Abstract
Aims
Aptamer BC 007, a 15‐mer single‐strand DNA oligonucleotide (5'‐GGTTGGTGTGGTTGG‐3'), was developed to neutralize functional autoantibodies that bind to the extracellular domains of G protein‐coupled receptors (GPCR‐AAB), leading to the modulation of receptor‐mediated signalling cascades that induce pathophysiological states. Among the GPCR‐AAB, there are those directed against the β1‐adrenergic receptor (β1‐AAB) that are highly present in patients with dilated cardiomyopathy (DCM) and are increasingly accepted as disease drivers.
Using Doberman Pinschers (DP) with DCM, which possess similarities with human DCM among these β1‐AAB positivity for that the disease‐driving role in DP DCM was demonstrated, the safety of BC 007, efficacy for neutralizing β1‐AAB, and the DP's outcome were investigated.
Methods and results
Fourteen client‐owned β1‐AAB‐positive DP with electrocardiographically and echocardiographically indicated DCM were treated with BC 007. For controlling, two groups were created: 14 β1‐AAB‐positive DP with DCM not treated with BC 007 (Control 1) and 14 DP with DCM closely matched to the BC 007‐treated DP (Control 2), retrospectively selected from the institutional database of DP. After treatment, DP were monitored both echocardiographically, and for β1‐AAB, and survival curves were calculated. Based on clinical and laboratory examination, no adverse effects associated with BC 007 treatment were observed during the study. Forty‐eight hours after treatment, the DP's blood was free of β1‐AAB, which led to a reduction or stabilization of left ventricular end‐systolic volume (ESVI) during β1‐AAB free time in 10 of the treated DP. In one DP, where β1‐AAB returned after 3 months and ESVI worsened again, a second BC 007 treatment after 9 months again cleared the blood from β1‐AAB and improved the ESVI. Compared with the controls, DP treated with BC 007 showed a significantly longer survival time [572 days, interquartile range (IQR) 442–840 days] vs. Control group 1 (266 days, IQR 97–438 days; logrank: P = 0.009) and Control group 2 (229 days, IQR 174–319 days; logrank: P = 0.012).
Conclusions
Treatment with BC 007 for β1‐AAB neutralization was safe, resulted in a long‐lasting reduction of β1‐AAB combined with improved cardiac function and prolonged the survival of DP with DCM. Using a natural large animal model of DCM considered superior to small animal models of immunization‐induced cardiomyopathy, combined with a study design comparable with clinical trials, we believe that our results provide the basis for optimism that treatment with BC 007 might also be effective in human patients with DCM.
Keywords: Anti‐beta1‐adrenergic receptor antibodies, BC 007, Cardiomyopathy, Doberman Pinscher, Heard failure, Treatment
Introduction
The aptamer BC 007 is a 15‐mer single‐strand DNA oligonucleotide (5'‐GGTTGGTGTGGTTGG‐3') that has recently been patented for its potency to neutralize autoantibodies directed against G protein‐coupled receptors (GPCRs), a new class of autoantibodies discovered in the second half of the 1970s.1, 2, 3 In contrast to ‘classic' autoantibodies, inflammatory or destruction injury is not invariably the consequence of the attack of autoantibodies on cells, tissues, or organs. This new type of autoantibody specifically binds to the extracellular domains of GPCR. After binding to GPCR, the related autoantibodies (GPCR‐AAB) exert predominantly but not exclusive agonistic effects,4, 5, 6, 7 leading to the modulation of receptor‐mediated signal cascades with an impact on physiological functions; these are therefore called ‘functional autoantibodies'. Finally, GPCR‐AAB induce disturbed metabolic balance and pathological conditions that are crucial in GPCR‐AAB‐associated autoimmunity. The related diseases could be summarized as ‘functional autoantibody disease', whereby cardiovascular diseases and diseases associated with vascular pathologies are clearly predominant in the presence of GPCR‐AAB.8
Among the GPCR‐AAB‐positive diseases, human dilated cardiomyopathy (DCM) belongs to those with the highest prevalence of GPCR‐AAB.
Originally, on the basis of the Olmstead country study, a DCM prevalence of nearly 1/2500 was reported in general reference books. However, recent re‐calculation of the Olmstead data revised the prevalence of DCM to almost 10‐fold higher: 1/250.9 DCM patients present highly prevalent (up to 80%) autoantibodies against the β1‐adrenergic receptor (β1‐AAB). Sometimes, β1‐AAB are combined with autoantibodies against the muscarinic M2 receptor (prevalence: up to 40%), both regarded as disease drivers.10 Not least for this reason, DCM pioneered the establishment and further development of the concept of ‘functional autoantibody disease', which, at the end of the last millennium, led to the testing of a treatment concept in which the blood of patients with DCM was cleared by extracorporeal immunoadsorption of GPCR‐AAB and in particular β1‐AAB. In the majority of these studies, clear short‐ and long‐term benefits were demonstrated through improved cardiac function and, above all, longer survival.11 However, cost factors, logistic problems, and the burden of immunoadsorption on patients should not be underestimated, and alternative treatment strategies were sought, such as the in vivo neutralization of GPCR‐AAB. To achieve such a treatment concept, BC 007, an aptamer that successfully neutralizes several cardiovascular‐pathogenic GPCR‐AAB in vitro, including β1‐AR‐AAB, has been introduced12 and successfully tested for the in vivo neutralization of β1‐AAB in spontaneously hypertensive rats13 and recently also in humans.14 However, studies to demonstrate the benefit of β1‐AAB neutralization by BC 007 in DCM are still lacking. To overcome this, we aimed to use the current animal study to test the efficacy of BC 007 to neutralize β1‐AAB in vivo, as well as testing its safety and the resulting outcome of the treated animals. For this study, we used client‐owned Doberman Pinschers (DP) with DCM (prevalence: 58.2% in a European DP population15). DP with DCM show many similarities to human DCM,16, 17, 18, 19, 20 and, most importantly, as for human DCM, DP DCM is closely associated with β1‐AAB (prevalence: 67.8%), with an indication for the disease‐driving role of β1‐AAB.21 In the present study, we show that i.v. treatment with BC 007 induced no adverse effects, effectively reduced β1‐AAB in DP with DCM and resulted in improved long‐term outcome of the dogs.
Methods
Study design
The study was conducted in accordance with the German animal welfare law. The study protocol was approved by ‘Regierung von Oberbayern—Sachgebiet 54, Verbraucherschutz und Veterinärwesen (approval number 55.2‐1‐54‐2532‐35‐2016)'. This study was a prospective, clinical, controlled exposure study.
Animals
Client‐owned purebred DP attending the Cardiology Department of ‘Medizinische Kleintierklinik, Ludwig‐Maximilians‐Universität München' for routine check‐up, cardiomyopathy diagnostics or cardiomyopathy follow‐up were analysed for DCM between October 2013 and January 2017 and consecutively enrolled in the study after signed consent was obtained from the owners.
