Abstract
Aims
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex multisystem disease. Evidence for disturbed vascular regulation comes from various studies showing cerebral hypoperfusion and orthostatic intolerance. The peripheral endothelial dysfunction (ED) has not been sufficiently investigated in patients with ME/CFS. The aim of the present study was to examine peripheral endothelial function in patients with ME/CFS.
Methods and results
Thirty‐five patients [median age 40 (range 18–70) years, mean body mass index 23.8 ± 4.2 kg/m2, 31% male] with ME/CFS were studied for peripheral endothelial function assessed by peripheral arterial tonometry (EndoPAT2000). Clinical diagnosis of ME/CFS was based on Canadian Criteria. Nine of these patients with elevated antibodies against β2‐adrenergic receptor underwent immunoadsorption, and endothelial function was measured at baseline and 3, 6, and 12 months follow‐up. ED was defined by reactive hyperaemia index ≤1.81. Twenty healthy subjects of similar age and body mass index were used as a control group. Peripheral ED was found in 18 of 35 patients (51%) with ME/CFS and in 4 healthy subjects (20%, P < 0.05). Patients with ED, in contrast to patients with normal endothelial function, reported more severe disease according to Bell score (31 ± 12 vs. 40 ± 16, P = 0.04), as well as more severe fatigue‐related symptoms (8.62 ± 0.87 vs. 7.75 ± 1.40, P = 0.04) including a higher demand for breaks [9.0 (interquartile range 7.0–10.0) vs. 7.5 (interquartile range 6.0–9.25), P = 0.04]. Peripheral ED showed correlations with more severe immune‐associated symptoms (r = −0.41, P = 0.026), such as sore throat (r = −0.38, P = 0.038) and painful lymph nodes (r = −0.37, P = 0.042), as well as more severe disease according to Bell score (r = 0.41, P = 0.008) and symptom score (r = −0.59, P = 0.005). There were no differences between the patient group with ED and the patient group with normal endothelial function regarding demographic, metabolic, and laboratory parameters. Further, there was no difference in soluble vascular cell adhesion molecule and soluble intercellular adhesion molecule levels. At baseline, peripheral ED was observed in six patients who underwent immunoadsorption. After 12 months, endothelial function had improved in five of these six patients (reactive hyperaemia index 1.58 ± 0.15 vs. 2.02 ± 0.46, P = 0.06).
Conclusions
Peripheral ED is frequent in patients with ME/CFS and associated with disease severity and severity of immune symptoms. As ED is a risk factor for cardiovascular disease, it is important to elucidate if peripheral ED is associated with increased cardiovascular morbidity and mortality in ME/CFS.
Keywords: Chronic fatigue syndrome, Peripheral endothelial dysfunction, Cardiovascular risk factor, Reactive hyperaemia index, Immune score
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a multisystem disorder characterized by severe fatigue with the hallmark symptom of exertion intolerance. This condition of symptom worsening called post‐exertion malaise typically shows a delayed recovery over more than 24 h.1 The pathophysiological mechanism of ME/CFS is not resolved yet, but there is ample evidence of dysregulation of immune and autonomic nervous systems.2, 3 The difficulties of diagnosing of ME/CFS are related to the complexity of symptoms and an absence of validated diagnostic tests available to date.
Endothelial dysfunction (ED) is an established cardiovascular (CV) risk factor that contributes to the development of atherosclerosis and predicts CV events.4 ED describes abnormal function and alteration of endothelial cells leading to reduced vasodilatative response. Diminished availability of nitric oxide and other endothelium relaxing and contracting factors are considered as a pathophysiological mechanism.5, 6 ED is found in various conditions including diabetes mellitus, arterial hypertension, chronic heart failure, and stroke.5, 7, 8, 9 CV symptoms such as autonomic dysregulation, postural hypotension, postural tachycardia syndrome, and reduced cardiac and blood volume have been observed in patients with ME/CFS.2, 10, 11 There is growing evidence that ED is frequent in autoimmune disease and is associated with chronic inflammation.12, 13
There are consistent reports of vascular dysfunction in ME/CFS. Cerebral hypoperfusion was diagnosed by using single photon emission computed tomography and other techniques by various groups.14, 15 Cerebral hypoperfusion has been shown to correlate with fatigue and orthostatic intolerance.16, 17 Previously, ED of large and small arteries was shown using flow‐mediated dilatation.18 A diminished post‐occlusive hyperaemic response of the brachial artery and of forearm skin microcirculation was observed. In contrast, a recent study comparing cytokine levels with various CV parameters including endothelial function assessed by EndoPAT reported no difference in patients with ME/CFS and controls.19 However, another study reported an enhanced acetylcholine‐induced vasodilatory response of the peripheral microvasculature in ME/CFS.20
The aim of the present study was to investigate peripheral endothelial function in patients with ME/CFS and to correlate it with symptom severity and biomarkers. Endothelial function was assessed by non‐invasive finger plethysmograph EndoPAT2000. Using this method, changes of peripheral arterial tone in the forefinger are assessed as a response to reactive hyperaemia induced by forearm ischaemia.21, 22
Materials and methods
Study participants
Thirty‐five patients with post‐infectious ME/CFS diagnosed at the Institute of Medical Immunology at the Charité Berlin between 2014 and 2016 were included. Diagnosis of ME/CFS was based on Canadian Criteria1 and exclusion of other medical or neurological diseases, which may cause fatigue. Patients had chronic disease ranging from <1 to 40 years. Nine patients with elevated β2‐adrenergic receptor antibodies participated in a prospective clinical study of immunoadsorption performed at five consecutive days to remove IgG from plasma as described previously.23 Twenty healthy subjects of similar age (range 24–75 years) and body mass index were used as a control group. The study was approved by the Ethics Committee of Charité ‐ Universitätsmedizin Berlin in accordance with the 1964 Declaration of Helsinki and its later amendments. All study participants gave written informed consent.
