Abstract
Therapeutic resistance remains a persistent challenge for patients with malignant tumors. Here, we reveal that endothelial cells (ECs) acquire transformation into mesenchymal stem cell (MSC)–like cells in glioblastoma (GBM), driving tumor resistance to cytotoxic treatment. Transcriptome analysis by RNA sequencing (RNA-seq) revealed that ECs undergo mesenchymal transformation and stemness-like activation in GBM microenvironment. Furthermore, we identified a c-Met-mediated axis that induces β-catenin phosphorylation at Ser675 and Wnt signaling activation, inducing multidrug resistance–associated protein-1(MRP-1) expression and leading to EC stemness-like activation and chemoresistance. Last, genetic ablation of β-catenin in ECs overcome GBM tumor resistance to temozolomide (TMZ) chemotherapy in vivo. Combination of Wnt inhibition and TMZ chemotherapy eliminated tumor-associated ECs, inhibited GBM growth, and increased mouse survival. These findings identified a cell plasticity–based, microenvironment-dependent mechanism that controls tumor chemoresistance, and suggest that targeting Wnt/β-catenin–mediated EC transformation and stemness activation may overcome therapeutic resistance in GBM.
INTRODUCTION
Sustained angiogenesis is a hallmark of cancer. Angiogenesis proceeds by sprouting and outgrowth of endothelial cells (ECs) that form the linings of the blood vessels. Newly formed blood vessels deliver oxygen and nutrients and produce paracrine factors that support the cancer microenvironment, fueling tumor growth, progression, and metastasis (1-4). However, current antivascular therapies that primarily target proangiogenic factors, albeit initially groundbreaking, have encountered major difficulties and have ultimately failed as treatments for most malignant solid tumors, likely due to inefficient eradication of or insufficient functional inhibition of tumor-associated ECs (5, 6). The development of new therapies that are efficient at eradicating ECs, therefore, is crucial for cancer treatment.
Glioblastoma (GBM), a grade IV glioma, is the most common and aggressive type of malignant brain tumor in adults. GBM is among the most lethal of human malignancies, with a current median survival of about 14 to 16 months (7, 8). Most GBM tumors are refractory to conventional cytotoxic therapies (9). Accumulating evidence indicates that stemness activation in GBM cells contributes to therapy resistance (10-13), which is subjected to regulation by key developmental signaling pathways such as wingless-related integration site (Wnt), Notch, and Hedgehog (14-17).
GBM is characterized by prominent vascularity and extraordinary vascular abnormality. Antiangiogenic therapies, mainly targeting a vascular endothelial growth factor (VEGF) and its receptors, have been developed and exploited in recent years; however, the therapeutic benefits are limited and transient in GBM (18, 19). We recently revealed that GBM-associated ECs acquire fibroblast-like phenotypes including high motility and proliferation to generate excessive abnormal vasculature, suggesting a potential role for EC plasticity in GBM progression and treatment resistance (6, 20). Here, we identified robust transformation of ECs into mesenchymal stem cell (MSC)–like cells in GBM, which induces chemoresistance through Wnt/β-catenin activation. Genetic ablation of β-catenin in ECs sensitized GBM to chemotherapy in mice. Thus, endothelial transformation represents a cellular mechanism that controls tumor chemoresistance, and targeting Wnt-mediated EC transformation and stemness activation may serve as a next-generation antivascular therapeutic strategy in cancer.
RESULTS
Glioma-associated ECs are chemoresistant
To analyze treatment responses in tumor-associated ECs, we took advantage of a genetic endothelial lineage tracing system based on Tie2-Cre– or Cdh5-CreERT2–driven tdTomato expression (Fig. 1A), in which EC-derived cells are fluorescently labeled. The labeling is independent of EC-specific surface marker expression that may be altered by endothelial plasticity in the tumor microenvironment (20, 21). We induced GBM in a native microenvironment by implanting tumor cells isolated from a genetically engineered murine GBM model that was generated by RCAS/N-tva–mediated somatic Pdgfb gene transfer in Ink4a-Arf−−/−;Pten−/− neural stem/progenitor cells (22-24). We initially used Tie2-Cre;Rosa-LSL-tdTomato mice that exhibited robust fluorescence in ECs. In addition, non-EC myeloid cells and, to a lesser extent, pericytes also express Tie2 (25-27), which may contribute to the tumor-associated tdTomato+ cells. However, our previous analysis shows a minimal contribution of CD11b+ monocytes/macrophages and NG-2+ pericytes to the Tie2-Cre+ GBM cell population, suggesting the endothelial specificity of Tie2-Cre–based lineage tracing in our GBM model (20).
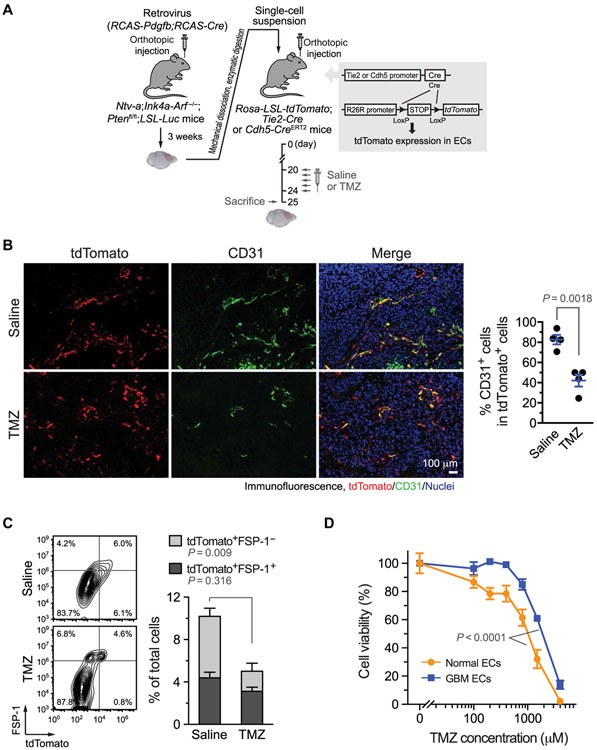
Fig. 1. Glioma-associated ECs are chemoresistant.
(A to C) GBM was induced by RCAS-mediated somatic gene transfer in Ntv-a;Ink4a-Arf−/−;Ptenfl/fl;LSL-Luc donor mice. Single-cell tumor suspensions were injected into Rosa-LSL-tdTomato;Tie2-Cre or Rosa-LSL-tdTomato;Cdh5-CreERT2 mice. After tumor induction, the mice were treated with saline or with TMZ (100 mg/kg) for 5 days. (A) Schematic approach. (B) Tumor sections were stained with anti-tdTomato and anti-CD31 antibodies. Left: Representative images. Right: Quantified results (n = 4, means ± SEM). Statistical analysis by Student’s t test. (C) Single-cell suspensions isolated from tumors were stained with anti–FSP-1 antibody and analyzed by flow cytometry. Left: Representative sorting. Right: Quantitative data for tdTomato+FSP-1+ and tdTomato+FSP-1− in total cells (n = 5 mice, means ± SEM). Statistical analysis by two-way ANOVA. (D) ECs were treated with TMZ and subjected to cell viability analysis (n = 3, means ± SEM). Statistical analysis by Student’s t test.
Consistent with extensive studies showing that cytotoxic treatments disrupt tumor-associated vasculature (28, 29), our data indicate that chemotherapy with temozolomide (TMZ) markedly eliminated CD31high ECs in GBM (Fig. 1B). TMZ treatment did not efficiently eradicate tdTomato+ cells, and a prominent population of CD31lowtdTomato+ cells was retained after the treatment, suggesting that EC-derived CD31low cells, but not CD31high cells, are treatment resistant. Our recent work suggests that GBM-associated ECs undergo mesenchymal transformation, as evidenced by increased expression of mesenchymal genes including S100a4 [fibroblast-specific protein 1 (FSP-1)] and ACTA2 [α-smooth muscle actin (α-SMA)] but reduced expression of endothelial-specific genes including PECAM1 (CD31) (20), which implicates that EC transformation may contribute to the treatment resistance in ECs. Consistent with the role for EC transformation in chemoresistance, flow cytometric analysis of tumor-associated tdTomato+ cells revealed that TMZ did not affect the FSP-1+tdTomato+ population, but it substantially reduced the FSP-1−tdTomato+ population (Fig. 1C). In addition, our analysis of GBM tumors in Cdh5-CreERT2;Rosa-LSL-tdTomato mice confirms that there is a cell population of CD31lowFSP-1high in the tumor-associated tdTomato+ cells (fig. S1).
