Abstract
Aims
Changes in echocardiographic parameters and biomarkers of cardiac and venous pressures or estimated plasma volume during hospitalization associated with decongestive treatments in acute heart failure (AHF) patients with either preserved left ventricular ejection fraction (LVEF) (HFPEF) or reduced LVEF (HFREF) are poorly assessed.
Methods and results
From the metabolic road to diastolic heart failure: diastolic heart failure (MEDIA‐DHF) study, 111 patients were included in this substudy: 77 AHF (43 HFPEF and 34 HFREF) and 34 non‐cardiac dyspnea patients. Echocardiographic measurements and blood samples were obtained within 4 h of presentation at the emergency department and before hospital discharge. In AHF patients, echocardiographic indices of cardiac and venous pressures, including inferior vena cava diameter [from 22 (16–24) mm to 13 (11–18) mm, P = 0.009], its respiratory variability [from 32 (8–44) % to 43 (29–70) %, P = 0.04], medial E/e' [from 21.1 (15.8–29.6) to 16.6 (11.7–24.3), P = 0.004], and E wave deceleration time [from 129 (105–156) ms to 166 (128–203) ms, P = 0.003], improved during hospitalization, similarly in HFPEF and HFREF patients. By contrast, no changes were seen in non‐cardiac dyspnea patients. In AHF patients, all plasma biomarkers of cardiac and venous pressures, namely B‐type natriuretic peptide [from 935 (514–2037) pg/mL to 308 (183–609) pg/mL, P < 0.001], mid‐regional pro‐atrial natriuretic peptide [from 449 (274–653) pmol/L to 366 (242–549) pmol/L, P < 0.001], and soluble CD‐146 levels [from 528 (406–654) ng/mL to 450 (374–529) ng/mL, P = 0.003], significantly decreased during hospitalization, similarly in HFPEF and HFREF patients. Echocardiographic parameters of cardiac chamber dimensions [left ventricular end‐diastolic volume: from 120 (76–140) mL to 118 (95–176) mL, P = 0.23] and cardiac index [from 2.1 (1.6–2.6) mL/min/m2 to 1.9 (1.4–2.4) mL/min/m2, P = 0.55] were unchanged in AHF patients, except tricuspid annular plane systolic excursion (TAPSE) that improved during hospitalization [from 16 (15–19) mm to 19 (17–21) mm, P = 0.04]. Estimated plasma volume increased in both AHF [from 4.8 (4.2–5.6) to 5.1 (4.4–5.8), P = 0.03] and non‐cardiac dyspnea patients (P = 0.01). Serum creatinine [from 1.18 (0.90–1.53) to 1.19 (0.86–1.70) mg/dL, P = 0.89] and creatinine‐based estimated glomerular filtration rate [from 59 (40–75) mL/min/1.73m2 to 56 (38–73) mL/min/1.73m2, P = 0.09] were similar, while plasma cystatin C [from 1.50 (1.20–2.27) mg/L to 1.78 (1.33–2.59) mg/L, P < 0.001] and neutrophil gelatinase associated lipocalin (NGAL) [from 127 (95–260) ng/mL to 167 (104–263) ng/mL, P = 0.004] increased during hospitalization in AHF.
Conclusions
Echocardiographic parameters and plasma biomarkers of cardiac and venous pressures improved during AHF hospitalization in both acute HFPEF and HFREF patients, while cardiac chamber dimensions, cardiac output, and estimated plasma volume showed minimal changes.
Keywords: Acute heart failure, Congestion, Biomarker, Echocardiography, Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction
1. Introduction
In his landmark paper, WC Little and his group showed that patients admitted with cardiogenic pulmonary edema and high blood pressure had preserved left ventricular (LV) ejection fraction (EF) and increased LV filling pressure, and improvement in clinical and radiological signs of pulmonary edema during hospital stay was associated with improved LV filling pressure but no change in LVEF.1 This was the first demonstration that pulmonary congestion in acute heart failure (AHF) is related to afterload mismatch.2 However, WC Little's paper did not explore right heart function nor effects of ‘decongestive’ therapies on cardiac parameters or plasma volume during hospital stay. Several novel biomarkers were identified in recent years, including natriuretic peptides3 and endothelial soluble CD146,4 which are associated with cardiac and/or venous pressures. To this day, changes in cardiac function, plasma biomarkers, and plasma volume during hospital stay associated with decongestive therapy in AHF still remain elusive.
Therefore, in the present study, we sought to assess changes in echocardiographic parameters, plasma biomarkers of cardiac and venous pressures, and estimated plasma volume during hospital stay in AHF patients, with special attention to differences between acute HFPEF, HFREF, and patients with non‐cardiac dyspnea.
