Abstract
Background:
Although people who inject drugs (PWID) having the highest incidence and prevalence of hepatitis C virus (HCV) in the US, HCV treatment is rarely provided to PWID due to assumptions about poor adherence and reinfection risk. As direct-acting antiviral agents (DAAs) have achieved sustained virologic response (SVR) rates of 95% or more, evidence-based strategies are urgently needed to demonstrate real-world effectiveness in marginalized patient populations such as PWID. The objectives of this study are: 1) to determine whether either of two patient-centered treatment models - patient navigation (PN) or modified directly observed therapy (mDOT) - results in more forward movement along the HCV care cascade including treatment initiation, adherence, and SVR; 2) using quantitative and qualitative methods, to understand factors associated with lack of treatment uptake, poor adherence (< 80%), failure to achieve SVR, DAA resistance, and HCV reinfection.
Methods:
The HERO study is a multi-site, pragmatic randomized clinical trial conducted in eight states where 754 HCV-infected PWID were randomly assigned to either PN or mDOT.
Conclusions:
This study addresses an urgent need for timely and accurate information on optimal models of care to promote HCV treatment initiation, adherence, treatment completion and SVR among PWID, as well as rates and factors associated with reinfection and resistance after treatment. This clinical trial has the potential to provide valuable information on how to reduce the burden of the HCV epidemic in PWID.
Keywords: Injection drug use, Hepatitis C, Antiviral therapy, Sustained virologic response
1. Introduction
Chronic hepatitis C virus (HCV) infection is one of the leading causes of morbidity and m ortality in the US [1]. The prevalence of HCV infection is a growing burden that rose by 133% from 2004 to 2014 [2], mainly due to the growing epidemic of injection drug use (IDU) and the opioid crisis. The highest prevalence of HCV infection is found among people who inject drugs (PWID), which has been estimated to be up to 77% [3]. Facilitating access to curative HCV treatment in PWID is an urgent public health priority [4]. Guidelines from the American Association for the Study of Liver Diseases (AASLD), Infectious Diseases Society of America (IDSA), European Association for the Study of the Liver (EASL), International Network of Hepatitis in Substance Users (INHSU), and the World Health Organization (WHO), recommend treatment for HCV infection among PWID [5–8]. It is estimated that treatment scale-up among PWID could reduce HCV prevalence by up to 75% within 15 years [9]. Unfortunately, HCV treatment uptake is extremely low among PWID (1–2%) [10].
Multiple interrelated barriers exist to direct-acting antiviral agent (DAA) treatment among PWID (Fig. 1). At the patient level, these may include general barriers, such as difficulties in accessing health care (e.g., lack of care provider), competing comorbidities (e.g., mental health), and stability factors (e.g., unemployment, homelessness), and HCV-specific barriers, such as limited knowledge of treatment and lack of symptoms [11–15]. At the provider level, barriers relate to provider concerns of ongoing substance use leading to poor adherence and reinfection [15–17]. Finally, system-level barriers can be related to the health care system, such as the price of the medications, insurance restrictions on prescribing DAAs especially to active drug users, and insufficient locations for HCV testing and treatment [17,18], and to workforce issues, such as an inadequate supply of HCV treatment providers [19].
Fig. 1.
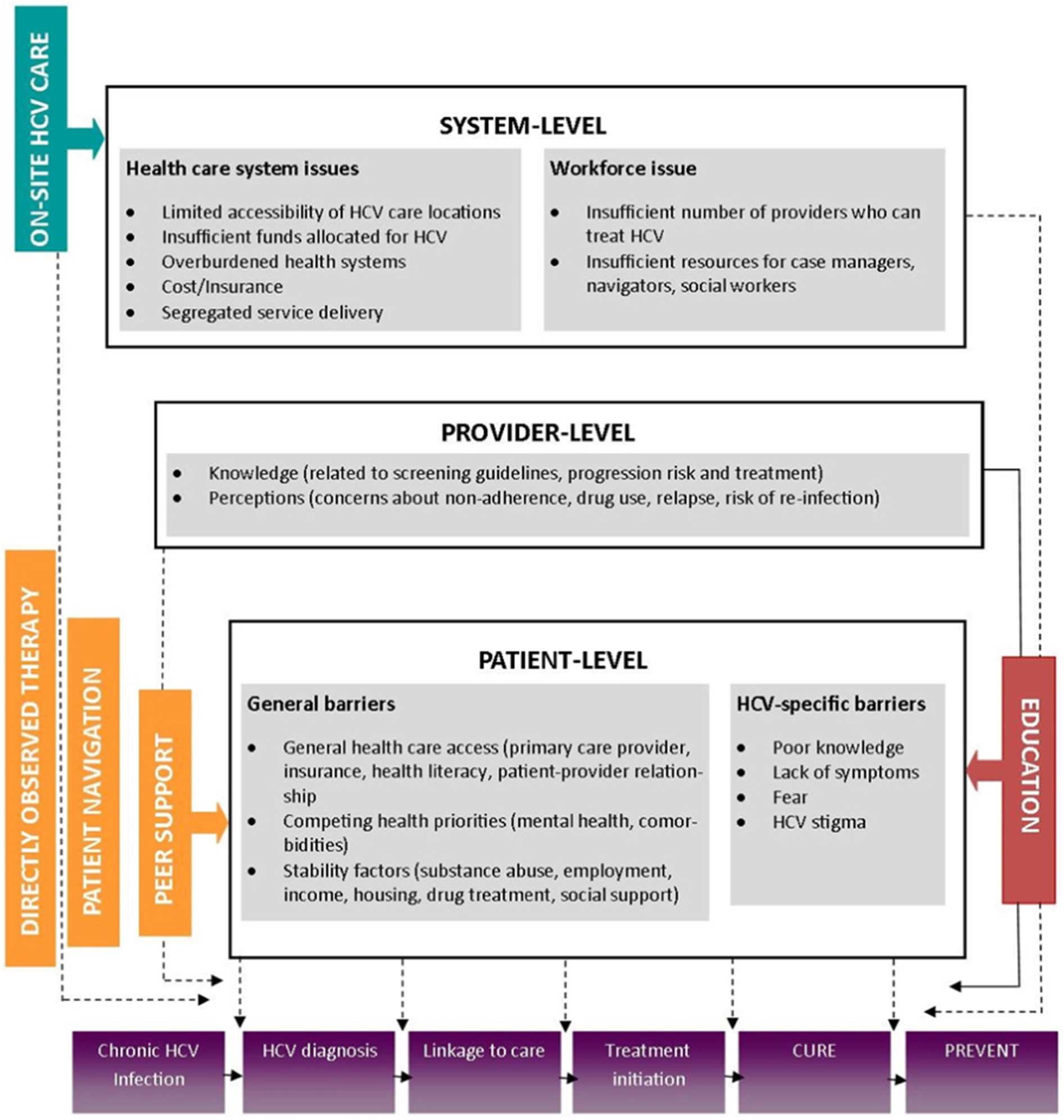
Barriers to treatment.
As DAAs have achieved sustained virologic response (SVR) rates of ≥ 95%, evidence-based strategies are urgently needed to demonstrate real-world effectiveness among PWID. Multidisciplinary approaches that combine antiviral and addiction treatment have shown comparable rates of treatment uptake and SVR among PWID compared to non-PWID populations [20–26]. Similarly, interventions based in primary care settings have demonstrated that patients with ongoing drug use and psychiatric comorbidity can be effectively linked to HCV care [12,27–31]. Peer-driven interventions including weekly support groups and patient navigation have also demonstrated high treatment uptake and SVR rates among PWID [32–34]. A modified directly-observed therapy (DOT) approaches have successfully supported HCV treatment in opioid agonist treatment programs [21,23,35], prison settings [36], and community health centers [32–33].