Furthermore, human patient-derived, GBM-associated ECs exhibited greater treatment resistance to chemotherapy drugs, including TMZ, doxorubicin, and etoposide, compared with normal brain ECs (Fig. 1D and fig. S2). These cells were previously verified for their EC identity: All of the cells uptaken acetylated low-density lipoprotein (Ac-LDL) and were negative for pericyte marker NG-2 staining, and these ECs acquired robust mesenchymal transformation (20). Collectively, our data show that GBM-associated ECs are chemoresistant, which may be linked to EC transformation in the tumor microenvironment.
Stemness-like activation and mesenchymalization are induced in ECs under glioma conditions
To investigate the regulatory mechanism(s) that control EC plasticity and chemoresistance, we treated normal human brain ECs with glioma–conditioned medium (glioma-CM), followed by transcriptome analysis. RNA sequencing (RNA-seq) analysis of these ECs suggested a possible cell lineage alteration, as indicated by nonlinear dimension reduction analysis with t-distributed stochastic neighbor embedding (t-SNE) algorithm (Fig. 2A), and showed a robust change in global gene expression (Fig. 2, B and C). This unbiased analysis indicated an increase in expression of mesenchymal gene S100A4 (FSP-1) (Fig. 1D), consistent with our previous work showing that glioma-CM induces EC mesenchymalization in vitro (20, 30). Moreover, our RNA-seq analysis showed expression of a potential stemness-associated signature in the treated ECs, as indicated by enhanced gene expression of THY1 (CD90) (Fig. 2D). Furthermore, either glioma-CM treatment or coculture with U251 glioma cells induced robust expression of the MSC marker Stro-1 in normal human brain ECs (Fig. 2E), suggesting that ECs acquire MSC-like gene expression signature under glioma conditions.
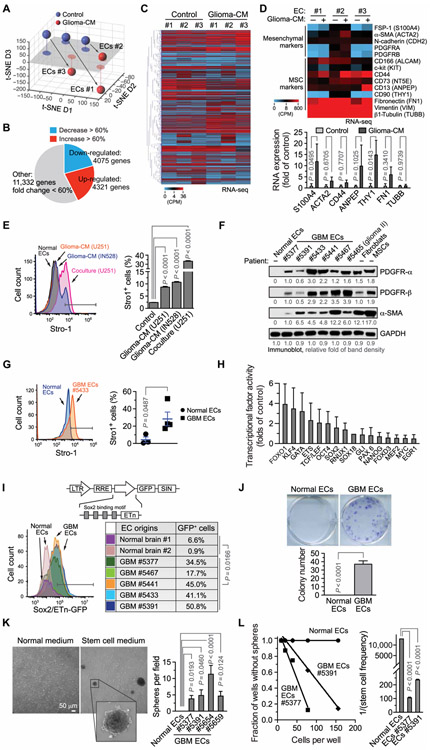
Fig. 2. Stemness-like activation in GBM ECs.
(A to D) RNA-seq analysis of ECs treated with or without glioma-CM. (A) t-SNE analysis of gene expression. (B) Summary of global changes in gene expression. (C) Heat map for genes with up-regulated or down-regulated expression. Counts per million (CPM). (D) Gene expression was analyzed for mesenchymal and MSC-associated genes. Top: Heat map. Bottom: Expression of fold for a subset of these genes (n = 3, means ± SEM). Statistical analysis by two-way ANOVA. (E) ECs were treated with glioma-CM or were cocultured with GFP-expressing U251 glioma cells, immunostained with anti–Stro-1 antibody or with control isotype immunoglobulin G (IgG), and analyzed by flow cytometry. Left: Representative sortings are shown. Right: Quantified results (n = 3, means ± SEM). Statistical analysis by one-way ANOVA. (F to L) ECs were isolated from GBM tumors of different human patients or from normal human brain. (F) Immunoblot analysis of cell lysates. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (G) Tumor ECs and normal brain ECs were immunostained with anti–Stro-1 antibody or with control isotype IgG and analyzed by flow cytometry. Left: Representative sortings. Right: Quantified results (n = 3 to 5, means ± SEM). Statistical analysis by Student’s t test. (H) ECs were subjected to multiplex transcriptional factor activity analysis. The activity in GBM ECs was normalized to SOX18 activity and expressed as the folds of normal ECs (n = 3, means ± SEM). (I) ECs were transduced with lentivirus to express a Sox2/ETn-GFP probe, followed by flow cytometric analysis. Statistical analysis by Student’s t test. (J) Single cells were cultured in MSC medium, followed by crystal violet staining and imaging. Top: Representative images. Bottom: Colony numbers were counted (n = 5, means ± SEM). Statistical analysis by Student’s t test. (K) ECs were treated with Neurobasal stem cell medium under hypoxia (2% O2), followed by imaging. Left: Representative images. Right: Quantified results (n = 3 to 10, means ± SEM). Statistical analysis by one-way ANOVA. (L) Spheres in ECs cultured with Neurobasal medium were counted and analyzed. Left: Linear regression analysis. Right: Stem cell frequency (n = 3, means ± SEM). Statistical analysis by one-way ANOVA.
We next investigated the potential stemness-like activation and mesenchymalization in human GBM-associated ECs. Immunoblot analysis showed that GBM ECs, but not normal ECs, expressed multiple mesenchymal proteins including PDGFR-α (platelet-derived growth factor receptor-α), PDGFR-β, and α-SMA, comparable to fibroblasts and MSCs (Fig. 2F). These GBM-associated ECs also showed increased expression of the MSC marker Stro-1 (Fig. 2G), as well as c-Kit and CD44 (fig. S3). Moreover, multiplex analysis of transcriptional factor activity, based on DNA binding activity, indicated potential activation of multiple stemness-associated transcriptional factors, including FOXO1, KLF4, GATA, ETS, and TCF/LEF in GBM ECs (Fig. 2H). The ECs were transduced to express a genetic probe to trace cells with stemness activation (Fig. 2I, top), wherein green fluorescent protein (GFP) expression is driven by an early transposon promoter (ETn) with Sox2 binding motifs (15, 31). Our data revealed activation of Sox2/ETn transcription in multiple GBM-associated ECs (Fig. 2I), collectively suggesting stemness-like activation in these tumor ECs.
We further analyzed stemness-associated functions in GBM ECs. Our data show that GBM ECs, but not normal brain ECs, generated single-cell–derived colonies in MSC medium (Fig. 2J). Moreover, GBM ECs formed spheres in serum-free Neurobasal medium but not in endothelial culture medium (Fig. 2K). The cells in the spheres could still uptake Dil-Ac-LDL, which is a key feature of ECs (fig. S4), suggesting that self-renewal potential is acquired in GBM ECs, unlikely due to possible contamination of glioma stem cells during EC sorting. Limited dilution analysis confirmed sphere-forming stemness feature specifically in GBM ECs (Fig. 2L). In addition, we tested the potential differentiation capacity of GBM ECs into mesenchymal cells. We show that, upon incubation with different stimulus (fig. S5A), GBM ECs expressed smooth muscle cell–specific markers including α-SMA, calponin, and Myh11 (fig. S5B); fibroblast-specific protein FSP-1 (fig. S5C); and pericyte-specific marker NG-2 (fig. S5D), implicating potential multipotency in these GBM ECs. Moreover, GBM ECs exhibited MSC-like capacity for potential trilineage differentiation into adipocytes, osteoblasts, and chondrocytes (fig. S6). Last, both cell mesenchymalization (as indicated by FSP-1 expression) and Sox2/ETn transcriptional activation (as indicated by Sox2/ETn promoter-derived GFP expression) correlated with cell resistance to TMZ treatment in multiple ECs (fig. S7). Together, these findings provide evidence for stemness-like activation and mesenchymalization in GBM-associated ECs, which likely contributes to their chemoresistance.
