Managing the immediate demands of the current coronavirus disease 2019 (COVID-19) global pandemic has tested many healthcare systems across the world, to their limits. As we move forward, new challenges resulting from the impact of this must be faced. In the months since the initial outbreak of COVID-19 in December 2019, worldwide there have been more than 7.5 millions cases of infection with the severe acute respiratory syndrome coronavirus-2 (SARS-Cov-2) reported.1 However, the rapidity of spread appears to be slowing, the curve is flattening in many countries, and attention is now turning towards how the international healthcare community will address the ongoing needs of those most significantly affected by the pandemic. Recent UK data (covering February–April 2020) suggests 17% of cases admitted to hospital require support in high dependency or critical care environments, and of those more than 50% require mechanical ventilation.2 About 20% of those requiring mechanical ventilation will be discharged with a further 27% receiving ongoing care. Critical care survival in other countries including Italy, USA, and China has been reported as 16–37%, although many cohorts include those receiving ongoing care in ICU.3, 4, 5 Given the number of global infections, this suggests a cohort of critically ill survivors of unprecedented size.
The treatment needs of COVID-19 survivors are not yet fully appreciated. Although initially assumed to be a respiratory disease, it is now clear that it affects a variety of systems. Multi-organ failure can occur, with reports of cardiac, renal, haematological, and neurological effects in the acute stages. It is likely, therefore, that these survivors will have significant multi-domain impairment requiring ongoing support. There has been a recent ‘call to action’ amongst the rehabilitation community to act quickly to ensure adequate resources to provide early phase, multidisciplinary interventions to promote physical and psychological recovery.6
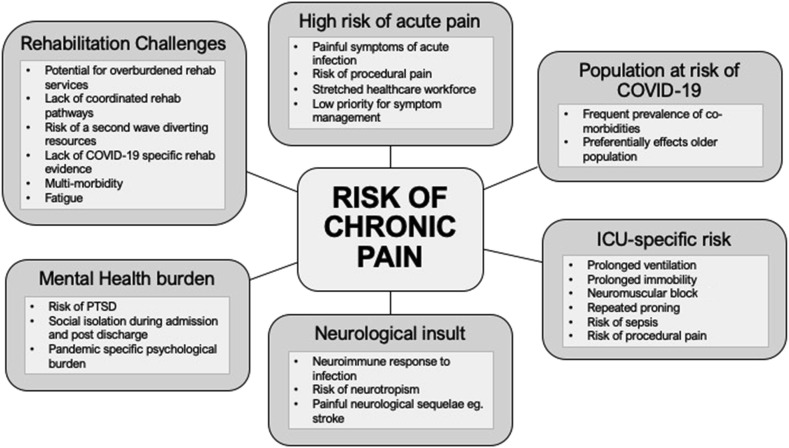
We can perhaps learn from previous studies of critical care survivorship, which has been relatively neglected until recently. This complex challenge has been termed post-intensive care syndrome (PICS).7 It incorporates the cognitive, physical, and psychological dysfunction reported after ICU discharge that can have profound effects on quality of life. Chronic pain is often part of this, but how this additional co-morbidity affects critical care survivors is poorly understood. Estimates of chronic pain prevalence after ICU vary from 14% to 77% depending on timescale, method of measurement, and population.8 Pain also appears to be an important factor affecting ability to return to work and quality of life up to 5 yr after discharge.9 It is likely that those surviving critical illness with COVID-19 will be at particular risk of developing chronic pain. There are a number of reasons why this may be the case (Fig 1 ).
Fig 1.
Potential risk factors for development of chronic pain after COVID-19. COVID-19, coronavirus disease 2019; PTSD, post-traumatic stress disorder.