Assessment of symptoms
The presence and severity of symptoms was assessed using a questionnaire developed by Fluge et al.24 based on the Canadian Criteria. Symptoms were classified quantitatively according to a scale ranging from ‘1’ to ‘10’, there ‘1’ indicates no symptoms and ‘10’ indicates very severe symptoms. The fatigue score was calculated as the mean of fatigue, malaise after exertion, demand for breaks in daily life and daily functioning, cognitive score as mean of memory disturbance, concentration ability and mental tiredness and immune score as mean of painful lymph nodes, sore throat, and flu‐like symptoms. Furthermore, the questionnaire assessed muscle pain and the cumulative severity of reported symptoms (disease severity). Symptom classification was based on patient's self‐report. Symptoms of autonomic dysfunction were assessed by the Composite Autonomic Symptom Score (COMPASS‐31) questionnaire25 examining autonomic functions in the domains of orthostatic, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor regulation. Based on these domains, a weighted total score ranging from ‘0’ indicating no to ‘100’ indicating severe symptoms of autonomic dysfunction was calculated. In addition, disease severity was examined by Bell score focusing on the level of restriction in daily functioning.26 Completely bedridden patients are classified as ‘0’, and patients with unrestricted daily functioning as ‘100’.
Assessment of endothelial function
Peripheral endothelial function was evaluated by the peripheral arterial tonometry (PAT) device (EndoPAT2000, Itamar, Israel). Assessments were performed at baseline under standardized conditions after at least 15 min of supine rest in a quiet, air‐conditioned room. EndoPAT quantifies the endothelium‐mediated changes in vascular tone, elicited by a 5 min occlusion of the brachial artery using a standard blood pressure cuff. When the cuff is released, the surge of blood flow causes a dilatation. The post‐occlusion dilatation in relation to pre‐occlusion is calculated as reactive hyperaemia index (RHI). ED was defined as RHI ≤1.81 based on previous cohort studies.5, 27 In patients who were treated by immunoadsorption,23 peripheral endothelial function was additionally assessed at 12 months follow‐up.
Blood sampling
Venous blood samples were obtained in all patients at a morning visit to the outpatient clinic. Standard biochemical parameters were assessed by routine laboratory measurement. Soluble vascular cell adhesion molecule (sVCAM) and soluble intercellular adhesion molecule (sICAM) were assessed using ELISA technology (R&D Systems, Minneapolis).
Statistical analysis
Statistical data analyses were performed using IBM SPSS Statistics 22.0 and the software GraphPad Prism 6.0. For variables with n > 30, parametric tests were used according to central limit theorem. Variables with n < 30 were tested for normal distribution using Shapiro–Wilk test. All data were presented as mean ± standard deviation, median (interquartile range), median (range), or percentage as appropriate. For comparative analysis, unpaired t‐test was used. Not normally distributed parameters were logarithmically transformed (ln). Correlation analysis was performed using parametric Pearson coefficient and non‐parametric Spearman coefficient as appropriate to distribution. Analysis of bivariate parameters was performed using χ 2‐test. A one‐tailed P‐value of <0.05 was considered statistically significant for clinical scores, and a two‐tailed P‐value of <0.05 for laboratory parameters. Due to multiple testing, P‐values are descriptive.
Results
Clinical characteristics of the study groups are presented in Table 1. There was no difference between the ME/CFS patients and control subjects regarding demographic and clinical characteristics except heart rate, albumin, and creatinine (Table 1). However, RHI was significantly reduced in patients compared with healthy controls (1.95 ± 0.47 vs. 2.21 ± 0.37, P < 0.05, Figure 1 A).
Table 1.