Wnt/β-catenin is critical for stemness activation and chemoresistance in GBM ECs
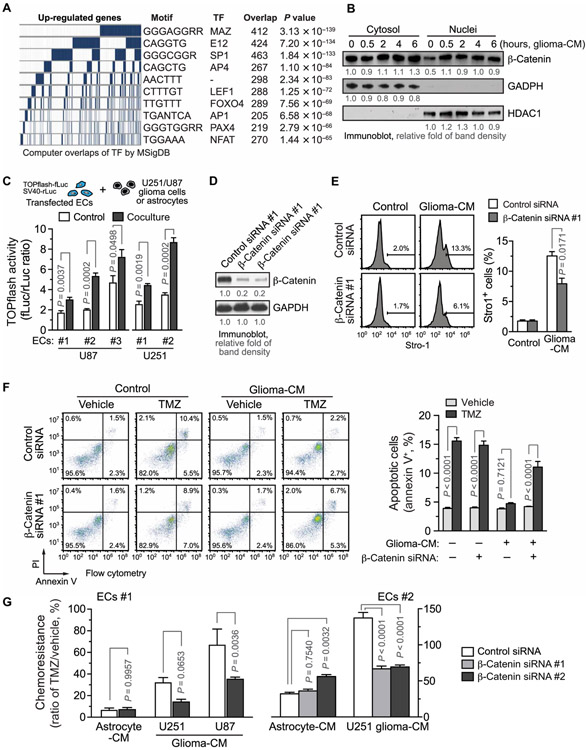
We then explored the molecular mechanism(s) underlying stemness-like activation in GBM ECs. Computational bioinformatics analysis of the top 1000 up-regulated gene promoter sequences in ECs pretreated with glioma-CM identified consensus DNA motifs that are known to be recognized by several transcriptional factors, including MAZ, E12, SP1, AP4, LEF1, FOXO4, AP1, PAX4, and NFAT (Fig. 3A). LEF1 and FOXOs are known to be associated with stemness activation (14, 32). However, small interfering RNA (siRNA)–mediated knockdown of FOXO1 or KLF4, whose transcriptional activity was enhanced in GBM ECs, did not affect glioma-CM–induced chemoresistance in ECs (fig. S8). Therefore, we focused our studies on LEF1. LEF1 is the master transcriptional factor that controls Wnt signaling activation, which is initiated by β-catenin nuclear translocation and has a well-established role in stemness and tumor treatment resistance (14, 33-36). We showed that glioma-CM induced the rapid nuclear translocation of β-catenin in ECs (Fig. 3B), which is an essential step in LEF1/Wnt activation. Moreover, TOPflash luciferase-based transcriptional reporter assays confirmed that coculture of normal human brain ECs with U251 or patient-derived glioma cells induced LEF1 activation in the ECs (Fig. 3C and fig. S9). Furthermore, glioma-CM induced a time-dependent expression of Wnt target genes including DAB2, CCND2, MYC, BTRC, FOSL1, and PPARD in human brain ECs (fig. S10). siRNA-mediated knockdown of β-catenin inhibited glioma-CM–induced Stro-1 expression in ECs (Fig. 3, D and E), suggesting a critical role for β-catenin in stemness-like activation in ECs under glioma conditions. In addition, β-catenin knockdown restored TMZ-induced cell apoptosis in ECs pretreated with glioma-CM (Fig. 3F). Glioma-CM derived from U251 or U87 glioma cell lines or primary GBM cells induced chemoresistance in ECs, which is inhibited by β-catenin knockdown (Fig. 3G and fig. S11). Together, these findings suggest that Wnt/β-catenin induces stemness-like activation and chemoresistance in GBM ECs.
Fig. 3. β-Catenin/Wnt is critical for stemness activation and chemoresistance to TMZ in ECs under GBM conditions.
(A) ECs were treated with glioma-CM and analyzed by RNA-seq (n=3). The promoter sequences of the top 1000 up-regulated genes were analyzed against the Molecular Signatures Database (MSigDB), and the most common motifs were identified. The corresponding transcriptional factors are shown. (B) ECs were treated with glioma-CM. Cytosolic and nuclear fractions were isolated and immunoblotted. HDAC1, histone deacetylase 1. (C) ECs were transfected with plasmids that encode TOPflash-fLuc and SV40-rLuc and cocultured with U87 glioma cells or with control human brain astrocytes, or with U215 glioma cells, or with control EC medium. Cells were lysed and subjected to dual-luciferase activity analysis. TOPflash-fLuc values are expressed as the ratios of SV40-rLuc (n = 3, means ± SEM). Statistical analysis by Student’s t test. (D to G) ECs were transfected with siRNAs targeting β-catenin or with a scrambled siRNA. (D) Cell lysates were immunoblotted. (E) ECs were treated with glioma-CM, stained with anti–Stro-1 antibody or with control isotype IgG, and analyzed using flow cytometry. Left: Representative sortings. Right: Quantified results (n=3, means ± SEM). Statistical analysis by Student’s t test. (F and G) ECs were pretreated with glioma-CM, followed by 1 mM TMZ treatment. (F) Cells were stained with annexin V and propidium iodide (PI) and analyzed by flow cytometry. Left: Representative cell sortings. Right: Quantitative results (n = 3, means ± SEM). Statistical analysis by two-way ANOVA. (G) Cell viability was determined by MTS assay. Chemoresistance was expressed by the cell viability percentage of TMZ-treated and vehicle-treated ECs (n = 3, means ± SEM). Statistical analysis by two-way ANOVA.
HGF/c-Met induces Wnt activation through β-catenin phosphorylation at Ser675
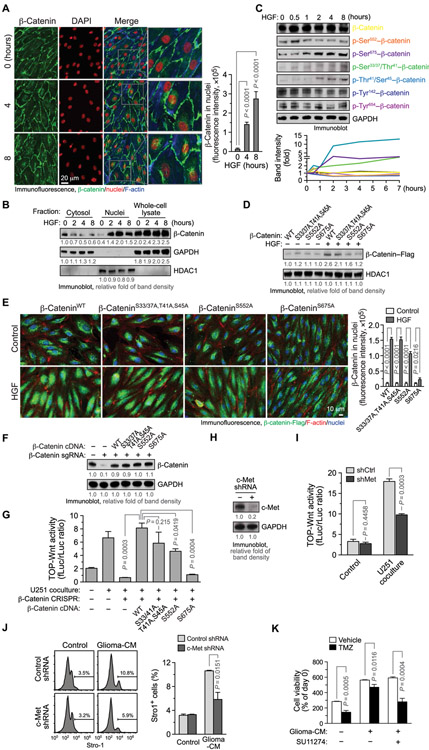
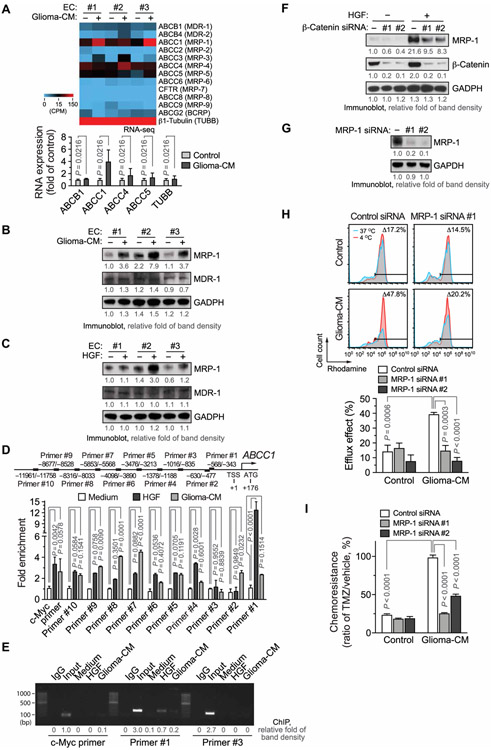
To investigate stimulus-inducible Wnt activation in ECs, cells were treated with multiple growth factors including basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), and VEGF followed by β-catenin translocation analysis. Our immunofluorescence data showed that HGF induced time-dependent β-catenin nuclear translocation (Fig. 4A). Immunoblot analysis of different subcellular fractions validated the rapid nuclear translocation of β-catenin by HGF (Fig. 4B). β-Catenin translocation to nuclei is known to be induced by cytosolic accumulation and facilitated transport, both regulated by β-catenin phosphorylation (37). HGF did not induce cytosol accumulation of β-catenin (Fig. 4B) but evoked rapid β-catenin phosphorylation at Ser552, followed by more sustained phosphorylation at Ser675 and slightly increased phosphorylation at Ser33/Ser37/Thr41/Ser45 (Fig. 4C). Consistent with previous reports showing direct β-catenin phosphorylation by HGF receptor kinase c-Met (38, 39), HGF induced a transient β-catenin phosphorylation at Tyr142 and Tyr654, peaking at 10 to 20 min after treatment but did not affect the phosphorylation after 0.5 hour (Fig. 4C). Considering the established role of β-catenin phosphorylation at Ser552 and Ser675 for its transport into nuclei (40, 41), we tested whether the phosphorylation at these sites regulates HGF-induced β-catenin nuclei translocalization in ECs. Our data showed that phosphorylation-dead Ala mutation at Ser675 or Ser552, but not mutations at Ser33/Ser37/Thr41/Ser45, abrogated HGF-induced β-catenin nuclear translocalization, as revealed by immunoblot analysis of nuclear fractions (Fig. 4D). Moreover, immunofluorescence analysis validated that Ser675 to Ala mutation and, to a lesser extent, Ser552 to Ala mutation, but not mutations at Ser33/Ser37/Thr41/Ser45, blocked β-catenin nuclear translocalization in HGF-treated ECs (Fig. 4E). In addition, consistent with previous reports (42, 43), overexpressed Flag-tagged β-catenin did not exhibit preferential localization at cell junctions (Fig. 4E) as endogenous β-catenin does (Fig. 4A), but HGF stimulated β-cateninWT–Flag nuclear translocalization (Fig. 4E), suggesting its intact translocalization function. Expression of β-catenin with the Ala mutation at Ser675, but not at Ser552 or Ser33/Ser37/Thr41/Ser45, failed to rescue LEF1 transcriptional activity in CRISPR-mediated β-catenin knockout ECs (Fig. 4, F and G, and fig. S12). These findings suggest that HGF/c-Met induces β-catenin phosphorylation at Ser675, leading to β-catenin nuclear translocation, which may be critical for Wnt signaling activation in GBM ECs.
