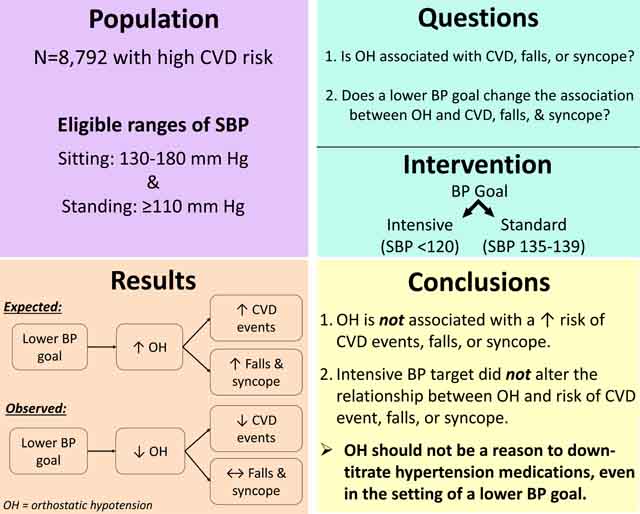
Abstract
Orthostatic hypotension (OH) is frequently observed with hypertension treatment, but its contribution to adverse outcomes is unknown. The Systolic Blood Pressure Intervention Trial (SPRINT) was a randomized trial of adults, age ≥50years at high risk for cardiovascular disease (CVD) with a seated systolic BP (SBP) of 130–180 mmHg and a standing SBP ≥110 mmHg. Participants were randomized to a SBP treatment goal of either <120 mmHg or <140 mmHg. OH was defined as a drop in SBP≥20 or diastolic BP ≥10 mmHg one minute after standing from a seated position. We used Cox models to examine the association of OH with CVD or adverse study events by randomized BP goal. During the follow-up period (median 3years), there were 1,170 (5.7%) instances of OH among those assigned a standard BP goal, and 1,057 (5.0%) among those assigned the intensive BP goal. OH was not associated with higher risk of CVD events (primary outcome: HR 1.06; 95%CI: 0.78,1.44). Moreover, OH was not associated with syncope, electrolyte abnormalities, injurious falls, or acute renal failure. OH was associated with hypotension-related hospitalizations or emergency department visits (HR 1.77; 1.11,2.82) and bradycardia (HR 1.94; 1.19,3.15), but these associations did not differ by BP treatment goal. OH was not associated with a higher risk of CVD events and BP treatment goal had no effect on OH’s association with hypotension and bradycardia. Symptomless OH during hypertension treatment should not be viewed as a reason to down-titrate therapy even in the setting of a lower BP goal.
Keywords: orthostatic hypotension, hypertension treatment, trial, fall, cardiovascular disease, syncope, blood pressure
Graphical Abstract
Summary
Orthostatic hypotension should not be a reason for down-titration of medications, even in the setting of a lower blood pressure goal.
Orthostatic hypotension (OH), a drop in blood pressure (BP) after standing, is common among older adults1 and has been reported to be an important predictor of falls, syncope, cardiovascular disease (CVD), and death.1–3 OH is highly prevalent among older adults with hypertension4 and in the setting of hypertension treatment.5 These observations have contributed to concerns that aggressive BP lowering, especially in older adults, might increase OH and its sequelae.
Recently, SPRINT (Systolic Blood Pressure Intervention Trial) demonstrated that a lower systolic BP (SBP) treatment goal reduced the risk of CVD events and total mortality in middle-aged and older adults without diabetes when compared to conventional goals.6 SPRINT also found that the lower BP target reduced risk of OH, despite increasing risk of hypotension and possibly syncope without increasing injurious falls.7 It was later shown that CVD risk factors were associated with OH8 and that baseline OH in SPRINT was associated with falls,7 but whether OH identified during the post-randomization period, overall or with intensive BP intervention, was related to CVD or adverse events has not been reported.
Our objectives were to determine: (1) the association of OH with CVD or adverse events; and (2) whether OH detected in the setting of intensive hypertension treatment was associated with greater risk of events than OH detected in the setting of standard BP treatment goal. We hypothesized that OH would be associated with CVD and adverse events and that OH in the setting of an intensive BP treatment goal would be associated with a higher risk of CVD and adverse events.
Methods
The data that support the findings of this study are available from the National Heart, Lung, and Blood Institute BioLINCC repository.
SPRINT was a NIH-funded, prospective, randomized, controlled, and open-label outcome trial with blinded end point determination conducted at 102 clinical sites throughout the United States and Puerto Rico between November, 2010, and August 2015.6,9,10 The trial compared intensive treatment to a SBP target <120 mmHg and standard treatment to a SBP target <140 mmHg. Institutional Review Boards (IRB) at each site approved the original protocol, including subsequent analyses. All participants provided written informed consent. This analysis is based on SPRINT’s final expanded data set.
Participants
SPRINT recruited adults (≥50 years) with a SBP of 130–180 mmHg and a high CVD risk based on CVD history, chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR) of 20–59 mL/min/1.73m2, or 10-year Framingham Risk Score ≥15%. The study enhanced recruitment of adults aged ≥75 years, with CKD, and African Americans. Nursing home residents or persons with diabetes mellitus, prior stroke, dementia, symptomatic or severe heart failure (or measured left ventricular ejection fraction <35%), or a 1-minute standing SBP <110 mmHg were excluded. Of the original 9,361 participants, 569 were excluded due to a missing covariate of interest at baseline.