Because a consistent risk factor for chronic pain development is the occurrence of acute pain, it is worth considering how this is managed in the ICU. Those recalling higher pain and distress during ICU admission appear to be at higher risk of developing chronic pain after discharge.10 Unfortunately, even in quiet periods on ICU, pain is an often neglected symptom receiving low priority and surprisingly poor assessment and management given the highly staffed, well-skilled environment.11 Guidelines to improve pain assessment and management in ICU have been developed in the USA and Europe, and initiatives such as the ICU Liberation ABCDEF bundles of care have been adopted in some centres. These are aimed at improving long-term outcomes through multidisciplinary management of symptoms, mobility, and communication.12 , 13
However, these processes, which often involve non-pharmacological strategies, are labour intensive and realistically may be unachievable in current pandemic conditions. Furthermore, during this outbreak, the ICU workforce has been stretched beyond its capacity with patients being treated, through necessity, by staff with rapidly scaled-up training in units with reduced staffing ratios.14 There is therefore the potential that non-lifesaving symptomatic control may have been further neglected. The critically ill undergo a significant pain burden during everyday procedures in ICU, such as tracheal tube suctioning, turning, positioning, and line insertion.15 Owing to the severity of COVID-19 critical illness, it is likely that survivors will have undergone multiple pain-associated interventions.
COVID-19 survivors are likely to have sustained a prolonged period of immobilisation, sedation, and ventilation,5 putting them at high risk of associated ICU-acquired weakness (ICUAW). Commonly manifesting as any combination of critical illness myopathy (CIM), critical illness polyneuropathy (CIN), and muscle atrophy, known risk factors include the use of neuromuscular block and corticosteroids, the presence of sepsis and multi-organ dysfunction, and prolonged mechanical ventilation.16 Neuromuscular block is now highlighted in several guideline publications as a strategy to improve ventilation in those with acute respiratory distress syndrome (ARDS) associated with COVID-1917 , 18; although there is no consensus, some recommendations also include use of corticosteroids in certain populations.19 The prevalence of ICUAW in the general ARDS population is estimated at 25–96%,20 and although reported after the Middle East respiratory syndrome (MERS) epidemic21 it is yet to be determined in those critically ill with COVID-19. Although the focus of ICUAW is often the motor component, there is growing evidence for sensory disruption and associated pain. Weakness can lead to rapid deconditioning, joint-related pain, and contractures and, although mechanisms remain unclear, shoulder pain in particular has been highlighted as a significant problem in the post-ICU population.22
A mainstay of respiratory support through the COVID-19 pandemic has been use of repeated patient proning to improve ventilation.17, 18, 19 Complications associated with proning sedated patients include brachial plexopathy, joint subluxation, and soft tissue damage. These have the potential to result in persistent neuropathic and musculoskeletal pain.23
Neuropathic symptoms including numbness, paraesthesia, and pain are well documented after critical illness with abnormalities in nerve conduction studies demonstrated up to 5 yr after ICU discharge.24 Even in the absence of electrophysiological abnormalities, small nerve fibre impairment associated with neuropathic symptoms can persist for several months.25 Reports of neurological sequelae of COVID-19 infection are emerging, indicating both central and peripheral nervous system involvement; symptoms such as confusion, headache and dizziness, and anosmia, ageusia, and nerve pain are now described in retrospective cohorts and case reports.26 This has led to speculation of potential neurotropism, with both muscle and neural tissue expressing angiotensin-converting enzyme 2 (ACE2) receptor, the functional receptor for SARS-CoV-2.27 The related SARS-CoV virus is also associated with neural injury, including axonopathic polyneuropathy,28 and has been detected in both the CSF and brain tissue.29 There are ongoing efforts to determine which human cells are susceptible to SARS-CoV-2 infection, but direct neural invasion has not yet been demonstrated.30
Regardless of direct neural entry, SARS-CoV-2, like SARS and MERS, appears to have the capacity to induce painful para-infectious neurological disease as shown by a number of case reports of Guillain–Barré syndrome31 and polyneuritis.32 Thrombotic, hypotensive, and hypoxaemic consequences of infection can also contribute to longstanding, potentially painful neurological sequelae such as stroke. Renal dysfunction is also common and may be associated with a peripheral neuropathy, particularly if renal impairment persists after the acute injury. A further aspect to consider is neuropathic pain as a side-effect of putative therapeutic agents currently under investigation for modifying disease severity, such as lopinavir/ritonavir and hydroxychloroquine.