Fig. 4. HGF/c-Met induces β-catenin phosphorylation at Ser675 and nuclear translocalization, which is critical for stemness activation and TMZ chemoresistance in ECs under GBM conditions.
(A to C) ECs were treated with HGF (100 ng/ml). (A) Cells were stained with anti–β-catenin antibody and analyzed by immunofluorescence. Left: Representative images. Right: Quantified results (n = 20, means ± SEM). Statistical analysis by one-way ANOVA. DAPI, 4′,6-diamidino-2-phenylindole. (B) Cytosolic and nuclear fractions were isolated and immunoblotted. (C) Cell lysates were subjected to immunoblot analysis. (D and E) ECs were transfected with plasmids that encode Flag-tagged WT and mutated β-catenin and treated with HGF. (D) Nuclear fractions were extracted and analyzed by immunoblot. (E) Cells were fixed and stained with anti-Flag antibody and analyzed by immunofluorescence. Left: Representative images. Right: Quantified results (n = 20, means ± SEM). Statistical analysis by two-way ANOVA. (F and G) ECs were transfected to express CRISPR single guide RNA (sgRNA) that targets β-catenin or a control scrambled sequence. Cells were transfected with plasmids that encode Flag-tagged WT and mutated β-catenin complementary DNA (cDNA). (F) Cell lysates were immunoblotted. (G) Cells were transfected with plasmids that encodes TOPflash-fLuc and SV40-rLuc and cocultured with U251 glioma cells or with control medium. Cells were subjected to dual-luciferase activity analysis. TOPflash-fLuc values are expressed as the ratios of SV40-rLuc (n = 3, means ± SEM). Statistical analysis by one-way ANOVA. (H to J) ECs were transduced with lentivirus to express shRNA targeting c-Met or a scrambled shRNA sequence. (H) Cell lysates were immunoblotted. (I) Cells were transfected with plasmids that encodes TOPflash-fLuc and SV40-rLuc and cocultured with U251 glioma cells or control medium. Cells were subjected to bioluminescence analysis. TOPflash-fLuc values are expressed as the ratios of SV40-rLuc (n = 3, means ± SEM). Statistical analysis by Student’s t test. (J) Cells were treated with glioma-CM and immunostained with anti–Stro-1 antibody or with control isotype IgG, followed by flow cytometry analysis. Left: Representative sortings. Right: Quantitative results (n = 3, means ± SEM). Statistical analysis by Student’s t test. (K) ECs were treated with glioma-CM with or without SU11274, followed by treatment with TMZ. Cell viability was analyzed and expressed as percentage of pretreatment (n = 3, means ± SEM). Statistical analysis by Student’s t test.
We next investigated the role of HGF/c-Met for Wnt activation, stemness-like activation, and chemoresistance in ECs under glioma conditions. Our data showed that short hairpin RNA (shRNA)–mediated knockdown of c-Met inhibited LEF1 transcriptional activation induced by coculture with U251 cells (Fig. 4, H and I). Moreover, c-Met knockdown suppressed glioma-CM–induced stemness-like activation, as indicated by reduced Stro-1 expression in the ECs (Fig. 4J). Furthermore, pretreatment with glioma-CM induced EC resistance to TMZ treatment, and pharmacological inhibition of c-Met by SU11274 reversed glioma-CM–induced chemoresistance in ECs (Fig. 4K). Consistently, incubation of neutralizing antibody against HGF, but not Wnt-5, a Wnt-activating ligand that was previously shown overexpressed in GBM (44), abrogated glioma-CM–induced chemoresistance in ECs (fig. S13). These results collectively suggest that HGF/c-Met is required for Wnt activation, stemness-like activation, and chemoresistance in ECs under glioma conditions.
HGF induces Wnt-mediated MRP-1 expression
It is well established that ATP-binding cassette (ABC) subfamily proteins induce drug pumping and efflux, contributing to chemoresistance in cancer cells and stem cells (45-48). We analyzed gene expression of these ABC transporters in ECs under glioma conditions by RNA-seq. We detected a threefold increase in ABCC1 [multidrug resistance-associated protein 1 (MRP-1)], but not in other family members (Fig. 5A). Glioma-CM–inducible up-regulation of MRP-1 expression was verified by immunoblot analysis (Fig. 5B). Moreover, our data showed that HGF induced MRP-1 expression in ECs (Fig. 5C). Furthermore, chromatin immunoprecipitation (ChIP) analysis indicated that HGF induced robust LEF1 binding to MRP-1 promoter at the DNA region of −568 to −343 in ECs (Fig. 5, D and E). Last, siRNA-mediated knockdown of β-catenin inhibited HGF-induced MRP-1 expression (Fig. 5F), suggesting that Wnt/β-catenin activation plays a key role in this process. Treatment with glioma-CM robustly enhanced efflux of Rhodamine 123 in ECs; knockdown of MRP-1 abrogated glioma-CM–stimulated efflux and chemoresistance (Fig. 5, G to I), suggesting a critical role of MRP-1 in EC drug efflux and chemoresistance under glioma condition. Collectively, these findings suggest that HGF induces β-catenin phosphorylation at Ser675 and stimulates its nuclear translocation; subsequent LEF1-mediated Wnt activation induces stemness-like activation as well as MRP-1 expression and drug efflux, eventually leading to EC chemoresistance under glioma conditions (fig. S14).
Fig. 5. HGF induces Wnt/β-catenin–mediated MRP-1 expression and TMZ chemoresistance in ECs.
(A) Gene expression in glioma-CM–treated ECs was analyzed for ABC family proteins by RNA-seq. Top: Heat map. Bottom: Expression (n = 3, means ± SEM). Statistical analysis by two-way ANOVA. (B) ECs were treated with glioma-CM, followed by immunoblot analysis. (C) ECs were treated with HGF. Cell lysates were immunoblotted. (D and E) ECs were treated with control medium, HGF, or glioma-CM. Nuclei protein was immunoprecipitated with anti-LEF1 antibody or IgG and subjected to ChIP analysis with different primers. (D) Quantitative real-time polymerase chain reaction analysis (n = 3, means ± SEM). Statistical analysis by two-way ANOVA. (E) DNA was resolved by agarose electrophoresis and imaged. bp, base pair. (F) ECs were transfected with siRNAs targeting β-catenin or with a control scrambled siRNA, treated with HGF, and analyzed by immunoblotting. (G to I) ECs were transfected with siRNAs targeting MRP-1 or with a control siRNA. (G) Cell lysates were immunoblotted. (H and I) Transfected cells were treated with glioma-CM or control astrocyte-CM. (H) Cells were loaded with Rhodamine 123, followed by flow cytometry analysis. Top: Representative sortings. Bottom: Quantified results (n = 3, means ± SEM). Statistical analysis by two-way ANOVA. (I) Cells were treated with 1 mM TMZ, and cell viability was determined by MTS assay. Chemoresistance was expressed by the cell viability percentage of TMZ-treated and vehicle-treated ECs. Statistical analysis by two-way ANOVA.
Endothelial Wnt activation is critical for EC chemoresistance and glioma response to TMZ treatment
To rigorously determine the in vivo role of endothelial Wnt activation in EC chemoresistance and tumor treatment response, we generated an EC-specific β-catenin knockout mouse line, Cdh5-CreERT2;Ctnnb1fl/fl, by crossing Ctnnb1fl/fl mice with mice expressing Cre DNA recombinase under the control of the EC-specific promoter Cdh5ERT2 with tamoxifen-inducible activation (fig. S15A). Postnatal deletion of β-catenin in ECs was lethal in adult mice, as evidenced by that all Cdh5-CreERT2;Ctnnb1fl/fl mice had a median survival time of 16.5 days after tamoxifen injection (fig. S15B). We therefore used a viable, heterozygous knockout mouse line, Cdh5-CreERT2;Ctnnb1+/fl, in which β-catenin expression is markedly knocked down in an EC-specific manner (fig. S15C). In addition, we incorporated the Rosa-LSL-tdTomato system to allow for visualization of ECs in tissues.
