Abstract
Aims
This study aims to investigate the association of resting heart rate (RHR) measured in late adolescence with long‐term risk of cause‐specific heart failure (HF) and subtypes of cardiomyopathy (CM), with special attention to cardiorespiratory fitness.
Methods and results
We performed a nation‐wide, register‐based cohort study of all Swedish men enrolled for conscription in 1968–2005 (n = 1 008 363; mean age = 18.3 years). RHR and arterial blood pressure were measured together with anthropometrics as part of the enlistment protocol. HF and its concomitant diagnoses, as well as all CM diagnoses, were collected from the national inpatient, outpatient, and cause of death registries. Risk estimates were calculated by Cox‐proportional hazards models while adjusting for potential confounders. During follow‐up, there were 8400 cases of first hospitalization for HF and 3377 for CM. Comparing the first and fifth quintiles of the RHR distribution, the hazard ratio (HR) for HF associated with coronary heart disease, diabetes, or hypertension was 1.25 [95% confidence interval (CI) = 1.13–1.38] after adjustment for body mass index, blood pressure, and cardiorespiratory fitness. The corresponding HR was 1.43 (CI = 1.08–1.90) for HF associated with CM and 1.34 (CI = 1.16–1.54) for HF without concomitant diagnosis. There was an association between RHR and dilated CM [HR = 1.47 (CI = 1.27–1.71)] but not hypertrophic, alcohol/drug‐induced, or other cardiomyopathies.
Conclusions
Adolescent RHR is associated with future risk of HF, regardless of associated aetiological condition. The association was strongest for HF associated with CM, driven by the association with dilated CM. These findings indicate a causal pathway between elevated RHR and myocardial dysfunction that warrants further investigation.
Keywords: Adolescence, Population, Epidemiology, Resting heart rate, Heart failure, Cardiomyopathy
Introduction
Heart failure is a common disease and a major cause of disability and mortality among the adult and older population. Although the overall incidence and prevalence is declining in Western countries, 1 , 2 increasing rates of first time hospitalizations among younger individuals have been found in Sweden 1 and Denmark. 2 Furthermore, heart failure with concomitant cardiomyopathy has more than doubled in Sweden from 1987 to 2006. 1 This development is alarming considering the severe nature and prognosis of the disease, as one in 10 patients still does not survive the first year after heart failure diagnosis. 1 The cause for the diverging trends in older and younger people is unknown, but increasing obesity rates may constitute one factor. Among young Swedish men, we have recently found marked increases in the longitudinal risk for heart failure 3 and cardiomyopathy 4 with increasing levels of body mass index (BMI). In parallel, low levels of physical activity and cardiorespiratory fitness, now increasingly common, are associated with increased risk of heart failure. 5
We previously established an association between resting heart rate, measured in late adolescence, with future risk of heart failure among Swedish men enrolling for military service, 6 an association that proved independent of arterial blood pressure, BMI, and fitness. While it has been suggested that the association between elevated resting heart rate and heart failure is mediated via coronary heart disease, previous studies have shown that the association persists even after adjustments for preceding coronary events. 7 , 8 Furthermore, heart failure constitutes an advanced stage of a variety of cardiovascular disorders. While coronary heart disease and hypertension are predominant factors in Western populations, including Sweden, other causes include acquired or congenital heart disease, arrhythmias, or primary disease of the myocardium such as the cardiomyopathies. Because of this, we sought to investigate the association between resting heart rate in late adolescence with heart failure according to various aetiological conditions, as well as various types of cardiomyopathy, over an extended follow‐up period.
Methods
Participants
A cohort comprising all Swedish men that enlisted for conscription between 1968 and 2005 was created, using data from the Swedish Military Service Conscription Registry. Until the abolishment of the mandatory enlistment in 2005, all Swedish men were obliged to participate, with serious, chronic medical or mental conditions or disabilities, or incarceration constituting the only exemptions (2–3% yearly). The background population comprised all men that enlisted (n = 1 874 651, Supporting Information, Figure S1 ). Exclusion criteria used were based on age (<16 or >25 years; n = 57 095), missing data on resting heart rate (n = 633 889), or values >145 and <35 (n = 493), fitness (n = 23 275), BMI (n = 150 662), invalid systolic and diastolic blood pressure (n = 503), or pre‐existing diagnoses of myocardial infarction, heart failure, or cardiomyopathy (n = 208). Subsequently, a total of 1 008 363 conscripts were included. The study received approval from the regional ethics committee of the University of Gothenburg, which is conducted in concordance with the Declaration of Helsinki.