This report describes the HERO Study: Hepatitis C Real Options, a randomized pragmatic trial aimed at determining which model provides better HCV outcomes among PWID while also exploring which model is preferred by PWID. The study objectives are: 1) determining which of two patient-centered treatment models (patient navigation (PN) or modified directly observed therapy (mDOT)) results in greater treatment initiation, adherence and SVR; and 2) determining the incidence and factors associated with lack of treatment initiation, poor adherence, failure to achieve SVR, drug resistance, and HCV reinfection, using both quantitative and qualitative methods.
2. Methods
2.1. Study design
The HERO Study is a multi-site pragmatic clinical trial conducted in eight states, where 754 HCV-infected PWID who injected any illicit substances within 90 days of screening were randomized to either PN or mDOT. Quantitative data were collected from 1) participant interviews; 2) blood specimens for HCV viral load and resistance assays; 3) urine toxicology tests at research visits; 4) electronic pill packages for assessing adherence; and 5) clinic records for methadone or buprenorphine dose, clinical tests and medical history. Qualitative data on study implementation and outcomes were collected from patient, research team and staff interviews.
2.2. Study objectives
The primary objective is to determine whether SVR, defined as the absence of HCV ribonucleic acid (RNA) in the blood 12 weeks following the completion of treatment, is higher among PWID randomized to PN or those randomized to mDOT. The secondary objectives are to examine the following outcomes overall and by arm: 1) treatment initiation within 12 weeks of enrollment; 2) longitudinal biweekly adherence rates of prescribed medication measured using electronic Med-ic® blister packs; 3) treatment completion defined as finishing 100% of the prescribed 12-week course regardless of adherence to the medication; 4) the proportion who develop drug resistance; and 5) reinfection, defined as the rate (incidence) and the proportion who become HCV-infected 3 years after treatment completion.
2.3. Study setting
The study is being conducted in sites across eight states: New York City (NY), Providence (RI), Albuquerque (NM), San Francisco (CA), Boston (MA), Baltimore (MD), Seattle (WA), and Morgantown (WV), in eight opioid treatment programs (OTPs) that provide opioid agonist treatment (including methadone and buprenorphine) and 15 community health centers (CHCs). It is estimated that these OTPs provide care for 5000 HCV-infected patients and the CHCs provide care for 10,000 HCV-infected patients. The study is directed from Clemson University (SC).
2.4. Eligibility criteria
Eligibility criteria include: 1) age 18–70 years; 2) HCV viremia; 3) aspartate aminotransferase CAST), alanine aminotransferase CAL T), and platelets measured within the past 12 months; 4) self-report of actively injecting any substance within 90 days of screening; 5) not previously treated with HCV DAAs; 6) willing to receive treatment with sofosbuvir/velpatasvir; 7) willing to be randomized to PN or mDOT; 8) if receiving methadone treatment for opioid use disorder, then attending or willing to attend that program a minimum of 5 times per week if randomized to mDOT; 9) able to provide written informed consent; and 1 0) fluent in English or Spanish. Participants are excluded if 1) they are pregnant or breastfeeding, or 2) have been previously diagnosed with hepatocellular carcinoma. Co-infection with Human Immunodeficiency Virus (HIV) or hepatitis B is permitted, as is a diagnosis of compensated cirrhosis.
2.5. Approvals, confidentiality, and data safety and monitoring
The trial protocol was reviewed and approved by the Institutional Review Boards (IRB) of the 10 participating institutions: Clemson University / Prism a Health, Harvard School of Medicine/Massachusetts General Hospital, Albert Einstein College of Medicine/Montefiore Medical Center, Johns Hopkins University, University of Rhode Island, Partners Health Care, West Virginia University, University of California at San Francisco, University of New Mexico Health Sciences Center, and University of Washington. Written informed consent is obtained from all participants. A certificate of confidentiality was obtained by each site from the National Institutes of Health (NIH) to protect participants from being identified in any social, civil, criminal, or legislative dealings at the local, state, or federal level.
The principal investigator (PI), A.H.L., is responsible for monitoring the safety and efficacy of this study, executing the Data and Safety Monitoring (DSM) plan, and complying with all reporting requirements. The PI provides a summary of the DSM report to Patient-Centered Outcomes Research Institute (PCORI) on an annual basis as part of the progress report. Because the intervention is low risk and is integrated within usual clinical care, the main element of the monitoring plan is continuous, close monitoring by study staff and investigators, with prompt identification and reporting of serious adverse events (SAEs).
Each site maintains appropriate medical and research records in compliance with the International Council for Harmonization (ICH) of Technical Requirements for Pharmaceutical for Human Use E6, Section 4.9, and regulatory and institutional requirements for the protection of subject confidentiality. Forms for use as source documents are derived from the electronic case report forms (eCRFs) provided by the University of New Mexico Health Sciences Center Statistical and Data Coordinating Center (SDCC). Source documents are only accessible by study staff and all research records are kept in a locked file cabinet in a locked research office at each site.
2. 6. Stakeholder engagement
Each of the eight enrolling research sites formed a Local Stakeholder Advisory Board (LSAB) which consists of a site PI, site Project Director (PD), patients, local advocacy organizations, and local representatives from participating venues such as OTPs and CHCs. During the course of the study, the LSAB meets on a quarterly basis to discuss study implementation, recruitment, participant tracking, intervention delivery, outcome assessment, and dissemination.
At a national level, a National Stakeholder Advisory Board (NSAB) was created. The NSAB consists of the site Pis, a local representative of each site, and representatives from governmental organizations; professional policy, educational, and advocacy organizations; professional patient organizations; and industry. The NSAB meets on a quarterly basis through the duration of the study to review outcomes related to the study implementation, progress, and dissemination.
2. 7. Recruitment; enrollment; and reimbursement
Potential participants are identified through chart reviews of existing patients at the clinics, community-based outreach, and medical provider referrals. Participants are screened to see if they meet basic eligibility criteria and are asked if they are currently seeing a healthcare provider. If they have a provider, a release of information to view clinical records is obtained, data to assess eligibility are extracted, and study eligibility is documented on the electronic case report forms (eCRF). If they do not have a provider, they are referred to one at the CHC or OTP (if also interested in opioid agonist treatment) prior to obtaining a release of information for clinical records. Following chart review to assess study eligibility, a research staff member meets with the potential participant to confirm eligibility, discuss study procedures including randomization to the mDOT or PN interventions, risks and benefits of participation, and to obtain written informed consent. Eligible participants are enrolled within 1 month of screening. If enrollment did not occur within 1 month, eligibility is reassessed.
Participants are reimbursed $20 for the time and effort to attend each of 17 research visits and an additional $5 for returning weekly electronic blister packs for a total of $400 per participant over the course of the study. Participants are followed for 168 weeks. Enrollment commenced in September 2016, and treatment initiation began in October 2016. Enrollment concluded in August 2018, and the trial is expected to be completed in 2022.
2.8. Randomization
Seven hundred and fifty-four patients were enrolled, with patients randomized to PN or mDOT in a 1:1 ratio in variable block sizes of 2–6 via central, computer-generated randomization provided by the SDCC (Fig. 2). Randomization occurred in blocks to ensure comparison groups of approximately equal size. As the interventions are not blinded, block size will vary to prevent anticipation of treatment arm assignment.
Fig. 2.