2. Methods
2.1. Study population
The study population consisted of a subset of patients included in the metabolic road to diastolic heart failure: diastolic heart failure (MEDIA‐DHF study, Clinical Trials. gov, NCT02446327). MEDIA‐DHF enrolled patients with acute dyspnea of non‐cardiac origin, AHF, and chronic HF. For this specific substudy, patients who were admitted for acute dyspnea of cardiac and non‐cardiac origin as the main symptom at two university hospitals in France (Hôpital Lariboisière, Paris and Hôpital Universitaire Jean Minjoz, Besançon) and who were 18 years or older, were included between February 2011 and April 2014. Exclusion criteria were shown elsewhere.5 Adjudication of the final diagnosis (AHF or non‐cardiac dyspnea, using clinical exams, echocardiography, and natriuretic peptides) was performed independently by two cardiologists blinded from patients' management. When the two evaluators disagreed regarding the final diagnosis, a third physician adjudicated the final diagnosis.6 AHF patients were divided into two subgroups based on their LVEF on admission: HFREF (defined as LVEF<40%) and HFPEF (defined as LVEF≥40%). Data on AHF with LVEF ≥ 50% were also investigated. For the purposes of this study, we included patients whose echocardiographic parameters or plasma biomarkers were available within 4 h of presentation for dyspnea in the emergency department and before hospital discharge [at median (interquartile range) 6 (5–8) days after hospital admission].
Total intravenous furosemide dose during hospital stay was collected from the medical record in AHF patients. Forty milligrames of furosemide was considered equivalent to 1 mg of bumetanide. AHF patients were divided into two groups (high and low) using median value of total intravenous furosemide dose during hospital stay.
The study protocol complied with the Declaration of Helsinki and was approved by the ethical committee of the participating institutions (Commission Nationale de l'Informatique et des Libertes 910198; Comité d'Evaluation de l'Ethique des projets de Recherche Biomedicale 10–017). All patients provided written informed consent.
2.2. Echocardiography
Echocardiographic measurements of cardiac chamber, cardiac systolic and diastolic functions, and inferior vena cava (IVC) diameters were obtained according to the guidelines by experienced cardiologists (RP and MFS) within 4 h of presentation for dyspnea at the emergency department and before hospital discharge, using Philips CX50® or Philips iE33® ultrasound systems.7 Investigators involved in image acquisition were blinded to plasma biomarker values or patients' management and were not involved in further statistical data analysis.
2.3. Biomarkers
During initial presentation at the emergency department and before hospital discharge, blood samples were collected in plastic tubes containing ethylenediaminetetra‐acetic acid. Aliquots of plasma samples were stored at −80°C for further analysis. Several plasma biomarkers were assessed as indicators of cardiac filling pressures [B‐type natriuretic peptide (BNP; Abbott), mid‐regional pro‐atrial natriuretic peptide (MR‐proANP; BRAHMS AG–ThermoFisher)], venous pressures [the endothelial soluble cluster of differentiation 146 (sCD146; Biocytex)], or renal function [NGAL (Abbott) and cystatin C (Abbott)]. Other biomarkers of myocardial ischemia [high sensitive troponin I (hsTnI; Roche Diagnostics)], cardiovascular [soluble suppression of tumorigenecity‐2 (sST‐2; Critical Diagnostics)], and systemic inflammation [C‐reactive protein (CRP; Siemens) and procalcitonin (BRAHMS AG–ThermoFisher)] are also measured. Estimated glomerular filtration rate (eGFR) was calculated from creatinine values using the modified diet in renal disease formula. Relative changes in estimated plasma volume (∆ estimated plasma volume) between at admission and before discharge were calculated using the Straus formula: ∆ estimated plasma volume = 100 * [(haemoglobin at admission) / (haemoglobin at discharge)] * [(1‐haematocrit at discharge) / (1‐haematocrit at admission)].8, 9 Because estimated plasma volume was thought to be proportional to this value, the instantaneous estimated plasma volume was defined using this formula as previously described: estimated plasma volume = (1‐haematocrit) / haemoglobin (g/dL) * 0.01.10
2.4. Primary and secondary outcome
The primary outcome of the present study was changes in echocardiographic parameters and plasma biomarkers of cardiac and venous pressures during hospital stay in acute HFPEF, HFREF, and non‐cardiac dyspnea patients. The secondary outcomes were (i) changes in echocardiographic parameters of cardiac chamber dimensions and function, (ii) estimated plasma volume, and (iii) changes in plasma renal biomarkers during hospital stay in acute HFPEF, HFREF, and non‐cardiac dyspnea patients.
2.5. Statistical analysis
Continuous variables were summarized as the median (interquartile range). Comparisons of changes within group were performed using Wilcoxon signed‐rank test and changes between group were Mann–Whitney U‐test.
Analyses were performed with PASW Statistics 18 (SPSS Japan Inc., an IBM company, Tokyo, Japan). A P value <0.05 was considered statistically significant.
