± 2.4/47.2 ± 2.3 | mSMP + MTX 10 + LEF 10 | MTX 10 + LEF 10 | 60 | Effective rate ESR MST AEs | Wind and dampness-heat |
Note: mSMP: modified Si-Miao pill; TCM: traditional Chinese medicine; MTX : Methopterin mg/week; LEF: Leflunomide mg, bid; Voltaren: mg/day; Loxoprofen sodium: mg/day; Meloxicam: mg/day; SASP: Salazosulfapyridine, mg, bid; HCQ: hydroxychloroquine g/day; Diclofenac sodium: mg/day; AEs: adverse events; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; RF: rheumatoid factors; MST: morning stiffness time; SJC: swollen joint count; TJC: tender joint count.
Table 2.
The components of mSMP.
| Studies | Formulations | Components of mSMP |
|---|---|---|
| Chen [23] | Decoction | Atractylodis rhizome, achyranthis bidentatae radix, Coicis Semen, atractylodes macrocephalae rhizome, alismatis rhizome, sinomenii caulis, piperis kadsurae caulis, dioscoreae nipponicae rhizome, lonicerae japonicae flos, lonicerae japonicae flos, glycyrrhizae radix et rhizome |
| Hu [29] | Decoction | Phellodendri chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen |
| Li [27] | Decoction | Phellodendri chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, coicis semen, lonicerae japonicae caulis, smilacis glabbrae rhizome, angelicae sinensis radix, paeoniaeradix rubra, salviae miltiorrhizae radix et rhizome, plantaginis semen, alismatis rhizome, saposhnikoviae radix, astragali radix, gleditsiae apina, glycyrrhizae radix et rhizome |
| Li and Gao [19] | Decoction | Phellodendri Chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen, piperis kadsurae caulis, sinomenii caulis, atractylodes macrocephalae rhizome, smilacis glabbrae rhizome |
| Liu [22] | Decoction | Phellodendri chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen, gentianae macrophyllae radix, |
| Liu and Yuan [26] | Decoction | Phellodendri chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen, sinomenii caulis, glycyrrhizae radix et rhizome |
| Liu [22] | Decoction | Phellodendri Chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen, forsythia fructus, clematidis radix et rhizome, paeoniae radix alba, lysimachiae herba, saposhnikoviae radix, stephaniae tetrandrae radix, lonicerae japonicae caulis, hedyotis diffusa, mori ramulus, violae herba, pheretima, pheretima, glycyrrhizae radix et rhizome |
| Qian [18] | Decoction | Phellodendri Chinensis cortex, Coicis Semen, stephaniae tetrandrae radix, stephaniae tetrandrae radix, pheretima, lysimachiae herba, violae herba, lonicerae japonicae caulis, mori ramulus, paeoniae radix alba, clematidis radix et rhizoma |
| Wang [24] | Decoction | Atractylodis macrocephalae, corydalis rhizome, sinomenii caulis, piperis kadsurae caulis, dioscoreae nipponicae rhizome, alismatis rhizome, lonicerae japonicae caulis, lonicerae japonicae flos, trachelospermi caulis et folium, glycyrrhizae radix et rhizome |
| Wei [21] | Decoction | Phellodendri chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen, mori ramulus, chaenomelis fructus, spatholobi caulis, trachelospermi caulis et folium, glycyrrhizae radix et rhizome, |
| Yang [28] | Decoction | Phellodendri Chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen |
| Zeng [17] | Pill | Phellodendri Chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen |
| Zhang [31] | Pill | Phellodendri Chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen |
| Zhang [25] | Decoction | Phellodendri Chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen, semen persicae, rhizoma pinelliae, paeoniae radix rubra, angelicae sinensis radix, bombyx batryticatus, pericarpium citri reticulatae, paeoniae radix alba, radix glycyrrhizae preparata |
| Zhao [30] | Decoction | Phellodendri Chinensis cortex, atractylodes rhizome, achyranthis bidentatae radix, Coicis Semen, forsythia fructus, clematidis radix et rhizome, paeoniae radix alba, lysimachiae herba, saposhnikoviae radix, stephaniae tetrandrae radix, lonicerae japonicae caulis, hedyotis diffusa, mori ramulus, violae herba, pheretima, pheretima, glycyrrhizae radix et rhizome |
3.2. Quality Assessment of Included Studies
Most of the included RCTs were of poor quality according to the Cochrane Collaboration's risk of bias tool criteria shown in Figure 2. Eight [17–19, 21, 22, 25, 28, 31] of the included studies indicated random sequence generation, two [19, 21] of which are based on treatment order, one [18] of which was based on sortition randomization method, and the remaining five [17, 22, 25, 28, 31] were based on the table of random numbers. None of the articles mentioned allocation concealment or blind method, as well as intentional analysis. All patients completed the experiments, and no one lost the interview or dropped out. None of the trials reported other biases.