Baseline characteristics and biochemical parameters of the study groups
| Parameter |
Controls N = 20 |
All patients N = 35 |
P‐value |
RHI > 1.81 N = 17 |
RHI ≤ 1.81 N = 18 |
P‐value |
|---|---|---|---|---|---|---|
| Age, mean ± SD (years) | 48 ± 14 | 42 ± 11 | 0.9 | 43 ± 14 | 41 ± 9 | 0.8 |
| Female sex, N (%) | 10 (50) | 24 (69) | 0.2 | 13 (77) | 11 (61) | 0.3 |
| Body mass index, mean ± SD (kg/m2) | 24.4 ± 3.3 | 23.8 ± 4.2 | 0.6 | 24.4 ± 4.2 | 23.2 ± 4.3 | 0.4 |
| Heart rate, mean ± SD (b.p.m.) | 61 ± 8 | 68 ± 13 | 0.03 | 68 ± 15 | 68 ± 10 | 0.9 |
| Systolic blood pressure, mean ± SD (mmHg) | 123 ± 14 | 126 ± 15 | 0.5 | 128 ± 19 | 124 ± 11 | 0.4 |
| Diastolic blood pressure, mean ± SD (mmHg) | 74 ± 8 | 79 ± 12 | 0.1 | 79 ± 14 | 79 ± 9 | 1.0 |
| Biochemical parameters | ||||||
| C‐reactive protein, mean ± SD (mg/dL) | 1.5 (0.7–2.2) | 0.6 (0.3–1.2) | 0.9 | 0.6 (0.3–3.3) | 0.7 (0.3–1.1) | 1.0 |
| Albumin, mean ± SD (g/L) | 38.6 ± 3.1 | 45.2 ± 3.0 | <0.001 | 45.0 ± 3.3 | 45.3 ± 2.8 | 0.8 |
| Haemoglobin, mean ± SD (g/dL) | 13.6 ± 0.8 | 14.2 ± 1.3 | 0.2 | 14.2 ± 1.1 | 14.2 ± 1.4 | 1.0 |
| Creatinine, mean ± SD (mg/dL) | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.01 | 0.8 ± 0.1 | 0.79 ± 0.11 | 0.8 |
| Aspartate transaminase, mean ± SD (U/L) | 26.6 ± 8.2 | 25.4 ± 7.3 | 0.5 | 25.4 ± 8.0 | 24.4 ± 6.9 | 1.0 |
| Alanine transaminase, mean ± SD (U/L) | 21.2 ± 10.9 | 26.7 ± 17.1 | 0.3 | 24.8 ± 19.7 | 28.4 ± 14.8 | 0.6 |
| Gamma‐glutamyltransferase, mean ± SD (U/L) | 23.9 ± 13.8 | 21.5 ± 21.1 | 0.3 | 23.5 ± 25.4 | 19.5 ± 15.0 | 0.6 |
| Haemoglobin A1c, median (IQR) [ln (%)] | 5.3 ± 0.4 | 5.4 ± 1.0 | 0.9 | 5.4 ± 0.6 | 5.4 ± 1.3 | 1.0 |
IQR, interquartile range; RHI, reactive hyperaemia index; SD, standard deviation.
Figure 1.
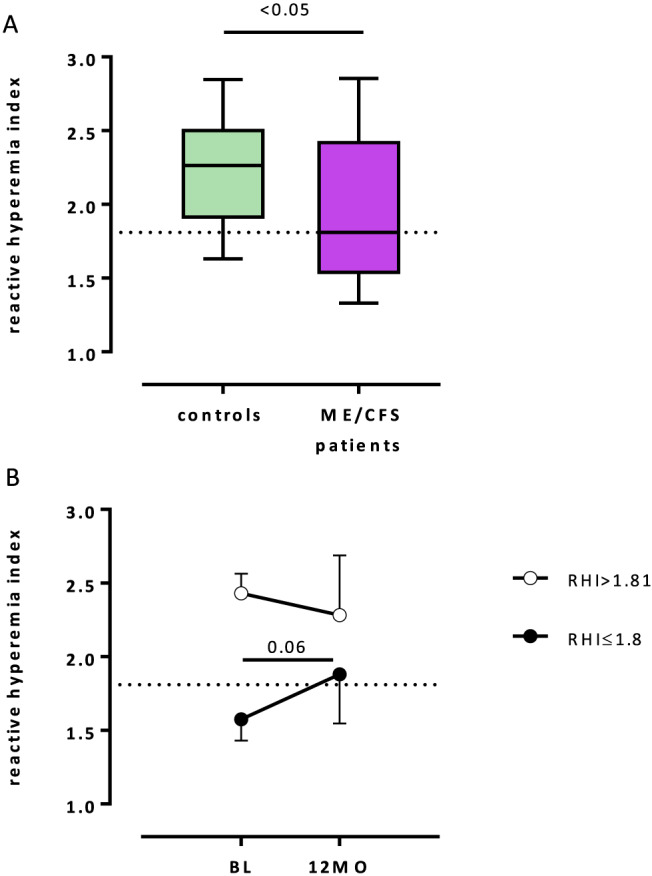
(A) Reactive hyperaemia index (RHI) was assessed in 35 patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and 20 healthy subjects by peripheral arterial tonometry (EndoPAT2000). Significantly reduced RHI was observed in patients compared with the healthy controls. (B) RHI was assessed in nine patients with ME/CFS at baseline (BL) before treatment with one cycle of immunoadsorption and at 12 months. Endothelial dysfunction defined as a diminished RHI ≤1.81 was found in six patients at BL (black circle). Patients with endothelial dysfunction showed improvement of RHI at 12 months as a trend (P = 0.06).