It is now clear that COVID-19 itself is associated with painful symptoms, including myalgia, arthralgia, abdominal pain, headache, and chest pain, and even those not admitted to critical care environments may have pain requiring opioids for symptom management.33
An important area to recognise is the psychological impact of COVID-19, with the unique social restrictions likely to create an additional burden. Severe psychological sequelae have been reported in ICU survivors with up to 30% of ARDS survivors developing post-traumatic stress disorder (PTSD).34 In COVID-19 this may be augmented by separation from personal protective equipment (PPE) adding to the already alien environment, breakdown of social networks, and fear of mortality; this increases the potential for development of PTSD, anxiety, and depression, as observed in the SARS outbreak.35 Pain is thought to have a bidirectional relationship with such psychological factors: in the acute phase it may be a risk factor contributing to the development of mental health co-morbidities, with chronic pain being a well-recognised co-morbidity. Even baseline patient characteristics, identified as factors associated with the development of severe COVID-19, overlap with those associated with chronic pain after critical illness, including multi-morbidity and increasing age.36 It is also likely that those with pre-existing multi-morbidity were at higher risk of chronic pain before infection, which may predispose them to exacerbation of current or development of new pain conditions.37
Emerging reports from Wuhan, which is now operating several rehabilitation institutions for COVID-19 survivors, and Italy indicate a significant symptom burden in COVID-19 survivors including anxiety, sleep disorders, fatigue, limited exercise tolerance, and memory and executive function impairment.38 Such symptoms are likely to be exacerbated or even attributed to pain, although this is yet to be explored. What remains unclear is the level of rehabilitation that will be possible for different countries in the early phase of recovery. Early intervention including adequate pain management, and psychological and physical therapy has the potential to reduce the risk of long-term pain and other features of PICS.39 However, currently resources are focused on frontline services, which may leave limited support for such an unprecedented cohort of patients.
There is conflicting evidence on the beneficial effects of post-ICU rehabilitation strategies in general on exercise tolerance and health-related quality of life in the pre-COVID era.40 , 41 Qualitative evaluation suggests increased patient satisfaction and reduced anxiety.42 Although pain forms a component of health-related quality of life measures, specific research into the effect of post-ICU rehabilitation on pain has never been formally evaluated. The majority of studies on efficacy of pain management and post-critical illness rehabilitation have focused on face-to-face delivery, often in a group-based setting. Such traditional models of care may not be possible for some time, with ongoing social distancing and diversion of healthcare resources. We therefore must develop and assess innovative ways to deliver therapy that is accessible to those who need it. Telemedicine and promotion of self-management programmes are being explored for this cohort, and may become part of the ‘new normal’ for delivery of this type of service. Yet for some vulnerable patient groups (e.g. older, cognitively impaired, high deprivation), access may be problematic.
Stratifying patients to high-intensity or speciality-specific rehabilitation through a stepped care model may be required but is difficult given the lack of specific COVID-19 research and experience. Extrapolation of best practice evidence from other cohorts will be required. Historically, rehabilitation for survivors of critical illness has been disease specific. For example cardiac patients may get streamed to a cardiac rehabilitation pathway; those with chronic respiratory disease to pulmonary rehabilitation; those with a stroke to post-stroke resources. However, this was problematic for two reasons: firstly, these classes and pathways were not designed to address the additional burden of PICS in addition to the patients underlying condition, and secondly, there was a large proportion of patients that did not fall into these categories, ‘slipped through the net’, and received sub-optimal care.
Several models of more contemporary general ICU follow-up clinics currently exist,43 but they are by no means universal. It is likely that these have not been subject to the number of patients who will need their services in the foreseeable future. The make-up of such services may also need to be adjusted to address COVID-19-specific sequelae, and this may represent an opportunity to develop better links between pain and ICU survivorship programmes, and improving dialogue with other specialties such as renal, respiratory, and mental health to build existing collaborations and manage multi-morbidity. Pain services are traditionally multidisciplinary, incorporating physical and psychological expertise with the goal of improving function and quality of life, and could therefore have a great deal to offer overwhelmed critical care services. Such integrated follow-up pathways also provide an opportunity to develop embedded research and registries to learn more about the features, aetiology, risk factors, and therapeutic interventions for chronic pain after critical illness, an as yet neglected area of critical care survivorship.
