Abstract
Background:
Challenges to effective pharmacologic management of symptomatic diabetic peripheral neuropathy include the limited effectiveness of available medicines, frequent side effects, and the need for ongoing symptom assessment and treatment titration for maximal effectiveness.
Aims:
We present here the rationale and implementation challenges of the Diabetes Telephone Study, a randomized trial designed to improve medication treatment, titration and quality of life among patients with symptomatic diabetic peripheral neuropathy.
Methods:
We implemented a pragmatic cluster randomized controlled trial to test the effectiveness of an automated interactive voice response tool designed to provide physicians with real-time patient-reported data about responses to newly prescribed diabetic peripheral neuropathy medicines. 1,830 primary care physicians treating patients in the diabetes registry at Kaiser Permanente Northern California were randomized into the intervention or control arm in September 2014. Patients assigned to physicians in the intervention group receive three brief interactive calls every 2 months after a medication is prescribed to alleviate diabetic peripheral neuropathy symptoms. These calls provide patients with the opportunity to report on symptoms, side effects, self-titration of medication dose and overall satisfaction with treatment. We plan to compare changes in self-reported quality of life between the intervention group and patients in the control group who receive three non-interactive automated educational phone calls.
Results:
Successful implementation of this clinical trial required robust stakeholder engagement to help tailor the intervention and to address pragmatic concerns such as provider time constraints. As of October 27, 2015, we had screened 2,078 patients, 1,447 of whom were eligible for participation. We consented and enrolled 1,206 or 83% of those eligible. Among those enrolled, 53% are women and the mean age is 67 (sd 12). The racial ethnic make-up is 56% white, 8% Asian, 13% black or African American, and 19% Hispanic or Latino.
Conclusions:
Innovative strategies are needed to guide improvements in healthcare delivery for patients with symptomatic diabetic peripheral neuropathy. This trial aims to assess whether real-time collection and clinical feedback of patient treatment experiences can reduce patient symptom burden. Implementation of a clinical trial closely involving clinical care required researchers to partner with clinicians. If successful, this intervention provides a critical information feedback loop that would optimize diabetic peripheral neuropathy medication titration through widely available interactive voice response technology.
Keywords: diabetic peripheral neuropathy, treatment titration, quality of life, stakeholder engagement research
Introduction
Diabetic peripheral neuropathy is a painful and prevalent complication that affects over 5.5 million people with diabetes, including up to 50% of diabetes patients with long-standing disease.[1] Diabetic peripheral neuropathy is characterized by pain, burning, pins and needles sensations, and/or numbness in the toes and feet.[2] Symptoms related to diabetic peripheral neuropathy are associated with lower quality of life, limited mobility, depression, and social dysfunction.[3,4]
A major challenge to effective treatment of diabetic peripheral neuropathy symptoms is the lack of strong clinical evidence to guide which treatments are likely to work for individual patients. There are currently a wide range of available medications to treat diabetic peripheral neuropathy symptoms including tricyclic antidepressants, anticonvulsants, serotonin norepinephrine reuptake inhibitors, and opioid analgesics. [2,5,6] First line therapies require intensive step-wise titration to reach a dose that optimally balances symptom relief with side effect burden. Ultimately, many of these treatments relieve only 20 to 30% of pain symptoms. Moreover, all available treatments have significant side effects such as dizziness, somnolence, nausea and confusion that may be intolerable to some patients.[2,5–8] Given the complexity of diabetic peripheral neuropathy medication management, new tools are needed to support more effective prescription and titration of available medicines.
For conditions like diabetic peripheral neuropathy, where there is a high degree of individual patient variability in treatment tolerance and efficacy, physicians must rely more extensively on patient preferences and experiences to inform symptom management.[9] Research suggests that facilitating timely communication and information exchange between patients and physicians can contribute to patient satisfaction with treatment decisions and improve quality of life.[10]
We designed a study to implement and evaluate a new care tool that uses an automated interactive voice response system to extend the window of opportunity for more effective patient-provider communication. This interactive voice response system gathers patient responses to help guide physicians in timely dose titration and medication changes. Our primary research goal is to facilitate more effective medication titration by collecting and transferring patient responses about new medication to the physician’s electronic health record. We hypothesize that this technology-enabled communication feedback tool will improve patient quality of life by promoting clinically appropriate treatment changes. The purpose of this article is to describe the study protocol and the specific challenges we addressed in implementing this pragmatic trial.