Disease‐specific characteristics of the patient cohort are presented in Table 2. Median disease duration was 5 years (range <1–40), and median disease severity according to the Bell score was 35 (range 10–70). Peripheral ED as defined by RHI ≤1.81 was observed in 18 patients and in 4 healthy subjects (51% vs. 20%, P < 0.05, respectively). Accordingly, patients were divided into two subgroups. Patients with ED showed more severe disease as assessed by Bell score (median 30 vs. 40) and by symptom score (mean 8.6 vs. 7.0) compared with patients with normal endothelial function (both P < 0.05, Table 2). In addition, patients with ED reported more severe fatigue‐related symptoms (mean 8.62 vs. 7.75, P < 0.05) compared with patients with normal endothelial function. None of the biochemical parameters and immunological markers except basal thyrotropin were significantly different in patients with normal peripheral endothelial function and patients with ED (Tables 1 and 2).
Table 2.
Symptom scores and disease‐specific and immunological characteristics of the myalgic encephalomyelitis/chronic fatigue syndrome patients
| Parameter | All patients | N | RHI > 1.81 | N | RHI ≤ 1.81 | N | P‐value |
|---|---|---|---|---|---|---|---|
| Infectious disease onset, N (%) | 25 (71.4) | 35 | 12 (70.6) | 17 | 13 (72.2) | 18 | 0.9 |
| Disease duration, mean ± SD (years) | 8a ± 8 | 35 | 8a ± 1 | 17 | 7a ± 5 | 18 | 0.6 |
| Recurrent respiratory tract infections, N (%) | 22 (64.7) | 34 | 9 (52.9) | 17 | 13 (76.5) | 17 | 0.2 |
| Bell score, median (IQR) | 35 (20–41) | 34 | 40 (30–52) | 17 | 30 (20–40) | 17 | 0.04 |
| COMPASS‐31 score, mean ± SD | 47.24 ± 13.83 | 24 | 46.84 ± 12.03 | 10 | 47.53 ± 15.43 | 14 | 0.5 |
| Symptom scores | |||||||
| Fatigue score, mean ± SD | 8.24 ± 1.19 | 23 | 7.75 ± 1.40 | 10 | 8.62 ± 0.87 | 13 | 0.04 |
| Fatigue, mean ± SD | 7.83 ± 1.53 | 23 | 7.40 ± 1.43 | 10 | 8.15 ± 1.57 | 13 | 0.1 |
| Post‐exertion malaise, median (IQR) (ln) | 9.0 (8.0–10.0) | 23 | 9.00 (6.75–9.25) | 10 | 9.0 (8.0–10.0) | 13 | 0.1 |
| Impaired daily functioning, median (IQR) (ln) | 9.0 (8.0–9.0) | 23 | 8.5 (7.75–9.25) | 10 | 9.0 (8.0–9.0) | 13 | 0.1 |
| Demand for breaks, median (IQR) (ln) | 8.0 (7.0–10.0) | 23 | 7.5 (6.0–9.25) | 10 | 9.0 (7.0–10.0) | 13 | 0.04 |
| Immune score, mean ± SD | 5.43 ± 2.71 | 23 | 4.43 ± 2.69 | 10 | 6.2 ± 2.6 | 13 | 0.06 |
| Flu‐like symptoms, median (IQR) (ln) | 7.0 (6.0–8.0) | 23 | 7.0 (5.25–7.25) | 10 | 8.0 (6.0–9.0) | 13 | 0.3 |
| Sore throat, median (IQR) (ln) | 5.0 (1.0–7.0) | 23 | 3.0 (0.75–6.5) | 10 | 6.0 (3.5–8.5) | 13 | 0.2 |