Conscription register data
During the study period (1968–2005), a standardized 2‐day protocol was used for assessment of aptitude for military service, including physical and physiological evaluations. Conscripts were first seen by a nurse practitioner, performing basic somatic assessment including measurement of height, weight, and collection of medical history. After a resting period of 5–10 min, resting heart rate and arterial blood pressure were measured in supine position with an appropriately sized cuff at heart level. Following examination by a physician, fitness testing was performed using stationary cycle ergometry. The maximum work capacity expressed in Watts (Wmax) was divided by body weight and transformed into nine levels that were digitized and served as a measure of fitness. 5 For further information, see Supporting Information.
Follow‐up procedures
From 1968, hospital discharge diagnoses are reported to the Swedish National Inpatient Registry. The coverage increased gradually and is considered complete from 1987. Recording of diagnoses in hospital outpatient care started in 2001, referred to as the outpatient registry. The Cause of Death Registry keeps records of the cause of death for all Swedish residents and is complete from 1952. Linkage between the national health registries was made using the 12‐digit personal identification number, unique to every Swedish citizen. Follow‐up started at date of conscription, and participants were followed until a first discharge after hospitalization for heart failure, or death, registered in the inpatient and cause of death registries according to the contemporary version of the International Classification of Diseases (ICD).
Definitions of heart failure and mutually exclusive associated conditions
The main outcome in this study was hospitalization for heart failure. A large proportion of patients with heart failure had other primary diagnoses; thus, the first‐ever heart failure diagnosis code in any position was accepted as heart failure. From 1968 to 1986, ICD‐8 was in use; from 1987 to 1996, ICD‐9; and thereafter, ICD‐10. Heart failure was defined as 427.00 and 427.10 (ICD‐8); 428 (ICD‐9); and I50 (ICD‐10).
The following associated co‐morbidities were included in the analysis until the first heart failure discharge diagnosis: diabetes 250 (ICD‐8 and ICD‐9) and E10–E14 (ICD‐10); hypertension 401–405 (ICD‐8 and ICD‐9) and I10–I15 (ICD‐10); acute myocardial infarction 410 (ICD‐8 and ICD‐9) and I21 (ICD‐10); ischaemic heart disease 410–414 (ICD‐8 and ICD‐9) and I20–I25 (ICD‐10); cardiomyopathy 425 (ICD‐8 and ICD‐9) and I42, I43 (ICD‐10); valvulopathies 394, 395, 396, 398, 424 (ICD‐8), 394–398, 424 (ICD‐9), and I05–I09, I33–I39 (ICD‐10); congenital heart disease 746–747 (ICD‐8), 745–747 (ICD‐9), and Q20–Q28, Q87, Q89 (ICD‐10); atrial fibrillation 427, 92 (ICD‐8), 427D (ICD‐9), and I48 (ICD‐10); stroke 431, 433, 434, 436 (ICD‐8), 431, 434, 436, 432X (ICD‐9), and I61, I63, I64, I62.9 (ICD‐10); ischaemic stroke 433, 434, 436 (ICD‐8), 434, 436 (ICD‐9), and I63, I64 (ICD‐10); alcohol‐related disorders 291, 303; 291, 303, 305.0 (ICD‐8 and ICD‐9); F10 (ICD‐10); other substance use disorders 294.3, 304; 292, 304, 305.1‐8 (ICD‐8 and ICD‐9); F11–F19 (ICD‐10).
Because of overlapping heart failure aetiologies, we assigned mutually exclusive causes of heart failure in the following hierarchical order: (1) congenital heart disease and valvulopathies; (2) ischaemic heart disease, diabetes, or hypertension; (3) cardiomyopathy; and (4) other causes. 1
Cardiomyopathies
Four mutually exclusive categories of cardiomyopathy were created as follows: (1) dilated cardiomyopathy, (2) hypertrophic cardiomyopathy, (3) alcohol/drug‐induced cardiomyopathy, and (4) other cardiomyopathies, which was a composite of less common forms (Supporting Information, Table S1 ). Drug‐induced cardiomyopathies were categorized together with alcoholic cardiomyopathies and formed one single group. Drug‐induced cardiomyopathies were classified by 425X (secondary cardiomyopathy, ICD9) in combination with a prior registered substance use disorder [209, 303, 304 (ICD‐8); 303, 304, 305, 965A, 969G, 969H (ICD‐9)]; I42.7 (cardiomyopathy due to drugs or external agents), I42.8 (other cardiomyopathies), and I42.9 (cardiomyopathy, unspecified), if prior substance use disorder (as before but additionally F10–19, ICD‐10).
