Abstract
The healthy prostate contains the highest concentration of mobile zinc in the body. As this level decreases dramatically during the initial development of prostate cancer, in vivo detection of prostate zinc content may be applied for diagnosis of prostate cancer. Using 19F ion chemical exchange saturation transfer magnetic resonance imaging (iCEST MRI) and TF-BAPTA as a fluorinated Zn-binding probe with micromolar sensitivity, we show that iCEST MRI is able to differentiate between normal and malignant prostate cells with a 10-fold difference in contrast following glucose-stimulated zinc secretion in vitro. The iCEST signal decreased in normal prostate cells upon downregulation of the ZIP1 zinc transporter In vivo, using an orthotopic prostate cancer mouse model and a transgenic adenocarcinoma of the mouse prostate (TRAMP) model, a gradual decrease of > 300% in iCEST contrast following the transition of normal prostate epithelial cells to cancer cells was detected.
Keywords: imaging agents, MRI, prostate cancer, zinc
Introduction
According to the American Cancer Society, prostate cancer (PCa) is still the most common type of cancer and the second leading cause of cancer death in American men.[1] This high morbidity primarily results from the absence of symptoms and lack of robust screening methods in the early curable stages, calling for the development of sensitive and accurate methods for an early diagnosis of PCa before incurable metastasis occurs. Methods for clinical diagnosis include the well-established digital rectal examination and prostate-specific antigen (PSA) blood test,[2] targeted magnetic resonance imaging (MRI)/ultrasound fusion prostate biopsy,[3] and conventional radiological imaging.[4] Nevertheless, these tests are limited by their specificity, invasiveness, and low accuracy, even for the PSA assay that has about 30 % specificity. Hence, a further exploration of alternative PCa biomarkers beyond PSA is warranted.
A unique feature of the normal prostate is that it exhibits the highest concentration of mobile zinc amongst all soft tissues in the body.[5] Consistent with the downregulation of zinc influx transporters, i.e., ZIP1, the zinc concentration becomes much lower in prostate tumor, even at the T1 stage, when the zinc content decreases by 82%.[5b,6] Since other prostatic pathologies such as prostatitis and benign hyperplasia do not display such a substantial zinc decrease, it has been suggested that mobile zinc may serve as an alternative biomarker of PCa.[5b,7] Therefore, in vivo imaging approaches that are able to probe zinc content may possibly be used to detect the progression of prostate cancer. To the best of our knowledge, there are only two reports describing such an imaging approach.[8]
Ghosh et al. have used a fluorescent ditopic zinc sensor ZPP1 for optical imaging of malignant cell transformation in a transgenic mouse model of PCa.[8a] Tumor progression correlated with a decreasing fluorescence intensity in an age-dependent manner. However, optical imaging is difficult to translate clinically, owing to the limits of light penetration in whole-body deeper tissues, necessitating the use of invasive endoscopic imaging. In contrast, MRI is widely used clinically and has no whole-body imaging limitations. Jordan et al. have used a zinc-binding gadolinium-based paramagnetic contrast agent to detect extracellular zinc with proton (1H) MRI following glucose-stimulated zinc secretion (GSZS).[8b]
Fluorescent 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) dyes have been used for over three decades to probe metals in biologicals systems in vitro,[9] having frequency-specific fluorescent emissions for distinct metal ion species. In this study, we present an alternative MRI method to detect zinc in vivo using a metal-free BAPTA-based probe, termed ion chemical exchange saturation transfer (iCEST) MRI.[10] This method is not based on conventional 1H MRI, but uses a combination of 19F MRI and CEST to specifically monitor Zn2+ with the metal-binding agent 5,5′,6,6′-tetrafluoro-BAPTA (TF-BAPTA) (Scheme 1 a). Owing to the slow dynamic exchange between the zinc-bound TF-BAPTA and non-zinc bound (“free”) TF-BAPTA, and the shift in the Δω of 19F upon ion binding, zinc-specific iCEST MRI can be generated with a specific saturation pulse for the zinc-bound probe.
Scheme 1.
Conceptual design of Zn-specific cellular ICEST MRI. a) Chemical structure of free and zinc-bound TF-BAPTA. b) Schematic of glucose-stimulated extracellular zinc secretion with subsequent chelation by TF-BAPTA, which generates an ICEST signal In normal but not In cancerous prostate cells.
