Abstract
In the absence of a cure or vaccine for HIV/AIDS, small molecule inhibitors remain an attractive choice for antiviral therapeutics. Recent structural and functional studies of the HIV-1 surface envelope glycoprotein gp120 have revealed sites of vulnerability that can be targeted by small molecule and peptide inhibitors, thereby inhibiting HIV-1 infection. Here we describe a series of small molecule entry inhibitors that were designed to mimic the sulfated N-terminal peptide of the HIV-1 coreceptor CCR5. From a panel of hydrazonothiazolyl pyrazolinones, we demonstrate that compounds containing naphthyl di- and trisulfonic acids inhibit HIV-1 infection in single round infectivity assays with the disulfonic acids being the most potent. Molecular docking supports the observed structure activity relationship, and SPR confirmed binding to gp120. In infectivity assays treatment with a representative naphthyl disulfonate and a disulfated CCR5 N-terminus peptide results in competitive inhibition, with combination indices >2. In total this work shows that gp120 and HIV-1 infection can be inhibited by small molecules that mimic the function of, and are competitive with the natural sulfated CCR5 N-terminus.
Keywords: Tyrosine sulfate, Molecular docking, HIV co-receptors, CCR5 N-terminus, Competitive inhibition
1. Introduction
HIV entry into CD4+ host cells represents the first stage of HIV infection and entails a sequential series of binding events that lead to conformational changes along the entry pathway. These include docking of the HIV envelope (Env) glycoprotein gp120 to CD4 that induces a conformational change in HIV Env to unmask the co-receptor binding site.1,2 Engagement with co-receptors CCR5 or CXCR4 induces further conformational changes that ultimately lead to membrane fusion and virus infection.3–5 Sites of vulnerability that can be targeted by small molecule, peptide and protein inhibitors are exposed on HIV Env before and during the entry process.6 These include the CD4- and coreceptor-binding sites, the prehairpin intermediate conformation of the transmembrane protein gp41, as well as the HIV co-receptors themselves.1,6–8 Examples of small molecule HIV-1 entry inhibitors are pictured in Figure 1, and include BMS-378806 that stabilizes gp120 and prevents CD4-induced conformational changes,9 the CD4-binding site inhibitors NBD-55610 and (+)-DMJ-II-121,11 recently described co-receptor binding site inhibitors 1 and 2, and the clinically approved CCR5 antagonist maraviroc.12
Figure 1.

Structures of small molecule HIV entry inhibitors and CCR5 N-terminus tyrosine sulfate mimetics (1, 2).
The coreceptor-binding site on gp120 is located at the base of the V3 loop and extends toward the bridging sheet.13–15 It is among the most conserved sites on the viral envelope making it interesting from the point of therapeutics. In addition to containing the epitope for monoclonal antibodies such as X514 and 412d,13 it is also the binding site for the sulfated N-terminus of CCR5.16–18 The CCR5 N-terminus (Nt) contains four Tyr residues, and sulfation on Tyr10 and Tyr14 are essential for binding to CD4-activated gp120 and facilitating viral entry.17,18 Remarkably, a number of monoclonal antibodies (mAbs) have been found to possess sulfotyrosine residues in their third complementary determining heavy chain regions (CDRH3’s) and to target the coreceptor binding site on gp120 through these residues.19 In previous work employing NMR, X-ray crystallography, and molecular docking we solved the structure of a monomeric gp120 construct in complex with CD4 and sulfated antibody 412d. At the same time we determined the solution structure of a disulfated CCR5 Nt peptide (residues 2–18) in the CD4-gp120-bound conformation and generated a docked model of this complex.13 Upon binding sulfated Nt or 412d, a conserved pocket lined with hydrophobic and positively charged amino acids is formed on gp120, and surrounds the Tyr-SO4 moiety. A more recent NMR study employing full-length trisulfated CCR5 Nt (27 residues) suggested that additional positively charged residues located proximal to the sulfotyrosine-binding pocket likely expand the coreceptor binding site by providing a greater surface for interactions with additional sulfotyrosine moieties.20
The CCR5 Nt binding site on gp120 represents a novel pocket that can be targeted by small molecules. Previously, using fragments of the CD4-gp120-bound CCR5-Nt structure or the sulfated CDRH3 of 412d as search queries, we carried out in silico screening of the ZINC chemical library, relying primarily on a shape- and chemical-similarity matching routine (ROCS).21 We identified two 4-sulfophenylhydrazonothiazolyl-pyrazolinones (1, 2) that bound gp120 in a CD4 dependent manner, and could compete with sulfated antibody 412d for binding to CD4-bound gp120. Here we describe medicinal chemistry efforts to improve on those entry inhibitors by increasing potency and/or breadth. Building upon our preliminary findings for compounds 1 and 2, we show that incorporation of di- and tri-sulfonyl naphthalene groups leads to compounds that inhibit HIV-1 in single round infectivity assays and are competitive with sulfated CCR5 Nt. Using molecular docking, we identify distinct modes of binding that are consistent with the observed potencies.
2. Chemistry and biological evaluation
Our previously described inhibitors 1 and 2 were obtained from commercial libraries. To explore the structure activity relationship around this scaffold, we developed a synthetic route where commercially available reagents could be incorporated to generate a small library. A retrosynthetic analysis showed that the core ring system could be obtained by reacting a 1,3-dioxobutan-2-ylidene intermediate with thiosemicarbazide to form the thiocarboxamido pyrazolinone, and subsequent treatment with various bromoacetates would form the thiazole ring bearing different substituents.22,23 Starting with 4-aminobenzenesulfonic acid, we generated 4-(2-(1-ethoxy-1,3-dioxobutan-2-ylidene)hydrazinyl) benzenesulfonic acid (Scheme 1a). Treatment with thiosemicarbazide in the presence of acetic acid gave thiocarboxamido-sulfophenylhydrazono pyrazolinone 3.23 A Hantzsch thiazole reaction24 with thioamide 3 and commercially available phenyl acetyl bromides, employing minor modifications to published procedures (Schemes 1 and 2), gave reference compound 2 and analogs 4a–f containing the desired heterocyclic scaffold.
In previous work we showed that CCR5 Nt peptides containing the hydrolytically stable methane sulfonate isostere of sulfotyrosine could bind and recognize the CD4-activated coreceptor-binding site on gp120, albeit with lower affinity.25 To compare these groups in the context of our small molecule inhibitors we modified the first steps of the synthesis by converting 1-(chloromethyl)-4-nitrobenzene into the desired 4-aminophenyl methane-sulfonate (Na2SO3, H2O:MeOH, reflux; (d) H2(g) 1 atm, H2O:MeOH). Treatment with ethyl 3-oxobutanoate followed by thiosemicarbazide gave thiocarboxamide 5 that was in turn converted to desired compound 6 (Scheme 1b).
We evaluated compounds 2, 4a–f and 6 in an HIV-1 infectivity assay to test their ability to inhibit HIV-1 entry using the CCR5-tropic primary isolate YU2 (Table 1). Though none of the substituents on C-5 of the thiazole ring improved the IC50 compared to compound 2, the inhibition data showed that incorporation of phenyl groups containing ortho or meta substituents (4a,b,e) decreased potency, as did inclusion of charged or bulkier para substituents (4d,f). Indeed compound 4c containing a para −CF3 substituent was the only equipotent analog identified. Surprisingly, methane-sulfonate 6 was inactive, as was disulfonate 4f.
Table 1.
HIV neutralization by phenyl sulfonates
Not active at concentrations as high as 500 μM.
We next turned our attention to modifying the aryl sulfonate portion of the structures. Although a crystal structure of gp120 in complex with CCR5 N-terminus has yet to be determined, NMR and molecular docking studies suggest that additional sulfotyrosine and/or acidic residues present in the N-terminus of CCR5 are likely to contact a positively charged region on gp120 located at the edge of the coreceptor binding site.20 With this in mind, we envisioned expanding the inhibitor scaffold by incorporating di- and tri-sulfonated naphthylene moieties in place of the phenylsulfonate. Earlier studies have shown that naphthalene sulfonate polymers can inhibit gp120–CD4 binding and inhibit HIV-1 infection26 offering the possibility that inclusion of one naphthalene sulfonate moiety in a small molecule could augment binding to gp120. As summarized in Scheme 2a and b, starting with commercially available 8-aminonaphthalene-1,3,6-trisulfonic acid or 6-aminonaphthalene-1,3-disulfonic acid, we followed a synthetic route similar to that shown in Scheme 1. Reaction of the naphthyldiazonium tetrafluoroborate salts with either ethyl 3-oxobutanoate, ethyl 3-oxo-3-phenylpropanoate, ethyl 4,4,4-trifluoro-3-oxobutanoate or ethyl methyl malonate, followed by a Hantzsch thiazole synthesis with various alkyl bromides (Scheme 2) gave 18 new tri- or di-sulfonate derivatives each of which was tested in HIV neutralization assays. We were encouraged to find that incorporation of the naphthalene trisulfonate group (Table 2, 9a–f) improved the HIV neutralization activities by approximately five to eight-fold as compared to the phenylsulfonates. Still greater improvements in efficacy were observed for the 6-aminonaphthalene-derived disulfonates that lowered the IC50’s to single digit micromolar against 5 different HIV-1 strains (Table 3). The inhibition values for the disulfonates revealed structure activity relationships for substitution on the pyrazolinone and thiazole rings. First, incorporation of the bulky, electron rich phenyl group at the 5-position of the pyrazolinone ring compared to a methyl group markedly enhanced HIV inhibitory activity (compare compounds 11a vs 11d, 11b vs 11e, and 11c vs 11f, Table 3). In contrast, the presence of a small electron-withdrawing group, −CF3, at this position led to an order of magnitude decrease in potency (11m vs 11d). Second, the data indicate that inhibitory activity is enhanced by aromatic ring substitution on the thiazole ring and further enhanced by a factor of two by the presence of hydrogen-bond acceptor or electron-donating group, such as −OMe or −NMe2 in the para position (11a vs 11c; 11d vs 11f or 11j).
