Abstract
Aims
In the coming decade, heart failure (HF) represents a major global healthcare challenge due to an ageing population and rising prevalence combined with scarcity of medical resources and increasing healthcare costs. A transitional care strategy within the period of clinical worsening of HF before hospitalization may offer a solution to prevent hospitalization. The outpatient treatment of worsening HF with intravenous or subcutaneous diuretics as an alternative strategy for hospitalization has been described in the literature.
Methods and results
In this systematic review, the available evidence for the efficacy and safety of outpatient treatment with intravenous or subcutaneous diuretics of patients with worsening HF is analysed. A search was performed in the electronic databases MEDLINE and EMBASE. Of the 11 included studies 10 were single‐centre, using non‐randomized, observational registries of treatment with intravenous or subcutaneous diuretics for patients with worsening HF with highly variable selection criteria, baseline characteristics, and treatment design. One study was a randomized study comparing subcutaneous furosemide with intravenous furosemide. In a total of 984 unique individual patients treated in the reviewed studies, only a few adverse events were reported. Re‐hospitalization rates for HF at 30 and 180 days were 28 and 46%, respectively. All‐cause re‐hospitalization rates at 30 and 60 days were 18–37 and 22%, respectively. The highest HF re‐hospitalization was 52% in 30 days in the subcutaneous diuretic group and 42% in 30 days in the intravenous diuretic group.
Conclusions
The reviewed studies present practice‐based results of treatment of patients with worsening HF with intravenous or subcutaneous diuretics in an outpatient HF care unit and report that it is effective by relieving symptoms with a low risk of adverse events. The studies do not provide satisfactory evidence for reduction in rates of re‐hospitalization or improvement in mortality or quality of life. The conclusions drawn from these studies are limited by the quality of the individual studies. Prospective randomized studies are needed to determine the safety and effectiveness of outpatient intravenous or subcutaneous diuretic treatment for patient with worsening HF.
Keywords: Acute heart failure, Worsening heart failure, Outpatient treatment, Ambulatory treatment, Intravenous diuretics, Subcutaneous diuretics
Introduction
In the coming decade, heart failure (HF) represents a major global healthcare challenge as due to an ageing of the population1 and rising prevalence. The outcome of HF is dismal, despite advances in treatment options.2 Worldwide, the prevalence of HF is estimated to be approximately 60 million patients. Annually, in the United States and Europe, more than one million patients are hospitalized with HF.3, 4 Approximately, 50% of these patients are readmitted within 6 months of discharge, and almost 30% die within 1 year.5, 6 Approximately, 1–2% of the total national healthcare budget in the developed society is consumed by HF, mostly due to hospitalizations.1
HF is a progressive condition. For most patients with HF, the clinical course is characterized by clinical stability interrupted by episodes of worsening of symptoms.7 While some hospitalizations are due to acute HF, most patients with chronic HF have worsening signs and symptoms after a period of clinical stability that requires escalation of therapy (worsening HF).8, 9 Worsening HF is associated with markedly worse prognosis and diminished quality of life.10 Signs and symptoms of congestion are the main reason why patients with worsening and acute HF seek urgent care.11 Current HF guidelines therefore recommend intravenous loop diuretics as starting treatment to alleviate signs and symptoms of congestion.12 The majority of patients with acute decompensated HF are hospitalized and treated with intravenous diuretics.13
In order to improve quality of care for individual HF patients, healthcare professionals are trying to improve HF management by looking for effective, value‐based alternative treatment strategies that would avoid HF hospitalization. The extended period of clinical worsening before hospitalization offers a time window for intervention before hospitalization would be required.9, 14 One of these interventions is the outpatient treatment of worsening HF with intravenous or subcutaneous diuretics as an alternative strategy for hospitalization15 (Figure 1 ). In this systematic review, the available data for efficacy and safety of the outpatient treatment with intravenous diuretics or subcutaneous of patients are described and critically appraised.
Figure 1.
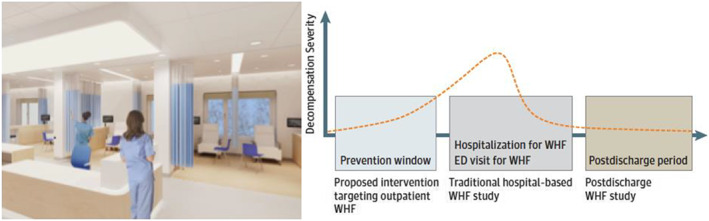
Example of an outpatient treatment centre for patients with worsening heart failure (left) and schematic representation of time course of cardiac decompensation (right, with permission of JAMA Cardiology9). ED, emergency department; WHF, worsening heart failure.
Methods
Objectives
This systematic review aims to determine the efficacy of outpatient treatment with intravenous or subcutaneous diuretics compared to standard of care comprised of hospitalization with intravenous diuretics. Studies were searched on non‐hospitalized patients with HF with reduced ejection fraction (EF) (HFrEF) or HF with preserved EF (HFpEF) with signs and symptoms of worsening HF who were treated with intravenous or subcutaneous diuretics in an outpatient setting. The outcomes of interest were efficacy [symptomatology, New York Heart Association (NYHA) classification] and HF and total re‐hospitalization (primary endpoint in the analysis). Safety of the intervention (adverse events) and all‐cause mortality were regarded as secondary endpoint. In addition, patient‐reported outcome measures were of interest (quality of life by validated questionnaire).