Figure 2.
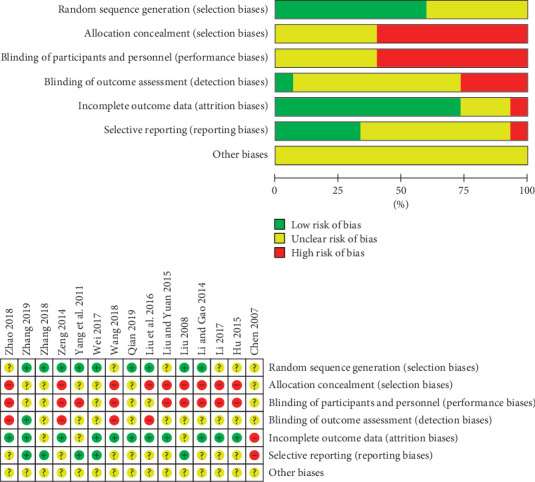
Risk of bias graph.
3.3. Publication Bias
Egger's publication bias test showed that there were negligible publication biases for four outcomes in terms of RF (P=0.309), morning stiffness time (P=0.065), swollen joint count (P=0.092), and tender joint count (P=0.734) while there were significant publication biases for four outcomes in terms of ESR (P=0.001), CRP (P=0.014), effective rate (P=0.04), and AEs (P=0.032) for the evaluation rules that studies with P values over 0.05 in Egger's test were deemed low heterogeneity. The effects of lowering ESR and reducing need further exploration, as presented in Appendix 2 in Supplementary Materials.
3.4. TEs of mSMP
3.4.1. ESR (mm/h), CRP (mg/L), RF (IU/mL), Swollen Joint Count, and Tender Joint Count
ESR was reported in eleven trials (involving 892 patients) [17–19, 21, 22, 24, 25, 27, 28, 30, 31]. The number of trial participants ranged from 40 to 190, with the trial duration varying from 30 days to 90 days. The mSMP groups were superior to the control groups regarding decreasing the ESR (MD = −10.61, 95% CI [−12.19, −9.03]). In order to reduce the high heterogeneity (I2 = 85%, P < 0.00001) and explore the effect of treatment time, we stratified studies based on trial duration (30 d, 30–60 d, 90 d). The analysis of the subgroups showed that trial duration could be one of the potential sources of heterogeneity (I2 = 73.1%, P=0.02). Additionally, the treatment effect of mSMP seemed to be time-dependent (30 d: MD −7.68, CI [−9.45, −5.92]; 30–60 d: MD −8.53, CI [−9.40, −7.67]; 90 d: MD −13.63, CI [−17.54, −9.73]), as illustrated in Figure 3(a).
Figure 3.

Forest plots for the comparison of ESR (a), CRP (b), RF (c), swollen joint count (d), and tender joint count (e) of mSMP treatment in combination with western drugs (DMARDs and NSAIDs) (experimental) and western drugs (control) only. Note: ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; RF: rheumatoid factors; AEs: adverse events; mSMP: modified Si-Miao pill; DMARDs: disease-modifying antirheumatic drugs; NSAIDs: nonsteroidal anti-inflammatory drugs (NSAIDs).
CRP was determined in seven trials (involving 477 patients) [17, 19, 21, 25, 27, 28, 31]. The number of trial participants ranged from 40 to 98, with the trial duration varying from 30 days to 90 days. The experimental groups were superior to the control groups regarding reducing the CRP CRP (MD = -6.50, 95% CI [−8.43, −4.56]). There was statistical heterogeneity between the studies based on the random-effects model (I2 = 90%, P < 0.00001), as illustrated in Figure 3(b).
