Abstract
Congenital heart defect is one of the most common structural birth defects in the human population. It is highly associated with heterotaxy, a birth defect involving randomized left–right patterning of visceral organ situs. Large scale mouse forward genetics have led to the finding of a central role for cilia in CHD pathogenesis, with some cilia and non-cilia mutations causing CHD with heterotaxy. Interestingly, many of the mutations causing CHD with heterotaxy can give rise to three laterality outcomes comprising normal situs solitus, mirror symmetric situs inversus totalis, or randomized situs with heterotaxy. Given CHD is largely observed only with heterotaxy, this suggests a new paradigm is needed for investigating the genetics of CHD associated with heterotaxy. Furthermore, analysis of data from multiple large birth cohorts have independently confirmed a broader involvement of laterality disturbance in CHD. This was demonstrated by the common cooccurrence of rare laterality defects with CHD lesions of a wide spectrum. These findings suggest left–right patterning is tightly intertwined with the developmental processes that regulate cardiac morphogenesis and its disturbance may contribute to all types of CHD even in the absence of laterality defects.
Keywords: cilia, congenital heart disease, heterotaxy, left–right patterning
1 |. CRITICAL CONGENITAL HEART DISEASE
Congenital heart defect (CHD) is one of the most common structural birth defects, affecting up to 1% of live births (Liu et al., 2019; van der Linde et al., 2011). Simple defects such as ventricular septal defects (VSD) and atrial septal defects (ASD) encompass a large majority of CHD (Reller, Strickland, Riehle-Colarusso, Mahle, & Correa, 2008). These are usually benign and require minimal or no clinical intervention. In contrast, critical or complex CHD lesions encompassing only 20% of all CHD drives the majority of disease burden in CHD (Hoffman, Kaplan, & Liberthson, 2004). Patients with critical CHD usually require clinical intervention in the neonatal period with surgical palliation. The lethality of such CHD lesions stems from disruption of efficient blood oxygenation built around the unique left–right asymmetry of the cardiovascular system. This asymmetry allows for two independent circuits of blood flow connected in series. The pulmonary circuit conducts deoxygenated blood returning from the body for reoxygenation in the lung, while the systemic circuit delivers the oxygenated blood from the lung to the rest of the body. Structural heart defects with anatomical perturbations affecting this left–right asymmetry are largely incompatible with postnatal survival. However, with recent clinical advances in critical care and congenital cardiac surgeries, most patients with complex CHD now survive into adulthood. In fact, currently there are more adult CHD patients then children born every year with CHD in the United States (Gilboa et al., 2016). However, as adult CHD patients continue to suffer high morbidity, this has created new challenges with the management of clinical care for adult CHD patients (Marelli, Miller, Marino, Jefferson, & Newburger, 2016; Saha et al., 2019).
2 |. CONGENITAL HEART DISEASE AND LATERALITY DEFECTS
Congenital heart defect is highly associated with heterotaxy (HTX), a birth defect involving randomization of left–right visceral organ situs (Lin et al., 2014). In contrast to CHD, HTX is a rare disorder with an incidence estimated at one in 10,000 (Bedard et al., 2012; Khoshnood et al., 2012; Lin et al., 2014; Lin, Ticho, Houde, Westgate, & Holmes, 2000). It can involve alteration of left–right patterning not only of the heart, but other visceral organs, such as left–right lung and liver lobation. In human, typically there are three lung lobes on the right and two on the left. The stomach and spleen are usually situated on the body’s left side, while normal gut looping entails a developmentally regulated 270° rotation around the superior mesenteric artery. If all the visceral organs show mirror symmetric reversal of visceral organ situs, it is referred to as situs inversus totalis, and such individuals often go undetected clinically. However, if there is randomization of visceral organ situs, this can result in life threatening congenital defects of the heart and other organs. Thus, while alterations in lung or liver lobation are largely inconsequential, abnormal gut looping can cause duodenal obstruction or midgut volvulus that can be life threatening. The latter is observed with an incidence of one in 6,000 (Berseth, 1998). Another life-threatening visceral organ laterality defect is biliary atresia, also a relatively rare birth defect with an incidence of one in 10,000–15,000(Bates, Bucuvalas, Alonso, & Ryckman, 1998; Lupo et al., 2017). Biliary atresia causes bile to back into the liver, resulting in jaundice and ensuing liver cirrhosis and liver failure. Biliary atresia is often found in association with polysplenia, dextrocardia, and gut malrotation, indicating the disturbance of left–right patterning (Mathur, Gupta, Soni, Ahmed, & Goyal, 2014). With HTX, there may be not only abnormal positioning of the heart in the chest cavity, such as dextrocardia or mesocardia, but also more problematic complex CHD involving left–right atrioventricular discordance or ventricular-arterial discordance, such as seen with D or L-transposition of the great arteries.
