Abstract
This article presents a new strategy for achieving regiocontrol over the endo versus exo modes of cycloisomerizations of epoxide-containing alcohols, which leads to the formation of five- or six-membered cyclic ethers. Unlike traditional methods relying on achiral reagents or enzymes, this approach utilizes chiral phosphoric acids to catalyze the regiodivergent selective formations of either tetrahydrofuran- or tetrahydropyran-containing products. By using methyl ester of epoxide-containing antibiotic mupirocin as the substrate, it is demonstrated that catalytic chiral phosphoric acids (R)-TCYP and (S)-TIPSY could be used to achieve the selective formation of either the six-membered endo product (95:5 r.r.) or the five-membered exo product (77:23 r.r.), correspondingly. This cyclization was found to be unselective under the standard conditions involving various achiral acids, bases, or buffers. The subsequent mechanistic studies using state-of-the-art quantum chemical solutions provided the description of the potential energy surface, which is fully consistent with the experimental observations. Based on these results, highly detailed reaction paths are obtained and a concerted and highly synchronous mechanism is proposed for the formation of both exo and endo products.
Keywords: chiral phosphoric acids, cyclic ethers, epoxides, mupirocin, regioselectivity
Introduction
Three-membered cyclic ethers, called epoxides or oxiranes, are of great importance in both manmade applications and biosynthesis of natural products. An almost endless number of naturally occurring epoxides with a wide range of interesting biological activities are found in nature, which has further increased the interest of epoxides for biologists, pharmacologists, and synthetic chemists.[1] Epoxides are highly strained heterocycles,[2] which are relatively easy to synthesize in their enantiopure form by using asymmetric catalytic techniques such as the Sharpless asymmetric epoxidation of allylic alcohols,[3] the Jacobsen epoxidation,[4,5] the Shi epoxidation[6,7] as well as a plethora of other methods.[8,9] Thus, epoxide chemistry is dominated by ring-opening reactions, which relieve the vast potential energy that is captured in the ring strain.
Epoxide-based intermediates play an important role in the biosynthesis of various classes of natural products, and, in particular, cyclic ether-containing natural products such as polyether ionophores, the annonaceous acetogenins, and marine polyethers.[10] In 1983, Cane, Celmer, and Westley proposed a unified stereochemical model of polyether antibiotic biogenesis, which implied that such natural products are formed through the cascade cyclization of the epoxide-containing biosynthetic precursors.[11] Later, Nakanishi invoked the cascade cyclization of polyepoxide precursors in the biosynthesis of ladder polyether natural product brevetoxin B (Figure 1A).[12] Since then, epoxide intermediates have become routinely proposed and observed in the biosynthesis of other cyclic ethers. The factors affecting the regioselectivity of the epoxide opening are not well understood, as such biosynthetic cascade reactions may often produce seemingly unfavored regioisomers. As demonstrated by Jamison and co-workers (Scheme 1C),[13] the medium of such transformations plays an important role, and the use of water as the solvent was found to promote the formation of the kinetically less favored endo products. At the same time, enzymes are known to play an important role in controlling the selectivity of these reactions. For example, it was found that epoxide hydrolase Lsd19 is the enzyme that promotes 6-endo-cyclization in the biosynthesis of natural lasalocid A whereas the 5-exo-product isolasalocid A is formed under standard acidic conditions (Figure 1B).[14]
Figure 1.

Biosynthetic epoxide cyclizations leading to the formation of cyclic ether containing natural products. A) Nakanishi’s cascade hypothesis for the formation of brevetoxin A. B) Epoxide hydrolase Lsd19-controlled cyclization
Scheme 1.

A) The endo vs. exo modes of cyclization. B) Examples of substituent-directed endo cyclizations. C) endo-Selective cascade cyclization by Jamison and co-workers.
Inspired by the aforementioned processes observed in nature, chemists have extensively used epoxyalcohol cycloisomerizations to generate various cyclic ethers (Scheme 1), in particular tetrahydrofurans (THFs) and tetrahydropyrans (THPs). However, for such an approach to be a reliable strategy in the synthetic toolbox, effective ways to control the regioselectivity of the epoxide ring-opening (ERO) is mandatory. Baldwin's rules,[15] a set of empirical generalizations that help discern kinetically favored intramolecular cyclizations, suggest that the regiochemistry of intramolecular ERO favors exo processes that proceed through a spiro transition state. Indeed, with a few exceptions, intramolecular ERO leads to the formation of five-membered rings (exo products) instead of six-membered rings (endo products; Scheme 1A).[16] For this reason, methods that facilitate endo control for intramolecular ERO are of high interest to synthetic chemists.
Most of the strategies developed to control the regiochemistry of intramolecular EROs have relied on the use of directing groups covalently present in the substrates. A variety of modifications have been developed, including alkenyl,[17] alkynyl,[18] alkyl,[19] and silyl (Scheme 1B).[20] The majority of these methods justify their regioselectivity through electronic perturbations of the epoxide that stabilize the 6-endo-tet transition state (TS) or disfavor the 5-exo-tet TS. For instance, the Nicolaou group, pioneers of the regioselective ERO, used epoxyalcohols containing a simple vinyl substituent on the epoxide such as 1 to stabilize the nascent allylic carbocation intermediate 2, which leads to endo-product 3 (Scheme 1B).[17c,d] Similarly, Schaumann and co-workers achieved a similar stereoelectronic bias by using silyl-substituted epoxides 4, which preferentially produce endo-product 5 (Scheme 1B).[20b] In addition, the Jamison group discovered an effective strategy that is based on the use of neutral water in combination with a preinstalled THP template 7, which results in the formation of poly-THP subunits such as 8 (Scheme 1C).[13,21]
These substrate-controlled strategies offered a breakthrough in intramolecular ERO. However, the use of covalently linked directing groups as the sole source of regiocontrol has some evident drawbacks. The most obvious one is that these methods are restricted in scope and tend to lack generality. In view of the recent successes in chiral catalyst-controlled selective functionalization of natural products,[22] chiral catalyst-controlled regioselective intramolecular ERO could provide a more robust method that is applicable to a variety of substrates. Our group has a long-standing interest in the application of chiral phosphoric acids (CPAs) to control the regio- and stereoselective functionalization of natural products.[23] In our recent efforts, we demonstrated that CPAs could mimic enzymatic processes and control the regioselectivity and/or stereoselectivity of acetalization,[23d] spiroketalization,[23c,e,f] and glycosylation[23a,b] reactions. Inspired by the endo regiocontrol exhibited by the epoxide hydrolase Lsd19, which catalyzes the selective formation of lasalocid A rather than its kinetically favored regioisomer isolacid A (Figure 1B),[19] we sought to investigate the possibility of controlling the formation of the exo and endo products through CPA catalysis. As an encouraging precedent, both the Sun and the List groups independently demonstrated the usefulness of CPAs 10 and 12 in the desymmetrizative intermolecular ERO of meso-epoxide 9 with thiols and carboxylic acids as nucleophiles, respectively (Scheme 2).[24,25] However, no applications of CPAs for controlling the regioselectivity of the epoxide opening have been reported to date.
