Abstract
Expression of the receptor tyrosine kinase HER3 is negatively correlated with survival in ovarian cancer, and HER3 overexpression is associated with cancer progression and therapeutic resistance. Thus, improvements in HER3-targeted therapy could lead to significant clinical impact for ovarian cancer patients. Previous work from our group established multivalency as a potential strategy to improve the therapeutic efficacy of HER3-targeted ligands, including affibodies. Others have established HER3 affibodies as viable and potentially superior alternatives to monoclonal antibodies for cancer therapy. Here, bivalent HER3 affibodies were engineered for optimized production, specificity, and function as evaluated in an ovarian cancer xenograft model. Enhanced inhibition of HER3-mediated signaling and increased HER3 downregulation associated with multivalency could be achieved with a simplified construct, potentially increasing translational potential. Additionally, functional effects of affibodies due to multivalency were found to be specific to HER3 targeting, suggesting a unique molecular mechanism. Further, HER3 affibodies demonstrated efficacy in ovarian cancer xenograft mouse models, both as single agents and in combination with carboplatin. Overall, these results reinforce the potential of HER3-targeted affibodies for cancer therapy and establish treatment of ovarian cancer as an application where multivalent HER3 ligands may be useful. Further, this work introduces the potential of HER3 affibodies to be utilized as part of clinically relevant combination therapies (e.g., with carboplatin).
Keywords: HER3s, ErbB3, affibody, carboplatin, ovarian cancer
INTRODUCTION
The transmembrane receptor tyrosine kinase HER3 is overexpressed in a variety of cancers (1), and HER3 signaling has been implicated as a key mediator of acquired resistance to numerous FDA-approved therapeutics, including trastuzumab (2), lapatinib (3), erlotinib (4), and paclitaxel (5). Thus, a multitude of strategies to directly target HER3 for cancer therapy have been conceived, some of which have progressed to clinical trials (e.g., seribantumab (6,7), patritumab (8,9), and others (10)). However, none of these has yet received approval by the US FDA, with seribantumab clinical trials being terminated in late 2018 due to lack of efficacy. Thus, improved therapeutic strategies targeting HER3 have the potential to benefit a wide range of cancer patients, especially those that develop resistance to existing therapies.
Previously, our group demonstrated that multivalency could be utilized to enhance the efficacy of HER3-targeted therapeutics, employing first the native protein neuregulin-1B (NRG) (11) and later Z05413 affibodies (12) as HER3 binding domains. Specifically, we identified that multivalent HER3 affibodies more effectively inhibited NRG-mediated HER3 activation compared with the monovalent versions of the affibodies (12). Further, multivalent affibodies, but not their monovalent analogs, induced rapid and prolonged HER3 downregulation (12), indicating a potentially useful mechanism of action to limit HER3-mediated pro-mitogenic signaling and acquired resistance. While the specific nature of this HER3 downregulation mechanism is unclear, prior data indicate that it is at least partially post-translational (12), and a combination of receptor internalization and degradation as well as ectodomain shedding may be involved.
In this report, we aimed to further develop HER3-directed therapeutics specifically for the treatment of ovarian cancer. HER3 expression is negatively correlated with ovarian cancer patient survival (13), and HER3 overexpression has been found in 16% of patients with ovarian cancer (14). Moreover, NRG-HER3 autocrine signaling has been identified as a key driver of some ovarian cancers (15), thus HER3 inhibition may be particularly effective in this setting. Herein, we describe the optimization of multivalent affibodies designed to promote HER3 downregulation and inhibition of HER3 phosphorylation. We further examined the potential utility of multivalent ligand-induced receptor downregulation as a general phenomenon beyond HER3 (e.g., epidermal growth factor receptor (EGFR) downregulation by multivalent EGFR affibodies) and evaluated HER3 affibody efficacy in vivo in an ovarian cancer xenograft model alone and in combination with carboplatin, a first-line chemotherapeutic for ovarian cancer. Our results confirm and extend previous findings that multivalent affibodies are robustly amenable to molecular engineering (16,17), as modulation of linker length, valency, and modification through molecular fusion (e.g., albumin-binding domain fusion for improved half-life) allowed for near-complete retention of ligand bioactivity, indicating the versatility and modular potential of these ligands for myriad biomedical applications. Most importantly, HER3 affibodies were shown to significantly reduce tumor progression in vivo in adriamycin-resistant ovarian cancer xenograft models, providing persuasive evidence for the further development of HER3 affibodies and HER3 ligands in general for the treatment of ovarian cancer.
METHODS
Protein Production and Purification
Coding DNA sequences for affibody constructs were inserted into a pET45b vector (Merck Millipore), and Hifi DNA Assembly and Q5 Directed mutagenesis (New England Biolabs) were used to alter linker length and valency and to generate albumin-binding domain fusion and bispecific constructs. Expected DNA sequence identities were confirmed by Sanger sequencing (Genewiz). pET45b-affibody constructs were transformed into Escherichia coli strain Bl21 (DE3) (New England Biolabs). For protein expression, 1000 mL LB broth with 100 μg/mL ampicillin was inoculated from three 3 mL overnight starter cultures, incubated at 37°C shaking at 220 rpm, grown to an OD600 of 0.6–0.8, induced with 0.4 μM IPTG, and grown for 4 h at 30°C. The cell pellet was isolated by centrifugation at 3600 rpm for 15 min and frozen at − 20°C. Soluble protein lysates were generated using B-PER complete protein extraction reagent (Thermo Fisher Scientific) following the manufacturer’s protocol and purified by immobilized metal-affinity chromatography using TALON metal affinity resin (Clontech), and refined/buffer exchanged in PBS using ENrich SEC 650 size exclusion (Bio-Rad) highperformance liquid chromatography NGC Quest 10 Plus system (Bio-Rad).