3. Results
3.1. Study population
Of 146 patients in the MEDIA‐DHF cohort, 111 patients fulfilled the inclusion criteria and were analysed in this substudy comprised of 43 HFPEF [age 83 (76–89) years, LVEF 50 (40–60) %, BNP 732 (380–1183) pg/mL, systolic blood pressure (SBP) at admission 149 (134–181) mmHg], 34 HFREF [age 63 (57–76) years, LVEF 27 (20–31) %, BNP 1027 (874–2781) pg/mL, SBP 132 (116–159) mmHg], and 34 non‐cardiac dyspnea [age 76 (55–86) years, LVEF 60 (54–65) %, BNP 137 (29–254) pg/mL, SBP 143 (124–162) mmHg] patients. Dyspnea improved in all patients, and they all left the hospital alive.
3.2. Changes in echocardiographic parameters during hospital stay in acute dyspneic patients
Concerning LV filling pressures, medial E/e' decreased from 21.1 (15.8–29.6) at admission to 16.6 (11.7–24.3) at discharge (P = 0.004), and E wave deceleration time increased during hospitalization in AHF patients from 129 (105–156) ms at admission to 166 (128–203) ms at discharge (P = 0.003), suggesting improved LV filling pressures (Figure 1 A and Supporting Information, Table S1 ). By contrast, most left heart chamber dimensions and parameters of LV systolic function, including left atrial areas, LV end‐diastolic volume, LVEF, and cardiac index, were similar during hospital stay (Figure 2 A, 2 B and Table S1 ). Echocardiographic indices of right heart showed marked decrease in venous pressures during hospitalization: IVC diameter at rest decreased from 22 (16–24) mm at admission to 13 (11–18) mm at discharge (P = 0.009) and respiratory variability of IVC diameter increased from 32 (8–44) % at admission to 43 (29–70) % at discharge (P = 0.04), an improvement of TAPSE that increased [from 16 (15–19) mm at admission to 19 (17–21) mm at discharge, P = 0.04], and no changes in right atrial area (Figures 1 A, 2 A, 2 B and Table S1 ).
Figure 1.
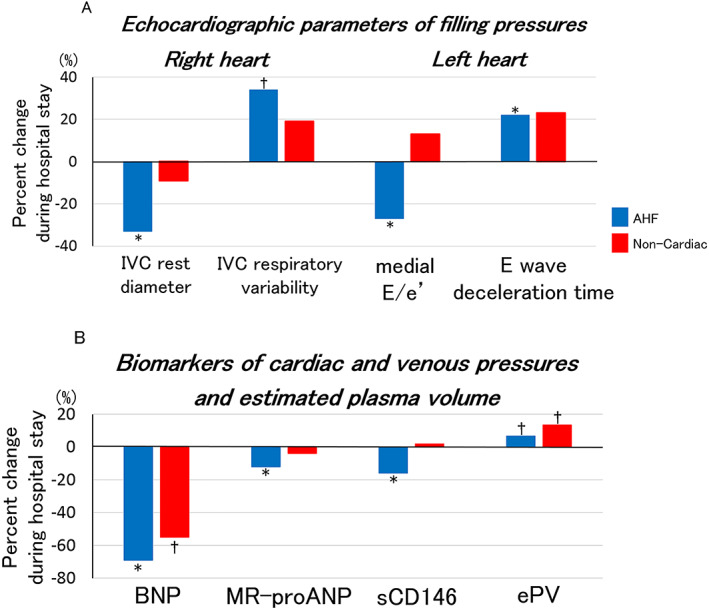
Percent changes in echocardiographic parameters and plasma biomarkers of cardiac and venous pressures and estimated plasma volume during hospital stay for AHF. Figure 1(A) shows median values of percent changes in echocardiographic parameters of right and left cardiac filling pressures (IVC rest diameter, IVC respiratory variability, medial E/e', and E wave deceleration time) during hospital stay in AHF. Figure 1(B) shows median values of percent changes in plasma biomarkers of cardiac and venous pressures (BNP, MR‐proANP, and sCD146) and estimated plasma volume during hospital stay in AHF. *P < 0.01, †P < 0.05. AHF, acute heart failure; BNP, B‐type natriuretic peptide; ePV, estimated plasma volume; IVC, inferior vena cava; MR‐proANP, mid‐regional pro‐atrial natriuretic peptide; sCD146, soluble cluster of differentiation 146.
Figure 2.