3 |. PREVALENCE OF CHD ASSOCIATED WITH LATERALITY DEFECTS IN BIRTH DEFECTS COHORT
A retrospective study of cases with laterality defects from data curated in the National Birth Defects Prevention Study (NBDPS) yielded 517 nonsyndromic laterality defect cases, of which 378 had heterotaxy (HTX) and 130 had situs inversus totalis (SIT; Lin et al., 2014). Among the HTX cases, 90.2% had CHD, with 82.8% being complex CHD (Lin et al., 2014). Interestingly, among the SIT cases, only 41% had CHD and of these, 25% were simple CHD (Lin et al., 2014). Detailed examination of the phenotypes showed all types of CHD lesions are seen in conjunction with laterality defects, with the highest incidence in double outlet right ventricle (DORV), atrioventricular cushion defect (AVSD), total anomalous pulmonary venous return (TAPVR), and also single ventricle (SV) lesions (Table 1A; Lin et al., 2014). Overall, these observations confirm the well described association of CHD with HTX. They further show CHD can cooccur with SIT, although with much lower prevalence. Together these findings suggest the disturbance of left–right patterning may play a pivotal role in CHD pathogenesis and this is not restricted to specific CHD lesion types.
TABLE 1.
Prevalence of laterality defects among different types of congenital heart defectsa
| HLHS (%) |
SV (%) |
TA (%) |
PTA (%) |
PA (%) |
TGA (%) |
DORV (%) |
AVSD/ ECD (%) | TAPVR (%) |
TOF (%) |
EB (%) |
Total (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. CHD associated with heterotaxy and situs inversus in national birth defects prevention study by Lin et al. (2014) | ||||||||||||
| Situs inversus | 1.4 | 5.8 | 0.7 | 0 | 1.4 | 2.4 | 7.9 | 5.0 | 2.2 | 2.9 | 0.7 | 43.2 |
| Heterotaxy | 3.2 | 14.0 | 2.1 | 1.1 | 9.8 | 3.7 | 25.7 | 48.4 | 31.2 | 4.2 | 0.3 | 96.6 |
| All laterality | 2.7 | 11.8 | 1.7 | 0.8 | 4.9 | 3.1 | 20.9 | 36.8 | 23.4 | 3.9 | 0.4 | 82.2 |
| B. Situs-related anomalies associated with CHD in birth defects registry study focused on congenital malformations by Pradat et al.(2003) | ||||||||||||
| Situs inversus | 4.7 | 17.6 | 0 | 1.0 | 9.8 | 5.7 | 13.6 | 9.4 | 4.8 | 3.7 | 5.0 | 5.6 |
| Gut malrotation | 6.5 | 11.8 | 0 | 7.1 | 4.9 | 2.4 | 11.9 | 10.4 | 6.3 | 4.9 | 0 | 6.2 |
| Spleen abnormal | 7.7 | 23.5 | 4.1 | 6.1 | 4.9 | 4.9 | 8.5 | 11.3 | 12.7 | 5.8 | 0 | 7.5 |
HLHS, hypoplastic left heart syndrome; SV, single ventricle; TA, tricuspid atresia; PTA, persistent truncus arteriosus; PA, pulmonary atresia; TGA, transposition of the great arteries; DORV, double outlet right ventricle; AVSD/ECD, atrioventricular septal defect/endocardial cushion defect; TAPVR, total anomalous pulmonary venous return; TOF, tetralogy of Fallot; EB, Ebstein’s anomaly.
While this study on the NBDPS cohort focused on the incidence of CHD in cases with known laterality defects, it is interesting to note data on laterality defects in another study that examined the incidence of CHD phenotypes in three large registries of congenital malformations. This encompassed data from over 12,000 infants with CHD from 4.4 million live births (Pradat, Francannet, Harris, & Robert, 2003). The co-occurrence of other birth defects including laterality disturbance was examined, including situs inversus, gut malrotation, and spleen abnormalities (Pradat et al., 2003). As laterality defects are rare, left–right patterning defects co-occurring by chance among cases with critical CHD is expected to be even more rare. Interestingly, all three laterality defects were found in all the same critical CHD lesions seen in the NBDPS study (Table 1B). It should be noted the prevalence of the critical CHD lesions were similar to that reported in other studies, ranging from 0.4 to 2 per 10,000 (Julien IE Hoffman & Kaplan, 2002). Notable is the association of 23.5% of SV cases with spleen abnormalities (Lin et al., 2014; Pradat et al., 2003). The four CHD lesions (SV, DORV, AVSD, TAPVR) with the highest incidence of laterality defects in the NBDPS study also had the highest incidence of laterality defects in the latter CHD focused study (Table 1). Also notable was the high incidence of laterality defects among the HLHS and PA cases in the CHD study (Table 1B), with a striking finding that 10% of PA cases have situs inversus (Pradat et al., 2003). These findings suggest CHD cases with high cooccurrence of rare laterality phenotypes are unlikely to arise from random chance associations. Indeed, the latter study of CHD cases reported an odds ratio of 3.57 (95%CI 2.56–4.98) for the association of complex CHD with situs inversus, 3.52 (95% CI 2.62–4.73) for CHD with malformations of the spleen, and 1.86 (95% CI 1.20–2.89) for CHD with gut malrotation (Pradat et al., 2003). Together these findings confirm left–right patterning plays an important role in a wide spectrum of CHD. They further suggest the possibility that left–right patterning may be involved in CHD pathogenesis beyond its well described association with heterotaxy.