Registration
This review was registered at PROSPERO (International prospective register of systematic reviews) in 2018 ahead of data extraction (registration number CRD42019118821). The protocol is available upon request.
Literature search strategy
A search was performed in the electronic Ovid databases MEDLINE and EMBASE using non‐controlled vocabulary search terms and the following controlled vocabulary indexation terms (Medical Subject Headings, MeSH for MEDLINE, and Emtree for EMBASE): Heart Failure, Diuretics, Outpatients, intravenous, and subcutaneous. The search strategy was developed with the support of a medical information specialist to ensure a comprehensive approach incorporating all key search terms. An overview of the complete search strategy is available upon request. Eligibility criteria for inclusion in this review required that studies were full‐text articles written in English, studies in humans conducted in adults with acute or worsening HF, and concerned outpatient treatment with intravenous or subcutaneous diuretics. We included studies without limitation on sample size (no case reports, n > 1) or follow‐up duration. Excluded were studies with treatment in the emergency department or observation units or review studies or studies where only the abstract was available. References were checked from the studies out of the search strategy and a citation index search was performed in Web of Science. There are currently two trials studying this subject registered at http://ClinicalTrials.gov, including the SUBQ‐HF trial (Subcutaneous Furosemide in Acute Decompensated Heart Failure, NCT03170219).
Study selection and data extraction
Titles and/or abstracts of studies retrieved using the search strategy were independently screened by two reviewing authors (E. W., Y. dR.) to identify studies that potentially met the inclusion criteria. Full texts of relevant studies was retrieved and independently assessed for eligibility. Eighty‐one articles were excluded, 11 studies were selected to be of sufficient quality to be included in this systematic review for data extraction. See Figure 2 for a flowchart of article selection on eligibility and inclusion of studies for data extraction. The eligible studies were used to plan a narrative descriptive synthesis with patient data structured around patient characteristics, selection criteria, study protocol, and treatment regimens and treatment outcome. Data from selected studies were extracted by single data extraction with verification (E. W., Y. dR). Patient characteristics, selection criteria, study protocol and treatment regimens, treatment outcome (symptomatology, all‐cause mortality, re‐hospitalizations for HF, and adverse events), and the limitations of these studies were tabulated. To ensure systematic and complete reporting of the studies, the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist was followed that is available upon request.16 The risk of bias and methodological quality of included studies was independently assessed by two reviewing authors (E. W., Y. dR.) using the Quality in Prognosis Studies tool.
Figure 2.
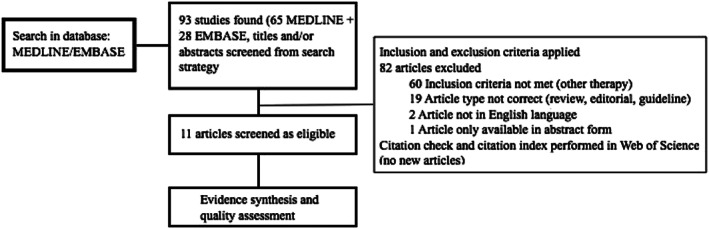
Flowchart of article selection and inclusion process.
Results
Ten of the 11 included studies were single‐centre studies, non‐randomized, observational registries of treatment with intravenous or subcutaneous diuretics for patients with worsening HF with highly variable selection criteria, baseline characteristics, and treatment design. One study was a randomized study comparing subcutaneous furosemide with intravenous furosemide.
Selection criteria
Inclusion criteria did not differ substantially across studies. Most studies included patients with symptoms of HF, either described as hypervolemia, (pulmonary) congestion, decompensated HF, or worsening HF. Inclusion was at the discretion of a healthcare professional. Three studies mentioned specific weight gain in a certain time period that was refractory to uptitration of oral diuretic treatment. One study excluded patients with advanced chronic kidney disease (defined as serum creatinine >200 micromol/l), and four studies excluded patients with an acute significant co‐morbid condition, such as unstable angina pectoris, myocardial infarction, heart rhythm disorder, or hypertensive crisis.
Patient characteristics
The number of patients included in the studies range from 7 to 283 in the study with the largest population. Mean age across the studies ranged from 57 to 75 years. In all the studies, most study participants were male, with 89% male participants as the highest percentage. For five studies that reported EF, mean LVEF ranged from 28–39%. Five studies differentiated between HF with reduced EF (HFrEF) and preserved EF (HFpEF) with 57–94% of the patient having HFrEF. Five studies mentioned that patients with HFrEF had ischemic cardiomyopathy as an underlying aetiology. When mentioned, most patients were in NYHA classification III and IV, although in two studies a considerable number of patients were in NYHA classification II (26 and 44%).17, 18 Only one study reported if patients were on maximally tolerated guideline‐directed medical therapy. An overview of all selection criteria and patient characteristics is shown in Table 1.