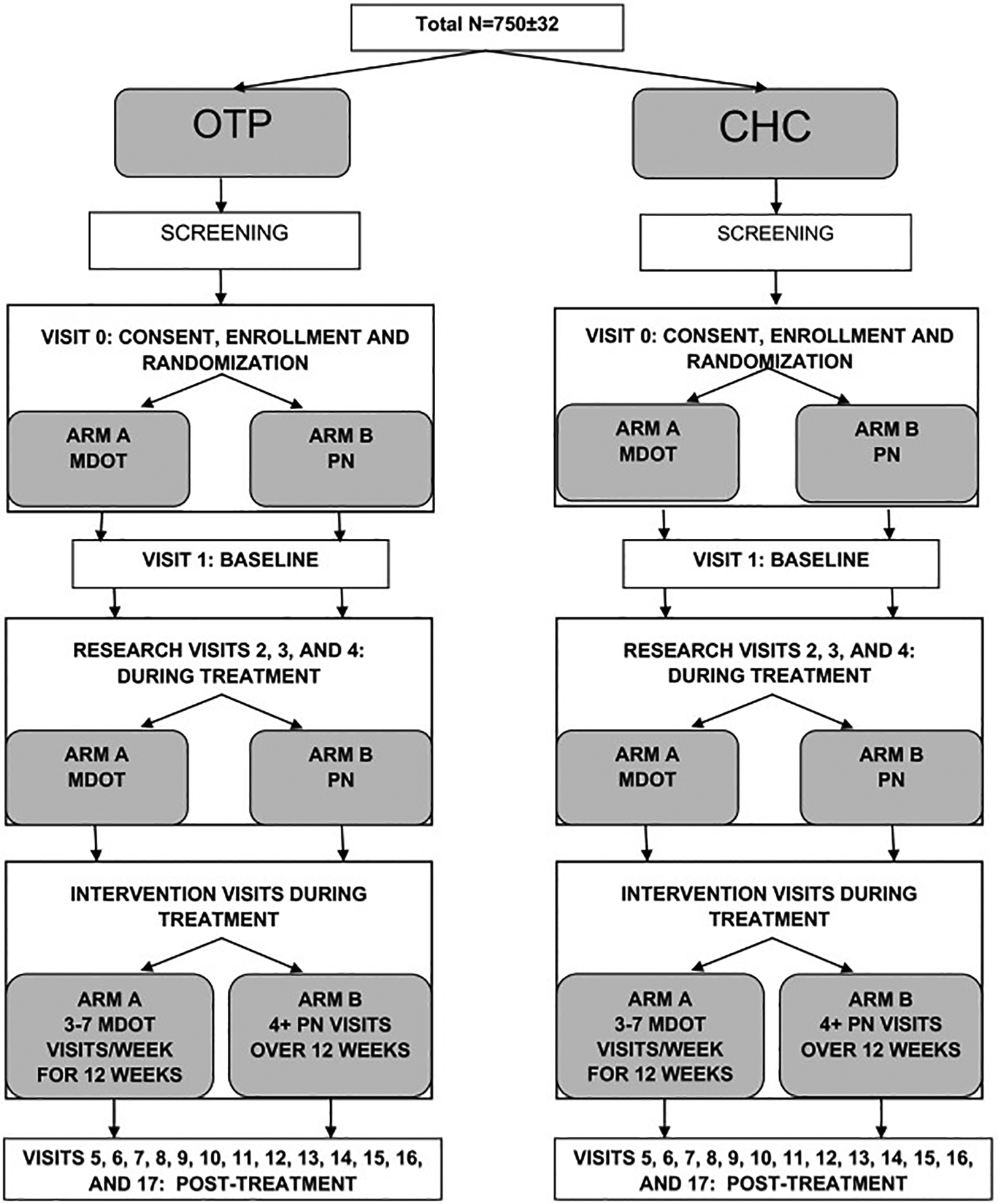
Schematic of the study design.
Note: OTP: opioid treatment program; CHC: community health center; MDOT: modified directly-observed therapy; PN: patient navigation.
Two randomization strategies (stratification and blocking) are used to avert imbalances in prognostic factors or treatment settings, and to ensure comparison groups of approximately equal size. Participants are stratified by 3 factors: city, OTP vs. CHC, and stage of liver disease (cirrhosis vs. no cirrhosis).
To ensure a balance at the end of the trial in the primary analytic sample sizes of participants who initiated the HCV treatment between the PN and mDOT arms, an adaptive re-weighting of random allocation in a pseudo urn randomization fashion [37] will be applied. As we hypothesize that the number of randomized subjects who will initiate HCV treatment will be greater in the PN arm, adjustment of random allocation ratio for a potential imbalance between the arms will be made based on the observed allocation rates at the middle of the trial, i.e., when 300 participants had initiated treatment. If the observed difference is > 5% (i.e. < 47.5% vs. 52.5%) between the two arms, we will accordingly re-weight the 1:1 random allocation so that final sample sizes will be balanced. Of note, a randomized subject will not necessarily be a clinical treatment subject but will be included in the comparison of treatment initiation.
2. 9. Baseline visit
The baseline visit occurs within 2 weeks of enrollment and prior to treatment initiation. Several different factors, including HCV clinical factors and psychosocial factors hypothesized to predict treatment initiation, adherence and treatment completion, are assessed using the following instruments: Modified Addiction Severity Index (ASI) - Baseline (drug use) [38] [38], Behavior Risk Assessment (injection behaviors) [39], Alcohol Use Disorders Identification Test (AUDIT-C, alcohol use) [38] [40], Substance Use Treatment (past and present experience with treatment), Patient Health Questionnaire (PHQ-9, depression) [39] [41], Generalized Anxiety Disorder Assessment (GAD-7, anxiety) [40] [42], EuroQOL five dimensions questionnaire (EQ-5D, quality of life) [41] [43], Stigma scale [44], Shame scale [45], Health efficacy, and Medical Outcomes Social Support Scale [46] (Table 1). Survey results are captured in REDCap, a secure, HIPAA-compliant web application for building and managing online surveys and databases. Participants provide a urine sample and blood is drawn. Counseling on avoidance of HCV transmission and HIV infection is provided, as well as information on local drug treatment, syringe services programs, and other referrals as needed. After all baseline instruments have been administered, research staff reveal the treatment delivery arm to which the participant has been randomized.
Table 1.
Core assessment measures.
| Research visit activities | Time (minutes) | Pre-treatment | Treatment visits | Post-treatment visits | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-screening | Enrollment | Baseline | Week 4, ± 1 wk | Week 8, ± 1 wk | Week 12, ± 1 wk | Week 24, ± 2wks | Week 36, ± 2 wks | Week 48, ± 2 wks | ||
| Visit number | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| TIME (in minutes) | 40 | 70 | 37 | 22 | 90 | 70 | 20 | 65 | ||
| Release of information | X | X | ||||||||
| Informed consent | 30 | X | ||||||||
| Randomization | X | |||||||||
| Instruments | ||||||||||
| Socio-demographic survey | 10 | X | ||||||||
| Short socio-demographic survey | 5 | X | ||||||||
| Behavioral risk assessment | 10 | X | X | X | X | X | X | |||
| Depression (PHQ-9) | 5 | X | X | X | X | |||||
| Anxiety (GAD-7) | 4 | X | X | X | X | |||||
| Alcohol use (AUDIT-C) | 3 | X | X | X | X | |||||
| Modified ASI - baseline | 10 | X | ||||||||
| Modified ASI - follow-up | 5 | X | X | X | X | |||||
| Substance use treatment | 3 | X | X | X | X | X | X | |||
| Quality of life (EQ-5D) | 8 | X | X | X | X | |||||
| Adherence (visual analogue scale) | 2 | X | X | X | ||||||
| Medical outcomes social support scale | 5 | X | X | X | ||||||
| Brief revised working alliance (PN arm only) | 5 | X | ||||||||
| Stigma scale | 5 | X | ||||||||
| Shame scale | 2 | X | X | |||||||
| Health efficacy scale | 3 | X | X | |||||||
| Satisfaction scale | 1 | X | ||||||||
| Peer support | 1 | X | X | X | X | X | ||||
| Labs | ||||||||||
| HCV antibody | MC | MC | ||||||||
| HCV viremia | MC | MC | MC | MC | MC | MC | X | X | ||
| HCV genotype/subtype | MC | MC | ||||||||
| HIV test | MC | |||||||||
| HIV viral load* | MC | MC | MC | MC | ||||||
| HIV CD4* | MC | MC | MC | MC | ||||||
| AST/ALT/Platelets/FIB-4 calculation | MC | MC | MC | MC | MC | MC | ||||
| Total bilirubin | MC | |||||||||
| Albumin | MC | |||||||||
| Prothrombin time | MC | |||||||||
| FibroSure/Fibroscan/Liver biopsy | MC | MC** | MC** | |||||||
| Imaging study** | MC | MC | ||||||||
| Child-Pugh score (exclude B,C) | MC | MC | MC | MC | ||||||
| Pregnancy test* | MC | |||||||||
| Archiving sample | X | X | X | X | ||||||
| HCV GenoSure NS5A/NS5B Assay* | X | |||||||||
| HBV panel | MC | |||||||||
| HAV status | MC | |||||||||
| Urine toxicology POC assay | X | X | X | X | X | X | ||||
| Research visit activities | Post-treatment visits | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 60, ± 2 wks | Week 72, ± 2 wks | Week 84 ± 2 wks | Week 96, ± 2 wks | Week 108, ± 2 wks | Week 120, ± 2 wks | Week 132, ± 2 wks | Week 144, ± 2 wks | Week 156, ± 2 wks | Week 168, ± 2 wks | |
| Visit number | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| TIME (in minutes) | 15 | 45 | 15 | 25 | 15 | 90 | 15 | 25 | 15 | 90 |
| Release of information | ||||||||||
| Informed consent | ||||||||||
| Randomization | ||||||||||
| Instruments | ||||||||||
| Socio-demographic survey | ||||||||||
| Short socio-demographic survey | X | X | X | |||||||
| Behavioral risk assessment | X | X | X | X | X | X | X | X | X | X |
| Depression (PHQ-9) | X | X | ||||||||
| Anxiety (GAD-7) | X | X | ||||||||
| Alcohol use (AUDIT-C) | X | X | X | X | X | |||||
| Modified ASI - baseline | ||||||||||
| Modified ASI - follow-up | X | X | X | |||||||
| Substance use treatment | X | X | ||||||||
| Quality of life (EQ-5D) | X | X | ||||||||
| Adherence (visual analogue scale) | ||||||||||
| Medical outcomes social support scale | X | X | ||||||||
| Brief revised working alliance (PN arm only) | ||||||||||
| Stigma scale | ||||||||||
| Shame scale | X | X | ||||||||
| Health efficacy scale | ||||||||||
| Satisfaction scale | ||||||||||
| Peer support | ||||||||||
| Labs | ||||||||||
| HCV antibody | ||||||||||
| HCV viremia | X | X | X | X | X | X | X | X | X | X |
| HCV genotype/subtype | ||||||||||