4 |. INTERROGATING THE GENETICS OF CHD PATHOGENESIS WITH MOUSE FORWARD GENETICS
Insights into the connection between heterotaxy, left–right patterning and CHD pathogenesis may come from better understanding of the genetic etiology of CHD. Supporting a genetic etiology for CHD is the well described association of CHD with various chromosomal abnormalities and copy number variants (Zaidi & Brueckner, 2017). Particularly notable are the known association of CHD with trisomy 21 in Down’s syndrome, 22q11 deletion in DiGeorge syndrome, and loss of the X-chromosome in Turner syndrome (Bondy, 2008; Freeman et al., 1998; Ryan et al., 1997). Further supporting a genetic etiology for CHD is the finding of a high recurrence risk for CHD in familial studies (Fesslova et al., 2011; Øyen et al., 2009). However, the confounding effects of genetic heterogeneity in the human population have complicated investigations into the genetics of CHD. Clinical studies have shown CHD is largely sporadic, and is associated with incomplete penetrance and variable expressivity (Blue et al., 2017). Together, these challenges have hampered progress in elucidating the genetic landscape of CHD.
These challenges can be largely overcome with genetic studies conducted in animal models such as inbred mice. Mice have the same four chamber cardiovascular anatomy that is the substrate for human CHD. Moreover, inbred mice essential for genetic analysis are readily available and the mouse genome is well characterized and nearly identical to that of the human genome (Rosenthal & Brown, 2007). To interrogate the genetic landscape of CHD, we conducted a large scale chemical mutagenesis screen with ethylnitrosourea to recover CHD causing mutations (Li et al., 2015). This phenotype based screen used high throughput noninvasive fetal echocardiography for cardiovascular phenotyping, an imaging modality highly sensitive for the detection and diagnosis of CHD (Liu et al., 2014). Follow up histological reconstructions were used to confirm the ultrasound CHD diagnosis. Mutation recovery was carried out using whole exome sequencing (WES) analysis with sequence alterations in the mutant identified by comparison to the reference genome.
Ultrasound cardiovascular phenotyping of 100,000 fetal mice yielded over 300 mutant lines with a wide spectrum of CHD that included outflow septation defects such as persistent truncus arteriosus (PTA), outflow tract malalignment defects such as double outlet right ventricle (DORV) and transposition of the great arteries (TGA), and also right sided lesions such as hypoplastic right heart syndrome (HRHS) or left sided lesions such as hypoplastic left heart syndrome (HLHS; Figure 1;Li et al., 2015; Liu et al., 2017). Unexpectedly, 30% of the CHD mutant lines recovered exhibited laterality defects and half of the pathogenic CHD causing mutations were in cilia related genes (Li et al., 2015). This included genes essential for motile or non-motile primary cilia structure/function (Li et al., 2015). In addition, also recovered were many mutations required for cilia transduced cell signaling, and genes regulating vesicular trafficking, a pathway important for ciliogenesis and cilia transduced cell signaling. Together these findings point to a central role for cilia in CHD pathogenesis (Li et al., 2015). Interestingly, both motile and nonmotile primary cilia have essential roles in left–right patterning. However, not all the laterality mutants harbored cilia mutations, and conversely not all cilia related CHD causing mutations were associated with laterality defects. Together these findings suggest the role of cilia in CHD pathogenesis extends beyond the regulation of left– right patterning.