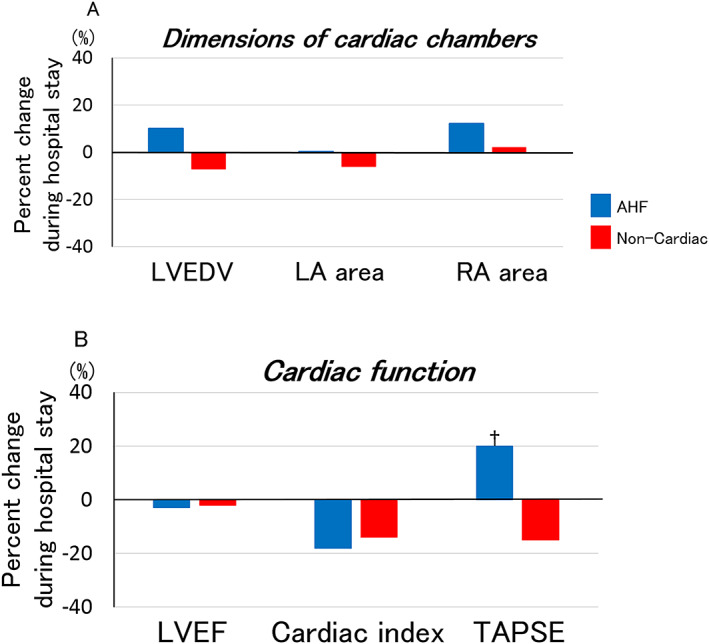
Percent changes in cardiac chamber dimensions and function during hospitalization for AHF. Figure 2(A) shows median values of percent changes in cardiac chamber dimensions assessed by echocardiography during hospital stay in AHF. Figure 2(B) shows median values of percent changes in echocardiographic parameters of cardiac function during hospital stay in AHF. *P < 0.01, †P < 0.05. AHF, acute heart failure; LA, left atrial; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; RA, right atrial; TAPSE, tricuspid annular plane systolic excursion.
Echocardiographic changes during hospital stay were similar in HFPEF and HFREF (Table 1). Echocardiographic findings of AHF with LVEF ≥ 50% were similar to those with LVEF ≥ 40% (Table S2 ). Echocardiographic changes were also similar (i) between AHF patients receiving high (>median cumulative dose of 280 mg during hospital stay) and low (≤280 mg) total intravenous furosemide dose and (ii) between those receiving intravenous furosemide alone and those receiving the combination of intravenous furosemide and vasodilators during hospital stay (Tables S3 and S4 ).
Table 1.
Changes of echocardiographic parameters during hospitalization in patients with acute heart failure with preserved left ventricular ejection fraction and heart failure with reduced left ventricular ejection fraction
| HFPEF | HFREF | Difference in percent change between groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Admission | Discharge | P | n | Admission | Discharge | P | P | |
| Cardiac geometry | |||||||||
| LVEDD (mm) | 16 | 51 [45–55] | 49 [42–55] | 0.98 | 14 | 68 [61–72] | 66 [61–71] | 0.21 | 0.51 |
| LVESD (mm) | 16 | 33 [27–42] | 31 [29–40] | 0.95 | 14 | 56 [48–61] | 55 [44–59] | 0.16 | 0.18 |
| LVEDV (mL) | 14 | 88 [68–103] | 93 [68–103] | 0.36 | 15 | 138 [131–173] | 171 [147–183] | 0.51 | 0.97 |
| LVESV (mL) | 13 | 42 [36–60] | 43 [31–51] | 0.07 | 13 | 108 [78–126] | 124 [75–147] | 0.62 | 0.14 |
| Left atrial area (cm2) | 23 | 22 [18–26] | 23 [19–27] | 0.73 | 15 | 27 [25–34] | 29 [24–34] | 0.98 | 0.78 |
| Right atrial area (cm2) | 8 | 17 [12–20] | 17 [16–18] | 0.48 | 11 | 18 [17–23] | 22 [17–27] | 0.48 | 0.74 |
| Cardiac function | |||||||||
| Heart rate (bpm) | 22 | 80 [72–98] | 74 [67–81] | 0.04 | 16 | 96 [88–110] | 75 [62–95] | 0.01 | 0.28 |
| LVEF (%) | 23 | 48 [40–60] | 54 [48–61] | 0.17 | 18 | 30 [25–36] | 29 [23–32] | 0.24 | 0.06 |
| Stroke volume (mL) | 14 | 34 [29–49] | 48 [37–66] | 0.10 | 13 | 52 [36–61] | 45 [35–58] | 0.75 | 0.24 |
| Cardiac index (mL/min/m2) | 10 | 1.6 [1.4–2.2] | 2.1 [1.5–2.4] | 0.80 | 9 | 2.5 [2.1–3.3] | 1.9 [1.4–2.4] | 0.21 | 0.14 |
| TAPSE (mm) | 11 | 17 [13–20] | 17 [16–19] | 0.34 | 10 | 16 [15–18] | 19 [18–25] | 0.06 | 0.67 |
| E wave (cm/s) | 24 | 98 [80–117] | 83 [69–114] | 0.08 | 18 | 91 [70–131] | 89 [74–111] | 0.83 | 0.20 |
| E wave deceleration time (ms) | 22 | 145 [107–184] | 177 [133–226] | 0.09 | 19 | 121 [102–139] | 159 [127–179] | 0.008 | 0.77 |
| A wave (cm/s) | 13 | 91 [57–103] | 81 [68–110] | 0.53 | 11 | 31 [25–62] | 46 [32–67] | 0.35 | 0.19 |