Scheme 2.

CPA-catalyzed epoxide desymmetrization by the Sun and List groups.
This article describes the development and studies of CPA-controlled endo- and exo-selective cyclization of antibiotic mupirocin methyl ester, leading to the selective formation of either the THP- or THF-containing derivatives. To the best of our knowledge, this is the first example of a strategy that achieves regiocontrol by using chiral catalysts rather than achiral reagents or enzymes. The mechanisms of these transformations were investigated by using a combination of experimental techniques as well as computational methods based on the single-ended growing string (SE-GSM), a quantum chemical tool developed by the Zimmerman group.[26] These mechanistic studies provide a description of the potential energy surface and suggest a concerted and highly synchronous mechanism for the formation of both the exo and endo products.
Results and Discussion
To test our hypothesis that CPAs could be used to control the regioselectivity of intramolecular ERO in a complex setting, we investigated the cyclization of the methyl ester of natural antibiotic mupirocin (14).[27] The control experiments showed that the use of acidic, neutral, and basic conditions, which have been previously reported in the literature,[28] resulted in either poor conversion or the unselective formation of both the endo and exo products (15 and 16, Table 1). The use of LiCl as the Lewis acid did not result in successful cyclization (entry 1), whereas ZnCl2 and Sc(OTf)3 catalyzed the unselective formation of 15 and 16 (entries 2 and 3). Interestingly, the suspension of 14 in deionized water underwent slow cyclization over the course of 4 days and provided a 71:29 mixture of 15/16 in 24% conversion (entry 4). The reaction rate could be significantly improved if pH 7 phosphate buffer was used instead (entry 5); however, this reaction proceeded with no selectivity and produced an equimolar mixture of 15 and 16. The use of more basic conditions did not produce the desired cyclization products (entries 6 and 7). However, using catalytic amounts of the acidic phosphoric acid (p-NO2-C6H4O)2PO2H, resulted in complete conversion of mupirocin methyl ester 14, and low levels of endo selectivity were observed (entry 8). This suggests that, contrary to what might be expected by Baldwin's rules, phosphoric acid catalysis has small inherent endo selectivity for the intramolecular ERO of mupirocin methyl ester, but high selectivity for either 15 or 16 is unlikely to be achieved when using achiral catalysts or conditions.
Table 1.
Evaluation of mupirocin methyl ester (14) cyclization leading to 15 and 16 with achiral catalysts and promoters.
 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst/conditions | Solvent | Time | Conversion [%] | 15/16[b] |
| 1[a] | LiCl | CH2Cl2 | 12 h | < 5 | - |
| 2[a] | ZnCl2 | CH2Cl2 | 12 h | 82 | 48:52 |
| 3[c] | Sc(OTf)3 | CH2Cl2 | 12 h | 77 | 57:43 |
| 4[d] | - | H2O | 4 d | 24 | 71:29 |
| 5 | KH2PO4 buffer (pH 7) | H2O | 4 d | > 98 | 48:52 |
| 6[e] | Cs2CO3 | CH3OH | 4 d | - | - |
| 7[f] | LiHMDS | THF | 4 d | - | - |
| 8[g] | (p-NO2-C6H4O)2PO2H | CH2Cl2 | 5 d | > 98 | 70:30 |
Conditions: 14 (0.02 mmol), catalyst (20 mol%), CH2Cl2 (0.01 m).
Determined by RP HPLC.
Conditions: catalyst (20 mol%), CH2Cl2 (0.2 m).
70°C.
Cs2CO3 (10 equiv), CH2Cl2 (0.02 m).
LiHMDS (3equiv), CH2Cl2 (0.01 m).
Catalyst (20 mol%), CH2Cl2 (0.2 m).
Following these control studies, we then investigated if the use of CPAs could further enhance the formation of 15 or even reverse the observed trend and favor the exo-product 16. To this end, we screened a number of BINOL-derived CPAs (Table 2).[29] We selected methylene chloride dried with 4 Å MS as the starting solvent owing to its excellent solvating properties and good compatibility with phosphoric acid catalysis. Subsequently, we evaluated an array of BINOL-based CPAs (see Tables S2-S6 in the Supporting Information for the full list of catalysts and conditions). Encouragingly, we found that the axial chirality of the BINOL backbone had a significant impact on the regioselectivity of this transformation, with (R)-CPAs consistently favoring endo-product 16 formation more than the (S)-enantiomers (Table 2, entries 1–9). Although CPAs bearing 3,5-substituted aryl groups at the 3,3′-positions of the BINOL scaffold were not effective (catalysts 17a–c, entries 1–5), the 2,4,6-substituted CPAs such as (R)-TRIP (17d) and (R)-TCYP (17e) were found to be superior catalysts for the formation of endo-product 15 (entries 6–10).
Table 2.