As TF-BAPTA has limited cell permeability, being only able to bind extracellular zinc, we adopted the GSZS method for our iCEST MRI approach. Scheme 1 b illustrates how, following stimulation of normal human epithelial prostate cells by D-glucose, secreted zinc ions are chelated by the extracellular TF-BAPTA, switching the iCEST signal from “off” to “on”. In contrast, for zinc-deficient prostate cancer cells, the iCEST signal is off due to the near absence of intracellular zinc ion.
Results
In Vitro CEST MRI Phantom Studies to Validate Zinc Specificity
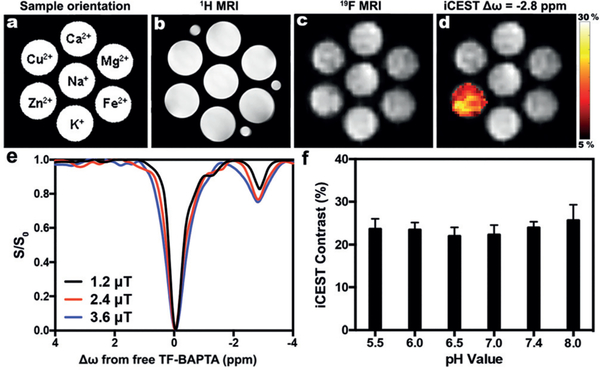
We first validated the specificity of zinc-induced iCEST contrast by mixing different metal ions with TF-BAPTA in secretion-assay buffer (SAB). Figure 1a–d and Figure S1 in the Supporting Information show that only Zn2+ produced an iCEST peak at the specific offset frequency Δω = −2.8 ppm. Based on the results shown in Figure 1e, we chose B1 = 2.4 μT for all further experiments, as it shows a comparable CEST signal at circa −2.8 ppm to that for 3.6 μT, but with a less broad spectrum. The iCEST contrast is relatively stable for a wide range of pH values from 5.5 to 8.0 (Figure 1f).
Figure 1.
ICEST MRI only detects zinc at the Zn-TF-BAPTA-specific offset frequency. a) Orientation of sample phantoms containing 10 mm TF-BAPTA and 100 μM Ion in SAB. b) 1H MRI, c) 19F MRI, and d) ICEST signal (Δω = −2.8 ppm, B1 = 2.4 μT) overlaid on 19F MRI. e) ICEST spectra of 10 mm TF-BAPTA + 100 μM Zn2+ for three saturation pulses with different powers. f) ICEST contrast (−2.8 ppm) of 10 mm TF-BAPTA + 100 μM Zn2+ as a function of pH.
In Vitro iCEST MRI Cell Studies
We studied four human prostate cell lines to assess the ability of iCEST MRI to differentiate between normal and cancerous prostate cells after GSZS and addition of TF-BAPTA. The two normal prostate epithelial cell lines were RWPE1 (normal expression of the zinc influx protein transporter ZIP1) and RWPE2 (downregulated ZIP1); for the malignant cells we used LNCaP (androgen-dependent) and DU145 (androgen-independent). We first validated the lack of toxicity of TF-BAPTA for all four lines using a 3-(4,5-dimethylthiazol−2-yl)−2,5-diphenyltetrazolium bromide (MTT) assay. No toxicity of the iCEST probe could be observed up to 10 mM (Supporting Information, Figure S2), which is well above the concentration used in our cell experiments.
