Abstract
A public health emergency of current international concern is the outbreak of a severe respiratory illness, that is, coronavirus disease (COVID-19). The disease initially started in Wuhan, China, and it rapidly spread to most regions of the world. Herein, we report a case of critical COVID-19 pneumonia treated with extracorporeal membrane oxygenation from symptom onset day 19 (SOD#19) to SOD#30. We describe the patient's clinical course, from mild symptoms at the time of illness onset to symptoms of severe pneumonia as the illness progressed. We provide important information regarding our clinical experience for further understanding of management discrepancies, as treatment with extracorporeal membrane oxygenation or pharmacotherapy (e.g., antivirals, immunomodulators, and glucocorticoids) is often dependent on the severity of symptoms.
Keywords: Coronavirus, Acute respiratory distress syndrome, Gamma-globulin, Organizing pneumonia
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease; ECMO, extracorporeal membrane oxygenation; IVIG, intravenous immune globulin; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Highlights
-
•
A critical case of 76-year-old female with COVID-19 pneumonia.
-
•
No significant clinical benefits of lopinavir-ritonavir and peramivir treatment.
-
•
The pneumonia rapidly progressed to acute respiratory distress syndrome.
-
•
Extracorporeal membrane oxygenation from illness day 21–31 lead to recovery.
1. Introduction
An outbreak of a severe respiratory illness, coronavirus disease (COVID-19), occurred in Wuhan, China, in December 2019. The virus that causes COVID-19, which has rapidly spread to most regions of the world, is called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. So far, there are few reports on critical patients with COVID-19 [2,[5], [6], [7]]. Here, we report the clinical course of a patient with a severe case of COVID-19 complicated with acute respiratory distress syndrome (ARDS). We report the patient's response to intensive care, including invasive ventilation in the early stage of the illness and extracorporeal membrane oxygenation (ECMO) with antiviral, immunomodulatory, and glucocorticoid therapies as the illness progressed.
2. Case presentation
On February of 2020, a 76-year-old woman was referred to our hospital in Matsumoto, Japan, from another hospital in Japan, where she was admitted for sore throat, dry cough, and fever that started on February 7, 2020 (symptom onset day 1; SOD#1). (The term “symptom onset day” is used to illustrate the patient's clinical course, and the term “hospital day” is used to describe treatment measures.) Past medical history was significant for diabetes mellitus, hypertension, and glaucoma, but she was otherwise healthy and did not smoke. The patient, an American living in the United States, was visiting Japan and arrived at Yokohama Harbor aboard the Diamond Princess cruise ship. Due to a COVID-19 outbreak inside the cruise ship, she was kept in the cruise ship and underwent viral testing as part of quarantine inspection. A reverse transcription polymerase chain reaction (RT-PCR) test, performed by the Japan Ministry of Health, Labour and Welfare, produced a positive result for SARS-CoV-2.
One day before admission to our hospital, the patient was started on lopinavir-ritonavir (400 mg/100 mg twice daily orally) and moxifloxacin (400 mg once a daily orally). She was transferred to our hospital on SOD#12 (hospital day 1; HD#1). On admission, her body temperature was 38.3 °C, and her oxygen saturation (SpO2) by pulse oximetry was 93% on 8 L/min of supplemental oxygen via mask. Physical examination revealed coarse crackles in the upper chest on the right. Laboratory examination revealed peripheral blood lymphopenia (350/μL) and elevated levels of blood urea nitrogen (BUN, 28.9 mg/dL), creatinine (1.62 mg/dL), C-reactive protein (CRP, 12.90 mg/dL), and lactate dehydrogenase (LDH, 325 U/L) (Table 1).
Table 1.
Laboratory examination results on admission.