Table 1.
Selection criteria and patient characteristics
| Author | Inclusion | Exclusion | Patient# | Age (yr) | Sex | Aetiology | Mean LVEF (%)19 | NYHA | Renal function |
|---|---|---|---|---|---|---|---|---|---|
| Ryder et al., 200820 | Weight gain >2 kg in 2 days and symptoms | NA | 107 | 71 ± 11 | 80% male | 70% HFrEF, 30% HFpEF | 38 ± 15 | 8% II 83% III 8% IV | NA |
| Freimark et al., 200921 | Congestion, recent hospitalization | UAP | 190 | 65 ± 12 | 89% male | 77% iCMP | 25 ± 11 | 100% III and IV | Creatinine 1.7 ± 0.7 mg/ml |
| Hebert et al., 201117 | Weight gain >2.2 kg and symptoms | NA | 130 | 58 ± 13 | 73% male | 35% iCMP | 23 ± 10 | 12% I 26% II 29% III 33% IV | eGFR ml/min 70 |
| Lazkani and Ota 201222 | Weight gain >2.2 kg and symptoms | NA | 7 | NA | 57% male | 57% HFrEF, 43% HFpEF | NA | 100% III | NA |
| Banerjee et al., 201223 | Congestion | Advanced chronic kidney disease | 17 | 70 ± 6 | 71% male | 94% HFrEF, 58% iCMP | NA | NA | NA |
| Makadia et al., 201518 | Pulmonary congestion | NA | 106 | 68 ± 13 | 53% male | 64% HFrEF (iCMP), 36% HFpEF | 39 ± 18 | 3% I 44% II 42% III 11% IV | Creatinine 127 micromol/l |
| Buckley et al., 201624 | Pulmonary congestion | Significant acute comorbid condition | 60 | 70 ± 10 | 57% male | 60% HFrEF, 40% HFpEF |
25% in HFrEF 55% in HFpEF |
12% II 58% III 20% IV 10% unknown | Creatinine 115 micromol/l |
| Al‐Ani et al., 201925 | Hypervolemia | NA | 19 | 65 | 57% male | 27% HFrEF | NA | NA | 60% eGFR <60 ml/min |
| Buckley et al., 201926 | Pulmonary congestion | Significant acute comorbid condition | 283 | 68 ± 14 | 62% male | NA | 49% LVEF <40% | NA | NA |
| Zatarain et al., ,201327 | Decompensated heart failure (s.c. Furosemide) | NA | 24 | 75 ± 10 | 79% male | 38% iCMP | 58% LVEF <45% | 93% III and IV | Creatinine 1.59 ± 0.59 mg/ml |
| Gilotra et al., 201828 | Worsening HF (s.c. Furosemide) | Chance of hospitalization | 41 | 57 ± 13 | 78% male | 69%, HFrEF, 33% HFpEF | 25% (range 15‐55%) |
30% II 60% III 10% IV |
eGFR 62 ± 53 ml/min |
EF, ejection fraction; eGFR, estimated glomerular filtration rate; iCMP, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction;NA, not available; NYHA, New York Heart Failure Association; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HT, hypertension; s.c., subcutaneous; UAP, unstable angina pectoris.
Study protocols and treatment regimens
All of the 11 studies used the loop diuretic furosemide as main intravenous or subcutaneous diuretic treatment in the outpatient setting. In five studies, the oral diuretic dosage prior to ambulatory intravenous or home subcutaneous treatment was specified, with a mean range from 160 to 396 mg. One study used either furosemide or bumetanide22; two studies combined the use of furosemide with metolazone (thiazide diuretic).17, 22 All of the studies with intravenous furosemide used a bolus of furosemide (40–160 mg) either alone or followed by continuous infusion (maximum 240 mg). The total dosage differed considerably between the different study protocols, ranging from 40 to 240 mg. One study mentioned diuretic dosage was chosen at the discretion of the physician.22 One study used a treatment protocol with the chosen dosage based on the used oral diuretic dosage.24 Two studies used a specific dosage of the bolus therapy for all patients.25 All studies with intravenous diuretics in the outpatient setting mentioned the number of visits made to the outpatient clinic. Between 14–45% of patients had multiple outpatient clinic visits. An overview of the study protocols and treatment regimens is shown in Table 2.
Table 2.