RF was determined in seven trials (involving 607 patients) [17, 21, 24, 25, 27, 28, 31]. The number of trial participants ranged from 40 to 190, with a 90-day trial duration. As illustrated in Figure 3(c), there was statistical heterogeneity between the studies based on the random-effects model (I2 = 91%, P < 0.00001). The experimental groups were superior to the control groups regarding reducing the RF (MD = −17.31, 95% CI [−24.34, −10.27]).
The swollen joint count was determined in six trials (involving 566 patients) [17, 25, 27–29, 31]. The number of trial participants ranged from 40 to 190, with a 90-day trial duration. As illustrated in Figure 3(d), there was statistical heterogeneity between the studies based on the random-effects model (I2 = 88%, P < 0.00001). The experimental groups were superior to the control groups regarding reducing the swollen joint count (MD = −1.63, 95% CI [−2.29, −0.97]).
The tender joint count was determined in four trials (involving 348 patients) [17, 25, 28, 31]. The number of trial participants ranged from 40 to 190, with a 90-day trial duration. As illustrated in Figure 3(e), there was no statistical heterogeneity between the studies based on the fixed-effects model (I2 = 0%, P=0.78). The experimental groups were superior to the control groups regarding reducing the tender joint count (MD = −1.98, 95% CI [−2.34, −1.62]).
3.4.2. Effective Rate and Morning Stiffness Time (min)
Concerning mSMP treatment in combination with DMARDs and NSAIDs (or no NSAIDs), fifteen trials (involving 1349 patients) compared the effective rate of mSMP groups with control groups [17–31]. Patients reaching ACR20 or efficient according to the criteria for diagnosis and efficacy of TCM diseases and syndromes were considered as effective. The trial duration varied from 30 days to 90 days. There was no statistical heterogeneity between the studies based on the fixed-effects model (I2 = 0%, P=0.98). The experimental groups were more effective in improving the condition (OR = 3.69, 95% CI [2.64, 5.14]), as illustrated in Figure 4(a).
Figure 4.

Forest plots for the comparison of effective rate (a), morning stiffness time (b), and adverse events (c) of mSMP treatment in combination with western drugs (DMARDs and NSAIDs) (experimental) and western drugs only. mSMP: modified Si-Miao pill; DMARDs: disease-modifying antirheumatic drugs; NSAIDs: nonsteroidal anti-inflammatory drugs (NSAIDs).
Morning stiffness time was determined in five trials (involving 428 patients) [21, 27–30]. The number of trial participants ranged from 40 to 146, with the trial duration varying from 42 days to 90 days. As illustrated in Figure 4(b), there was statistical heterogeneity between the studies based on the random-effects model (I2 = 76%, P < 0.00001). The experimental groups were superior to the control groups regarding the reduction of the morning stiffness time (MD = −24.37, 95% CI [−29.41, 19.33]).
3.5. AEs
AEs were reported in eight trials (involving 651 patients) [17, 23–25, 27, 28, 30, 31]. As illustrated in Table 3, the number of cases ranged from 0 to 18. Gastrointestinal discomfort was the most common adverse reaction, of which 18 cases were reported in the control groups and 10 cases in the experimental groups (mSMP + western medicine) according to Chen, Li, Qian, Zeng, Zhao, and Zhang's studies. There were six cases of erythema or itch of skin reported in control groups (western medicine) and only two cases in experimental groups in Zhao's study. Three trials reported hyperleukocytosis with a total of 3 cases in the control groups and no case in the experimental groups, the same as a dental ulcer. Two cases of abnormal liver function were reported in the control groups and only one case occurred in the experimental groups, as illustrated in Figure 4(c). There was no statistical heterogeneity between the studies based on the fixed-effects model (I2 = 0%, P=0.77). Compared to the control groups, the mSMP groups could reduce the side effects apparently (OR = 0.49, 95% CI [0.30, 0.81]).
Table 3.