Methods
Study Setting
Kaiser Permanente Northern California is an integrated health care system serving more than 3.6 million people in California, including more than 260,000 members with diabetes who are automatically enrolled in the Kaiser Permanente Northern California diabetes registry.[11]
Usual care for patients with diabetes and related chronic conditions in this setting includes a robust panel management approach that leverages performance feedback, system-wide efficiencies, disease registries and evidence-based practice.[12] The electronic medical record at Kaiser Permanente Northern California is a critical component of this comprehensive disease management strategy.[13,14]
Stakeholder Engagement Strategy
Given the pragmatic nature of this trial, we engaged patients with diabetic peripheral neuropathy and clinical stakeholders in all aspects of the research from the identification of the research question to the design of the intervention and dissemination of research findings. Our stakeholder panel includes three patients, three primary care physicians (one of whom is an expert in electronic medical record physician prompts), one nurse case manager, two pharmacists, one health educator, and one endocrinologist. Our approach to stakeholder engagement is grounded in a framework developed by one of the authors (Schmittdiel) on system-based participatory research in health care.[15] The four principles guiding our efforts are: 1) partnership and collaboration in all phases of research; 2) building upon existing resources and goals, 3) creating and investing in long term robust partnerships and 4) engaging in research as a cyclical and iterative process.[15]
Physician-Level Randomization, Participant Eligibility, and Consent
We randomized at the physician level to reduce the likelihood of contamination among patients seen by the same physician. One thousand eight hundred and thirty four Kaiser Permanente Northern California primary care physicians with adult patients in the Kaiser Permanente Northern California diabetes registry were randomized to the intervention or control group prior to patient eligibility determination and recruitment using a randomization procedure available in the SAS® software package.[16] Of those randomized, 1,714 physicians had patients who met the initial inclusion and exclusion criteria. In October 2014, we notified these physicians that their patients may be eligible for a new clinical trial, explained the trial, and invited them to opt out any patient due to illness or other factors that would make them ineligible for participation. Among diabetes patients not opted out by their physicians, we use the electronic health record to prospectively identify patients who start a new prescription (i.e., no use in the previous 12 months) for medications commonly used to treat diabetic peripheral neuropathy symptoms (amitriptyline, nortriptyline, imipramine, desipramine, duloxetine, paroxetine, citalopram, pregabalin, venlafaxine, or gabapentin) between September 2014-November 2015 and who have screened positive for DPN symptoms at least once during a routine visit occurring between April 2012 (when the screener was implemented system-wide) and the time they started medication.
Research associates send recruitment letters describing the study and potential risks and benefits of participation. The materials sent to patients in the treatment and control arms were identical. Patients are contacted by an interviewer by phone one week later to obtain phone consent and to conduct the baseline interview. To reduce the risk of selection bias, research associates and interviewers are blinded to treatment assignment from consent through outcomes assessment. Patients are blinded to their treatment status at the time of recruitment. The procedures followed are in accordance with the ethical standards of the responsible committee on human experimentation, the Kaiser Permanente Internal Review Board, which has reviewed and approved the study protocol. The study is registered at ClinicalTrials.gov (CE-1304-7250).
To be eligible for inclusion, identified patients have to be 18 years old at the time they start therapy, be continuously enrolled in Kaiser Permanente Northern California during the 12 months prior to starting study medications, and speak English or Spanish. We exclude patients who have a diagnosis of gestational diabetes, dementia or substance abuse disorder during the previous 12 months or who have any opioid use during the 90 days prior to starting diabetic peripheral neuropathy treatment. [Figure I]
Figure I.