Overview of study protocols and treatment regimens
| Author | Diuretic dosage before | Therapy | Treatment regiment | 1 visit | >1 visit | Visit per patient | Length of follow‐up |
|---|---|---|---|---|---|---|---|
| Ryder | NA | Furosemide i.v. | 40‐80 mg bolus | 72% | 28% | NA | NA |
| Freimark | NA | Furosemide i.v. ± metolazone ± inotropic medication | NA | NA | NA | NA | 12 months |
| Hebert | NA | Furosemide i.v. ± metolazone | 40 mg bolus + infusion 160 mg | NA | NA | 1‐14 | 17 months |
| Lazkani | NA | Furosemide/bumetanide i.v. + metolazone | Dosage not mentioned | 86% | 14% | 1‐2 | 30 days |
| Banerjee | NA | Furosemide i.v. | 80‐100 mg bolus | 83% | 17% | 1‐2 | 30 days |
| Makadia | Mean oral 160 mg furosemide | Furosemide ± metolazone | Continuous infusion furosemide (mean 100 mg) | NA | NA | 1‐22 | NA |
| Buckley | Mean oral 240 mg (80‐800 mg) furosemide | Furosemide i.v. | Bolus furosemide and continuous infusion | 55% | 45% | NA | 60 days |
| Al‐Ani | Mean oral 160 mg furosemide or equivalent | Furosemide i.v. | Bolus 80 mg | NA | NA | 30 visits for 19 patients | NA |
| Buckley | Mean 396 mg ± 375 furosemide or equivalent | Furosemide i.v. | Bolus furosemide and continuous infusion | NA | NA | 483 visits for 283 patients | NA |
| Zatarain | NA | Furosemide s.c. | Furosmide continuously s.c., mean 146 mg/day | NA | NA | 41 episodes for 24 patients, mean 9 ± 4 days | NA |
| Gilotra | Mean 246 mg ± 167 mg furosemide | Furosemide s.c. or i.v. | Randomization between fixed single bolus of furosemide s.c. 80 mg and intravenous bolus of 80‐160 mg (mean 123 ± 47 mg) | NA | NA | NA | 30 days |
i.v., intravenous; NA, not available; s.c., subcutaneously
Endpoints and clinical study outcomes
Although the primary and secondary endpoints differed and only one of the studies used re‐hospitalization as primary endpoint, almost all studies described the safety of the intervention. Most studies monitored vital parameters and urine output during intervention and electrolyte status prior to intervention. In a total of 984 unique individual patients treated in the reviewed studies, only a few adverse events were reported (see Table 4). Almost all studies mentioned weight loss, which was between 1.0 and 6.0 kg. Four studies reported a decrease in functional NYHA classification. One study mentioned a significant decrease in BNP.20
Table 4.
Quality assessment of the studies
| Author | Selection bias | Information bias | Bias in the analysis | Quality assessment |
|---|---|---|---|---|
| Ryder |
Exclusion criteria not clear GDMT not mentioned RoB: moderate |
RoB: low | RoB: low | Moderate |
| Freimark | RoB: low |
Dose of study intervention not clear RoB: moderate |
RoB: low | Moderate |
| Hebert |
Exclusion criteria not clear Recruitment period not clear RoB: moderate |
Dose of study intervention not clear RoB: moderate |
Duration of follow‐up not clear RoB: moderate |
Poor |
| Lazkani |
Response rate inadequate RoB: moderate |
RoB: low | RoB: low | Moderate |
| Banerjee |
Inclusion criteria not clear Recruitment period not clear RoB: moderate |
Dose and type of study intervention not clear Number of visits not clear RoB: moderate |
Definition re‐hospitalization not clear Duration of follow‐up not clear RoB: moderate |
Poor |
| Makadia | RoB: low | RoB: low | RoB: low | Good |
| Buckley | RoB: low | RoB: low | RoB: low | Good |
| Al‐Ani |
Mean EF not mentioned RoB: low |
RoB: low | RoB: low | Good |
| Buckley |
Type of HF not mentioned, mean EF not mentioned GDMT not mentioned RoB: moderate |
RoB: low | RoB: low | Moderate |
| Zatarain |
Specific inclusion criteria not mentioned Mean EF not mentioned RoB: moderate |
RoB: low | RoB: low | Moderate |
| Gilotra | Randomized protocol | Good | ||
| RoB: low | RoB: low | RoB: low |
EF, ejection fraction; HF, heart failure; GDMT, guideline‐directed medical therapy; RoB, risk of bias.
With respect to the objective of this systematic review, only one study studied HF re‐hospitalization as a primary endpoint, four studies as one of the secondary endpoints.26 Re‐hospitalization rates for HF at 30 and 180 days amounted 28 and 46% respectively. All‐cause re‐hospitalization rates at 30 and 60 days amounted 18–3723, 24, 25 and 22%,24 respectively. The highest HF re‐hospitalization was demonstrated by Gilotra et al.28 and amounted to 52% in 30 days in the subcutaneous diuretic group and 42% in 30 days in the intravenous diuretic group. An overview of the endpoints and clinical study outcomes is shown in Table 3.
Table 3.