| Painful lymph nodes, mean ± SD | 4.7 ± 3.1 | 23 | 3.8 ± 3.1 | 10 | 5.5 ± 2.9 | 13 | 0.1 |
| Cognitive score, median (IQR) (ln) | 7.66 (6.0–8.0) | 23 | 7.33 (5.75–8.0) | 10 | 7.66 (6.33–8.17) | 13 | 0.4 |
| Muscle pain, median (IQR) (ln) | 7.5 (5.0–9.0) | 22 | 8.00 (5.00–8.5) | 9 | 6.0 (4.0–9.5) | 13 | 0.2 |
| Disease severity, mean ± SD | 8.2 ± 1.4 | 18 | 7.0 ± 1.7 | 5 | 8.6 ± 1.0 | 13 | 0.02 |
| Immunological markers | |||||||
| Basal thyrotropin, mean ± SD (mU/L) | 1.70 ± 0.87 | 26 | 1.3 ± 0.8 | 12 | 2.04 ± 0.81 | 14 | 0.03 |
| Immunoglobulin G, mean ± SD (g/L) | 9.75 ± 2.22 | 34 | 9.95 ± 2.75 | 17 | 9.95 ± 2.75 | 17 | 0.6 |
| Immunoglobulin M, mean ± SD (g/L) | 1.32 ± 1.01 | 34 | 1.56 ± 1.19 | 17 | 1.56 ± 1.19 | 17 | 0.2 |
| Immunoglobulin A, mean ± SD (g/L) | 1.93 ± 0.74 | 34 | 1.89 ± 0.65 | 17 | 1.89 ± 0.65 | 17 | 0.8 |
| Interleukin‐8, mean ± SD (pg/mL) | 117.8 ± 48.0 | 32 | 109.1 ± 48.4 | 15 | 125.4 ± 47.8 | 17 | 0.3 |
| Soluble interleukin‐2 receptor, median (IQR) [ln (IU/mL)] | 357 (274–466) | 24 | 422 (293–478) | 12 | 325 (261–382) | 12 | 0.9 |
| Soluble VCAM, mean ± SD (ng/mL) | 132.9 ± 35.0 | 35 | 137.3 ± 28.5 | 17 | 137.3 ± 28.5 | 18 | 0.5 |
| Soluble ICAM, mean ± SD (ng/mL) | 54.3 ± 14.3 | 35 | 55.0 ± 12.4 | 17 | 55.0 ± 12.4 | 18 | 0.8 |
| β2‐adrenergic receptor antibody, mean ± SD (U/mL) | 9.21 ± 9.57 | 34 | 9.6 ± 10.3 | 17 | 9.6 ± 10.3 | 17 | 0.8 |
| M3 acetylcholine receptor antibody, mean ± SD (U/mL) | 7.04 ± 10.41 | 34 | 8.4 ± 13.1 | 17 | 8.4 ± 13.1 | 17 | 0.5 |
ICAM, intercellular adhesion molecule; IQR, interquartile range; RHI, reactive hyperaemia index; SD, standard deviation; VCAM, vascular cell adhesion molecule.
Correlation analyses
In the whole patient cohort, a correlation was found between lower RHI and more severe disease according to symptom score (r = −0.59, P = 0.005) and Bell score (r = −0.41, P = 0.008), more severe immune symptoms (r = −0.41, P = 0.026) including sore throat (r = −0.38, P = 0.038), painful lymph nodes (r = −0.39, P = 0.042), and flu‐like symptoms (r = −0.31, P = 0.073) as a trend (Figure 2 ). No significant correlation was found neither with cognitive scores, muscle pain, nor with COMPASS‐31 score assessing autonomous dysfunction (not shown). Further, age, sex, infectious disease onset, and disease duration did not correlate with RHI.
Figure 2.
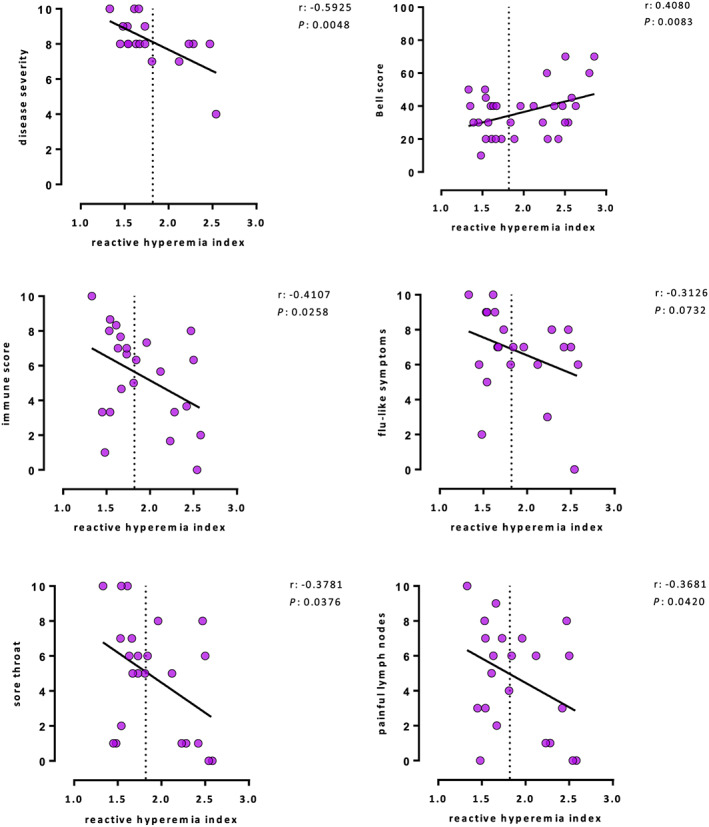
Patients with endothelial dysfunction reported more severe disease assessed by a disease severity score and Bell score and a significantly higher immune symptom score including sore throat and painful lymph nodes, as well as a tendency for more severe flu‐like symptoms.