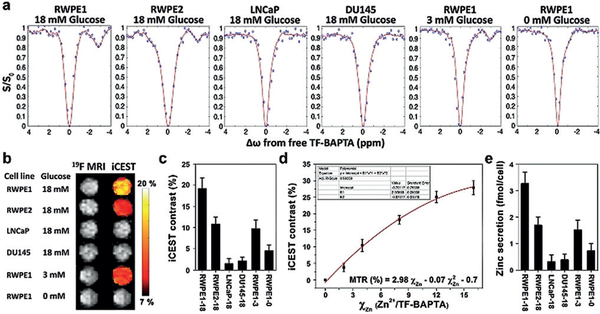
Cells were first pre-treated with 75 μM ZnSO4 for 72 h to achieve proper intracellular zinc concentrations mimicking those in vivo. After co-incubation of 5 mM TF-BAPTA and 18 mM glucose in SAB, the supernatant of each cell line was collected by centrifugation and analyzed by iCEST MRI. The strongest iCEST signal (19.2%) was observed for 18 mM glucose-stimulated RWPE1 cells having a normal ZIP1 level, followed by ZIP1-downregulated RWPE2 cells (10.8%). In contrast, owing to their ZIP1 deficiency, the iCEST signal from DU145 and LNCaP cells was below the detection level (Figure 2a–c). Using a zinc-iCEST signal calibration curve (Figure 2d), the secreted zinc concentration from RWPE1, RWPE2, LNCaP, and DU145 cells following 18 mM glucose stimulation was calculated to be 41.5 ± 5.5, 21.4 ± 4.1, 3.9 ± 3.2, and 4.8 ± 2.7 μM, corresponding to values of 3.27 ± 0.43, 1.69 ± 0.32, 0.31 ± 0.25, and 0.38 ± 0.21 fmol Zn cell−1 respectively (Figure 2e). To validate that zinc secretion only occurs after stimulation of glucose, the effect of a lower concentration of 3 mM and no glucose was tested for RWPE1 cells. The respective iCEST signal intensities were 9.7 % and 4.5 % (Figure 2c), that is, a 2- to 4-fold reduction of the value at 18 mM glucose (19.2%). The zinc secreted from RWPE1 cells was measured to be 1.51 or 0.72 fmol cell−1 when 3 or 0 mM glucose was used. This confirms that zinc secretion is glucose-induced and that the GSZS/iCEST MRI method can be used to distinguish normal cells from cancerous cells in vitro.
Figure 2.
ICEST MRI can distinguish between normal and cancerous prostate cells In vitro. a) 19F ICEST spectra, b) 19F MRI and ICEST signal overlaid on 19F MRI. c) ICEST signal of supernatants of cells treated with 5 mM TF-BAPTA and 18 mM, 3, or 0 mM D-glucose. d) Calibration curve of χZn (Zn2+/TF-BAPTA, μM mM−1) vs. ICEST contrast. Various zinc concentrations were added to 5 mM TF-BAPTA (R2 = 0.994). e) Amount of cellular glucose-induced zinc secretion calculated from the equation shown in (d). All measurements were performed at Δω = −2.8 ppm.
In Vitro Fluorescence Studies to Validate iCEST MRI Detection of Zinc Secretion
To verify that zinc ion is abundant in normal prostate cells and deficient in cancerous cell lines, a commercial zinc-sensitive fluorescent dye N-(6-methoxy−8-quinolyl)-p-carboxybenzoyl-sulphonamide (TFLZn) potassium salt was used to assess zinc content. Similar to the iCEST MRI experiments, cells were first pretreated with 75 μM ZnSO4 for 72 h, and then with 200 μM TFLZn for 1 h. Unlike TF-BAPTA, TFLZn is cell permeable and detects intracellular zinc rather than secreted zinc; hence, no glucose stimulation is needed. A strong fluorescence was observed for RWPE1 cells (Figure 3a and Figure S3). Fluorescence intensity was much weaker in RWPE2 cells and undetectable in LNCaP and DU145 cell lines. These results confirmed that the difference in intracellular zinc levels correspond to those secreted upon glucose stimulation.
Figure 3.
a) Zinc-activated TFLZn (green) fluorescent images of RWPE1, RWPE2, LNCaP, and DU145 cells, counterstained with propidium iodide (PI, red). Scale bar = 50 μm. b) Calibration curve of zinc fluorescence in SAB for 500 μm TFLZn (λex/λem = 380/510 nm). c) Calculated secreted zinc levels for D-glucose at different concentrations.
The applicability of GSZS was further validated by zinc-activated TFLZn fluorescence. A linear correlation of fluorescence intensity with zinc concentration could be obtained up to 60 μM (Figure 3b), which allowed us to quantify the zinc secretion in cell supernatants (Figure 3c, corresponding values are shown in Table S1). These fluorescent measurements of glucose-stimulated zinc secretion are consistent with the iCEST MRI results, which directed us to further test this in vivo.