Endpoints and clinical study outcomes
| Ryder | Freimark | Hebert | Lazkani | Banerjee | Makadia | Buckley | Al‐Ani | Buckley | Zatarain | Gilotra | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosage | Furosemide i.v. 40–80 mg | NA | Furosemide i.v. 200 mg | NA | Furosemide i.v.80–100 mg | Furosemide i.v. 100 mg | NA | Furosemide i.v. 80 mg | NA | NA | Furosemide s.c. 146 mg |
| Primary endpoint | Clinical stability | NA | Utilization | NA | Symptoms | Days admission | Weight loss | NA | HF hospitalization | NA | Urine output |
| Secondary endpoint | NA | NA | Safety | NA | Re‐hospitalization | Weight loss | Re‐hospitalization | NA | Mortality | NA | Weight loss |
| HF hospitalization | NA | 0.6 hospitalizations per patient per year (mostly HF) | NA | 0% 30 days | NA | NA |
18% 30 days 22% 60 days |
37% 30 days |
28% 30 days 46% 180 days |
NA | 52% s.c. 42% i.v. 30 days |
| All‐cause hospitalization | NA | 0.6 hospitalizations per patient per year (mostly HF) | NA | NA | 18% 30 days | NA | NA | NA | NA | NA | NA |
| Mortality | NA | 29% 1 year | NA | 0% 30 days | 0% 30 days | NA | 2% 60 days | NA | 3% 30 days, 13% 180 days | NA | NA |
| Adverse events | None | None | None | NA | None | 1 hypotension | None | 1 hypotension, 1 hypokalaemia | 19% hypokalaemia, 14% kidney deterioration | 1 hypokalaemia | 1 hypokalaemia |
| BNP/pro BNP pre–post | BNP 1063–892 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| NYHA pre‐post | 3.0 ± 0.4‐2.5 ± 0.6 | 100%III/IV‐26%II | NA | NA | Half class lower in 71%, one class lower in 24% | NA | NA | NA | NA | NA |
AE, adverse events; HF, heart failure; i.v., intravenous; NA, not available; NYHA, New York Heart Association; s.c., subcutaneously.
Qualitative synthesis
The risk of bias and quality of individual studies was independently assessed by two reviewing authors (E. W., Y. dR.) using the Quality in Prognosis Studies tool.29 The summary of this assessment is shown in Table 4. When assessing the risk of metabias in this systematic review, specifically selection bias and comprehensiveness of the literature search, there is a risk that data are available on this subject that has not been published before. We performed a systematic literature search in two electronic databases (MEDLINE and EMBASE) but did not screen conference abstracts or book chapters, nor did we contact the authors of the studies included in this systematic review for possible gathering of unpublished data from patients treated in the period after the studies were conducted and reported upon. It is also possible that other centres treating patients with worsening HF in an outpatient setting did not report on their data (selective outcome reporting). With regards to information bias, we performed single data extraction with verification and not independent data extraction, which is possibly less accurate and is associated with a higher error rate.30 The author performing data extraction (E. W.) however was unknown to the authors or the results of the individual studies before data extraction.
All studies were performed in the United States or Europe between 2006 and 2016. The conclusions drawn from these studies are limited by the quality of the individual studies. The overall study population is small (total of 984 unique individual patients); there is considerable heterogeneity in the study population (including type of HF), and the subsequent number of events is small. There is also a significant degree of statistical heterogeneity in the choice for outpatient treatment (assessment of worsening HF and discretion of doctor or nurse and the compliance and failure of escalation of oral diuretic therapy), the specifics of intervention (type of diuretics, bolus vs. continuous therapy), and the duration of follow‐up.
We did not perform a meta‐analysis (standard pair‐wise meta‐analysis with comparison of the interventions) of the individual studies because of the difference in study characteristics (including primary and secondary endpoints) without a comparable measured and reported treatment effect or association.
Discussion: putting the findings into context
Safety and efficacy of outpatient treatment of worsening HF
This is the first systematic review to examine the effectiveness of outpatient intravenous or subcutaneous diuretic use for treatment of worsening HF. Based on this evidence, the reviewed studies (all but one non‐randomized) report that outpatient treatment with intravenous or subcutaneous diuretics of patients with worsening HF is effective by relieving symptoms and has a low risk of adverse events but do not provide satisfactory evidence for reduction in rates of re‐hospitalization or improvement in mortality or quality of life. In a total of 984 unique individual patients treated in the reviewed studies, only a few adverse events were reported, mostly electrolyte disturbance (hypokalaemia).