| HIV test | ||||||||||
| HIV viral load* | ||||||||||
| HIV CD4* | ||||||||||
| A ST/ALT/Pla te 1 ets/FIB-4 calculation | ||||||||||
| Total bilirubin | ||||||||||
| Albumin | ||||||||||
| Prothrombin time | ||||||||||
| FibroSure/Fibroscan/Liver biopsy | ||||||||||
| Imaging study** | ||||||||||
| Child-Pugh score (exclude B,C) | ||||||||||
| Pregnancy test* | ||||||||||
| Archiving sample | X | X | X | X | X | X | X | X | X | X |
| HCV GenoSure NS5A/NS5B Assay* | ||||||||||
| HBV panel | ||||||||||
| HAV status | ||||||||||
| Urine toxicology POC assay | X | X | X | X | X | |||||
Note: MC = Medical chart;
= if applicable;
= if available.
2.10. Study medication
Medications are dispensed in Med-ic® blister packs (Information Mediary Corp., Otawa, Canada). These electronic blister packs have a fine wire that is disrupted when the medication is removed and the circuit records the precise time and date of the removal, permitting assessment of adherence.
2. 11. Interventions: mDOT vs. PN
2. 11.1. mDOT
2.11.1.1. mDOT in the OTP clinics.
This intervention is considered a modified version of DOT as observation of dosing is not daily. Participants in the mDOT arm receive: 1) five to seven directly observed doses per week at the same time as they receive methadone or buprenorphine, 2) individually packaged take-home doses for self-administration on weekends at the discretion of the OTP, and 3) two rescue doses to be used in the event they cannot attend the OTP. Since mDOT is linked to OTP visits, the number of directly observed oral doses varies based on the number of days the participant attends the clinic to receive directly observed methadone or buprenorphine.
2. 11.1.2. mDOT in the CHC.
Participants are offered mDOT via the mobile health app miDOT (developed by emocha Mobile Health, Inc., Baltimore, MD) to document study drug administration on a smartphone. All 7 doses a week are observed through submission of videos via the app. Participants are either provided with a smartphone that is pre-loaded with a data/min plan and the mDOT app, or download the mDOT app to their own smartphone. Research staff train the participant on how to use the app. Participants record a video of themselves taking their HCV medication, then upload the video to the database maintained by emocha. Staff review these videos within 72 h to confirm the participant took their medication. This intervention is still considered mDOT since doses are not necessarily observed by study staff on the same day doses are taken. Subjects using the mobile health app miDOT receive seven doses of medication on a weekly basis at their CHC. Subjects who decline the app are offered DOT in the health center and receive: 1) three to five directly observed doses in the health center or affiliated organization administered by staff, and 2) individually packaged take-home doses for self-administration on weekends and other clinic days.
2.11.2. PN
The study follows a PN model developed by the New York City Department of Health (NYDOH) in collaboration with Montefiore Medical Center and the community [43,44] [47,48]. HCV patient navigators assist with: 1) coordination of treatment, 2) health education and promotion, 3) overcoming barriers, and 4) psychosocial support. Details of the PN protocol and materials are documented in a patient navigator manual.
Study medication is packaged in Med-ic® blister packs and dispensed as a 2-week supply every other week. If the participant has difficulty on the biweekly schedule, the healthcare provider may assess changing the dispensing frequency to weekly, and document the reason (s) for changing. No doses are observed even if the patient is coming to the clinic and picking up medications weekly.
2.12. Visit schedule, measures, and laboratory samples
Research visits are the same for all participants, regardless of assigned treatment arm (Table 1). In addition to the enrollment baseline visit, 16 additional visits will take place at weeks 4, 8, 12, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, and 168 for participants who initiate treatment. At each visit, research staff administer questionnaires, provide counseling on avoidance of HCV and HIV infection, offer information on drug treatment and syringe services programs, and other referrals as needed, such as health and social services. Urine specimens are collected at baseline and weeks 4, 8, 12, 24, 48, 72, 96, 120, 144 and 168 and tested for drugs, including amphetamine, barbiturate, benzodiazepine, buprenorphine, cocaine, THC/Cannabinoids, methadone, methamphetamine, opiate, and oxycodone (Multi-Drug Screen Dip Card 10 Panel; American Bio Medica Corp.,Kinderhook, NY), The oxycodone assay has a higher sensitivity than the opiate assay in detecting certain opioids in urine, including oxycodone (OxycContin ®), hydrocodone (Vicodin®) and hydromorphone (Dilaudid ®). At weeks 4, 8, 12, and 24 medical records are reviewed for evidence of HCV viremia, as the participant’s healthcare provider may order this test to monitor the result of treatment. A blood sample (20 mL of whole blood) is collected at visit 1 (baseline visit) for archiving. Additional blood samples (20 mL whole blood for viremia testing and archiving) are collected at weeks 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, and 168.
2.13. Planned statistical analyses
The six outcome variables are: 1) SVR rates among those randomized to mDOT compared to those randomized to PN (primary out-come), 2) treatment initiation, 3) longitudinal biweekly treatment adherence rates, 4) treatment completion, 5) development of drug resistance, and 6) reinfection. First, the distributions of all variables will be examined using graphical or descriptive statistics to identify any values out of range. When identified, out of range values will be verified by checking the original record, compared and corrected if needed. Second, although each arm will equally be distributed across city, clinical site (OTP and CHC) and stage of liver disease (cirrhosis vs. no cirrhosis) by the stratified randomization design, the success of randomization will be monitored as a way of checking key assumptions by comparing the PN and mDOT groups on key variables, including demographic, behavioral, and clinical characteristics. Continuous variables will be compared between arms using the t-test or Mann-Whitney test, and categorical variables will be compared using chi-square or Fisher’s exact test. When necessary, normal assumptions will be checked for continuous variables by applying a formal Kolmogorov-Smirnov test, and if violated, Box-Cox transformations will be considered. All statistical analyses will be conducted using SAS v9.4 or updated version (SAS Institute Inc. Cary, NC).