Table 2.
HIV neutralization activity of trisulfonaphthyl moleculesa
 | |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | IC50 (μM) | ||
| YU2 | JRCSF | HxB2 | |||
| 9a | Me | Ph | NAb | 47.6 | 36.8 |
| 9b | Me | 4-CF3-Ph | 91.9 | 26.1 | 19.7 |
| 9c | Me | 4-OMe-Ph | 47.6 | 27.0 | 22.5 |
| 9d | Ph | Ph | 148.1 | 35.5 | 29.3 |
| 9e | Ph | 4-OMe-Ph | 80.8 | 32.3 | 18.7 |
| 9f | Ph | 4-CF3-Ph | 80.6 | 30.8 | 28.9 |
HIV-1 strains include CCR5-tropic strains YU2 and JRCSF and CXCR4-tropic strain HxB2.
NA, not active at concentrations as high as 400 μM.
Table 3.
HIV neutralization activity of trisulfonaphthyl moleculesa
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | IC50 (μM) | ||||
| YU2 | JRCSF | 89.6 | HxB2 | NL4–3 | |||
| 11a | Me |  |
53.8 | 22.1 | 9.2 | 8.1 | 7.0 |
| 11b | Me | CF3 | NAb | 83.3 | 79.7 | NA | 80.1 |
| 11c | Me | Ph | 137.4 | 64.8 | 100.9 | 51.1 | 33.1 |
| 11d | Ph |  |
24.7 | 10.2 | 3.2 | 7.5 | 5.8 |
| 11e | Ph | CF3 | 27.9 | 5.8 | 2.4 | 6.8 | 6.3 |
| 11f | Ph | Ph | 43.7 | 30.7 | 8.4 | 3.6 | 7.1 |
| 11g | Ph |  |
30.7 | 9.9 | 2.9 | 2.5 | 3.6 |
| 11h |  |
74.9 | 36.2 | 22.3 | 10.1 | 15.6 | |
| 11i | Ph |  |
22.3 | 14.9 | 8.9 | 5.2 | 7.7 |
| 11j | Ph | 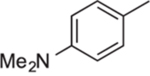 |
23.8 | 8.0 | 3.8 | 1.1 | 2.9 |
| 11k | Ph |  |
30.9 | 19.5 | 6.7 | 12.0 | 10.2 |
| 11l | Ph |  |
52.8 | 29.8 | 14.8 | 14.2 | 10.7 |
| 11m | CF3 |  |
297.7 | NAb | NA | 80.0 | 103.7 |
| 11n |  |
 |
62.4 | 24.1 | 14.6 | 9.4 | 11.2 |
HIV-1 strains include CCR5-tropic strains YU2 and JRCSF, dual CXCR4/CCR5-tropic strain 89.6 and CXCR4-tropic strain HxB2.
NA, not active at concentrations as high as 400 μM.
3. Hydrazonopyrazolinones have the Z configuration
Prior to docking the inhibitors to gp120, it was necessary to determine the configuration about the hydrazono pyrazolinone double bond. Using standard 2D 1H–1H and 1H–13C correlations experiments (COSY, HSQC, HMBC), we assigned all proton and carbon resonances for compound 2 summarized in Table S1 (Supporting information). In spectra recorded in DMSO-d6, the hydrazine NH appeared as a singlet at 13.1 ppm. We assigned the Z configuration to the double bond on the basis of the following observations: (i) in both NOESY and ROESY spectra the hydrazine NH singlet showed correlations only to H-3 of the phenylsulfonate ring; correlations to the C-7 methyl group were never observed. (ii) The large downfield shift (δH 13.1) for the NH proton suggested it was making a hydrogen bond, which is only possible with the Z configuration. (iii) The ROESY and NOESY spectra for compound 1 containing a phenyl rather than methyl pyrazolinone showed no NOEs between the NH and phenyl group protons. This is in agreement with a crystallographic study that showed ethyl 2-[(4-chlorophenyl)hydrazono]3-oxobutanoate to possess the Z configuration with an intramolecular NH⋯O hydrogen bond.27
4. Molecular docking
Neutralization data showed clear differences in HIV-1 inhibitory activity among various scaffolds. To help explain the observed SAR, we carried out molecular docking experiments on each of the diand tri-sulfonates binding to YU2 gp120. Because binding of sulfated tyrosine-containing peptides or antibodies to the coreceptor binding site on gp120 requires CD4-activation, we used the coordinates of YU2 gp120 taken from a ternary complex of gp120–CD4–mAb 412d (PDB entry: 2QAD). Naphthalene sulfonates were subjected to a long-term docking protocol using the program Autodock4 to give an ensemble of 100 docked models for inhibitors 9a–f and 11a–m. All of the disulfonate and trisulfonate naphthalenes docked in the CCR5 Nt binding site on gp120 making hydrophobic interactions and/or hydrogen bonds with residues Arg298 to Thr303 comprising the base of the V3 loop, and Pro438 to Gln442 (Fig. 2, SI Fig. S1), and demonstrated respectable binding energies ranging from −8.8 to −12.6 kcal/mol (Autodock). However, in all cases the disulfonates and trisulfonates docked to gp120 in opposite orientations relative to one another (SI Fig. S1). As shown in Figure 2a, disulfonate 11j binds directly inside the base of the V3 loop on gp120 where the C-3 sulfonate group closely overlaps with the tyrosine-sulfate group of sulfated monoclonal antibody 412d. The two sulfonate groups on 11j are positioned to form 9 hydrogen bonds (residues Arg298, Pro299, Asn300, Asn302, Thr202, Arg440 and Gly441) within the binding pocket anchoring the inhibitor in the coreceptor binding site (Fig. 2c). By comparison, trisulfonate 9c binds gp120 in the opposite orientation leading to fewer hydrogen bonds and intermolecular van der Waals interactions (Figs. 2C and S1 in Supporting information). The models suggest that this orientation occurs because of the close proximity of the sulfonate and amino groups on the naphthyl ring. In particular, the presence of the C-1 sulfonate and C-8 amino group on the same face of the naphthyl ring prevents the trisulfonates from docking in the same orientation as the disulfonates, forcing the trisulfonate inhibitors to orient to the back side of gp120 (as viewed in Fig. S1, Supporting information). The docked models and predicted inhibition constants are consistent with the HIV neutralization results where the trisulfonyl naphthalenes are weaker inhibitors than the disulfonyl naphthalenes.
Figure 2.

Molecular docking of tyrosine sulfate mimetics to gp120 CCR5 co-receptor binding site. (A) Rendering of the docked model of compound 11j bound to the co-crystal structure of YU2 gp120–mAb 412d. gp120 and its V3 loop are shown respectively as gray and orange surfaces, sulfated mAb 412d is shown as a blue ribbon, and compound 11j is shown in red sticks. The C-1 sulfonate on 11j overlaps with the tyrosine sulfate group of mAb 412d via docking in the CCR5 Nt binding site. Key contact residues of gp120 are labeled and shown as sticks. (B) Superimposed models of 11j (red) and 9c (blue) docked to gp120 (412d coordinates removed) showing the distinct binding modes of the di- versus tri-sulfonate inhibitors. (C) Ligand interaction map of 11j (red) and 9c (blue) showing interactions with gp120 residues (gray circles). The ensembles of all docked compounds are shown in Supporting information, Figure S1.