| Measurement | Result | Reference Range | Measurement | Result | Reference Range |
|---|---|---|---|---|---|
| Hemotology | BNP | 122.9 pg/mL | 0.0–20.0 pg/mL | ||
| White blood cell count | 6620/μL | 3300–8600/μL | Serology | ||
| Absolute neutrophil count | 6130/μL | 1170–6130/μL | C-reactive protein | 12.90 mg/dL | 0.00–0.14 pg/mL |
| Absolute lymphocyte count | 350/μL | 350–900/μL | KL-6 | 407 U/mL | 105–435 U/mL |
| Red blood cell count | 317 × 104/μL | 386–492 × 104/μL | Anti-nuclear antibody | negative | – |
| Hemoglobin | 9.7 g/dL | 11.6–14.8 g/dL | Rheumatoid factor | negative | – |
| Hematocrit | 29.3% | 35.1–44.1% | Blood Coagulation | ||
| Platelet count | 14.9 × 104/μL | 15.8–34.8 × 104/μL | PT | 14.7 s | 10.0–40.0 s |
| Blood Chemistry | PT-INR | 1.33 | 0.85–1.15 | ||
| Total protein | 5.1 g/dL | 6.6–8.1 g/dL | APTT | 32.5 s | 20.0–80.0 s |
| Albumin | 2.2 g/dL | 4.1–5.1 g/dL | Fibrinogen | 614.0 mg/dL | 100.0–700.0 mg/dL |
| Blood urea nitrogen | 28.9 mg/dL | 8.0–20.0 mg/dL | D-dimer | 3.2 μg/mL | 0.0–30.0 μg/mL |
| Creatinine | 1.62 mg/dL | 0.65–1.07 | Arterial Blood Gas After Intubation (FiO20.5) | ||
| AST | 30 U/L | 13–30 U/L | pH | 7.296 | 7.340–7.450 |
| ALT | 16 U/L | 10–42 U/L | PaCO2 | 48.0 Torr | 32.0–45.0 Torr |
| LDH | 325 U/L | 124–222 U/L | PaO2 | 95.5 Torr | 75.0–100.0 Torr |
| Total bilirubin | 0.79 mg/dL | 0.40–1.50 mg/dL | HCO3− | 21.7 mmol/L | 22.0–28.0 mmol/L |
| γ-glutamyl transferase | 9 U/L | 13–64 U/L | PaO2/FiO2 | 191 | – |
| Amylase | 70 U/L | 44–132 U/L |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; FiO2, fraction of inspired oxygen; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; PT, prothrombin time; INR, international normalized ratio.
Chest computed tomography (CT) images showed ground-glass opacities (GGOs) and consolidation (Fig. 1A and B).
Fig. 1.
Chest computed tomography (CT) images. (A–B) Images taken on SOD#9. (A) Ground-glass opacities (GGOs) in the anterior segment of the right upper lobe. (B) Partial consolidation and GGOs in the right middle and lower lobes and distribution of lesions in the subpleural area and periphery of the lung, showing “crazy-paving” pattern. (C–D) Images taken on SOD#33. (C) Subpleural consolidation in the right lung. (D) Posterior consolidation with air bronchogram in the posterior segments of the lower lobes of both lungs.
Due to possible community-acquired pneumonia caused by bacteria and influenza virus, the patient was treated with piperacillin-tazobactam and peramivir (at a loading dosage of 300 mg, reduced to 150 mg every 24 hours due to renal failure). Her respiratory failure progressed, leading to endotracheal intubation. An endotracheal aspirate obtained through the intubation tube was positive for SARS-CoV-2 on RT-PCR. Laboratory examination revealed a low gamma-globulin level on HD#3 (SOD#14); intravenous immune globulin (IVIG) (at a dosage of 5000 mg every 24 hours intravenously) was administered from HD#3 (SOD#14) to HD#8 (SOD#19).
On HD#8 (SOD#19), the patient's respiratory status progressively deteriorated; she exhibited dyspnea despite fever alleviation. The respirator setting at the time was assist/control (volume-controlled ventilation), tidal volume was 300 ml, positive end-expiratory pressure (PEEP) was 14 cmH2O, and respiratory frequency was 13 per minute. Her hypoxemia was not improved even if we regulated PEEP. We considered whether this deterioration might be due to increased airway dead space caused by pulmonary embolism or acute pulmonary edema; however, these diagnoses could not be confirmed with evidence on chest CT scans, which were unobtainable because there was concern about the risk of infecting other patients in the radiology unit. We conducted a compression ultrasound, but were not able to point out deep vein thrombosis. The patient's respiratory status continued to worsen and, despite optimal ventilator settings, we were unable to maintain her PaO2/FiO2 above 70 torr. Therefore, we decided to proceed to venous-venous extracorporeal membrane oxygenation (V–V ECMO) on HD#8 (SOD#19). For V–V ECMO, venous catheters were placed in the right common femoral vein (for drainage) and right internal jugular vein (for infusion). Her hemodynamics were maintained using vasoactive agents; we were able to normalize her body water with diuretics and stabilize her oxygenation by ECMO. On HD#16 (SOD#27), to prevent bacterial infection related to catheterization, the previous antibiotics were replaced with vancomycin. Her respiratory status gradually improved. On HD#16 (SOD#27), viral testing of an endotracheal aspirate sample showed that SARS-CoV-2 was undetectable using RT-PCR. A tracheotomy was performed on HD#18 (SOD#29); ECMO was discontinued on HD#19 (SOD#30). Intravenous meropenem was administered for ventilator-associated pneumonia (VAP) prophylaxis.