In Vivo iCEST MRI of Zinc Secretion in an Orthotopic PCa Mouse Model
An orthotopic PCa mouse model in the anterior prostate (AP)[11] was used to assess the ability of iCEST MRI to distinguish between non-cancerous and cancerous prostate in vivo. To this end, luciferase-transfected LNCaP or DU145 cells were xenografted into the left AP of immunodeficient (NSG) mice. PBS injection was used as sham control to preserve the normal prostate. Bioluminescence imaging (BLI) was then used to track the growth of the AP tumor (Figure S4), which became visible to the naked eye at three weeks post-injection. To promote glucose-induced zinc secretion from prostate cells, tumor-bearing mice were fasted for 12 h before iCEST MRI.[8b] A micro polyethylene catheter was then inserted into the left AP for TF-BAPTA injection, as outlined in Figure S4. A second catheter was inserted into the abdominal cavity for intraperitoneal (i.p.) injection of D-glucose and anesthetics. The location of the prostate was first determined by 1H MRI, and then 19F MRI and iCEST MRI were sequentially performed immediately after TF-BAPTA injection before and after glucose treatment. In contrast to the low iCEST signal (4.9 ± 3.4%) before injection of glucose, a dramatically increased signal (21.7 ± 5.1%) was observed in the AP of PBS-injected mice after glucose stimulation (Figure 4a), as a result from zinc secretion by normal prostate cells. By comparison, a significantly lower iCEST response was seen for both the androgen-responsive LNCaP tumor and the androgen-unresponsive DU145 tumor, in agreement with the zinc-deficient environment of poorly differentiated adenocarcinoma. H&E staining confirmed the presence of LNCaP and DU145 tumors in the AP (Figure 4b).
Figure 4.
a) 19F MRI, iCEST MRI, and iCEST spectra of control NSG mice (normal prostate) before and after glucose injection, and of LNCaP and DU145-bearing NSG mice after glucose injection. All mice were injected with 0.15 g kg−1 of TF-BAPTA, GSZS was induced by i.p. injection of 80 μL of 20% (w/v) D-glucose. All measurements were performed at Δω = −2.8 ppm. b) H&E staining of normal and tumor-bearing NSG mice.
In Vivo iCEST MRI of Zinc Secretion in a TRAMP Model
Having established that our iCEST approach can differentiate prostate adenocarcinoma from the normal prostate, we then investigated a transgenic adenocarcinoma of the mouse prostate (TRAMP) model, a clinically more relevant scenario in which the transition of the normal prostate to the cancerous stage can be monitored over time within the same individual, from the initiation of prostatic intraepithelial neoplasia (PIN) to the well-differentiated stage. TRAMP mice were imaged at 10, 17, and 24 weeks of age. At week 10, there was a clear difference in the iCEST signal before and after D-glucose injection (Figure 5a), suggesting the presence of a normal prostate. The iCEST contrast decreased over time, changing from 20.4 ± 4.3% to 17.9 ± 3.8% and 9.6 ± 3.1 % for 10,17, and 24-week-old TRAMP mice, respectively (Figure 5b). H&E staining and staining for the PCa marker proliferating cell nuclear antigen (PCNA)[12] demonstrated that the iCEST signal reduction corresponded to the formation of tumors (Figure 5c).
Figures 5.
a) 19F MRI, iCEST MRI, and iCEST spectra of 10-week old TRAMP mice before and after glucose injection, and of 17-week and 24-week old TRAMP mice after glucose injection. b) Quantification of iCEST signal after glucose stimulation. Data are shown as mean ± SEM (n = 4). All mice were injected with 0.15 gkg−1 of TF-BAPTA, GSZS was induced by i.p. injection of 80 μL of 20% (w/v) D-glucose. All measurements were performed at Δω = −2.8 ppm. c) H&E and PCNA staining (green = PCNA, blue = DAPI) of AP in 10-week, 17-week, and 24-week old TRAMP mice.
Discussion
For some time, zinc has been recognized as a potential clinical biomarker for prostate cancer, with decreasing zinc levels correlating to an increased Gleason score.[13] The drastic reduction of zinc levels in prostate adenocarcinoma (but not benign prostate hyperplasia)[14] compared to healthy prostate tissue has spurred interest to develop zinc-sensing probes for screening and diagnosis of malignant prostate cancer. Near all epidemiologic studies on the relation between zinc content and prostate cancer have been conducted retrospectively, and thus the possible influence of disease pathology on zinc levels limits its interpretation).[15] Taking advantage of “hot spot” 19F MRI[16] having zero tissue background signal and zinc-specific iCEST MRI for serial monitoring before and after tumor development, we were able to detect early malignant cell transformation in vivo.