Based on the guidelines of the European Society of Veterinary Cardiology (ESVC),22 DCM was diagnosed by echocardiograph indicative for cardiac dysfunction: left ventricular end‐systolic volume index (ESVI) (>55 mL/m2) and end‐diastolic volume index (EDVI) (>95 mL2) indexed to body surface area based on Simpson's method. After the owner gave consent, blood was sampled for the measurement of β1‐AAB.
Measurement of β1‐AAB
For the measurement of β1‐AAB, 2 mL of venous blood was taken. The serum was immediately centrifuged and frozen at −80°C until analysis. To measure β‐AABs, a bioassay established by Wallukat and Wollenberger was used,23 which was modified and standardized as described in Wallukat et al.24 In this bioassay, the chronotropic response of spontaneously beating cultured neonatal rat cardiomyocytes to the IgG prepared from the dogs' serum was recorded. For this purpose, six fields with synchronic and rhythmic beating cardiomyocytes were marked on the culture flask. The basal beating rate of the six fields was counted for 15 s and averaged. After addition of the IgG preparation to the culture flasks and incubation for 40 to 60 min at 37°C, the beating rate in the six fields were counted again for 15 s and averaged (one unit of β1‐AAB activity = 1 beat/min frequency increase; lower limit of detection (LLD) = 4.0 U; cut‐off β 1‐AAB positivity ≥8.0 U). Through the use of bisoprolol for specific blocking of the β1‐adrenergic receptor, the cells' chronotropic response can be attributed to β1‐AAB.
For comprehensive information about the IgG preparation, bioassay test set‐up, and procedure for the measurement of β1‐AAB, see other works.25, 26
Study groups
Three study groups (the BC 007 group, Control group 1, and Control group 2) were created. Each group consisted of 14 dogs. The BC 007 group and the Control group 1 were prospectively generated. β1‐AAB were measured in every dog of the BC 007 group and Control group 1 before study inclusion. Dogs were included in the BC 007 group if they were β1‐AAB‐positive (≥8.0 U) and presented DCM related to the inclusion criteria. If a dog was β1‐AAB‐positive and fulfilled the inclusion criteria for DCM but the owner declined the treatment with BC 007 or could not commit to bringing the dog to all follow‐up appointments, it was assigned to Control group 1. Control group 2 was retrospectively generated. For the selection of related dogs, the database of all DP participating in the continuing prospective longitudinal study from March 2005 to January 2017 was sorted for EDVI and ESVI. Dogs that were the closest in the database to the dogs in the BC 007 group were selected for Control group 2.
Exclusion criteria
Doberman Pinschers diagnosed with significant systemic diseases or congenital or acquired heart diseases other than DCM were excluded from the study. Dogs that had atrial fibrillation and/or signs of congestive heart failure (CHF) were excluded.
Examinations
Detailed clinical history was gathered, including information about sex, age, body weight, medication, and known systemic diseases. A complete general clinical examination, echocardiography, and Holter electrocardiogram (ECG) were performed on all dogs. In the BC 007 group, complete blood count analyses and chemistry screens as well as blood pressure measurements were undertaken to monitor adverse events. On the day of the infusion of BC 007, blood coagulation was monitored. In case of clinical signs of CHF, X‐ray examinations were undertaken on dogs. Table 1 shows an overview and timeline of the performed examinations.
Table 1.
Study design
| Screening/baseline Day −28 to Day 0 | Infusion Day 0 | Day 1 | Day 10 | Day 30 | 3 months | 6 months | 9 months | |
|---|---|---|---|---|---|---|---|---|
| Clinical history | X | A | A | A | A | X | X | X |
| Clinical examination | X | A | A | A | A | X | X | X |
| Intensive adverse event assessment | — | A | A | — | — | — | — | — |
| Blood coagulation | A | A | — | — | — | — | — | — |
| Blood count | A | A | A | A | A | A | A | A |
| Serum chemistry | A | A | A | A | A | A | A | A |
| Echocardiography | X | A | A | A | A | X | X | X |
| Holter monitoring | X | A | A | A | A | X | X | X |
| Blood pressure | A | A | A | A | A | A | A | A |
A, dogs treated with BC 007; X, all dogs.
Echocardiography
A complete echocardiogram with simultaneous ECG was performed and assessed by cardiology residents or diplomats in right and left lateral recumbency on unsedated dogs using a 2.0–3.5 MHz (Vivid 7 dimensions; General Electric Medical Systems, Waukesha, WI) or a 1–5 MHz (EPIQ 7C; Philips GmbH Market DACH, Germany) transducer according to official recommendations.27 The left ventricular EDVI and ESVI were measured using Simpson's method of disc. The left ventricular EDVI and ESVI were obtained by normalizing the values to the body surface area. The ratio of the left atrial dimension to the aortic annulus dimension (LA:Ao) was obtained from the right parasternal short‐axis two‐dimensional view.
Holter electrocardiogram
Twenty‐four‐hour ambulatory Holter recordings were performed on all dogs. Two different commercially available Holter analysis software programs (Amedtech ECGpro Holter software, EP 810 digital Recorder; Medizintechnik Aue GmbH, Aue, Germany; Custo tera; Arcon Systems GmbH, Starnberg, Germany) were used. Manual verification of the arrhythmias recognized by the software programs was performed. For statistical analysis, the total number of ventricular premature complexes (VPCs), the fastest rate of all VPCs, and the number of ventricular tachycardia events were used.
Administration of BC 007
BC 007 was administered via an i.v. infusion over 20 min. During the infusion, the dog was lying on a blanket on the floor in a position it preferred. Heart rate and rhythm were monitored continuously via ECG. In addition, all vital parameters were checked every for 5 min.
To find the effective dose that can completely neutralize all β1‐AABs, the dose was gradually reduced from 4 mg via 2 to 1 mg/kg body weight (b.w.).
Concomitant treatment
All concomitant medications that DP received during the course of the study are recorded in Table 2. All DP were treated with pimobendan, except one dog in the BC 007 group (see following discussion). DP that developed CHF during the course of the study also received furosemide. Depending on the malignancy criteria in the Holter ECG, dogs received different antiarrhythmic drugs. The following concomitant drugs were prescribed: Sotalol hydrochloride, Amiodarone hydrochloride, Mexiletine hydrochloride, Flecainide acetate, and Ramipril. The same criteria for the prescription of medication were applied to all DP.
Table 2.