Soluble intercellular adhesion molecule and soluble vascular cell adhesion molecule
No significant differences for sVCAM levels and sICAM levels between ME/CFS patients and healthy controls were observed (not shown). Further, levels of both adhesion molecules were not different between patients with normal and diminished RHI of ≤1.81 (Table 2).
Peripheral endothelial function after immunoadsorption
Nine patients with elevated levels of autoantibodies against β2‐adrenergic receptor underwent immunoadsorption. In five of six patients with peripheral ED at baseline, endothelial function was normalized at 12 months follow‐up (RHI 1.58 ± 0.15 vs. 2.02 ± 0.46, P = 0.06), while in three patients with normal endothelial function before treatment, no significant change was observed over 12 months (Figure 1 B).
Discussion
Our study showed that half of the patients with ME/CFS had diminished RHI indicating the presence of peripheral ED. We found a correlation between the peripheral ED and disease severity as well as severity of immune symptoms. Further, we have first evidence that peripheral ED in patients, who had elevated levels of autoantibodies against β2‐adrenergic receptor, can improve following immunoadsorption.
Our results of ED in ME/CFS shown by finger plethysmography are in line with a previous study by Newton et al.18 They had shown a significantly diminished post‐occlusive hyperaemic response of the brachial artery and of forearm skin microcirculation compared with healthy controls. In a recent study by Moneghetti and colleagues comparing cytokine levels with various CV parameters, a trend of diminished endothelial function assessed by EndoPAT in patients with ME/CFS compared with controls was observed.19
Endothelial dysfunction is known as an important risk factor for CV events. Several studies have shown peripheral ED in patients with various diseases including chronic heart failure, stroke, and pulmonary arterial hypertension based on EndoPAT measurements of RHI.5, 28, 29, 30
ED is characterized by the incapability of endothelial cells in the regulation of vascular tone. Besides traditional CV risk factors inflammatory cytokines, reactive oxygen species, and anti‐endothelial autoantibodies can activate endothelial cells, leading to ED.31 No evidence for an association of ED with pro‐inflammatory or metabolic biomarker was found in the present study. Further, no association between soluble endothelial adhesion molecules sVCAM and sICAM with ED was observed. sICAM was shown to correlate with atherosclerosis and sVCAM with endothelial dysregulation in aged persons.32 Previously, we showed that levels of the long non‐coding RNA myocardial infarction‐associated transcript were significantly elevated in ME/CFS patients as compared with healthy controls.33 Myocardial infarction‐associated transcript regulates ED and can be up‐regulated by inflammatory stimuli.34
We observed, however, a relation between immune symptoms and endothelial function in our study. Indeed, ME/CFS patients with lower RHI reported more severe immune‐associated symptoms and more severe disease. Although the pathophysiological mechanism of the immune symptoms in ME/CFS is not well understood yet, there is evidence that it is a hallmark symptom of the disease. A recent study by Ohanian et al. showed that immune symptoms were the best discriminating symptoms of ME/CFS and MS‐related fatigue.35 In many patients, immune symptoms occur or increase after exertion. Our findings suggest that the pathomechanism of immune symptoms is related to ED. This is in line with studies showing increased oxidative stress, nitric oxide, and a pro‐inflammatory phenotype in ED.36 It has been shown that exercise can amplify pre‐existing immune abnormalities in ME/CFS. Studies have reported exercise in ME/CFS patients resulting in enhanced production of cytokines, C4 complement activation, and enhanced oxidative stress.37, 38 Further, there may be a direct link between fatigue and ED. In a study in apparently healthy subjects without risk factors for CV disease, it was shown that ED is associated with the level of subjective fatigue.39
Finally, we observed a normalization of endothelial function in patients with elevated levels of β2‐adrenergic antibodies who underwent immunoadsorption. There is evidence from experimental studies indicating a role of β‐adrenergic autoantibodies in the development of ED.40, 41
In conclusion, the presence of ED in ME/CFS as a major risk factor for CV disease is in accordance with reports of an earlier CV mortality reported in these patients.42 Therefore, ED may be a prognostic marker to assess risk of CV morbidity and mortality in ME/CFS, which needs to be further analysed in clinical trials. Furthermore, it is important to elucidate the mechanisms leading to ED in order to understand its role in the pathomechanism of ME/CFS.
Limitations
The present study is limited by a relatively small sample size: 35 patients and 20 controls of similar age and body mass index were included. However, this observational clinical trial has a sample size similar to other comparable studies described in the literature.18, 20 In our study, the presence of ED was shown in half of the patients. Our results clearly showed a significantly higher RHI in the control group compared with the patients. In addition, the presence of ED was observed more frequently in patients with ME/CFS as in the control group (51% vs. 20%, P < 0.05, respectively). The results of our clinical trial should be confirmed in future studies with a larger sample size.
Conflict of interest
None declared.
Funding
M.S. receives a scholarship from the Lost Voices Foundation e.V. (sponsored by Gabriele Spreter). This work was supported by a grant from the Weidenhammer‐Zöbele Foundation.