The adverse events about all included RCTs.
| Adverse events | Experimental (mSMP + western medicine) | Control (western medicine) | ||
|---|---|---|---|---|
| N | Total | N total | Total | |
| Abnormal liver function [27] | 1 | 26 | 2 36 | 36 |
| Hyperleukocytosis [18, 23, 30] | 0 | 115 | 3 115 | 71 |
| Acratia ([28]) | 0 | 20 | 2 20 | 20 |
| Gastrointestinal discomfort [18, 23, 25, 27, 30] | 10 | 190 | 18 190 | 107 |
| Erythema or itch of skin [18, 30] | 2 | 80 | 6 80 | 36 |
| Dental ulcer [17] | 0 | 20 | 2 20 | 20 |
Note: RCTs: randomized, controlled trials; mSMP: modified Si-Miao pill.
3.6. Sensitivity Analysis
In the sensitivity analysis, no significant changes of heterogeneity related to ESR, CRP, and swollen joint count were observed. In terms of RF and morning stiffness time, the study by Li [27]was most likely to be the sources of heterogeneity study. When it was omitted, the change of heterogeneity was significant (RF: I2 reducing from 91% to 55%, morning stiffness time: I2 reducing from 76% to 44%), and the general conclusion remained the same, as presented in Appendix 3 in Supplementary Materials.
4. Discussion
Although mSMP has been applied in treating RA for several years and there were massive clinical reports and clinical trials in China, it is the first time to evaluate the efficacy and safety of mSMP in the treatment of RA by systematic reviews and meta-analyses. Biomarkers play an important role in guiding the clinical trials and therapies of RA. ESR and CRP are the most common experimental indicators reflecting the inflammatory activity of RA, which is useful to evaluate the condition and prognosis. RF is an antibody correlated with RA and predicts bone erosion and severe disease progression [32]. It generally does not change much for a short time, but its change can explain the disease to a certain extent. ACR20 and criteria for diagnosis and efficacy of TCM diseases and syndromes are the most common and authoritative standards to evaluate the efficacy of drug therapy. Swollen joint count, tender joint count, and morning stiffness time are the characteristic manifestations of RA which played a great role in assessing the disease activity. According to the 2018 Chinese guideline for diagnosis and treatment of rheumatoid arthritis, ESR, CRP, RF, anti-cyclic citrulline antibody, swollen joint count, and tender joint count should be taken into account in the treatment of RA. Since there were few studies on the measurement of anti-cyclic citrulline antibody, ESR, CRP, RF, swollen joint count, and tender joint count were regarded as primary outcomes. This statistical analysis revealed that mSMP in combination with western medicine seemed to be more effective and significant in reducing the levels of ESR, CRP, and RF, ameliorating morning stiffness time, swollen joint count, tender joint count, and the incidence of AEs incidence. Due to the limited studies included, we could not conduct subgroups' analysis in terms of dosage, treatment durations, the difference of control drugs to remove the high heterogeneity of CRP, and swollen joint count. We speculated on several possible reasons, such as patients with different disease activities, different regions, and not enough samples, which needs more and better quality clinical trials to explore. Furthermore, although the included article did not analyze whether the adverse events were caused by mSMP or western medicine in experimental groups, mSMP combined with western medicine had fewer adverse events than western medicine.
RA belongs to “bi” in traditional Chinese medicine, while a large number of active RAs vest in “dampness-heat of bi” [7]. TCM syndrome of most of the patients included in the RCTs was dampness-heat. SMP was written by Zhang Bingcheng in the Qing Dynasty famous for the definite effect of clearing heat and dampness, reducing swelling, and relieving pain [33]. The prescription medicine mainly includes cortex Phellodendri, rhizoma atractylodes, radix achyranthis bidentatae, and Semen Coicis, modified according to the individual symptom for being a more-targeted treatment, which is a mere coincidence with precision medical treatment. On the other hand, the difference in composition and dosage of the prescriptions is also a disadvantage of standard evaluation.
mSMP in the treatment of RA has also been confirmed according to modern pharmacology in vivo and in vitro. SMP was found to significantly inhibit the expression of IL-1β, IL-6, and TNF-α in adjuvant arthritis (AA) rats [34]. mSMP extract inhibited the release of inflammatory mediators, like NO and TNF-α, via the suppression of ERK and NF-κB-dependent pathways from lipopolysaccharide-stimulated mouse macrophages [14]. Wang found that SMP could downregulate the expression of VEGF in AA rats, thus inhibiting the formation of synovia pannus [35]. In addition, cortex Phellodendri, rhizoma atractylodes, and Semen Coicis had obvious anti-inflammatory and analgesic effects. Total saponins of Achyranthes bidentata could attenuate the acute inflammatory reaction and regulate immunity [36, 37]. Moreover, SMP and one of the main components, berberine, were confirmed to regulate the blood lipids, increase the level of high-density lipoprotein, which is thought to improve rheumatism and benefit the cardio-cerebrovascular disease in terms of the common view that patients with rheumatoid arthritis are at increased risk of cardiovascular disease [38–40]. In condition, mSMP may lower CVD occurrences by lipid regulation and well work in hormonal-dependent RA [41]. One trial reported that mSMP could lower the recurrence rate during the one-year follow-up period.
