Highlights
-
•
The elderly patients of COVID-19 have higher mortality rate.
-
•
Patients with underlying diseases of lung, heart and kidney are more vulnerable.
-
•
Dyspnea, age, neutrophilia, elevated ultra-TnI and D-dimers are risk factors.
Keywords: Coronavirus disease 2019 (COVID-19), Elderly, Clinical features, Short-term outcomes
Abstract
Background
The outbreak of Coronavirus Disease 2019 (COVID-19) has become a global public health emergency.
Methods
204 elderly patients (≥60 years old) diagnosed with COVID-19 in Renmin Hospital of Wuhan University from January 31st to February 20th, 2020 were included in this study. Clinical endpoint was in-hospital death.
Results
Of the 204 patients, hypertension, diabetes, cardiovascular disease, and chronic obstructive pulmonary disease (COPD) were the most common coexisting conditions. 76 patients died in the hospital. Multivariate analysis showed that dyspnea (hazards ratio (HR) 2.2, 95% confidence interval (CI) 1.414–3.517; p < 0.001), older age (HR 1.1, 95% CI 1.070–1.123; p < 0.001), neutrophilia (HR 4.4, 95% CI 1.310–15.061; p = 0.017) and elevated ultrasensitive cardiac troponin I (HR 3.9, 95% CI 1.471–10.433; p = 0.006) were independently associated with death.
Conclusion
Although so far the overall mortality of COVID-19 is relatively low, the mortality of elderly patients is much higher. Early diagnosis and supportive care are of great importance for the elderly patients of COVID-19.
On January 30th, with thousands of new cases of coronavirus disease 2019 (COVID-19) in China and the evidence of person-to-person spread in the United States and other countries emerging, the World Health Organization (WHO) declared the outbreak of COVID-19 constituted a Public Health Emergency of International Concern (World Health Organization, 2020a). As of 11th March 2020, there are more than 118,000 cases in 114 countries, and 4291 people have lost their lives, according to WHO (World Health Organization, 2020b). Considering the alarming levels of spread and severity, WHO characterized COVID-19 as a pandemic.
While the majority of patients (80%) experienced mild illness, the elderly patients (≥60 years old) tended to have more severe illness conditions. The reports (Wu and McGoogan, 2020, Wang et al., 2020, Chen et al., 2020, Huang et al., 2020) to date, indicated that elderly patients of COVID-19 were likely to have more serious illness, a selective analysis of clinical features for COVID-19 in the elderly remains to be performed. This retrospective case series study is designed to describe the clinical features and short-term outcomes of older patients with COVID-19 in Wuhan, China.
Methods
All patients with confirmed COVID-19 admitted to Renmin Hospital of Wuhan University from January 31 to February 20, 2020, were enrolled. This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University. All patients were diagnosed with COVID-19 according to the World Health Organization interim guidance (World Health Organization, 2020c). Of these, 204 patients were 60 years of age or older. Data, including age, gender, comorbid conditions, exposure history, symptoms, and initial chest radiographic presentation, hospitalization days, laboratory findings, survival data were collected from the electronic medical records. The clinical outcomes (ie, discharges, mortality, hospitalization days) were monitored up to February 20, 2020. The real-time reverse transcriptase-polymerase chain reaction (RT-PCR) test was performed to diagnose COVID-19 by testing respiratory samples (nasopharyngeal aspirates, throat swabs, or tracheal aspirates) as well as a stool sample if diarrhea was present. The RT-PCR assay was performed using a 2019-nCoV nucleic acid detection kit according to the manufacturer’s protocol (Shanghai bio-germ Medical Technology Co Ltd). A high-resolution CT scan of the thorax was performed for early diagnosis of pneumonic changes in cases where there was a definite history of exposure with respiratory symptoms. Classification of the Severity of all cases was made at the time of admission. The severity of symptoms variable was categorized as mild, severe, or critical. Mild cases included non-pneumonia and mild pneumonia cases. Severe was characterized by dyspnea, respiratory frequency ≥30/minute, blood oxygen saturation ≤93%, PaO2/FiO2 ratio <300, and/or lung infiltrates >50% within 24–48 h. Critical cases were those that exhibited respiratory failure, septic shock, and/or multiple organ dysfunction/failure (The Novel Coronavirus Pneumonia Emergency Response Epidemiology T, 2020).