Cell Lines and Reagents
The OvCAR8 cell line was obtained from Dr. Christina Annunziata (National Cancer Institute). OvCAR8 cells were maintained in RPMI 1640 (ATCC) with 1% penicillin/streptomycin and 10% FBS. Luciferase expressing ADR-RES (adriamycin-resistant) ovarian cancer cells were obtained from the University of Maryland School of Medicine. The ADR-RES cell line is a derivative of the OvCAR8 cell line (from the same patient donor) (18). Cell line identity and absence of mycoplasma was confirmed through Laragen Sequencing and Genotyping service.
Cell Signaling Studies
Cells were serum starved for 4 h, treated with the indicated concentrations of affibody or the small molecule pan-HER kinase inhibitor N-(4-((3-chloro-4-fluorophenyl)amino)pyrido[3,4-d]pyrimidin-6-yl)2-butynamide (Millipore 324,840) (total inhibitor) for 30 min, stimulated with 10 nM human heregulin-1β (NRG) (Peprotech) for 10 min (except for no treatment media only control), lysed, and probed by immunoblot for pHER3 and pAkt. For immunoblotting, membranes were probed with primary antibodies (21D3 pHER3, D9E pAkt, and 13E5 β-actin) all from Cell Signaling, and secondary antibody IRDye 800 CW (Licor), and scanned using an Odyssey CLx image system (Licor).
HER3 and EGFR Downregulation Assays
To examine the receptor levels, cells were treated with the indicated concentrations of affibody or no treatment (media alone) control, lysed at the indicated time points, and the immunoblots probed for HER3 and EGFR as previously described using primary antibodies (D22C5 HER3, C74B9 EGFR, and 13E5 β-actin; Cell Signaling Technology). EGFR was chosen for these experiments based on its structural and functional similarities with fellow ErbB receptor family member HER3 as well as the previous demonstration of EGFR expression and ligand-induced downregulation in OvCAR8 cells (19).
In Vitro Combination Studies
OvCAR8 cells were seeded at 18,000 cells per well in media supplemented with 2% FBS, 1% Pen-Strep, and 200 pM NRG in 24 well plates, treated with the indicated concentration of drug (carboplatin, cisplatin, doxorubicin, paclitaxel) alone or in combination with 10 nM bivalent HER3 affibody or the equivalent concentration of monovalent HER3 affibody (equivalent moles of affibody domain), and analyzed using Alamar Blue assay 5 days post-treatment.
In Vivo Efficacy Studies
To compare efficacy of monovalent and bivalent HER3 affibodies, 15 female nude mice were injected with 12.2 × 106 luciferase-expressing ADR-RES cancer cells by intraperitoneal injection (i.p.). Ten days after injection, mice were imaged with a Xenogen In Vivo Imaging System (IVIS) (Perkin Elmer, Bridgeport CT) to monitor disease burden using bioluminescence, and then the mice were sorted into three groups of five with equivalent mean tumor volumes. Mice were then injected i.p. with (1) PBS (vehicle); (2) 80 μg bivalent HER3 affibody; or (3) an equivalent dose of monovalent HER3 affibody (equivalent moles of the affibody domain) three times weekly for 4 weeks total (28 days). Tumor burden was monitored weekly by bioluminescent imaging using IVIS (Xenogen). In combination studies with the bivalent HER3 affibodies and carboplatin, female nude mice were injected i.p. with 15 × 106 luciferase-expressing ADR-RES cancer cells and 20 mice were sorted into four groups of five prior to start of treatment. Mice were injected i.p. with (1) PBS (vehicle), (2) 20 mg/kg carboplatin, (3) 80 μg bivalent HER3 affibody, or (4) 20 mg/kg carboplatin + 80 μg bivalent HER3 affibody. Carboplatin was dosed 1×/week while affibodies were dosed 3×/week for 4 weeks in total (28 days). Tumor burden was again monitored weekly by IVIS. All animal work was carried out in accordance with the NIH guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland Medical School.
Statistical Analysis
Graphed data are shown as mean ± SEM; group sizes are as indicated in the figure captions. Data were analyzed by one-way ANOVA with Bonferroni post hoc test or nonparametric Kruskal-Wallis test with Dunn’s post hoc test as indicated.