Intervention
Participants were randomly assigned to either intensive or standard hypertension treatment. Over the first 3 months, visits occurred monthly. Afterward, visits occurred every 3 months. Among the intensive group, participant visits continued monthly until SBP was <120 mmHg or the investigator decided not to intensify further. Titration of antihypertensive therapy was based on BP measurements at each visit. All major classes of antihypertensive medications were available to target a SBP of <120 mmHg (intensive) or a SBP between 135–139 mmHg (standard). Among those assigned the standard treatment, antihypertensive medications were reduced if SBP was <130 mmHg on a single visit or <135 mmHg on two consecutive visits.
Orthostatic Hypotension
OH was assessed at screening, baseline, 1-month, 6-month, 12-month, and then yearly visits. While there was no exclusion for history of OH, during the screening visit, adults with a 1-minute standing SBP <110 mmHg were excluded from participation. Seated BP was measured three times after a 5-minute quiet rest, using an automated, oscillometric sphygmomanometer (Omron 907XL, Omron Healthcare, Lake Forest, IL). Participants were then instructed to stand, and after 1 minute BP was re-measured.8,11 OH was defined as a difference in standing and mean sitting SBP ≥20 mmHg or diastolic BP (DBP) ≥10 mmHg. Participants completing the standing BP exam were further asked about dizziness; however, we did not differentiate between dizziness status due to small numbers (Table 1, Supplement Table S1). OH detected during the randomization visit prior to starting the trial intervention was considered baseline OH, while OH detected after randomization during follow-up visits was considered post-randomization OH.
Table 1.
Baseline characteristics, overall and by baseline orthostatic hypotension status
| Overall (N = 8792) | No Baseline OH (N = 8156) | Baseline OH (N = 636) | |
|---|---|---|---|
| Age, years | 67.6 (9.3) | 67.5 (9.3) | 69.4 (9.3) |
| Age ≥75 years, % | 26.9 | 26.3 | 34.6 |
| Female, % | 35.0 | 34.6 | 40.9 |
| Black, % | 31.3 | 31.9 | 23.7 |
| Mean SBP, mmHg | 139.6 (15.6) | 139.1 (15.5) | 145.2 (16.5) |
| Mean DBP, mmHg | 78.2 (11.9) | 78.1 (11.9) | 79.9 (12.3) |
| eGFR, mL/min/1.73 m2 | 72.2 (20.4) | 72.6 (20.2) | 66.3 (21.3) |
| Body mass index, kg/m2 | 29.9 (5.8) | 30.0 (5.8) | 29.2 (5.6) |
| HDL cholesterol, mg/dL | 52.8 (14.4) | 52.7 (14.4) | 53.4 (14.2) |
| Total cholesterol, mg/dL | 187.0 (42.5) | 187.1 (42.4) | 185.3 (43.5) |
| Statin use, % | 43.3 | 43.0 | 48.0 |
| Chronic kidney disease, % | 27.4 | 26.4 | 40.1 |
| Subclinical or clinical CVD, % | 19.9 | 19.7 | 22.3 |
| Smoking status, % | |||
| Never | 43.8 | 44.0 | 42.0 |
| Former | 42.8 | 42.6 | 45.6 |
| Current | 13.4 | 13.5 | 12.4 |
| Number of hypertensive agents prescribed at baseline, % | |||
| 0 | 9.1 | 9.1 | 8.2 |
| 1 | 34.8 | 34.9 | 33.0 |
| 2 | 34.0 | 34.1 | 33.6 |
| 3 | 17.9 | 17.6 | 21.1 |
| ≥4 | 4.2 | 4.2 | 4.1 |
| Self-reported dizziness with standing, %* | 4.3 | 4.2 | 5.5 |
Abbreviations: CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; OH, orthostatic hypotension; SBP, systolic blood pressure
Note: Total N for those reporting dizziness was 8791/8155/636.
Study Outcomes
The primary outcome combined the first episode of myocardial infarction, non-myocardial infarction acute coronary syndrome (ACS), stroke, acute decompensated heart failure, or death from cardiovascular causes. The individual components of the primary outcome or all-cause mortality were secondary. All outcomes were adjudicated by a committee blinded to treatment assignment.
Study Adverse Events
At each visit, staff asked participants about hospitalizations, emergency department visits, and adverse events. The following expected events were specifically collected by investigators to monitor safety: hypotension, syncope, injurious falls, electrolyte abnormalities, bradycardia, and acute kidney injury or acute renal failure. A hypotension event was based on mention of symptomatic low BP (without specific BP cut‐offs) in admission history and physicals or discharge summaries of hospitalization records.7 The definition of an injurious fall was a fall resulting in an emergency department evaluation or a hospitalization. A bradycardia event was based on the mention of symptomatic low heart rate in hospitalization records.
Other covariate measurements and definitions
Age, sex, race, smoking status (never, former, current), baseline statin use, and antihypertension medication use were ascertained via self-report; eGFR was determined using the four variable Modification of Diet in Renal Disease equation based on serum creatinine assessments. CKD was defined as an eGFR <60 mL/min/1.73 m2. Body mass index (BMI) was derived from standardized measurements of height and weight. High density lipoprotein (HDL) cholesterol and total cholesterol were measured in serum using standard assays. Diagnosis of subclinical or clinical CVD was based on a combination of self-report, prior studies, and documentation of CVD or CVD equivalent events.6,9,10
Statistical analysis
Study population characteristics were described using means (SD) and proportions overall and according to baseline OH status. We also determined the prevalence of OH at baseline and at each follow-up visit. We used Poisson regression, adjusted for BP target assignment, age, sex, and race, to estimate incidence rates overall and by baseline OH status.