Data analysis
Medians and interquartile ranges were calculated as summaries of continuous variables. For categorical variables, percentages of patients in each category were determined. Poor outcome was defined as the earliest of death. The time to death were investigated using survival analysis with follow up starting at hospital admission and ending on 20 February 2020. The primary end point was death. Patients were censored if at the end of the end of follow up they were still alive (for mortality). The Kaplan-Meier method was used for time-to-death plot. Comparisons between groups of time-to-death data were made using the Cox proportional hazards model. A multivariate Cox proportional hazards model was used to analyze independent risk factors for mortality from the three variables: age, onset of dyspnea, presence of any comorbidities. The same method was used to identify risk factors for adverse outcomes. SPSS (version 22.0) was used for all statistical analyses. A P value of less than 0.05 is considered statistically significant.
Results
The initial study cohort comprised of 209 elder patients. Five patients who contracted COVID-19 while in the hospital during a prolonged stay that began well before the COVID-19 outbreak (eg, for cancer) were excluded from this cohort because of difficulty in determining the inception of their disease, leaving 204 patients for subsequent analysis. The vast majority of patients (96%) received empirical antibiotic therapy during their hospitalization.
In these studies, 54 patients were discharged and 76 died. Most cases were classified as mild (64.7%) on admission. However, 33.3% were severe, and 2% were critical. The median age was 68 years (interquartile range (IQR),64–75; range, 60–95 years), and 104 (51%) were women.140 (68.8%) had one or more comorbidities. Hypertension (74 [36.3%]), diabetes (36 [17.6%]), cardiovascular disease (20 [14.5%]), and chronic obstructive pulmonary disease (COPD) (21 [10.3%]) were the most common coexisting conditions (Table 1 ). Nine patients had cancer and five had chronic renal failure.
Table 1.
Demographic Characteristics of the COVID-19 Patients.
| Study Population (N = 204) | No. (%) of Individuals |
|---|---|
| Median Age, y | 68 (60–95) |
| women | 104 (51) |
| Comorbidities | |
| Hypertension | 74 (36.3) |
| Cardiac disease | 44 (21.6) |
| Diabetes | 36 (17.6) |
| COPD | 21 (10.3) |
| Cancer | 9 (4.4) |
| Chronic renal failure | 5 (2.5) |
| Hospital Exposure history | 5 (2.5) |
| Time to admission(IQR), d | 10 (7–14) |
Abbreviations: COPDchronic obstructive pulmonary disease; COVID19coronavirus disease 2019.
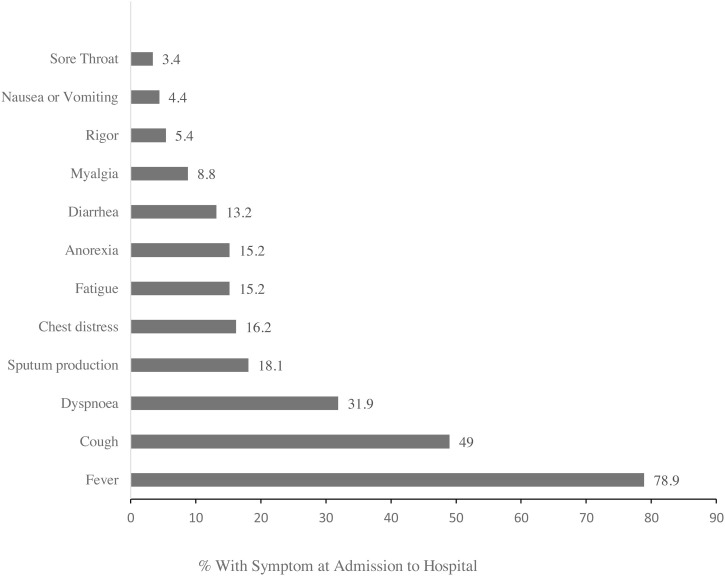
The main presenting symptoms included fever (78.9%), cough (49%), dyspnea (31.9%), sputum production (18.1%), chest distress (16.2%), fatigue (15.2%), anorexia (15.2%), diarrhea (13.2%), myalgia (8.8%). The relative frequencies of all reported symptoms at admission are shown in Fig. 1 .