5. Small molecules bind gp120 and are competitive inhibitors to sulfated CCR5 Nt
To test the mode of inhibition of the naphthyl sulfonates we carried out combination assays using HIV-1 infection assays. In these experiments viruses were treated with inhibitor alone (11d, 11i, 11j selected for potency and solubility, and CCR5 Nt sulfated peptide), or a constant ratio combination of inhibitor to sulfated peptide at two-fold serial dilutions starting around 30 times their IC50 values (Tables 2 and 3). The inhibition data were quantitatively analyzed using the formalism of Chou and Talalay where combination indices (CI) equal to, greater than, or less than 1 are indicative of additive, antagonistic, and synergistic effects, respectively.27,28 Summarized in Table 4, the CIs for each of the inhibitors in combination with CCR5 Nt range from 1.9 to 2.7, corresponding to antagonistic inhibition. This indicates that the small molecules are binding at or very near to the CCR5 Nt binding site. To provide additional evidence that the small molecules are acting at the level of HIV-1 entry, we used surface Plasmon resonance (SPR) to test for binding to trimeric gp120. As seen in Figure S2 (SI), representative inhibitors 11d and 11f bind to immobilized gp120 with respective Kd values of 80 and 350 μM. Finally we tested several of the more potent compounds of each structural type for cytotoxicity (Table 5) against two mammalian cell lines. While no cellular toxicity was observed for compounds 1, 9e and 11j at concentrations as high as 500 micromolar, compound 11i was toxic at low micromolar concentrations. The remaining compounds varied in toxicity with effects observed between 200 and 500 micromolar.
Table 4.
Combination effects of disulfonate inhibitors and sulfated CCR5 Nt(2–18)
| Inhibitor | Combination ratioa |
Dose reduction index (DRI) |
Combination index (CI)b | |
|---|---|---|---|---|
| Inhibitor: Nt(2–18) |
Inhibitor | Nt(2–18) | ||
| 11d | 1:10 | 1.3 ± 0.4 | 1.3 ± 0.4 | 2.3 |
| 11i | 1:10 | 1.2 ± 0.4 | 1.7 ± 0.5 | 1.9 |
| 11j | 1:10 | 1.2 ± 0.4 | 1.1 ± 0.3 | 2.5 |
The ratio of inhibitor concentrations approximates the ratio of the IC50 values of the inhibitors alone.
The CI represents the combined effect of two inhibitors in combination where CI values <1, 1 and >1 correspond to synergistic, additive and antagonistic effects, respectively.
Table 5.
Effects of inhibitors on target cells
| Inhibitor | LC50a,b (μM) | TId |
|---|---|---|
| 9a | 200 | <10 |
| 9e | Non-toxicc | >30–60 |
| 11a | Non-toxic | >30–60 |
| 11d | 200 | 10–60 |
| 11e | 370 | 13–150 |
| 11g | Non-toxic | 16–170 |
| 11i | 70 | <10 |
| 11j | 330 | 13–300 |
Effects on host cell line (TZM-bl).
Standard deviations averaged 15%.
Non-toxic at 500 μM.
Range takes into account differing potencies toward various strains.
6. Discussion and conclusions
In summary, we have generated a series of naphthyl sulfonic acids that inhibit HIV-1 entry. The inhibitors were designed to bind to the viral envelope protein gp120 via the CCR5 Nt binding site. Gratifyingly functional neutralization assays showed that the small molecules are competitive inhibitors to the natural sulfated CCR5 Nt peptide, consistent with the notion that they bind to the core-ceptor-binding site. Several other noteworthy findings were made. Molecular docking supported small molecule binding at the coreceptor binding site and revealed two distinct binding orientations that depend on the number and arrangement of sulfonate moieties on the naphthyl ring, and the position of the amino group used to install the naphthyl group. In light of the docking results it is important to point out that although two binding orientations are observed, the same primary binding site accommodates the naphthyl group present in both structural types. We also found that the naphthyl sulfonates inhibit not only CCR5- but also CXCR4-using viruses. Though the mechanisms for inhibiting R5 and X4 viruses at the coreceptor binding site are not known, there is precedent for broad activity at this site. For example antibody-peptide chimeras containing CCR5-derived sequences have been shown to inhibit large panels of viruses of different tropisms (i.e., X4, R5 and X4R5).28 Although there is little homology between the N-terminal sequences of CCR5 and CXCR4, both contain sulfotyrosine modifications. In addition, the coreceptor binding site is among the most conserved regions on gp120, yet it must accommodate diverse virus strains in order to survive changes in cellular tropism that occur early in the course of HIV infection.29,30 It is possible that the coreceptor binding sites present conserved features capable of recognizing specific chemical moieties such as a sulfate group. Finally we note that due to the composition of the original hits obtained from our in silico screen (that were triaged using a gp120 binding assay25) the inhibitors contain potentially undesirable moieties such as the keto pyrazole unit. Nevertheless we found that many of the analogs in this study are non-toxic to normal cell lines at millimolar concentrations and do not inhibit infection by amphotropic viruses such as murine leukemia virus and vesicular stomatitis virus. This outlines areas for further improvement of the small molecules.
7. Experimental methods
7.1. General methods
All chemical reagents and organic solvents were purchased from Sigma–Aldrich (St. Louis, MO). All reactions were performed under a N2 stream in flame-dried flasks using SureSeal™ anhydrous solvents. 1H and 13C NMR spectra were recorded on 400 or 500 MHz NMR spectrometers (1H/13C at 400/101 MHz or 500/126 MHz) at 298 K (Bruker Biospin, Switzerland). Chemical shifts (d) were reported in parts per million (ppm) relative to the internal standard TMS. 3JH–H values are reported in Hz with 1H multiplicities abbreviated as follows: s, singlet; d, doublet; dd, double doublet; t, triplet; q, quadruplet; m, multiplet, br s, broad singlet. ROESY, NOESY and 1D difference NOE spectra were recorded with mixing times of 200, 400 and 400 ms, respectively. HRESI-MS data were measured on a Waters LCT Premier time-of-flight (TOF) mass spectrometer. Purity of all final derivatives for biological testing was confirmed to be >95% as determined by HPLC with MS detection using an Agilent LC–ESIMS instrument with diode array detection (230, 254, 280 nm) and a Waters RP-C18 column compounds were eluted with a linear gradient of 10–60% acetonitrile in 0.01% TFA in water in 40 min at a flow rate of 0.5 mL min−1, with the column maintained at 25 °C. MS detection further confirmed peak identity.
7.1.1. General procedure for the synthesis of 3-(substituted)-5-oxo-4-(2-(substituted)-hydrazono)-4,5-dihydro-1H-pyrazole-1-carbothioamides 3, 5, 8a,b and 10a–c
HBF4 (48 wt % solution in H2O, 11.00 mL) was added to cold aryl aniline (11.55 mmol). After stirring of the sample for 1 h at 0 °C, NaNO2 (1.2 g in 3 mL H2O) was added drop wise to the reaction mixture, followed by 10 min of stirring at room temperature. The precipitate was collected by filtration to afford the sulfonic acid aryldiazonium tetrafluoroborate salts. NaOAc (2.84 g, 34.65 mmol) was added to a solution of the desired 3-oxo-3-substituted ethylacetate (11.55 mmol) in 1:1 DMF/H2O (20 mL) and the mixture stirred at room temperature for 1 h. After cooling to 0 °C the sulfonic acid tetrafluoroborate salt was added and this mixture was stirred at 0 °C for 10 min, then allowed to warm to room temperature for a period of 3 h with stirring. The mixture was extracted with EtOAc and the aqueous layer was dried in vacuo to yield arylhydrazono sulfonic acid intermediates. Without further purification an arylhydrazono sulfonic acid intermediate (1.59 mmol) and thiosemicarbazide (0.29 g, 3.182 mmol) were suspended in 10 mL glacial acetic acid and heated to 70 °C for 4 h. The reaction was allowed to cool to room temperature. The precipitate formed was filtered, rinsed with AcOH, and dried in vacuo to yield the corresponding thiocarboxamido hydrazono pyrazolinone aryl sulfonic acid.
7.1.2. General procedure for the synthesis of sulfoaryl hydrazonothiazolyl-pyrazolinones (4a–f, 6, 9a–f, 11a–m)
The desired acetyl bromide (25.4 μmol), thioamide intermediate (2.1 μmol), and a catalytic amount of tetrabutyl ammonium iodide (0.78 mg, 0.21 μmol) were dissolved in DMF (1.5 mL) and stirred for 5 h at room temperature. Each reaction mixture was concentrated in vacuo and purified by preparative HPLC (10–60% acetonitrile in 0.05% TFA–water in 40 min) to yield hydrazono substituted thiazolyl-pyrazolone aryl sulfonic acids (known compound 2, and compounds 4a–f, 6).
7.1.3. 4-(2-(1-Carbamothioyl-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)benzenesulfonic acid (3)
Yellow solid, 68% yield. 1H NMR (400 MHz, DMSO-d6) δH 7.72 (d, J = 8.5 Hz, 2H), 7.62 (d, J = 8.4 Hz, 2H), 2.29 (s, 3H). 13C NMR (101 MHz, DMSO) δC 176.8, 156.6, 149.6, 145.9, 141.1, 126.9, 126.6, 115.9, 11.6. HRMS (ES): m/z calculated for C11H12N5O4S2, (M−H)−, 342.0331 amu; found: 342.0335.