Why do normal prostate secretory epithelial cells contain so much zinc? One of the primary functions of these highly specialized glandular cells is to produce and secrete citrate, which delays or abolishes blood clotting as well as coagulation of sperm. Prostate fluid has an extremely high citrate concentration, ranging from 40000–150000 nmol g−1 wet weight compared to only 90–110 and 150–450 nmol g−1 for blood plasma and other non-prostate soft tissue.[17] A high level of zinc in mitochondria is essential to inhibit m-aconitase activity,[18] which catalyzes the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process. The ZIP zinc transporter family is responsible for cytoplasmic zinc influx from either the extracellular milieu or intracellular vesicles.[19] Downregulation of ZIP1 in prostate cancer cells results in a marked decrease in intracellular zinc levels.[20] As a control for testing iCEST MRI zinc specificity, we did include a ZIP1 zinc transporter-downregulated normal prostate cell line, i.e., RWPE2. This cell line, its non-downregulated counterpart RWPE1, and the two malignant cell lines DU145 and LNCaP all have varying zinc storage capacities, which was confirmed with iCEST MRI.
The iCEST contrast was found to be highly specific for Zn2+ compared to other similar metal species at physiological pH levels (Figure 1). The iCEST contrast remained stable over a broad range of pH values (5.5–8.0), thereby excluding a potential confounding effect of pH on CEST signal intensity when comparing the healthy prostate gland in a neutral pH environment versus the acidic microenvironment of prostate tumors. The results of the in vitro cell experiments and in vivo orthotopic tumor studies showed that the iCEST MRI contrast has an excellent correlation to the zinc concentration as measured by the fluorescent TFLZn (Figure 3, Table S1). Our calculated zinc secretion after 18 mM glucose stimulation for RWPE1 (3.27 ± 0.43), RWPE2 (1.69 ± 0.32), LNCaP (0.31 ± 0.25) and DU145 (0.38 ± 0.21 fmol cell−1) are in good agreement with the relative internal ratios reported by Ghosh et al.[8a] as measured by ICP-MS for RWPE1 (20 ± 0.2), RWPE2 (9 ± 0.4), LNCaP (3 ± 0.1) and DU145 (3 ± 0.3 fmol cell−1). The 5- to 10-fold difference in absolute values can be explained by the differences in the assay setup, as Ghosh et al. measured the total (intracellular and extracellular) zinc content by ICP-MS, whereas we only measured the extracellular (secreted) fraction. Androgen-dependent LNCaP and androgen-independent DU145 cells did not show a significant difference in iCEST contrast between them, suggesting that decreased zinc content is not androgen-related.
A minimal but noticeable iCEST signal was detected in vivo before i.p. injection of glucose, which we attribute to the presence of unbound ZnII in the extracellular matrix of tissues.[8b,21] The glucose-stimulated 19F MRI-based iCEST contrast enhancement in normal mice was 343 % (Figure 4), which is much higher than the reported 40% obtained by using the paramagnetic Gd-HP-DO3A 1H MRI-based method.[8b] Even though the exact mechanism of glucose-induced extracellular zinc secretion is not clear, this strategy allows the use of cell-impermeable probes to image zinc, with minimum intracellular toxicity (Figure S1). The cell-impermeable probe TF-BAPTA used in this study is commercially available, facilitating other studies to be compared to our findings.
Upon confirming the sensitivity and specificity of Zn-specific iCEST MRI, we concluded our studies with in vivo studies in TRAMP mice. The TRAMP model has been widely used to mimic the progression of prostate cancer in man. Compared to the orthotopic model, it represents a genetically well-defined model developing an androgen-independent aggressive metastatic cancer, which histologically and biochemically resembles the human disease.[22] Expression of transgenes is restricted to prostate epithelial cells, with adenocarcinomas arising with 100 % incidence after a short latency.[23] Previously, the prostate was shown to keep high levels of zinc constant for 28 weeks in normal mice.[8a] In TRAMP mice, the iCEST contrast decreased from 20.4 % to 9.6 % as the age of mice increased from 10 weeks to 24 weeks (Figure 5), at a time point when normal prostate epithelial cells transform into malignant cells. This was confirmed by PCNA and H&E staining showing that the AP of TRAMP mice went from a PIN grade at 17 weeks to a well-differentiated tumor at 24 weeks.