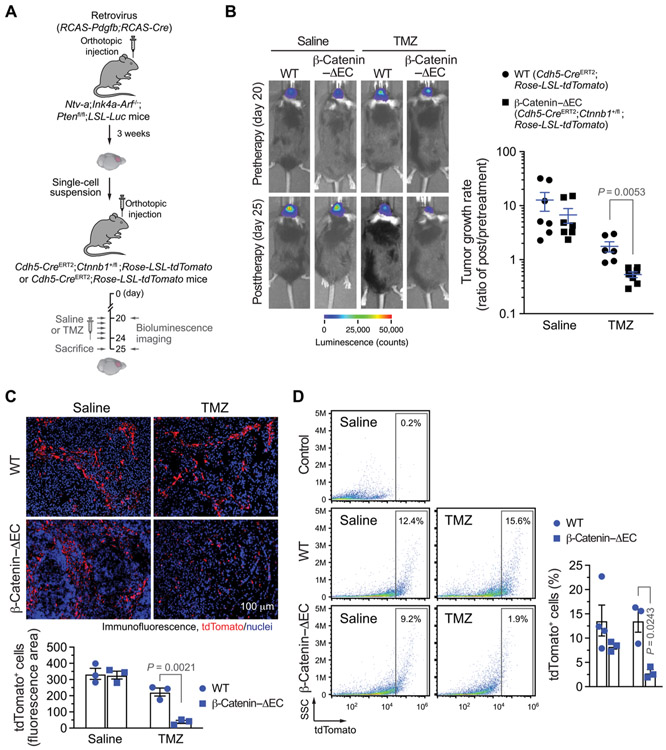
We then challenged the transgenic mice with an orthotopic injection of tumor cells isolated from the RCAS-mediated genetic GBM model, followed by treatment with TMZ or saline (Fig. 6A). Knockdown of β-catenin in ECs did not affect basal tumor growth (fig. S16); however, it sensitized the tumors to TMZ chemotherapy: During the therapeutic window (days 20 to 25), tumors grew by around 10-fold in volume in both wild-type (WT) (Cdh5-CreERT2;Rosa-LSL-tdTomato) and β-catenin–ΔEC (Cdh5-CreERT2;Rosa-LSL-tdTomato; Ctnnb1+/fl) mice treated with saline; TMZ treatment abrogated tumor growth but did not reduce tumor volume in WT mice during this period, as evidenced by similar tumor bioluminescence values observed before and after treatment; in contrast, TMZ treatment robustly reduced tumor volume by 90% in β-catenin–ΔEC mice (Fig. 6B). TMZ treatment only eliminated tumor-associated ECs in β-catenin–ΔEC mice but not in WT mice, as indicated by a decrease in tumor-associated tdTomato+ ECs in the β-catenin–ΔEC mice after TMZ treatment (Fig. 6, C and D). β-Catenin has dual roles for regulation of cell adhesion junctions and Wnt signaling activation (14, 35); however, β-catenin knockdown in Cdh5-CreERT2;Ctnnb1+/fl mice did not disturb F-actin cytoskeleton or affect the expression and distribution of VE-cadherin, a key β-catenin–interacting adhesion protein (fig. S15D), suggesting that the β-catenin knockdown–induced in vivo effects may mainly act through suppression of Wnt signaling. Collectively, these findings suggest that Wntβ-catenin is critical for EC chemoresistance, which determines tumor response to TMZ treatment.
Fig. 6. Wnt/β-catenin is critical for EC chemoresistance and GBM resistance to TMZ in vivo.
GBM was induced by RCAS-mediated gene transfer in Ntv-a;Ink4a-Arf−/−;Ptenfl/fl;LSL-Luc mice. Single-cell tumor suspensions were injected into Cdh5-CreERT2;Ctnnb1+/fl;Rosa-LSL-tdTomato or into Cdh5-CreERT2;Rosa-LSL-tdTomato mice that were pretreated with tamoxifen. After tumor induction, the mice were treated with saline or with TMZ (50 mg/kg). (A) Schematic approach. (B) Tumor volumes were analyzed by bioluminescence. Left: Representative images before and after TMZ therapy. Right: Quantitative results. Tumor growth rates were expressed as the ratios of integrated bioluminescence values before versus after TMZ therapy (n = 6 to 7, means ± SEM). Statistical analysis by Student’s t test. (C) Tumor sections were stained with anti-tdTomato antibody and subjected to immunofluorescence analysis. Top: Representative images. Bottom: Quantified results (n = 3, means ± SEM). Statistical analysis by Student’s t test. (D) Single-cell tumor suspensions were analyzed by flow cytometry. Side scatter (SSC). As a control, tumors were isolated from Cdh5-CreERT2;Rosa-LSL-tdTomato mice that were not pretreated with tamoxifen. Left: Representative sortings. Right: Quantitative results (n = 3 to 4, mean ± SEM). Statistical analysis by Student’s t test.
Wnt inhibition abrogates EC stemness activation and sensitizes GBM to TMZ therapy
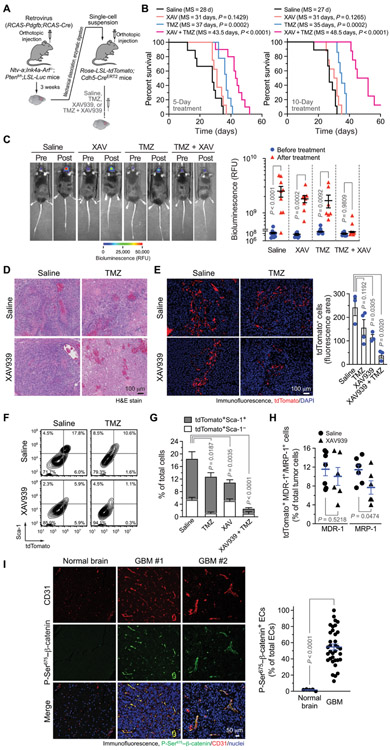
Last, we explored the therapeutic potential of a combination of Wnt inhibition and chemotherapy. We genetically induced GBM model in the Cdh5-Cre;Rosa-LSL-tdTomato mouse model, followed by treatment with Wnt inhibitor XAV939 and/or TMZ (Fig. 7A). XAV939 treatment alone did not affect animal survival (Fig. 7B). TMZ treatment for 5 or 10 days alone moderately improved animal survival by 8 to 9 days. Moreover, neither XAV939 nor TMZ treatment inhibited tumor growth during the 10-day treatment period (Fig. 7C). The combined treatment of XAV939 and TMZ extended mouse survival (+15.5 days by 5-day treatment and +21.5 days by 10-day treatment; Fig. 7B); the combined treatment also markedly inhibited tumor growth, as indicated by no increase in tumor volume during the 10-day treatment period (Fig. 7C), suggesting that XAV939 treatment sensitizes GBM to TMZ chemotherapy.
Fig. 7. Wnt inhibition abrogates EC stemness and sensitizes GBM and tumor-associated ECs to TMZ chemotherapy.
(A to H) GBM was induced by RCAS-mediated gene transfer in Ntv-a;Ink4a-Arf−/−;Ptenfl/fl;LSL-Luc mice. Single-cell tumor suspensions were injected into Rosa-LSL-tdTomato;Cdh5-CreERT2 mice. After tumor induction, the mice were treated with saline, TMZ, and/or XAV939. (A) Schematic approach. (B) Median Survival (MS) was monitored for 60 days. Left: Treatment with saline or TMZ (100 mg/kg) with or without XAV939 (40 mg/kg) for 5 days (n = 8 to 10). Right: Treatment with saline or TMZ (50 mg/kg) with or without XAV939 (40 mg/kg) for 10 days (n = 7 to 9). Statistical analysis by log-rank test. (C) Tumor volume was analyzed by bioluminescence imaging before and after 10-day treatment. Left: Representative images. Right: Bioluminescence values of mice before and after treatment (n = 7 to 9, means ± SEM). Statistical analysis by two-way ANOVA. RFU, relative fluorescence units. (D) Tumor sections were stained with hematoxylin and eosin (H&E) dyes. Representative images are shown (n = 5). (E) Tumor sections were stained with anti-tdTomato antibody, followed by immunofluorescence analysis. Left: Representative sortings. Right: Quantitative results (n = 3 to 4, means ± SEM). Statistical analysis by one-way ANOVA. (F and G) Single-cell tumor suspensions were stained with anti–Sca-1 and anti-tdTomato antibodies and analyzed by flow cytometry. (F) Representative sortings. (G) Quantitative results (n = 3 to 6, means ± SEM). Statistical analysis by one-way ANOVA for total tdTomato+ cells. (H) Single-cell tumor suspensions were stained with anti–MDR-1 and anti–MRP-1 antibodies and analyzed by flow cytometry (n = 6 to 7, means ± SEM). Statistical analysis by Student’s t test. (I) Tissue sections of surgical samples from human normal brain (n = 5) and from patients with GBM (n = 35) were probed with anti–P-Ser675–β-catenin and anti-CD31 antibodies, followed by immunofluorescence analysis. Left: Representative images. Right: Quantified results (means ± SEM). Statistical analysis by Student’s t test.