Patients with registrations for both dilated and hypertrophic cardiomyopathies during the follow‐up period were not included in either subgroup (indeterminable cardiomyopathy). Patients with a diagnosis of acute myocardial infarction [410 (ICD‐8 and ICD‐9) and I21 (ICD‐10)] before cardiomyopathy diagnosis were not accepted as cardiomyopathy, as defined by diagnostic guidelines that exclude heart disease of ischaemic origin, and were therefore excluded from all analyses.
Statistical methods
Incidence rates and their corresponding 95% confidence intervals (CIs) were calculated using Poisson regression. We used Cox‐proportional hazards regression analyses to estimate the association between resting heart rate at conscription with the risk of future hospitalization for heart failure and cardiomyopathy, while considering potential confounders. The follow‐up period started at date of conscription and ended at first hospitalization; death from other causes; emigration from Sweden; or at the end of the follow‐up period on 31 December 2014. Pre‐existing co‐morbidities, conscription year and test centre, age at conscription, BMI, systolic and diastolic blood pressure, and fitness were included as covariates for the multivariate analysis. Scores of fitness (1–9) were trichotomized. Due to the low number of participants in stanines 1–3, low fitness was classified by stanines 1–4, medium by stanines 5–7, and high by stanines 8–9. Age at conscription, BMI, body height, and systolic and diastolic blood pressure were used as continuous variables. The proportional hazards assumptions were assessed using plots based on weighted residuals. As a result of the large number of observations, the P values were very small and were not therefore reported. In all analyses when the 95% CI did not include 1, the majority of P values were <0.0001. All statistical analyses were performed using SAS, version 9.4 and R, version 3.3.2.
Results
Baseline characteristics of study participants (n = 1 008 363, mean age = 18.3) across quintiles of resting heart rate are shown in Table 1 . Quintiles of resting heart rate ranged from 35 to 62 (quantile 1); from 63 to 69 (quintile 2); from 70 to 75 (quintile 3); from 76 to 83 (quintile 4); and from 84 to 145 (quintile 5). Resting heart rate was associated with increasing systolic and diastolic blood pressure and inversely with level of fitness. Prior conditions and co‐morbidities are shown in Supporting Information, Table S2 .
Table 1.
Baseline characteristics by quintiles of resting heart rate in 1 008 485 male conscripts (Sweden, 1968–2005)
| Resting heart rate | ||||||
|---|---|---|---|---|---|---|
| All | Q1 (35–62) | Q2 (63–69) | Q3 (70–75) | Q4 (76–83) | Q5 (84–145) | |
| n (%) | 1 008 363 (100) | 219 351 (22) | 204 974 (20) | 188 909 (19) | 199 197 (20) | 195 932 (19) |
| Age (years) (SD) | 18.3 (0.633) | 18.3 (0.624) | 18.3 (0.629) | 18.3 (0.632) | 18.3 (0.633) | 18.3 (0.645) |
| Length (cm) (SD) | 179 (6.55) | 180 (6.50) | 179 (6.52) | 179 (6.54) | 179 (6.58) | 179 (6.59) |
| Weight (kg) (SD) | 70 (10.6) | 70.3 (9.37) | 69.9 (10.02) | 69.8 (10.47) | 69.9 (11.15) | 70.0 (12.11) |
| BMI (kg/m2) (SD) | 21.8 (2.92) | 21.8 (2.49) | 21.7 (2.70) | 21.7 (2.86) | 21.8 (3.08) | 21.9 (3.42) |
| SBP (mmHg) (SD) | 128 (11.1) | 125 (10.5) | 127 (10.5) | 128 (10.6) | 130 (10.7) | 133 (11.3) |
| DBP (mmHg) (SD) | 68.8 (9.49) | 67.7 (9.08) | 68.1 (9.23) | 68.4 (9.35) | 68.9 (9.54) | 70.8 (9.98) |
| Cardiorespiratory fitness | ||||||
| Low (1–4) | 171 185 (17) | 22 610 (10) | 29 622 (14) | 32 606 (17) | 40 058 (20) | 46 289 (24) |
| Medium (5–7) | 586 868 (58) | 115 449 (53) | 117 641 (57) | 111 643 (59) | 119 971 (60) | 122 164 (62) |
| High (8–9) | 250 310 (25) | 81 292 (37) | 57 711 (28) | 44 660 (24) | 39 168 (20) | 27 479 (14) |
Data presented as mean ± SD values or n (%). BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
During follow‐up [median 34 (interquartile range 19–40) years], there were 8400 cases of heart failure and 3377 cases of cardiomyopathy. Incidence rates of heart failure subdivided according to mutually exclusive associated conditions, and of subgroups of cardiomyopathy, are shown in Table 2 . For all heart failure categories, incidence rates increased with level of resting heart rate. For heart failure associated with congenital heart disease or acquired valvular disease, the incidence ranged from 2.6 (2.3–3.1) in Q1 to 3.8 (3.4–4.3) in Q5. The corresponding rates for heart failure associated with coronary heart disease, hypertension, or diabetes were 10.3 (9.5–11.1) and 20.3 (19.1–21.4); 1.4 (1.1–1.7) and 2.3 (2.0–2.8) for heart failure associated with cardiomyopathy, and 6.0 (5.4–6.6) and 9.2 (8.4–9.9) for cases from any other cause. For dilated cardiomyopathy, incidence rates ranged from 5.1 (4.5–5.6) in Q1 to 9.4 (8.6–10.2) in Q5. No significant difference was seen for other subgroups of cardiomyopathy.