RESULTS
Functional Effects of Bivalency Can Be Achieved with a 3-Glycine Linker
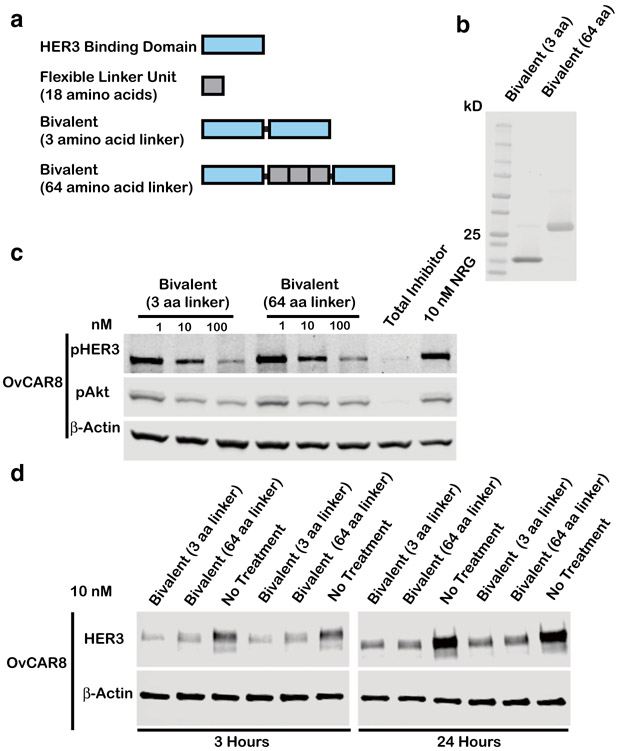
We sought to engineer HER3 ligands for optimal pHER3 inhibition and HER3 downregulation by modulating the parameters of linker domain size and valency. Our previous generation of multivalent affibodies was engineered with a flexible glycine-rich linker approximately 20 nm in length, theoretically long enough to sequester a cross-facing HER3 homodimer where binding sites are separated by a distance of 10 nm (11). Interestingly, reducing the linker domain size to approximately 1 nm in length (three glycine residues) did not impair affibody-mediated pHER3/pAkt inhibition or HER3 downregulation (Fig. 1a-d, S1, S6) as evaluated in OvCAR8 ovarian cancer cells previously identified to be highly responsive to HER3 affibody therapeutics (12). These results demonstrate that linker length (and by extension, the potential for cross-facing homodimer sequestration) has limited impact on multivalent HER3 ligand efficacy. A potential advantage of multivalent ligands utilizing a minimalistic linker may be their lower immunogenicity and higher translational potential compared with our first-generation bivalent affibodies, and therefore we moved forward with multivalent ligands incorporating a three-glycine linker for subsequent studies.
Fig. 1.
Linker length has limited impact on bivalent affibody-mediated pHER3 inhibition and HER3 downregulation. a Schematic representing multivalent affibody constructs with 3 or 64 amino acid (aa) linker size. b Purified protein products are displayed on a Coomassie stained gel loaded at 10 μg/lane. Bivalent affibodies with 3 aa linker and 64 aa linker ligands have respective theoretical molecular weights of 14.4 and 19.1 kDa. c OvCAR8 cells were treated with the indicated concentrations of bivalent affibody 3 or 64 aa linker or 100 nM pan-HER kinase inhibitor (total inhibitor), for 30 min, then stimulated with 10 nM NRG for 10 min, lysed, and probed for pHER3, pAkt, and β-actin by immunoblot. d OvCAR8 cells were treated with 10 nM bivalent affibody with 3 and 64 aa linker, lysed at the indicated time points, and probed by immunoblot for HER3 and β-actin. All multivalent ligand concentrations refer to the concentration of individual affibody domain. Results shown are representative of three independent experiments. All blots were cropped; blots for pHER3 and pAkt are presented at different exposures for clarity
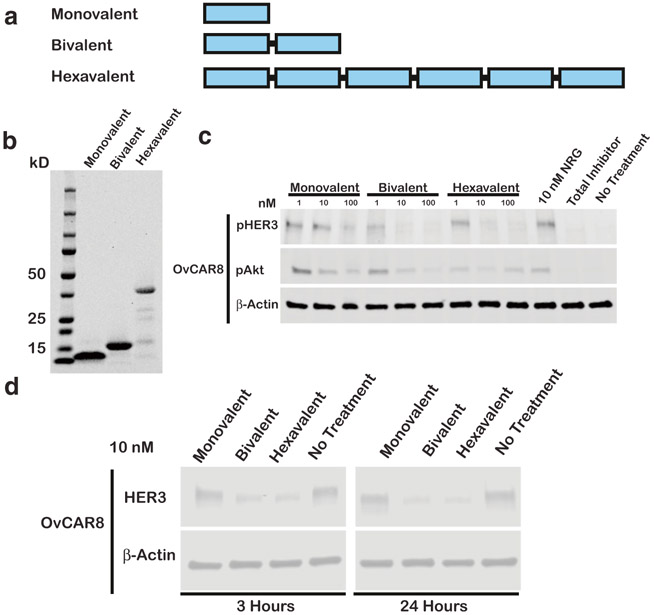
Hexavalent and Bivalent HER3 Affibodies Show Similar Activity
We hypothesized that engineered multivalency could improve HER3 ligand bioactivity through enhanced avidity effects and HER3 sequestration. Previously, we compared the bioactivity of monovalent, bivalent, and trivalent affibody formats and observed that bivalent and trivalent ligands had comparable bioactivity with regard to pHER3/pAkt inhibition, and HER3 downregulation (12). To more conclusively assess the impact of valency on affibody bioactivity, we examined the utility of hexavalent ligands in comparison with our bivalent ligands with three-glycine linkers (Fig. 2a-b). We likewise observed that valency above two had limited impact on pHER3 inhibition and HER3 downregulation (Fig. 2c-d, S2, S7). Thus, we focused further investigation on bivalent affibodies with three-glycine linkers (unless otherwise described).
Fig. 2.