7.1.4. 4-(2-(1-(5-(2,5-Dimethoxyphenyl)thiazol-2-yl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)benzenesulfonic acid(4a)
Light yellow solid, 82% yield. IR (film) 3414, 2949, 2836, 1648, 1450, 1229, 1120, 1031, 715 cm−1. 1H NMR (400 MHz, DMSO-d6) δH 7.93 (s, 1H), 7.77 (d, J = 3.2 Hz, 1H), 7.72 (d, J = 8.7 Hz, 2H), 7.66 (d, J = 8.5 Hz, 2H), 7.11 (d, J = 8.9 Hz, 1H), 6.95 (dd, J = 9.0, 3.2 Hz, 1H), 3.91 (s, 3H), 3.80 (s, 3H), 2.40 (s, 3H). 13C NMR (101 MHz, DMSO) δC 155.0, 153.3, 153.1, 151.1, 150.9, 146.1, 145.8, 126.9, 126.5, 122.9, 115.8, 114.9, 113.5, 113.0, 112.7, 55.9, 55.5, 11.7. HRMS (ES): m/z calculated for C21H18N5O6S2, (M−H)−, 500.0699 amu; found: 500.0708.
7.1.5. 4-(2-(1-(5-(3,4-Dihydroxyphenyl)thiazol-2-yl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)benzenesulfonic acid (4b)
Light yellow solid, 77% yield. 1H NMR (400 MHz, DMSO-d6) δH 7.72 (d, J = 8.7 Hz, 2H), 7.66 (d, J = 8.6 Hz, 2H), 7.54 (s, 1H), 7.44 (d, J = 2.1 Hz, 1H), 7.27 (dd, J = 8.2, 2.2 Hz, 1H), 6.82 (d, J = 8.2 Hz, 1H), 2.40 (s, 3H). 13C NMR (101 MHz, DMSO) δC 154.8, 153.9, 150.5, 146.0, 145.5, 145.2, 136.4, 126.9, 125.7, 117.2, 115.8, 113.5, 105.9, 11.7. HRMS (ES): m/z calculated for C19H14N5O6S2, (M−H)−, 472.0386 amu; found: 472.0389.
7.1.6. 4-(2-(3-Methyl-5-oxo-1-(5-(4-(trifluoromethyl)phenyl)thiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)benzenesulfonic acid (4c)
Light yellow solid, 64% yield. 1H NMR (400 MHz, DMSO-d6) δH 8.20 (d, J = 8.1 Hz, 2H), 8.06 (d, J = 2.6 Hz, 1H), 7.84 (d, J = 8.2 Hz, 2H), 7.72 (d, J = 8.4 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H), 2.39 (s, 3H). 13C NMR (101 MHz, DMSO) δC 155.0, 151.4, 148.4, 146.1, 141.2, 137.6, 126.9, 126.4, 125.7, 115.9, 111.5, 11.7. HRMS (ES): m/z calculated for C20H13N5O4S2F3, (M−H)−, 508.0361 amu; found: 508.0354.
7.1.7. 4-(2-(3-Methyl-5-oxo-1-(5-(4-(trifluoromethoxy)phenyl)thiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)benzenesulfonic acid (4d)
Light yellow solid, 56% yield. 1H NMR (400 MHz, DMSO-d6) δH 8.10 (d, J = 8.3 Hz, 2H), 7.92 (s, 1H), 7.73 (d, J = 8.3 Hz, 2H), 7.67 (d, J = 8.3 Hz, 2H), 7.47 (d, J = 8.3 Hz, 2H), 2.39 (s, 3H). 13C NMR (101 MHz, DMSO) δC 169.0, 168.9, 158.2, 158.1, 157.9, 155.1, 154.9, 152, 148.6, 147.9, 145.8, 141.2, 133.2, 127.7, 126.9, 121.3, 115.9, 110, 11.7. HRMS (ES): m/z calculated for C20H13N5O5S2F3, (M−H)−, 524.0310 amu; found: 524.0315.
7.1.8. 4-(2-(1-(5-(3-Chlorophenyl)thiazol-2-yl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)benzenesulfonic acid (4e)
Light yellow solid, 79% yield. 1H NMR (400 MHz, DMSO-d6) δH 8.02 (d, J = 15.6 Hz, 2H), 7.96 (d, J = 7.7 Hz, 1H), 7.72 (d, J = 8.5 Hz, 2H), 7.67 (d, J = 8.4 Hz, 2H), 7.52 (t, J = 7.9 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 2.40 (s, 3H). 13C NMR (126 MHz, DMSO) δC 155.1, 154.9, 151.4, 148.4, 146.1, 141.3, 135.9, 133.6, 130.7, 127.8, 126.9, 126.4, 125.5, 124.4, 115.9, 110.5, 11.8. HRMS (ES): m/z calculated for C20H17N5O3S2Cl, (M−H)−, 474.0461 amu; found: 474.0452.
7.1.9. 4-(2-(3-Methyl-5-oxo-1-(5-(4-sulfophenyl)thiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)benzenesulfonic acid (4f)
Light yellow solid, 65% yield. 1H NMR (400 MHz, DMSO-d6) δH 7.97 (d, J = 7.9 Hz, 2H), 7.91 (s, 1H), 7.75–7.64 (m, 6H), 2.41 (s, 3H). 13C NMR (126 MHz, DMSO) δC 155.1, 154.6, 151.2, 149.6, 147.8, 146.1, 141.2, 133.8, 126.9, 126.4, 125.9, 125.1, 115.8, 109.3, 11.7. HRMS (ES): m/z calculated for C19H14N5O7S3, (M−H)−, 520.0055 amu; found: 520.0048.
7.1.10. (4-(2-(1-Carbamothioyl-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)phenyl)methanesulfonic acid (5)
Light yellow solid, 62% yield. 1H NMR (400 MHz, DMSO-d6) δH 7.52 (d, J = 8.1 Hz, 1H), 7.43 (d, J = 8.4 Hz, 1H), 7.41–7.35 (m, 2H), 3.79 (s, 2H), 2.18 (d, J = 5.0 Hz, 3H). 13C NMR (126 MHz, DMSO) δC 180.3, 153.1, 133.3, 119.3, 57.3, 28.9, 28.5. HRMS (ES): m/z calculated for C12H13N5O4S2, (M−H)−, 356.0484 amu; found: 356.0487.
7.1.11. (4-(2-(1-(5-(4-Methoxyphenyl)thiazol-2-yl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)phenyl)methanesulfonic acid (6)
Light yellow solid, 76% yield. IR (film) 3456, 1645, 1490, 1178, 1045 cm−1. 1H NMR (400 MHz, DMSO-d6) δH 7.92 (d, J = 8.3 Hz, 2H), 7.69 (d, J = 2.4 Hz, 1H), 7.60 (d, J = 8.0 Hz, 2H), 7.42 (d, J = 7.9 Hz, 4H), 7.04 (d, J = 8.2 Hz, 2H), 3.82 (s, 3H), 2.39 (s, 3H). 13C NMR (126 MHz, DMSO) δC 159.1, 155.2, 154.5, 151, 150, 139.7, 134.1, 131.3, 131.3, 127.2, 126.8, 125.7, 116, 115.7, 114.1, 106.8, 57, 55.2, 11.6. HRMS (ES): m/z calculated for C21H19N5O5S2, (M−H)−, 484.0749 amu; found: 484.0754.
7.1.12. 8-(2-(1-Ethoxy-1,3-dioxobutan-2-ylidene) hydrazinyl)naphthalene-1,3,6-trisulfonic acid (7a)
Light yellow solid, 65% yield. 1H NMR (500 MHz, DMSO-d6) δH 13.63 (s, 1H), 8.06 (d, J = 1.7 Hz, 2H), 7.86 (d, J = 1.4 Hz, 1H), 4.30 (dd, J = 1.3, 7.2 Hz, 2H), 2.39 (d, J = 1.1 Hz, 3H), 1.27 (td, J = 1.2, 7.2 Hz, 3H). 13C NMR (126 MHz, DMSO) δC 193.4, 162.4, 146, 144.1, 141.5, 138.1, 134.5, 127.2, 125.5, 121.2, 113.1, 60.9, 24.3, 13.6. HRMS (ES): m/z calculated for C16H16N2O12S3, (M−H)−, 522.9787 amu; found: 522.9783.
7.1.13. 8-(2-(1-Ethoxy-1,3-dioxo-3-phenylpropan-2-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (7b)
Yellow solid, 72% yield. 1H NMR (500 MHz, DMSO-d6) δH 13.70 (s, 1H), 8.07 (t, J = 1.5 Hz, 1H), 7.99–7.93 (m, 4H), 7.86 (dt, J = 1.7, 10.7 Hz, 2H), 7.50 (t, J = 7.6 Hz, 3H), 4.36 (dd, J = 1.4, 7.2 Hz, 2H), 1.31 (td, J = 1.4, 7.2 Hz, 3H). 13C NMR (126 MHz, DMSO) δC 188.1, 174.2, 162.6, 162.2, 146.1, 141.5, 138.2, 134.5, 133.24, 131.9, 129.8, 128.6, 128, 127.1, 125.6, 121.5, 119, 114.2, 61.1, 24.5. HRMS (ES): m/z calculated for C21H18N2O12S3, (M−H)−, 584.9944 amu; found: 584.9955.