XAV939 treatment alone did not affect tumor pathological appearance; administration with TMZ induced moderate tumor necrosis; and combined treatment induced extensive tumor necrosis (Fig. 7D). Furthermore, immunofluorescence analysis showed that either XAV939- or TMZ-alone treatment slightly reduced tumor vascularity, but combined treatment markedly inhibited tumor vascularization as indicated by a substantial decrease in EC-derived tdTomato+ cells in the tumors (Fig. 7E). Likewise, flow cytometry analysis of tumor-derived single-cell suspensions verified that combined treatment with XAV939 and TMZ induced a robust reduction of tdTomato+ EC population in the tumors (Fig. 7F and G).
Consistent with that TMZ treatment did not decrease FSP-1+tdTomato+ cell population, TMZ treatment did not alter Sca-1+tdTomato+ population but markedly reduced Sca-1−tdTomato+ population (Fig. 7G), suggesting a role for cell transformation in EC chemoresistance. In contrast, XAV939 treatment reduced Sca-1+tdTomato+ population by half but did not affect Sca-1−tdTomato+ population; XAV939 treatment sensitized Sca-1+tdTomato+ cells to TMZ chemotherapy, leading to a marked reduction in these Sca-1+ chemoresistant populations after the combined XAV939 and TMZ treatment. Flow cytometry analysis revealed that XAV939 treatment reduced the expression of MRP-1, but not multidrug resistance-1 (MDR-1), in tumor-associated tdTomato+ cells (Fig. 7H), suggesting that Wnt activation induces MRP-1 expression in tumor ECs, contributing to EC chemoresistance. In addition, XAV939 treatment sensitized human GBM-associated ECs to TMZ chemotherapy in vitro in a dose-dependent manner (fig. S17A), supporting a critical role for Wnt in EC chemoresistance. XAV939 treatment did not improve U251 glioma cells’ responses to TMZ treatment but abrogated EC-induced chemoresistance in glioma cells (fig. S17B), suggesting that Wnt inhibition–induced in vivo tumor responses may primarily act through its effects on EC and EC-medicated tumor chemoresistance, rather than direct effects on glioma cells.
Last, our immunofluorescence analysis of human GBM patient–derived tumor specimens showed that β-catenin phosphorylation at Ser675 was preferentially localized in GBM-associated ECs (Fig. 7I), suggesting a potentially EC-selective mechanism for Wnt/β-catenin–mediated chemoresistance in GBM. In summary, our results suggest that ECs transform into MSC-like cells through Wnt/β-catenin signaling, which renders ECs and tumors resistant to chemotherapy. We identified a c-Met/Wnt/β-catenin axis, which induces MRP-1 expression and stemness-like activation in glioma ECs.
DISCUSSION
Malignant solid tumors are characterized by prominent vascularity (49). Newly formed blood vessels deliver oxygen and nutrients and produce paracrine factors to fuel tumor growth, progression, and metastasis; therefore, antivascular therapy has been served as a fundamental approach in cancer therapy over decades (1-4). However, recent antiangiogenic clinical trials showed limited therapeutic benefit in most malignant solid tumors including GBM, which challenges proangiogenic factors and their downstream pathways as valuable anticancer therapeutic targets. Here, we reveal that GBM ECs undergo transformation to stem cell-like cells, driving EC and tumor resistance to chemotherapy, which suggests that endothelial detransformation can sensitize ECs to cytotoxic treatments, providing an alternative avenue in cancer therapy.
EC plasticity has been well characterized in embryogenesis and pathological settings including cardiac, renal and liver fibrosis, pulmonary hypertension, vascular inflammation, and cerebral cavernous malformation (50-61). Previous work reveals conversion of ECs into MSC-like cells by an activin-like kinase-2–dependent mechanism in fibrodysplasia ossificans progressive (62). Here, our work characterizes EC transformation into stem cell–like cells in the tumor microenvironment, that is, endothelial mesenchymalization and stemness-like activation in GBM ECs, rendering ECs resistant to treatment. In contrast to the previously proposed concept that ECs undergo endothelial-mesenchymal transition to generate de novo fibroblasts in cancer (21, 63), our recent work reveals that GBM-associated ECs retain key endothelial functions and acquire mesenchymal phenotypes including increased migration and proliferation to induce vascular abnormalities and generate excessive, aberrant vasculature (20). Likewise, we suggest that ECs do not undergo a cell fate shift but acquire mesenchymalization and stemness-like activation in the GBM microenvironment, inducing EC resistance to treatments.
In addition to their classical functions for vessel delivery, cancer-associated ECs act as a niche that fuels tumor progression and metastasis and induces treatment resistance by producing paracrine factors to the tumor microenvironment (5, 22, 64-68). Hence, perivascular niche is critical for stemness maintenance and self-renewal in cancer stem cells (69-71). Moreover, our recent work reveals a vascular niche that regulates macrophage-mediated GBM immunity (72), collectively providing therapeutic rationale for eradicating tumor ECs in cancer. Here, our lineage tracing analysis revealed that most GBM ECs, but not the CD31high subpopulation, are highly chemoresistant. Likewise, a major therapeutic barrier in current anticancer therapy is the inefficient eradication or insufficient functional inhibition of tumor ECs. We show that treatment resistance in GBM ECs is, at least partially, driven by Wnt/β-catenin–mediated cell transformation, which plays a role in tumor chemoresistance. Consistently, growing evidence suggests a critical role of mesenchymalization and stemness activation for tumor treatment resistance (10, 11). Thus, it is tempting to speculate that EC transformation into MSC-like cells preserves vascular niches that induce tumor cell resistance during/after cancer treatment, therefore serving as a critical therapeutic target in cancer.
A critical role for Wnt/β-catenin signaling has been well established in both developmental and pathological angiogenesis: regulating EC proliferation, migration, vascular sprouting, and stability (73-78). Recent work revealed that β-catenin regulates adhesion protein expression in brain ECs and the integrity of the blood-brain barrier (79-83). Consistent with these findings, we showed that β-catenin knockout in ECs was lethal in adult mice. On the basis of our results, we suggest that moderate activity is critical for brain angiogenesis and the blood-brain barrier under physiological conditions and that further enhanced Wnt/β-catenin activity induces EC genetic reprograming, leading to stemness-like activation and chemoresistance. In addition, we showed that HGF/c-Met stimulates Wnt/β-catenin activity, inducing MRP-1 expression, stemness activation, and chemoresistance in GBM ECs, representing a mechanism for Wnt-mediated chemoresistance. This finding is supportive of a recent work showing that glioma cells release Wnt7 to activate ECs (84). Overactivation of Wnt/β-catenin signaling reduced density of CD31+ ECs and normalized tumor vasculature (44). Here, we showed that pharmacological Wnt inhibition does not increase EC-derived cell population in GBM but sensitized these cells to TMZ chemotherapy, suggesting Wnt/β-catenin as a critical therapeutic target for GBM.
HGF induces β-catenin accumulation and translocation into nuclei and Wnt signaling activation by disrupting c-Met-β-catenin association and by modulating glycogen synthase kinase 3 (GSK3) phosphorylation in hepatocytes, epithelial cells, and tumor cells (85-88). In contrast to the N-terminal phosphorylation by GSK3 that triggers β-catenin degradation, phosphorylation of several sites on β-catenin C terminus at Ser552 and Ser675 appeared to induce its nuclear transportation and β-catenin–dependent transcription (40, 41). Our data showed that HGF stimulates Wnt/β-catenin activity in brain ECs by inducing β-catenin phosphorylation at Ser675 and its nuclear translocation, but not through the cytosol accumulation–mediated mechanism.
In addition, previous studies showed that glioma stem cells transdifferentiate into pericyte lineages (89) and that Wnt signaling activation induced differentiation of glioma stem cells into ECs (90, 91), leading to invasive tumor phenotypes. Here, we revealed that GBM-associated ECs transform into MSC-like cells, leading to chemoresistance. These results may suggest a dynamic transition status between ECs and stem cells in the tumor microenvironment.
Regarding the limitations of this study, therapeutic exploitation of Wnt/β-catenin inhibition in GBM was conducted using one pharmacological inhibitor, XAV939. Preclinical investigation with other inhibitors targeting different Wnt/β-catenin signaling components may provide further information for clinical treatment in humans. The effects of tumor cells from different subtypes of human GBM on endothelial transformation are also unclear. Validation of our results with more human GBM-derived cell lines will be helpful for the development of relevant clinical trials. Another challenge for GBM therapy with Wnt inhibitors is the drug delivery across the blood-brain barrier, which needs to be evaluated in human patients in vivo.
In summary, our study reveals that ECs undergo transformation into stem cell–like cells in the GBM microenvironment, inducing EC and tumor treatment resistance, through a c-Met/β-catenin/MRP-1–dependent mechanism. Thus, targeting Wnt-mediated EC transformation may offer promising opportunities for efficient eradication of tumor ECs and overcoming cancer chemoresistance.