Fig. 1.
Symptoms of COVID-19 Reported at Admission to Hospital (N = 204).
The median time between the onset of symptoms and admission was 10 days (7−14 (7–14 days). The IQR time from self-reported earliest known exposure to onset of symptoms was 11days (7−15days) for prodrome (fatigue or myalgia), 10 days (7–14 days) for self-reported fever, 11 days (8–14 days) for diarrhea, and 10 days (7–14 days) for cough or dyspnea.
Laboratory data on admission are shown in Table 2 . 49.10% of patients of the cohort presented with lymphopenia. The median (IQR) lymphocyte count was 0.90 (0.55–1.39) ×109/L on admission. Most patients had mild anemia, hemoglobin levels decreased in 117 (84.8%) patients. 152 (89.9%) patients’ albumin levels decreased. 63% of patients had high serum concentration of procalcitonin (PCT), related to severe bacterial and fungal infection.
Table 2.
Laboratory Data upon Hospital Admission.
| Reference Values | Median Value (IQR) | No./Total (%) Abnormal |
|
|---|---|---|---|
| White blood cell count, ×109/L | 3.5−9.5 | 5.94 (4.22–8.53) | 53/173 (30.64) |
| Neutrophil count, ×109/L | 1.8−6.3 | 4.30 (2.69–7.36) | 68/173 (33.33) |
| Lymphocyte count, ×109/L | 1.1−3.2 | 0.90 (0.55–1.39) | 85/173 (49.10) |
| Monocyte count, ×109/L | 0.1−0.6 | 0.40 (0.26−0.58) | 40/171 (23.40) |
| hemoglobin, g/L | 130−175 | 121 (111–132) | 117/173 (84.8) |
| Platelet count, ×109/L | 125−350 | 203 (138–258) | 49/173 (28.3) |
| Alanine aminotransferase, U/L | 9−50 | 24 (17–40) | 83/171 (48.5) |
| Aspartate aminotransferase, U/L | 15−40 | 30 (21–47) | 76/171 (44.4) |
| Total bilirubin, mmol/L | 0−23 | 11.30 (8.55) | 21/169 (12.4) |
| Albumin, g/L | 40−55 | 35.20 (32.29–39.25) | 152/169 (89.9) |
| Creatinine, μmol/L | 57−111 | 63.00 (50.00–81.00) | 80/171 (46.8) |
| Procalcitonin, ng/mL | <0.1 | 0.17 (0.05–2.71) | 92/146 (63.0) |
| INR | 0.76−1.24 | 1.06 (0.99–1.13) | 11/161 (6.8) |
| D-Dimer, mg/L | 0−0.55 | 1.09 (0.47–4.82) | 112−161 (69.6) |
| CKMB, ng/mL | 0−5 | 1.60 (0.90–2.71) | 15/148 (10.1) |
| ultra-TnI, ng/mL | 0−0.04 | 0.84 (0.03–2.85) | 60/81 (74.1) |
| PH | 7.35−7.45 | 7.41 (7.36–7.46) | 49/109 (44.95) |
| Lactate, mmol/L | 0.5−1.5 | 2.30 (1.90–3.15) | 92/109 (84.4) |
| pO2, mmHg | 80−100 | 73.00 (52.00–93.50) | 91/109 (83.5) |
| pCO2, mmHg | 35−45 | 39.00 (34.00–43.50) | 52/109 (47.7) |
| O2Sat, % | 95−98 | 94 (87–97) | 56/109 (51.4) |
Abbreviations: IQR, interquartile range; INR, international normalized ratio; CKMB, creatine kinase-MB; ultra-TnI, Ultrasensitive cardiac troponin I; PH, Pondus Hydrogenii; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; O2Sat, oxygen saturation.
Chest radiography images on the admission of most patients were abnormal. The unilateral and bilateral infiltrates were observed in 5.8% and 94.2% of patients respectively. Although there were various patterns of the infiltrates (focal, lobar, diffuse), most patients had multifocal opacities.
Up to February 20,2020, 74 patients (36.3%) were still hospitalized. A total of 54 patients (26.5%) had been discharged, and 76 patients (37.3%) had died. Of the 76 patients who died, 6 received invasive ventilation and 17 to noninvasive ventilation.