Chest CT scans obtained on HD#22 (SOD#33) showed enlarged abnormal shadows, GGOs, and consolidation in the upper lobe of the right lung, suggesting organizing pneumonia (Fig. 1C and D), which could result in chronic respiratory failure after ECMO cessation. On HD#25 (SOD#36), the patient was started on methylprednisolone 250 mg daily for three days with improvement of her organizing pneumonia on chest x-ray (Fig. 2E and F). On HD#40 (SOD#51), the patient was taken off the respirator, and her condition was stable.
Fig. 2.
(A–C) Chest X-rays showing the progression of ground-glass opacification with consolidative abnormalities. (D–F) Slow improvement of shadows.
3. Discussion
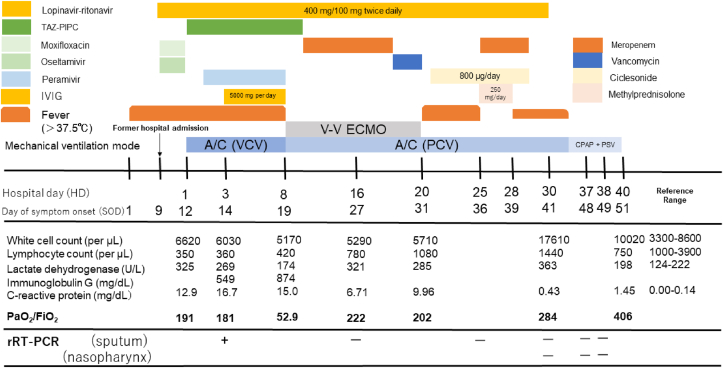
We herein report the clinical features of COVID-19 in a critical patient who met the criteria of ARDS with refractory hypoxemia. In accordance with the first report of COVID-19 from China, the patient initially presented with mild cough and intermittent fever before rapid progression to severe pneumonia that required mechanical ventilation [3]. Detection of SARS-CoV-2 RNA in a sputum sample on SOD#14 suggests long-term persistence of viral shedding and potential transmissibility (Fig. 3).
Fig. 3.
Diagram showing the patient's clinical course by day of illness and day of hospitalization. A/C, assist/control; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; IVIG, intravenous immune globulin; PaO2, partial pressure of arterial oxygen; PCV, pressure-controlled ventilation; PSV, pressure support ventilation; rRT-PCR, real-time reverse transcriptase polymerase chain reaction; TAZ-PIPC, piperacillin-tazobactam; V–V ECMO, veno-venous extracorporeal membrane oxygenation; VCV, volume-controlled ventilation.
So far, recent studies have described relatively mild cases of COVID-19 in multiple countries [[8], [9], [10], [11]]. In a report from the Chinese Center for Disease Control and Prevention, five percent of patients with COVID-19 were critical and required intensive care [4]. In critical cases, ARDS, septic shock, difficulty in correcting metabolic acidosis, and coagulation disorders may develop rapidly [3]. The current case did not respond to treatment with lopinavir-ritonavir, necessitating ECMO. Accumulating evidence strongly indicates that old age and comorbidities like diabetes mellitus and hypertension are risk factors for poor outcome [5,6], as seen in this patient. In particular, old age increases risk of ARDS and death associated with COVID-19 [23].
The laboratory abnormalities in this case (elevated serum CRP, LDH, BUN, creatinine, and D-dimer levels) coincide with those noted in previous reports [5,6]. The persistent peripheral blood lymphopenia in this case might be indicative of COVID-19 severity, as previous reports have shown lymphopenia to be common in patients with COVID-19 requiring intensive care [6,31]. Therefore, it was debated whether IVIG therapy should be performed. However, a few reports have shown that the efficacy and adverse effects of IVIG therapy are unclear [3,5,6].