Table 2.
Incidence rates for cause‐specific heart failure and cardiomyopathies by quintiles of resting heart rate
| Resting heart rate | ||||||
|---|---|---|---|---|---|---|
| All | Q1 (35–62) | Q2 (63–69) | Q3 (70–75) | Q4 (76–83) | Q5 (84–145) | |
| Heart failure | ||||||
| Heart failure with valvular or congenital heart disease (n) | 985 | 167 | 177 | 166 | 239 | 236 |
| Cases per 100 000 person‐years | 3.2 (3.0–3.4) | 2.6 (2.3–3.1) | 2.9 (2.5–3.3) | 2.9 (2.4–3.3) | 3.9 (3.4–4.4) | 3.8 (3.4–4.3) |
| Heart failure with coronary heart disease, diabetes, or hypertension (n) | 4570 | 649 | 776 | 878 | 1018 | 1249 |
| Cases per 100 000 person‐years | 15.0 (14.5–15.4) | 10.3 (9.5–11.1) | 12.7 (11.8–13.6) | 15.2 (14.2–16.2) | 16.6 (15.6–17.6) | 20.3 (19.1–21.4) |
| Heart failure with cardiomyopathy (n) | 595 | 90 | 103 | 107 | 150 | 145 |
| Cases per 100 000 person‐years | 1.9 (1.8–2.1) | 1.4 (1.1–1.7) | 1.7 (1.4–2.0) | 1.8 (1.5–2.2) | 2.4 (2.1–2.9) | 2.3 (2.0–2.8) |
| Heart failure, any other cause (n) | 2264 | 379 | 410 | 397 | 513 | 565 |
| Cases per 100 000 person‐years | 7.4 (7.1–7.7) | 6.0 (5.4–6.6) | 6.7 (6.1–7.4) | 6.9 (6.2–7.6) | 8.3 (7.6–9.1) | 9.2 (8.4–9.9) |
| Cardiomyopathies | ||||||
| Dilated cardiomyopathy (n) | 2089 | 311 | 360 | 389 | 468 | 561 |
| Cases per 100 000 person‐years | 7.1 (6.8–7.4) | 5.1 (4.5–5.7) | 6.1 (5.5–6.7) | 6.9 (6.3–7.7) | 7.9 (7.2–8.6) | 9.4 (8.6–10.2) |
| Hypertrophic cardiomyopathy (n) | 490 | 103 | 99 | 90 | 102 | 96 |
| Cases per 100 000 person‐years | 1.7 (1.5–1.8) | 1.7 (1.4–2.0) | 1.7 (1.4–2.0) | 1.6 (1.3–2.0) | 1.7 (1.4–2.1) | 1.6 (1.3–2.0) |
| Alcohol/drug‐induced cardiomyopathy (n) | 404 | 59 | 74 | 87 | 99 | 85 |
| Cases per 100 000 person‐years | 1.4 (1.2–1.5) | 1.0 (0.7–1.2) | 1.2 (1.0–1.6) | 1.6 (1.2–1.9) | 1.7 (1.4–2.0) | 1.4 (1.1–1.8) |
| Other cardiomyopathies (n) | 394 | 89 | 68 | 65 | 81 | 91 |
| Cases per 100 000 person‐years | 1.3 (1.2–1.5) | 1.5 (1.2–1.8) | 1.1 (0.9–1.5) | 1.2 (0.9–1.5) | 1.4 (1.1–1.7) | 1.5 (1.2–1.9) |
Data presented as frequencies (n), and cases registered per 100 000 person‐years with corresponding 95% confidence interval.