Respiratory failure was the most frequently observed complication, followed by sepsis, acute respiratory distress syndrome (ARDS), heart failure, and septic shock, coagulopathy, acidosis. All the patients died of respiratory failure. Besides the lung, the most commonly damaged organs were the heart (27 patients’ serum ultrasensitive cardiac troponin I (ultra-TnI) or/and creatine kinase MB (CKMB) levels increased), kidney (29 patients’ creatinine (Cr) levels increased) and liver (14 patients’ serum Alanine transaminase (ALT) and 29 patients’ aspartate aminotransferase (AST) levels increased). Besides, all the patients' albumin levels decreased.
The vast majority of patients (98.5%) received some sort of antiviral therapies within the first 48 h of hospitalization such as ribavirin, interferon, lopinavir/ ritonavir, arbidol, ganciclovir, oseltamivir. Most patients (83.8%) received broad-spectrum antibiotic therapy during their hospitalization including a β-lactam/β-lactamase inhibitor or a cephalosporin, together with a macrolide. Most patients (74%) required oxygen therapy. Glucocorticoid therapy was used to treat 70 (34.3%) patients if the clinical condition, radiological presentation, or oxygen saturation worsened (at least two out of the three) and lymphopenia persisted. Intravenous immunoglobulin was used to enhance the ability of anti-infection for 77 patients. Three patients with end-stage kidney disease received kidney replacement therapy. In addition to conventional treatment, appropriate partial parenteral nutrition support was given for critically ill patients.
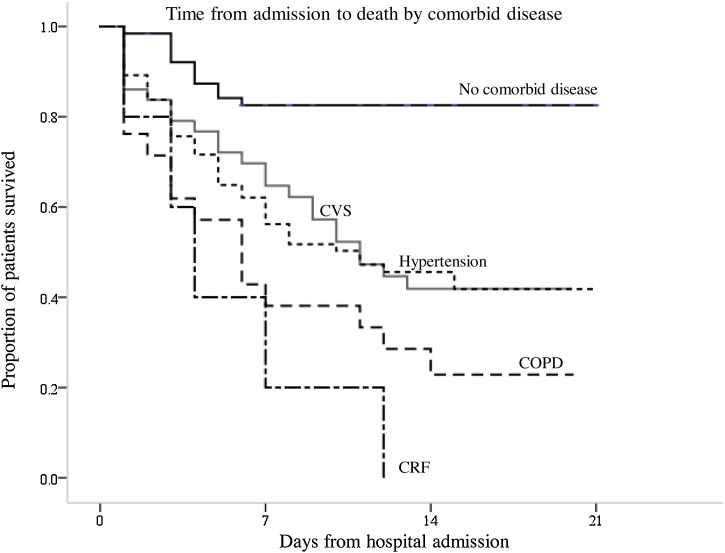
The univariate Cox proportional hazards model showed that the mortality risk was 5.3 times of that in those aged above 70 (95% confidence interval (CI) 3.1–9.0; p < 0.001). For every 5 years increase in age, the risk of death increased by 1.55. However, there was no difference in the proportion of men and women with mortality. The initial State of COVID-19 at admission is closely associated with mortality in the elderly. Severe or critical cases were associated with an increased mortality risk of 10 (95% CI 5.4–14.6; p < 0.001).The presence of any comorbidities increased the mortality risk (hazards ratio (HR) 3.1, 95% CI 1.6–5.8; p = 0.001), with COPD (HR 3.1, 95%CI 1.8–5.4 ; p < 0.001) and chronic renal failure (HR 4.2, 95% CI 1.7–10.5; p = 0.002), being the most important comorbidities (Table 2). The presence of cardiovascular disease with an increased mortality of 1.8 (95% CI 1.1–2.9; p = 0.016), similar to hypertension with an increased mortality of 2.3 (95% CI 1.5–3.6; p < 0.001) (Fig. 2 ).Other comorbidities factors (cancer and diabetes mellitus) had no significant effects on mortality. In addition, onset of dyspnea before admission increased the mortality risk (HR 2.0, 95% CI 1.3–3.2; p = 0.002). Leucocytosis, neutrophilia, pH values, CKMB levels, ultra-TnI, d-dimer, albumin, procalcitonin, partial pressure of carbon dioxide, the partial pressure of oxygen, and oxygen saturation were also associated with death (Table 3 ). The multivariate Cox proportional hazard model was used to analyzed independent risk factors for mortality. Using a model with age, onset of dyspnea, presence of any comorbidities, it was found that the onset of dyspnea (HR 2.2, 95% CI 1.414–3.517; p < 0.001),age (HR 1.1, 95% CI 1.070–1.123; p < 0.001), neutrophilia (HR 4.4, 95% CI 1.310–15.061; p = 0.017) and elevated ultra-TnI (HR 3.9, 95% CI 1.471–10.433; p = 0.006) were all independent predictors of death.