Baseline characteristics of the Doberman Pinschers of the treatment and control groups at enrolment (mean ± standard deviation or number and percentage)
| Parameter | Aptamer BC 007 | Control 1 | Control 2 | P‐value C1 | P‐value C2 |
|---|---|---|---|---|---|
| Basic parameters | |||||
| Gender (male/female) | 8/6 (57%/43%) | 7/7 (50%/50%) | 9/5 (64%/36%) | 0.705 | 0.699 |
| Age (years) | 7.2 ± 2.1 | 6.9 ± 2.3 | 7.3 ± 2.7 | 0.765 | 0.913 |
| Weight (kg) | 36.7 ± 6.5 | 37.2 ± 5.8 | 37.3 ± 5.0 | 0.824 | 0.784 |
|
β1‐AAB (Δ beats/min) |
21 ± 3 | 20 ± 6 | 0.400 | ||
| Cardiac treatment | |||||
| Pimobendan | 13/14 (93%) | 14/14 (100%) | 14/14 (100%) | 1.000 | 1.000 |
| Ramipril | 12/14 (86%) | 12/14 (86%) | 13/14 (93%) | 1.000 | 1.000 |
| Sotalol | 5/14 (36%) | 4/14/28%) | 5/14 (36%) | 1.000 | 1.000 |
| Mexiletine | 1/14 (7%) | 0/14 (0%) | 0/14 (0%) | 1.000 | 1.000 |
| Flecainide | 5/14 (36%) | 4/14 (28%) | 2/14 (14%) | 1.000 | 0.385 |
| Amiodarone | 2/14 (14%) | 4/14 (28%) | 4/14 (28%) | 0.648 | 0.648 |
| Furosemide | 1/14 (7%) | 0/14 (0%) | 0/14 (0%) | 1.000 | 1.000 |
| Dogs treated with antiarrhythmics | 7/14 (50%) | 8/14 (57%) | 9/14 (64%) | 0.705 | 0.445 |
| Time since start of pimobendan (months) | 4.6 ± 4.9 | 3.3 ± 1.6 | 4.8 ± 2.0 | 0.352 | 0.854 |
| Echocardiography | |||||
| EDVI (mL/m2) | 117 ± 33 | 108 ± 17 | 120 ± 33 | 0.349 | 0.814 |
| ESVI (mL/m2) | 81 ± 31 | 69 ± 16 | 80 ± 28 | 0.218 | 0.883 |
| LA:Ao | 1.36 ± 0.28 | 1.38 ± 0.09 | 1.36 ± 0.21 | 0.845 | 0.952 |
| 24 h Holter ECG | |||||
| VPCs per 24 h | 721 ± 1019 | 1821 ± 3159 | 503 ± 892 | 0.226 | 0.554 |
| FR of VPCs (b.p.m.) | 259 ± 36 | 270 ± 49 | 248 ± 42 | 0.510 | 0.455 |
| VTach per 24 h | 0.14 ± 0.54 | 2.21 ± 6.66 | 0.14 ± 0.54 | 0.256 | 1.000 |
| Number of dogs with VTach | 1/14 (7%) | 3/14 (21%) | 1/14 (7%) | 0.596 | 1.000 |
Δ PR/min, change in pulse rate per minute; b.p.m., beats per minute; EDVI, end‐diastolic volume index; ESVI, end‐systolic volume index; FR, the fastest rate of all VPCs; LA:Ao, left atrial dimension to the aortic annulus dimension; VPCs, ventricular premature complexes; VTach, number of ventricular tachycardia.
Endpoint
The endpoint was defined as death from cardiac reasons. This included either sudden cardiac death or death because of CHF.
Statistical analysis
Statistical analysis was performed using PASW Statistics, Version 18.0; IBM Corporation, Armonk, NY. Undetectable β1‐AAB activities (below the LLD) were numerically expressed as values representing one‐half of the LLD. Continuous variables are presented as mean ± standard deviation unless specified otherwise. For categorical data, the frequency of occurrence was determined. Baseline characteristics of the three study groups were summarized and assessed for homogeneity between the BC 007 group and each of the control groups. Continuous baseline variables were compared by an unpaired Student's t‐test. For categorical variables, Pearson's x2 test or Fisher's exact test was used. The proportion of DP experiencing the endpoint was compared between groups using a x2 test. The median time to endpoint was estimated, and survival curves were generated using the Kaplan–Meier method. A comparison of the median time to endpoint was performed using a logrank test. DP that died of non‐cardiac causes or were still alive on the last day of follow‐up were censored. Additionally, an all‐cause mortality, analysis was performed using the Kaplan–Meier method to estimate overall survival. Survival time was compared by the logrank test. The univariate Cox regression analysis was performed to identify variables that are independently associated with cardiac mortality. For the analysis, the following baseline variables were included: treatment with BC 007, age, sex, ESVI, LA:Ao, VPCs, the fastest rate of VPCs, and ventricular tachycardia. All baseline variables that had a P‐value of <0.1 in the univariate analysis were included in the multivariable Cox regression analysis. The multivariable analysis was performed in a backward stepwise manner. The variable with the highest P‐value was eliminated at each subsequent step until in the final model all variables had a P‐value ≤0.05.
For all analyses, a P‐value ≤0.05 was considered significant.
Results
Basic characteristics and β1‐AAB activity at enrolment
Enrolment in the study began in October 2013 and finished in January 2017. Follow‐up was continued until the study was closed in January 2018. In total, 28 DP with DCM were enrolled for prospective examination, 14 in each of the two study groups (BC 007 group/Control group 1). Table 1 shows the timeline of the examinations performed in both groups. Control group 2 was retrospectively designed. The baseline variables of the three groups are summarized in Table 2. The analyses for homogeneity of groups showed no significant difference between the BC 007 group and each of the control groups at baseline. With respect to β1‐AAB activity, the BC 007 group and Control group 1 were not significantly different.
Safety of BC 007
Based on clinical examination and the clinical chemistry, haematology and haemostaseology parameters, no adverse effects strongly associated with BC 007 treatment were observed in any of the treated dogs during the course of the study. In the BC 007 group (four dogs), as well as in Control groups 1 (two dogs) and 2 (three dogs), dogs died because of neoplasia in the follow‐up period; this difference was not significantly different.
Efficacy of BC 007 for β1‐AAB neutralization
For Dogs 1 to 9 of the BC 007 group, β1‐AAB were measured on every visit; the first measurement was 5 min after starting the treatment, and the last was after 24 months in Dog 3. For Dogs 10 to 14 of the BC 007 group, β1‐AABwere measured at least until Day 30.
For the nine dogs being closely monitored for β1‐AAB for 48 h after starting BC 007 treatment, Figure 1 shows that the β1‐AAB level was reduced below the LLD. This was despite the BC 007 dose being lowered from 4 mg/kg b.w. (Dogs 1–6) to 2 mg/kg b.w. (Dog 7) and to 1 mg/kg b.w. (Dogs 8 and 9), which was consequently applied for DP 10 to 14. Among the treated DP, β1‐AAB return was observed at the earliest after 9 months. All dogs were pre‐treated with pimobendan at least 2 weeks before study inclusion.
Figure 1.
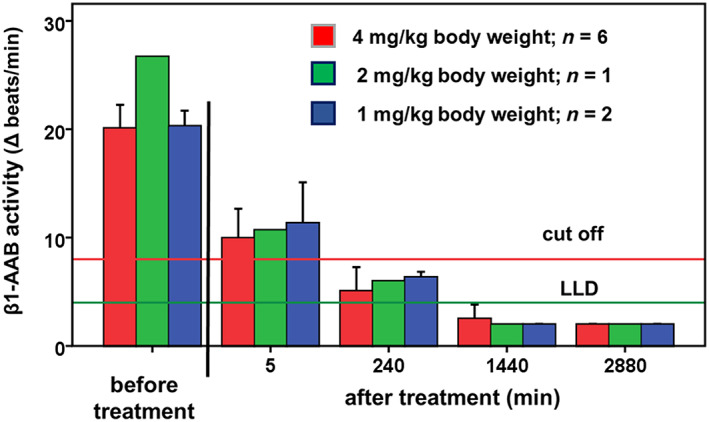
Time‐dependent β1‐AAB activity in the serum of dogs before and after treatment of BC 007. LLD, lower limit of detection
Only one DP was already positive again for β1‐AAB at 3 months. This dog fulfilled the admission criteria with an ESVI = 55.3 mL/m2 immediately at the time of enrolment. However, its systolic dysfunction was only very mild and the authors decided to give BC 007 without starting pimobendan treatment beforehand. Consequently, this dog was the only one that did not receive pimobendan at the beginning of the study. Because of the early return of β1‐AAB, the dog was treated with BC 007 a second time after 9 months, which in turn led to a lowering of β1‐AAB below the LLD for the next 3 months.