| E/A | 12 | 1.03 [0.67–1.96] | 1.25 [0.82–1.50] | 0.64 | 10 | 2.15 [1.50–2.81] | 2.17 [1.26–2.69] | 0.33 | 0.26 |
| Medial e' | 22 | 4 [4–7] | 6 [4–7] | 0.09 | 12 | 4 [3–4] | 5 [4–6] | 0.007 | 0.22 |
| Lateral e' | 22 | 7 [5–8] | 7 [5–8] | 0.25 | 17 | 7 [5–8] | 8 [5–11] | 0.53 | 0.66 |
| Medial E/e' | 25 | 21.3 [16.0–30.5] | 14.3 [11.5–24.3] | 0.007 | 17 | 21.0 [14.0–25.5] | 19.2 [12.0–22.0] | 0.20 | 0.29 |
| Lateral E/e' | 26 | 15.0 [11.4–19.8] | 14.0 [9.4–17.4] | 0.85 | 22 | 13.0 [8.8–16.0] | 13.4 [8.8–19.0] | 0.71 | 0.79 |
| Mean E/e' | 23 | 19.8 [16.1–27.0] | 17.4 [11.1–20.1] | 0.08 | 16 | 18.0 [11.3–21.5] | 18.0 [10.6–23.5] | 0.44 | 0.44 |
| Parameters of venous congestion | |||||||||
| IVC rest diameter (mm) | 11 | 22 [16–24] | 13 [11–18] | 0.09 | 12 | 22 [18–25] | 14 [12–20] | 0.04 | 1.00 |
| IVC sniff diameter (mm) | 11 | 11 [9–22] | 6 [5–11] | 0.04 | 10 | 19 [10–24] | 7 [0–13] | 0.03 | 0.62 |
| Respiratory variability of IVC diameter (%) | 11 | 40 [9–44] | 40 [31–65] | 0.11 | 10 | 24 [8–43] | 50 [23–100] | 0.11 | 0.70 |
HFPEF, heart failure with preserved left ventricular ejection fraction; HFREF, heart failure with reduced left ventricular ejection fraction; IVC, inferior vena cava; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVESV, left ventricular end‐systolic volume; TAPSE, tricuspid annular plane systolic excursion.
Data are presented as median [interquartile range]. Bold numbers mean statistically siginificant.
In patients with non‐cardiac dyspnea, echocardiographic parameters were normal at admission and unaltered during hospitalization (Figures 1 A, 2 A, 2 B and Table S1 ).
3.3. Changes in plasma cardiovascular biomarkers and estimated plasma volume during hospital stay in acute dyspneic patients
All plasma biomarkers of cardiac and venous pressures improved during hospitalization in AHF patients: BNP decreased from 935 (514–2037) pg/mL at admission to 308 (183–609) pg/mL at discharge (P < 0.001), MR‐proANP decreased from 449 (274–653) pmol/L at admission to 366 (242–549) pmol/L at discharge (P < 0.001), sCD146 decreased from 528 (406–654) ng/mL at admission to 450 (374–529) ng/mL at discharge (P = 0.003) (Figure 1 B and Table S5 ). Estimated plasma volume significantly increased during hospitalization in AHF patients from 4.8 (4.2–5.6) at admission to 5.1 (4.4–5.8) (P = 0.03) (Figure 1 B and Table S5 ).
High sensitive troponin I [from 36 (18–87) pg/mL at admission to 22 (11–66) pg/mL at discharge, P = 0.02] and sST2 [from 83 (61–132) ng/mL at admission to 42 (36–59) ng/mL at discharge, P < 0.001] significantly decreased, while CRP and procalcitonin were similar during hospitalization (Table S5 ).
The decrease in circulating biomarkers during hospital stay was similar (i) in HFPEF and HFREF (Table 2 and Table S6 for LVEF ≥ 50%), (ii) in AHF patients receiving high and low dose of intravenous furosemide (Table S7 ), and (iii) in AHF patients receiving intravenous furosemide alone and the combination of intravenous furosemide and vasodilators (Table S8 ).
Table 2.