MATERIALS AND METHODS
Study design
The overall objectives were to define the role of endothelial plasticity in tumor chemoresistance and to develop optimal combinatorial therapies to treat GBM. We aimed to characterize tumor-associated ECs and determine their chemoresistance using human patient–derived cells in vitro and mouse cells with genetic lineage tracing in vivo. We analyzed gene expression signature in GBM ECs by RNA-seq and investigated their stemness-like activation by marker expression– and stem cell function–based analyses. We further identified the mechanisms underlying MSC-like transformation in GBM ECs by genetic and pharmacological manipulations, as well as by mutagenesis and cell-based approaches. The in vitro experiments were carried out with at least three replicates. We tested the role of endothelial stemness activation in tumor chemoresistance by using an EC specifically targeting transgenic mouse line. Last, we interrogated the efficacy of combined therapy with pharmacological Wnt inhibition and chemotherapy in a genetically engineered mouse GBM model. Animals of similar age, weight, and sex were grouped randomly, and the number of animals per group was 6 to 10.
Statistical analysis
All grouped data were presented as box plot in figures. Statistical analysis was performed using unpaired Student’s t test or analysis of variance (ANOVA) analysis for experiments with two groups or more than two groups, respectively. Linear regression was performed by using Prism 8.0 software. Kaplan-Meier survival curves were generated using Prism software, and log-rank test was performed to assess statistical significance between groups. A two-sided P value lower than 0.05 was considered significant.
Supplementary Material
Fig. S1. FSP-1 expression in GBM-associated ECs.
Fig. S2. Chemoresistance in GBM-associated ECs.
Fig. S3. Expression of c-Kit and CD44 in GBM ECs.
Fig. S4. Dil-Ac-LDL uptake in sphere-forming GBM ECs.
Fig. S5. Differentiation of GBM ECs into mesenchymal cells.
Fig. S6. MSC-like trilineage differentiation of GBM ECs.
Fig. S7. Correlation of stemness activation and mesenchymalization with chemoresistance in GBM ECs.
Fig. S8. Effects of FOXO1 or FLF4 knockdown on chemoresistance in ECs.
Fig. S9. LEF1 transcriptional activation in ECs cocultured with GBM cells.
Fig. S10. Expression of Wnt target genes in ECs.
Fig. S11. Chemoresistance in ECs treated with glioma-CM.
Fig. S12. CRISPR-mediated β-catenin knockdown in ECs.
Fig. S13. Effects of HGF or Wnt5a neutralization on chemoresistance in ECs.
Fig. S14. A schematic model.
Fig. S15. Cdh5-CreERT2–mediated β-catenin knockdown in ECs.
Fig. S16. Effects of β-catenin knockdown in ECs on GBM response to chemotherapy.
Fig. S17. Effects of Wnt inhibition on EC chemoresistance and EC-mediated glioma cell chemoresistance.
Data file S1. Raw data.
Acknowledgments:
We are grateful to R. Adams and B. Ding for providing the Cdh5-CreERT2 mice, and to C. Simon for the helpful discussions.
Funding: This work was supported in part by NIH grants R01NS094533 and R01NS106108 (to Y.F.), the American Association for Cancer Research Judah Folkman Award (to Y.F.), and the McCabe award (to Y.F.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials. Raw sequencing data files are deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (GSE115850).
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Adams RH, Alitalo K, Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol 8, 464–478 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Kerbel RS, Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Jain RK, Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergers G, Hanahan D, Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 8, 592–603 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Y, Vascular detransformation for cancer therapy. Trends Cancer 5, 460–463 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med 352, 987–996 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Meco FD, Lieberman F, Zhu J-J, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim C-Y, Paek S-H, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z, Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 318, 2306–2316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huse JT, Holland EC, Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer 10, 319–331 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Reya T, Morrison SJ, Clarke MF, Weissman IL, Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Li Y, Yu T-S, McKay RM, Burns DK, Kernie SG, Parada LF, A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488, 522–526 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN, Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Xu H, Liu T, Huang M, Butter P-P, Li C, Zhang L, Kao GD, Gong Y, Maity A, Koumenis C, Fan Y, Temporal DNA-PK activation drives genomic instability and therapy resistance in glioma stem cells. JCI Insight 3, 10.1172/jci.insight.98096, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clevers H, Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Xu H, Huang M, Ma W, Saxena D, Lustig RA, Alonso-Basanta M, Zhang Z, O’Rourke DM, Zhang L, Gong Y, Kao GD, Dorsey JF, Fan Y, Circulating glioma cells exhibit stem cell-like properties. Cancer Res. 78, 6632–6642 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li Y-M, Maciaczyk J, NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28, 5–16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amakye D, Jagani Z, Dorsch M, Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med 19, 1410–1422 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr., M. P. Mehta, A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med 370, 699–708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK, AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang M, Liu T, Ma P, Mitteer RA Jr., Zhang Z, Kim HJ, Yeo E, Zhang D, Cai P, Li C, Zhang L, Zhao B, Roccograndi L, O’Rourke DM, Dahmane N, Gong Y, Koumenis C, Fan Y, c-Met-mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma. J. Clin. Invest 126, 1801–1814 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R, Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 67, 10123–10128 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC, Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 6, 141–152 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciznadija D, Liu Y, Pyonteck SM, Holland EC, Koff A, Cyclin D1 and cdk4 mediate development of neurologically destructive oligodendroglioma. Cancer Res. 71, 6174–6183 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Y, Potdar AA, Gong Y, Eswarappa SM, Donnola S, Lathia JD, Hambardzumyan D, Rich JN, Fox PL, Profilin-1 phosphorylation directs angiocrine expression and glioblastoma progression through HIF-1α accumulation. Nat. Cell Biol 16, 445–456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Österreicher CH, Penz-Österreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA, Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl. Acad. Sci. U.S.A 108, 308–313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L, Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, Ruckdeschel T, Hasanov Z, Srivastava K, Hu J, Hertel S, Bartol A, Schlereth K, Augustin HG, Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun 8, 16106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R, Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300, 1155–1159 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Soultati A, Mountzios G, Avgerinou C, Papaxoinis G, Pectasides D, Dimopoulos MA, Papadimitriou C, Endothelial vascular toxicity from chemotherapeutic agents: Preclinical evidence and clinical implications. Cancer Treat. Rev 38, 473–483 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Ma W, Xu H, Huang M, Zhang D, He Z, Zhang L, Brem S, O’Rourke DM, Gong Y, Mou Y, Zhang Z, Fan Y, PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat. Commun 9, 3439 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotta A, Cheung AY, Farra N, Vijayaragavan K, Seguin CA, Draper JS, Pasceri P, Maksakova IA, Mager DL, Rossant J, Bhatia M, Ellis J, Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat. Methods 6, 370–376 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Eijkelenboom A, Burgering BMT, FOXOs: Signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol 14, 83–97 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Anastas JN, Moon RT, WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Clevers H, Loh KM, Nusse R, Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Clevers H, Nusse R, Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 (2012). [DOI] [PubMed] [Google Scholar]
- 36.MacDonald BT, Tamai K, He X, Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daugherty RL, Gottardi CJ, Phospho-regulation of Beta-catenin adhesion and signaling functions. Physiology 22, 303–309 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng G, Apte U, Micsenyi A, Bell A, Monga PS, Tyrosine residues 654 and 670 in beta-catenin are crucial in regulation of Met-beta-catenin interactions. Exp. Cell Res 312, 3620–3630 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Náger M, Santacana M, Bhardwaj D, Valls J, Ferrer I, Nogués P, Cantí C, Herreros J, Nuclear phosphorylated Y142 β-catenin accumulates in astrocytomas and glioblastomas and regulates cell invasion. Cell Cycle 14, 3644–3655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z, Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem 282, 11221–11229 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO, Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J. Biol. Chem 281, 9971–9976 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Ge X, Jin Q, Zhang F, Yan T, Zhai Q, PCAF acetylates {beta}-catenin and improves its stability. Mol. Biol. Cell 20, 419–427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He P, Shen Y, Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer’s disease. J. Neurosci 29, 6545–6557 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, Heck R, Thom S, Macas J, Bockamp E, Fruttiger M, Taketo MM, Dimmeler S, Plate KH, Liebner S, Endothelial Wnt/β-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J. Exp. Med 209, 1611–1627 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Z, Zhu J, Zhao L, Luo Q, Jin X, Expression and clinical significance of multidrug resistance proteins in brain tumors. J. Exp. Clin. Cancer Res 29, 122 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dean M, Fojo T, Bates S, Tumour stem