The Consolidated Standards of Reporting Trials (CONSORT) and ICH-9 statistical analysis guidelines suggest that, any baseline characteristics, even if significantly unbalanced between arms, should not be included in the statistical models for the outcome analysis since baseline imbalance could be due to chance rather than systematic bias in randomized studies but include as the covariates the randomization stratification variables. In the HERO study, these variables are: site/city, clinic type (OTP and CHC) and stage of liver disease (cirrhosis vs. no cirrhosis). The primary analytic sample, nonetheless, might no longer represent a randomized pool of participants since the characteristics of participants who initiated treatments may be unequal between the two models of care which may have differential effects on triggering treatment initiations due to difference in modalities of treatment initiation and delivery. Therefore, baseline characteristics could exist that are unbalanced beyond chance among participants initiating treatment between the two arms. We will declare an imbalance if a baseline characteristic is significantly different between the two arms with a stringent two-sided p-value < .01, considering the multiplicity of testing. As such, we will include unbalanced baseline characteristics for adjusting purposes in the statistical models in addition to the randomization stratification variables.
Specifically, to test the overall effectiveness of PN or mDOT on the binary (success vs. failure) primary outcome such as SVR, we will apply multivariable logistic regression models which will include unbalanced baseline characteristics (if any) in addition to the three randomization stratifying variables for adjusting purposes. PN or mDOT will be the predictor for each outcome. With respect to the longitudinal adherence rates, however, repeatedly measured adherence will be analyzed using 6 post-baseline time points and adherence as a continuous measure. We will apply a multi variable mixed effects linear model to test if the two arms are significantly different. This model accounts for within-subject longitudinal outcome correlation by taking the subject-level intercept as random. Multivariable mixed effects logistic regression will also be applied to test the significance of PN and mDOT on repeatedly-measured undetectable HCV viral loads throughout the intervention period. Changes in illicit drug use will be analyzed using urine toxicology data from alternating visits, counting the “person-month” as a unit of analysis, and analyzing the percentage of person-months that are positive for use of drugs including amphetamine, barbiturate, benzodiazepine, buprenorphine, cocaine, THC, methadone, methamphetamine, opiate, and oxycodone during the study period using the t-test or Mann-Whitney test.
Mediation analysis will be conducted to identify potential mediators between mDOT/PN effect and each of the outcomes. A mediator will be a variable whose value changes or occurs between the baseline visit and the end of the study; for example, reduced shame, extent of peer support, changes in social support, and increased self-efficacy. The potential mediating effects will be assessed by differences in mDOT or PN effect sizes depending on outcome with and without a potential mediator variable in statistical models. The significance of mediating effects will be tested following the Baron and Kenny mediation test principle [49].
To further document the statistical analysis plans including data sources, definitions of analytic samples, algorithms determining the study outcomes, and subgroup analysis strategies, we utilized a statistical analysis plan (SAP).
2.14. Power analysis for SVR
Considering the base 80% SVR rate in PN arm, a minimum of 9% difference between the two arms can be detected (i.e. 89% vs. 80%, odds ratio (OR) = 2.1) in a multivariable regression model in which confounding variables will explain 10% of variation in the predictor variable. This study posits that a > 9% difference in SVR between the PN and mDOT groups will be clinically significant based on prior studies that showed that SVR rates in treatment naive patients were > 90%. Mixed effects logistics regression models for the undetectable HCV viral load will be able to detect an even smaller percent point difference since they will utilize repeated measures for each subject no matter how large within-subject outcome correlations are.
2.15. Reinfection, relapse, and resistance
Reinfection will be defined by post-treatment quantifiable HCV RNA with any of the following criteria: 1) viremia with different genotype/subtype; 2) date following documentation of SVR; 3) the same genotype/subtype but with phylogenetic evidence of reinfection. For the latter, next-generation sequencing of the HVR1 sequences will compare baseline and post-treatment viremia specimens with a genetic diversity threshold set at nucleotide substitutions per site > 3% [50], verified by cluster analysis. Those with post-treatment quantifiable HCV RNA > 1000 IU/mL and not classified as reinfection will be classified as relapse and resistance will be measured for baseline and post-treatment samples with genotypes 1a, 1b, and 3 (representing over 90% of the cohort) by ultra-deep sequencing of NS5A with reporting of relevant substitutions at the 10% level (Monogram Biosciences, South San Francisco, CA). The proportion of those developing resistance or reinfection will each be calculated using exact binomial methods.
For the analysis of resistance, bivariate analysis using logistic regression with continuous and dummy-coded categorical variables will be performed; factors significant in bivariate analysis will be included in a multi variable logistic regression. For the analysis of reinfection, the time to reinfection from SVR will be determined and the date of reinfection will be estimated by using the midpoint date between the date of the last undetectable HCV RNA test and the date of the first detectable HCV RNA test. The incidence of reinfection will be calculated by the number of incident infections divided by the number of person-years (py) of follow-up. Log-rank test will be applied to test difference in time to reinfection between two arms and also to identify categorical factors that are associated with reinfection. Bivariate cox proportional hazards regression models will be used to identity continuous variables and then a multivariable cox regression models will be applied primarily to test significance of the study arm in the presence of potential confounding factors. Both the adjusted and unadj usted Kaplan-Meier curves will be presented.
2.16. Qualitative interviews, methods and analysis
2.16. 1. PN qualitative study
2.16. 1.1. Recruitment.
Between 10 and 15 patient navigators, with at least one navigator from each site, will be recruited in Year 1 to complete an in-depth interview. Data are utilized as part of a formative evaluation, to identify emerging barriers to program success and facilitate problem solving [46] [51].
2.16. 1.2. Data collection.
After informed consent is obtained, patient navigators complete a 30-min semi-structured interview with a trained qualitative expert focusing on their experience and perception of the intervention, to capture the perspectives of these front-line patient navigators on the barriers to successful patient outcomes (Table 2).
Table 2.
Data collection plan for assessing implementation outcomes.
| Variables | Data collection and source | Responsible | Method of data analysis | Responsible |
|---|---|---|---|---|
| Acceptability of intervention | ||||
| Patient. satisfaction with treatment | 1 treatment satisfaction question | Site RA | Quantitative | Site RA |
| Positive/negative experiences with treatment | Brief treatment experiences interview at various time points | Site RA | Structured, ‘quantitated’ thematic analysis w/qual data analysis program | Bronx Implementation Team |
| Feasibility of intervention | ||||
| Recruitment milestones | Research database | Site staff | Quantitative | Local/New Mexico |
| Retention in intervention | Research database | Site staff | Quantitative | Local/New Mexico |
| Execution of work plans | Work plans for each arm and milestones and dates achieved | Bronx Project Director | Qualitative | Bronx Implementation Team |
| Intervention dose | ||||
| mDOT | ||||
| # Observed doses/# Planned observed doses | Nurse calendar/log or patient log | Frontline staff | Qualitative | Local/NM |
| Patient navigation | ||||
| 2 in Person meetings/3 follow-up calls | Case notes or other tecords | Frontline staff | Qualitative assessment and quantitative tabulation | Site Staff Supervisor |
| Fidelity of PN intervention to be discussed with DOH PN team | ||||
| Care plan completed | Navigation care plan | Frontline staff | Qualitative, assessment, tabulation | PN records |
| Initial medical evaluation completed | Case notes | Frontline staff | Qualitative, assessment, tabulation | PN records |
| Referrals made | Case notes | Frontline staff | Qualitative, assessment, tabulation | PN records |
| Referrals followed up | Case notes | Frontline staff | Qualitative, assessment, tabulation | PN records |
| Education sessions 1 and 2 provided | Attendance records/groups, case notes/ind. # Minutes | Frontline staff | Qualitative, assessment, tabulation | PN records |
| Consent | Data collection instruments |
|---|---|
| Site leader oral consent |
|
| Staff oral consent |
|
| Patient oral consent |
|
| Request waiver of consent-anonymous on worksite culture |
|
| Regular study consent form-to be embedded in 12-week follow-up interview |
|
2.16. 1.3. Analysis.