While hospitalization for some patients with worsening HF is undoubtedly in some cases necessary (in case of cardiogenic shock or respiratory failure), the decision to hospitalize a patient is subjective and influenced by many non‐clinical factors31 (patient preference, level of caregiver support, local outpatient healthcare infrastructure, and local hospital re‐imbursement structure). There is no clear evidence that hospitalization improves outcome.32 Treatment with intravenous diuretics in an outpatient setting could represent a viable alternative strategy for hospitalization. Patients with worsening HF have relatively slow onset and progression of symptoms that provides a window during which outpatient treatment may relieve symptoms without the need for emergency department presentation or hospitalization.9
The possibility of transition of care of patients with worsening HF from an inpatient to an outpatient setting is a patient‐centred alternative to hospitalization, maximizes time spent outside the hospital and possibly reduces healthcare costs.33 The so called ‘home time’ of patients (time alive and out of a healthcare institution) is an important patient‐centred outcome. One study showed that 91% patients prefer the outpatient treatment above treatment at the emergency department and 89% of patients showed a 100% satisfaction visual score.25 In the United States, approximately 19% of hospitals have the possibility of outpatient treatment with intravenous or subcutaneous diuretics, and these treatments comprise 1.4% of all outpatient visits for HF..31 Outpatient treatment of patients with worsening HF could avoid delays when presenting to the emergency department and streamline standard disciplinary HF care and early follow‐up after treatment.32 Furthermore, complications of hospitalization could be prevented, such as venous thrombosis, phlebitis, hospital‐acquired pneumonia, ulcers due to lack of mobility, loss of control, and depression, estimated to be as high as 25%.34
Risk stratification and clinical decision making: choosing the right patient and right treatment for outpatient treatment of worsening HF
Although inclusion and exclusion criteria were similar across studies, there was considerable heterogeneity in the patient characteristics and ambulatory treatment regimens. Average dosages of diuretic therapy ranged from 40 to 240 mg. In some studies, patients with chronic kidney disease were excluded. These patients might need higher doses of diuretic treatment.32 Orally administered, furosemide has limited and variable bioavailability,35 particularly when absorption is hampered by bowel oedema.36 Intravenous doses of furosemide are approximately twice as potent as oral doses.37 When hospitalized for acute and worsening HF and treated with intravenous loop diuretics, 76% of patients report dyspnea improvement within 6 h of presentation.38 90% of patients with acute HF receive intravenous diuretics.39 Intravenous administration of diuretic therapy is more effective in managing congestion than escalating doses of orally administered diuretic agents.37 Recently, some studies showed the effect of treatment with subcutaneous furosemide for worsening HF.
It is not possible to extract from the data out of the reviewed studies selectively which patients (clinical characteristics: demographics, type of HF, and co‐existing conditions) with worsening HF would benefit the most from outpatient treatment. There are some data available in the literature assisting in this risk stratification and clinical decision making. The BIOSTAT‐CHF study included patients with worsening HF in both hospitalized and outpatient settings. Subdividing patients into low, intermediate, and high‐risk categories (with a multivariate risk model using age, HF hospitalization in the last 12 months, edema, blood pressure, kidney function, urea, N‐terminal pro BNP, HDL, anaemia, sodium, and lack of beta‐blockers), the primary composite outcome (of death or HF hospitalization) event rate was 8.4, 29.9, and 43.3% for these categories. A comparable risk score could be developed to select patients who would benefit the most from outpatient treatment and to choose hospitalization for high‐risk patients.40
Cost‐effectiveness of outpatient treatment of worsening HF
The outpatient treatment option with intravenous diuretics for patients with worsening HF started in the United States when the US health care system started to move away from a volume‐based payment system to a quality‐ and value‐based system including giving financial penalties to hospitals with higher than average HF re‐hospitalization rate.41, 42 Although recent data show that the implementation of this Hospital Readmissions Reduction Program possibly leads to higher mortality, there is no data suggestive of the relation of this higher HF mortality and the use of outpatient HF care units.4 A recent study showed that in the United States 18.8% of hospitals are using the outpatient clinics to treat patients with worsening HF with intravenous diuretics. These hospitals demonstrated a modestly lower rate of 30‐day mortality.31 It is estimated that in the United States savings of more USD 650 million to more than USD 2 billion could be realized (on a total cost of treatment for worsening HF of USD 12 billion) by shifting hospitalizations for worsening HF towards outpatient treatment.43 In the Netherlands, the cost for one acute HF hospitalization is around € 6000 (USD 6600). For each episode of worsening HF, it is estimated that an average of two treatments (1–5) is necessary. Based on the differences in costs (around € 1900 for a maximum of five outpatient treatments, USD 2100), the yearly incidence of hospitalizations in the Netherlands (31 000), and the possibility of preventing 10–25% of all hospitalizations when implementing outpatient treatment, in a budget period of 4 years, total savings would be estimated € 33–82 million (USD 36–90 million). The advantage with outpatient treatment with intravenous or subcutaneous diuretics is that while there are costs per intervention (medication, intravenous or subcutaneous delivery equipment, facility costs, laboratory costs, and follow‐up costs) the treatment is built‐in in existing clinical care without additional costs for staffing.
Conclusion and future directions
This is the first systematic review to examine the effectiveness of outpatient intravenous or subcutaneous diuretic use to treat worsening HF. Outpatient intravenous or subcutaneous diuretic treatment of worsening HF may be a patient‐centred alternative to inpatient treatment of patients with worsening HF. It can both be of benefit to patients (better quality of life when hospitalization is prevented) as well as healthcare providers (increased capacity for hospitalization for other causes than HF) and healthcare policy makers (for macroeconomic benefit because of cost reduction when hospitalization is prevented). The reviewed studies report that outpatient treatment with intravenous or subcutaneous diuretics of patients with worsening HF is effective by relieving symptoms and has a low risk of adverse events but do not provide satisfactory evidence for reduction in rates of re‐hospitalization or improvement in mortality or quality of life. It is not possible to extract from these data more selectively that patients with worsening HF would benefit the most from outpatient treatment (clinical characteristics: demographics, type of HF, and co‐existing conditions) nor which specific intervention (type and dosage of diuretic therapy) would be most beneficial. Prospective randomized studies are needed to determine the safety and effectiveness of outpatient intravenous or subcutaneous diuretic treatment for patient with worsening HF.