7.1.14. 8-(2-(1-Carbamothioyl-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (8a)
Light orange solid, 45% yield. 1H NMR (400 MHz, DMSO-d6) δH 8.57 (d, J = 1.8 Hz, 1H), 8.27 (d, J = 2.3 Hz, 1H), 8.23 (d, J = 1.9 Hz, 1H), 8.00 (d, J = 1.8 Hz, 1H), 2.29 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δC 178.1, 155.2, 150.7, 144.8, 141.1, 137.9, 134.2, 127.1, 126.1, 125.4, 119.8, 12. HRMS (ES): m/z calculated for C15H13N5O10S4, (M−H)−, 549.9467 amu; found: 549.9459.
7.1.15. 8-(2-(1-Carbamothioyl-5-oxo-3-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (8b)
Yellow solid, 52% yield. 1H NMR (500 MHz, DMSO-d6) δH 15.26 (s, 1H), 9.51 (s, 1H), 9.31 (s, 1H), 8.19 (d, J = 15.8 Hz, 3H), 8.15–8.09 (m, 3H), 7.96 (s, 1H), 7.48 (d, J = 6.1 Hz, 4H). 13C NMR (126 MHz, DMSO-d6) δC 177.7, 155.3, 148.1, 146.1, 144.8, 141, 138.1, 134.2, 130, 129.9, 126.4, 127.2, 126.2, 125.8, 120.7, 30.9, 30.6. HRMS (ES): m/z calculated for C20H15N5O10S4, (M−H)−, 611.9624 amu; found: 611.9618.
7.1.16. 8-(2-(3-Methyl-5-oxo-1-(4-phenylthiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (9a)
Light orange solid, 72% yield. IR (film) 3454, 3204, 1544, 1444, 1177, 1103, 1043, 677, 454 cm−1. 1H NMR (400 MHz, DMSO-d6) δH 8.57 (s, 1H), 8.27 (d, J = 1.9 Hz, 1H), 8.22 (d, J = 1.7 Hz, 1H), 8.05–7.98 (m, 3H), 7.84 (d, J = 3.7 Hz, 1H), 7.49 (t, J = 7.6 Hz, 2H), 7.38 (t, J = 7.3 Hz, 1H), 2.39 (s, 3H). 13C NMR (101 MHz, DMSO) δC 154.8, 153.6, 151.7, 149.8, 145.8, 144.6, 141.1, 138.1, 134.3, 134.1, 128.6, 127.8, 127.3, 127.1, 126.1, 125.9, 125.3, 120.7, 119.7, 108.5, 99.5, 12.1. HRMS (ES): m/z calculated for C23H16N5O10S4, (M−H)−, 649.9780 amu; found: 649.9786.
7.1.17. 8-(2-(3-Methyl-5-oxo-1-(4-(4-(trifluoromethyl)phenyl)thiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (9b)
Light orange solid, 69% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.57 (s, 1H), 8.27 (s, 1H), 8.24 (d, J = 8.9 Hz, 3H), 8.07 (d, J = 6.6 Hz, 1H), 8.03 (s, 1H), 7.85 (d, J = 8.2 Hz, 2H), 2.39 (s, 3H). 13C NMR (126 MHz, DMSO) δC 155.1, 153.6, 152.1, 148.2, 145.8, 144.6, 141.1, 138.1, 137.8, 134.3, 128.1, 127.7, 127.3, 127, 126.5, 126.1, 125.7, 125.4, 123.2, 120.7, 119.7, 111.2, 12.2. HRMS (ES): m/z calculated for C24H15N5O10F3S4, (M−H)−, 717.9654 amu; found: 717.9645.
7.1.18. 8-(2-(1-(4-(4-Methoxyphenyl)thiazol-2-yl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (9c)
Light orange solid, 85% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.56 (s, 1H), 8.22 (d, J = 22.0 Hz, 2H), 8.01 (s, 1H), 7.95 (d, J = 8.3 Hz, 2H), 7.67 (s, 1H), 7.05 (d, J = 8.5 Hz, 2H), 3.84 (s, 3H), 2.38 (s, 3H). 13C NMR (126 MHz, DMSO) δC 154.3, 153.3, 149.4, 144.4, 137.8, 134.1, 127, 126.8, 126.7, 125.8, 125.1, 120.4, 119.4, 113.7, 106.2, 54.8, 11.9. HRMS (ES): m/z calculated for C24H18N5O11S4, (M−H)−, 679.9886 amu; found: 679.9898.
7.1.19. 8-(2-(5-Oxo-3-phenyl-1-(4-phenylthiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (9d)
Orange solid, 81% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.58 (s, 1H), 8.28 (s, 1H), 8.24 (s, 1H), 8.20 (dd, J = 6.5, 3.1 Hz, 2H), 8.06 (d, J = 7.7 Hz, 2H), 8.03 (s, 1H), 7.92 (d, J = 3.3 Hz, 1H), 7.60–7.54 (m, 3H), 7.52 (t, J = 7.7 Hz, 2H), 7.40 (t, J = 7.5 Hz, 1H). 13C NMR (126 MHz, DMSO) δC 154.5, 153.6, 149.7, 148.9, 145.7, 144.5, 140.8, 137.9, 134.1, 133.8, 129.8, 128.4, 128.4, 127.6, 127.3, 127.1, 125.9, 125.7, 125.2, 120.5, 120.1, 108.9. HRMS (ES): m/z calculated for C28H18N5O10S4, (M−H)−, 711.9937 amu; found: 711.9940.
7.1.20. 8-(2-(1-(4-(4-Methoxyphenyl)thiazol-2-yl)-5-oxo-3-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (9e)
Light yellow oil, 86% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.58 (d, J = 4.2 Hz, 1H), 8.28 (d, J = 3.9 Hz, 1H), 8.24 (d, J = 4.1 Hz, 1H), 8.20 (q, J = 4.1 Hz, 2H), 8.03 (d, J = 3.9 Hz, 1H), 7.99 (dd, J = 8.7, 3.2 Hz, 2H), 7.73 (d, J = 3.1 Hz, 1H), 7.59–7.51 (m, 3H), 7.07 (dd, J = 8.7, 3.4 Hz, 2H), 3.84 (d, J = 3.3 Hz, 3H). 13C NMR (126 MHz, DMSO) δC 158.8, 154.4, 153.5, 149.6, 148.8, 145.7, 144.5, 140.9, 137.9, 134.1, 129.8, 129.7, 128.4, 127.3, 127.1, 126.7, 125.9, 125.7, 125.2, 120.5, 120.1, 113.8, 106.8, 54.8. HRMS (ES): m/z calculated for C29H20N5O11S4, (M−H)−, 742.0042 amu; found: 742.0029.
7.1.21. 8-(2-(5-Oxo-3-phenyl-1-(4-(4-(trifluoromethyl)phenyl)thiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3,6-trisulfonic acid (9f)
Yellow solid, 63% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.32–8.26 (m, 3H), 8.25 (d, J = 1.8 Hz, 1H), 8.20 (dd, J = 6.8, 3.0 Hz, 2H), 8.13 (s, 1H), 8.03 (d, J = 1.9 Hz, 1H), 7.87 (d, J = 8.2 Hz, 2H), 7.57 (q, J = 3.2, 2.5 Hz, 4H). 13C NMR (126 MHz, DMSO) δC 154.9, 153.6, 149.1, 148.1, 145.8, 144.5, 140.8, 137.9, 137.5, 134.1, 129.8, 129.7, 128.4, 127.8, 127.5, 127.4, 127.1, 126.3, 125.9, 125.6, 125.4, 125.3, 123.1, 120.5, 120.2, 114.2, 111.5. HRMS (ES): m/z calculated for C29H17N5O10F3S4, (M−H)−, 779.9810 amu; found: 779.9811.
7.1.22. 6-(2-(1-Carbamothioyl-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (10a)
Yellow solid, 48% yield. 1H NMR (400 MHz, DMSO-d6) δH 8.89 (d, J = 9.3 Hz, 1H), 8.23 (d, J = 13.3 Hz, 3H), 7.95 (d, J = 9.3 Hz, 1H), 2.35 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δC 177.1, 145.4, 144, 133.2, 129.2, 127.1, 124.9, 122.7, 35.2. HRMS (ES): m/z calculated for C15H13N5O7S3, (M−H)−, 469.9899 amu; found: 469.9897.