The dose of glucose (0.64 gkg−1 i.p.) we injected in this study for GSZS translates to a human equivalent dose of 0.05 gkg−1. This is well below that used in clinical practice in diabetic patients, in which an insulin response is measured in response to 1.0 gkg−1 glucose i.v.[24] For human CEST imaging using glucose as contrast agent, the dose injected is about 0.33 gkg−1.[25] Our method is different from most other 19F reporters in that the TF-BAPTA is cell-impermeable and can be transferred from the prostate to the bladder within a short period of time (< 45 min as deduced from the 19F MRI results). Therefore, a relatively high local concentration of TF-BAPTA may not necessarily induce toxic side effects. Based on the calibration curve of 19F MRI signal-to-noise ratio (SNR) versus TF-BAPTA concentration (Figure S6), we estimate that the TF-BAPTA concentration injected into the prostate is about 13 mM. The relatively short acquisition time for in vivo MRI studies ensures a sufficiently high concentration of TF-BAPTA in the AP for iCEST imaging during the procedure. Although the intracellular toxicity results in Figure S2 show no toxicity after incubating cells with 10 mM TF-BAPTA for 24 h, further toxicity studies of TF-BAPTA will be needed to determine dose safety when injected i.v. As for transrectal or transperineal intraprostatic injection of TF-BAPTA, this minimally invasive procedure would be comparable to a routine fine needle aspiration and core biopsy.
Conclusion
Summarized, we have demonstrated a specific and sensitive method for detection of prostate cancer based upon 19F iCEST MRI. To the best of our knowledge, this is the first attempt to use 19F iCEST as a novel diagnostic tool for in vivo imaging. Since both CEST MRI and 19F MRI are clinically used, zinc iCEST MRI has translational potential for clinical diagnosis of prostate cancer.
Supplementary Material
Acknowledgements
This project was supported by R03 EB018882 and the Pearl and Yueh-Heng Yang Foundation. We thank Dr. Julie E. Pickett for the luciferase transfected LNCaP and DU145 cells, and Dr. Guanshu Liu and Dr. Jiadi Xu for experimental assistance.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Yue Yuan, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA); Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine Baltimore, MD (USA).
Zhiliang Wei, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Chengyan Chu, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA); Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine Baltimore, MD (USA).
Jia Zhang, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Xiaolei Song, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA); Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine Baltimore, MD (USA).
Piotr Walczak, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA); Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine Baltimore, MD (USA).
Jeff W. M. Bulte, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA) Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine Baltimore, MD (USA); Department of Oncology, Department of Biomedical Engineering, Department of Chemical Biomolecular Engineering, The Johns Hopkins University School of Medicine Baltimore, MD (USA).
References
- [1].Siegel RL, Miller KD, Jemal A, CA Cancer J Clin. 2018, 68, 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, DeKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL, Waters WB, MacFarlane MT, Southwick PC, J. Urol. 1994,151, 1283–1290. [DOI] [PubMed] [Google Scholar]
- [3].Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, Wood BJ, Pinto PA, JAMA J Am. Med. Assoc. 2015, 313, 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Kurhanewicz J, Vigneron D, Carroll P, Coakley F, Curr. Opin. Urol. 2008,18, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Halpern EJ, Rev. Urol. 2006,8, S29–37; [PMC free article] [PubMed] [Google Scholar]; c) Shreve PD, Grossman HB, Gross MD, Wahl RL, Radiology 1996,199, 751–756. [DOI] [PubMed] [Google Scholar]
- [5].a) Eggleton WG, Biochem. J. 1940, 34, 991–997; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zaichick V, Sviridova TV, Zaichick SV, Int. Urol. Nephrol 1997,29, 565–574. [DOI] [PubMed] [Google Scholar]