Conflict of interest
None declared.
Wierda, E. , Dickhoff, C. , Handoko, M. L. , Oosterom, L. , Kok, W. E. , de Rover, Y. , de Mol, B. A. J. M. , van Heerebeek, L. , and Schroeder‐Tanka, J. M. (2020) Outpatient treatment of worsening heart failure with intravenous and subcutaneous diuretics: a systematic review of the literature. ESC Heart Failure, 7: 892–902. 10.1002/ehf2.12677.
References
- 1. Berry C, Murdoch DR, McMurray JJ. Economics of chronic heart failure. Eur J Heart Fail 2001; 3: 283–291. [DOI] [PubMed] [Google Scholar]
- 2. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KKL, Murabito JM, Vasan RS. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med 2002; 347: 1397–1402. [DOI] [PubMed] [Google Scholar]
- 3. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L, Heart Failure Association of the European Society of Cardiology (HFA) . EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 4. Gupta A, Fonarow GC. The Hospital Readmissions Reduction Program—learning from failure of a healthcare policy. Eur J Heart Fail 2018; 20: 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007; 154: 260–266. [DOI] [PubMed] [Google Scholar]
- 6. Butler J, Yang M, Manzi MA, Hess GP, Patel MJ, Rhodes T, Givertz MM. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol 2019; 73: 935–944. [DOI] [PubMed] [Google Scholar]
- 7. Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 2005; 96: 11G–17G. [DOI] [PubMed] [Google Scholar]
- 8. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greene SJ, Mentz RJ, Felker GM. Outpatient worsening heart failure as a target for therapy: a Review. JAMA Cardiol 2018; 3: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skali H, Dwyer EM, Goldstein R, Haigney M, Krone R, Kukin M, Lichstein E, McNitt S, Moss AJ, Pfeffer MA, Solomon SD. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT‐CRT. Eur J Heart Fail 2014; 16: 560–565. [DOI] [PubMed] [Google Scholar]
- 11. Harjola V‐P, Mullens W, Banaszewski M, Bauersachs J, Brunner‐La Rocca H‐P, Chioncel O, Collins SP, Doehner W, Filippatos GS, Flammer AJ, Fuhrmann V, Lainscak M, Lassus J, Legrand M, Masip J, Mueller C, Papp Z, Parissis J, Platz E, Rudiger A, Ruschitzka F, Schäfer A, Seferovic PM, Skouri H, Yilmaz MB, Mebazaa A. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2017; 19: 821–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 14. Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008; 118: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 15. DeVore AD, Allen LA, Eapen ZJ. Thinking outside the box: treating acute heart failure outside the hospital to improve care and reduce Admissions. J Card Fail 2015; 21: 667–673. [DOI] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hebert K, Dias A, Franco E, Tamariz L, Steen D, Arcement LM. Open access to an outpatient intravenous diuresis program in a systolic heart failure disease management program. Congest Heart Fail 2011; 17: 309–313. [DOI] [PubMed] [Google Scholar]
- 18. Makadia S, Simmons T, Augustine S, Kovell L, Harris C, Chibungu A, Parakh K. The diuresis clinic: a new paradigm for the treatment of mild decompensated heart failure. Am J Med 2015; 128: 527–531. [DOI] [PubMed] [Google Scholar]
- 19. Durango LF. Continuous furosemide infusion is safe and effective in outpatient treatment of refractory heart failure. Circulation 2006; 114: 375–376. [Google Scholar]
- 20. Ryder M, Murphy NF, McCaffrey D, O'Loughlin C, Ledwidge M, McDonald K. Outpatient intravenous diuretic therapy; potential for marked reduction in hospitalisations for acute decompensated heart failure. Eur J Heart Fail 2008; 10: 267–272. [DOI] [PubMed] [Google Scholar]
- 21. Freimark D, Arad M, Matetzky S, DeNeen I, Gershovitz L, Morag NK, Hochberg N, Makmal Y, Shechter M. An advanced chronic heart failure day care service: a 5 year single‐center experience. Isr Med Assoc J 2009; 11: 419–425. [PubMed] [Google Scholar]
- 22. Lazkani M, Ota KS. The role of outpatient intravenous diuretic therapy in a transitional care program for patients with heart failure: a case series. J Clin Med Res 2012; 4: 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banerjee P, Tanner G, Williams L. Intravenous diuretic day‐care treatment for patients with heart failure. Clin Med (Lond) 2012; 12: 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buckley LF, Carter DM, Matta L, Cheng JW, Stevens C, Belenkiy RM, Burpee LJ, Young MA, Weiffenbach CS, Smallwood JA, Stevenson LW, Desai AS. Intravenous diuretic therapy for the management of heart failure and volume overload in a multidisciplinary outpatient unit. JACC Heart Fail 2016; 4: 1–8. [DOI] [PubMed] [Google Scholar]