7.1.23. 6-(2-(1-Carbamothioyl-5-oxo-3-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (10b)
Orange solid, 46% yield. 1H NMR (400 MHz, DMSO-d6) δH 8.93 (d, J = 9.3 Hz, 1H), 8.30 (d, J = 7.5 Hz, 2H), 8.26–8.15 (m, 3H), 7.96 (d, J = 9.2 Hz, 1H), 7.63 (dt, J = 17.2, 7.3 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δC 176.5, 157.1, 146.4, 145.5, 144.1, 133.2, 130.3, 129.5, 128.7, 127.7, 127.2, 125.5, 125.1, 122.8, 115.6, 115. HRMS (ES): m/z calculated for C20H15N5O7S3, (M−H)−, 532.0055 amu; found: 532.0052.
7.1.24. 6-(2-(1-Carbamothioyl-3-(methoxymethyl)-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (10c)
Orange solid, 56% yield. 1H NMR (400 MHz, DMSO-d6) δH 8.89 (d, J = 9.5 Hz, 1H), 8.29–8.14 (m, 3H), 7.93 (d, J = 9.4 Hz, 1H), 4.58 (s, 2H), 3.47 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δC 177.2, 156.5, 148.9, 145.5, 144.1, 133.1, 129.3, 127.1, 125.3, 124.9, 122.8, 115.7, 114.9, 65.1, 58.3, 20.9. HRMS (ES): m/z calculated for C16H15N5O8S3, (M−H)−, 500.0005 amu; found: 500.0006.
7.1.25. 6-(2-(1-(4-(4-Methoxyphenyl)thiazol-2-yl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11a)
Light yellow solid, 81% yield. IR (film) 3439, 1641, 1509, 1183, 1039, 670 cm−1. 1H NMR (500 MHz, DMSO-d6) δH 8.90 (d, J = 9.3 Hz, 1H), 8.24 (s, 1H), 8.21 (s, 1H), 8.15 (d, J = 3.1 Hz, 1H), 7.95 (t, J = 11.1 Hz, 3H), 7.70 (s, 1H), 7.06 (d, J = 8.1 Hz, 2H), 3.84 (s, 3H), 2.46 (s, 3H). 13C NMR (126 MHz, DMSO) δC 159.3, 155.3, 154.8, 151.5, 150.1, 145.1, 143.8, 139.1, 133.5, 131.5, 129.3, 127.4, 127.2, 127, 126.6, 125.5, 122.7, 116.3, 114.7, 114.3, 107.1, 55.3, 11.9. HRMS (ES): m/z calculated for C24H18N5O8S3, (M−H)−, 600.0318 amu; found: 600.0315.
7.1.26. 6-(2-(3-Methyl-5-oxo-1-(4-(trifluoromethyl)thiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11b)
Pale yellow solid, 66% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.83 (d, J = 9.6 Hz, 1H), 8.24 (s, 2H), 8.16 (d, J = 10.3 Hz, 2H), 7.96 (d, J = 9.3 Hz, 1H), 2.37 (s, 3H). 13C NMR (126 MHz, DMSO) δC 157.5, 156.1, 152.2, 145.2, 143.9, 133.2, 129.1, 127.1, 125.2, 125.1, 123, 122.7, 116.1, 114.8, 101.1, 11.7. HRMS (ES): m/z calculated for C18H11N5O7F3S3, (M−H)−, 561.9773 amu; found: 561.9771.
7.1.27. 6-(2-(3-Methyl-5-oxo-1-(4-phenylthiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11c)
Yellow solid, 80% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.85 (t, J = 9.5 Hz, 1H), 8.27 (d, J = 8.9 Hz, 1H), 8.17 (t, J = 10.1 Hz, 2H), 8.00 (q, J = 8.4, 7.8 Hz, 3H), 7.90 (d, J = 8.9 Hz, 1H), 7.50 (dt, J = 14.8, 7.4 Hz, 2H), 7.39 (dt, J = 14.4, 7.2 Hz, 1H), 2.46 (s, 3H). 13C NMR (126 MHz, DMSO) δC 155.4, 154.9, 154.8, 151.8, 151.7, 150.3, 150.1, 145.1, 144.5, 143.8, 143.4, 139.3, 134.1, 134.1, 133.5, 129.3, 129.1, 129.1, 128.4, 127.2, 127.1, 126.7, 126.1, 125.9, 125.7, 122.8, 116.7, 116.4, 114.7, 109.2, 12.1. HRMS (ES): m/z calculated for C23H16N5O7S3, (M−H)−, 570.0212 amu; found: 570.0213.
7.1.28. 6-(2-(1-(4-(4-Methoxyphenyl)thiazol-2-yl)-5-oxo-3-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11d)
Yellow solid, 80% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.95 (d, J = 7.9 Hz, 1H), 8.34–8.25 (m, 3H), 8.22 (s, 1H), 8.18 (s, 1H), 8.03–7.94 (m, 3H), 7.78 (d, J = 2.9 Hz, 1H), 7.70 (dt, J = 7.5, 4.5 Hz, 2H), 7.66–7.59 (m, 1H), 7.12–7.04 (m, 2H), 3.85 (s, 3H). 13C NMR (126 MHz, DMSO) δC 158.9, 155.3, 154.3, 149.9, 148.1, 145.2, 143.8, 133, 130.1, 129.3, 128.7, 127.3, 127.1, 126.9, 126.5, 125.1, 124.9, 122.6, 120.3, 118.7, 115.5, 114.8, 114.2, 113.8, 107.2, 103.9, 54.9. HRMS (ES): m/z calculated for C29H20N5O8S3, (M−H)−, 662.0474 amu; found: 662.0475.
7.1.29. 6-(2-(5-Oxo-3-phenyl-1-(4-(trifluoromethyl)thiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11e)
Light orange solid, 69% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.98–8.87 (m, 1H), 8.30–8.11 (m, 4H), 7.97 (t, J = 9.2 Hz, 1H), 7.74–7.57 (m, 4H), 3.79 (t, J = 10.7 Hz, 2H). 13C NMR (126 MHz, DMSO) δC 157.8, 156.6, 149.3, 145.3, 144.1, 139.1, 133.2, 130.5, 129.4, 129.3, 128.9, 127.6, 127.2, 125.2, 124.6, 122.9, 122.8, 120.7, 115.9, 115.5, 101.4, 101.2, 100.9, 100.7, 38.4. HRMS (ES): m/z calculated for C23H15N5O8F3S3, (M−H)−, 624.0035 amu; found: 624.0048.
7.1.30. 6-(2-(5-Oxo-3-phenyl-1-(4-phenylthiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11f)
Light yellow solid, 79% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.95 (d, J = 9.3 Hz, 1H), 8.32 (d, J = 7.6 Hz, 2H), 8.28 (s, 1H), 8.23 (s, 1H), 8.19 (s, 1H), 8.05 (d, J = 7.7 Hz, 2H), 8.00 (d, J = 9.4 Hz, 1H), 7.95 (s, 1H), 7.71 (t, J = 7.6 Hz, 2H), 7.63 (t, J = 7.4 Hz, 1H), 7.52 (t, J = 7.6 Hz, 2H), 7.41 (t, J = 7.3 Hz, 1H). 13C NMR (126 MHz, DMSO) δC 155.6, 154.7, 150.2, 148.4, 145.4, 144.1, 133.9, 133.2, 130.4, 129.6, 129.5, 128.9, 128.8, 128.1, 127.6, 127.2, 125.9, 125.3, 125.2, 122.8, 115.9, 115.2, 109.5. HRMS (ES): m/z calculated for C28H18N5O7S3, (M−H)−, 632.0368 amu; found: 632.0379.
7.1.31. 6-(2-(1-(4-(2,5-Dimethoxyphenyl)thiazol-2-yl)-5-oxo-3-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene) hydrazinyl)naphthalene-1,3-disulfonic acid (11g)
Yellow solid, 86% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.95 (d, J = 9.3 Hz, 1H), 8.32 (d, J = 7.0 Hz, 2H), 8.27 (d, J = 2.3 Hz, 1H), 8.23 (d, J = 1.7 Hz, 1H), 8.19 (d, J = 1.7 Hz, 1H), 8.03–7.97 (m, 2H), 7.84 (d, J = 3.2 Hz, 1H), 7.70 (t, J = 7.6 Hz, 2H), 7.63 (dd, J = 8.5, 6.2 Hz, 1H), 7.12 (d, J = 9.0 Hz, 1H), 6.97 (dd, J = 8.9, 3.3 Hz, 1H), 3.93 (s, 3H), 3.82 (s, 3H). 13C NMR (126 MHz, DMSO) δC 155.6, 153.4, 153.1, 150.9, 148.2, 146.1, 145.3, 144, 138.9, 133.2, 130.4, 129.6, 129.5, 129.1, 127.6, 127.1, 125.4, 125.2, 122.9, 122.8, 115.9, 115.1, 113.6, 113.4, 112.7, 55.9, 55.5. HRMS (ES): m/z calculated for C30H22N5O9S3, (M−H)−, 692.0580 amu; found: 692.0594.