The present patient was treated with invasive ventilation, which was later switched to ECMO to manage respiratory failure. An overabundance of body fluid may contribute to refractory hypoxemia in patients with ARDS. In this case, ECMO showed great effectiveness in treating the patient's rapidly deteriorating respiratory status due to pneumonia. The World Health Organization generally recommends referring patients with refractory hypoxemia to expert centers that are capable of providing ECMO for treatment of severe ARDS due to COVID-19 [17,22]. Recent evidence has suggested that ECMO use in the most severe ARDS cases was associated with reduced mortality [21]. In contrast, the potential harm of ECMO in patients with COVID-19 was described by Yang et al., who reported death in five of six patients with severe COVID-19 who were treated with ECMO [7]. Risk factors such as advanced age or other complications (e.g., hypertension, diabetes) may be associated with mortality after ECMO induction rather than ECMO induction itself. While the role of ECMO in the management of COVID-19 remains unclear, we suggest that intensive treatment with ECMO offered clinical benefits in the present case.
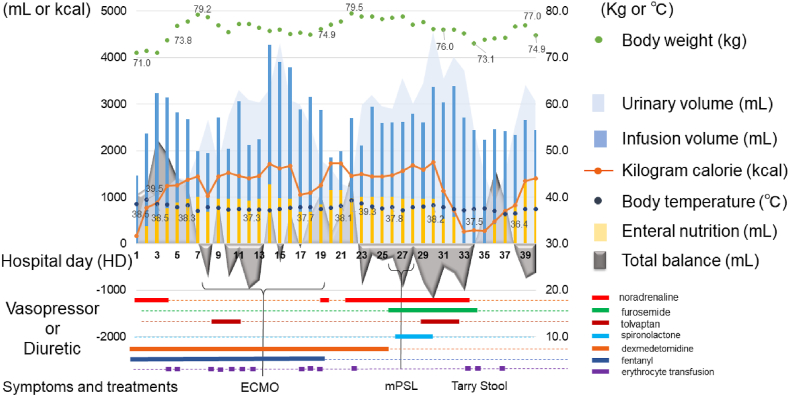
The present case of COVID-19 pneumonia rapidly progressed to ARDS. The patient's worst respiratory status was observed on HD#8 (SOD#19), as vascular hyperlucency caused pulmonary edema due to uncontrollable inflammation. ECMO was introduced at that time to manage respiratory failure. Continued ECMO was tied to successful treatment in this patient. We assumed that intensive management of body fluid levels and proper enteral nutrition in the critical stage of the illness were strongly associated with our successful treatment (Fig. 4).
Fig. 4.
Diagram showing the fluid strategies by the day of hospitalization.
The patient's respiratory status was significantly improved until we started administering glucocorticoids for organizing pneumonia on HD#25 (SOD#36). In light of the comorbidities of diabetes mellitus and infectious disease in this patient, glucocorticoids were administered for three days. Of note, a recent report suggested that treatment with methylprednisolone in the recovery phase might have been beneficial for patients with COVID-19 who developed ARDS [23]. As we experienced in the treatment of this patient, glucocorticoids may be effective for organizing pneumonia due to COVID-19. In contrast, a study by Lansbury et al. demonstrated that glucocorticoids were associated with an increased risk of mortality in patients with influenza [19]. Although glucocorticoids have been widely used in the management of severe acute respiratory syndrome (SARS), some studies have demonstrated adverse effects with both short- and long-term administration [[18], [19], [20]].
No randomized controlled trials have yet resulted in a recommendation for antiviral treatment for patients with suspected or confirmed COVID-19. Management of patients with COVID-19 currently consists of ensuring appropriate infection control and supportive care [17]. In clinical practice, antiviral agents, including neuraminidase inhibitors (oseltamivir, peramivir, zanamivir, etc.) and ribavirin, in combination with protease inhibitors (like lopinavir-ritonavir) are often used tentatively [15]. Lopinavir-ritonavir has been proposed for the treatment of COVID-19 due to its potential effectiveness in treating SARS, as reported in a series of studies [12,13]. Moreover, lopinavir has shown an inhibitive effect on Middle East respiratory syndrome coronavirus in an in vitro study [14]. Our patient was treated with lopinavir-ritonavir and peramivir, but the clinical benefits did not seem significant. Further studies, including randomized controlled trials, are urgently needed to examine the effectiveness of lopinavir-ritonavir in treating COVID-19 [15].