FIGURE 1.
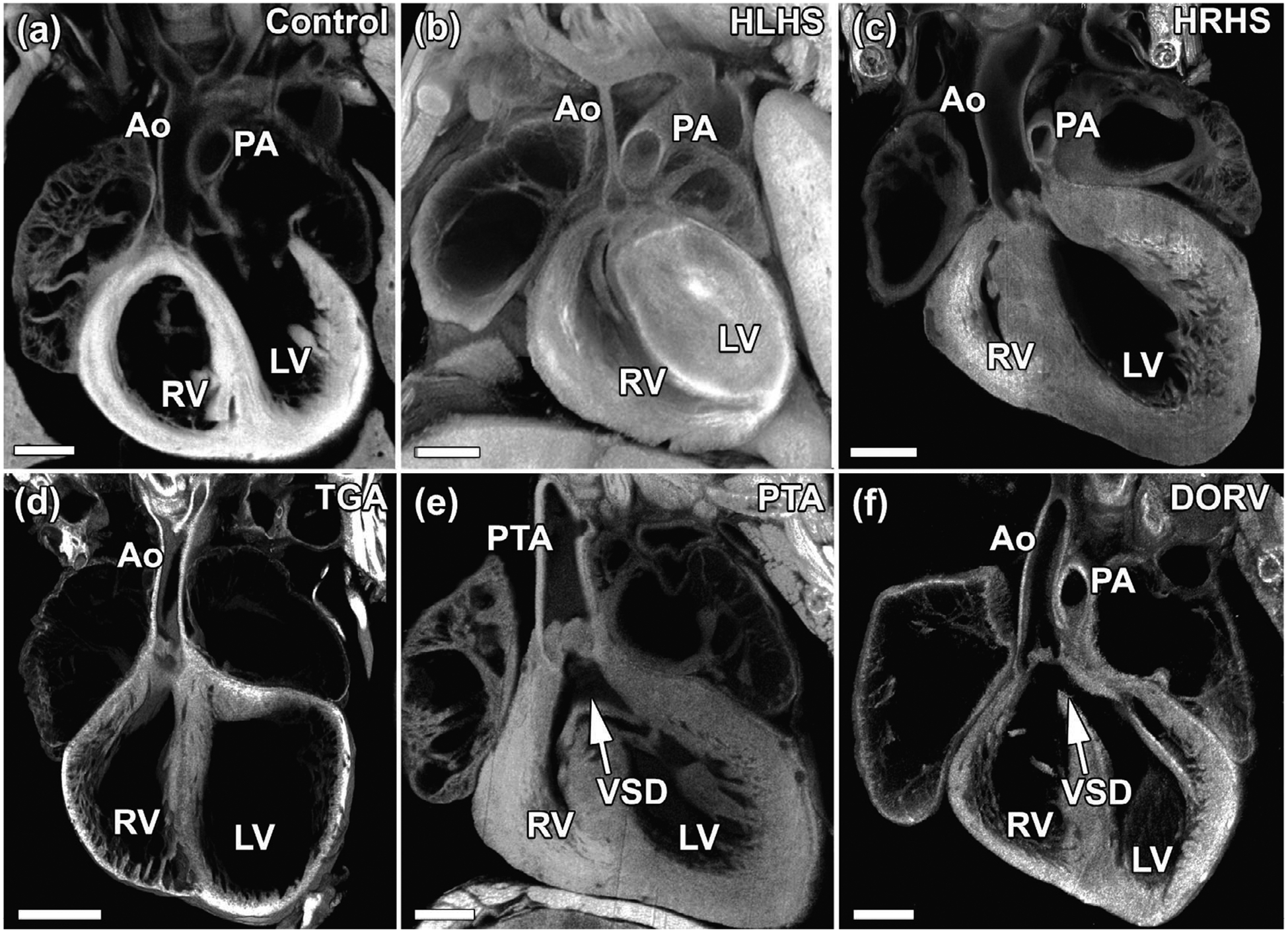
Examples of congenital heart disease phenotypes identified in newborn mice. (a) Compared to wildtype newborn mouse heart, mutants recovered in our forward-genetic ENU screen exhibit a spectrum of cardiac phenotypes including (b) hypoplastic left heart syndrome (HLHS) in which left sided heart structures including the left ventricle, aorta, and mitral valve are severely hypoplastic, (c) hypoplastic right heart syndrome (HRHS) in which right sided heart structures including the right ventricle, pulmonary artery, and tricuspid valve are hypoplastic, (d) transposition of the great arteries (TGA) in which the aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle, (e) persistent truncus arteriosis (PTA) in which a single outflow tract arises from the heart, and (f) double outlet right ventricle (DORV) in which both the aorta and the pulmonary artery arise from the right ventricle. Ao, aorta; PA, pulmonary artery; RV, right ventricle; LV, left ventricle; VSD, ventricular septal defect. Scale bar represents 0.5 mm
5 |. ROLE OF CILIA AND LATERALITY DISTURBANCE IN CHD PATHOGENESIS
Given the mouse mutagenesis screen was phenotype driven, the preponderance of cilia related genes was unexpected. The motile cilia genes recovered included many known to cause primary ciliary dyskinesia (PCD), a sinopulmonary disease due to mucociliary clearance deficits arising from dyskinetic or immotile cilia in the airway (Noone et al., 2004). Importantly, all the CHD mutant lines with pathogenic mutations in known PCD genes also exhibited laterality defects, a reflection of the requirement for motile cilia function in the embryonic node for left–right patterning (McGrath, Somlo, Makova, Tian, & Brueckner, 2003). Clinically PCD patients are also known to have laterality defects, such as Kartagener’s syndrome, a birth defect involving mirror symmetric reversal of visceral organ left–right asymmetry (Leigh et al., 2009). More recently, PCD patients also have been shown to be at risk for HTX, and for CHD associated with HTX (Kennedy et al., 2007; Shapiro et al., 2014). Importantly, in all the mouse lines harboring mutations in PCD related genes, a single mutation typically can yield three alternative phenotypic outcomes: SS, SIT, and HTX, with CHD mostly seen with HTX (Figure 2). Similar to findings in mice, PCD mutation can give rise to three possible laterality outcomes, with CHD predominantly seen with HTX.
FIGURE 2.
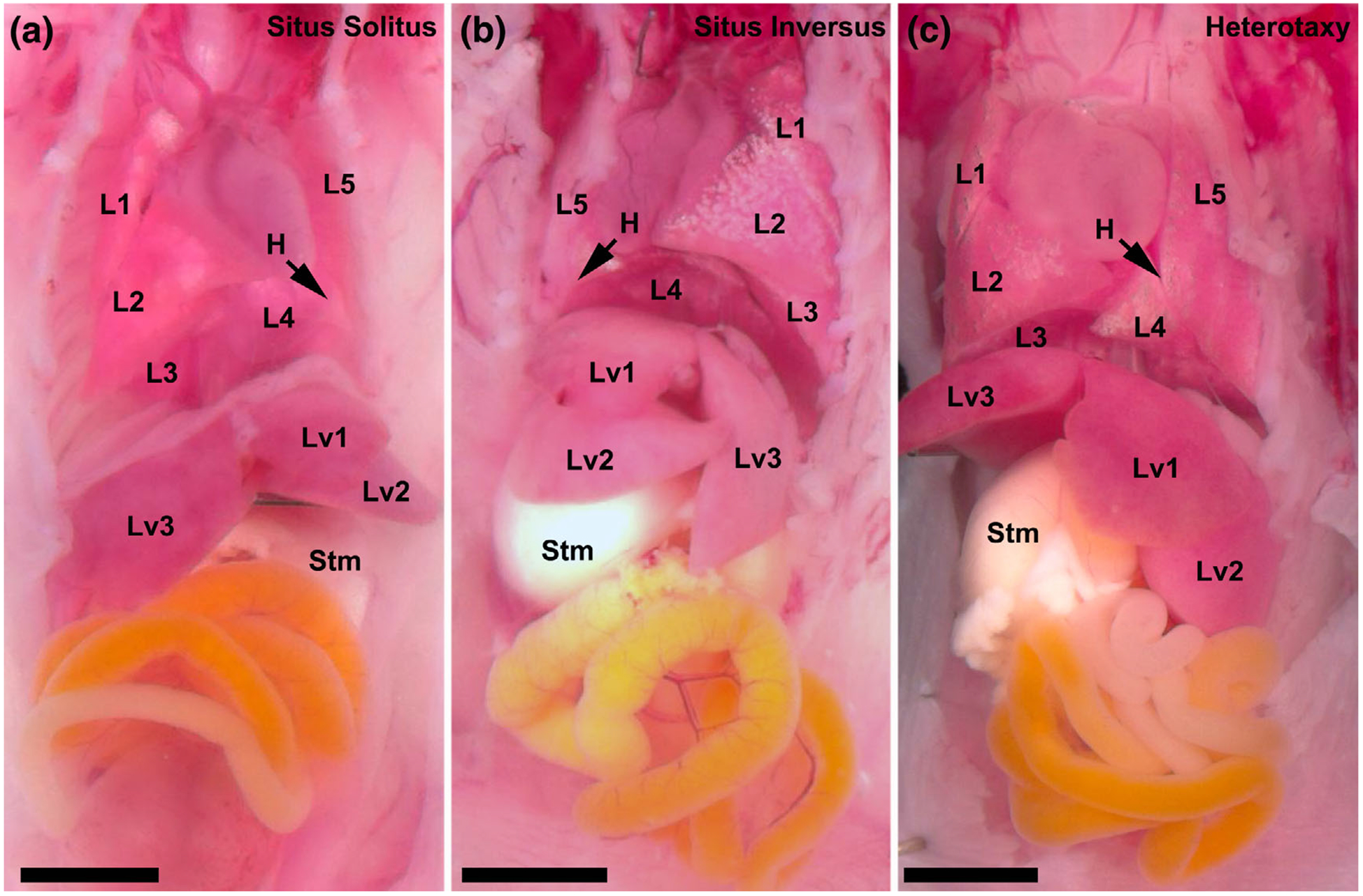
Three phenotypic outcomes associated with mutations causing left–right patterning defects. Mutations causing left–right patterning defects can result in three laterality phenotypes. (a) An example of situs solitus, or the normal arrangement of visceral organ situs, in which the heart apex points toward the left, the stomach is left sided, there are three lung lobes visible on the right with two lung lobes visible on the left, and there are two liver lobes visible on the left and one liver lobe visible on the right. (b) An example of situs inversus totalis in which there is a mirror reversal of visceral organ situs and the heart apex points toward the right, the stomach is right sided, there are three lung lobes visible on the left with two lung lobes visible on the right, and there are two liver lobes visible on the right and one liver lobe visible on the left. (c) An example of heterotaxy in which there is randomization of visceral organ situs and the heart apex points toward the left, the stomach is right sided, there are three lung lobes visible on the left with two lung lobes visible on the right, and there are two liver lobes visible on the left and one liver lobe visible on the right. H, heart; L1–5, lung lobation; Liv1–3, liver lobation; stm, stomach. Scale bar represent 2.5 mm