Hexavalent and bivalent HER3 affibodies show similar activity. a Schematic representing monovalent, bivalent, and hexavalent affibody constructs. b Purified protein products are displayed on a Coomassie stained gel loaded at 10 μg/lane. Monovalent, bivalent, and hexavalent affibody constructs have respective theoretical molecular weights of 7.6, 14.4, and 41.6 kDa, respectively. c OvCAR8 cells were treated with the indicated concentrations of monovalent, bivalent, or hexavalent affibodies, 100 nM pan-HER kinase inhibitor (total inhibitor), or media alone (no treatment) for 30 min, then stimulated with 10 nM NRG for 10 min (except for no treatment negative control), lysed, and probed for pHER3, pAkt, and β-actin by immunoblot. d OvCAR8 cells were treated with 10 nM monovalent, bivalent, or hexavalent linker, pan-HER kinase inhibitor, or media alone, lysed at the indicated time points, and probed by immunoblot for HER3 and β-actin. All multivalent ligand concentrations refer to the concentration of individual affibody domains. Results shown are representative of three independent experiments. All blots were cropped; blots for pHER3 and pAkt are presented at different exposures for clarity
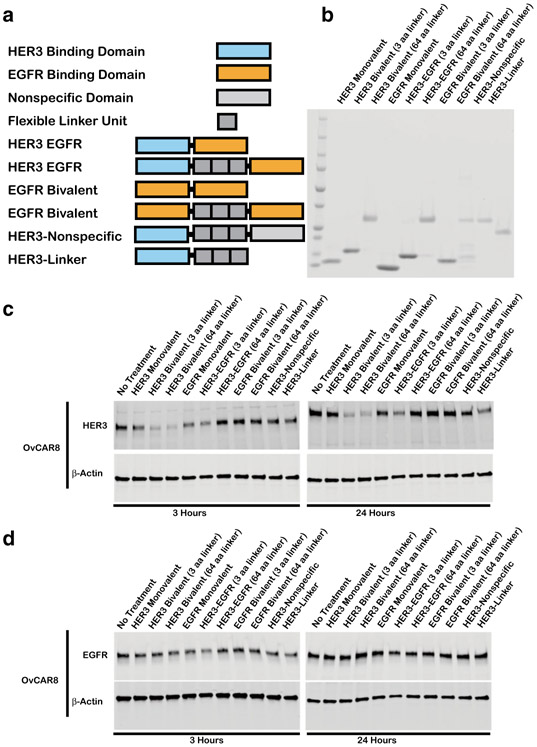
Bivalent Affibody-Mediated HER3 Downregulation Is a Specific Phenomenon
Given that multivalent HER3 affibodies promote HER3 receptor downregulation, we sought to examine to what extent multivalency can be employed as a general tool to induce ErbB receptor downregulation. Using a Z-EGFR1907 domain developed by Friedman et al. (20), we generated bivalent EGFR-EGFR affibodies as well as bispecific, bivalent HER3-EGFR affibody molecules (Fig. 3a-b) and assessed their ability to downregulate HER3 and EGFR receptors (by immunoblot in the absence of NRG and EGF) compared with bivalent HER3 affibodies. We observed that only HER3 bivalent ligands induced HER3 receptor downregulation, while EGFR levels were unaffected by any of the molecules (Fig. 3c-d, S3), indicating that HER3 downregulation by multivalent ligands may be a specific phenomenon. HER3 and EGFR levels were normalized to β-actin levels and the results of three independent experiments were quantified in Fig. S3.
Fig. 3.
HER3 downregulation by bivalent affibodies is a specific phenomenon. a Schematic of various affibody constructs: monovalent HER3 (7.6 kDa), HER3 bivalent affibody with 3 or 64 amino acid linker (14.4 and 19.1 kDa), monovalent EGFR (7.6 kDa), HER3 EGFR bivalent bispecific affibody with 3 and 64 aa linker (14.3 and 18.9 kDa), EGFR bivalent affibody with 3 or 64 aa linker (14.4 and 18.2 kDa), bivalent bispecific HER3 wild-type affibody with 64 aa linker (19.1 kDa), monovalent HER3 affibody with 64 aa linker tail (12.5 kDa). b Purified protein products are displayed on a Coomassie stained gel loaded at 10 μg/lane with respective molecular weights listed in Figure 3a. c OvCAR8 cells were treated with 10 nM of the indicated affibody construct or media alone (no treatment), lysed at the indicated time points and probed by immunoblot for HER3 and β-actin or d EGFR and β-actin. All multivalent ligand concentrations refer to the concentration of individual affibody domains. Results shown are representative of three independent experiments. All blots were cropped
Further, we hypothesized that HER3 downregulation by multivalent ligands is a direct result of HER3 sequestration by simultaneous engagement of multiple HER3 receptors. However, one general mechanism for HER3 inhibition is the blockade of heterodimer formation, and the possibility exists that multivalent ligands demonstrate improved bioactivity compared with monovalent analogs due to engagement of a single HER3 receptor by one HER3 affibody domain, while the unengaged HER3 binding domain acts through HER3-independent steric blockade to inhibit receptor heterodimerization and induce HER3 downregulation. To examine this possibility, we assessed the activity of a bivalent HER3-nonspecific affibody that incorporated one HER3-binding domain as well as a structurally similar affibody domain that does not have an affinity for HER3 (Fig. 3c-d, S3, S8). No significant HER3 downregulation was observed in OvCAR8 cells upon stimulation with HER3-nonspecific affibodies nor with a control monovalent HER3 affibody containing an attached linker domain, suggesting that HER3 downregulation is not idiosyncratic to bivalent molecules but rather requires simultaneous engagement of multiple HER3 receptors (Fig. 3c-d, S3).