In the rapidly changing clinical environment, flexibility and changes to health and social care delivery are required. Whilst the trajectory of this pandemic has not given us the luxury of developing a high-quality evidence base on which to base our management decisions, it is beholden on us to critically assess what we are doing. Perhaps now more than ever we need to work collaboratively to assess interventions used in rehabilitation of post-COVID-19 patients. There is the opportunity to use a similar approach to that of some clinical trials of acute interventions (such as RECOVERY; https://www.recoverytrial.net/), where adaptive trial design allows rapid evaluation of a range of potential COVID-19 treatments. Although the acute challenges of managing COVID-19 have been significant, it may be the long-term effects, including pain, that will have the greatest impact on survivors and society, As an academic community, understanding post-COVID-19 effects and ensuring a strong evidence base for how to manage these is vital for patients, health and social care systems, and for policy makers.
Authors' contributions
Devised the topic of the manuscript: LC, HK
Drafted sections and final version of the manuscript: HK, LC, EC
Funding
National Institute for Health Research (HK).
Declarations of interest
LC is an editor for the British Journal of Anaesthesia. HK is a fellow with the British Journal of Anaesthesia Peer Review Fellowship Programme.
References
- 1.Johns Hopkins University & Medicine. Coronavirus Resource Centre. Available at: https://coronavirus.jhu.edu/map.html (accessed 11th June 2020).
- 2.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO clinical characterisation protocol. COVID-19 SARS-CoV-2 bioRxiv. April 2020 doi: 10.1101/2020.04.23.20076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020 doi: 10.1056/nejmoa2004500. Epub March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stam H.J., Stucki G., Bickenbach J. Covid-19 and post intensive care syndrome: a call for action. J Rehabil Med. 2020;52:jrm000044. doi: 10.2340/16501977-2677. [DOI] [PubMed] [Google Scholar]
- 7.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 8.Kemp H.I., Laycock H., Costello A., Brett S.J. Chronic pain in critical care survivors: a narrative review. Br J Anaesth. 2019;123:e372–e384. doi: 10.1016/j.bja.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuthbertson B.H., Roughton S., Jenkinson D., Maclennan G., Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntillo K.A., Max A., Chaize M., Chanques G., Azoulay E. Patient recollection of ICU procedural pain and post ICU burden: the Memory Study. Crit Care Med. 2016;44:1988–1995. doi: 10.1097/CCM.0000000000001875. [DOI] [PubMed] [Google Scholar]
- 11.Kemp H.I., Bantel C., Gordon F., et al. Pain Assessment in INTensive Care (PAINT):an observational study of physician-documented pain assessment in 45 intensive care units in the United Kingdom. Anaesthesia. 2017;72:737–748. doi: 10.1111/anae.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devlin J.W., Skrobik Y., Gélinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Car Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 13.Das-Tasforce 2015, Baron R., Binder A., et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Ger Med Sci. 2015;13:1612–1654. doi: 10.3205/000223. Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunhill L. Intensive care staffing ratios dramatically diluted. Health Serv J. Mar 2020;23 https://www.hsj.co.uk/exclusive-intensive-care-staffing-ratios-dramatically-diluted/7027214 [article] [Google Scholar]
- 15.Puntillo K.A., Max A., Timsit J.F., et al. Determinants of procedural pain intensity in the intensive care unit: the Europain® study. Am J Respir Crit Care Med. 2014;189:39–47. doi: 10.1164/rccm.201306-1174OC. [DOI] [PubMed] [Google Scholar]
- 16.De Jonghe B., Sharshar T., Lefaucheur J.-P., et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 17.Phua J., Weng L., Ling L., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorbello M., El-Boghdadly K., Di Giacinto I., et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75:724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 19.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Int Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zorowitz R.D. ICU–acquired weakness: a rehabilitation perspective of diagnosis, Treatment, and functional management. Chest. 2016;150:966–971. doi: 10.1016/j.chest.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Kim J.E., Heo J.H., Kim H.O., et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafson O.D., Rowland M.J., Watkinson P.J., McKechnie S., Igo S. Shoulder impairment following critical illness. Crit Care Med. 2018;46:1769–1774. doi: 10.1097/CCM.0000000000003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goettler C.E., Pryor J.P., Reilly P.M. Brachial plexopathy after prone positioning. Crit Care. 2002;6:540–542. doi: 10.1186/cc1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher S.N., Kennedy D.D., Ghosh I.R., et al. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012–1016. doi: 10.1097/01.CCM.0000053651.38421.D9. [DOI] [PubMed] [Google Scholar]