7.1.32. 6-(2-(3-(Methoxymethyl)-1-(4-(4-methoxyphenyl)thiazol-2-yl)-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11h)
Light orange solid, 81% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.90 (d, J = 9.3 Hz, 1H), 8.30–8.18 (m, 2H), 8.15 (s, 1H), 7.96 (dd, J = 12.3, 8.5 Hz, 3H), 7.73 (s, 1H), 7.06 (d, J = 8.4 Hz, 2H), 4.67 (s, 2H), 3.84 (s, 3H), 3.50 (s, 3H). 13C NMR (126 MHz, DMSO) δC 158.9, 154.7, 154.2, 149.9, 149.7, 145.1, 143.7, 138.7, 132.9, 129, 127.1, 126.9, 126.5, 124.8, 122.5, 115.7, 114.7, 114.2, 113.8, 106.9, 64.9, 58.1, 54.9. HRMS (ES): m/z calculated for C25H20N5O9S3, (M−H)−, 630.0423 amu; found: 630.0433.
7.1.33. 6-(2-(1-(4-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)thiazol-2-yl)-5-oxo-3-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11i)
Yellow solid, 84% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.95 (d, J = 9.3 Hz, 1H), 8.31 (d, J = 7.7 Hz, 2H), 8.27 (d, J = 2.1 Hz, 1H), 8.23 (s, 1H), 8.20 (s, 1H), 8.00 (dd, J = 9.3, 2.3 Hz, 1H), 7.79 (d, J = 2.2 Hz, 1H), 7.70 (t, J = 7.6 Hz, 2H), 7.63 (t, J = 7.3 Hz, 1H), 7.52 (d, J = 8.8 Hz, 2H), 6.97 (d, J = 8.3 Hz, 1H), 4.31 (s, 4H). 13C NMR (126 MHz, DMSO) δC 155.6, 154.5, 149.9, 148.3, 145.3, 144, 143.5, 138.8, 133.2, 130.4, 129.5, 129.1, 127.6, 127.5, 127.2, 125.4, 125.2, 122.8, 119.1, 117.34, 115.9, 115.1, 114.5, 107.9, 64.2, 64.1. HRMS (ES): m/z calculated for C30H20N5O9S3, (M−H)−, 690.0423 amu; found: 690.0410.
7.1.34. 6-(2-(1-(4-(4-(Dimethylamino)phenyl)thiazol-2-yl)-5-oxo-3-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11j)
Tan solid, 80% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.89–8.77 (m, 2H), 8.30–8.14 (m, 4H), 8.14–8.00 (m, 2H), 7.97–7.86 (m, 1H), 7.86–7.71 (m, 1H), 7.70–7.50 (m, 3H), 7.10–6.96 (m, 2H), 3.01 (s, 6H). 13C NMR (126 MHz, DMSO) δC 155.6, 154.4, 150.7, 148.2, 145.4, 144.1, 138.8, 133.2, 130.4, 129.5, 128.9, 127.6, 127.1, 126.9, 125.4, 125.1, 122.8, 115.8, 115.1, 57.4. HRMS (ES): m/z calculated for C30H23N6O7S3, (M−H)−, 675.0790 amu; found: 675.0782.
7.1.35. 6-(2-(1-([2,4′-Bithiazol]-2′-yl)-5-oxo-3-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11k)
Yellow solid, 73% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.95 (d, J = 9.3 Hz, 1H), 8.29 (d, J = 7.4 Hz, 3H), 8.24 (s, 1H), 8.20 (s, 1H), 8.08 (s, 1H), 8.01 (d, J = 9.4 Hz, 1H), 7.96 (d, J = 3.2 Hz, 1H), 7.82 (d, J = 3.4 Hz, 1H), 7.70 (t, J = 7.5 Hz, 2H), 7.63 (t, J = 7.3 Hz, 1H). 13C NMR (126 MHz, DMSO) δC 161.9, 155.7, 155.3, 148.8, 145.3, 144.5, 143.9, 143.9, 138.9, 133.2, 130.5, 129.5, 129.4, 129.1, 127.7, 127.2, 125.3, 125.2, 122.8, 120.9, 116, 115.3, 112.1. HRMS (ES): m/z calculated for C25H15N6O7S4, (M−H)−, 638.9885 amu; found: 638.9882.
7.1.36. 6-(2-(5-Oxo-3-phenyl-1-(4-(4-(trifluoromethoxy)phenyl)thiazol-2-yl)-1,5-dihydro-4H-pyrazol-4-ylidene) hydrazinyl)naphthalene-1,3-disulfonic acid (11l)
Light yellow oil, 62% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.95 (d, J = 9.3 Hz, 1H), 8.31 (d, J = 7.7 Hz, 2H), 8.28 (s, 1H), 8.24 (s, 1H), 8.20 (s, 1H), 8.16 (d, J = 8.4 Hz, 2H), 8.06–7.97 (m, 2H), 7.70 (t, J = 7.5 Hz, 2H), 7.63 (t, J = 7.3 Hz, 1H), 7.51 (d, J = 8.2 Hz, 2H). 13C NMR (126 MHz, DMSO) δC 155.6, 155.1, 148.7, 148.5, 147.9, 145.3, 144.1, 139.1, 133.2, 133.2, 130.4, 129.5, 129, 127.8, 127.6, 127.2, 125.3, 125.2, 122.8, 121.4, 115.9, 115.2, 110.5. HRMS (ES): m/z calculated for C29H17N5O8F3S3, (M−H)−, 716.0191 amu; found: 716.0208.
7.1.37. 6-(2-(1-(4-(4-Methoxyphenyl)thiazol-2-yl)-5-oxo-3-(trifluoromethyl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11m)
Light yellow viscous oil, 58% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.82 (d, J = 9.3 Hz, 1H), 8.21 (s, 1H), 8.16 (s, 1H), 8.14–8.06 (m, 1H), 7.95 (d, J = 8.4 Hz, 2H), 7.87 (d, J = 9.2 Hz, 1H), 7.63 (s, 1H), 7.05 (d, J = 8.5 Hz, 2H), 3.83 (s, 3H). 13C NMR (126 MHz, DMSO) δC 158.7, 154.4, 148.9, 143.6, 142.3, 133.1, 128.2, 127.7, 127, 126.9, 125.2, 122.5, 116.7, 114.2, 113.7, 106.8, 54.9. HRMS (ES): m/z calculated for C24H15N5O8F3S3, (M−H)−, 654.0035 amu; found: 654.0034.
7.1.38. 6-(2-(1-(4-(4-Methoxyphenyl)thiazol-2-yl)-5-oxo-3-(phenoxymethyl)-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)naphthalene-1,3-disulfonic acid (11n)
Yellow solid, 79% yield. 1H NMR (500 MHz, DMSO-d6) δH 8.85 (d, J = 9.2 Hz, 1H), 8.18 (d, J = 17.5 Hz, 2H), 8.11 (s, 1H), 7.99–7.91 (m, 2H), 7.89 (d, J = 9.5 Hz, 1H), 7.74 (s, 1H), 7.40 (t, J = 7.3 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 7.11–6.99 (m, 3H), 5.39 (s, 2H), 3.84 (d, J = 2.0 Hz, 3H). 13C NMR (126 MHz, DMSO) δC 158.9, 158, 154.2, 149.6, 145.1, 143.7, 132.9, 129.3, 128.9, 127.1, 126.9, 126.5, 124.8, 122.5, 121.3, 115.8, 114.8, 114.2, 113.8, 107.1, 61.6, 54.9. HRMS (ES): m/z calculated for C30H22N5O9S3, (M−H)−, 692.0580 amu; found: 692.0585.
8. HIV-1 neutralization assays
HIV envelope pseudotype viruses were prepared and single round neutralization assays performed as described previously.31,32 Briefly, 293T cells (American Type Culture Collection) were transfected with plasmid SG3denv and HIV-1 env expressing plasmid (NIH AIDS Reagent Program) using X-treme GENE HP DNA (Roche). The media was changed after 24 h and at 48 h post-transfection, the supernatant was collected, passed through a 0.45 μm filter and stored at −80 °C until further use in neutralization assays. Two-fold serial dilutions of the inhibitors were prepared in a 96-well plate after which pseudotyped virus was added followed by TZM-bl cells (NIH AIDS Reagent Program). The plates were incubated at 37 °C, 5% CO2, and fresh media added at 24 h. The cells were then lysed and the plates read for luciferase activity (Bright Glo, Promega) at 48 h post-infection.
9. Combination experiments
The effect of the small molecule CCR5 Nt mimetics, 11d and 11j in combination with synthetic, sulfated CCR5 N-terminal peptide 2–18 were investigated using the CCR5-tropic strain YU2 in single round neutralization assays. Combination effects were analyzed for their combination indices (CI) according to the method of Chou and Talalay.33,34 Briefly, the dose reduction index (DRI) of inhibitor x in combination with inhibitor y is given by DRIx = (IC50)x/(IC50)x,y where (IC50)x and (IC50)y are the IC50 values of x alone and in combination with y, respectively. The combination effect of the two inhibitors is determined by the equation CI = (DRI)x−1 + (DRI)y−1 + (DRI)x(DRI)y−1 , where the last term which makes a small contribution to CI accounts for the state at which both inhibitors are bound (4, 5). CI values equal to 1, less than 1, and greater than 1 correspond to additive, synergistic, and antagonistic modes of inhibition, respectively.