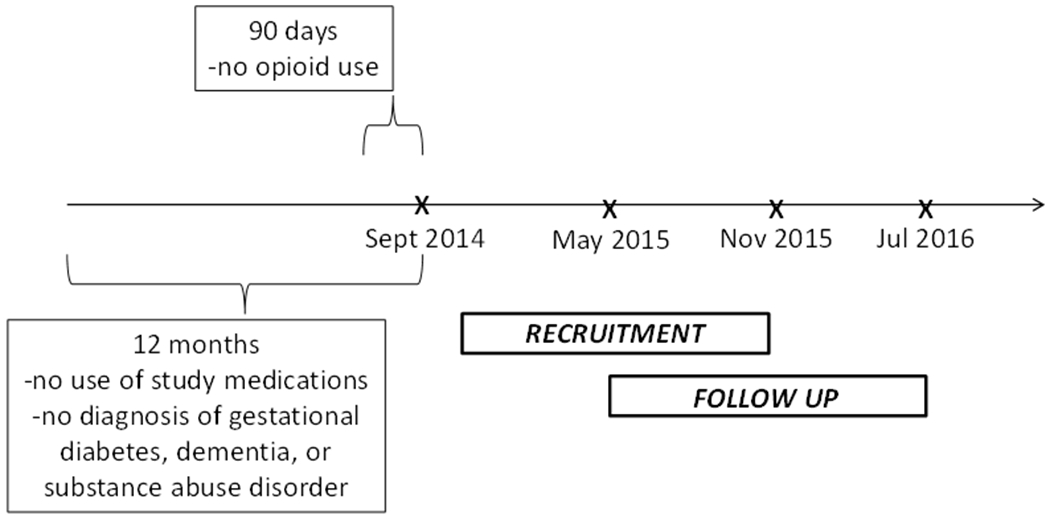
Timeline for Study Recruitment and Follow-up
As we identify potential participants, study telephone interviewers confirm that they have active diabetic peripheral neuropathy symptoms and that the prescribed study medications are for diabetic peripheral neuropathy and not some other condition. In addition, the interviewers identify other factors that may impede participation in this telephone based study such as undiagnosed cognitive deficits or use of rotary phones.
The Intervention
Patients of physicians allocated to the intervention arm receive all usual care for diabetes and diabetic peripheral neuropathy, plus three five-minute interactive voice response calls spaced two months apart over the six-month period following start of diabetic peripheral neuropathy treatment. These interactive calls systematically collect patient information on symptom relief, medication use, titration, discontinuation and side effects. Patient responses are then manually entered into the electronic health record by a trained research assistant. Responses indicating dissatisfaction with the level of symptom relief, side effects, and/or discontinuation of (or failing to start) the medication are forwarded directly to the physician for more rapid physician notification and response. Responses from patients who report no major problems with their diabetic peripheral neuropathy medication are entered into the health record but not forwarded to the physician since no immediate follow-up is required. (Table 1)
Table 1.