Figure 1 shows hazard ratios (HRs) with 95% CIs for hospitalizations for categories of heart failure across quintiles of resting heart rate, using Q1 as reference. After adjustment for pre‐existing co‐morbidities, conscription year and test centre, age at conscription, and baseline BMI, there was an association between high resting heart rate and heart failure with valvular or congenital heart disease [HR = 1.24 (CI = 1.02–1.52)] for the highest compared with the lowest quintile. The association was, however, no longer significant after further adjustment for systolic and diastolic blood pressure [HR = 1.19 (CI = 0.96–1.47)]. The corresponding model adjustments produced HRs of 1.55 (CI = 1.40–1.71) and 1.41 (CI = 1.27–1.55) for heart failure associated with coronary heart disease, diabetes, or hypertension; 1.49 (CI = 1.13–1.95) and 1.53 (CI = 1.16–2.02) for heart failure associated with cardiomyopathy; and 1.36 (CI = 1.19–1.56) and 1.42 (CI = 1.23–1.62) for heart failure from any other cause.
Figure 1.
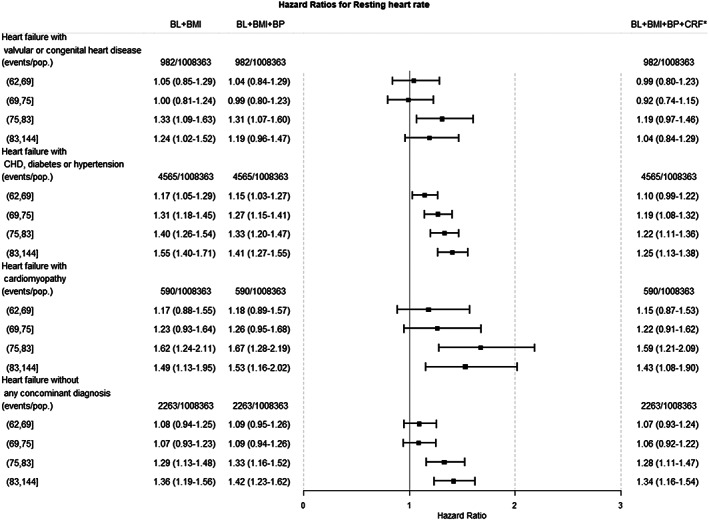
Hazard ratios (HRs) with corresponding 95% confidence intervals for heart failure categories by quintiles of resting heart rate. BL, baseline; BMI, body mass index; BP, arterial blood pressure; CRF, cardiorespiratory fitness.
The HR for dilated cardiomyopathy was 1.58 (1.37–1.82) in the least adjusted model and 1.47 (1.27–1.71) after further adjustments for arterial blood pressure and fitness (Figure 2 ). For cardiomyopathy of any other cause, there was no significant association. Notably, when additionally adjusting for fitness, a well‐known determinant of resting heart rate, the observed associations were slightly attenuated but remained significant (Figure 2 ).
Figure 2.
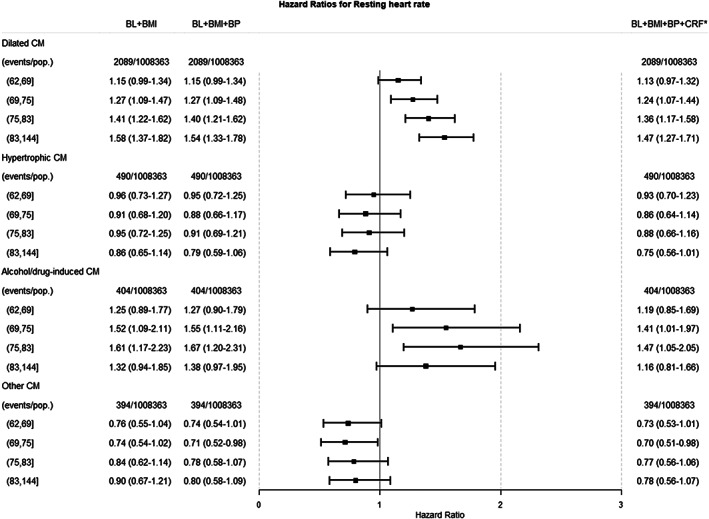
Hazard ratios (HRs) with corresponding 95% confidence intervals for categories of cardiomyopathy (CM) by quintiles of resting heart rate. BL, baseline; BMI, body mass index; BP, arterial blood pressure; CRF, cardiorespiratory fitness.