There were also a lot of shortcomings in this study. First, all the included RCTs were conducted in China and might cause selection bias. Most of the included RCTs were of poor quality according to the Cochrane Collaboration's risk of bias tool criteria for a missing message of allocation concealment, blind method, and intentional analysis. Second, the form, compositions, dosages, and treatment duration of mSMP were complex and changeable, which might undermine the credibility. Furthermore, the studies included in the systematic review were few in number and there was no unified standard for disease evaluation. Finally, the lack of relevant grey literature may lead to publication bias.
5. Conclusions
Overall, mSMP combined with western medicine was more effective and safer than western medicine in the treatment of RA. mSMP may play the pharmacological action by anti-inflammation, regulating immunity, analgesia, and lipid regulation. In consideration of the low quality, single area of the given trials, and variable interventions, more large randomized controlled, double-blind, multicenter clinical trials with good methodological quality are needed to recommend mSMP as an alternative remedy for RA.
Acknowledgments
The authors would like to thank all authors of references for performing RCTs of mSMP for RA treatment. This work was supported by the National Natural Science Foundation of China (grant nos. 81503426 and 81573802).
Contributor Information
Zhe Chen, Email: zhepi2006@163.com.
Shenghao Tu, Email: shtu@tjh.tjmu.edu.cn.
Conflicts of Interest
There are no conflicts of interest.
Authors' Contributions
HW and YAH collected the data and carried out the analysis. YW and KQ gave advice of the study, YiH, PS, and XB helped amend the paper. S-H T and ZC resolved the discrepancy and provided funding. All authors read and approved the paper.
Supplementary Materials
Appendix 1: Checklist. Appendix 2: Figure 1: Egger's publication bias in the included trails. A: erythrocyte sedimentation; B: C-reactive protein; C: rheumatoid factors; D: effective rate; E: morning stiffness time; F: swollen joint count; G: tender joint count; H: adverse events. Appendix 3: Figure 2: Sensitivity analysis of ESR (A), CRP (B), RF (C), morning stiffness time (D), and swollen joint count (E). Note: ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; RF: rheumatoid factors.
References
- 1.McInnes I. B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet. 2017;389(10086):2328–2337. doi: 10.1016/s0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 2.Cross M., Smith E., Hoy D., et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases. 2014;73(7):1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 3.Smolen J. S., Aletaha D., Bijlsma J. W., et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Annals of the Rheumatic Diseases. 2010;69(4):631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W., Zhou H., Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. European Journal of Medicinal Chemistry. 2018;158:502–516. doi: 10.1016/j.ejmech.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Bongartz T., Sutton A. J., Sweeting M. J., Buchan I., Matteson E. L., Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies. JAMA. 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 6.Jin S. Chinese Registry of rheumatoid arthritis (CREDIT): II. prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Research & Therapy. 2017;19(1):p. 251. doi: 10.1186/s13075-017-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lü S., Wang Q., Li G., Sun S., Guo Y., Kuang H. The treatment of rheumatoid arthritis using Chinese medicinal plants: from pharmacology to potential molecular mechanisms. Journal of Ethnopharmacology. 2015;176:177–206. doi: 10.1016/j.jep.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Huang M.-C., Pai F.-T., Lin C.-C., et al. Characteristics of traditional Chinese medicine use in patients with rheumatoid arthritis in Taiwan: a nationwide population-based study. Journal of Ethnopharmacology. 2015;176:9–16. doi: 10.1016/j.jep.