Cox proportional hazards models stratified by clinic site were used to examine the association of OH as a time-varying covariate detected at baseline or during post-randomization visits with the trial’s primary and secondary outcomes and serious adverse events. Models were adjusted for age, sex, race, and treatment assignment (Model 1). In a second model (Model 2), we adjusted for the following baseline covariates: age, sex, race, treatment assignment, SBP, DBP, BMI, HDL cholesterol, total cholesterol, statin use, chronic kidney disease, eGFR, subclinical or clinical CVD, smoking status, and number of hypertensive medications. Both models were stratified by research site. For these analyses, participants were censored when an event occurred or at the end of follow-up if no event occurred.
We also examined the association of time-varying OH detected during the follow-up period (post-randomization OH only) with trial outcomes and adverse events by randomized BP goal assignment to isolate the effects of treatment on OH. Models were stratified by research site and adjusted for the covariates in Model 2 above along with baseline OH. We used interaction terms to compare associations across BP goal assignments.
Analyses were conducted with Stata v15.1 (StataCorp, College Station, TX). P-values were two-sided and not adjusted for multiple comparisons.
Results
Baseline characteristics of the 8,792 SPRINT participants included in our analysis are shown overall and by baseline OH status in Table 1. Overall, the mean age of participants was 67.6±9.3 years; 35.0% were female, and 31.3% were black. The mean baseline SBP was 139.6±15.6 mmHg and the mean baseline DBP was 78.2±11.9 mmHg; 636 participants had OH at baseline (Figure 1).
Figure 1.
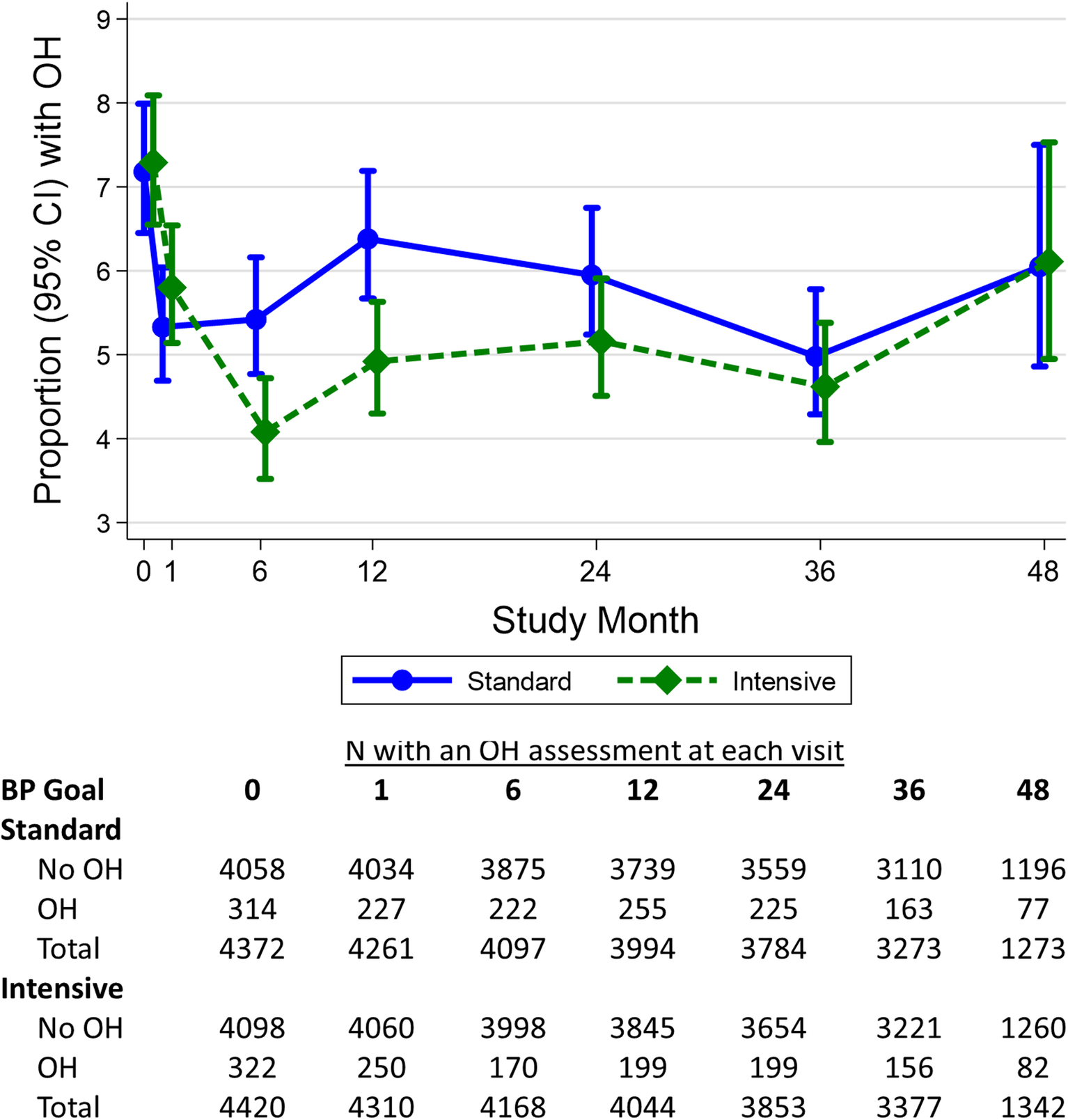
The proportion with orthostatic hypotension during the trial and number of participants contributing to orthostatic hypotension assessments by treatment assignment.