Discussion
The main finding of the present study is that a high resting heart rate, measured in adolescence, is a risk factor for heart failure regardless of underlying aetiological condition and furthermore, largely independent of confounders including BMI, arterial blood pressure, and fitness. These results are supportive of the body of evidence that found a positive association between elevated resting heart rate and heart failure among middle‐aged and older populations. 7 , 8 We also found an association between elevated resting heart rate and dilated, but not other types of, cardiomyopathy that has not been demonstrated before but where there are clear clinical implications.
Elevated resting heart rate has been associated with development of risk factors including diabetes 9 and hypertension. 10 It has previously been suggested that the association between resting heart rate and heart failure is driven by accelerated coronary atherosclerosis via increased inflammation, endothelial dysfunction, and ventricular remodelling due to increased metabolic stress. 11 While we found that increased resting heart rate was associated with heart failure associated with coronary heart disease, diabetes, or hypertension, the association persisted regardless of aetiology. In a recent study of Swedish conscripts from our group, there was, however, no significant association between resting heart rate and incident myocardial infarction, after adjustment for blood pressure and BMI. 6 Our findings provide support to previous studies where an elevated risk for heart failure in subjects with high resting heart rate was not explained by intercurrent coronary heart disease. 6 , 7 , 8 Together, these results indicate that resting heart rate increases heart failure risk through mechanisms at least partly separate from those linking resting heart rate to coronary heart disease.
The association between congenital or acquired valvular heart disease and heart failure was weak and did not persist after adjustment for arterial blood pressure. It is possible that this reflects the differential origin of elevated resting heart rate that is secondary to congenital heart disease. Our analyses investigating the association of resting heart rate with various subtypes of cardiomyopathy revealed that this association is driven by an association with dilated cardiomyopathy. This is, to our knowledge, a novel finding, but the observational design of the present study warrants caution when drawing conclusions from this observation. However, a long‐standing elevated resting heart rate may elicit direct effects on the myocardium or constitute a mediator on the pathway between genetic predisposition and myocardial disease. Indeed, genetic factors have been suggested to play an important part in the pathogenesis of both sporadic and familial dilated cardiomyopathies, although to what extent remains unknown. 12 Even so, long‐standing tachycardia is well known to cause heart failure in animal models and in humans, although the mechanisms are unknown. 13 It has been suggested that elevated resting heart rate is a marker of autonomic imbalance where sympathetic overactivity associated with heart failure has been found to be reversible by aerobic conditioning. 14 Aerobic conditioning decreases resting heart rate and increases cardiac vagal output 15 but seemingly does not attenuate cardiovascular sympathetic function in young, healthy adults, 16 strengthening the link between fitness‐related disease states and autonomic imbalance. In the present study, we found no association between resting heart rate and incident hypertrophic cardiomyopathy. This indicates that the strong genetic component previously observed for this condition 17 is mediated via pathways separate from that of an elevated resting heart rate.
The physiological stress response has been suggested as cause of the association between resting heart rate and cardiovascular disease, and self‐perceived stress has been linked to the development of heart failure. 18 Acute episodes of stress can trigger cardiac events such as myocardial infarction or arrhythmia 19 and have recently been linked to a syndrome of transient systolic dysfunction, known as Takotsubo cardiomyopathy. 20 Uncertainty remains regarding the possible direct effects of chronic stress and stress reactivity on myocardial function. Even so, it has been proposed that the protective effect of physical activity can be partly explained by attenuation of the physiological stress response, 21 although there are also conflicting reports. 22 In healthy young men, impaired resilience to stress, as assessed by a clinical psychologist, has also been longitudinally associated with heart failure development, 23 supporting this idea.
An elevated resting heart rate has also been associated with measures of structural remodelling of the heart. The SHIFT trial showed that rate‐lowering therapy in heart failure with ivabradine, a selective sinoatrial node inhibitor, not only improves clinical outcomes but also reverses indices of cardiac remodelling, providing new insights into the impact of resting heart rate on disease progression. 24 In young adults participating in the CARDIA study, baseline resting heart rate has been associated with left ventricular mass and left atrial dimension, 25 as well as longitudinal changes in left ventricular mass. 26 However, these studies did not assess functional indices including left ventricular ejection fraction, global longitudinal strain, and measures of diastolic function that may help differentiate pathological remodelling from physiological hypertrophy due to conditioning (athletes' heart). 27 A recent trial exploring the effects of ivabradine in a small sample of paediatric patients with dilated cardiomyopathy and chronic heart failure did show significant reductions in resting heart rate accompanied by increased left ventricular ejection fraction, left ventricular end‐systolic volume, and clinical status estimated by New York Heart Association class, when compared with placebo treatment. 28 Whether rate‐lowering therapy in healthy populations would similarly elicit protective effects remains speculative.