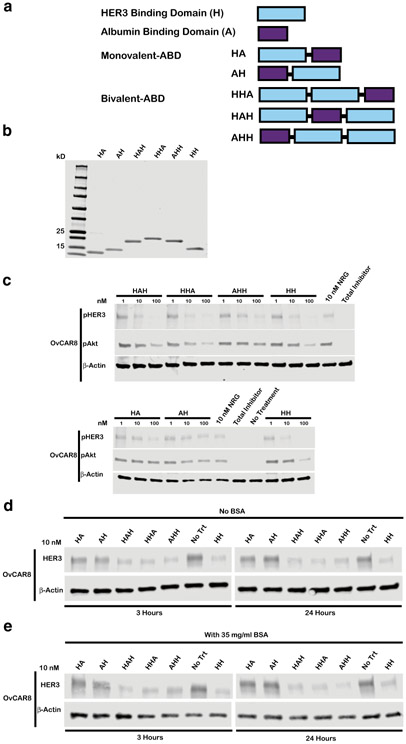
Arrangement of HER3 Affibodies Around an Albumin-Binding Domain Has Minimal Impact on Bioactivity
Affibodies, despite their advantages over mAbs such as increased tissue penetration, stability, and ease of manufacturing, have pharmacokinetic limitations that are likely to hinder their therapeutic efficacy in vivo (21). Albumin-binding domain (ABD) fusions have been widely exploited to extend circulating half-life by increasing molecular hydrodynamic radius, enabling FcRn-mediated recycling, and reducing renal clearance (21-24). Thus, we generated various monovalent and bivalent HER3 affibody fusions with albumin-binding domains (Fig. 4a-b). Specifically, we generated monovalent ligands HA and AH and bivalent ligands HHA, HAH, and AHH where “H” denotes HER3 binding domain and “A” denotes albumin-binding domain; molecular domains were connected by three-glycine minimal linkers. We selected ABD035 as the albumin-binding domain for this study (22). The entire panel of HER3 affibody ABD fusions retained bioactivity similar to unmodified affibodies with regard to pHER3/pAkt inhibition (Fig. 4c, S4) and induction of HER3 downregulation, both in the presence and absence of bovine serum albumin (Fig. 4d-e, S5, S9). The retained bioactivity of HHA and AHH molecules indicates that HER3 receptor engagement is efficient even with a centralized HER3 binding domain that is at a greater risk of being sterically inhibited from interacting with HER3. Given that ABD domain location had limited impact on protein bioactivity, we selected monovalent HA and bivalent HHA HER3 affibody-ABD fusions for subsequent in vivo efficacy evaluation.
Fig. 4.
Arrangement of HER3 affibody domains and albumin-binding domains in a fusion protein has minimal impact on bioactivity. a Schematic representing monovalent and bivalent albumin-binding domain fusion constructs where “H” denotes HER3 binding domain and “A” denotes albumin-binding domain b Purified protein products are displayed on a Coomassie stained gel loaded at 10 μg/lane. Monovalent ABDs HA and AH have respective theoretical molecular weights of 12.9 and 13.0 kDa, bivalent ABDs HHA, HAH, and AHH have MW of 19.7, 19.7, and 19.8 kDa respectively, and HH-bivalent HER3 affibody positive control lacking ABD has MW of 14.4 kDa. c OvCAR8 cells were treated with the indicated concentration affibody construct, 100 nM pan-HER kinase inhibitor (total inhibitor), or media alone (no treatment) for 30 min, then stimulated with 10 nM NRG for 10 min (except for no treatment negative control), lysed and probed for pHER3, pAkt, and β-actin by immunoblot. In the d absence of bovine serum albumin (BSA) or e presence of 35 mg/ml BSA, OvCAR8 cells were treated with 10 nM of the indicated affibody construct or media alone (no treatment), lysed at the indicated time points and probed by immunoblot for HER3 and β-actin. All multivalent ligand concentrations refer to the concentration of individual affibody domains. Results shown are representative of three independent experiments. All blots were cropped; blots for pHER3 and pAkt are presented at different exposures for clarity
HER3 Affibodies Reduce Tumor Progression In Vivo in an Ovarian Cancer Xenograft Model Alone and in Combination with Carboplatin
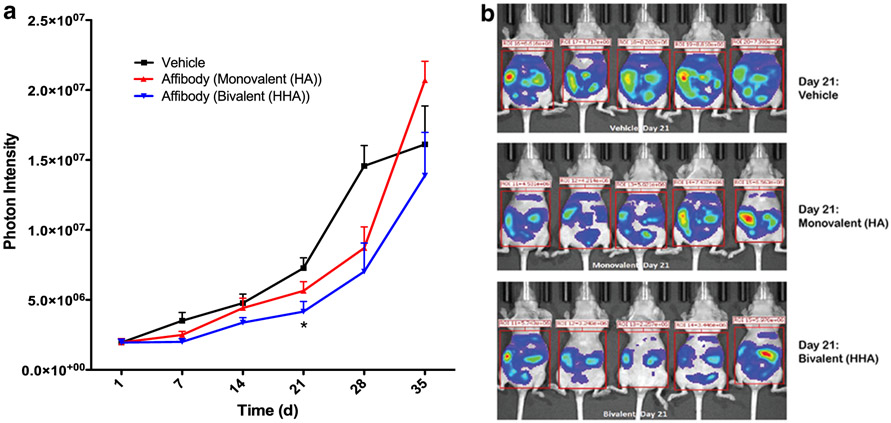
We next performed pilot studies to assess the effect of the affibodies at slowing tumor growth in a xenograft mouse model of the human ovarian cancer. The ADR-RES cell line was chosen for these studies as it is a derivative of the OvCAR8 cell line (from the same patient donor) (18). Both monovalent (HA) and bivalent (HHA) HER3 affibody-ABD fusions were evaluated as single agents in this model, with only the bivalent affibodies inducing significant reduction in tumor progression (Fig. 5a-b).
Fig. 5.