During a median of 3.0 years of post-randomization follow-up there were 2,227 instances of OH, representing 1,627 (18.5% of 8,792) participants. The distribution of change in BP was similar across baseline, intensive, and standard treatment visits (Supplement Figure S1). In both treatment groups, the visit with the higher proportion of OH was the baseline visit (>7% vs <6.5% during post-randomization visits). Furthermore, OH was more common among those assigned standard treatment at most visits over the course of the trial compared with those assigned intensive treatment (Figure 1).
In both treatment groups combined, OH detected during baseline or follow-up was not associated with the primary outcome (adjusted HR of 1.06; 95%CI: 0.78,1.44) or any secondary outcomes (Table 2, Supplement Table S2). With regard to serious adverse events, OH was only associated with hypotension events (HR 1.77; 95%CI: 1.11,2.82) and bradycardia (HR 1.94; 95%CI: 1.19,3.15) (Table 3).
Table 2.
Adjusted* incidence rates (per 10,000 person-years) of trial outcomes or serious adverse events, overall and by baseline orthostatic hypotension status
| Overall | No baseline OH | Baseline OH | ||||||
|---|---|---|---|---|---|---|---|---|
| Event/No Event | IR (95% CI) | Event/No Event | IR (95% CI) | Event/No Event | IR (95% CI) | |||
| Outcomes | ||||||||
| Primary outcome | 649/8089 | 5.7 (5.2,6.1) | 588/7515 | 5.6 (5.2,6.1) | 61/574 | 5.8 (5.4,6.2) | ||
| Secondary outcomes | ||||||||
| Myocardial infarction | 246/8495 | 2.2 (2.0,2.5) | 225/7881 | 2.2 (2.0,2.5) | 21/614 | 2.3 (2.1,2.6) | ||
| Acute coronary syndrome | 84/8659 | 0.8 (0.7,1.0) | 72/8036 | 0.9 (0.7,1.0) | 12/623 | 0.8 (0.6,1.0) | ||
| Stroke | 156/8590 | 1.6 (1.3,1.8) | 141/7970 | 1.6 (1.3,1.8) | 15/620 | 1.7 (1.5,1.9) | ||
| Heart failure | 187/8555 | 1.5 (1.2,1.8) | 172/7935 | 1.5 (1.2,1.8) | 15/620 | 1.7 (1.4,1.9) | ||
| Death from cardiovascular causes | 122/8624 | 0.8 (0.7,1.0) | 111/8000 | 0.8 (0.7,1.0) | 11/624 | 0.9 (0.7,1.1) | ||
| Death from any cause | 364/8381 | 2.6 (2.3,2.9) | 322/7788 | 2.6 (2.3,2.8) | 42/593 | 2.8 (2.5,3.0) | ||
| Primary outcome or death | 856/7880 | 5.5 (5.2,5.9) | 774/7327 | 5.5 (5.1,5.9) | 82/553 | 5.6 (5.3,6.0) | ||
| Serious Adverse Events | ||||||||
| Hypotension | 220/8562 | 2.4 (2.1,2.7) | 201/7945 | 2.4 (2.1,2.7) | 19/617 | 2.4 (2.1,2.7) | ||
| Syncope | 247/8539 | 2.3 (2.1,2.6) | 227/7923 | 2.3 (2.1,2.6) | 20/616 | 2.5 (2.2,2.8) | ||
| Bradycardia | 176/8610 | 1.7 (1.4,2.0) | 154/7997 | 1.7 (1.4,2.0) | 22/613 | 1.9 (1.6,2.2) | ||
| Electrolyte abnormality | 298/8488 | 2.8 (2.5,3.1) | 269/7881 | 2.8 (2.5,3.1) | 29/607 | 3.0 (2.7,3.3) | ||
| Injurious Fall | 654/8122 | 5.5 (5.0,6.0) | 590/7551 | 5.4 (5.0,5.9) | 64/571 | 6.4 (5.9,6.8) | ||
| Acute kidney injury or acute renal failure | 317/8465 | 2.7 (2.4,3.0) | 294/7853 | 2.7 (2.4,3.0) | 23/612 | 2.7 (2.5,3.0) | ||
Abbreviations: CI, confidence interval; IR, incidence rate; OH, orthostatic hypotension
Adjusted for blood pressure assignment, age, sex, and race
Note: Total number varies by outcome or serious adverse event depending on whether orthostatic hypotension assessments were missing before the outcome or serious adverse event occurred.
Table 3.