Development of CPA-controlled endo-selective cyclization of mupirocin methyl ester (14).
 | ||||
|---|---|---|---|---|
| Entry[a] | Catalyst | Solvent[b] | Conversion [%] | 15/16[b] |
| 1 | (S)-17a | CH2Cl2 | >98 | 66:34 |
| 2 | (R)-17a | CH2Cl2 | >98 | 74:26 |
| 3 | (S)-17b | CH2Cl2 | 95 | 62:38 |
| 4 | (S)-17c | CH2Cl2 | >98 | 66:34 |
| 5 | (R)-17c | CH2Cl2 | >98 | 73:27 |
| 6 | (S)-17d | CH2Cl2 | >98 | 57:43 |
| 7 | (R)-17d | CH2Cl2 | >98 | 93:7 |
| 8 | (S)-17e | CH2Cl2 | >98 | 45:55 |
| 9 | (R)-17e | CH2Cl2 | >98 | 94:6 |
| 10 | (R)-17f | CH2Cl2 | >98 | 48:52 |
| 11[c] | (R)-17e | CH2Cl2 | 67 | 90:10 |
| 12[c] | (R)-17e | CyH | 39 | 77:23 |
| 13[c] | (R)-17e | PhCF3 | >98 | 92:8 |
| 14[c] | (R)-17e | PhMe | >98 | 95:5 |
| 15[c,d] | (R)-17e | PhMe | >98 | 97:3 |
| 16[c,e] | (R)-17e | PhMe | > 98 | 95:5 |
| 17[c,f] | (R)-17e | PhMe | >98 | 95:5 |
| 18[c, g] | (R)-17e | PhMe | 93% | 93:7 |
Conditions: 14 (0.02 mmol), solvent (0.2 m), 4 Å molecular sieves (MS), room temperature, 12 h.
Determined by RP HPLC.
Reactions were performed without 4 Å MS.
Reaction was performed with 100 mol% of the catalyst.
Reaction was performed with 10 mol% of the catalyst.
Reaction was performed with 5 mol% of the catalyst on a 0.20 mmol scale for 18 h.
Reaction was performed with 5 mol% of the catalyst in the presence of 5 mol% of water.
As before, the (S)-enantiomers of 17d and 17e did not provide good selectivities and were not further investigated. Similarly, the use of more acidic N-triflyl phosphoramide 17 f did not result in a selective reaction, and no further attempts to investigate this class of Brønsted acids was pursued. Other Brønsted acids and hydrogen bond donor (HBD) catalysts were also tested without success (see the Supporting Information, Tables S5). Based on these results, catalyst 17e was selected and the reaction conditions were further optimized (entries 11–17). The evaluation of other non-polar non-coordinating solvents in the absence of 4 Å MS led to the selection of toluene as the solvent of choice, which resulted in an increase in regioselectivity (95:5, entry 14). Under these optimized conditions, we found that the catalyst loading could be lowered to 5 mol% on a larger reaction scale (0.20 mmol) with minimal detriment to the regioselectivity (entry 17). As expected, an increase in the catalyst loading results in faster reaction times and higher 15/16 ratios (entry 16). In addition, this reaction tolerated the presence of 5 mol% of water without any significant effect on the selectivity (entry 18).
Having been successful in finding conditions for the preparation of the endo-product 15 with excellent conversion and regioselectivity, we conjectured that we could bias the selectivity towards the formation of the exo-product 16 through the judicious choice of a CPA, especially after having observed that in all cases BINOL-derived catalysts with (S)-chirality gave better exo selectivity than achiral phosphoric acids (Table 2, entries 1, 3, 4, 6, 8, compared with Table 1, entry 7). To this end, we evaluated (S)-CPAs (see the Supporting Information, Tables S4-S6) using toluene as the solvent and identified (S)-TiPSY (18) as the catalyst that enhances the formation of the exo-product 16 (Table 3). The selectivity of this transformation was found to be concentration dependent, and gradual decrease of the concentration (0.2 m to 0.01 m) led to the enhancement of the exo/endo selectivity to 74:26 (entries 1–5). To our delight, we found that the use of 5 mol% of (S)-TiPSY does not erode the selectivity, and 75% of the exo-product 16 was obtained after stirring the reaction mixture for 72 h (77:23, Table 3, entry 6). Unlike the reaction with (R)-TCYP (Table 2, entry 19), this transformation was highly sensitive to the presence of water as the presence of even 5 mol% of water resulted in an unselective pathway and slower reaction rates (Table 3, entry 7).
Table 3.
Development of (S)-TiPSY (18)-controlled exo-selective cyclization of mupirocin methyl ester (14).
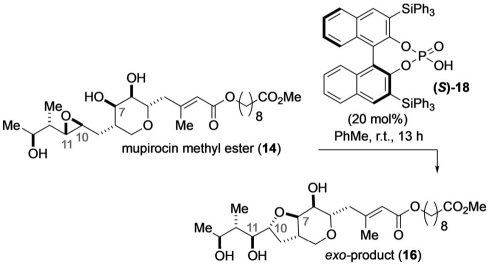 | ||||
|---|---|---|---|---|
| Entry[a] | Concentration [M] | Loading [mol%] | Conversion [%] | 15/16[b] |
| 1 | 0.5 | 20 | 94 | 55:45 |
| 2 | 0.2 | 20 | 90 | 48:52 |
| 3 | 0.1 | 20 | 85 | 38:62 |
| 4 | 0.025 | 20 | 83 | 35:65 |
| 5 | 0.01 | 20 | 95 | 26:74 |
| 6[c] | 0.01 | 5 | 77 | 23:77 |
| 7[d] | 0.01 | 5 | 44 | 45:55 |
Conditions: 14 (0.02 mmol), toluene, room temperature, 12 h.
Determined by 1H NMR spectroscopy.
14 (0.2 mmol), toluene, room temperature, 72 h.
This reaction was attempted in the presence of 5 mol% of H2O.
With the catalyst-controlled pathways leading to both 15 and 16, some experimental studies to probe the potential reaction mechanism were performed (Figure 2A). To rule out the CPA-catalyzed equilibration, both 15 and 16 were subjected to (R)-17e and (S)-18, and no isomerization, retrocyclization, or material degradation was noted at room temperature. The selectivities of the cyclizations leading to both endo-product (15) and exo-product (16) were found to be conversion-dependent and increased with the progression of these reactions (Figure 2B and Supporting Information, Tables S7 and S8). However, the addition of 0.5 equivalents of either the endo- or exo-products 15 or 16 to the initial reaction mixture did not affect the selectivity versus conversion profiles. Similarly, neither 15 nor 16 acted as the catalysts when stirred with mupirocin methyl ester (14). These results coupled with the observation that catalytic quantities of water diminish the reaction selectivity strongly suggest the formation of hydrogen-bond complexes between 14 and catalysts (R)-17e or (S)-18.
Figure 2.

A) Control experiments to probe the isomerization of 15 and 16 under the reaction conditions. B) Dependence of the endo/exo selectivity on reaction progression for the (R)-17e catalyzed cyclization of 14.