Bivalent HER3 affibodies reduce tumor progression in vivo in an ADR-RES xenograft model. a Whole body photon intensity at indicated time points after treatment initiation. b Raw IVIS images 21 days after treatment initiation. For a and b, nude mice were injected i.p. with 12.2 million luciferase expressing ADR-RES cancer cells. Ten days after injection, mice were imaged to monitor disease burden and sorted into three groups of five with equivalent mean tumor volumes. Mice were then injected i.p. with 80 μg bivalent affibody, an equivalent dose of monovalent affibody or PBS alone (vehicle) 3×/week for 4 weeks (treatment ended on day 28). Tumor burden was monitored weekly by bioluminescent imaging and results shown are mean ± SEM; n = 5. *P < 0.05 indicates significance compared with PBS only (control) group by one-way ANOVA with Bonferroni post hoc test
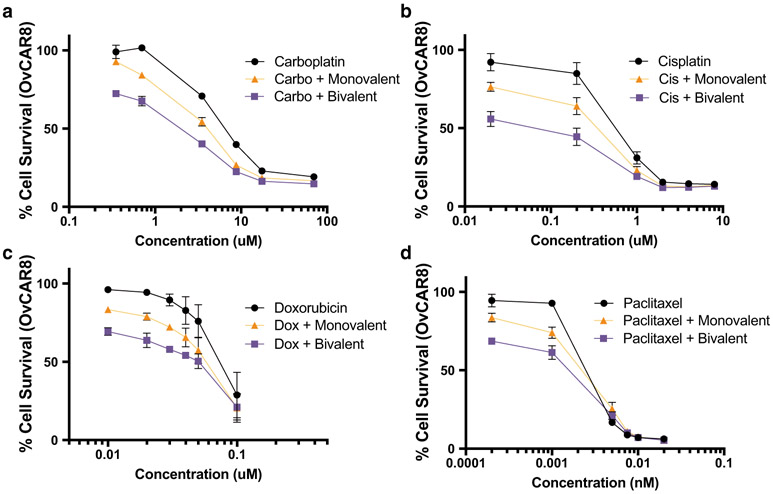
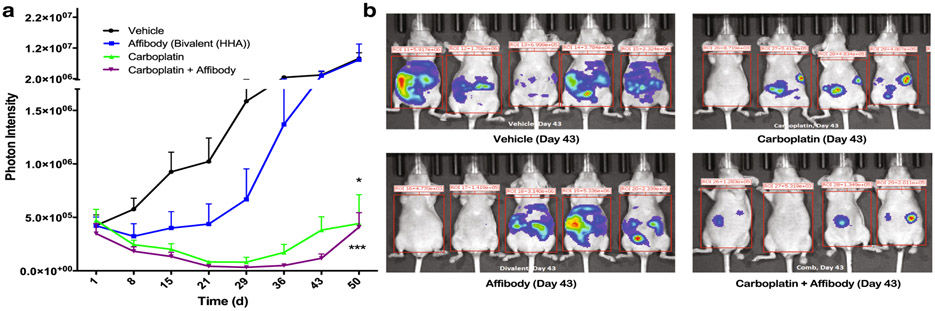
Both constructs were then evaluated in vitro in combination with chemotherapeutic drugs that have been applied to ovarian cancer patients (carboplatin, cisplatin, doxorubicin, paclitaxel). In all cases, combination of the chemotherapeutics with HER3 affibodies led to a leftward shift of the cell survival curve, indicative of reduced cancer cell survival at lower doses of the chemotherapeutics (Fig. 6a-d). Further, the magnitude of these effects was consistently higher with bivalent compared with monovalent affibodies. Thus, additional pilot in vivo studies to assess HER3 affibodies in combination with chemotherapeutics were carried out with bivalent HER3 affibody-ABD fusions (HHA). These studies revealed that both carboplatin alone and the combination of carboplatin with HER3 affibodies induced significant reduction of tumor progression compared with the vehicle control group (Fig. 7a-b). Further, there was a consistent trend of enhanced tumor reduction in the combination group compared with carboplatin alone, although the low statistical power of these pilot studies limits the significance of the findings. Nevertheless, the data obtained are highly consistent and indicate that HER3 affibodies have therapeutic potential for ovarian cancer treatment, justifying further studies with higher statistical power and in additional ovarian cancer models.
Fig. 6.
HER3 affibodies enhance therapeutic effects of chemotherapeutic drugs. OvCAR8 cells were seeded at 18,000 cells per well in media supplemented with 2% FBS, 1% Pen-Strep, and 200 pM NRG in 24-well plates, treated with the indicated concentration of drug (a carboplatin, b cisplatin, c doxorubicin, d paclitaxel) alone or in combination with 10 nM bivalent HER3 affibody or the equivalent concentration of monovalent HER3 affibody and analyzed using Alamar Blue assay 5 days post-treatment
Fig. 7.
Bivalent HER3 affibodies reduce tumor progression in vivo in combination with carboplatin. a Whole body photon intensity at indicated time points after treatment initiation. b Raw IVIS images 43 days after treatment initiation. For a and b, nude mice were injected i.p. with 15 million luciferase expressing ADR-RES cancer cells. Ten days after injection, mice were imaged and sorted into four groups of five mice with equivalent mean tumor volumes. Mice were then injected i.p. with PBS (vehicle), 20 mg/kg carboplatin, 80 μg bivalent HER3 affibody, or 20 mg/kg carboplatin + 80 μg bivalent HER3 affibody. Carboplatin was dosed 1×/week for 4 weeks while affibodies were dosed 3×/week for 4 weeks total (treatment ended on day 28). Tumor burden was monitored weekly by bioluminescent imaging and results shown are mean ± SEM; n = 5. ***P < 0.001, *P < 0.05 indicate significance compared with the vehicle (control) group by Kruskal-Wallis test with Dunn’s post hoc test
DISCUSSION
The development of new therapeutic approaches for ovarian cancer is imperative due to the generally high rate of recurrence and poor prognosis associated with this disease. Building on elegant work in pancreatic cancer models (16,17,25), we show here that HER3 affibodies have therapeutic effects in ovarian cancer mouse models. Critically, we demonstrate that these molecules can be used effectively in combination with carboplatin, a first-line ovarian cancer chemotherapeutic, and thus have significant translational potential as part of clinically relevant combination therapies.