Changes of biomarkers during hospitalization in patients with acute heart failure with preserved left ventricular ejection fraction and heart failure with reduced left ventricular ejection fraction
| HFPEF | HFREF | Difference in percent change between groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Admission | Discharge | P | n | Admission | Discharge | P | P | |
| Markers of cardiovascular stress and congestion: | |||||||||
| BNP (pg/mL) | 19 | 848 [414–1577] | 228 [161–520] | <0.001 | 19 | 989 [901–3039] | 313 [194–598] | <0.001 | 0.67 |
| MR‐proANP (pmol/L) | 34 | 386 [267–626] | 362 [230–502] | 0.02 | 25 | 515 [305–737] | 376 [254–587] | 0.003 | 0.41 |
| sCD146 (ng/mL) | 34 | 509 [379–624] | 420 [320–519] | 0.01 | 24 | 606 [448–705] | 483 [423–590] | 0.12 | 0.96 |
| hsTnI (pg/mL) | 34 | 29 [16–75] | 19 [10–66] | 0.03 | 20 | 45 [21–111] | 31 [15–89] | 0.20 | 0.89 |
| Markers of inflammation and fibrosis | |||||||||
| Soluble ST2 (ng/mL) | 34 | 81 [61–131] | 50 [40–59] | <0.001 | 25 | 89 [59–133] | 41 [33–53] | <0.001 | 0.46 |
| CRP (mg/L) | 34 | 11.8 [4.7–37.3] | 10.7 [6.4–34.4] | 0.86 | 24 | 8.0 [3.8–17.9] | 4.0 [2.5–27.9] | 0.14 | 0.37 |
| Procalcitonin (ng/mL) | 32 | 0.11 [0.08–0.14] | 0.10 [0.07–0.14] | 0.34 | 24 | 0.10 [0.08–0.17] | 0.11 [0.07–0.17] | 0.75 | 0.60 |
| Biological markers of renal function: | |||||||||
| Creatinine (mg/dL) | 34 | 1.05 [0.87–1.39] | 1.05 [0.79–1.90] | 0.17 | 25 | 1.27 [1.05–1.60] | 1.34 [1.13–1.64] | 0.46 | 0.35 |
| eGFR (mL/min/1.73m2) | 34 | 59 [40–74] | 56 [36–74] | 0.19 | 25 | 59 [45–76] | 56 [41–68] | 0.41 | 0.35 |
| Cystatin C (mg/L) | 33 | 1.55 [1.23–2.35] | 1.92 [1.51–2.73] | 0.002 | 24 | 1.40 [1.14–1.83] | 1.49 [1.30–1.88] | 0.002 | 0.46 |
| Urea nitrogen (mmol/L) | 33 | 8.9 [6.8–12.3] | 10.2 [7.5–16.0] | 0.02 | 24 | 8.5 [7.2–14.1] | 10.4 [7.9–16.4] | 0.16 | 0.99 |
| Uric acid (μmol/L) | 33 | 358 [331–476] | 478 [343–544] | <0.001 | 24 | 449 [394–679] | 481 [401–635] | 0.95 | 0.01 |
| NGAL (ng/mL) | 32 | 176 [104–273] | 217 [111–273] | 0.02 | 22 | 107 [85–147] | 132 [84–188] | 0.09 | 0.97 |
| Markers of plasma volume: | |||||||||
| Haemoglobin (g/dL) | 27 | 12.2 [10.9–13.3] | 11.5 [11.0–12.4] | 0.10 | 26 | 13.5 [12.5–14.4] | 13.1 [11.9–13.9] | 0.14 | 0.75 |
| Haematocrit (%) | 27 | 37 [35–40] | 35 [34–38] | 0.08 | 26 | 41 [38–44] | 39 [37–43] | 0.12 | 0.63 |
| Estimated plasma volume | 27 | 5.2 [4.5–5.9] | 5.7 [5.1–6.2] | 0.12 | 26 | 4.3 [3.9–4.9] | 4.7 [4.1–5.1] | 0.16 | 0.71 |
AHF, acute heart failure; BNP, B‐type natriuretic peptide, CRP: C‐reactive protein; eGFR, estimated glomerular filtration rate; HFPEF, heart failure with preserved left ventricular ejection fraction; hsTnI, high sensitive troponin I; MR‐proANP, mid‐regional pro‐atrial natriuretic peptide; NGAL, neutrophil gelatinase associated lipocalin; sCD146, soluble cluster of differentiation 146; ST‐2, suppression of tumorigenecity‐2.
Data are presented as median [interquartile range]. Bold numbers mean statistically significant.
Regarding patients with non‐cardiac dyspnea, BNP, hsTnI, sST2, and CRP levels decreased, whereas MR‐proANP and sCD146 levels were unchanged during hospitalization (Figure 1 B and Table S5 ). Estimated plasma volume increased in patients with non‐cardiac dyspnea as in AHF patients (Figure 1 B and Table S5 ).
3.4. Changes in renal function during hospital stay in AHF
In patients with AHF, serum creatinine and creatinine‐based eGFR seemed similar during hospitalization: creatinine from 1.18 (0.90–1.53) at admission to 1.19 (0.86–1.70) mg/dL at discharge (P = 0.89), eGFR from 59 (40–75) mL/min/1.73m2 at admission to 56 (38–73) mL/min/1.73m2 at discharge (P = 0.09), while urea nitrogen increased from 8.9 (6.9–13.2) mmol/L at admission to 10.2 (7.6–16.0) mmol/L at discharge (P = 0.006), cystatin C increased from 1.50 (1.20–2.27) mg/L at admission to 1.78 (1.33–2.59) mg/L at discharge (P < 0.001), and plasma NGAL increased from 127 (95–260) ng/mL at admission to 167 (104–263) ng/mL at discharge (P = 0.004) (Figure 3 and Table S5 ).