Based on these experimental observations, we turned to computation to help explain the reaction mechanism and observed selectivities.[30] In particular, Zimmerman group's reaction exploration tools were used to facilitate the accurate and fast search of relevant reactions paths and transition states.[25] These tools have previously been used in the elucidation of fine mechanistic details of phosphoric acid-catalyzed spiroketalizations, glycosylations, and intramolecular aza-Michael reactions.[23b,c,31] Here, the single-ended growing string method (GSM) was employed to detail the mechanism of phosphoric acid-catalyzed intramolecular ERO.
To alleviate computational costs, a truncated model of the mupirocin methyl ester (20) was used alongside biphenyl phosphoric acid (BPA, 19) as the catalyst (Figure 3). Given that CPAs possess both Brønsted acidic and Lewis basic sites in proximity, it is possible that CPAs simultaneously activate the electrophilic epoxide and the nucleophilic alcohol.[25d,32] Several arrangements that allow such concerted operation were modeled, leading to the products 21 (exo reaction) and 22 (endo reaction).
Figure 3.

Energy diagram for the intramolecular ERO of a truncated mupirocin model.
These simulations (Figure 3) revealed that the formation of a substrate-BPA complex [20-BPA] was thermodynamically favored by 3.5 kcal mol−1 over separated reactant/catalyst, giving three hydrogen bonds between the C6, C7, and C13 hydroxy groups and the phosphoric acid. Upon transforming this complex, the endo-product 22 was found to be 12.6 kcalmol−1 downhill from the substrate-BPA complex [20-BPA], making it preferred by 5.1 kcalmol−1 over the exo product. Concerted reaction pathways for the formation of endo and exo products were readily found by using GSM (Figure 3). These revealed that the preferred regiotopic pathways, although topologically distinct, shared almost identical activation barriers of around 19.1 kcal mol−1. The endo pathway was preferred minutely by 0.16 kcal mol−1, a number that, although within the error of the computational methods, agrees with the small intrinsic endo selectivity that is observed with achiral phosphoric acids experimentally (Figure 3, TSendo vs. TSexo and Table 1, entry 7). An alternative mechanism through epoxide opening and involving a phosphate intermediate[23b,31 was ruled out because the barrier was substantially higher (≈8.3 kcal mol−1, TSP) than the concerted pathways.
The reverse reactions for the concerted mechanism have activation barriers of 36.0 and 32.1 kcal mol−1 for endo and exo product formation, respectively, indicating the transformation is irreversible at room temperature. This is consistent with the observation that no interconversion was observed between the exo product (21) or endo product (22) in the control experiments (Figure 2A). As mentioned earlier, the preferred endo and exo pathways are close in energy and share some common features. For instance, in both cases, the hydroxyl group at C13 is needed to serve as a proton donor/acceptor relay for the concerted pathway to be operative (Figure 4). Indeed, models lacking this functional group require a step-wise sequence of events (protonation, rotation, and cyclization) to yield the product and have a higher activation barrier. Another shared feature for this pathway is that protonation of the epoxide (red arrows) and cyclization (blue arrows) occur simultaneously in the TS. There are, however, important differences between the endo and exo pathways. The geometrical demands of 5-exo-tet cyclizations require a more closed and tight transition state, and the C13 hydroxyl group serves as a relay to protonate the epoxide, whereas the phosphoric acid activates the C7 alcohol. On the other hand, the 6-endo-tet mechanism involves a more open transition state, and the C13 hydroxyl group serves as a relay to activate the C7 alcohol, whereas the phosphoric acid protonates the epoxide directly.
Figure 4.

Quadrant-based perspective for key TSs. A) Quadrant analysis for (R)-BINOL-derived CPAs. B) Perspective taken. C) 6-Endo-tet pathway in a (R)-CPA quadrant framework. D) 5-Exo-tet pathway in a (R)-CPA quadrant framework. E) 6-Endo-tet pathway in a (S)-CPA quadrant framework. F) 5-Exo-tet pathway in a (S)-CPA quadrant framework. The biphenyl backbone of the catalyst is omitted from the perspective for clarity.
The quadrant-based analysis, developed by the groups of Himo and Terada to explain the selectivity of BINOL-based CPAs,[33] has been previously used to describe the steric profile imposed by these acids (Figure 4A).[23c] Herein, we use a similar analysis to explain the observed selectivity pattern. This qualitative model uses the TS for endo and exo cyclization obtained with BPA (Figures 3, 4B) as a template to juxtapose the steric profile of (R)- or (S)-BINOL-derived CPAs. Figure 4C and E show that the 6-endo-tet pathway leading to 22 would not have significant contacts with either (R)- or (S)-CPAs, and most of the interactions are with torsionally flexible parts of the substrate, colored blue in Figure 4. This is consistent with the more open and flexible TS compared with the 5-exo-tet pathway and suggests that the choice of chirality of the CPA does not have a drastic impact on the 6-endo-tet cyclization activation barrier. On the other hand, the tighter TS of the 5-exo-tet mechanism places an inflexible and bulky group (colored in red in Figure 4) in the bottom right quadrant pointing towards the phosphoric acid (Figure 4D and F). This implies that (R)-BINOL-derived CPAs would disfavor the exo pathway owing to steric clashes in this quadrant. On the other hand, CPAs with (S)-chirality, which have an inverse steric profile, would not suffer from these interactions and would therefore have lower barriers for the formation of exo-product 21 (Figure 4D). Taking both considerations together, this model explains why catalysts with (R)-chirality favor endo product formation more than those with (S)-chirality and is in agreement with the experimental results (Table 2 and Table 3).
Conclusion
We have developed a catalyst-controlled method for the regioselective intramolecular epoxide ring-opening of mupirocin derivatives. After an extensive screening, we found that (R)-TCYP could catalyze the formation of endo-product 15 in 95:4 r.r., whereas (S)-TIPSY yielded the exo-product 16 in 77:23 r.r. Importantly, we investigated the mechanism by using state-of-the-art quantum chemical solutions developed by the Zimmerman group. We found that our method could rapidly describe a potential energy surface fully consistent with the experimental observations. On the basis of our results, we postulate that a concerted and highly synchronous mechanism is likely for this reaction. We obtained highly detailed reaction paths for the formation of exo and endo products, which, although similar in activation barrier, showed key structural dissimilarities that helped us establish the origin of the regiocontrol of these reactions; this is likely due to hindrance of the 5-exo-tet cyclization caused by steric clashes of the epoxide alkyl substituents with the 3- and 3′- substituents of BINOL-derived CPAs with (R)-chirality. Catalyst-controlled regiodivergent methods for epoxide opening are scarce but synthetically useful and versatile. We hope that our mechanistic insights can assist in developing this methodology further to make it more broadly applicable to cases useful to the organic chemist. We also envision that CPAs could be broadly applied to affect other intramolecular ERO reactions such as the ones depicted in Figure 1 and Scheme 1, and these studies are currently ongoing in our laboratories.