In the PCD mutant mouse lines, typically 40% of the homozygous mutants have heterotaxy, while the rest are distributed approximately equally between normal SS or SIT. CHD was largely only seen in mutants with HTX, and these are underrepresented at birth, a reflection of the prenatal lethality of many HTX mutants with complex CHD (Li et al., 2015; Tan et al., 2007). Mutant lines harboring mutations in PCD related genes were found to have respiratory motile cilia defects, both in the airway and in the embryonic node where motile cilia are required for orchestrating normal left–right patterning. It should be noted some of the cilia mutations causing CHD are primary cilia related and do not affect motile cilia function (Li et al., 2015). They also can give rise to three phenotypic outcomes as observed for the PCD causing mutations affecting motile cilia function. In addition, we also recovered mutations in non-cilia related genes causing CHD associated with heterotaxy, and most of these laterality mutants also yielded the same three laterality phenotypic outcomes (Li et al., 2015). However, we note in all these laterality mutant lines, CHD is typically only seen with heterotaxy. More rarely, CHD was observed with either SS or SIT, but usually these comprise relatively mild phenotypes such as VSDs. Conversely, heterotaxy mutants typically had complex CHD, and only very rarely had mild CHD. Surviving heterotaxy mice with no CHD phenotypes are almost never found.
The findings from our mouse forward genetic screen would suggest the perturbation of laterality has an important role to play in CHD pathogenesis. This accounted for some of the enrichment in cilia related mutations, although as noted above, most cilia mutations causing CHD did not cause laterality defects and conversely not all mutations causing CHD associated with laterality defects were cilia related. Overall, findings in the mouse screen are in agreement with the human birth cohort studies. For example, analysis of the NBDPS birth cohort showed higher prevalence of complex CHD associated with HTX as compared to cases with SIT (Table 1A; Lin et al., 2014). However, in the analysis focused on CHD cases, some complex CHD lesions were more highly associated with situs inversus as compared to gut malrotation or splenic anomalies that are indicative of heterotaxy (Table 1B; Pradat et al., 2003). It should be noted the designation was situs inversus, not situs inversus totalis, as reported in the NBDPS study. Hence, these cases may only involve situs reversal of a single visceral organ indicating HTX with overall visceral organ situs discordance. Another difference to note is the fact our mouse screen focused on monogenic causes of CHD, while clinically CHD is more likely to involve complex genetics with an oligogenic disease etiology (Gelb & Chung, 2014). These differences could account for some of the apparent discrepancies between our findings and the observations seen in the clinical setting.