Scherbakov, N. , Szklarski, M. , Hartwig, J. , Sotzny, F. , Lorenz, S. , Meyer, A. , Grabowski, P. , Doehner, W. , and Scheibenbogen, C. (2020) Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Failure, 7: 1064–1071. 10.1002/ehf2.12633.
References
- 1. Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles AC, Speight N, Vallings R, Bateman L, Baumgarten‐Austrheim B, Bell DS, Carlo‐Stella N, Chia J, Darragh A, Jo D, Lewis D, Light AR, Marshall‐Gradisnik S, Mena I, Mikovits JA, Miwa K, Murovska M, Pall ML, Stevens S. Myalgic encephalomyelitis: international consensus criteria. J Intern Med 2011; 270: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newton JL, Okonkwo O, Sutcliffe K, Seth A, Shin J, Jones DE. Symptoms of autonomic dysfunction in chronic fatigue syndrome. QJM 2007; 100: 519–526. [DOI] [PubMed] [Google Scholar]
- 3. Sotzny F, Blanco J, Capelli E, Castro‐Marrero J, Steiner S, Murovska M. Scheibenbogen C; European Network on ME/CFS (EUROMENE). Autoimmun Rev 2018; 17: 601–609. [DOI] [PubMed] [Google Scholar]
- 4. Gimbrone MA Jr, García‐Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 2016; 118: 620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scherbakov N, Sandek A, Martens‐Lobenhoffer J, Kung T, Turhan G, Liman T, Ebinger M, von Haehling S, Bode‐Böger SM, Endres M, Doehner W. Endothelial dysfunction of the peripheral vascular bed in the acute phase after ischemic stroke. Cerebrovasc Dis 2012; 33: 37–46. [DOI] [PubMed] [Google Scholar]
- 6. Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol (Oxf) 2017; 219: 22–96. [DOI] [PubMed] [Google Scholar]
- 7. Dos Santos MR, Saitoh M, Ebner N, Valentova M, Konishi M, Ishida J, Emami A, Springer J, Sandek A, Doehner W, Anker SD, von Haehling S. Sarcopenia and endothelial function in patients with chronic heart failure: results from the Studies Investigating Comorbidities Aggravating Heart Failure (SICA‐HF). J Am Med Dir Assoc 2017; 18: 240–245. [DOI] [PubMed] [Google Scholar]
- 8. Giraldo‐Grueso M, Echeverri D. From endothelial dysfunction to arterial stiffness in diabetes mellitus. Curr Diabetes Rev 2018; 14 10.2174/1573399814666181017120415. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9. Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev Cardiovasc Ther 2018; 16: 879–887. [DOI] [PubMed] [Google Scholar]
- 10. Schondorf R, Benoit J, Wein T, Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst 1999; 75: 192–201. [DOI] [PubMed] [Google Scholar]
- 11. Newton JL, Finkelmeyer A, Petrides G, Frith J, Hodgson T, Maclachlan L, MacGowan G, Blamire AM. Reduced cardiac volumes in chronic fatigue syndrome associate with plasma volume but not length of disease: a cohort study. Open Heart 2016; 3: e000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steyers CM 3rd, Miller FJ Jr. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 2014; 15: 11324–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moroni L, Selmi C, Angelini C, Meroni PL. Evaluation of endothelial function by flow‐mediated dilation: a comprehensive review in rheumatic disease. Arch Immunol Ther Exp (Warsz) 2017; 65: 463–475. [DOI] [PubMed] [Google Scholar]
- 14. Boissoneault J, Letzen J, Lai S, O'Shea A, Craggs J, Robinson ME, Staud R. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: an arterial spin‐labeling fMRI study. Magn Reson Imaging 2016; 34: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Staud R, Boissoneault J, Craggs JG, Lai S, Robinson ME. Task related cerebral blood flow changes of patients with chronic fatigue syndrome: an arterial spin labeling study. Fatigue 2018; 6: 63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 1998; 29: 1876–1881. [DOI] [PubMed] [Google Scholar]
- 17. Boissoneault J, Letzen J, Robinson M, Staud R. Cerebral blood flow and heart rate variability predict fatigue severity in patients with chronic fatigue syndrome. Brain Imaging Behav 2018; 13: 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newton DJ, Kennedy G, Chan KK, Lang CC, Belch JJ, Khan F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int J Cardiol 2012; 154: 335–336. [DOI] [PubMed] [Google Scholar]
- 19. Moneghetti KJ, Skhiri M, Contrepois K, Kobayashi Y, Maecker H, Davis M, Snyder M, Haddad F, Montoya JG. Value of circulating cytokine profiling during submaximal exercise testing in myalgic encephalomyelitis/chronic fatigue syndrome. Sci Rep 2018; 8: 2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan F, Spence V, Kennedy G, Belch JJ. Prolonged acetylcholine‐induced vasodilatation in the peripheral microcirculation of patients with chronic fatigue syndrome. Clin Physiol Funct Imaging 2003; 23: 282–285. [DOI] [PubMed] [Google Scholar]