In summary, we highlight the clinical course of severe COVID-19 in this critical patient and share important clinical information pertaining to our experience, particularly regarding the effective management of acute respiratory failure using ECMO.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the patient as well as the nurses and clinical staff who provided intensive care for the patient. We would also like to thank the following members of the first SARS-CoV-2 treatment teams at Shinshu University Hospital: Junpei Akahane, MD; Shuhei Nozawa, MD; Ryosuke Machida, MD; Takashi Ichiyama, MD, PhD; Taisuke Araki, MD; Naoya Uehara, MD; Daisuke Matsui, MD; Satoshi Nakao, MD; Soichiro Ebisawa, MD, PhD; Toru Murakami, MD; Yukio Nakamura, MD, PhD; Hiroshi Murakami, MD; Yuki Sato, MD, PhD; and Tsuyoshi Notake, MD, PhD. We would like to thank Editage (www.editage.com) for English language editing. Finally, we would like to thank Kenichi Nishie, MD, PhD, and Akihiro Tsukadaira, MD, for their initial treatment of the patient at Iida Municipal Hospital, Iida, Nagano, Japan.
Contributor Information
Yuichi Ikuyama, Email: ikuyama@shinshu-u.ac.jp.
Yosuke Wada, Email: yosuke@shinshu-u.ac.jp.
Kazunari Tateishi, Email: tateishi@shinshu-u.ac.jp.
Yoshiaki Kitaguchi, Email: kitaguti@shinshu-u.ac.jp.
Masanori Yasuo, Email: yasumasa@shinshu-u.ac.jp.
Atsuhito Ushiki, Email: atsuhito@shinshu-u.ac.jp.
Kazuhisa Urushihata, Email: ichiju@shinshu-u.ac.jp.
Hiroshi Yamamoto, Email: yama5252@shinshu-u.ac.jp.
Hiroshi Kamijo, Email: hkamijo@shinshu-u.ac.jp.
Atsuyoshi Mita, Email: mita@shinshu-u.ac.jp.
Hiroshi Imamura, Email: imamura@shinshu-u.ac.jp.
Masayuki Hanaoka, Email: masayuki@shinshu-u.ac.jp.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. China novel coronavirus investigating and research team, A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., H Liang W., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., C Hui D.S., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China medical treatment expert group for covid-19, clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. pii:S2213-2600(20) 300795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. Washington state 2019-nCoV case investigation team. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020 Mar 5;382(10):929–936. doi: 10.1056/NEJMoa2001191.Epub.2020.Jan.31. PubMed PMID: 32004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng S.C., Chang Y.C., Fan Chiang Y.L., Chien Y.C., Cheng M., Yang C.H., Huang C.H., Hsu Y.N. First case of coronavirus disease 2019 (COVID-19) pneumonia in taiwan. J. Formos. Med. Assoc. 2020 Feb 26;(20):30044–30049. doi: 10.1016/j.jfma.2020.02.007. pii: S0929-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K., Anderson D.E., Chan K.S., Tan T.Y., Ng T.Y., Cui L., Said Z., Kurupatham L., Chen M.I., Chan M., Vasoo S., Wang L.F., Tan B.H., Lin R.T.P., Lee V.J.M., Leo Y.S., Lye D.C. Novel coronavirus outbreak research team. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. J. Am. Med. Assoc. Singapore 2019 doi: 10.1001/jama.2020.3204. 2020 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in lombardy, Italy: early experience and forecast during an emergency response. J. Am. Med. Assoc. 2020 Mar 13 doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 12.Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M., Tse M.W., Que T.L., Peiris J.S., Sung J., Wong V.C., Yuen K.Y. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003;9:399–406. [PubMed] [Google Scholar]
- 13.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y., HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014 Aug;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020 Feb 13 doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected interim guidance 13 march 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Publication. acessed 15 March 2020.
- 18.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020 Feb15;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansbury L.E., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Shen Lim W. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated cochrane systematic review and meta-analysis. Crit. Care Med. 2020 Feb;48(2):e98–e106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Interim clinical guidance for management of patients with confirmed 2019 novel coronavirus (2019-nCoV) infection. February 12, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html Updated. accessed 14 March 2020.
- 21.Goligher E.C., Tomlinson G., Hajage D., Wijeysundera D.N., Fan E., Jüni P., Brodie D., Slutsky A.S., Combes A. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. J. Am. Med. Assoc. 2018;4(320):2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 22.MacLaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 23.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019. Pneumonia in wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]