6 |. CLINICAL IMPLICATIONS FOR PATIENTS WITH CHD ASSOCIATED WITH HETEROTAXY
Our screen being a prenatal fetal screen, can recover CHD causing mutations even if they caused prenatal lethality. However, clinical studies largely only entail the examination of liveborn infants and hence have inevitable selection bias due to the loss of mutations and phenotypes associated with prenatal lethality. Indeed, studies examining stillborn fetuses have shown a much higher prevalence of CHD and CHD associated with HTX then observed in the clinical population (Buca et al., 2018; Hoffman, 1995). One other point of importance to consider in clinical practice is the fact that many mutations causing CHD with heterotaxy can potentially yield three different situs phenotypic outcomes. This is observed not only for cilia related genes, but also non-cilia related genes. Hence, in genetic analysis, the inclusion of sequencing data from normal individuals as controls can be problematic, especially if they comprise unaffected siblings or relatives. Moreover, for genetic counseling, individuals with SIT without CHD, or unaffected situs solitus family members of HTX/CHD cases may also be carriers of genetic risk for CHD even if they do not exhibit either CHD or laterality defects.
It should be noted CHD patients with HTX have been shown to have a high prevalence of respiratory ciliary dysfunction and this is significantly associated with a higher risk for postsurgical respiratory complications (Harden et al., 2014; Nakhleh et al., 2012). Interestingly, further studies showed the respiratory ciliary dysfunction seen in CHD patients with HTX, is also seen with equal prevalence in CHD patients without HTX (Garrod et al., 2014). As with HTX patients, ciliary dysfunction in the non-HTX patients was also associated with more postsurgical respiratory complications (Stewart et al., 2018). Overall, these findings suggest there may be a high prevalence of cilia related mutations associated with CHD pathogenesis, and these may contribute to increased mucociliary clearance dysfunction driving postsurgical respiratory complications.
7 |. ROLE OF LEFT–RIGHT PATTERNING IN CONGENITAL HEART DISEASE
The high prevalence of laterality defects in patients with CHD would suggest the disturbance of left–right patterning may have a central role in the pathogenesis of CHD, and this may be driven by cilia related disturbances. Given the realization that many mutations causing CHD with HTX can also give rise to SS and SIT, is it possible that patients with isolated CHD without laterality defects might represent individuals with mutations disturbing laterality, but with the chance outcome of SS? Could this account for some of the CHD burden in individuals with isolated CHD without laterality defects? In our mouse screen, homozygous mutants with SS outcome usually did not exhibit CHD (Li et al., 2015). However, differing from the human population, our mice are entirely inbred and the screen was designed to recover only recessive mutations. In the human population, CHD is more likely mediated by complex genetics involving oligogenic interactions (Akhirome, Walton, Nogee, & Jay, 2017; Gifford et al., 2019). This could also explain the observation of complex CHD in patients with SIT, which is seldom seen in inbred mice.
It is interesting to note that among the different types of CHD lesions, there are some complex CHD that show distinct left vs. right asymmetry, and these often result in single ventricle physiology with only one functional pumping chamber comprising either the right or left ventricle. Notable among these is the highly lethal lesion, HLHS, yielding only a functional right ventricle. These and other left vs. right sided ventricular outflow obstructive lesions referred to collectively as LVOTO/ RVOTO are interesting to consider in the context of the possible essential involvement of left–right disturbance in the pathogenesis of CHD. Is it possible that these striking left or right sided lesions are a reflection of left–right patterning disturbance driving the pathogenesis of CHD? Precisely how the regulation of left–right patterning is integrated and translated by the developing cardiovascular system is still not well understood, although the nodal signaling cascade and downstream activation of Pitx2 is well described to play important roles in looping of the heart tube and the regulation of heart development (Bamforth et al., 2004; Franco, Sedmera, & Lozano-Velasco, 2017; Patel, Isaac, & Cooke, 1999). Elucidating how these left vs. right sided cardiac lesions arise will require a better understanding of the overall molecular mechanisms regulating left–right patterning. Insights into the developmental etiology of severe lesions such as HLHS may help elucidate the evolution of these distinct left vs. right sided cardiac lesions and whether these lesions may involve the primary disturbance of left–right patterning. Such advances in knowledge may help guide future development of new therapies that can help improve outcome for patients with complex CHD who continue to suffer from high morbidity and mortality.
ACKNOWLEDGEMENTS
This work was supported by NIH grant 1F30HD097967 (GCG), NIH grants HL142788 and HL132024 (CWL), and DOD grants W81XWH-15-1-0649 and W81XWH-16-1-0613 (CWL).