Follow‐up
Left ventricular end‐diastolic volume/body surface area and left ventricular systolic volume/body surface area
After BC 007 administration and the disappearance of β1‐AAB for at least 3 month, 10 dogs presented with decreased or stabilized left ventricular sizes compared with the sizes before BC 007 treatment. Figure 2 A shows the results for a DP remaining free of β1‐AAB following BC 007 treatment until the study end (12 months), with EDVI and ESVI decreasing continuously from 93.48 and 56.68 mL/m2 to 57.78 and 34.67 mL/m2, respectively. None of the dogs had relevant mitral valve insufficiency secondary because of volume overload. Figure 2 B shows the dog that was treated twice. This DP presented with reduced EDVI (89.70 vs. 81.45 mL/m2) and ESVI (55.28 vs. 49.97 mL/m2) 1 month after the first BC 007 treatment; for ESVI, there was a further reduction to 49.07 mL/m2 at the follow‐up examination after 3 months. However, thereafter, EDVI and ESVI started to increase until the second treatment at 9 months to EDVI of 97.97 mL/m2 and ESVI of 62 mL/m2. After the second BC 007 treatment, EDVI and EDVI decreased again to 73.71 mL/m2 and ESVI to 40.79 mL/m2 until 15 months after the first and 6 months after the second treatment, respectively. EDVI (88.10 mL/m2) and ESVI (59.00 mL/m2) re‐increased thereafter, but neither exceeded the pre‐study levels.
Figure 2.
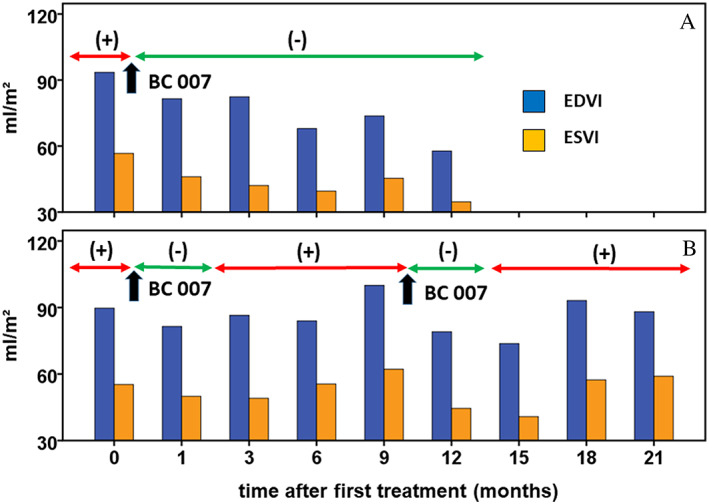
Left ventricular end‐diastolic volume/body surface area (EDVI) and left ventricular systolic volume/body surface area (ESVI) after BC 007 treatment related to the β1‐AAB positivity (+) and β1‐AAB negativity (−) demonstrated exemplarily for two Doberman Pinschers
Survival
The median time in the study for all DP was 289 days. Twenty‐nine of the 42 dogs reached the endpoint, giving an overall event rate of 69%. Fifty per cent of the DP in the BC 007 group, 85.7% of those in Control group 1, and 71.4% of those in Control group 2 reached the endpoint. The proportion of DP that reached the endpoint was not significantly different between the BC 007 group and Control group 1 (P = 0.103), or between the BC 007 group and Control group 2 (P = 0.246). However, dogs in Control group 1 experienced sudden cardiac death more often than dogs in the BC 007 group (P = 0.008). The outcome of the 42 dogs is summarized in Table 3.
Table 3.
Outcome of all under study
| Outcome | BC 007 (n = 14) | Control 1 (n = 14) | Control 2 (n = 14) |
|---|---|---|---|
| Sudden cardiac death | 4 (29%) | 11 (79%) | 7 (50%) |
| Congestive heart failure | 3 (21%) | 1 (7%) | 3 (21%) |
| Neoplasia | 4 (29%) | 2 (14%) | 3 (21%) |
| Other non‐cardiac deaths | 0 (0%) | 0 (0%) | 1 (7%) |
| Still alive | 3 (21%) | 0 (0%) | 0 (0%) |
No DP was lost to follow‐up. The estimated median time to endpoint was significantly longer for DP that received BC 007 [572 days, interquartile range (IQR) 442–840 days] than for DP in Control group 1 (266 days, IQR 97–438 days; logrank: P = 0.009) and Control group 2 (229 days, IQR 174–319 days; logrank: P = 0.012) (Figures 3 and 4 ). The median time to reach the endpoint for DP in the BC 007 group was approximately 10 and 11 months longer, respectively, compared with the control groups.
Figure 3.
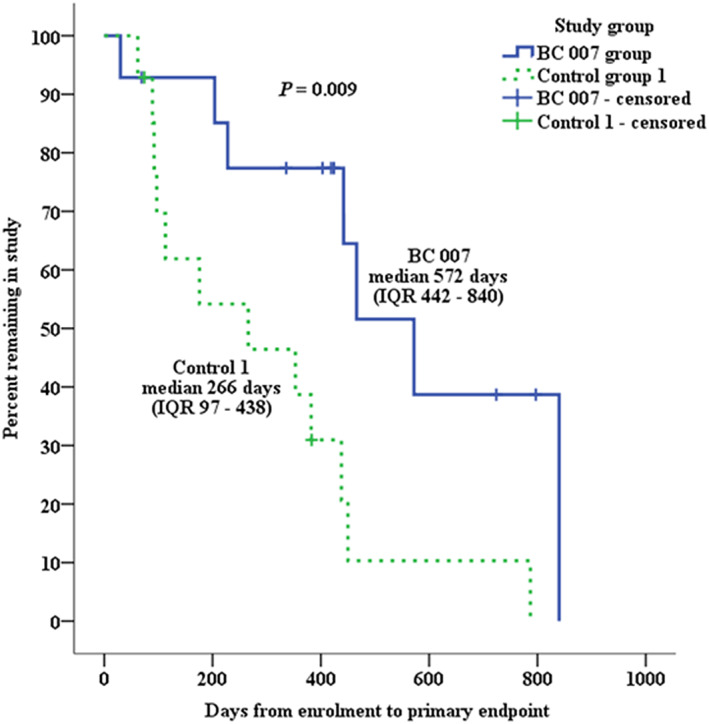
Kaplan–Meier survival curves plotting the estimated percentage of dogs in the BC 007 group and Control group 1 that have not yet met the primary endpoint, against time. IQR, interquartile range
Figure 4.