- 25. Al‐Ani MA, Schwartz C, Winchester D, Barry J, Cerda M, Aranda JM, Ahmed MM. Outpatient intravenous diuretic therapy for acute heart failure: a simplified solution to a formidable problem. J Card Fail 2019. https://www.ncbi.nlm.nih.gov/pubmed/31449962 [DOI] [PubMed] [Google Scholar]
- 26. Buckley LF, Stevenson LW, Cooper IM, Knowles DM, Matta L, Molway DW, Navarro‐Velez K, Rhoten MN, Shea EL, Stern GM, Weintraub JR, Young MA, Mehra MR, Desai AS. Heart ambulatory treatment of worsening heart failure with intravenous loop diuretics: a four‐year experience. J Card Fail 2019. https://www.ncbi.nlm.nih.gov/pubmed/31704197 [DOI] [PubMed] [Google Scholar]
- 27. Zatarain‐Nicolás E, López‐Díaz J, de la Fuente‐Galán L, García‐Pardo H, Recio‐Platero A, Román‐Calvar S, José A. Subcutaneous infusion of furosemide administered by elastomeric pumps for decompensated heart failure treatment: initial experience. Rev Esp Cardiol (Engl Ed) 2013; 66: 1002–1004. [DOI] [PubMed] [Google Scholar]
- 28. Gilotra NA, Princewill O, Marino B, Okwuosa IS, Chasler J, Almansa J, Cummings A, Rhodes P, Chambers J, Cuomo K, Russell SD. Efficacy of intravenous furosemide versus a novel, pH‐neutral furosemide formulation administered subcutaneously in outpatients with worsening heart failure. JACC Heart Fail 2018; 6: 65–70. [DOI] [PubMed] [Google Scholar]
- 29. Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006; 144: 427–437. [DOI] [PubMed] [Google Scholar]
- 30. Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol 2006; 59: 697–703. [DOI] [PubMed] [Google Scholar]
- 31. Greene SJ, Wilson LE, Abbasi SA, Yusuf AA, Hammill BG. Outpatient intravenous diuretic therapy for heart failure in the United States. J Am Coll Cardiol 2019; 73: 1101–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee C, Beleznai T, Hassan S, Rawat A, Douglas H, Kanagala P, Sankaranarayanan R. Ambulatory management of acute decompensation in heart failure. Br J Hosp Med (Lond) 2019; 80: 40–45. [DOI] [PubMed] [Google Scholar]
- 33. Feltner C, Jones CD, Cené CW, Zheng Z‐J, Sueta CA, Coker‐Schwimmer EJL, Arvanitis M, Lohr KN, Middleton JC, Jonas DE. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med 2014; 160: 774–784. [DOI] [PubMed] [Google Scholar]
- 34. Stiell IG, Clement CM, Brison RJ, Rowe BH, Borgundvaag B, Aaron SD, Lang E, Calder LA, Perry JJ, Forster AJ, Wells GA. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad Emerg Med 2013; 20: 17–26. [DOI] [PubMed] [Google Scholar]
- 35. Shankar SS, Brater DC. Loop diuretics: from the Na‐K‐2Cl transporter to clinical use. Am J Physiol Renal Physiol 2003; 284: F11–F21. [DOI] [PubMed] [Google Scholar]
- 36. Gottlieb SS, Khatta M, Wentworth D, Roffman D, Fisher ML, Kramer WG. The effects of diuresis on the pharmacokinetics of the loop diuretics furosemide and torsemide in patients with heart failure. Am J Med 1998; 104: 533–538. [DOI] [PubMed] [Google Scholar]
- 37. Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med 2017; 377: 1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, Andre S, Mark Courtney D, Hasa J, Spinar J, Masip J, Frank Peacock W, Sliwa K, Gayat E, Filippatos G, Cleland JGF, Gheorghiade M. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT‐dyspnoea study. Eur Heart J 2010; 31: 832–841. [DOI] [PubMed] [Google Scholar]
- 39. Peacock WF, Costanzo MR, De Marco T, Lopatin M, Wynne J, Mills RM, Emerman CL, ADHERE Scientific Advisory Committee and Investigators . Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology 2009; 113: 12–19. [DOI] [PubMed] [Google Scholar]
- 40. Ferreira JP, Metra M, Mordi I, Gregson J, Ter Maaten JM, Tromp J, Anker SD, Dickstein K, Hillege HL, Ng LL, van Veldhuisen DJ, Lang CC, Voors AA, Zannad F. Heart failure in the outpatient versus inpatient setting: findings from the BIOSTAT‐CHF study. Eur J Heart Fail 2019; 21: 112–120. [DOI] [PubMed] [Google Scholar]
- 41. Srinivasan D, Desai NR. The impact of the transition from volume to value on heart failure care: implications of novel payment models and quality improvement initiatives. J Card Fail 2017; 23: 615–620. [DOI] [PubMed] [Google Scholar]
- 42. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med 2016; 374: 1543–1551. [DOI] [PubMed] [Google Scholar]
- 43. Fitch K, Lau J, Engel T, Medicis JJ, Mohr JF, Weintraub WS. The cost impact to Medicare of shifting treatment of worsening heart failure from inpatient to outpatient management settings. Clinicoecon Outcomes Res 2018; 10: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]