Association of orthostatic hypotension as time-varying covariate detected during baseline or post-randomization follow-up with trial outcomes, N = 8,792
| Orthostatic Hypotension (Model 1) | Orthostatic Hypotension (Model 2) | |||||
|---|---|---|---|---|---|---|
| Total N of Events | HR (95% CI) | P | HR (95% CI) | P | ||
| Outcomes | ||||||
| Primary outcome | 649 | 1.16 (0.85, 1.57) | 0.36 | 1.06 (0.78, 1.44) | 0.72 | |
| Secondary outcomes | ||||||
| Myocardial infarction | 246 | 0.97 (0.57, 1.66) | 0.92 | 0.92 (0.54, 1.58) | 0.77 | |
| Acute coronary syndrome | 84 | 1.87 (0.91, 3.83) | 0.09 | 1.68 (0.81, 3.49) | 0.16 | |
| Stroke | 156 | 0.95 (0.47, 1.91) | 0.89 | 0.86 (0.43, 1.73) | 0.68 | |
| Heart failure | 187 | 1.09 (0.63, 1.88) | 0.76 | 0.96 (0.55, 1.68) | 0.89 | |
| Death from cardiovascular causes | 122 | 0.73 (0.32, 1.69) | 0.46 | 0.63 (0.27, 1.47) | 0.29 | |
| Death from any cause | 364 | 1.24 (0.84, 1.83) | 0.29 | 1.13 (0.76, 1.68) | 0.53 | |
| Primary outcome or death | 856 | 1.20 (0.92, 1.56) | 0.17 | 1.11 (0.85, 1.44) | 0.46 | |
| Serious Adverse Events | ||||||
| Hypotension | 220 | 1.99 (1.26, 3.14) | 0.003 | 1.77 (1.11, 2.82) | 0.02 | |
| Syncope | 247 | 1.49 (0.93, 2.39) | 0.10 | 1.38 (0.86, 2.23) | 0.18 | |
| Bradycardia | 176 | 2.10 (1.30, 3.39) | 0.002 | 1.94 (1.19, 3.15) | 0.008 | |
| Electrolyte abnormality | 298 | 1.08 (0.66, 1.75) | 0.76 | 0.99 (0.60, 1.62) | 0.97 | |
| Injurious Fall | 654 | 1.24 (0.91, 1.68) | 0.17 | 1.20 (0.88, 1.63) | 0.24 | |
| Acute kidney injury or acute renal failure | 317 | 1.35 (0.86, 2.13) | 0.20 | 1.14 (0.72, 1.81) | 0.58 | |
Model 1: Adjusted for age, sex, race
Model 2: Adjusted for age, sex, race and the following baseline characteristics: treatment assignment, systolic blood pressure, diastolic blood pressure, body mass index, high density lipoprotein cholesterol, total cholesterol, statin use, chronic kidney disease, estimated glomerular filtration rate, subclinical or clinical cardiovascular disease, smoking status, or number of hypertensive medications
Stratified by research site.
In SPRINT, a serious adverse event was defined as events that (1) were fatal or life-threatening, (2) resulted in clinically significant or persistent disability, (3) required or prolonged a hospitalization, or (4) were judged by the investigator to represent a clinically significant hazard or harm to the participant that might require intervention (medical or surgical) to prevent one of three previously mentioned events listed above.
The association of post-randomization OH with trial outcomes and serious adverse events by BP goal assignment is shown in Table 4. There was evidence of a higher hazard for non-myocardial infarction ACS among participants assigned the intensive BP goal versus the standard BP goal (HR 2.56; 95%CI: 1.04,6.31 for intensive vs 0.35; 95%CI: 0.05,2.73 for standard; P-interaction=0.03; see Supplement Table S3). After adjustment, OH was associated with hypotension events among those assigned the standard BP goal (HR 3.26; 95%CI: 1.56,6.81), but not among those assigned the intensive goal (HR 1.35; 95%CI: 0.71,2.59); however, the interaction did not achieve statistical significance.
Table 4.
Association of post-randomization orthostatic hypotension as a time-varying covariate with trial outcomes and serious adverse events by blood pressure goal assignment, N=8,792.
| Standard Blood Pressure Goal | Intensive Blood Pressure Goal | P-interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | HR (95% CI) | P | Events | HR (95% CI) | P | ||||
| Outcomes | |||||||||
| Primary outcome | 369 | 0.94 (0.62, 1.44) | 0.79 | 280 | 1.04 (0.63, 1.72) | 0.87 | 0.52 | ||
| Secondary outcomes | |||||||||
| Myocardial infarction | 144 | 1.01 (0.51, 1.99) | 0.98 | 102 | 0.87 (0.33, 2.26) | 0.78 | 0.81 | ||
| Acute coronary syndrome | 44 | 0.35 (0.05, 2.73) | 0.32 | 40 | 2.56 (1.04, 6.31) | 0.04 | 0.03 | ||
| Stroke | 85 | 0.52 (0.18, 1.50) | 0.23 | 71 | 1.18 (0.42, 3.32) | 0.75 | 0.35 | ||
| Heart failure | 105 | 1.33 (0.66, 2.69) | 0.42 | 82 | 0.34 (0.10, 1.23) | 0.10 | 0.15 | ||
| Death from cardiovascular causes | 72 | 0.72 (0.25, 2.09) | 0.55 | 50 | 0.53 (0.12, 2.40) | 0.41 | 0.79 | ||
| Death from any cause | 201 | 1.40 (0.85, 2.29) | 0.18 | 163 | 0.72 (0.35, 1.48) | 0.37 | 0.19 | ||
| Primary outcome or death | 475 | 1.09 (0.76, 1.55) | 0.65 | 381 | 1.01 (0.66, 1.56) | 0.95 | 0.88 | ||
| Serious Adverse Events | |||||||||
| Hypotension | 74 | 3.26 (1.56, 6.81) | 0.002 | 146 | 1.35 (0.71, 2.59) | 0.36 | 0.31 | ||
| Syncope | 103 | 1.57 (0.72, 3.43) | 0.26 | 144 | 1.55 (0.82, 2.95) | 0.18 | 0.87 | ||
| Bradycardia | 78 | 1.96 (0.92, 4.18) | 0.08 | 98 | 1.85 (0.89, 3.86) | 0.10 | 0.63 | ||
| Electrolyte abnormality | 125 | 0.99 (0.46, 2.10) | 0.97 | 173 | 0.91 (0.46, 1.80) | 0.79 | 0.92 | ||
| Injurious Fall | 305 | 1.17 (0.74, 1.84) | 0.50 | 349 | 1.14 (0.73, 1.78) | 0.56 | 0.72 | ||
| Acute kidney injury or acute renal failure | 118 | 1.77 (0.88, 3.55) | 0.11 | 199 | 0.99 (0.51, 1.93) | 0.97 | 0.19 | ||
Models were adjusted for baseline orthostatic hypotension and the following baseline characteristics: age, sex, race, treatment assignment, systolic blood pressure, diastolic blood pressure, body mass index, high density lipoprotein cholesterol, total cholesterol, statin use, chronic kidney disease, estimated glomerular filtration rate, subclinical or clinical cardiovascular disease, smoking status, or number of hypertensive medications
Stratified by research site.