Experimental Section
General methods and materials
All reactions were carried out under an atmosphere of nitrogen in flame- or oven-dried glassware with magnetic stirring. Mupirocin was purchased from Sigma–Aldrich and used as such. Chiral phosphoric acids (R)-17e and (S)-18 are commercially available or could be synthesized by using known procedures. Deionized water was used in the preparation of all aqueous solutions and for all aqueous extractions. Solvents used for extraction and chromatography were ACS or HPLC grade. Purification of reaction mixtures was performed by flash chromatography using a SiliCycle SiliaFlash P60 (230–400 mesh). Diastereomeric ratios were determined by RP HPLC analysis by using a Shimadzu SBM-20A Separations Module with a photodiode array detector equipped with C18 Nova-Pack® column (60 Å, 4 mm, 3.9 × 150 mm). All spectra were recorded with Varian vnmrs 700 (700 MHz), Varian vnmrs 500 (500 MHz), Varian MR400 (400 MHz), Varian Inova 500 (500 MHz) spectrometers and chemical shifts (δ) are reported in parts per million (ppm) and referenced to the 1H signal of the internal tetramethylsilane according to IUPAC recommendations. Data are reported as (br=broad, s=singlet, d=doublet, t=triplet, q=quartet, qn=quintet, sext=sextet, m=multiplet; coupling constant(s) in Hz; integration). High-resolution mass spectra (HRMS) were recorded with Micromass AutoSpecUltima or VG (Micromass) 70–250-S Magnetic sector mass spectrometers at the University of Michigan mass spectrometry laboratory. Infrared (IR) spectra were recorded as thin films on NaCl plates with a PerkinElmer Spectrum BX FTIR spectrometer and are reported in wavenumbers (cm−1).
Computational details
All quantum chemical calculations were performed by using the Q-Chem 4.3 package.[34] Geometry optimizations were evaluated by using the B97-D density functional[35] using the double-ζ- quality basis set with polarization functions on all atoms, 6-31G**[36] Pictorial representations of important stationary points were generated with the Discovery Studio 4.1 Visualizer. The electronic contributions to the Gibbs free energy of all stationary points were computed through solvent-corrected (dichloromethane) single-point energies by using the SMD model.[37] For these calculations, the ωB97X-D3 exchange functional[38] was employed with a 6-31G** basis set. The final Gibbs free energy values were obtained by correcting the electronic free energy with the enthalpic and entropic contributions from vibrations, rotations, and translations at 298.15 K. These frequency computations were performed by using the B97-D functional and 6-31G** basis set.
Synthesis and characterization
Synthesis of mupirocin methyl ester 14:
Mupirocin (2.0 g, 4.0 mmol) was initially dissolved in toluene (16 mL) and methanol (4 mL). Then, trimethylsilyldiazomethane (2.4 mL, 4.8 mmol, 1.2 equiv) was added dropwise. The reaction mixture was stirred for 1 h at room temperature under N2. After the reaction is completed, the reaction mixture was diluted with EtOAc (15 mL), quenched with 10% v/v AcOH (15 mL) and extracted with EtOAc (15 mL × 3). The combined organic extract was washed with brine and dried with Na2SO4 and concentrated in vacuo to obtain a yellow oil. The crude mixture was purified by recrystallization in hexane (10 mL) and diethyl ether (20 mL) and filtered through a Buchner funnel to afford 14 (1.93 g, 85%) as a white solid. 1H NMR (700 MHz, CDCl3): δ = 5.76 (d, J = 1.6 Hz, 1H), 4.07 (t, J = 6.7 Hz, 2H), 3.93 (s, 1H), 3.86 (d, J = 3.1 Hz, 1H), 3.82 (t, J = 6.4 Hz, 1H), 3.76 (td, J = 8.7, 3.4 Hz, 1H), 3.67 (s, 3H), 3.56 (dd, J = 11.7, 2.8 Hz, 1H), 3.48 (d, J = 7.7 Hz, 1H), 2.80 (ddd, J = 6.9, 5.0, 2.2 Hz, 1H), 2.70 (dd, J = 8.0, 2.2 Hz, 1H), 2.61–2.54 (m, 1H), 2.38 (s, 1H), 2.33–2.18 (m, 8H), 2.01 (dq, J = 7.2, 3.9 Hz, 1H), 1.79–1.67 (m, 2H), 1.40–1.27 (m, 10H), 1.22 (d, J = 6.3 Hz, 3H), 0.94 ppm (d, J = 7.0 Hz, 3H); 13C NMR (176 MHz, CDCl3): δ = 174.4, 166.7, 156.6, 117.6, 77.2, 77.0, 76.8, 74.8, 71.4, 70.4, 69.0, 65.3, 63.8, 61.3, 55.6, 51.5, 42.84, 42.82, 39.5, 34.1, 31.5, 29.1, 29.03, 29.01, 28.6, 25.9, 24.9, 20.8, 19.0, 12.7 ppm; IR (thin film): 3564, 3399 (br), 1737, 1712, 1647, 1218, 1142, 1110, 1075, 941, 922, 890, 814, 754, 669 cm−1; [α]D25 = −9.4 (c = 2.06, CHCl3); HRMS (ESI+) (m/z): [M+Na]+ calcd for C27H46O9: 537.3040; found: 537.3014.