10. Molecular docking
The 3D structure of gp120 was extracted from the gp120– CD4–412d complex structure of 2QAD and hydrogen atoms were added via Molecular Operation Environment (MOE) 2008.10 (Chemical Computing Group, Montreal, Montreal, Canada; http://www.chemcomp.com/). The molecular structures of the small molecules were generated and subjected to energy minimization using the Merck Molecular Force Field 94 program available in MOE. The minimum conformation reached by the simulation was subjected to docking experiments with the program Autodock4 (The Scripps Research Institute, La Jolla, CA; http://autodock.scripps.edu) against gp120. Docking was carried out using genetic algorithm parameters (population size: 250; maximum number of evaluation: 50,000,000; maximum number of generations: 50,000) and the center of the protein structure as the grid center (100 × 100 × 100 Å) with 100 output structures. The docking mode and scores were evaluated by AutoDockTools 1.5.4 (The Scripps Research Institute, La Jolla, CA; http://autodock.scripps.edu) and figures were prepared using PyMOL (Schrödinger, LLC, New York, NY; http://schrodinger.com/); ligand–gp120 interaction maps were generated using the MOE software package.
Supplementary Material
Scheme 1.

Synthesis of phenyl and phenyl methylene sulfonic acids. Reagents and conditions: (a) thiosemicarbazide, AcOH, 70 °C, 4 h; (b) n-Bu4NI(cat), DMF; (c) (1) HBF4, H2O, 0 °C, 1 h; (2) NaNO2, H2O, 0 °C, 10 min; (d) NaOAc, DMF/H2O (1:1), 0 °C, 10 min.
Scheme 2.

Synthesis of di- and tri-sulfonaphthyl tyrosine sulfate mimetics. Reagents and conditions: (a) (1) HBF4, H2O, 0 °C, 1 h; (2) NaNO2, H2O, 0 °C, 10 min; (b) NaOAc, DMF/H2O (1:1), 0 °C, 10 min; (c) thiosemicarbazide, AcOH, 70 °C, 4 h; (d) n-Bu4NI(cat), DMF.
Acknowledgements
This work was supported by the NIH Intramural Research Program (NIDDK) and the Intramural AIDS Targeted Antiviral Program, Office of the Director, NIH (C.A.B.). C.D.-I. acknowledges an NIH Intramural AIDS Research Fellowship.
Abbreviations:
- CD4
cluster of differentiation 4
- CCR5
C–C chemokine receptor type 5
- CXCR4
C–X–C chemokine receptor type 4
- CCR5 Nt
CCR5 N-terminus
- CDRH3
complementarity determining region heavy 3
- HBF4
tetrafluoroboric acid
- DMSO
dimethylsulfoxide
Footnotes
Supplementary data
Supplementary data (additional NMR data and figures illustrating docked ensembles and constant ratio inhibition curves) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2016.02.044.
References and notes
- 1.Eckert DM; Kim PS Ann. Rev. Biochem 2001, 70, 777. [DOI] [PubMed] [Google Scholar]
- 2.Freed EO; Martin MA J. Biol. Chem 1995, 270, 23883. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y; Broder CC; Kennedy PE; Berger EA Science 1996, 272, 872.8629022 [Google Scholar]
- 4.Moore JP; Trkola A; Dragic T Curr. Opin. Immunol 1997, 9, 551. [DOI] [PubMed] [Google Scholar]
- 5.Trkola A; Dragic T; Arthos J; Binley JM; Olson WC; Allaway GP; ChengMayer C; Robinson J; Maddon PJ; Moore JP Nature 1996, 384, 184. [DOI] [PubMed] [Google Scholar]
- 6.Acharya P; Lusvarghi S; Bewley CA; Kwong PD Expert Opin. Ther. Targets 2015, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray N; Doms RW Curr. Top. Microbiol. Immunol 2006, 303, 97. [DOI] [PubMed] [Google Scholar]
- 8.Schols D Curr. Top. Med. Chem 2004, 4, 883. [DOI] [PubMed] [Google Scholar]
- 9.Lin PF; Blair W; Wang T; Spicer T; Guo Q; Zhou N; Gong YF; Wang HG; Rose R; Yamanaka G, et al. Proc. Natl. Acad. Sci. U.S.A 2003, 100, 11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q; Ma L; Jiang S; Lu H; Liu S; He Y; Strick N; Neamati N; Debnath AK Virology 2005, 339, 213. [DOI] [PubMed] [Google Scholar]
- 11.Courter JR; Madani N; Sodroski J; Schon A; Freire E; Kwong PD; Hendrickson WA; Chaiken IM; LaLonde JM; Smith AB 3rd Acc. Chem. Res 2014, 47, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T; Zhang Z; Wallace OB; Deshpande M; Fang H; Yang Z; Zadjura LM; Tweedie DL; Huang S; Zhao F, et al. J. Med. Chem 2003, 46, 4236. [DOI] [PubMed] [Google Scholar]
- 13.Huang CC; Lam SN; Acharya P; Tang M; Xiang SH; Hussan SS; Stanfield RL; Robinson J; Sodroski J; Wilson IA, et al. Science 2007, 317, 1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CC; Tang M; Zhang MY; Majeed S; Montabana E; Stanfield RL; Dimitrov DS; Korber B; Sodroski J; Wilson IA, et al. Science 2005, 310, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyatt R; Kwong PD; Desjardins E; Sweet RW; Robinson J; Hendrickson WA; Sodroski JG Nature 1998, 393, 705. [DOI] [PubMed] [Google Scholar]
- 16.Cormier EG; Persuh M; Thompson DA; Lin SW; Sakmar TP; Olson WC; Dragic T Proc. Natl. Acad. Sci. U.S.A 2000, 97, 5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzan M; Chung S; Li W; Vasilieva N; Wright PL; Schnitzler CE; Marchione RJ; Gerard C; Gerard NP; Sodroski J, et al. J. Biol. Chem 2002, 277, 40397. [DOI] [PubMed] [Google Scholar]
- 18.Cormier EG; Tran DN; Yukhayeva L; Olson WC; Dragic TJ Virol 2001, 75, 5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choe H; Li W; Wright PL; Vasilieva N; Venturi M; Huang CC; Grundner C; Dorfman T; Zwick MB; Wang L, et al. Cell 2003, 114, 161. [DOI] [PubMed] [Google Scholar]
- 20.Schnur E; Noah E; Ayzenshtat I; Sargsyan H; Inui T; Ding FX; Arshava B; Sagi Y; Kessler N; Levy R, et al. J. Mol. Biol 2011, 410, 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acharya P; Dogo-Isonagie C; LaLonde JM; Lam SN; Leslie GJ; Louder MK; Frye LL; Debnath AK; Greenwood JR; Luongo TS, et al. ACS Chem. Biol 2011, 6, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chunduru VSR; Vedula RR Synth. Commun 2012, 42, 1154. [Google Scholar]
- 23.Shi S; Han L; Zhou M; Li Y; Liu Z; Yu B; Wang R Chin. J. Chem 2013, 1133. [Google Scholar]
- 24.Wang Z Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons: Hoboken, NJ, 1999. [Google Scholar]
- 25.Lam SN; Acharya P; Wyatt R; Kwong PD; Bewley CA Bioorg. Med. Chem 2008, 16, 10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusconi S; Moonis M; Merrill DP; Pallai PV; Neidhardt EA; Singh SK; Willis KJ; Osburne MS; Profy AT; Jenson JC, et al. Antimicrob. Agents Chemother 1996, 40, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fun HK; Chantrapromma S; Padaki M; Radhika; Isloor AM Acta Crystallogr., Sect. E Struct. Rep. Online 2009, 65, o1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner MR; Kattenhorn LM; Kondur HR; von Schaewen M; Dorfman T; Chiang JJ; Haworth KG; Decker JM; Alpert MD; Bailey CC, et al. Nature 2015, 519, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartley O; Klasse PJ; Sattentau QJ; Moore JP AIDS Res. Hum. Retroviruses 2005, 21, 171. [DOI] [PubMed] [Google Scholar]
- 30.Pontow S; Ratner LJ Virol. 2001, 75, 11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustchina E; Louis JM; Bewley CA; Clore GM J. Mol. Biol 2006, 364, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M; Gao F; Mascola JR; Stamatatos L; Polonis VR; Koutsoukos M; Voss G; Goepfert P; Gilbert P; Greene KM, et al. J. Virol 2005, 79, 10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou TC; Talalay P Eur. J. Biochem 1981, 115, 207. [DOI] [PubMed] [Google Scholar]
- 34.Chou TC; Talalay P Adv. Enzyme Regul 1984, 22, 27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.