Interviews will be recorded and transcribed. Data are analyzed using Miller and Crabtree’s “Editing” approach [47,52]. A preliminary coding scheme will be created and revised in iterative fashion as it was applied to new subsets of data. The complete data set will be coded in NVivo (QSR International, Pty Ltd., 2017), a qualitative data analysis program that facilitates the rapid organization and retrieval of thematically related data [53–56]. Themes related to successes and failures, and sources of stress and satisfaction will be summarized. Data summaries will be presented to the NSAB to discuss implications, identify emerging problems, and find solutions.
2.16.2. Patient qualitative study
The focus of the patient study, conducted in years 2–6, is to understand barriers to successful outcomes along the pathway to care, including lack of treatment initiation, poor adherence (< 80%), failure to achieve SVR, development of drug resistance, and HCV reinfection. The patient study incorporates a comparative design, whereby structured comparisons are made between successful and non-successful patients at each stage along the care cascade with the goal of identifying barriers to successful treatment.
2.16.2. 1. Recruitment.
Approximately 10 participants who successfully initiated treatment and 10 who did not initiate treatment will be recruited and interviewed, with the goal of including at least one patient in each category by site. Similarly, participants who adhered/did not adhere, achieved SVR/did not achieve SVR; and those who become reinfected/remained HCV-free will be recruited and interviewed. In keeping with standard qualitative methodological procedures, sample sizes are approximate, and sampling will continue until theoretical saturation is achieved.
2.16.2.2. Data collection.
Participants will be telephone interviewed by a trained interviewer, using an interview guide focused on barriers and facilitators to successful treatment, perceptions of the intervention, perceived need for treatment, relationships with research staff, and treatment experiences. The guide includes questions on contextual barriers to successful participation such as economic, social, and psychological stressors, as well as facilitators such as social support and other resources. The interviews are recorded and transcribed for analysis.
2.16.2.3. Qualitative data analysis.
Data analysis will proceed in two steps. The first step entails the standard data analysis approach described above. In the second step, the analysis team will make structured comparisons across successful and unsuccessful patients, searching for patterns in the data that reveal core differences across groups and provide insight into why some patients are able to succeed with treatment and some are not. Data from these comparative analyses will be summarized and presented to the Stakeholder team, with the goal of improving the capacity of the intervention team to provide patient-centered care and better meet patient needs. Data collected in later years of the project will be used as part of a summative evaluation, to understand the successes and failures of the intervention with the goal of improving the effectiveness of future interventions.
3. Discussion
This study represents the first randomized pragmatic trial comparing the effectiveness of mDOT compared to PN in treating HCV in PWID who are actively injecting. Studies of HCV treatment outcomes in PWID have included various subpopulations, including those who are no longer injecting drugs and those who are enrolled in OTPs. There are, to date, no published prospective studies which restrict the PWID population to those actively injecting any substance within 90 days of enrollment.
The HERO study will provide valuable information on optimal models of care to promote HCV treatment initiation, adherence, treatment completion and SVR among PWID. We will also examine rates of resistance development and reinfection up to 3 years. Understanding these factors is crucial to addressing the HCV epidemic in this high-risk population. Reviews and guidelines emphasize that further research on the effects of different models of care is needed to provide recommendations on the most effective way to provide curative HCV therapy to PWID. The HERO study addresses this critical need [6–8,14]. We expect that the results of this study will advance knowledge regarding the effectiveness of both mDOT and PN for HCV treatment support for PWID and inform modifications and variations to both models of care.
The use of electronic blister pack technology to monitor adherence is innovative. In the interferon (IFN) era, strict adherence to combination IFN and ribavirin (RBV) was necessary to optimize SVR rates. In the DAA era, adherence remains a critical issue for achievement of SVR and reducing risk of developing resistance [57], but as there are currently limited data describing associations between DAA adherence and virologic outcomes, optimal adherence to DAA regimens is not known. Accurate measurement of adherence will be an indispensable element to understand the direct effect of nonadherence on treatment failure. Previous randomized trials that focused on DOT for pegylated-IFN and RBV utilized diaries and pill counts to measure adherence [58–60]. Use of electronic blister packs in HERO will build on this literature by measuring adherence using electronic monitors that have been shown to better predict virologic outcomes in HIV-infected populations [7,20,61].
The HERO study has several strengths. First, it is focuses on people who are actively injecting substances as defined by injection drug use within 90 days prior to enrollment. Prior studies have not used specific criteria and often have mixed populations of people actively injecting drugs and those who injected in the past. It is necessary to treat people who are actively injecting in order to interrupt transmission and reduce incidence. Secondly, HCV treatment in this study is delivered in community-based clinical settings where PWID seek care. Community-based models of care are consistent with the current recommendations to expand treatment to primary care settings. Third, HERO sites have significant geographic and state policy diversity, including variable Medicaid restrictions of DAAs for PWID. Therefore, our results represent the implementation of the interventions in these diverse settings and as such provide valuable insights on real-world effectiveness given these heterogeneities. Fourth, we include PWID both maintained and not maintained on opioid agonist treatment. Fifth, our study is unique in that the eight sites provide a robust sample size for a study of PWID which will allow important subgroup analyses for heterogeneity. For example, additional analyses will be conducted to evaluate whether the mDOT and PN effects varies by subgroups stratified by the following characteristics: OTP vs. CHC; men vs. women; African-American/Latina vs. Caucasian; unstable housing vs. stably housed; illicit drug use during treatment vs. no illicit drug use during treatment; HIV / HCV co-infected vs. HCV mono-infected; cirrhotic vs. non-cirrhotic; severe to moderate anxiety symptoms vs. mild to minimal; and, severe to moderate depression symptoms vs. mild to minimal. Sixth, the use of a pan-genotypic medication (sofosbuvir/velpatasvir) allows inclusion of DAA-naïve PWID irrespective of their genotype. Seventh, we include several important patient-reported outcomes such as shame and stigma that have not been included in other HCV treatment studies in PWID. Finally, this is the largest study to measure rates of reinfection in active PWID who successfully complete HCV treatment. Our results will likely inform modeling studies regarding optimal HCV elimination strategies and should have important policy implications.
The HERO study does have some limitations. Most of our study sites are located in urban areas although two sites also serve rural participants seeking care in the adjoining urban areas (e.g. Albuquerque, New Mexico, and Morgantown, West Virginia); PN and mDOT models of care may have different effects in non urban settings. Second, our sites serve PWID who predominantly are of low socioeconomic status. It is unclear if these same interventions will be effective or preferred by PWID of higher socioeconomic status. Finally, the study is conducted in 23 different clinical settings in eight cities which may lead to variability in fidelity; as we are measuring fidelity (Table 2) to the mDOT and PN interventions we will attempt to account for these differences in the analyses.
The HERO study is the largest study to date investigating the effectiveness of different models of curative HCV treatment in people who are injecting drugs and will likely have important policy implications as we move towards the WHO goal of HCV elimination by 2030.
Acknowledgements
The HERO Research Group includes the PI and Co-Investigators from each of the 9 sites, Pis from the CDC and the NYC DOH, statisticians and key staff (such as project directors and patient representatives), and key stakeholders. HERO study sites include: Prisma Health and Clemson University, Albert Einstein College of Medicine/Montefiore Medical Center, University of Rhode Island, Johns Hopkins Bloomberg School of Public Health, Massachusetts General Hospital, University of California, San Francisco, University of New Mexico, University of Washington, and West Virginia University.
The opinions presented in this work are solely the responsibility of the author and do not necessarily represent the views of PCORI, its Board of Governors or Methodology Committee.
Funding sources
This research was funded through a Patient-Centered Outcomes Research Institute (PCORI) AwardHPC-1503-28122 with additional support by Gilead Sciences, Quest Diagnostics, Monogram Biosciences, and OraSure Technologies.