Content of Data Collected from Interactive Voice Response Calls in Intervention Group
| Call 1 | Calls 2 and 3 |
|---|---|
| Patient still taking the medication prescribed for diabetic peripheral neuropathy | Overall diabetic peripheral neuropathy symptom status |
| Reason(s) if patient no longer taking medication: | Patient still taking the medication prescribed for diabetic peripheral neuropathy |
| Medication not helpful for symptoms | Reason(s) if patient no longer taking medication: |
| Told by physician to stop the medication | Medication not helpful for symptoms |
| Symptoms got better | Told by physician to stop the medication |
| Taking too many medications, didn’t want to add another | Symptoms got better |
| Side effects | Taking too many medications, didn’t want to add another |
| Medication costs too much | Side effects |
| Other reasons | Medication costs too much |
| Patient usually takes medication daily | Switched medications |
| Patient decided to increase or decrease medication dosage | Other reasons |
| How well medication working to reduce symptoms | Patient started diabetic peripheral neuropathy medication in last two months |
| Medication working as well as patient hoped | In the last two months, patient decided to increase or decrease medication dosage |
| Diabetic peripheral neuropathy medication side effects in last 7 days | Patient taking more than one medication for diabetic peripheral neuropathy symptoms |
| Medication working as well as patient hoped | |
| Diabetic peripheral neuropathy medication side effects in last 7 days | |
| Patient considered stopping diabetic peripheral neuropathy medication because of side effects |
Control Arm
Patients of physicians allocated to the control arm receive all usual care for diabetes and diabetic peripheral neuropathy, plus three non-interactive, automated telephone calls spaced two months apart over the six months following start of a diabetic peripheral neuropathy treatment. The automated telephone calls consist of three separate diabetes-related educational messages about physical activity, dietary changes, and the importance of regular foot checks and last about two minutes or less. The goal of providing these non-interactive calls is to isolate the marginal impact of receiving an automated, diabetes-related phone call in the intervention arm. Intervention and control calls are offered in English and Spanish.
Outcome Measures
The primary outcome measure is quality of life as measured by the Global Health Scale, a 10-item PROMIS® measure developed and validated in patients with neuropathy as part of the Quality of Life in Neurological Disorders Measures.[16] The questionnaire includes questions about quality of life, mood, physical and social functioning and pain severity. Data are collected by trained researchers during 20-minute computer assisted telephone interviews at approximately 30 days before the first and 30 days after the final interactive voice response/control call. (Table 2)
Table 2:
Schedule of Data Collection
| Type | Instrument | Domain | Baseline | Follow Up |
|---|---|---|---|---|
| Primary Outcomes | Global Health Scale | Overall health status | Yes | Yes |
| Secondary | Pain Interference | Pain | Yes* | Yes |
| Sleep Disturbance | Sleep | Yes | Yes | |
| Depression | Depression | Yes* | Yes | |
| Ability to Participate | Social Functioning | Yes* | Yes | |
| Lower Extremity Functioning | Physical Functioning | Yes* | Yes | |
| 6 Item Doctor Communication Composite (CAHPS) | Communication | Yes | Yes | |
| Potential Effect Modifiers | SDM-Q-9 | Shared decision making | Yes | No |
| Adaptive Conjoint Analysis Tool | Treatment Preferences | Yes** | No |
Restricted to patients who indicated difficulty in that domain on the global health scale.
To be administered to a subset (~200) of patients .
To assess the mechanisms by which the intervention may impact quality of life, we are also capturing the following secondary outcome measures using instruments from the Quality of Life in Neurological Disorders Measures: Pain Interference, Sleep Disturbance, Depression, Ability to Participate, and Lower Extremity Functioning.[17] In addition, we will evaluate patient-perceived changes in communication between patients and their doctors using the six-item Doctor Communication Composite from the Consumer Assessment of Health Plans Survey. This instrument is used routinely in this setting and refers to the patient’s most recent visit.[18] Using the electronic health record, we will also assess changes in medication use and physician prescribing patterns.
Evaluation of the Intervention’s Effectiveness
We will evaluate the intention-to-treat effect of the interactive voice response intervention compared to the control intervention on the change in quality of life measurements collected at baseline and 30 days after the final interactive voice response/control call using generalized estimating equations to account for repeated (patient) measures within physician clusters .[19,20] Double robust estimation will be implemented in secondary analyses to address any observed imbalance in characteristics between the control and intervention groups at baseline [21] and to address any evidence of differential rates of missing outcomes due to dropout between the two arms .[22–24] Generalized estimating equations analyses will be conducted using the GENMOD procedure in SAS® for fitting a linear model with repeated measurements. Doubly robust estimation will be conducted using the ltmle R package (available at: https://cran.r-project.org/web/packages/ltmle/index.html).
Our protocol includes testing for heterogeneity in study outcomes by patient desire for shared decision making. However, the high response rate will enable for additional secondary analyses of physician characteristics such as age, gender, and years at Kaiser Permanente Northern California, as well as patient’s perception of physician communication skills, as potential moderators of intervention effects.