Fig. 2.
Survival analysis showing time from admission to death by comorbid. conditions. COPD = chronic obstructive pulmonary disease; CVS = cardiovascular disease; CRF = chronic renal failure.
Table 3.
Analysis of risk factors for mortality.
| Hazards ratio (95% CI) | p value | |
|---|---|---|
| Univariate analysis | ||
| Sex (female) | 0.7 (0.4–1.1) | 0.084 |
| Age(≥70years) | 5.3 (3.1–9.0) | <0.001 |
| Presence of any comorbidity | 3.1 (1.6–5.8) | 0.001 |
| Hypertension | 2.3 (1.5–3.6) | <0.001 |
| CVS | 1.8 (1.1–2.9) | 0.016 |
| Diabetes | 1.1 (0.6–1.9) | 0.877 |
| COPD | 3.1 (1.8–5.4) | <0.001 |
| Cancer | 1.2 (0.5–3.4) | 0.682 |
| Chronic renal failure | 4.2 (1.7–10.5) | 0.002 |
| Dyspnea | 2.0 (1.3–3.2) | 0.002 |
| White blood cell count (>9.5 × 109/L) | 7.0 (4.2–11.6) | <0.001 |
| Neutrophil count (>6.3 × 109/L) | 2.9 (1.8–4.7) | <0.001 |
| Lymphocyte count (<1.1 × 109/L) | 1.0 (0.6–1.6) | 0.894 |
| PH (<7.35) | 2.1(1.3–3.5) | 0.003 |
| pO2 (<80 mmHg) | 3.1 (1.3–7.8) | 0.015 |
| pCO2 (>45 mmHg) | 1.7 (1.1–2.9) | 0.028 |
| O2Sat (<95%) | 5.1 (2.9–9.1) | <0.001 |
| Procalcitonin (<0.1 ng/mL) | 28.9 (7.1–118.5) | <0.001 |
| D-Dimer (>0.55 mg/L) | 36.6 (5.1–264.2) | <0.001 |
| Ultra-TnI (>0.04 ng/mL) | 2.8(1.4–5.5) | 0.004 |
| CKMB (>5 ng/mL) | 5.3 (2.9–9.7) | <0.001 |
| Albumin (<40 g/L) | 4.2 (1.0–17.3) | 0.044 |
| Multivariable analysis | ||
| Age(≥70years) | 1.1 (1.070–1.123) | <0.001 |
| Dyspnea | 2.2 (1.414–3.517) | <0.001 |
| Neutrophil count (>6.3 × 109/L) | 4.4 (1.310–15.061) | 0.017 |
| Ultra-TnI (>0.04 ng/mL) | 3.9 (1.471–10.433) | 0.006 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CVS, Cardiovascular disease: 95% CI, 95% confidence interval; CKMB, creatine kinase-MB; ultra-TnI, Ultrasensitive cardiac troponin I; PH, Pondus Hydrogenii; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; O2Sat, oxygen saturation.
Discussion
We conducted a cohort of 204 elder patients over 60 years who were hospitalized with COVID-19 in Wuhan, China. We observed similar clinical features as recently reported COVID-19. The most common symptoms were fever, cough, and dyspnea. Fever was the first symptom reported by many patients (78.9%). The onset of dyspnea might help physicians identify the patients with poor prognosis.35.3% patients were classified as severe or even critical cases on admission. The overall rate of serious illness was higher than those reported in previous studies. In addition, a significant portion of patients showed bilateral infiltrate chest radiograph results on admission.