Funding information
National Institutes of Health, Grant/Award Numbers: 1F30HD097967, HL132024, HL142788; U.S. Department of Defense, Grant/Award Numbers: W81XWH-15-1-0649, W81XWH-16-1-0613
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Akhirome E, Walton NA, Nogee JM, & Jay PY (2017). The complex genetic basis of congenital heart defects. Circulation Journal, 81(5), 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth SD, Bragança J, Farthing CR, Schneider JE, Broadbent C, Michell AC, … Brown NA (2004). Cited2 controls left-right patterning and heart development through a nodal-Pitx2c pathway. Nature Genetics, 36(11), 1189–1196. [DOI] [PubMed] [Google Scholar]
- Bates MD, Bucuvalas JC, Alonso MH, & Ryckman FC (1998). Biliary atresia: Pathogenesis and treatment. Seminars in Liver Disease, 18 (3), 281–293. [DOI] [PubMed] [Google Scholar]
- Bedard T, Lowry RB, Sibbald B, Harder JR, Trevenen C, Horobec V, & Dyck JD (2012). Congenital heart defect case ascertainment by the Alberta congenital anomalies surveillance system. Birth Defects Research. Part A, Clinical and Molecular Teratology, 94(6), 449–458. 10.1002/bdra.23007 [DOI] [PubMed] [Google Scholar]
- Berseth C (1998). Disorders of stomach: Gastric perforation In Taeush WH & Ballard RA(Eds.), Avery’s diseases of the newborn. Philadelphia, PA: WB Saunders Co. [Google Scholar]
- Blue GM, Kirk EP, Giannoulatou E, Sholler GF, Dunwoodie SL, Harvey RP, & Winlaw DS (2017). Advances in the genetics of congenital heart disease: A clinician’s guide. Journal of the American College of Cardiology, 69(7), 859–870. [DOI] [PubMed] [Google Scholar]
- Bondy CA (2008). Congenital cardiovascular disease in Turner syndrome. Congenital Heart Disease, 3(1), 2–15. [DOI] [PubMed] [Google Scholar]
- Buca DIP, Khalil A, Rizzo G, Familiari A, Di Giovanni S, Liberati M, … Scambia G (2018). Outcome of prenatally diagnosed fetal heterotaxy: Systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology, 51(3), 323–330. [DOI] [PubMed] [Google Scholar]
- Fesslova V, Brankovic J, Lalatta F, Villa L, Meli V, Piazza L, & Ricci C (2011). Recurrence of congenital heart disease in cases with familial risk screened prenatally by echocardiography. Journal of Pregnancy, 2011, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco D, Sedmera D, & Lozano-Velasco E (2017). Multiple roles of Pitx2 in cardiac development and disease. Journal of Cardiovascular Development and Disease, 4(4), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, … Saker DM (1998). Population-based study of congenital heart defects in down syndrome. American Journal of Medical Genetics, 80(3), 213–217. [PubMed] [Google Scholar]
- Garrod AS, Zahid M, Tian X, Francis RJ, Khalifa O, Devine W, … Lo CW (2014). Airway ciliary dysfunction and sinopulmonary symptoms in patients with congenital heart disease. Annals of the American Thoracic Society, 11(9), 1426–1432. 10.1513/AnnalsATS.201405-222OC [DOI] [PubMed] [Google Scholar]
- Gelb BD, & Chung WK (2014). Complex genetics and the etiology of human congenital heart disease. Cold Spring Harbor Perspectives in Medicine, 4(7), a013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford CA, Ranade SS, Samarakoon R, Salunga HT, de Soysa TY, Huang Y, … Bui YK (2019). Oligogenic inheritance of a human heart disease involving a genetic modifier. Science, 364(6443), 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle-Colarusso T, Nembhard WN, … Marelli AJ (2016). Congenital heart defects in the United States: Estimating the magnitude of the affected population in 2010. Circulation, 134(2), 101–109. 10.1161/CIRCULATIONAHA.115.019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden B, Tian X, Giese R, Nakhleh N, Kureshi S, Francis R, … Leatherbury L (2014). Increased postoperative respiratory complications in heterotaxy congenital heart disease patients with respiratory ciliary dysfunction. The Journal of Thoracic and Cardiovascular Surgery, 147(4), 1291–1298. 10.1016/j.jtcvs.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Hoffman J (1995). Incidence of congenital heart disease: II. Prenatal incidence. Pediatric Cardiology, 16(4), 155–165. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, & Kaplan S (2002). The incidence of congenital heart disease. Journal of the American College of Cardiology, 39(12), 1890–1900. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S, & Liberthson RR (2004). Prevalence of congenital heart disease. American Heart Journal, 147(3), 425–439. 10.1016/j.ahj.2003.05.003 [DOI] [PubMed] [Google Scholar]
- Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, … Knowles MR (2007). Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation, 115(22), 2814–2821. 10.1161/CIRCULATIONAHA.106.649038 [DOI] [PubMed] [Google Scholar]
- Khoshnood B, Lelong N, Houyel L, Thieulin AC, Jouannic JM, Magnier S, … Group ES (2012). Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: A population-based study. Heart, 98(22), 1667–1673. 10.1136/heartjnl-2012-302543 [DOI] [PubMed] [Google Scholar]
- Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, … Zariwala MA (2009). Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genetics in Medicine, 11(7), 473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Klena NT, Gabriel GC, Liu X, Kim AJ, Lemke K, … Lo CW (2015). Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature, 521(7553), 520–524. 10.1038/nature14269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AE, Krikov S, Riehle-Colarusso T, Frias JL, Belmont J, Anderka M, … National Birth Defects Prevention, S. (2014). Laterality defects in the national birth defects prevention study (1998–2007): Birth prevalence and descriptive epidemiology. American Journal of Medical Genetics. Part A, 164A(10), 2581–2591. 10.1002/ajmg.a.36695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AE, Ticho BS, Houde K, Westgate MN, & Holmes LB (2000). Heterotaxy: Associated conditions and hospital-based prevalence in newborns. Genetics in Medicine, 2(3), 157–172. 10.1097/00125817-200005000-00002 [DOI] [PubMed] [Google Scholar]