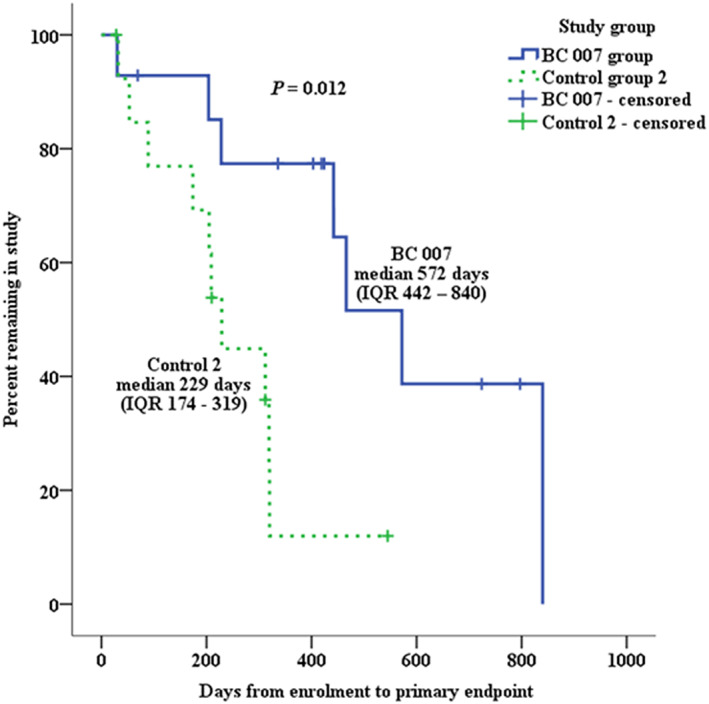
Kaplan–Meier survival curves plotting the estimated percentage of dogs in the BC 007 group and Control group 2 that have not yet met the primary endpoint, against time. IQR, interquartile range
When comparing the all‐cause mortality, the estimated median survival time in the BC 007 group was 442 days (IQR 228–724 days) vs. 176 days (IQR 92–383) in Control group 1 (logrank: P = 0.031) and 209 days (IQR 89–312) in Control group 2 (logrank: P = 0.002). In the univariate Cox regression analysis, when comparing the BC 007 group to Control groups 1 and 2, the following variables demonstrated an association with the time to the primary endpoint at a P‐value <0.1 and were entered in the first run of the multivariate analysis: treatment with BC 007, ESVI, LA:Ao, and VPCs (Tables 4 and 5). The final model of the multivariate Cox regression analysis only contained two variables in both comparisons. Treatment with BC 007 was the only variable showing a beneficial effect on mortality [hazard ratio (HR): 0.081 (95% confidence interval, CI: 0.016–0.406), P = 0.002 and HR: 0.107 (95% CI: 0.026–0.436), P = 0.002]. In contrast, ESVI showed a slight adverse effect on mortality [HR: 1.041 (95% CI 1.014–1.069), P = 0.002 and HR: 1.035 (95% CI: 1.017–1.055), P < 0.001].
Table 4.
Results of the univariate Cox regression analysis comparing the Aptamer BC 007 group and the Control group 1. Variables with a P‐value <0.1 that entered the multivariable analysis are displayed in bold
| Variable | HR | 95% CI of the HR | P‐value |
|---|---|---|---|
| Treatment with Aptamer BC 007 | 0.284 | 0.104–0.776 | 0.014 |
| Age | 1.118 | 0.886–1.409 | 0.347 |
| Sex | 1.271 | 0.490–3.300 | 0.622 |
| ESVI | 1.015 | 0.999–1.032 | 0.060 |
| LA:Ao | 18.110 | 0.939–349.139 | 0.055 |
| VPCs | 1.000 | 1.000–1.000 | 0.007 |
| FR of VPCs | 1.004 | 0.992–1.017 | 0.497 |
| VTach | 1.058 | 0.974–1.149 | 0.181 |
CI, confidence interval; ESVI, end‐systolic volume index; FR, the fastest rate of all VPCs; HR, hazard ratio; LA:Ao, left atrial dimension to the aortic annulus dimension; VPCs, ventricular premature complexes; VTach, number of ventricular tachycardia.
Table 5.
Results of the univariate Cox regression analysis comparing the Aptamer BC 007 group and the Control group 2. Variables with a P‐value <0.1 that entered the multivariable analysis are displayed in bold
| Variable | HR | 95% CI of the HR | P‐value |
|---|---|---|---|
| Treatment with Aptamer BC 007 | 0.257 | 0.084–0.789 | 0.018 |
| Age | 0.962 | 0.781–1.185 | 0.716 |
| Sex | 2.096 | 0.711–6.176 | 0.180 |
| ESVI | 1.008 | 1.008–1.040 | 0.003 |
| LA:Ao | 14.070 | 1.236–160.135 | 0.033 |
| VPCs | 1.001 | 1.000–1.001 | 0.002 |
| FR of VPCs | 1.000 | 0.986–1.014 | 0.991 |
| VTach | 1.054 | 0.379–2.928 | 0.920 |
CI, confidence interval; ESVI, end‐systolic volume index; FR, the fastest rate of all VPCs; HR, hazard ratio; LA:Ao, left atrial dimension to the aortic annulus dimension; VPCs, ventricular premature complexes; VTach, number of ventricular tachycardia.
Discussion
Autoimmunity associated with GPCR‐AAB, specifically with β1‐AAB, is increasingly accepted as a pathogenic driver of DCM in humans.8, 11 As a result, the search for treatment strategies to counteract these autoantibodies has been initiated.
With the finding of aptamers that inhibit the activity of β1‐AAB, new promising drugs for the in vivo neutralization of β1‐AABs has been discovered.28 Compared with antibodies, aptamers present a couple advantages in both biological and chemical properties, as well as in production and delivery; among these, their low immunogenicity favours aptamers for human treatment.29
BC 007 is an aptamer that highly effectively neutralized β1‐AAB isolated from patients with DCM in vitro, as well as several other GPCR‐AAB found in patients with cardiovascular disease and diseases associated with cardiovascular changes.13 BC 007 was therefore proposed for the treatment of patients with DCM, as well as for those patients with DCM who carry further GPCR‐AAB because of comorbidities. Compared to the already successfully tested extracorporeal immunoadsorption for the blood clearance of β1‐AAB, BC 007 treatment for the in vivo neutralization of β1‐AAB should be superior in terms of patient burden, logistics, and costs.
We present here for the first time an animal study showing that the neutralization of β1‐AABs after BC 007 treatment, in addition to the indication of its safety and efficacy for β1‐AAB neutralization, improved the outcomes in DCM.