cells and drug resistance. Nat. Rev. Cancer 5, 275–284 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM, Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov 5, 219–234 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D, Weinberg RA, Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V, Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: From cardiovascular development to disease. Circulation 125, 1795–1808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R, Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med 13, 952–961 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Li J, Qu X, Bertram JF, Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am. J. Pathol 175, 1380–1388 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R, Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol 19, 2282–2287 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen P-Y, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M, FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2, 1684–1696 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC, Boehm M, TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl. Med 6, 227ra234 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E, EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498, 492–496 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Eilken HM, Nishikawa S-I, Schroeder T, Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457, 896–900 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Boisset J-C, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C, In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Kissa K, Herbomel P, Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D, Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G, The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457, 892–895 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR, Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med 16, 1400–1406 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasperini P, Espigol-Frigole G, McCormick PJ, Salvucci O, Maric D, Uldrick TS, Polizzotto MN, Yarchoan R, Tosato G, Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through Notch-dependent signaling. Cancer Res. 72, 1157–1169 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Butler JM, Kobayashi H, Rafii S, Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 10, 138–146 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmeliet P, Jain RK, Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ, The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol 15, 807–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weis SM, Cheresh DA, Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med 17, 1359–1370 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Cao Z, Ding B-S, Guo P, Lee SB, Butler JM, Casey SC, Simons M, Tam W, Felsher DW, Shido K, Rafii A, Scandura JM, Rafii S, Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell 25, 350–365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, Mascre G, Drogat B, Dekoninck S, Haigh JJ, Carmeliet P, Blanpain C, A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478, 399–403 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ, A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, Maru DM, Hawke DH, Rak J, Mani SA, Zweidler-McKay P, Ellis LM, Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell 23, 171–185 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Q, He Z, Huang M, Liu T, Wang Y, Xu H, Duan H, Ma P, Zhang L, Zamvil SS, Hidalgo J, Zhang Z, O’Rourke DM, Dahmane N, Brem S, Mou Y, Gong Y, Fan Y, Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 9, 559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E, The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev. Cell 18, 938–949 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I, Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development 141, 1757–1766 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Scholz B, Korn C, Wojtarowicz J, Mogler C, Augustin I, Boutros M, Niehrs C, Augustin HG, Endothelial RSPO3 controls vascular stability and pruning through non-canonical WNT/Ca(2+)/NFAT Signaling. Dev. Cell 36, 79–93 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Min J-K, Park H, Choi H-J, Kim Y, Pyun B-J, Agrawal V, Song B-W, Jeon J, Maeng Y-S, Rho S-S, Shim S, Chai J-H, Koo B-K, Hong HJ, Yun C-O, Choi C, Kim Y-M, Hwang K-C, Kwon Y-G, The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J. Clin. Invest 121, 1882–1893 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E, The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J. Cell Biol 162, 1111–1122 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birdsey GM, Shah AV, Dufton N, Reynolds LE, Osuna Almagro L, Yang Y, Aspalter IM, Khan ST, Mason JC, Dejana E, Gottgens B, Hodivala-Dilke K, Gerhardt H, Adams RH, Randi AM, The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling. Dev. Cell 32, 82–96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Göthert JR, Malik AB, Valyi-Nagy T, Zhao Y-Y, Endothelial β-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation 133, 177–186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E, Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol 183, 409–417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA, Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U.S.A 106, 641–646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallego-Delgado J, Basu-Roy U, Ty M, Alique M, Fernandez-Arias C, Movila A, Gomes P, Weinstock A, Xu W, Edagha I, Wassmer SC, Walther T, Ruiz-Ortega M, Rodriguez A, Angiotensin receptors and β-catenin regulate brain endothelial integrity in malaria. J. Clin. Invest 126, 4016–4029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang J, Mancuso MR, Maier C, Liang X, Yuki K, Yang L, Kwong JW, Wang J, Rao V, Vallon M, Kosinski C, Zhang JJ, Mah AT, Xu L, Li L, Gholamin S, Reyes TF, Li R, Kuhnert F, Han X, Yuan J, Chiou S-H, Brettman AD, Daly L, Corney DC, Cheshier SH, Shortliffe LD, Wu X, Snyder M, Chan P, Giffard RG, Chang HY, Andreasson K, Kuo CJ, Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med 23, 450–460 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, Obernier K, Krishnan S, Lindberg OR, Yuen TJ, Tien A-C, Sabo JK, Wang N, Chen I, Kloepper J, Larrouquere L, Ghosh M, Tirosh I, Huillard E, Alvarez-Buylla A, Oldham MC, Persson AI, Weiss WA, Batchelor TT, Stemmer-Rachamimov A, Suva ML, Phillips JJ, M. K. Aghi, S. Mehta, R. K. Jain, D. H. Rowitch, A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell 33, 874–889.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Apte U, Zeng G, Muller P, Tan X, Micsenyi A, Cieply B, Dai C, Liu Y, Kaestner KH, Monga SP, Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology 44, 992–1002 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Monga SP, Mars WM, Pediaditakis P, Bell A, Mulé K, Bowen WC, Wang X, Zarnegar R, Michalopoulos GK, Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 62, 2064–2071 (2002). [PubMed] [Google Scholar]
- 87.Papkoff J, Aikawa M, WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem. Biophys. Res. Commun 247, 851–858 (1998). [DOI] [PubMed] [Google Scholar]
- 88.Koraishy FM, Silva C, Mason S, Wu D, Cantley LG, Hepatocyte growth factor (Hgf) stimulates low density lipoprotein receptor-related protein (Lrp) 5/6 phosphorylation and promotes canonical Wnt signaling. J. Biol. Chem 289, 14341–14350 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng JC, Chang H-M, Leung PCK, Transforming growth factor-β1 inhibits trophoblast cell invasion by inducing Snail-mediated down-regulation of vascular endothelial-cadherin protein. J. Biol. Chem. 288, 33181–33192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Binda E, Visioli A, Giani F, Trivieri N, Palumbo O, Restelli S, Dezi F, Mazza T, Fusilli C, Legnani F, Carella M, Di Meco F, Duggal R, Vescovi AL, Wnt5a drives an invasive phenotype in human glioblastoma stem-like cells. Cancer Res. 77, 996–1007 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Hu B, Wang Q, Wang YA, Hua S, Sauvé CG, Ong D, Lan ZD, Chang Q, Ho YW, Monasterio MM, Lu X, Zhong Y, Zhang J, Deng P, Tan Z, Wang G, Liao WT, Corley LJ, Yan H, Zhang J, You Y, Liu N, Cai L, Finocchiaro G, Phillips JJ, Berger MS, Spring DJ, Hu J, Sulman EP, Fuller GN, Chin L, Verhaak RGW, DePinho RA, Epigenetic activation of WNT5A drives glioblastoma stem cell differentiation and invasive growth. Cell 167, 1281–1295.e18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, Barberis A, Benjamin LE, Mäkinen T, Nobes CD, Adams RH, Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010). [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Yeh N, Zhu X-H, Leversha M, Cordon-Cardo C, Ghossein R, Singh B, Holland E, Koff A, Somatic cell type specific gene transfer reveals a tumor-promoting function for p21(Waf1/Cip1). EMBO J. 26, 4683–4693 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bajpai VK, Mistriotis P, Loh Y-H, Daley GQ, Andreadis ST, Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc. Res 96, 391–400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumar A, D’Souza SS, Moskvin OV, Toh H, Wang B, Zhang J, Swanson S, Guo LW, Thomson JA, Slukvin II, Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep. 19, 1902–1916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu Y, Smyth GK, ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 347, 70–78 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. FSP-1 expression in GBM-associated ECs.
Fig. S2. Chemoresistance in GBM-associated ECs.
Fig. S3. Expression of c-Kit and CD44 in GBM ECs.
Fig. S4. Dil-Ac-LDL uptake in sphere-forming GBM ECs.
Fig. S5. Differentiation of GBM ECs into mesenchymal cells.
Fig. S6. MSC-like trilineage differentiation of GBM ECs.
Fig. S7. Correlation of stemness activation and mesenchymalization with chemoresistance in GBM ECs.
Fig. S8. Effects of FOXO1 or FLF4 knockdown on chemoresistance in ECs.
Fig. S9. LEF1 transcriptional activation in ECs cocultured with GBM cells.
Fig. S10. Expression of Wnt target genes in ECs.
Fig. S11. Chemoresistance in ECs treated with glioma-CM.
Fig. S12. CRISPR-mediated β-catenin knockdown in ECs.
Fig. S13. Effects of HGF or Wnt5a neutralization on chemoresistance in ECs.
Fig. S14. A schematic model.
Fig. S15. Cdh5-CreERT2–mediated β-catenin knockdown in ECs.
Fig. S16. Effects of β-catenin knockdown in ECs on GBM response to chemotherapy.
Fig. S17. Effects of Wnt inhibition on EC chemoresistance and EC-mediated glioma cell chemoresistance.
Data file S1. Raw data.