Endo-selective cyclization leading to 15:
To a flame-dried, N2-flushed 1-dram vial, a stir bar and mupirocin methyl ester 14 (104 mg, 0.2 mmol) were added. This was followed by the addition of chiral phosphoric acid (R)-17e (10 mg, 0.01 mmol, 0.05 equiv) and dissolved in toluene (1.0 mL, 0.2 m). The reaction was stirred for 18 h at room temperature before being concentrated on a rotatory evaporator and purified by chromatography on silica gel by using 10:9:1 hexanes/dichloromethane/methanol (Rf=0.2). This provided 97 mg of purified endo-product 15 in 93% yield. 1H NMR (700 MHz, CDCl3): δ = 5.69 (d, J = 1.9 Hz, 1H), 4.22 (ddd, J = 10.0, 5.3, 1.4 Hz, 1H), 4.06 (t, J = 6.7 Hz, 2H), 3.79 (p, J = 6.5 Hz, 1H), 3.74 (t, J = 2.0 Hz, 1H), 3.65 (s, 3H), 3.63 (d, J = 2.0 Hz, 1H), 3.60 (ddd, J = 16.2, 10.8, 5.1 Hz, 2H), 3.33–3.24 (m, 2H), 2.57 (dd, J = 14.2, 9.9 Hz, 1H), 2.28 (t, J = 7.5 Hz, 2H), 2.24–2.17 (m, 5H), 2.17–2.08 (m, 1H), 2.01 (dt, J = 11.7, 3.9 Hz, 1H), 1.93 (pd, J = 7.1, 1.8 Hz, 1H), 1.66–1.55 (m, 5H), 1.37–1.27 (m, 10H), 1.25 (d, J = 6.3Hz, 3H), 0.96 ppm (d, J = 7.1 Hz, 3H); 13C NMR (176 MHz, CDCl3): δ = 174.31, 166.40, 155.05, 118.27, 82.03, 77.05, 75.94, 70.15, 69.11, 66.34, 64.16, 63.93, 53.41, 51.45, 39.96, 39.86, 34.58, 34.05, 32.90, 29.08, 29.03, 29.00, 28.62, 25.91, 24.87, 22.16, 18.28, 10.78 ppm; IR (thin film): 3407 (br), 2930, 2856, 1713, 1646, 1436, 1223, 1149, 1095, 1056, 997, 914, 862, 808, 753, 610 cm−1; [α]D25 = −8.5 (c = 1.91, CHCl3); HRMS (ESI+) (m/z): [M+Na]+ calcd for C27H46O9: 537.3040; found: 537.3054.
Exo-selective cyclization leading to 16:
To a flame-dried, N2-flushed 1-dram vial, a stir bar and mupirocin methyl ester 14 (100 mg, 0.2 mmol) were added. This was followed by the addition of chiral phosphoric acid (S)-18 (10 mg, 0.01 mmol, 0.05 equiv) and dissolved in toluene (20 mL, 0.01 m). The reaction was stirred for 72 h at room temperature before being concentrated on a rotatory evaporator and purified by chromatography on silica gel by using 10:9:1 hexanes/dichloromethane/methanol (Rf=0.2). This provided 77 mg of purified exo-product 16 in 77% yield. 1H NMR (700 MHz, CDCl3): δ = 5.70 (s, 1H), 4.28 (ddd, J = 9.4, 5.9, 3.3 Hz, 1H), 4.14 (ddd, J = 9.5, 5.4, 1.8 Hz, 1H), 4.07 (t, J = 6.7 Hz, 2H), 4.03–3.98 (m, 2H), 3.91 (dd, J = 9.5, 3.3 Hz, 1H), 3.89–3.83 (m, 1H), 3.66 (s, 3H), 3.64 (dd, J = 11.3, 3.1 Hz, 1H), 2.50 (dd, J = 14.2, 9.4 Hz, 1H), 2.41 (ddq, J = 17.2, 11.5, 5.4 Hz, 1H), 2.30 (t, J = 7.5 Hz, 2H), 2.25 (dd, J = 14.1, 5.4 Hz, 1H), 2.20 (d, J = 1.3 Hz, 3H), 1.91 (dt, J = 11.6, 5.9 Hz, 1H), 1.80 (td, J = 12.1, 9.8 Hz, 1H), 1.61 (hept, J = 6.8 Hz, 5H), 1.49 (dp, J = 9.1, 7.1 Hz, 1H), 1.38–1.27 (m, 10H), 1.19 (d, J = 6.2 Hz, 3H), 0.81 ppm (d, J = 6.9 Hz, 3H); 13C NMR (176 MHz, CDCl3): δ = 174.29, 166.42, 155.15, 118.27, 81.14, 80.59, 77.29, 76.16, 72.24, 68.98, 65.62, 63.88, 51.45, 41.98, 41.40, 36.27, 34.06, 29.09, 29.03, 29.01, 28.63, 26.36, 25.92, 24.88, 20.93, 18.38, 12.54 ppm; IR (thin film): 3407 (br), 2929, 2856, 1714, 1646, 1436, 1223, 1149, 1059, 993, 913, 842, 800, 755, 667, 610 cm−1; [α]D25 = −2.1 (c = 2.23, CHCl3); HRMS (ESI+) (m/z): [M+Na]+ calcd for C27H46O9: 537.3040; found: 537.3041.