- 25.Angel M., Bril V., Shannon P., Herridge M. Neuromuscular function in survivors of the acute respiratory distress syndrome. Can J Neurol Sci. 2007;34:427–432. doi: 10.1017/s0317167100007307. [DOI] [PubMed] [Google Scholar]
- 26.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. Epub Apr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L., Kitch D., Evans S., et al. Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology. 2007;68:2113–2119. doi: 10.1212/01.wnl.0000264888.87918.a1. [DOI] [PubMed] [Google Scholar]
- 28.Tsai L., Hsieh S., Chang Y. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. 2005;14:113–119. [PubMed] [Google Scholar]
- 29.Ding Y., He L., Zhang Q., et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis virus transmission pathways. J Pathol. 2004;203:622–623. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The human cell atlas. 2020. https://www.humancellatlas.org/covid-19/ Available at:
- 31.Zhao H., Shen D., Zhou H., et al. Guillain–Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutiérrez-Ortiz C., Méndez A., Rodrigo-Rey S., et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. Apr 2020 doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 33.Lovell N., Maddocks M., Etkind S.N., et al. Characteristics, symptom management and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manage. 2020 doi: 10.1016/j.jpainsymman.2020.04.015. Epub 15 Apr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikayin S., Rabiee A., Hashem M., et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS) J Trauma Stress. 2005;18:39–42. doi: 10.1002/jts.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battle C.E., Lovett S., Hutchings H. Chronic pain in survivors of critical illness: a retrospective analysis of incidence and risk factors. Crit Care. 2013;17:R101. doi: 10.1186/cc12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumbach P., Götz T., Günther A., Weiss T., Meissner W. Prevalence and characteristics of chronic intensive care-related pain: the role of severe sepsis and septic shock. Crit Care Med. 2016;44:1129–1137. doi: 10.1097/CCM.0000000000001635. [DOI] [PubMed] [Google Scholar]
- 38.Li J. Rehabilitation management of patients with COVID-19. Lessons learned from the first experiences in China. Eur J Phys Rehabil Med. 2020 doi: 10.23736/S1973-9087.20.06292-9. Epub Apr 2020. [DOI] [PubMed] [Google Scholar]
- 39.Fuke R., Hifumi T., Kondo Y., et al. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: a systematic review and meta-analysis. BMJ Open. 2018;8:1–10. doi: 10.1136/bmjopen-2017-019998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connolly B., O’Neill B., Salisbury L., et al. Physical rehabilitation interventions for adult patients with critical illness across the continuum of recovery: an overview of systematic reviews protocol. Syst Rev. 2015;4:881–890. doi: 10.1186/s13643-015-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDowell K., O’Neill B., Blackwood B., et al. Effectiveness of an exercise programme on physical function in patients discharged from hospital following critical illness: a randomised controlled trial (the REVIVE trial) Thorax. 2016;72:600–609. doi: 10.1136/thoraxjnl-2016-208723. [DOI] [PubMed] [Google Scholar]
- 42.Walker W., Wright J., Danjoux G., Howell S.J., Martin D., Bonner S. Project Post Intensive Care eXercise (PIX): a qualitative exploration of intensive care unit survivors’ perceptions of quality of life post-discharge and experience of exercise rehabilitation. J Intensive Care Soc. 2015;16:37–44. doi: 10.1177/1751143714554896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasiter S., Oles S., Mundell J., London S., Khan B. Critical care follow-up clinics: a systematic review. Clin Nurse Spec. 2016;30:227–237. doi: 10.1097/NUR.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]