- Liu X, Francis R, Kim AJ, Ramirez R, Chen G, Subramanian R, … Lo CW (2014). Interrogating congenital heart defects with noninvasive fetal echocardiography in a mouse forward genetic screen. Circulation. Cardiovascular Imaging, 7(1), 31–42. 10.1161/CIRCIMAGING.113.000451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yagi H, Saeed S, Bais AS, Gabriel GC, Chen Z, … Lo CW (2017). The complex genetics of hypoplastic left heart syndrome. Nature Genetics, 49(7), 1152–1159. 10.1038/ng.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen S, Zuhlke L, Black GC, Choy MK, Li N, & Keavney BD (2019). Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. International Journal of Epidemiology, 48, 455–463. 10.1093/ije/dyz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo PJ, Isenburg JL, Salemi JL, Mai CT, Liberman RF, Canfield MA, … The National Birth Defects Prevention, N. (2017). Population-based birth defects data in the United States, 2010–2014: A focus on gastrointestinal defects. Birth Defects Research, 109(18), 1504–1514. 10.1002/bdr2.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli A, Miller SP, Marino BS, Jefferson AL, & Newburger JW (2016). Brain in congenital heart disease across the lifespan: The cumulative burden of injury. Circulation, 133(20), 1951–1962. 10.1161/CIRCULATIONAHA.115.019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Gupta R, Soni V, Ahmed R, & Goyal RB (2014). Biliary atresia associated with polysplenia syndrome, dextrocardia, situs inversus totalis and malrotation of intestines. Journal of Neonatal Surgery, 3(1), 9. [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, & Brueckner M (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell, 114(1), 61–73. [DOI] [PubMed] [Google Scholar]
- Nakhleh N, Francis R, Giese RA, Tian X, Li Y, Zariwala MA, … Lo CW (2012). High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation, 125(18), 2232–2242. 10.1161/CIRCULATIONAHA.111.079780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, … Knowles MR (2004). Primary ciliary dyskinesia: Diagnostic and phenotypic features. American Journal of Respiratory and Critical Care Medicine, 169(4), 459–467. [DOI] [PubMed] [Google Scholar]
- Øyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen P, & Melbye M (2009). Recurrence of congenital heart defects in families. Circulation, 120(4), 295–301. [DOI] [PubMed] [Google Scholar]
- Patel K, Isaac A, & Cooke J (1999). Nodal signalling and the roles of the transcription factors SnR and Pitx2 in vertebrate left–right asymmetry. Current Biology, 9(11), 609–S601. [DOI] [PubMed] [Google Scholar]
- Pradat P, Francannet C, Harris J, & Robert E (2003). The epidemiology of cardiovascular defects, part I: A study based on data from three large registries of congenital malformations. Pediatric Cardiology, 24(3), 195–221. [DOI] [PubMed] [Google Scholar]
- Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, & Correa A (2008). Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. The Journal of Pediatrics, 153(6), 807–813. 10.1016/j.jpeds.2008.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N, & Brown S (2007). The mouse ascending: Perspectives for human-disease models. Nature Cell Biology, 9(9), 993–999. 10.1038/ncb437 [DOI] [PubMed] [Google Scholar]
- Ryan A, Goodship J, Wilson D, Philip N, Levy A, Seidel H, … Prieur M (1997). Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A European collaborative study. Journal of Medical Genetics, 34(10), 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, Potiny P, Rigdon J, Morello M, Tcheandjieu C, Romfh A, … Priest JR (2019). Substantial cardiovascular morbidity in adults with lower-complexity congenital heart disease. Circulation, 139(16), 1889–1899. 10.1161/CIRCULATIONAHA.118.037064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AJ, Davis SD, Ferkol T, Dell SD, Rosenfeld M, Olivier KN, … Wolf W (2014). Laterality defects other than situs inversus totalis in primary ciliary dyskinesia: Insights into situs ambiguus and heterotaxy. Chest, 146(5), 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart E, Adams PS, Tian X, Khalifa O, Wearden P, Zahid M, & Lo CW (2018). Airway ciliary dysfunction: Association with adverse postoperative outcomes in nonheterotaxy congenital heart disease patients. The Journal of Thoracic and Cardiovascular Surgery, 155(2), 755–763 e757. 10.1016/j.jtcvs.2017.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SY, Rosenthal J, Zhao XQ, Francis RJ, Chatterjee B, Sabol SL, … Lo CW (2007). Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. The Journal of Clinical Investigation, 117(12), 3742–3752. 10.1172/JCI33284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, & Roos-Hesselink JW (2011). Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. Journal of American College of Cardiology, 58(21), 2241–2247. 10.1016/j.jacc.2011.08.025 [DOI] [PubMed] [Google Scholar]
- Zaidi S, & Brueckner M (2017). Genetics and genomics of congenital heart disease. Circulation Research, 120(6), 923–940. 10.1161/CIRCRESAHA.116.309140 [DOI] [PMC free article] [PubMed] [Google Scholar]