Figure 3.
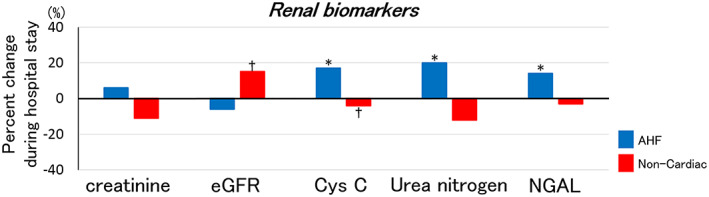
Percent changes in renal biomarkers during hospital stay for AHF. It shows median values of percent changes in renal biomarkers during hospital stay in AHF. *P < 0.01, †P < 0.05. AHF, acute heart failure; Cys C, cystatin C; eGFR, estimated glomerular filtration rate; NGAL, neutrophil gelatinase associated lipocalin.
The results were consistent in HFPEF, HFREF, and in AHF patients receiving low or high total intravenous furosemide dose (Table 2, Table S6 for LVEF ≥ 50%, and Table 7 ). Serum creatinine increased, and eGFR decreased in patients receiving the combination of intravenous furosemide and vasodilators (Table S8 ).
In patients with non‐cardiac dyspnea, most of renal parameters were similar, except that eGFR and cystatin C slightly but significantly improved during hospital stay (Figure 3 and Table S5 ).
4. Discussion
Our study shows that AHF is a single disease, regardless of LVEF, and surrogates for right and left cardiac filling pressures as assessed by echocardiography, and circulating cardiovascular biomarkers improve in both acute HFPEF and HFREF patients during hospital stay despite minimal changes in cardiac chamber dimensions, cardiac output, or estimated plasma volume.
Our study confirms the concept delineated by Gandhi and Little et al. that excess of congestion upstream the LV, rather than acute deterioration of LV systolic function, is critical in the pathogenesis of acute pulmonary edema.1 We recently showed that right cardiac function was also altered, with decreased TAPSE and increased IVC diameter, in both acute HFPEF and HFREF patients.5 The present study shows a marked and parallel improvement in left and right cardiac and venous pressures during hospital stay in AHF. The marked reduction in intracardiac and central venous pressures was associated with increased estimated plasma volume during hospitalization. Similarly, Konishi et al. showed haemoglobin decrease associated with improvement of acute pulmonary edema following diuretic treatments.11 Discrepancies between improvement in cardiac and venous pressures with minimal changes in estimated plasma volume in AHF patients suggest that venous capacitance better accommodate plasma volume during hospital stay compared with the early phase, as previously mentioned.12 A better cardio‐arterial coupling of both right and left ventricle may have improved venous congestion. Increased estimated plasma volume was also observed in non‐cardiac dyspnea, suggesting that it might be related to uncontrolled fluid balance during hospital stay. Although the impact of “decongestive” therapies on improvement in cardiac and venous pressures seemed minimal in our study, further studies are needed.
Our study further clarifies the role of echocardiography in the management of AHF patients. Recent recommendations mention that echocardiography should be performed at admission only in case of hemodynamic instability, including cardiogenic shock or acute valvular disease.13 However, there are no clear guidelines on when performing an echocardiography in the following days. Our study showed that cardiac dimensions, LV systolic indices of cardiac function, and cardiac output were roughly unchanged in the days following admission in both acute HFPEF and HFREF, suggesting that assessment of cardiac function by echocardiography could be performed during hospital stay. By contrast, elevated filling pressures of right and left ventricle as assessed by IVC parameters, medial E/e', and E wave deceleration time were significantly improved by decongestion therapy in AHF patients, again regardless of LVEF. These results suggest that serial echocardiographic measurement of congestion could be useful for the follow‐up during hospital stay.
Biomarkers of cardiac filling pressures, namely natriuretic peptides, are released by cardiac overstretch and are important tools for diagnosis and risk prediction in HF.3 Our study showed a striking reduction in BNP and MR‐proANP despite no changes in atrial and ventricular dimensions by echocardiography and even increase in estimated plasma volume. This is true in both acute HFPEF and acute HFREF. Our study also shows that circulating sCD146, the endothelial marker of venous pressure, decreased during hospital stay in AHF patients as previously described.4, 14 Our study confirms that natriuretic peptides and sCD146 are good markers of cardiac and venous pressures, independent from estimated plasma volume and may be measured at admission and during hospital stay to evaluate benefits of management on cardiac filling pressures.