Supplementary Material
Acknowledgments
The authors are grateful to the NIH (R01GM111476 and R35-GM-128830) for financial support.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Marco-Contelles J, Molina MT, Anjum S, Chem. Rev 2004, 104, 2857. [DOI] [PubMed] [Google Scholar]
- [2].Morgan KM, Ellis JA, Lee J, Fulton A, Wilson SL, Dupart PS, Dastoori R, J. Org. Chem 2013, 78, 4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Katsuki T, Sharpless KB, J. Am. Chem. Soc 1980, 102, 5974. [Google Scholar]
- [4].Chang S, Galvin JM, Jacobsen EN, J. Am. Chem. Soc 1994, 116, 6937. [Google Scholar]
- [5].Zhang W, Loebach JL, Wilson SR, Jacobsen EN, J. Am. Chem. Soc 1990, 112, 2801. [Google Scholar]
- [6].Wang ZX, Tu Y, Frohn M, Zhang JR, Shi Y, J. Am. Chem. Soc. 1997, 119, 11224. [Google Scholar]
- [7].Tu Y, Wang ZX, Shi Y, J. Am. Chem. Soc 1996, 118, 9806. [Google Scholar]
- [8].Das B, Damodar K, in Heterocycles in Natural Product Synthesis (Eds.: Majumdar KC, Chattopadhyay SK), Wiley-VCH, Weinheim, 2011, 63–95. [Google Scholar]
- [9].Padwa A, Murphree S, ARKIVOC (Gainesville, FL, U.S.) 2006, 2006, 6. [Google Scholar]
- [10].Gallimore AR, Nat. Prod. Rep 2009, 26, 266. [DOI] [PubMed] [Google Scholar]
- [11].Cane DE, Celmer WD, Westley JW, J. Am. Chem. Soc 1983, 105, 3594. [Google Scholar]
- [12].Nakanishi K, Toxicon 1985, 23, 473. [DOI] [PubMed] [Google Scholar]
- [13].Vilotijevic I, Jamison TF, Science 2007, 317, 1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14] a).Shichijo Y, Migita A, Oguri H, Watanabe M, Tokiwano T, Watanabe K, Oikawa H, J. Am. Chem. Soc 2008, 130, 12230; [DOI] [PubMed] [Google Scholar]; b) Matsuura Y, Shichijo Y, Minami A, Migita A, Oguri H, Watanabe M, Tokiwano T, Watanabe K, Oikawa H, Org. Lett 2010, 12, 2226. [DOI] [PubMed] [Google Scholar]
- [15].Baldwin JE, J. Chem. Soc. Chem. Commun 1976, 0, 734. [Google Scholar]
- [16].Vilotijevic I, Jamison TF, Angew. Chem. Int. Ed 2009, 48, 5250; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem 2009, 121,5352. [Google Scholar]
- [17] a).Matsukura H, Morimoto M, Koshino H, Nakata T, Tetrahedron Lett. 1997, 38, 5545; [Google Scholar]; b) Suzuki T, Sato O, Hirama M, Tetrahedron Lett. 1990, 31, 4747; [Google Scholar]; c) Nicolaou KC, Prasad CVC, Somers PK, Hwang CK, J. Am. Chem. Soc 1989, 111, 5330; [Google Scholar]; d) Nicolaou KC, Duggan ME, Hwang CK, Somers PK, J. Chem. Soc. Chem. Commun 1985, 0, 1359. [Google Scholar]
- [18].Mukai C, Sugimoto YI, Ikeda Y, Hanaoka M, Tetrahedron 1998, 54, 823. [Google Scholar]
- [19] a).Morimoto Y, Nishikawa Y, Ueba C, Tanaka T, Angew. Chem. Int. Ed 2006, 45, 810; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2006, 118, 824; [Google Scholar]; b) Bravo F, McDonald FE, Neiwert WA, Do B, Hardcastle KI, Org. Lett 2003, 5, 2123. [DOI] [PubMed] [Google Scholar]
- [20] a).Heffron TP, Jamison TF, Org. Lett 2003, 5, 2339; [DOI] [PubMed] [Google Scholar]; b) Adiwidjaja G, Florke H, Kirschning A, Schaumann E, Tetrahedron Lett. 1995, 36, 8771. [Google Scholar]
- [21] a).Sittihan S, Jamison TF, J. Am. Chem. Soc 2019, 141, 11239; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kelley EH, Jamison TF, Bioorg. Med. Chem. 2018, 26, 5327; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Czabaniuk LC, Jamison TF, Org. Lett 2015, 17, 774; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Byers JA, Jamison TF, Proc. Natl. Acad. Sci. USA 2013, 110, 16724; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Mousseau JJ, Morten CJ, Jamison TF, Chem. Eur. J 2013, 19, 10004; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Underwood BS, Tanuwidjaja J, Jamison TF, Tetrahedron 2013, 69, 5205; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Morten CJ, Byers JA, Van Dyke AR, Vilotijevic I, Jamison TF, Chem. Soc. Rev 2009, 38, 3175; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Tanuwidjaja J, Ng S-S, Jamison TF, J. Am. Chem. Soc 2009, 131, 12084; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Morten CJ, Jamison TF, J. Am. Chem. Soc 2009, 131, 6678; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Byers JA, Jamison TF, J. Am. Chem. Soc 2009, ISI, 6383; [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Van Dyke AR, Jamison TF, Angew. Chem. Int. Ed 2009, 48, 4430; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem 2009, 121, 4494. [Google Scholar]
- [22].Selected examples of chiral catalyst-controlled functionalization of natural products: a)Lewis CA, Miller SJ, Angew. Chem. Int. Ed 2006, 45, 5616; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2006, 118, 5744; [Google Scholar]; b) Sánchez-Roselló M, Puchlopek ALA, Morgan AJ, Miller SJ, J. Org. Chem 2008, 73, 1774; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lewis CA, Longcore KE, Miller SJ, Wender PA, J. Nat. Prod 2009, 72, 1864; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Jordan PA, Miller SJ, Angew. Chem. Int. Ed. 2012, 51, 2907; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem 2012, 124, 2961; [Google Scholar]; e) Pathak TP, Miller SJ, J. Am. Chem. Soc 2012, 134, 6120; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Fowler BS, Laemmerhold KM, Miller SJ, J. Am. Chem. Soc 2012, 134, 9755; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Lichtor PA, Miller SJ, Nat. Chem 2014. 4, 990; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Ueda Y, Mishiro K, Yoshida K, Furuta T, Kawabata T, J. Org. Chem 2012, 77, 7850; [DOI] [PubMed] [Google Scholar]; i) Pathak TP, Miller SJ, J. Am. Chem. Soc 2012. 