Animal model and study design
Rodent models, mainly those with cardiomyopathy artificially induced by immunization, were used to demonstrate the role of β1‐AAB as cardiomyopathy driver and consequently as treatment target.30, 31 However, based on ELISA experiments,32 the functional autoantibodies found in the immunization models seem to differ from human autoantibodies in both quality and quantity. Furthermore, because of several disadvantages between small animal models such as mice and rats, as well as humans, for example, in genetic regulation and cardiac performance listed in other works,33, 34 it was stated that ‘… for research aimed at clinical translation, it is imperative that initial results from small rodent studies be confirmed in a large animal model that more closely resembles humans …'.35
In accordance with this, we have selected DP as a model because of the high prevalence of DCM,15 with many similarities compared with the related human disease,16, 17, 18, 19, 20 including the indication for β1‐AAB as a DCM driver.21 With DP as the study model, and using the enrolment of client‐owned purebred DP attending a veterinary medical institution for routine check‐up, disease diagnostics, or follow‐up, we were able to design our study in a manner similar to common clinical studies. In addition, the DP of our study came from different breeding populations throughout Europe and therefore have greater genetic diversity, which can better reflect the pathogenic situation in human DCM.
Safety of BC 007
BC 007 was safe during treatment and immediately thereafter (Days 0 and 1), as well as in the follow‐up. In this context, and in addition to relevant markers of clinical chemistry and haematology, we specifically monitored the influence of BC 007 on coagulation because of its potency for thrombin inhibition that was primarily suggested as indication for the treatment with this aptamer. However, an insufficient dose–response relationship counteracted further developments in this area as cited in Keefe and Schaub.36
In the current study, blood clotting was closely observed on the day of BC 007 treatment, but clinically relevant prolongation of bleeding could be excluded. In our opinion, the much lower dose of BC 007 required for neutralization of β1‐AABs compared with the dose for anticoagulation, together with the very short half‐life of BC 007,36, 37, 38 prevented any clotting problems that might counteract the administration of BC 007 for GPCR‐AAB neutralization. This agreed with recently published data of the BC 007 clinical trial Phase 1.38
Additionally, the treated dogs were monitored closely for other adverse events during the complete duration of the study. Although four dogs in the BC 007 group died in the follow‐up because of neoplasia, this could not be attributed to any adverse effect of BC 007 because deaths because of neoplasia were in a comparable range in the untreated control groups (two dogs in Control group 1 and three dogs in Control group 2). Furthermore, it is known that DP possess a higher risk of neoplasia in general.39 No other BC 007‐related adverse events were observed, indicating that BC 007 administration is safe and tolerable. This confirmed the data from a standard battery of pharmacological and toxicological safety studies that had already been performed in rats and dogs and that allowed BC 007 to be clinically tested in humans.
Efficiency of BC 007 for β1‐AAB neutralization
To determine the effective dose of BC 007 for β1‐AAB neutralization, the administered dose of BC 007 was gradually reduced from 4 mg via 2 to 1 mg/kg b.w. Because the BC 007 dose of 1 mg/kg b.w. was effective for complete β1‐AAB neutralization, the majority of DP (seven dogs) were treated with this dose. We cannot completely rule out that the different doses of BC 007 will in principle have an influence on the outcome of the DP. Because of the short half‐life of BC 007 and the fact that all DP were β1‐AAB negative after treatment, such an effect seems rather unlikely, which is why we are skeptical about any relationship between the administered dose of BC 007 (2 mg/kg b.w.) and the very early return of β1‐AAB in DP 7. DP 7 was the only animal that did not receive pimobendan before BC 007 treatment. Pimobendan is a calcium sensitizer and an inhibitor of phosphodiesterase 3 with positive inotropic and vasodilatory potency, which prolonged the time to the onset of clinical signs in DP with preclinical DCM.40 However, to what extent the early return of β1‐AAB in DP 7 can be explained by the fact that this dog did not receive pimobendan must remain speculative.
Follow‐up
Left ventricular end‐diastolic volume/body surface area and left ventricular systolic volume/body surface area
For DCM patients, the increased left ventricular ejection fraction (LVEF) calculated from the EDVI and ESVI is a commonly used indicator for the benefit of β1‐AAB blood clearance by extracorporeal immunoadsorption whereby, with respect to the different study times, improved cardiac function visible by decreased LVED was seen 3, 6, and 12 months, respectively, after the treatment, as summarized in Becker et al.11
As demonstrated for 10 of the 14 treated DP, we have now used the measurement of EDVI and EVSI to show that the benefit of β1‐AAB blood clearance by immunoadsorption demonstrated in patients with DCM could also be achieved by the in vivo neutralization of β1‐AAB by BC 007. In addition, as clearly shown in Figure 2 A and B, the time interval with functional heart improvement was clearly associated with the β1‐AAB‐free time interval. This corroborated the data of β1‐AAB immunoadsorption studies, where the patient benefit (decreased NYHA class) disappeared after the return of β1‐AAB41 or only patients with complete β1‐AAB clearance profited from immunoadsorption.42
In accordance with the ESVC guidelines for the diagnosis of DCM in DP, we have used the EDVI and ESVI to diagnose and monitor the disease progression and the effect of BC007, whereas in human patients, the LVEF is usually used.22 However, the LVEF is just calculated form EDVI and ESVI and looking directly at the remodelling in diastole and systole allowed us to evaluate the remodelling and systolic function directly and not just by the LVEF. Patients with DCM have often severe mitral regurgitation secondary to LV dilation, although in our study the cardiac dilation was not yet so severe, and therefore, none of the DP had relevant mitral insufficiency. In patients with secondary mitral regurgitation, ESV can be relatively low, despite of a low stroke volume and LVEF calculation from the EDV and ESV can reveal misleadingly higher values because in fact, EF represents the fraction of blood that leaves the ventricle during systole (i.e. stroke volume + regurgitation volume into the left atrium).43
Focusing on LV volume changes instead of EF measurements is therefore a strategy if a regurgitation volume cannot be reliably measured, and thus, this strategy was chosen by the authors of the study before the start of the study.
Survival
The median time to the primary endpoint was significantly longer in the BC 007 group compared with the control groups, related to the univariate Cox regression analysis, because of the BC 007 treatment. In the multivariate analysis, this effect is strengthened further with a lower hazard ratio and narrower confidence intervals.
Furthermore, it can be concluded from the multivariate analysis that heart size is the main negative factor for survival time. This finding is consistent with another study that determined the predictors of sudden cardiac death in DP.44
The median time to the primary endpoint was prolonged for approximately 10–11 months. This prolongation approximately equalled the β1‐AAB‐free time until the recurrence of autoantibodies. This agrees with our recent study that β1‐AAB‐positive DP with DCM have a significantly shorter survival time than comparable DP without β1‐AAB.21
Last but not least, the longer survival time because of neutralizing β1‐AAB by BC 007 in DP with DCM strongly corresponded to the survival effects published for people with DCM who were treated with immunoadsorption for β1‐AAB removal.45
Limitations
The most relevant limitation is the small sample size, which prevented the exact analysis of BC 007 on the echocardiographic and electrocardiographic variables. In addition, because of the small number of dogs, the estimated IQRs and CIs are relatively wide. The results should therefore be interpreted with caution, before further studies have manifested the data.