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Shen P., Tu S., Wang H., Qin K., Chen Z. Simiao pill attenuates collagen-induced arthritis in rats through suppressing the ATX-LPA and MAPK signalling pathways. Evidence-Based Complementary and Alternative Medicine. 2019;2019:11. doi: 10.1155/2019/7498527.7498527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J.-J., Hu X.-W., Li P., Chen J. Global identification of chemical constituents and rat metabolites of Si-Miao-Wan by liquid chromatography-electrospray ionization/quadrupole time-of-flight mass spectrometry. Chinese Journal of Natural Medicines. 2017;15(7):550–560. doi: 10.1016/s1875-5364(17)30082-1. [DOI] [PubMed] [Google Scholar]
- 11.Luo T. J. Modified Si-Miao-San regulates adipokine expression and ameliorates insulin resistance by targeting IKKβ/Insulin receptor substrate-1 in mice. Chinese Journal of Integrative Medicine. 2014;20 doi: 10.1007/s11655-014-1802-x. [DOI] [PubMed] [Google Scholar]
- 12.Shang S.-W., Yang J.-L., Huang F., Liu K., Liu B.-L. Modified Si-Miao-San ameliorates pancreatic B cell dysfunction by inhibition of reactive oxygen species-associated inflammation through AMP-kinase activation. Chinese Journal of Natural Medicines. 2014;12(5):351–360. doi: 10.1016/s1875-5364(14)60043-1. [DOI] [PubMed] [Google Scholar]
- 13.Liu K., Luo T., Zhang Z., et al. Modified Si-Miao-San extract inhibits inflammatory response and modulates insulin sensitivity in hepatocytes through an IKKβ/IRS-1/Akt-dependent pathway. Journal of Ethnopharmacology. 2011;136(3):473–479. doi: 10.1016/j.jep.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Fan J., Liu K., Zhang Z., et al. Modified Si-Miao-San extract inhibits the release of inflammatory mediators from lipopolysaccharide-stimulated mouse macrophages. Journal of Ethnopharmacology. 2010;129(1):5–9. doi: 10.1016/j.jep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.-L., Wang J.-L., Huang F., Liu K., Liu B.-L. Modified Si-Miao-San inhibits inflammation and promotes glucose disposal in adipocytes through regulation of AMP-kinase. Chinese Journal of Natural Medicines. 2014;12(12):911–919. doi: 10.1016/s1875-5364(14)60134-5. [DOI] [PubMed] [Google Scholar]
- 16. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training, 2018, http://training.cochrane.org/handbook.
- 17.Zeng. Observation on the short-term curative effect of Si-miao Pill combined with Methotrexate tablet in the treatment of active Rheumatoid Arthritis. Chinese Health Nutrition (Chinese Journal) 2014;24(2):p. 714. [Google Scholar]
- 18.Qian. Effect of qubi decoction and simiao powder on immunity of rheumatoid arthritis patients with rheumatic heat depression. World Clinical Medicine. 2019;13(2):78–80. [Google Scholar]
- 19.Li. Modified Si-miao Powder in the treatment of rheumatoid arthritis. International Journal of Traditional Chinese Medicine. 2014;36(2):164–165. [Google Scholar]
- 20.Liu. Guizhi Shaoyao Anemarrhena decoction and Si-miao Pill in the treatment of active rheumatoid arthritis in 32 cases. Shandong Journal of Traditional Chinese Medicine. 2008;28(6):374–375. [Google Scholar]
- 21.Wei. Clinical observation on treatment of rheumatoid arthritis of dampness and heat blocking collaterals with Si-miao Er-teng decoction. Shaanxi Traditional Chinese Medicine. 2017;38(5):603–604. [Google Scholar]
- 22.Liu. Clinical observation on treatment of 35 cases of rheumatoid arthritis of damp-heat type with Si-miao San and Xuan-bi decoction combined with western medicine. World Journal of Integrated Chinese and Western Medicine. 2016;11(6):800–803. [Google Scholar]
- 23.Chen. Modified Si-miao San in the treatment of rheumatoid arthritis of damp-heat type in 35 cases. Shaanxi Traditional Chinese Medicine. 2007;28(9):1191–1192. [Google Scholar]
- 24.Wang. Observation on the clinical efficacy of simiao powder in the treatment of rheumatoid arthritis. Chinese Journal Medical Sciences. 2018;8(21):54–56. [Google Scholar]