Univariate analysis showed age of 70 years or older, comorbidity (hypertension, cardiovascular disease, COPD, chronic renal failure), onset of dyspnea, and several laboratory indices abnormalities were associated with poor outcome. In our multivariable Cox proportional hazards model, dyspnea, older age, neutrophilia and elevated ultra-TnI were independently associated with poor outcomes.
In this study, we reported 76 death of COVID-19. Most patients had pulmonary consolidation and hypoxemia which was difficult to recover. The clinical characteristics of these patients indicated that the age and underlying diseases were the most important risk factors for death. For every 5 years increase in age, the risk of death increased by 1.55. The most common underlying disease was hypertension, followed by cardiovascular disease, diabetes, COPD, malignant tumors, and kidney disease. The effects of age and comorbidities have also been addressed in other cohorts of patients with COVID-19 (Wang et al., 2020, Chen et al., 2020). The presence of cancer or diabetes mellitus was related to a higher mortality rate or an adverse outcome in other studies (Wang et al., 2020), which could predispose to superimposed nosocomial pneumonia due to Staphylococcus aureus or other agents in these already critically ill patients, although this was not observed in our patients. However, the presence of COPD, chronic renal failure, cardiovascular disease or hypertension was related to a higher mortality rate in our data.
The patients with underlying disease of lung, heart, and kidney are more vulnerable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), most likely because angiotensin converting enzyme II (ACE2) protein has an abundant expression in many kinds of cells, such as renal tubular epithelial cells, alveolar epithelial cells, heart, artery smooth muscle cells (Hamming et al., 2004). The coronavirus has a three-dimensional structure of spike protein, which is closely bound to human cell receptor ACE2. Therefore, the cells with ACE2 expression may act as target cells and be susceptible to COVID-19 infection, such as type II alveolar cells (AT2) in the lung (Zhou et al., 2020a).
In terms of laboratory tests, the count of lymphocytes in most patients was reduced. This result suggests that COVID-19 is a viral disease characterized by decreased lymphocyte count, like those recently reported (Wang et al., 2020, Chen et al., 2020, Zhou et al., 2020b). Although lymphopenia has been commonly observed, the absolute lymphocyte count was not associated with poor outcome in our study. Most patients’ albumin levels and 84.8% hemoglobin levels were decreased, which indicates that malnutrition is common to elderly patients. Elevated alanine aminotransferase and aspartate aminotransferase levels were prevalent on admission in our cohort. High levels of D-dimer was found in more than half of patients with infection. These findings are quite different from those associated with pneumonia caused by common bacterial pathogens, but similar to those previously observed in patients with SARS-CoV infection (Lee et al., 2003, Centers for Disease C, Prevention, 2003). The routine blood test and PCT were used to reflect changes in the inflammatory response in COVID-19. Increased white blood cell and neutrophils count were observed in 30.64% and 33.33% of patients. In particular, a high neutrophil count was an independent predictor for poor outcome. Neutrophilia was observed during the cytokine storm induced by virus infection (Guo and Thomas, 2017, Henderson et al., 2020, Barnes et al., 2020). In the study of Betsy et al, after autopsy they showed neutrophils infiltrating lung in the context of a cytokine storm triggered ARDS and caused organ damage and mortality in COVID-19 (Barnes et al., 2020). Once a cytokine storm is formed, the immune system will be over-activated and immune cells spread beyond infected body parts and start attacking healthy tissues which will seriously damage the lung function and resulted in dyspnea and acute respiratory failure when fluid builds up in the air sacs in lungs. Elevated levels of ultra-TnI was more commonly seen in COVID-19. Cardiac complications were common in patients with pneumonia in our cohort. The high level of ultra-TnI was another independent predictor for poor outcome. The present understanding of the human cardiovascular response to infections, including pneumonia, is derived mainly from studies of critically ill patients with septic shock (Corrales-Medina et al., 2013). Further research is needed to investigate the pathogenesis of sepsis in COVID-19 illness.