Declaration of competing interest
Authors: AHL is a consultant/advisor and has received research grants from AbbVie, Gilead Sciences, and Merck Pharmaceuticals. JJ, KW, MH, AK, SM, AK, LT, PL, and IPV have nothing to declare. JF is a consultant/advisor and has received research grants from Gilead. JT is the recipient of a Small Business Innovation Research (SBIR) grant from NIH/NIDA (R44DA044053; PI: Seiguer/Tsui) in partnership with a health technology company (emocha) to research the feasibility of using smartphone app to improve adherence to office-based buprenorphine treatment. KP has received research funding from Gilead Sciences for research unrelated to this project.
HERO Study Group: BN has received research grants from Merck Pharmaceuticals. WS, VL, MH, EM, LA, JA, DMM, PM, CB, OF-N, SW, CM-K, KT, ES, KB, JW, AA, NJ, AR have nothing to declare.
Abbreviations:
- HCV
Hepatitis C virus
- CHC
Community health centers
- DAA
Direct-acting antiviral agents
- IDU
Injection drug use
- HERO
Hepatitis C Real Options
- mDOT
Modified directly-observed therapy
- OTP
Opioid treatment program
- PN
Patient navigation
- PWID
People who inject drugs
- SVR
Sustained virologic response
References
- [1].Ly KN, Hughes EM, Jiles RB, Holmberg SD, Rising mortality associated with hepatitis C virus in the United States, 2003–2013, Clin. Infect. Dis 62 (10) (2016) 1287–1288, 10.1093/cid/ciwlll. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zibbell JE, Asher AK, Patel RC, et al. , Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014, Am. J. Public Health 108 (2) (2018) 175–181, 10.2105/AJPH.2017.304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nelson PK, Matllers BM, Cowie B, et al. , Global epidemiology of hepatitis Band hepatitis C in people who inject drugs: results of systematic reviews, Lancet. 78 (9791) (2011) 571–583, 10.1016/SOI40-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Day E, Hellard M, Treloar C, et al. , Hepatitis C elimination among people who inject drugs: challenges and recommendations for action withing a health systems framework, Liver Int. 39 (1) (2019) 20–30, 10.1111/liv.l3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C, American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, 2019, https://www.hcvguidelines.org (Accessed March 10, 2019).
- [6].Robaeys G, Grebely J, Mauss S, et al. , Recommendations for the management of hepatitis C virus infection among people who inject drugs, Clin. Infect. Dis 57 (Suppl. 2) (2013) S1 29–S1 37, 10.1093/cid/cit302. [DOI] [PubMed] [Google Scholar]
- [7].Meyer JP, Moghimi Y, Marcus R, Lim JK, Litwin AH, Altice FL, Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic hepatitis C care continuum, Int. J. Drug Policy 26 (10) (2015) 922–935, 10.1016/j.drugpo.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bruggmann P, Litwin AH, Models of care for the management of hepatitis C vims among people who inject drugs: one size does not fit all, Clin. Infect. Dis 57 (Suppl. 2) (2013) S56–S61, 10.1093/cid/cit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Martin NK, Vickerman P, Grebely J, et al. , Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals, Hepatology 58 (5) (2013) 1598–1609, 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Iversen J, Grebely J, Topp L, et al. , Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999–2011, J. Viral Hepat 21 (3) (2014) 198–207, 10.1111/jvh.l2129. [DOI] [PubMed] [Google Scholar]
- [11].Mehta SH, Lucas GM, Mirel LB, et al. , Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic, AIDS. 20 (18) (2006) 2361–2369, 10.1097/QAD.Oh013e32801086da. [DOI] [PubMed] [Google Scholar]
- [12].Mehta SH, Genberg BL, Astemborski J, et al. , Limited uptake of hepatitis C treatment among injection drug users, J. Community Health 33 (3) (2008) 126–133, 10.1007/sl0900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grebely J, Genoway KA, Raffa JD, et al. , Barriers associated with the treatment of hepatitis C vims infection among illicit drug users, Drug Alcohol Depend. 93 (1–2) (2008) 141–147, 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- [14].Osilla KC, Ryan G, Bhatti L, et al. , Factors that influence an HIV coinfected patient’s decision to start hepatitis C treatment, AIDS Patien.t Care STDs 23 (12) (2009) 993–999, https://doi.org/l0.1089/apc.2009.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Treloar C, Hull P, Dore GJ, Grebely J, Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs, Drug Alcohol Rev. 31 (17) (2012) 918–924, 10.1111/j.1465-3362.2012.00468.x. [DOI] [PubMed] [Google Scholar]
- [16].Mehta SH, Thomas DL, Sulkowski MS, et al. , A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users, AIDS 19 (2005) S179–S189, 10.1097/01.aids.0000192088.72055.90. [DOI] [PubMed] [Google Scholar]
- [17].Grebely J, Oser M, Taylor LE, Dore GJ, Breaking down the barriers to hepatitis C vims (HCV) treatment among individuals with HCV/HJV coinfection: action required at the system, provider, and patient levels, J. Infect. Dis 207 (Suppl. 1) (2013) S19–S25, 10.1093/infdis/jis928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Swan D, Long J, Carr O, et al. , Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration, AIDS Patient Care STDs 24 (12) (2010) 753–762, 10.1089/apc.2010.0142. [DOI] [PubMed] [Google Scholar]
- [19].McLaughlin MM, Marx KT, Terriff C, American academy of HIV medicine pharmacists committee. Improving patient access ro hepatitis C virus treatment, J. Am. Pharm. Assoc 58(1) (2018) 109–112, 10.1016/j.japh.2017.10.013. [DOI] [PubMed] [Google Scholar]
- [20].Alavi M, Grebely J, Micallef M, et al. , Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study, Clin. Infect. Dis 57 (Suppl. 2) (2013) S62–S69, 10.1093/cid/cit305. [DOI] [PubMed] [Google Scholar]
- [21].Bonkovsky HL, Tice AD, Yapp RG, et al. , Efficacy and safety of peginterferon alfa-2a/ribavirin in methadone maintenance patients: randomized comparison of direct observed therapy and self-administration, Am. J. Gastroenterol 103 (11) (2008) 2757–2765, 10.1111/j.1572-0241.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- [22].Belfiori B, Ciliegi P, Chiodera A, et al. , Peginterferon plus ribavirin for chronic hepatitis C in opioid addicts on methadonelbuprenorphine maintenance therapy, Dig. Liver Dis 41 (4) (2009) 303–307, 10.1016/j.dld.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [23].Bruce RD, Eiserman J, Acosta A, et al. , Developing a modified directly observed therapy intervention for hepatitis C treatment in a methadone maintenance program: implications for program replication, Am. J. Drug Alcohol Abuse 38 (3) (2012) 206–212, 10.3109/00952990.2011.643975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Litwin AH, Harris KA Jr., Nahvi S, et al. , Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program, J. Subst. Abus. Treat 37 (1) (2009) 32–40, 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Martinez AD, Dimova R, Marks KM, et al. , Integrated internist - addiction medicine - hepatology model for hepatitis C management for individuals on methadone maintenance, J. Viral Hepat 19 (1) (2012) 47–54, 10.1111/j.1365-2893.2010.0141l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dimova RB, Zeremski M, Jacobson IM, Hagan H, DesJarlais DC, Talal AH, Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis, Clin. Infect. Dis 56 (6) (2013) 806–816, 10.1093/cid/cis1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jack K, Willott S, Manners J, Varnam MA, Thomson BJ, Clinical trial: a primary-care-based model for the delivery of anti-viral treatment to injecting drug users infected with hepatitis C, Aliment. Pharmacol. Ther 29 (1) (2009) 38–45, 10.1111/j.l365-2036.2008.03872.x. [DOI] [PubMed] [Google Scholar]
- [28].McAllister G, Innes H, McLeod A, et al. , Uptake of hepatitis C specialist services and treatment following diagnosis by dried blood spot in Scotland, J. Clin. Virol 61 (3) (2014) 359–364, 10.1016/j.jcv.2014.09.004. [DOI] [PubMed] [Google Scholar]