The results here stand largely in agreement with a previous work in the field. Malm et al. showed that a bivalent affibody of molecular format ZHER3-ABD-ZHER3 (3A3), where domains are separated by a (S4G)4 linker domain, is capable of simultaneously binding both HER3 and albumin (21). Further, 3A3 showed improved pHER3 inhibition compared with monovalent affibody (21), similar to our own prior findings that affibody multivalency improves pHER3 inhibition (12). 3A3 was also shown to significantly reduce pancreatic cancer progression in mice (16) and, as reported by Orlova et al., allowed for tumor growth inhibition comparable to monoclonal antibody seribantumab (25). The Orlova et al. report also supports our results indicating that the efficacy of bivalent HER3 affibodies can be retained in a variety of formats (25), which is further confirmed by the work of Altai et al. (17). This work also showed that bivalent affibody-ABD fusions of multiple formats could effectively accumulate in the pancreatic tumors in mice (17). Thus, overall, multivalent HER3 affibodies are versatile and show in vivo therapeutic potential against cancers from a variety of source tissues.
A particularly surprising result was that domain linker length did not impact bivalent affibody function as expected (Fig. 1). Previously, Roovers et al. demonstrated the importance of linker length in the development of an optimized biparatopic nanobody against EGFR known as 7D12-9G8. In this construct, where two nanobody domains separated by a flexible linker bound a single EGFR molecule on non-overlapping epitopes, linker length was found to be inversely proportional with efficacy (26). Linker length and composition are typically important variables in multivalent ligand design, with a general principle that a linker separating target-binding domains in a multivalent construct should be sufficiently long and flexible enough to allow for simultaneous target engagement. However, increasing linker length increases the risk of steric hindrance and likewise increases binding entropy. While our data indicate that linker length is not a crucial determinant of efficacy of the HER3-targeted constructs reported here, this variable should be assessed on a case-by-case basis.
Mechanistically, our data provide valuable insights into the effects of multivalency on the enhanced downregulation of HER3 and other receptors. The possibility exists that receptor downregulation is a result of enhanced avidity effects, endowed through multivalency, and not necessarily a result of simultaneous engagement of multiple receptors. However, our previous results indicate that increasing ligand valency does not increase KD as measured by surface plasmon resonance (12). In the current study, neither monovalent nor bivalent EGFR ligands had an effect on the total EGFR levels (or HER3 levels), suggesting that enhanced avidity is not a general mechanism enabling ErbB receptor downregulation, and indeed HER3 downregulation requires simultaneous engagement of multiple HER3 receptors. Considering previous reports of receptor downregulation by multivalent ErbB therapeutics, to our knowledge, evidence of receptor downregulation by bispecific ligands is limited. Neither the HER2-HER3 bivalent bispecific antibody MM-111 (27), nor the two-in-one antibody HER3-EGFR MEHD7945A (28) were reported to induce downregulation or degradation of either target receptor. However, the HER3 IGF-1R tetravalent bispecific antibody MM-141 was observed to promote degradation of both HER3 and IGF-1R, as well as HER2 and insulin receptor (29). In our experiments, monospecific bivalent EGFR affibodies did not induce EGFR downregulation, suggesting that engineered multivalency cannot be applied universally as a strategy to downregulate ErbB family target receptors. It should be noted that previous investigations indicate that multivalency can be used as a tool to induce EGFR downregulation on a case-by-case basis (30), however this phenomenon appears highly dependent on molecular idiosyncrasies beyond the degree of valency and may include affinity, avidity, binding epitope, degree of epitopes targeted, and degree of EGFR crosslinking. In particular, the effects of specific epitope binding on downregulation should be more deeply explored in future investigations.
CONCLUSION
Overall, this work provides proof of principle for bivalent affibodies as a promising strategy to limit in vivo ovarian cancer progression. OvCAR8 and ADR-RES cell lines are representative models of a high-grade serous ovarian cancer (HGSOC), a highly aggressive and common ovarian cancer subtype. The results herein encourage a more extensive evaluation of multivalent HER3 ligands against a broad panel of HGSOC cell models, which may help identify biomarkers of susceptibility to HER3-targeted therapies and enable the strategic deployment of such therapeutics against ovarian cancer in a more personalized medicine approach.