Sample Size and Power Considerations
Estimating the minimum detectable effect was challenging in this prospective pragmatic trial because we could not know in advance how many patients would become eligible during the enrollment period. Based on our prior studies, we estimated that 118 patients would be newly started on diabetic peripheral neuropathy treatment each month (est. n=2,124 over 18 months) and that, on average, most physicians would have one patient enrolled only. Assuming 1,062 patients are approached to participate in each arm, that none of these patients share the same primary care physician, a 40% participation rate and 20% loss to follow-up (complete data for 340/arm), the minimum detectable mean score difference between the two groups with alpha=0.05, beta=0.2, and an outcome with standard deviation of 0.143 is 0.030.[25,26,27] Previous studies have estimated a minimally meaningful difference in the quality of life measure to be 0.074,[28] indicating that we should be able to evaluate clinically meaningful changes in quality of life. To ensure our ability to conduct secondary analyses, we set a target enrollment of 1,190 patients or 595 per arm.
Given an average cluster size of 1.5 patients per physician and a commonly assumed intraclass correlation coefficient of 0.05, accounting for clustering would amount to a negligible variance inflation factor of 1.025, resulting in a minimum detectable effect of 0.0305 (which also results in an increase of 17 patients for a total of 697 patients with complete data required for detecting an effect of 0.030). Given that we have already collected baseline data on more than 1,200 patients and have a drop-out rate of less than 2%, we should have sufficient sample size for both primary and secondary outcomes even when clustering is present.
Results
Recruitment
Recruitment of patients began in October 2014, approximately four months later than initially planned. However, the rate of new medication starts has been higher than anticipated. By October 27, 2015, we had screened 2,078 patients, 1,447 of whom were eligible for participation. Among those eligible, 877 unique primary care physicians were represented. The most common reasons for ineligibility were use of study medications for conditions other than diabetic peripheral neuropathy, absence of active symptoms, and lack of proficiency in Spanish or English language. We consented and enrolled 1,206 or 83% of those eligible, representing 792 unique primary care physicians and 54 facilities. Recruitment rates are similar across the two arms (control=82.7%; treatment=83.9%). Common reasons for refusal included lack of interest in participating in a study, being too sick to participate and being too busy.
Since the start of recruitment, twenty patients (<2%) have dropped out of the study. The most common reasons for drop out were worsening health status, caregiving responsibilities and challenges with the interactive voice response system such as missed calls.
Characteristics of Enrolled Subjects
Among the 1,206 enrolled as of October 27, 2015, 53% are women and the mean age is 67 (standard deviation: 12). The racial ethnic make-up is 56% white, 8% Asian, 13% black or African American, 19% Hispanic or Latino, 1% Native Hawaiian, Pacific Islander, or Native American, and 1% unknown. We have collected baseline data on 1,191 patients, 71 of whom conducted the survey in Spanish. Close out surveys have been completed by 92% (589) of those who have completed the intervention. We identified no statistically significant differences in the demographic characteristics or in baseline quality of life between the treatment and control arms.(Table 3)
Table 3:
Characteristics of Patients Enrolled in the Diabetes Telephone Study as of October 27, 2015
| ╲N (%) | All (N=1,191) | Control Group (N=623) | Treatment Group (N=568) | P value* |
|---|---|---|---|---|
|
Gender Female Male |
637 (53.5) 554 (46.5) |
329 (52.8) 294 (47.2) |
308 (54.2) 260 (45.8) |
0.6245 |
| Race/Ethnicity | 0.5476 | |||
| White | 672 (56.4) | 350 (56.2) | 322 (56.7) | |
| Asian | 98 (8.2) | 45 (7.2) | 53 (9.4) | |
| Black | 161 (13.5) | 87 (14.0) | 74 (13.0) | |
| Hispanic | 232 (19.5) | 124 (19.9) | 108 (19.0) | |
| HI/PI/NA | 14 (1.2) | 10 (1.6) | 4 (0.7) | |
| Unknown | 14 (1.2) | 7 (1.1) | 7 (1.2) | |
| Spanish Speaker | 0.9728 | |||
| Yes | 71 (6.0) | 37 (5.9) | 34 (6.0) | |
| No | 1,120 (94.0) | 586 (94.1) | 534 (94.0) | |
| Age Mean ± Standard Deviation | 67.1 ± 11.8 | 67.1 ± 11.5 | 67.2 ± 12.0 | 0.8355 |
|
Quality of Life (EQ5D) Mean ± Standard Deviation |
0.65 ± 0.1 | 0.65 ± 0.1 | 0.66 ± 0.1 | 0.4538 |
comparing control and treatment