- [29].Morano JP, Zelenev A, Lombard A, et al. , Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic, J. Conununity Health 39 (5) (2014) 922–934, 10.1007/s10900-014-9932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Newman AI, Beckstead S, Beking D, et al. , Treatment of chronic hepatitis C infection among current and former injection drug users within a multidisciplinary treatment model at community health centre, Can. J. Gastroeneterol 27 (4) (2013) 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seidenberg A, Rosemann T, Senn O, Patients receiving opioid maintenance treatment in primary care: successful chronic hepatitis C care in a real world setting, BMC Infect. Dis 13 (9) (2013), 10.1186/1471-2334-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Grebely J, Genoway K, Khara M, et al. , Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection, Int. J. Drug Policy 18 (5) (2007) 437–443, 10.1016/j.drugpo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- [33].Sylvestre DL, Zweben JE, Integrating HCV services for drug users: a model to improve engagen1ent and outcomes, Int. J. Drug Policy 18 (5) (2007) 406–410, 10.1016/j.drugpo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- [34].Stein MR, Soloway IJ, Jefferson KS, Roose RJ, Arnsten JH, Litwin AH, Concurrent group treatment for hepatitis C: implementation and outcomes in a methadone maintenance treatment program, J. Subst. Abus. Treat 43 (4) (2012) 424–432, 10.1016/j.jsat.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Litwin AH, Berg KM, Li X, Hidalgo J, Arnsten JH, Rationale and design of a randomized controlled trial of directly observed hepatitis C treatment delivered in methadone clinics, BMC Infect. Dis 11 (2011) 315, 10.1186/1471-2334-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saiz de Ia Hoya P, Portilla J, Marco A, et al. , Directly observed therapy for chronic hepatitis C: a randomized clinical trial in the prison setting, Gastroenterol. Hepatol 37 (8) (2014) 433–453, 10.1016/j.gastrohep.2014.03.004. [DOI] [PubMed] [Google Scholar]
- [37].Wei LJ, Lacltin JM, Properties of the urn randomization in clinical trials, Control. Clin. Trials 9 (4) (1988) 345–364. [DOI] [PubMed] [Google Scholar]
- [38].Calsyn DA, Saxon AJ, Bush KR, et al. , The Addiction Severity Index medical and psychiatric composite scores measure similar domains as the SF-36 in substance-dependent veterans: concurrent and discriminant validity, Drug Alcohol Depend. 76 (2) (2004) 165–171, 10.1016/j.drugalcdep.2004.04.018. [DOI] [PubMed] [Google Scholar]
- [39].Weatherby NL, Needle R, Cesari H, et al. , Validity of self-reported drug use among injection drug users and crack cocaine users recruited through street out-reach, Eval. Program Plan 17 (4) (1994) 347–355. [Google Scholar]
- [40].Saunders JB, Aasland OG, Babor TF, de Ia Fuente JR, Grant M, Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consurnption-11, Addiction 88 (6) (1993) 791–804, 10.1111/j.1360-0443.1993.th02093.x. [DOI] [PubMed] [Google Scholar]
- [41].Kroenke K, Spitzer RL, Williams JBW, The PHQ-9: validity of a brief depression severity measure, J. Gen. Intern. Med 16 (9) (2001) 606–613, 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Spitzer RL, Kroenke K, Williams JBW, Lowe B, A brief measure for assessing generalized anxiety disorder, Arch. Intern. Med 166 (10) (2006) 1092–1097, 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- [43].van Hout B, Jansenn MF, Feng YS, et al. , Interim scoring for the EQ-SD-SL: mapping the EQ-SD-SL to EQ-SD-3L value sets, Value Health 15 (5) (2012) 708–715, 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- [44].Wright K, Naar-King S, Lam P, Templin T, Frey M, Stigma scale revised: reliability and validity of a brief measure of stigma for HIV + youth, J. Adolesc. Health 40 (1) (2007) 96–98, 10.1016/j.jadohealth.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].L Fredrickson B, Tugade MM, Waugh CE, Larkin GR, What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001, J. Pers. Soc. Psychol 84 (2) (2003) 365–376, 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sherbourne CD, Stewart AL, The MOS social support survey, Soc. Sci. Med 32 (6) (1991) 705–714, 10.1016/0277-9536(91)90150-B. [DOI] [PubMed] [Google Scholar]
- [47].Ford MM, Johnson N, Desai P, Rude E, Larangue F, From care to cure: demonstrating a model of clinical patient navigation for hepatitis C care and treatment in high-need patients, Clin. Infect. Dis 64 (5) (2017) 685–691, 10.1093/cid/ciw806. [DOI] [PubMed] [Google Scholar]
- [48].Norton B, Voils C, Timberlake SH, et al. , Community-based HCV screening: knowledge and attitudes in a high risk urban population, BMC Infect. Dis 14 (74) (2014), 10.1186/1471-2334-14-74.10.1186/1471-2334-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baron RM, Kenny DA, The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations, J. Pers. Soc. Psychol 51 (6) (1986) 1173–1182, 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- [50].Sarrazin C, lsakov V, Svarovskaia ES, Hedskog C, Martin R, et al. , Late relapse versus hepatitis C virus reinfection in patients with sustained virologic response after Sofosbuvir-based therapies, Clin. Infect. Dis 64 (1) (2016) 44–52, 10.1093/cid/ciw676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Glasgow RE, What types of evidence are most needed to advance behavioral medicine? Ann. Behav. Med 35 (1) (2008) 19–25, 10.1007/s12160-007-9008-S. [DOI] [PubMed] [Google Scholar]
- [52].L Miller W, Crabtree BF, Qualitative analysis: how to begin making sense, Fam. Pract. Res. J 14 (3) (1994) 289–297. [PubMed] [Google Scholar]
- [53].Karasz A, McKee MD, Roybal K, Women’s experiences of abnormal cervical cytology: illness representations, care processes, and outcomes, Ann. Fam. Med 1 (4) (2003) 196–202, 10.1370/afm.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Garcia IA, Blank AE, Eastwood EA, Karasz A, Barriers and facilitators to the implementation of SPNS interventions designed to engage and retain HIV positive women of color in medical care, AIDS Behav. 19 (4) (2015) 655–665, 10.1007/s10461-014-0837-5. [DOI] [PubMed] [Google Scholar]
- [55].McKee MD, Karasz A, Weber CM, Health care seeking among urban minority adolescent girls: the crisis at sexual debut, Ann. Fam. Med 2 (6) (2004) 549–554, 10.1370/afm.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Karasz A, Cultural differences in conceptual models of depression, Soc. Sci. Med 60 (7) (2005) 1625–1635, 10.1016/j.socscimed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- [57].Sacks-Davis R, Grebely J, Osborn MK, et al. , HCV Reinfection and Spontaneous Clearance after Spontaneous Clearance of Primary HCV Infection: The InC3 Study, Presented at: HCV 2013 Symposium; October 6–10 (2013) Melbourne, Australia. [Google Scholar]
- [58].Vickerman P, Grebely J, Dore GJ, et al. , The more you look, the more you find: effects of hepatitis C virus testing interval on reinfection incidence and clearance and implications for future vaccine study design, J. Infect. Dis 205 (9) (2012) 1342–1350, 10.1093/infdis/jis213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hill A, Effects of SVR on the Risk of Liver Transplant, Hepatocellular Carcinoma, Death and Reinfection: Meta-analysis of 129 Studies in 34,563 Patients with Hepatitis C Infection, Presented at: 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11 (2014) Boston, MA. [Google Scholar]
- [60].Osburn WO, Fisher BE, Dowd KA, et al. , Spontaneous control of primary hepatitis C virus infection and irrununity against persistent reinfection, Gastroenterology 138 (1) (2010) 315–324, 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mehta SH, Cox A, Hoover DR, et al. , Protection against persistence of hepatitis C, Lancet 359 (9316) (2002) 1478–1483, 10.1016/50140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]