Discussion
In this cohort of hypertensive, middle-aged and older adults, we found that OH did not predict CVD events. While OH was associated with hypotension and bradycardia, these associations did not differ by randomized treatment group. Aside from non-myocardial infarction ACS, associations between OH and CVD outcomes or adverse events (including hypotension and bradycardia events) did not differ by assigned BP target.
OH was present in about 7% of participants at baseline and in less than 7% of participants through follow-up. Other blood pressure trials have reported a baseline prevalence of OH between 3–17%.12–16 OH is a manifestation of BP dysregulation upon change in position. While traditionally viewed as a neurogenic condition, emerging evidence suggests that CVD may also represent a common etiology of OH.17 This relationship was observed in a previously published analysis of SPRINT baseline characteristics and postural change in BP where a number of CVD risk factors, including age, CKD, and smoking were associated with larger reductions in standing BP.8 Moreover, both the primary SPRINT publication and the present study showed that intensive hypertension treatment, a CVD risk factor, lowered risk of OH.6 This is despite the greater number of antihypertensive medications and greater use of diuretics (chlorthalidone) in the intensive arm.6 This suggests that CVD may represent an important contributor to OH.
Whether OH contributes to CVD events remains controversial. Multiple studies have observed that OH is a predictor of CVD events,1,3,17–20 leading to the hypothesis that transient hypoperfusion of the heart from OH contributes to cumulative micro-ischemia over time.21–23 However, this relationship has not been observed in all studies,24–26 and was not observed for the majority of CVD outcomes in the present study. Some of these different observations between studies may be secondary to differences in study population or follow-up duration. There was some suggestion in our study that OH may be associated with non-myocardial infarction ACS. Participants with OH in the setting of intensive therapy had 2.5 times the risk of non-myocardial infarction ACS compared with those without OH, which could support a role for treatment-related OH in the pathogenesis of coronary ischemia. However, these findings are based on a small number of events and should be confirmed. Further, as previously reported,14,15 intensive treatment significantly reduced the incidence of OH, the incidence of which was 20 times more frequent than non-myocardial infarction ACS in SPRINT.6
OH was strongly associated with two serious adverse events, hypotension and bradycardia. Both hypotension events and bradycardia were based on mention of symptomatic low blood pressure or heart rate (without specific cut‐offs) in admission history and physicals or discharge summaries of hospitalization records. In a prior analysis, baseline OH was non-significantly associated with a higher odds of hypotension events (HR 1.58; 95% CI: 0.96–2.62).7 Since OH is a form of hypotension, the relationship between OH and hypotension events may be expected, although OH was not significantly associated with hypotension events in the intensive group. Bradycardia, on the other hand, is an under-recognized cause of OH.27,28 Given the importance of stroke volume augmentation in maintaining BP with change in position,29 it is biologically plausible that bradycardia might cause OH, especially in adults with underlying physical attributes (e.g. vascular stiffness) where HR is a compensatory mechanism.30 It is also possible that bradycardia reflects common upstream factors such as underlying cardiac ischemia or autonomic dysfunction. This is an important topic for future study.
Prior analyses of baseline OH in SPRINT demonstrated that while OH was not associated with syncope, it was associated with falls.7 Our analysis of post-randomization instances of OH confirmed the null association with syncope, but did not show an association between OH and falls. This observation suggests that symptomless OH in the setting of hypertension treatment is not a reliable predictor of falls. Ultimately, our finding conflicts with many observational studies, which have demonstrated that OH is a risk factor for syncope31–33 and falls.34–38 The exclusion of adults with a standing SBP <110 mmHg (which would exclude more severe cases of OH) and use of injurious falls for ascertainment (rather than more sensitive methods like fall calendars) may explain some of these differences with observational studies. It is also possible that differences in BP measurement account for differences across studies, as SPRINT followed a rigorous protocol to monitor BP. Despite these issues, it is important to note there was no difference in the associations between OH and serious adverse events across intensive and standard BP goals, suggesting that OH is not a consequence of more aggressive hypertension treatment that contributes to syncope or falls.
This study has limitations. First, OH was measured using a seated to standing protocol after 1 minute of standing. There is evidence that OH measurements within 1 minute of standing may be more predictive of subsequent falls.31,38 Also, seated versus supine-to-standing protocols can miss cases of OH as gravitational effects on BP are blunted.39 Second, our study did not include non-injurious falls. While inpatient or emergency department claims are specific, a substantial number of fall claims are found only in outpatient records or may never be reported to health professionals.34 Third, the study excluded people with a standing SBP <110 mmHg, diabetes, prior stroke, and dementia, conditions which have been associated with OH in other studies.15,40,41 As a result the most severe cases of OH may not be represented in our study. Moreover, about 93% of participants with OH were asymptomatic. As a result, these results may not be generalizable to all OH patients as OH is rarely screened for in asymptomatic patients. Fourth, our results were observed in the context of the treatment regimens used in SPRINT, which are consistent with previous and current US hypertension guidelines. It is possible that more frequent use of other classes of drugs might be associated with more OH and SAEs. Finally, given the observational design of our secondary analysis, the non-randomized contrasts in our study are subject to residual confounding.