Changes to the Initial Study Plan based on Stakeholder Feedback
This pragmatic, cluster randomized study is designed to be maximally generalizable to the patients and clinical settings in which the intervention is being tested.[29] However, the pragmatic nature of the study design also presented several challenges that were addressed through collaboration with patient and provider stakeholders:
1. Isolating the Impact of the Intervention:
While we use the interactive voice response as a mechanism for facilitating the feedback tool, we wanted to measure the impact of the feedback tool, not the receipt of an automated call. The creation of non-interactive automated educational calls for the control group ensures that any differences between the groups will be related to the feedback tool. Stakeholders helped to choose the content of these automated calls.
2. Avoiding Physician Information Overload:
Our clinician stakeholders emphasized the need to limit the data being forwarded to clinicians to clinically “actionable” information (e.g., information that would trigger changes in dose or medication class). Our triage system prioritizes patients for more rapid intervention if they report being dissatisfied with the level of symptom relief, are experiencing side effects, or are discontinuing medication. These triggers for action were selected by our clinician stakeholders.
3. Tailoring the Intervention to Patient Population:
Our patient population includes a disproportionate number of frail and disabled people, patients who are frequently excluded from traditional clinical trials. As a consequence, we found early on that some patients were having difficulty reaching the phone in time to receive the interactive voice response calls and interacting with the interactive voice response system. To address these issues, the interviewers now provide patients with a toll free number to call into the system should they miss a call. In addition, we increased the number of interactive voice response call attempts from 3 to 8 to maximize participation among all patients.
Discussion
This study tests the potential for an interactive voice response-based feedback intervention to improve quality of life for patients newly treated for diabetic peripheral neuropathy symptoms. While there is no cure for diabetic peripheral neuropathy, symptoms are treatable and ameliorable through medications and frequent monitoring of patient symptoms and side effects to facilitate clinically appropriate treatment changes for maximum benefit.[5–8] Unfortunately, many patients may never achieve the maximum benefit from their medications due to the absence of routine monitoring of symptoms and treatment effects.[2,3]
In this study, we use a cluster randomized trial design, endeavoring to reduce common threats to validity,[30] in order to evaluate the impact of an intervention to address this gap in care. As with any study, this approach has some limitations that deserve consideration. First, we chose to randomize at the physician level to reduce risk of contamination among patients treated by the same physician. However, the possibility of contamination among physicians in the same medical center remains. We estimate the risk of such contamination to be minimal due to the small number of patients affected over a long period of time and the absence of any changes in clinical workflows.
In addition, we randomized physicians to either the treatment or control condition prior to consenting patients to reduce the possibility of selection. However, this decision means that we have some attrition of physicians post randomization, as well as some physicians with no eligible patients. In addition, while patients are blinded to treatment assignment at the time they consent, it is possible that patients in the intervention may correctly guess their status due to the interactive nature of the intervention phone calls. Therefore, we will compare the characteristics of physicians and patients who drop out to assess whether they differ in systematic ways from those included in the study. However, our very low drop-out rates suggests that threats to validity related to this type of bias should be minimal.
Leveraging available technology to supplement patient-provider communication about symptoms has the potential to facilitate treatment titration and to improve patient treatment experiences and quality of life. If successful, the methods employed in the Diabetes Telephone Study trial could apply to other conditions where timely feedback of information on treatment efficacy and side effects are critical to achieving optimal outcomes.