Conclusions
Treatment with the aptamer BC 007 was safe in DP with DCM, showed a high efficiency in neutralizing β1‐AAB, and provided benefits because of prolonged survival time. As we collected the data in a natural large animal model of DCM, which is considered superior to small animal models of immunization‐induced cardiomyopathy, combined with the possibility of designing our DP study in a manner comparable with clinical studies, we believe that our results provide the basis for optimism that treatment with BC 007 might also be effective in human patients with DCM.
Conflict of interest
The authors declare that the study was not funded by any public, commercial, or private funds. G.W., N.‐P.B., K.W., J.M., and I.S. as employees and shareholder (G.W., J.M., and I.S.) of Berlin Cures GmbH declare that Berlin Cures GmbH only provided support in the form of salaries and research materials but did not have any additional role in the study design, data collection, analysis and statistical evaluation, decision to publish, or preparation of the manuscript. S.W. and G.W. declare that they have no conflict of interest.
Werner, S. , Wallukat, G. , Becker, N.‐P. , Wenzel, K. , Müller, J. , Schimke, I. , and Wess, G. (2020) The aptamer BC 007 for treatment of dilated cardiomyopathy: evaluation in Doberman Pinschers of efficacy and outcomes. ESC Heart Failure, 7: 844–855. 10.1002/ehf2.12628.
Contributor Information
Ingolf Schimke, Email: schimke@berlincues.de.
Gerhard Wess, Email: g.wess@medizinische-kleintierklinik.de.
References
- 1. Borda E, Pascual J, Cossio P, De La Vega M, Arana R, Sterin‐Borda L. A circulating IgG in Chagas' disease which binds to beta‐adrenoceptors of myocardium and modulates their activity. Clin Exp Immunol 1984; 57: 679–686. [PMC free article] [PubMed] [Google Scholar]
- 2. Smith BR, Pyle GA, Petersen VB, Hall R. Interaction of thyroid‐stimulating antibodies with the human thyrotrophin receptor. J Endocrinol 1977; 75: 401–407. [DOI] [PubMed] [Google Scholar]
- 3. Sterin‐Borda L, Cossio PM, Gimeno MF, Gimeno AL, Diez C, Laguens RP, Meckert PC, Arana RM. Effect of chagasic sera on the rat isolated atrial preparation: immunological, morphological and function aspects. Cardiovasc Res 1976; 10: 613–622. [DOI] [PubMed] [Google Scholar]
- 4. Jahns R, Boivin V, Lohse MJ. Beta 1‐adrenergic receptor‐directed autoimmunity as a cause of dilated cardiomyopathy in rats. Int J Cardiol 2006; 112: 7–14. [DOI] [PubMed] [Google Scholar]
- 5. Limas CJ, Goldenberg IF, Limas C. Influence of anti‐beta‐receptor antibodies on cardiac adenylate cyclase in patients with idiopathic dilated cardiomyopathy. Am Heart J 1990; 119: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 6. Wallukat G, Wollenberger A. Autoantibodies to beta 2‐adrenergic receptors with antiadrenergic activity from patients with allergic asthma. J Allergy Clin Immunol 1991; 88: 581–587. [DOI] [PubMed] [Google Scholar]
- 7. Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig 2003; 10: 82–93. [DOI] [PubMed] [Google Scholar]
- 8. Becker NP, Muller J, Gottel P, Wallukat G, Schimke I. Cardiomyopathy ‐ An approach to the autoimmune background. Autoimmun Rev 2017; 16: 269–286. [DOI] [PubMed] [Google Scholar]
- 9. Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 2013; 10: 531–547. [DOI] [PubMed] [Google Scholar]
- 10. Wallukat G, Schimke I. Agonistic autoantibodies directed against G‐protein‐coupled receptors and their relationship to cardiovascular diseases. Semin Immunopathol 2014; 36: 351–363. [DOI] [PubMed] [Google Scholar]
- 11. Becker NP, Goettel P, Mueller J, Wallukat G, Schimke I. Functional autoantibody diseases: basics and treatment related to cardiomyopathies. Front Biosci (Landmark Ed) 2019; 24: 48–95. [DOI] [PubMed] [Google Scholar]
- 12. Haberland A, Holtzhauer M, Schlichtiger A, Bartel S, Schimke I, Muller J, Dandel M, Luppa PB, Wallukat G. Aptamer BC 007 ‐ A broad spectrum neutralizer of pathogenic autoantibodies against G‐protein‐coupled receptors. Eur J Pharmacol 2016; 789: 37–45. [DOI] [PubMed] [Google Scholar]
- 13. Wallukat G, Muller J, Haberland A, Berg S, Schulz A, Freyse EJ, Vetter R, Salzsieder E, Kreutz R, Schimke I. Aptamer BC007 for neutralization of pathogenic autoantibodies directed against G‐protein coupled receptors: a vision of future treatment of patients with cardiomyopathies and positivity for those autoantibodies. Atherosclerosis 2016; 244: 44–47. [DOI] [PubMed] [Google Scholar]
- 14. Müller J, Haberland A, Wallukat G, Becker N‐P, Wenzel K, Göttel P, Schulze‐Rothe S, Schimke I, Yilmaz T, Abay A, Golor G, Grossmann M, Sinn A, Hönicke A‐S, Davideit H, Becker S. The DNA‐based drug BC 007 neutralizes agonistically acting autoantibodies directed against G protein–coupled receptors – successful mode of action demonstrated in clinical phase 1 trial. Chim Oggi 2019; 2: 65–67. [Google Scholar]
- 15. Wess G, Schulze A, Butz V, Simak J, Killich M, Keller LJ, Maeurer J, Hartmann K. Prevalence of dilated cardiomyopathy in Doberman Pinschers in various age groups. J Vet Intern Med 2010; 24: 533–538. [DOI] [PubMed] [Google Scholar]
- 16. Hamlin RL. Animal models of ventricular arrhythmias. Pharmacol Ther 2007; 113: 276–295. [DOI] [PubMed] [Google Scholar]
- 17. Hensley MT, Tang J, Woodruff K, Defrancesco T, Tou S, Williams CM, Breen M, Meurs K, Keene B, Cheng K. Intracoronary allogeneic cardiosphere‐derived stem cells are safe for use in dogs with dilated cardiomyopathy. J Cell Mol Med 2017; 21: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Grady MR, O'Sullivan ML. Dilated cardiomyopathy: an update. Vet Clin North Am Small Anim Pract 2004; 34: 1187–1207. [DOI] [PubMed] [Google Scholar]
- 19. Petric AD, Stabej P, Zemva A. Dilated cardiomyopathy in Doberman Pinschers: survival, causes of death and a pedigree review in a related line. J Vet Cardiol 2002; 4: 17–24. [DOI] [PubMed] [Google Scholar]
- 20. Smucker ML, Kaul S, Woodfield JA, Keith JC, Manning SA, Gascho JA. Naturally occurring cardiomyopathy in the Doberman Pinscher: a possible large animal model of human cardiomyopathy? J Am Coll Cardiol 1990; 16: 200–206. [DOI] [PubMed] [Google Scholar]