- 25.Zhang. Clinical Observation of Simiaosanwei in the Treatment of Dampness-Heat Bibi Rheumatoid Arthritis. Fuzhou, China: Fujian University of Traditional Chinese Medicine; 2019. [Google Scholar]
- 26.Liu. Clinical observation on the treatment of active rheumatoid arthritis with Si-miao decoction and western medicine. Shenzhen Journal of Integrated Chinese and Western Medicine. 2015;26(21):27–28. [PubMed] [Google Scholar]
- 27.Li. Clinical observation on 36 cases of rheumatoid arthritis with damp-heat arthralgia and obstruction treated with modified Si-miao pill and western medicine. Clinical Study of Traditional Chinese Medicine. 2017;9(16):31–33. [Google Scholar]
- 28.Yang. Clinical observation on 20 cases of active rheumatoid arthritis treated with Si-miao pill and western medicine. Journal of Traditional Chinese Medicine. 2011;(18):1566–1569. [Google Scholar]
- 29.Hu. Clinical study on the treatment of active rheumatoid arthritis with Si-miao pill. A Clinical Study of Traditional Chinese Medicine. 2015;28(7):103–104. [Google Scholar]
- 30.Zhao. The effect of self-made Qu-bi decoction and Si-miao San on immune index of rheumatoid arthritis patients of heat depression type. Exploration of Rational Use of Drugs in China. 2018;15(5):50–54. [Google Scholar]
- 31.Zhang. Papaya fengshi pills combined with western medicine in the treatment of 100 cases of damp-heat bibi rheumatoid arthritis. Chinese Journal of Integrated Traditional and Western Medicine. 2018;38(11):1336–1339. [Google Scholar]
- 32.Atzeni F., Talotta R., Masala I. F., Bongiovanni S., Boccassini L., Sarzi-Puttini P. Biomarkers in rheumatoid arthritis. The Israel Medical Association Journal: IMAJ. 2017;19(19):512–516. [PubMed] [Google Scholar]
- 33.Yin. Summarization of Si-miao pill in the treatment of acute gouty arthritis. Chinese Traditional Medicine Science and Technology. 2004;11(1):63–64. [Google Scholar]
- 34.Wang. Study on the mechanism of Si-miao pill on adjuvant arthritis in rats. Chinese Journal of Traditional Chinese Medicine. 2010;35(21):2889–2892. [PubMed] [Google Scholar]
- 35.Shi. Clinical observation on 57 cases of rheumatoid arthritis treated by syndrome differentiation. Modern Journal of Integrated Chinese and Western Medicine. 1999;9(8):p. 1463. [Google Scholar]
- 36.Yu. Achyranthes bidentata polysaccharide anti-tumor effect and immune mechanism experimental study. Chinese Journal of Cancer. 1995;17(4):275–278. [PubMed] [Google Scholar]
- 37.Oh Y., Kwon Y.-S., Jung B. D. Anti-inflammatory effects of the natural compounds cortex phellodendri and humulus japonicus on pelvic inflammatory disease in mice. International Journal of Medical Sciences. 2017;14(8):729–734. doi: 10.7150/ijms.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang. Effect of addition and subtraction of Si-miao powder on early pathological changes in rats with arteriosclerosis. Chinese Journal of Experimental Prescriptions. 2017;23(2):126–130. [Google Scholar]
- 39.Derosa G., D’Angelo A., Bonaventura A., Bianchi L., Romano D., Maffioli P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opinion on Biological Therapy. 2013;13(4):475–482. doi: 10.1517/14712598.2013.776037. [DOI] [PubMed] [Google Scholar]
- 40.England B. R., Thiele G. M., Anderson D. R., Mikuls T. R. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018;361:p. k1036. doi: 10.1136/bmj.k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan Z., Zhou Tripterygium wilfordii preparation combined with traditional Chinese medicine in the treatment of hormone-dependent rheumatoid arthritis. Rheumatology and Arthritis. 2012;1(4):8–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Checklist. Appendix 2: Figure 1: Egger's publication bias in the included trails. A: erythrocyte sedimentation; B: C-reactive protein; C: rheumatoid factors; D: effective rate; E: morning stiffness time; F: swollen joint count; G: tender joint count; H: adverse events. Appendix 3: Figure 2: Sensitivity analysis of ESR (A), CRP (B), RF (C), morning stiffness time (D), and swollen joint count (E). Note: ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; RF: rheumatoid factors.