- 21. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH. Udelson JE Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 2003; 146: 168–174. [DOI] [PubMed] [Google Scholar]
- 22. Truschel E, Jarczok MN, Fischer JE, Terris DD. High‐throughput ambulatory assessment of digital reactive hyperemia: concurrent validity with known cardiovascular risk factors and potential confounding. Prev Med 2009; 49: 468–472. [DOI] [PubMed] [Google Scholar]
- 23. Scheibenbogen C, Loebel M, Freitag H, Krueger A, Bauer S, Antelmann M, Doehner W, Scherbakov N, Heidecke H, Reinke P, Volk HD, Grabowski P. Immunoadsorption to remove ß2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLoS ONE 2018; 13: e0193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fluge Ø, Risa K, Lunde S, Alme K, Rekeland IG, Sapkota D, Kristoffersen EK, Sørland K, Bruland O, Dahl O, Mella O. B‐lymphocyte depletion in myalgic encephalopathy/chronic fatigue syndrome. An Open‐Label Phase II Study with Rituximab Maintenance Treatment. PLoS ONE 2015; 10: e0129898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 2012; 87: 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bell DS. The doctor's guide to chronic fatigue syndrome: understanding, treating and living with CFIDS. Boston: Da Capo Lifelong Books; 1995. [Google Scholar]
- 27. Scherbakov N, Sandek A, Ebner N, Valentova M, Nave AH, Jankowska EA, Schefold JC, von Haehling S, Anker SD, Fietze I, Fiebach JB, Haeusler KG, Doehner W. Sleep‐disordered breathing in acute ischemic stroke: a mechanistic link to peripheral endothelial dysfunction. J Am Heart Assoc 2017; 6 pii: e006010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peled N, Bendayan D, Shitrit D, Fox B, Yehoshua L, Kramer MR. Peripheral endothelial dysfunction in patients with pulmonary arterial hypertension. Respir Med 2008; 102: 1791–1796. [DOI] [PubMed] [Google Scholar]
- 29. Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008; 117: 2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 2010; 31: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 31. Physiology of the endothelium , Galley HF, Webster NR. Br J Anaesth 2004; 93: 105–113. [DOI] [PubMed] [Google Scholar]
- 32. Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM Jr, Boerwinkle E. Circulating adhesion molecules VCAM‐1, ICAM‐1, and E‐selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997; 96: 4219–4225. [DOI] [PubMed] [Google Scholar]
- 33. Yang CA, Bauer S, Ho YC, Sotzny F, Chang JG, Scheibenbogen C. The expression signature of very long non‐coding RNA in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med 2018; 16: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, Jiang Q. lncRNA‐MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res 2015; 116: 1143–1156. [DOI] [PubMed] [Google Scholar]
- 35. Ohanian D, Brown A, Sunnquist M, Furst J, Nicholson L, Klebek L, Jason LA. Identifying key symptoms differentiating myalgic encephalomyelitis and chronic fatigue syndrome from multiple sclerosis. Neurology (ECronicon) 2016; 4: 41–45. [PMC free article] [PubMed] [Google Scholar]
- 36. Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 2018; 100: 1–19. [DOI] [PubMed] [Google Scholar]
- 37. Light AR, Bateman L, Jo D, Hughen RW, Vanhaitsma TA, White AT, Light KC. Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. J Intern Med 2012; 271: 64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nijs J, Nees A, Paul L, De Kooning M, Ickmans K, Meeus M, Van Oosterwijck J. Altered immune response to exercise in patients with chronic fatigue syndrome/myalgic encephalomyelitis: a systematic literature review. Exerc Immunol Rev 2014; 20: 94–116 Review. [PubMed] [Google Scholar]
- 39. Ohno Y, Hashiguchi T, Maenosono R, Yamashita H, Taira Y, Minowa K, Yamashita Y, Kato Y, Kawahara K, Maruyama I. The diagnostic value of endothelial function as a potential sensor of fatigue in health. Vasc Health Risk Manag 2010; 24: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abdelkrim MA, Leonetti D, Montaudon E, Chatagnon G, Gogny M, Desfontis JC, Noireaud J, Mallem MY. Antibodies against the second extracellular loop of β₁‐adrenergic receptors induce endothelial dysfunction in conductance and resistance arteries of the Wistar rat. Int Immunopharmacol 2014; 19: 308–316. [DOI] [PubMed] [Google Scholar]
- 41. Liu X, Tan W, Liu Y, Lin G, Xie C. The role of the β2 adrenergic receptor on endothelial progenitor cells dysfunction of proliferation and migration in chronic obstructive pulmonary disease patients. Expert Opin Ther Targets 2013; 17: 485–500. [DOI] [PubMed] [Google Scholar]
- 42. McManimen SL, Devendorf AR, Brown AA, Moore BC, Moore JH, Jason LA. Mortality in patients with myalgic encephalomyelitis and chronic fatigue syndrome. Fatigue 2016; 4: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]