Strengths and limitations
The main strengths of the present study include a large sample size and number of cases with heart failure. Fitness was estimated objectively via a maximal ergometer testing, minimizing measurement error and allowing for confounder adjustment. There are, however, some limitations that must be acknowledged. Importantly, the diagnostic codes were registered in the Inpatient Registry for administrative purposes, and not formally validated. However, validation studies have been performed and showed generally high validity, with a positive predictive value of 82–88% for heart failure diagnoses. 29 Furthermore, in a recent study from our group validating hospital‐registered diagnoses of cardiomyopathy, the diagnoses were found to be highly accurate. 30 The single measurement of resting heart rate used does not allow for studying the effects of temporal changes of the exposure and introduces risk of regression to the mean, underestimating our results. The study has also some limitations in terms of generalizability. As follow‐up time is limited, the majority of observed cases occurred considerably earlier than most cases of heart failure, and it is therefore uncertain to which extent our results are applicable to cases occurring at an age more typical for heart failure cases. Furthermore, study participants were predominantly young Caucasian men, limiting generalizability towards women and other ethnicities. Residual confounding may be present, and cigarette smoking, which we could not adjust for, may be a factor that increases both resting heart rate and heart failure risk.
Conclusions
A high resting heart rate in late adolescence was associated with increased risk of heart failure, largely irrespective of underlying associated conditions, but was strongest in heart failure associated with cardiomyopathy, driven by an association with dilated cardiomyopathy. This finding is clinically relevant, with a potential for intervention that might be further explored. However, the underlying mechanisms remain uncertain and should be further investigated.
Conflict of interest
None declared.
Funding
This work was supported by grants from the following: the Swedish state under the agreement concerning research and education of doctors (grant number ALFGBG‐427301); the Swedish Society for Physicians, the Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Sweden, and the Swedish Heart and Lung Foundation (grant number 2015‐0438); the Swedish Research Council (grant numbers 2013‐5187 (SIMSAM), 2013‐4236); and the Swedish Council for Health, Working Life and Welfare (FORTE) (grant number 2013‐0325).
Supporting information
Data S1. Supporting Information
Lindgren, M. , Robertson, J. , Adiels, M. , Schaufelberger, M. , Åberg, M. , Torén, K. , Waern, M. , Åberg, N. D. , and Rosengren, A. (2020) Elevated resting heart rate in adolescent men and risk of heart failure and cardiomyopathy. ESC Heart Failure, 7: 1178–1185. 10.1002/ehf2.12726.
References
- 1. Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M, Rosengren A. Heart failure in young adults: 20‐year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J 2014; 35: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christiansen MN, Kober L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, Gislason GH, Torp‐Pedersen C, Andersson C. Age‐specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation 2017; 135: 1214–1223. [DOI] [PubMed] [Google Scholar]
- 3. Rosengren A, Aberg M, Robertson J, Waern M, Schaufelberger M, Kuhn G, Aberg D, Schioler L, Toren K. Body weight in adolescence and long‐term risk of early heart failure in adulthood among men in Sweden. Eur Heart J 2016; 38: –1926, 1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robertson J, Schaufelberger M, Lindgren M, Adiels M, Schioler L, Toren K, McMurray JJ, Sattar N, Aberg M, Rosengren A. Higher body mass index in adolescence predicts cardiomyopathy risk in midlife: a long term follow up amongst Swedish men. Circulation 2019; 140: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindgren M, Aberg M, Schaufelberger M, Aberg D, Schioler L, Toren K, Rosengren A. Cardiorespiratory fitness and muscle strength in late adolescence and long‐term risk of early heart failure in Swedish men. Eur J Prev Cardiol 2017; 24: 876–884. [DOI] [PubMed] [Google Scholar]
- 6. Lindgren M, Robertson J, Adiels M, Schaufelberger M, Aberg M, Toren K, Waern M, Aberg ND, Rosengren A. Resting heart rate in late adolescence and long term risk of cardiovascular disease in Swedish men. Int J Cardiol 2018; 259C: 109–115. [DOI] [PubMed] [Google Scholar]