Our findings have important clinical implications. SPRINT demonstrated a survival benefit from more aggressive hypertension treatment in both middle aged and older adults at increased risk for CVD.6,10 It has reshaped how hypertension is defined and established lower goals for therapy.42 One of the major impacts of SPRINT was that medical treatment of hypertension was recommended by some major guidelines for 82 million adults in the US, including many healthy adults aged ≥75 years with BP above 130/80 mmHg.43 These expanded treatment recommendations have led to the concern, particularly for geriatric populations, that more aggressive hypertension treatment might increase OH, contributing to falls, syncope, and even in some cases CVD events. The present study should allay these concerns. OH was not associated with a higher risk of CVD events, falls, or syncope. Further, there was no evidence that OH in the setting of intensive therapy was more strongly related to the majority of outcomes and adverse events examined. While further research is needed to examine the association between OH and non-myocardial infarction ACS, given the primary survival benefits from more aggressive hypertension treatment among older adults, the detection of OH does not represent a clear contraindication for treatment.
Supplementary Material
Perspectives.
In conclusion, in this population of middle-aged and older hypertensive adults, in addition to the previous observation that more aggressive hypertension treatment reduced (rather than increased) the risk of OH,6 we found that OH was not associated with a higher risk of CVD events, falls, or syncope. Moreover, BP goal did not alter the relationship between OH and risk of CVD events or adverse effects with the exception of non-myocardial infarction ACS. These findings provide strong evidence that the presence of symptomless OH should not be a reason for down-titration of medications, even in the setting of a lower BP goal.
Novelty and Significance.
What is New?
Orthostatic hypotension was not associated with higher risk of cardiovascular events, falls, or syncope. Hypertension treatment did not alter the association between orthostatic hypotension and cardiovascular outcomes or adverse events.
What is Relevant?
There are ongoing concerns that orthostatic hypotension in the setting of more intensive blood pressure treatment represents a greater risk of adverse events from treatment. Our data challenges this notion and the practice of reducing hypertension treatment in response to orthostatic hypotension.
Acknowledgements
The authors thank the participants of the SPRINT study for their important contributions.
For a full list of contributors to SPRINT, please visit www.sprinttrial.org.
Sources of Funding
SPJ is supported by NIH/NHLBI grant K23HL135273.
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420.
Abbreviations used:
- SPRINT
Systolic Blood Pressure Intervention Trial
- BP
blood pressure
- CI
confidence interval
Footnotes
Conflicts of Interest
The authors declare that there is no conflict of interest associated with this manuscript.
This trial is registered at clinicaltrials.gov, number: NCT01206062
References
- 1.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19:508–519. [DOI] [PubMed] [Google Scholar]
- 2.Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM. Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension. 2011;57:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Press Y, Punchik B, Freud T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J Hypertens. 2015; [DOI] [PubMed] [Google Scholar]
- 5.Di Stefano C, Milazzo V, Totaro S, Sobrero G, Ravera A, Milan A, Maule S, Veglio F. Orthostatic hypotension in a cohort of hypertensive patients referring to a hypertension clinic. J Hum Hypertens. 2015;29:599–603. [DOI] [PubMed] [Google Scholar]
- 6.SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sink KM, Evans GW, Shorr RI, Bates JT, Berlowitz D, Conroy MB, Felton DM, Gure T, Johnson KC, Kitzman D, Lyles MF, Servilla K, Supiano MA, Whittle J, Wiggers A, Fine LJ. Syncope, Hypotension, and Falls in the Treatment of Hypertension: Results from the Randomized Clinical Systolic Blood Pressure Intervention Trial. J Am Geriatr Soc. 2018;66:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend RR, Chang TI, Cohen DL, Cushman WC, Evans GW, Glasser SP, Haley WE, Olney C, Oparil S, Del Pinto R, Pisoni R, Taylor AA, Umanath K, Wright JT, Yeboah J, SPRINT Study Research Group. Orthostatic changes in systolic blood pressure among SPRINT participants at baseline. J Am Soc Hypertens. 2016;10:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT, Whelton PK, SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT, Pajewski NM, SPRINT Research Group. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, Lewis CE, Rahman M, Reboussin DM, Rocco MV, Oparil S, Wright JT, SPRINT Research Group. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostis WJ, Sargsyan D, Mekkaoui C, Moreyra AE, Cabrera J, Cosgrove NM, Sedjro JE, Kostis JB, Cushman WC, Pantazopoulos JS, Pressel SL, Davis BR. Association of orthostatic hypertension with mortality in the Systolic Hypertension in the Elderly Program. J Hum Hypertens. 2019;33:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanhanen H, Thijs L, Birkenhäger W, Bulpitt C, Tilvis R, Sarti C, Tuomilehto J, Staessen JA. Prevalence and persistency of orthostatic blood pressure fall in older patients with isolated systolic hypertension. Syst-Eur Investigators. J Hum Hypertens. 1996;10:607–612. [PubMed] [Google Scholar]