Acknowledgements
The authors would also like to thank of members of the Diabetes Telephone Study group, including Nancy Connolly, Bonnie Halpern, Michelle Lamet, Virginia Pozo, Kalliope Bellesis, Shirish Paranjpe, and Marvella Villasenor at the Kaiser Permanente Division of Research; Michael Shainline, Leslie Wright, and David Steffen at Kaiser Permanente Colorado Institute for Health Research; and Manueal Ballesca and David David Liu from the Permanente Medical Group for assistance in creating the feedback tool. We would also like to thank our patient [Joel Clark, Bonieta Cook, and David Willyoung] and clinical stakeholders [Dan Cheung, pharmacist, Gary Choy, pharmacist, Cynthia Mik, RN and diabetes care manager, and Nora Kurose, health educator] for their invaluable contributions to this work. In addition, we would like to thank our consultants: Drs. Brian Callaghan, Lisa Prosser and Eve Wittenberg for lending their expertise.
Dr. Adams conceptualized the work and drafted the manuscript. Drs. Jaffe, Young, Kim, Grant, Schmittdiel, Bayliss, Neugebauer and Altschuler and Ms. Dyer contributed to the conceptualization of the paper and revisions/edits to the manuscript.
Funding
This work was supported by the Patient-Centered Outcomes Research Institute [CE-1304-7250; SC14-1403-11992]; the Division of Diabetes Translation, Centers for Disease Control and Prevention [U58 DP002641]; the National Institute for Diabetes, Digestive and Kidney Disorders [R01DK099108], and the National Health, Lung, Blood Institute [R01 HL117939].
Funding for this work was supported through a Patient-Centered Outcomes Research Institute Assessment of Prevention, Diagnosis, and Treatment Options Program Award [CE-1304-7250]. Drs. Adams and Schmittdiel are also support by a grant from the Division of Diabetes Translation, Centers for Disease Control and Prevention [U58 DP002641]. Dr. Grant is supported by the National Institute for Diabetes, and Digestive and Kidney Diseases (R01DK099108), the National Heart, Lung and Blood Institute, (R01 HL117939) and Patient-Centered Outcomes Research Institute (SC14-1403-11992).
Footnotes
This study is registered at ClinicalTrials.gov, #CE-1304-7250.
Publisher's Disclaimer: DISCLAIMER: All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors or Methodology Committee.
Data Availability
Data collected as part of this randomized controlled trial were obtained via informed consent related to the aims of this project and are, therefore, not publicly available. However, the Diabetes Telephone Study research team welcomes collaborations and anyone interested in possible scientific collaboration should contact the corresponding author, Dr. Alyce Adams.
References
- 1.Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26 :1790–1795. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28 :956–962. [DOI] [PubMed] [Google Scholar]
- 3.Sadosky A, Schaefer C, Mann R, et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospective chart review and cross-sectional survey. Diabetes Metab Syndr Obes 2013; 6: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain R, Jain S, Raison CL, et al. Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators. Curr Diab Rep 2011; 11: 275–284.5. [DOI] [PubMed] [Google Scholar]
- 5.Boulton AJ, Malik RA, Arezzo JC, et al. Diabetic somatic neuropathies. Diabetes Care 2004; 27: 1458–1486. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay TJ, Rodgers BC, Savath V, et al. Treating diabetic peripheral neuropathic pain. Am Fam Physician 2010; 82 :151–158. [PubMed] [Google Scholar]
- 7.Greig M, Tesfaye S, Selvarajah D, et al. Insights into the pathogenesis and treatment of painful diabetic neuropathy In: Zochodne DW, Malik RA (eds) Diabetes and the Nervous System, Volume 126: Handbook of Clinical Neurology. 3rd series. Waltham: Elsevier Science, 2014; pp.559–78. [DOI] [PubMed] [Google Scholar]
- 8.Peltier A, Goutman SA, and Callaghan BC. Painful Diabetic Neuropathy. BMJ 2014; 348: g1799. [DOI] [PubMed] [Google Scholar]