In the largest case series to date of COVID-19, a total of 1023 deaths have occurred among 44,672 confirmed cases for an overall case fatality rate of 2.3% (Wu and McGoogan, 2020), and the ≥80 age group had the highest case fatality rate of all age groups at 14.8%. However, in our case series,≥ 60 years old age group had a case fatality rate of 37.3% which is much higher. The possible explanation is that the cases in our cohort are from the stage of the epidemic outbreak. Most of the patients have to be isolated at home without medical support due to a lack of inpatient beds in the hospital. Two-third of patients in our studies had received empirical therapy out of hospital over ten days before their admission with COVID-19. So that their illness was from mild to severe. Moreover, the case fatality rate of is unsurprisingly highest among critical cases at 75%. However, it is difficult to calculate the true mortality rate of the disease while the epidemic is continuing and it is impossible to ascertain which of the remaining patients will eventually die or be discharged.
Until now, no specific antiviral treatment has been recommended for coronavirus infection. Once infected, the Older patients are harder to treat without supportive care. Currently, the approaches to control this disease is to prevent the sources; use of personal protective equipment to reduce the transmission; and early diagnosis, isolation, and supportive treatments. This study suggests that the elderly patients of COVID-19 have a rapid course of the disease and a higher case fatality ratio. Severe cases on admission were often subjected to higher death rates. According to current diagnostic criteria, viral nucleic acid test results confirmed by RT-PCR assay play a vital role in determining whether to hospitalize a patient. The overall sensitivity of the RT-PCR test for coronavirus in patients was only 59% (Ai et al., 2020) which needs to be improved. This might be affected by sample quality, the methods of obtaining the samples, as well as the viral load. Early diagnosis and supportive care in the hospital are of great importance for the elderly COVID-19 patients. The elderly patient with acute dyspnea should seek medical attention immediately.
The results of this study must be interpreted with caution and this research is subject to several limitations. This was a retrospective case series study based on data from medical records. clinical notes and patient charts. Accordingly, certain information was missing for various patients, and certain data that may have been based on patient memory, such as details concerning exposure history and timing of onset of symptoms, maybe affected by recall bias. Not all laboratory tests were done in all patients. Laboratory parameter is not all documented in our studies. Finally, in an effort to quickly disseminate information to clinicians worldwide, we only assessed short-term outcomes. The follow-up evaluation to determine the long-term repercussions of this disease need to be done in the future.
In conclusion, the elderly patients of COVID-19 have a higher mortality rate. Our data and analysis suggest that elderly patients with comorbidities need more medical care. And dyspnea, age, neutrophilia, elevated ultra-TnI and elevated D-dimer at admission are risk factors for mortality for the elderly patients of COVID-19. Early diagnosis and supportive care are of great importance for the elderly COVID-19 patients.
Declaration of interests
We declare no conflict of interest.
Funding
This research was supported by grant No. 2014CFB394, 2019CFB721 from the N atural Science Foundation of Hubei Province (CN) and No. WJ2017M027 from H ealth and Family Planning Commission of Hubei Province (CN).
Ethical approval
This retrospective study was approved by the institutional review board of Renmin Hospital of Wuhan University (No. WDRY2020-K080).
Author contributions
LX and CF made substantial contributions to the study concept and design. LP was in charge of the manuscript draft. LX, WH, RS took responsibility for obtaining ethical approval and collecting samples. PJ, MQ, and ZD made substantial contributions to data acquisition. FZ and SQ processed statistical data. GH and XT reviewed the data. CL and LZ made substantial revisions to the manuscript.
References
- World Health Organization . 2020. WHO Timeline - COVID-19.https://wwwwhoint/news-room/detail/08-04-2020-who-timeline---covid-19 [Google Scholar]
- World Health Organization . 2020. Novel coronavirus(2019-nCoV): situation report 51.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;6736(20):30211–30217. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interimguidance. Published January 28.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected# Accessed January 31, 2020. [Google Scholar]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology T The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. China CDC Weekly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;1038(3):020–2012. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Centers for Disease C, Prevention Preliminary clinical description of severe acute respiratory syndrome. MMWR Morb Mortal Wkly Rep. 2003;52(12):255–256. [PubMed] [Google Scholar]
- Guo X.J., Thomas P.G. New fronts emerge in the influenza cytokine storm. Semin Immunopathol. 2017;39(5):541–550. doi: 10.1007/s00281-017-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.A., Canna S.W., Schulert G.S. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020 doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales-Medina V.F., Musher D.M., Shachkina S., Chirinos J.A. Acute pneumonia and the cardiovascular system. Lancet. 2013;381(9865):496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- Ai T., Yang Z., Hou H. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]