135, 8415; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Han S, Miller SJ, J. Am. Chem. Soc 2013, 135, 12414; [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Sun X, Lee H, Lee S, Tan KL, Nat. Chem 2013, 5, 790; [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Yoganathan S, Miller SJ, J. Med. Chem 2015, 58, 2367; [DOI] [PMC free article] [PubMed] [Google Scholar]; m) Ueda Y, Furuta T, Kawabata T, Angew. Chem. Int. Ed 2015, 54, 11966; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2015, 127, 12134; [Google Scholar]; n) Howell JM, Feng K, Clark JR, Trzepkowski LJ, White MC, J. Am. Chem. Soc 2015, 137, 14590; [DOI] [PMC free article] [PubMed] [Google Scholar]; o) He J, Hamann LG, Davies HML, Beckwith REJ, Nat. Commun 2015, 6, 5943; [DOI] [PubMed] [Google Scholar]; p) Sharma A, Hartwig JF, Nature 2015, 517, 600; [DOI] [PMC free article] [PubMed] [Google Scholar]; q) Yanagi M, Ninomiya R, Ueda Y, Furuta T, Yamada T, Sunazuka T, Kawabata T, Chem. Pharm. Bull 2016, 64, 907; [DOI] [PubMed] [Google Scholar]; r) Karimov RR, Sharma A, Hartwig JF, ACS Cent. Sci 2016, 2, 715; [DOI] [PMC free article] [PubMed] [Google Scholar]; s) Key HM, Miller SJ, J. Am. Chem. Soc 2017, 139, 15460; [DOI] [PMC free article] [PubMed] [Google Scholar]; t) Li J, Grosslight S, Miller SJ, Sigman MS, Toste FD, ACS Catal. 2019, 9, 9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23] a).Lee J, Borovika A, Khomutnyk Y, Nagorny P, Chem. Commun 2017, 53, 8976; [DOI] [PubMed] [Google Scholar]; b) Tay J-H, Arguelles AJ, DeMars MD II, Zimmerman PM, Sherman DH, Nagorny P, J. Am. Chem. Soc 2017, 139, 8570; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Khomutnyk YY, Arguelles AJ, Winschel GA, Sun Z, Zimmerman PM, Nagorny P, J. Am. Chem. Soc 2016, 138, 444; [DOI] [PubMed] [Google Scholar]; d) Mensah E, Camasso N, Kaplan W, Nagorny P, Angew. Chem. Int. Ed 2013, 52, 12932; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2013, 125, 13170; [Google Scholar]; e) Nagorny P, Sun Z, Winschel GA, Synlett 2013, 24, 661; [Google Scholar]; f) Sun Z, Winschel GA, Borovika A, Nagorny P, J. Am. Chem. Soc 2012, 134, 8074. [DOI] [PubMed] [Google Scholar]
- [24].Wang Z, Law WK, Sun J, Org. Lett 2013, 15, 5964. [DOI] [PubMed] [Google Scholar]
- [25] a).Monaco MR, Prevost S, List B, Angew. Chem. Int. Ed 2014, 53, 8142; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2014, 126, 8280; [Google Scholar]; b) Liao S, Leutzsch M, Monaco MR, List B, J. Am. Chem. Soc 2016, 138, 5230; [DOI] [PubMed] [Google Scholar]; c) Monaco MR, Prevost S, List B, J. Am. Chem. Soc 2014, 136, 16982; [DOI] [PubMed] [Google Scholar]; d) Monaco MR, Fazzi D, Tsuji N, Leutzsch M, Liao S, Thiel W, List B, J. Am. Chem. Soc 2016, 138, 14740. [DOI] [PubMed] [Google Scholar]
- [26] a).Zimmerman PM, J. Chem. Phys 2013, 138, 184102; [DOI] [PubMed] [Google Scholar]; b) Zimmerman PM, J. Comput. Chem 2013, 34, 1385; [DOI] [PubMed] [Google Scholar]; c) Zimmerman PM, J. Comput. Chem 2015, 36, 601; [DOI] [PubMed] [Google Scholar]; d) Zimmerman P, J. Chem. Theory Comput 2013, 9, 3043. [DOI] [PubMed] [Google Scholar]
- [27] a).Khoshnood S, Heidary M, Asadi A, Soleimani S, Motahar M, Savari M, Saki M, Abdi M, Biomed. Pharmacother 2019, 109, 1809; [DOI] [PubMed] [Google Scholar]; b) Thomas CM, Hothersall J, Willis CL, Simpson TJ, Nat. Rev. Microbiol 2010, 8, 281; [DOI] [PubMed] [Google Scholar]; c) Class YJ, DeShong P, Chem. Rev 1995, 95, 1843; [Google Scholar]; d) Ward A, Campoli-Richards DM, Drugs 1986, 32, 425. [DOI] [PubMed] [Google Scholar]
- [28].Clayton JP, Oliver RS, Rogers NH, J. Chem. Soc. Perkin Trans. 1 1979, 838. [Google Scholar]
- [29] a).Akiyama T, Itoh J, Yokota K, Fuchibe K, Angew. Chem. Int. Ed 2004, 43, 1566; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2004, 116, 1592; [Google Scholar]; b) Uraguchi D, Terada M, J. Am. Chem. Soc 2004, 126, 5356. [DOI] [PubMed] [Google Scholar]
- [30].Dewyer AL, Zimmerman PM, Org. Biomol. Chem 2017, 15, 501. [DOI] [PubMed] [Google Scholar]
- [31].Sun Z, Winschel GA, Zimmerman PM, Nagorny P, Angew. Chem. Int. Ed 2014, 53, 11194; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2014, 126, 11376. [Google Scholar]
- [32].Computational studies of related CPA-catalyzed transformations: a) Champagne PA, Houk KN, J. Am. Chem. Soc 2016, 138, 12356; [DOI] [PubMed] [Google Scholar]; b) Seguin TJ, Wheeler SE, ACS Catal. 2016, 6, 2681; [Google Scholar]; c) Maji R, Cham-pagne PA, Houk KN, Wheeler SE, ACS Catal. 2017, 7, 7332; [Google Scholar]; d) Duarte F, Paton RS, J. Am. Chem. Soc 2017, 139, 8886. [DOI] [PubMed] [Google Scholar]
- [33] a).Gridnev ID, Kouchi M, Sorimachi K, Terada M, Tetrahedron Lett. 2007, 48, 497; [Google Scholar]; b) Marcelli T, Hammar P, Himo F, Chem. Eur. J 2008, 14, 8562. [DOI] [PubMed] [Google Scholar]
- [34].Shao Y, Gan Z, Epifanovsky E, Gilbert ATB, Wormit M, Kussmann J, Lange AW, Behn A, Deng J, Feng X, et al. , Mol. Phys 2015, 113, 184. [Google Scholar]
- [35] a).Becke AD, J. Chem. Phys 1997, 107, 8554; [Google Scholar]; b) Grimme S, J. Comput. Chem 2006, 27, 1787. [DOI] [PubMed] [Google Scholar]
- [36] a).Hariharan PC, Pople JA, Theor. Chim. Acta 1973, 28, 213; [Google Scholar]; b) Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA, J. Chem. Phys 1982, 77, 3654. [Google Scholar]
- [37].Marenich AV, Cramer CJ, Truhlar DG, J. Phys. Chem. B 2009, 113, 6378. [DOI] [PubMed] [Google Scholar]
- [38].Da Chai J, Head-Gordon M, J. Chem. Phys 2008, 128, 084106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