- 14.Juraschek SP, Appel LJ, Miller ER, Mukamal KJ, Lipsitz LA. Hypertension Treatment Effects on Orthostatic Hypotension and Its Relationship With Cardiovascular Disease. Hypertension. 2018;72:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, Cutler JA, Grimm R, Pedley C, Peterson K, Pop-Busui R, Sperl-Hillen J, Cushman WC. Orthostatic Hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Blood Pressure Trial: Prevalence, Incidence, and Prognostic Significance. Hypertension. 2016;68:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters R, Anstey KJ, Booth A, Beckett N, Warwick J, Antikainen R, Rockwood K, Peters J, Bulpitt CJ. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur Heart J. 2018;39:3135–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juraschek SP, Daya N, Appel LJ, Miller ER, McEvoy JW, Matsushita K, Ballantyne CM, Selvin E. Orthostatic Hypotension and Risk of Clinical and Subclinical Cardiovascular Disease in Middle-Aged Adults. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verwoert GC, Mattace-Raso FUS, Hofman A, Heeringa J, Stricker BHC, Breteler MMB, Witteman JCM. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008;56:1816–1820. [DOI] [PubMed] [Google Scholar]
- 19.Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13:571–578. [DOI] [PubMed] [Google Scholar]
- 20.Fedorowski A, Hedblad B, Melander O. Early postural blood pressure response and cause-specific mortality among middle-aged adults. Eur J Epidemiol. 2011;26:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan X-H, Wang Y, Sun K, Zhang W, Wang H, Wu H, Zhang H, Zhou X, Hui R. Disorders of orthostatic blood pressure response are associated with cardiovascular disease and target organ damage in hypertensive patients. Am J Hypertens. 2010;23:829–837. [DOI] [PubMed] [Google Scholar]
- 22.Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T, Shimada K. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol. 2002;40:133–141. [DOI] [PubMed] [Google Scholar]
- 23.Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50:325–332. [DOI] [PubMed] [Google Scholar]
- 24.Fedorowski A, Wahlstrand B, Hedner T, Melander O. Systolic and diastolic component of orthostatic hypotension and cardiovascular events in hypertensive patients: the Captopril Prevention Project. J Hypertens. 2014;32:75–81. [DOI] [PubMed] [Google Scholar]
- 25.Hossain M, Ooi WL, Lipsitz LA. Intra-individual postural blood pressure variability and stroke in elderly nursing home residents. J Clin Epidemiol. 2001;54:488–494. [DOI] [PubMed] [Google Scholar]
- 26.Veronese N, De Rui M, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Maggi S, Crepaldi G, Perissinotto E, Manzato E, Sergi G. Orthostatic Changes in Blood Pressure and Mortality in the Elderly: The Pro.V.A Study. Am J Hypertens. 2015;28:1248–1256. [DOI] [PubMed] [Google Scholar]
- 27.Gupta R, Singh S, Rahman MA, Saeed M, Birnbaum Y. Symptomatic bradycardia and postural hypotension. Postgrad Med J. 2004;80:679–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah RV, Patel KP, Manion C, Runkana A, Hama Amin A, Jain A. Third-degree atrioventricular block followed by syncope, labile hypertension, and orthostatic hypotension in a patient with nasopharyngeal cancer: baroreflex failure. Am J Cardiovasc Dis. 2018;8:39–42. [PMC free article] [PubMed] [Google Scholar]
- 29.Feldstein C, Weder AB. Orthostatic hypotension: a common, serious and underrecognized problem in hospitalized patients. J Am Soc Hypertens. 2012;6:27–39. [DOI] [PubMed] [Google Scholar]
- 30.Mattace-Raso FUS, van der Cammen TJM, Knetsch AM, van den Meiracker AH, Schalekamp MADH, Hofman A, Witteman JCM. Arterial stiffness as the candidate underlying mechanism for postural blood pressure changes and orthostatic hypotension in older adults: the Rotterdam Study. J Hypertens. 2006;24:339–344. [DOI] [PubMed] [Google Scholar]
- 31.Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER, Windham BG, Griswold ME, Heiss G, Selvin E. Association of History of Dizziness and Long-term Adverse Outcomes With Early vs Later Orthostatic Hypotension Assessment Times in Middle-aged Adults. JAMA Intern Med. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan MP, Newton JL, Chadwick TJ, Parry SW. The relationship between carotid sinus hypersensitivity, orthostatic hypotension, and vasovagal syncope: a case-control study. Europace. 2008;10:1400–1405. [DOI] [PubMed] [Google Scholar]
- 33.O’Mahony D, Foote C. Prospective evaluation of unexplained syncope, dizziness, and falls among community-dwelling elderly adults. J Gerontol A Biol Sci Med Sci. 1998;53:M435–440. [DOI] [PubMed] [Google Scholar]
- 34.Juraschek SP, Daya N, Appel LJ, Miller ER, Windham BG, Pompeii L, Griswold ME, Kucharska-Newton A, Selvin E. Orthostatic Hypotension in Middle-Age and Risk of Falls. Am J Hypertens. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108:106–111. [DOI] [PubMed] [Google Scholar]
- 36.Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143:1129–1136. [DOI] [PubMed] [Google Scholar]
- 37.Heitterachi E, Lord SR, Meyerkort P, McCloskey I, Fitzpatrick R. Blood pressure changes on upright tilting predict falls in older people. Age Ageing. 2002;31:181–186. [DOI] [PubMed] [Google Scholar]
- 38.Finucane C, O’Connell MDL, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired Orthostatic Blood Pressure Recovery Is Associated with Unexplained and Injurious Falls. J Am Geriatr Soc. 2017;65:474–482. [DOI] [PubMed] [Google Scholar]
- 39.van Wijnen VK, Finucane C, Harms MPM, Nolan H, Freeman RL, Westerhof BE, Kenny RA, Ter Maaten JC, Wieling W. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med. 2017;282:468–483. [DOI] [PubMed] [Google Scholar]
- 40.Kim H-A, Lee H. Orthostatic hypotension in acute cerebellar infarction. J Neurol. 2016;263:120–126. [DOI] [PubMed] [Google Scholar]
- 41.Sonnesyn H, Nilsen DW, Rongve A, Nore S, Ballard C, Tysnes OB, Aarsland D. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28:307–313. [DOI] [PubMed] [Google Scholar]
- 42.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;HYP.0000000000000066. [Google Scholar]
- 43.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, Whelton PK. Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