- 9.Fraenkel L Incorporating patient’s preferences into medical decision making. Med Care Res Rev 2013; 70: 80s–93s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez LS, Schwartz JS, Freres D, etl al. Patient-clinician information engagement increases treatment decision satisfaction among cancer patients through feeling of being informed. Patient Educ Couns 2009; 77: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karter AJ, Schillinger D, Adams AS, et al. Elevated Rates of Diabetes in Pacific Islander and Asian and subgroups are Hidden by Aggregated Racial Categories: The Diabetes Study of Northern California (DISTANCE). Diabetes Care 2013; 36: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffe MG, Lee GA, Young JD, et al. Improved blood pressure control associated with a large-scale hypertension program. JAMA 2013; 310:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed M, Huang J, Graetz I, et al. Outpatient electronic health records and the clinical care and outcomes of patients with diabetes mellitus. Ann Intern Med. 2012; 157: 482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed M, Huang J, Brand R, et al. Implementation of an outpatient electronic health record and emergency department visits, hospitalizations, and office visits among patients with diabetes. JAMA 2013; 310: 1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmittdiel JA, Grumbach K, and Selby JV. System-based participatory research in health care: an approach for sustainable translational research and quality improvement. Ann Fam Med 2010; 8 :256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SAS Institute Inc. SAS OnlineDoc®, Version 9. Cary, NC, 2002-2006. [Google Scholar]
- 17.National Institute of Neurological Disorders and Stroke (NINDS). User Manual for the Quality of Life in Neurological Disorders (Neuro-QOL) Measures, Version 1.0, http://www.neuroqol.org/Resources/Resources%20documents/Neuro-QOL_User%20Manual%20v2_24Mar2015.pdf (2010, accessed 17 June 2015).
- 18.Hargraves JL, Hays RD, and Cleary PD. Psychometric Properties of the Consumer Assessment of Health Plans Study (CAHPS®) 2.0 Adult Core Survey. Health Services Research 2003; 38: 1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell MK, Grimshaw JM. Cluster randomised trials: time for improvement. The implications of adopting a cluster design are still largely being ignored. BMJ 1998; 317: 1171–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell MK, Mollison J, Steen N, et al. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract 2000; 17: 192–196. [DOI] [PubMed] [Google Scholar]
- 21.Moore KL, Neugebauer R, Valappil T, et al. Robust extraction of covariate information to improve estimation efficiency in randomized trials. Stat Med 2011; 30: 2389–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004; 15: 615–25. [DOI] [PubMed] [Google Scholar]
- 23.Scharfstein DO, Rotnitzky A, and Robins JM. Adjusting for Nonignorable Drop-Out Using Semiparametric Nonresponse Models. Available at: http://www.tandfonline.com/doi/abs/10.1080/01621459.1999.10473862#.VSxUY_nF81I.
- 24.Van der Laan M, Rose S. Targeted Learning: Causal Inference for Observational and Experimental Data. New York: Springer; 2011.21. [Google Scholar]
- 25.Revicki DA, Kawata AK, Harnam N, et al. Predicting EuroQol (EQ-5D) scores from the patient-reported outcomes measurement information system (PROMIS) global items and domain item banks in a United States sample. Qual Life Res 2009; 18: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerry SM, Bland JM. Sample size in cluster randomisation. BMJ 1998; 316:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerry SM, Bland JM. The intracluster correlation coefficient in cluster randomisation. BMJ 1998; 316: 1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Quality of Life Research 2005;14:1523–1532. [DOI] [PubMed] [Google Scholar]
- 29.Johnson KE, Tachibana C, Coronado GD, et al. A guide to research partnerships for pragmatic clinical trials. BMJ 2014; 349: g6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puffer S, Torgerson D, and Watson J. Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. BMJ 2003; 327: 785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]