- 21. Wess G, Wallukat G, Fritscher A, Becker NP, Wenzel K, Muller J, Schimke I. Doberman Pinschers present autoimmunity associated with functional autoantibodies: a model to study the autoimmune background of human dilated cardiomyopathy. PLoS ONE 2019; 14: e0214263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wess G, Domenech O, Dukes‐McEwan J, Haggstrom J, Gordon S. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman Pinschers. J Vet Cardiol 2017; 19: 405–415. [DOI] [PubMed] [Google Scholar]
- 23. Wallukat G, Wollenberger A. Effects of the serum gamma globulin fraction of patients with allergic asthma and dilated cardiomyopathy on chronotropic beta adrenoceptor function in cultured neonatal rat heart myocytes. Biomed Biochim Acta 1987; 46: S634–S639. [PubMed] [Google Scholar]
- 24. Wallukat G, Munoz Saravia SG, Haberland A, Bartel S, Araujo R, Valda G, Duchen D, Diaz Ramirez I, Borges AC, Schimke I. Distinct patterns of autoantibodies against G‐protein‐coupled receptors in Chagas' cardiomyopathy and megacolon. Their potential impact for early risk assessment in asymptomatic Chagas' patients. J Am Coll Cardiol 2010; 55: 463–468. [DOI] [PubMed] [Google Scholar]
- 25. Bornholz B, Wallukat G, Roggenbuck D, Schimke I. Chapter 3 ‐ Autoantibodies directed against G‐protein‐coupled receptors in cardiovascular diseases: basics and diagnostics In Nussinovitch U., ed. The Heart in Rheumatic, Autoimmune and Inflammatory Diseases. Academic Press; 2017. p 49–63. [Google Scholar]
- 26. Wallukat G, Pruss H, Muller J, Schimke I. Functional autoantibodies in patients with different forms of dementia. PLoS ONE 2018; 13: e0192778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas WP, Gaber CE, Jacobs GJ, Kaplan PM, Lombard CW, Moise NS, Moses BL. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993; 7: 247–252. [DOI] [PubMed] [Google Scholar]
- 28. Haberland A, Wallukat G, Schimke I. Aptamer binding and neutralization of beta1‐adrenoceptor autoantibodies: basics and a vision of its future in cardiomyopathy treatment. Trends Cardiovasc Med 2011; 21: 177–182. [DOI] [PubMed] [Google Scholar]
- 29. Bruno JG. Predicting the uncertain future of aptamer‐based diagnostics and therapeutics. Molecules 2015; 20: 6866–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, Lohse MJ. Direct evidence for a beta 1‐adrenergic receptor‐directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest 2004; 113: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsui S, Fu M, Hayase M, Katsuda S, Yamaguchi N, Teraoka K, Kurihara T, Takekoshi N. Transfer of rabbit autoimmune cardiomyopathy into severe combined immunodeficiency mice. J Cardiovasc Pharmacol 2003; 42: S99–S103. [DOI] [PubMed] [Google Scholar]
- 32. Wenzel K, Schulze‐Rothe S, Muller J, Wallukat G, Haberland A. Difference between beta1‐adrenoceptor autoantibodies of human and animal origin‐Limitations detecting beta1‐adrenoceptor autoantibodies using peptide based ELISA technology. PLoS ONE 2018; 13: e0192615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Recchia FA, Lionetti V. Animal models of dilated cardiomyopathy for translational research. Vet Res Commun 2007; 31: 35–41. [DOI] [PubMed] [Google Scholar]
- 34. Milani‐Nejad N, Brunello L, Gyorke S, Janssen PM. Decrease in sarcoplasmic reticulum calcium content, not myofilament function, contributes to muscle twitch force decline in isolated cardiac trabeculae. J Muscle Res Cell Motil 2014; 35: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Camacho P, Fan H, Liu Z, He JQ. Small mammalian animal models of heart disease. Am J Cardiovasc Dis 2016; 6: 70–80. [PMC free article] [PubMed] [Google Scholar]
- 36. Keefe AD, Schaub RG. Aptamers as candidate therapeutics for cardiovascular indications. Curr Opin Pharmacol 2008; 8: 147–152. [DOI] [PubMed] [Google Scholar]
- 37. Haberland A, Wallukat G, Berg S, Schulz AM, Freyse EJ, Vetter R, Salzsieder E, Muller J, Kreutz R, Schimke I. Neutralization of pathogenic beta1‐receptor autoantibodies by aptamers in vivo: the first successful proof of principle in spontaneously hypertensive rats. Mol Cell Biochem 2014; 393: 177–180. [DOI] [PubMed] [Google Scholar]
- 38. Mueller J, Haberland A, Becker N‐P, Wenzel K, Wallukat G, Goettel P, Schulze‐Rothe S, Schimke I, Golor G, Grossmann M, Sinn A, Steiper M, Yilmaz T, Wallukat A, Davideit H. The DNA‐based therapeutic agent BC 007 completely neutralizes agonistic autoantibodies directed against β1‐adrenoceptors: results of a phase 1 trial. J Am Coll Cardiol 2018; 71: A645. [Google Scholar]
- 39. Yau P, Dhand NK, Thomson PC, Taylor RM. Retrospective study on the occurrence of canine lymphoma and associated breed risks in a population of dogs in NSW (2001–2009). Aust Vet J 2017; 95: 149–155. [DOI] [PubMed] [Google Scholar]
- 40. Summerfield NJ, Boswood A, O'Grady MR, Gordon SG, Dukes‐McEwan J, Oyama MA, Smith S, Patteson M, French AT, Culshaw GJ, Braz‐Ruivo L, Estrada A, O'Sullivan ML, Loureiro J, Willis R, Watson P. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman Pinschers with preclinical dilated cardiomyopathy (the PROTECT Study). J Vet Intern Med 2012; 26: 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wallukat G, Reinke P, Dorffel WV, Luther HP, Bestvater K, Felix SB, Baumann G. Removal of autoantibodies in dilated cardiomyopathy by immunoadsorption. Int J Cardiol 1996; 54: 191–195. [DOI] [PubMed] [Google Scholar]
- 42. Baba A, Akaishi M, Shimada M, Monkawa T, Wakabayashi Y, Takahashi M, Nagatomo Y, Yoshikawa T. Complete elimination of cardiodepressant IgG3 autoantibodies by immunoadsorption in patients with severe heart failure. Circ J 2010; 74: 1372–1378. [DOI] [PubMed] [Google Scholar]
- 43. Hetzer R, Dandel M. Early detection of left ventricular dysfunction in patients with mitral regurgitation due to flail leaflet is still a challenge. Eur Heart J 2011; 32: 665–667. [DOI] [PubMed] [Google Scholar]
- 44. Kluser L, Holler PJ, Simak J, Tater G, Smets P, Rugamer D, Kuchenhoff H, Wess G. Predictors of sudden cardiac death in Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med 2016; 30: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dandel M, Wallukat G, Englert A, Lehmkuhl HB, Knosalla C, Hetzer R. Long‐term benefits of immunoadsorption in beta(1)‐adrenoceptor autoantibody‐positive transplant candidates with dilated cardiomyopathy. Eur J Heart Fail 2012; 14: 1374–1388. [DOI] [PubMed] [Google Scholar]