- [6].a) Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC, Mol. Cancer 2005, 4, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mawson CA, Fischer MI, Can. J. Med. Sci. 1952, 30, 336–339. [DOI] [PubMed] [Google Scholar]
- [7].Costello LC, Franklin RB, Prostate Cancer Prostatic Dis. 2009,12, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Ghosh SK, Kim P, Zhang XA, Yun SH, Moore A, Lippard SJ, Medarova Z, Cancer Res. 2010, 70, 6119–6127 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jordan MVC, Lo ST, Chen SW, Preihs C, Chirayil S, Zhang R, Kapur P, Li WH, De Leon-Rodriguez LM, Lubag AJM, Rofsky NM, Sherry AD, Proc. Natl. Acad. Sci. USA 2016, 113, E5464–E5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tsien RY, Biochemistry 1980,19, 2396–2404. [DOI] [PubMed] [Google Scholar]
- [10].Bar-Shir A, Yadav NN, Gilad AA, van Zijl PCM, T McMahon M, Bulte JWM, J. Am. Chem. Soc. 2015, 137, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Cifuentes FF, Valenzuela RH, Contreras HR, Castellon EA, Oncol. Lett. 2015, 10, 2142–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pavese J, Ogden IM, Bergan RC, J. Vis. Exp. 2013, e50873. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hughes RM, Simons BW, Hurley PJ, Biol. Protoc. 2017, 7, e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mulligan JM, Mai KT, Parks W, Gerridzen RG, Can. J. Urol. 1997, 4, 422–425. [PubMed] [Google Scholar]
- [13].a) Cortesi M, Chechik R, Breskin A, Vartsky D, Ramon J, Raviv G, Volkov A, Fridman E, Phys. Med. Biol. 2009, 54, 781–796 [DOI] [PubMed] [Google Scholar]; b) Cortesi M, Fridman E, Volkov A, Shilstein S, Chechik R, Breskin A, Vartsky D, Kleinman, Kogan G, Moriel E, Gladysh V, Huszar M, Ramon J, Raviv G, Prostate 2008, 68, 994–1006 [DOI] [PubMed] [Google Scholar]; c) Zhao J, Wu Q, Hu X, Dong X, Wang L, Liu Q, Long Z, Li L, Sci. Rep. 2016, 6, 25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Feustel A, Wennrich R, Steiniger D, Klauss P, Urol. Res. 1982, 10, 301–303. [DOI] [PubMed] [Google Scholar]
- [15].Platz EA, Helzlsouer KJ, Epidemiol. Rev. 2001, 23, 93–101. [DOI] [PubMed] [Google Scholar]
- [16].Bulte JW, Nat. Biotechnol. 2005, 23, 945–946. [DOI] [PubMed] [Google Scholar]
- [17].Costello LC, Feng P, Milon B, Tan M, Franklin RB, Prostate Cancer Prostatic Dis. 2004, 7, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Costello LC, Liu Y, Franklin RB, Kennedy MC, J. Biol. Chem. 1997, 272, 28875–28881. [DOI] [PubMed] [Google Scholar]
- [19].a) Zhang T, Sui D, Hu J, Nat. Commun. 2016, 7, 11979. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lichten LA, Cousins RJ, Annu. Rev. Nutr. 2009, 29, 153–176 [DOI] [PubMed] [Google Scholar]; c) Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D, Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB, Prostate 2011, 71,1518–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Costello LC, Liu Y, Zou J, Franklin RB, J. Biol. Chem. 1999, 274, 17499–17504 [DOI] [PubMed] [Google Scholar]; c) Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P, Costello LC, J. Inorg. Biochem. 2003, 96, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].a) Schroeder JJ, Cousins RJ, Proc. Natl. Acad. Sci. USA 1990, 87, 3137–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) De Lisle RC, Sarras MP, Hidalgo J, Andrews GK, Am. J. Physiol Cell Physiol 1996, 271, C1103–C1110. [DOI] [PubMed] [Google Scholar]
- [22].a) Gingrich JR, Barrios RJ, Foster BA, Greenberg NM, Prostate Cancer Prostatic Dis. 1999, 2, 70–75 [DOI] [PubMed] [Google Scholar]; b) Gelman IH, Cancer Res. 2016, 76, 6137–6139; [DOI] [PubMed] [Google Scholar]; c) Hensley PJ, Kyprianou N, J. Androl. 2012, 33, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].a) Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM, Cancer Res. 1997, 57, 3325–3330 [PubMed] [Google Scholar]; b) Gingrich JR, Greenberg NM, Toxicol. Pathol. 1996, 24, 502–504 [DOI] [PubMed] [Google Scholar]; c) Greenberg NM, Urol Oncol. 1996, 2, 119–122. [DOI] [PubMed] [Google Scholar]
- [24].Shapiro ET, Tillil H, Miller MA, Frank BH, Galloway JA, Rubenstein AH, Polonsky KS, Diabetes 1987, 36,1365–1371. [DOI] [PubMed] [Google Scholar]
- [25].Knutsson L, Seidemo A, Rydhog Scherman A, Markenroth Bloch K, Kalyani RR, Andersen M, Sundgren PC, Wirestam R, Helms G, van Zijl PCM, Xu X, Tomography 2018, 4, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.