Supplementary Material
Acknowledgments
FUNDING INFORMATION
This work was supported by funding from the National Institutes of Health (R00 HL112905 to SMJ), the Elsa U. Pardee Foundation (SMJ), the NCI-UMD Partnership for Integrative Cancer Research (JSS, SMJ and SL), the University of Maryland Greenebaum Comprehensive Care Center seed grant (SMJ, AH, and RGL), the Rivkin Center for Ovarian Cancer (SMJ), and the American Association of Pharmaceutical Scientists Foundation Graduate Student Fellowship (JSS). AH was supported by a Merit Review Award from the U.S. Department of Veterans Affairs (I01 BX000545). This research was also supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research (SL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
All animal work was carried out in accordance with the NIH guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland Medical School.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material The online version of this article (https://doi.org/10.1208/s12248-019-0318-x) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell. 2013;23(5):603–17. [DOI] [PubMed] [Google Scholar]
- 2.Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia W, Petricoin EF 3rd, Zhao S, Liu L, Osada T, Cheng Q, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15(5):R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonesaka K, Zejnullahu K, Lindeman N, Homes AJ, Jackman DM, Zhao F, et al. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res. 2008;14(21):6963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S, Li C, Armstrong EA, Peet CR, Saker J, Amler LC, et al. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2013;73(2):824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary JM, McRee AJ, Shapiro GI, Tolaney SM, O’Neil BH, Kearns JD, et al. A phase 1 study combining the HER3 antibody seribantumab (MM-121) and cetuximab with and without irinotecan. Investig New Drugs. 2017;35(1):68–78. [DOI] [PubMed] [Google Scholar]
- 7.Liu JF, Ray-Coquard I, Selle F, Poveda AM, Cibula D, Hirte H, et al. Randomized phase II trial of seribantumab in combination with paclitaxel in patients with advanced platinum-resistant or - refractory ovarian cancer. J Clin Oncol. 2016;34(36):4345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakui H, Yamamoto N, Nakamichi S, Tamura Y, Nokihara H, Yamada Y, et al. Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73(3):511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishio M, Horiike A, Murakami H, Yamamoto N, Kaneda H, Nakagawa K, et al. Phase I study of the HER3-targeted antibody patritumab (U3-1287) combined with erlotinib in Japanese patients with non-small cell lung cancer. Lung Cancer. 2015;88(3):275–81. [DOI] [PubMed] [Google Scholar]
- 10.Jacob W, James I, Hasmann M, Weisser M. Clinical development of HER3-targeting monoclonal antibodies: perils and progress. Cancer Treat Rev. 2018;68:111–23. [DOI] [PubMed] [Google Scholar]
- 11.Jay SM, Kurtagic E, Alvarez LM, de Picciotto S, Sanchez E, Hawkins JF, et al. Engineered bivalent ligands to bias ErbB receptor-mediated signaling and phenotypes. J Biol Chem. 2011. August;286(31):27729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schardt JS, Oubaid JM, Williams SC, Howard JL, Aloimonos CM, Bookstaver ML, et al. Engineered multivalency enhances affibody-based HER3 inhibition and downregulation in cancer cells. Mol Pharm. 2017;14(4):1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanner B, Hasenclever D, Stern K, Schormann W, Bezler M, Hermes M, et al. ErbB-3 predicts survival in ovarian cancer. J Clin Oncol. 2006;24(26):4317–23. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar T, Stamp GW, Hughes CM, Gullick WJ. C-erbB3 protein expression in ovarian cancer. Clin Mol Pathol. 1996;49(4):M199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng Q, Liu X, Fleming E, Yuan K, Piao H, Chen J, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell. 2010;17(3):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bass TZ, Rosestedt M, Mitran B, Frejd FY, Löfblom J, Tolmachev V, et al. In vivo evaluation of a novel format of a bivalent HER3-targeting and albumin-binding therapeutic affibody construct. Sci Rep. 2017. February;7:43118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altai M, Leitao CD, Rinne SS, Vorobyeva A, Atterby C, Ståhl S, et al. Influence of molecular design on the targeting properties of ABD-fused mono- and bi-valent anti-HER3 affibody therapeutic constructs. Cells. 2018;7(10):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005. July;436(7047):117–22. [DOI] [PubMed] [Google Scholar]
- 19.Ellebaek S, Brix S, Grandal M, Lantto J, Horak ID, Kragh M, et al. Pan-HER-an antibody mixture targeting EGFR, HER2 and HER3 abrogates preformed and ligand-induced EGFR homo- and heterodimers. Int J Cancer. 2016;139(9):2095–105. [DOI] [PubMed] [Google Scholar]
- 20.Friedman M, Orlova A, Johansson E, Eriksson TLJ, Höidén-Guthenberg I, Tolmachev V, et al. Directed evolution to low nanomolar affinity of a tumor-targeting epidermal growth factor receptor-binding affibody molecule. J Mol Biol. 2008. March;376(5):1388–402. [DOI] [PubMed] [Google Scholar]
- 21.Malm M, Bass T, Gudmundsdotter L, Lord M, Frejd FY, Ståhl S, et al. Engineering of a bispecific affibody molecule towards HER2 and HER3 by addition of an albumin-binding domain allows for affinity purification and in vivo half-life extension. Biotechnol J. 2014. September;9(9):1215–22. [DOI] [PubMed] [Google Scholar]
- 22.Nilvebrant J, Hober S. The albumin-binding domain as a scaffold for protein engineering. Comput Struct Biotechnol J. 2013;6:e201303009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werle M, Bernkop-Schnürch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30(4):351–67. [DOI] [PubMed] [Google Scholar]
- 24.Kontermann RE. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs. 2009. June;23(2):93–109. [DOI] [PubMed] [Google Scholar]
- 25.Orlova A, Bass TZ, Rinne SS, Leitao CD, Rosestedt M, Atterby C, et al. Evaluation of the therapeutic potential of a HER3-binding affibody construct TAM-HER3 in comparison with a monoclonal antibody, seribantumab. Mol Pharm. 2018. August 6;15(8):3394–403. [DOI] [PubMed] [Google Scholar]
- 26.Roovers RC, Vosjan MJWD, Laeremans T, El Khoulati R, De Bruin RCG, Ferguson KM, et al. A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth. Int J Cancer. 2011;129(8):2013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11(3):582–93. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell. 2011. October;20(4):472–86. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald JB, Johnson BW, Baum J, Adams S, Iadevaia S, Tang J, et al. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Ther. 2014;13(2):410–25. [DOI] [PubMed] [Google Scholar]
- 30.Arena S, Siravegna G, Mussolin B, Kearns JD, Wolf BB, Misale S, et al. MM-151 overcomes acquired resistance to cetuximab and panitumumab in colorectal cancers harboring EGFR extracellular domain mutations. Sci Transl Med. 2016;8(324):324ra14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.