- 7. Opdahl A, Ambale Venkatesh B, Fernandes VRS, Wu CO, Nasir K, Choi EY, Almeida ALC, Rosen B, Carvalho B, Edvardsen T, Bluemke DA, Lima JAC. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2014; 63: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw K‐T. Resting heart rate and incident heart failure in apparently healthy men and women in the EPIC‐Norfolk study. Eur J Heart Fail 2012; 14: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 9. Aune D, ó Hartaigh B, Vatten LJ. Resting heart rate and the risk of type 2 diabetes: a systematic review and dose‐response meta‐analysis of cohort studies. Nutr Metab Cardiovasc Dis 2015; 25: 526–534. [DOI] [PubMed] [Google Scholar]
- 10. Aladin AI, Al Rifai M, Rasool SH, Keteyian SJ, Brawner CA, Michos ED, Blaha MJ, Al‐Mallah MH, McEvoy JW. The association of resting heart rate and incident hypertension: the Henry Ford Hospital Exercise Testing (FIT) Project. Am J Hypertens 2016; 29: 251–257. [DOI] [PubMed] [Google Scholar]
- 11. Giannoglou GD, Chatzizisis YS, Zamboulis C, Parcharidis GE, Mikhailidis DP, Louridas GE. Elevated heart rate and atherosclerosis: an overview of the pathogenetic mechanisms. Int J Cardiol 2008; 126: 302–312. [DOI] [PubMed] [Google Scholar]
- 12. McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circ Res 2017; 121: 722–730. [DOI] [PubMed] [Google Scholar]
- 13. Heusch G. Heart rate and heart failure. Not a simple relationship. Circ J 2011; 75: 229–236. [DOI] [PubMed] [Google Scholar]
- 14. de Mello Franco FG, Santos AC, Rondon MU, Trombetta IC, Strunz C, Braga AM, Middlekauff H, Negrao CE, Pereira Barretto AC. Effects of home‐based exercise training on neurovascular control in patients with heart failure. Eur J Heart Fail 2006; 8: 851–855. [DOI] [PubMed] [Google Scholar]
- 15. Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN, McKinley PS, Slavov I, Fang Y, Myers MM. The effect of aerobic training and cardiac autonomic regulation in young adults. Am J Public Health 2009; 99: 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alex C, Lindgren M, Shapiro PA, McKinley PS, Brondolo EN, Myers MM, Zhao Y, Sloan RP. Aerobic exercise and strength training effects on cardiovascular sympathetic function in healthy adults: a randomized controlled trial. Psychosom Med 2013; 75: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 18. Rosengren A, Hawken S, Ôunpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case‐control study. The Lancet 2004; 364: 953–962. [DOI] [PubMed] [Google Scholar]
- 19. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 2010; 90: 513–557. [DOI] [PubMed] [Google Scholar]
- 20. Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol 2008; 124: 283–292. [DOI] [PubMed] [Google Scholar]
- 21. Huang CJ, Webb HE, Zourdos MC, Acevedo EO. Cardiovascular reactivity, stress, and physical activity. Front Physiol 2013; 4: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindgren M, Alex C, Shapiro PA, McKinley PS, Brondolo EN, Myers MM, Choi CJ, Lopez‐Pintado S, Sloan RP. Effects of aerobic conditioning on cardiovascular sympathetic response to and recovery from challenge. Psychophysiology 2013; 50: 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robertson J, Schiöler L, Torén K, Söderberg M, Löve J, Waern M, Rosengren A, Åberg M. Mental disorders and stress resilience in adolescence and long‐term risk of early heart failure among Swedish men. Int J Cardiol 2016; 243: 326–331. [DOI] [PubMed] [Google Scholar]
- 24. Tardif JC, O'Meara E, Komajda M, Bohm M, Borer JS, Ford I, Tavazzi L, Swedberg K. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J 2011; 32: 2507–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gidding SS, Carnethon MR, Daniels S, Liu K, Jacobs DR, Sidney S, Gardin J. Low cardiovascular risk is associated with favorable left ventricular mass, left ventricular relative wall thickness, and left atrial size: the CARDIA study. J Am Soc Echocardiogr 2010; 23: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gidding SS, Liu K, Colangelo LA, Cook NL, Goff DC, Glasser SP, Gardin JM, Lima JA. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging 2013; 6: 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pluim BM, Zwinderman AH, Van Der Laarse A, Van Der Wall EE. The athlete's heart: a meta‐analysis of cardiac structure and function. Circulation 2000; 101: 336–344. [DOI] [PubMed] [Google Scholar]
- 28. Bonnet D, Berger F, Jokinen E, Kantor PF, Daubeney PEF. Ivabradine in children with dilated cardiomyopathy and symptomatic chronic heart failure. J Am Coll Cardiol 2017; 70: 1262–1272. [DOI] [PubMed] [Google Scholar]
- 29. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Basic C, Rosengren A, Lindstrom S, Schaufelberger M. High validity of cardiomyopathy diagnoses in western Sweden (1989‐2009). ESC heart failure 2018; 5: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