Increased right and left filling pressures, in a context of altered endothelial properties, are major determinants of organ congestion, including lung and kidney and hospital admissions for AHF. It is, however, very difficult to assess the degree of right and left filling pressures and to follow its reduction during hospitalization.15 Many AHF patients are discharged with persistent signs and symptoms of congestion,16 and residual congestion has been shown to be associated with worse outcomes in hospitalized AHF patients.17, 18, 19, 20, 21 Our study showed that repeated measures of IVC, medial E/e', E wave deceleration time, and biomarkers allow to assess improvement in cardiac and venous pressures during hospital stay for AHF and might help to decide on the optimal time point for hospital discharge, which supports the concepts of the current practical guidance of the Heart Failure Association of the European Society of Cardiology.22, 23
The present study also shows that some biological markers of renal function such as serum creatinine and eGFR were similar, whereas other renal biomarkers including cystatin C and NGAL worsened during hospital stay for AHF. Recently, venous congestion has gathered increased attention in the pathogenesis of renal dysfunction in patients with HF, especially in patients with advanced decompensated HFREF who underwent right heart catheterization.24, 25, 26, 27 By contrast, other papers showed low central venous pressure at admission,28 or aggressive decongestive therapy29 was associated with worsening renal function. The recent subanalysis of Renal Optimization Strategies Evaluation (ROSE)‐AHF trial30 showed that cystatin C levels were elevated, though renal tubular biomarkers such as urine NGAL were unchanged or slightly improved during aggressive decongestive treatment. These differences between ROSE‐AHF and our study might be caused by differences in baseline renal function, follow‐up duration, or prevalence of HFPEF. ROSE‐AHF also reported that changes in renal tubular biomarkers were not associated with changes in urine output or body weight. Further studies are needed to clarify whether the improvements in cardiac filling pressures were associated with changes in renal function or renal tubular injury in the broad spectrum of AHF population including HFPEF.
4.1. Study limitations
Our study has several limitations. First, the present study included only a small number of AHF patients from two French university hospitals, and AHF patients were not consecutively recruited. Moreover, our cohort has missing values of echocardiographic parameters and plasma biomarkers. Selection bias might affect the results. Those results should be confirmed by larger AHF population enrolled consecutively, though obtaining echocardiographic parameters at emergency room in consecutive AHF patients has much difficulty in both clinical and research settings. Second, echocardiographic measurement and biomarkers were available only at admission and before discharge, and early changes in those parameters could not be ascertained. Third, we lack data regarding patients' urine output, body weight, or HF signs and symptoms during hospital stay and at discharge. Our study could not assess relationships between echocardiographic parameters and plasma biomarkers of congestion and classical HF signs and symptoms. Fourth, because estimated plasma volume was calculated using haemoglobin and haematocrit, repeated blood sampling tests might affect the results of changes in estimated plasma volume.
5. Conclusions
Our study showed that echocardiographic parameters and plasma biomarkers of cardiac and venous pressures improved during hospitalization for AHF in both acute HFPEF and HFREF, while echocardiographic parameters of cardiac chamber dimensions, cardiac output, and estimated plasma volume showed minimal changes. Serial assessment of cardiac filling pressures using echocardiography and plasma biomarkers could be useful for the follow‐up during hospital stay and determining benefits of management in AHF patients.
Conflict of interest
F.Z. has received personal fees for Steering Committee membership from Janssen, Bayer, Pfizer, Novartis, Boston Scientific, Resmed, Takeda, General Electric, and Boehringer Ingelheim, has had consultancy for Amgen, CVRx, Quantum Genomics, Relypsa, ZS Pharma, AstraZeneca, GSK, and is a founder of Cardiovascular Clinical Trialists (CVCT) and of CardioRenal. P.R. has received personal fees (consulting) from Novartis, Relypsa, AstraZeneca, Grünenthal, Idorsia, Stealth Peptides, Fresenius, Vifor Fresenius Medical Care Renal Pharma, Vifor and CTMA, lecture fees from Bayer and CVRx and is a cofounder of CardioRenal. A.C.S. has received grants or honoraria from Novartis, Servier, Daichii‐Sankyo, Vifor, Menarini and Cardiorentis. C.S.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Thermofisher, Medtronic, and Vifor Pharma; and has consulted for Bayer, Novartis, Takeda, Merck, Astra Zeneca, Janssen Research & Development, LLC, Menarini, Boehringer Ingelheim, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, Roche, and Amgen. J.T. has received consulting fees and research support from FIRE‐1. W.M. has received research grants from Novartis, Vifor, Medtronic, Biotronik, Abbott and Boston Scientific. A.M. received speaker's honoraria from Abbott, Orion, Roche and Servier and fee as member of advisory board and/or Steering Committee and/or research grant from BMS, Adrenomed, Neurotronik, Roche, Sanofi, and Sphyngotec.
Funding
This study was supported by a grant from the European Union funded by the Seventh Framework Programme for Health in 2010 (FP7‐HEALTH‐2010‐MEDIA; Luxembourg) (F.Z., P.R., A.M) and research fellowship from Japan Heart Foundation (E.A.). P.R., N.G., T.C., and F.Z. are supported by a public grant overseen by the French National Research Agency (ANR) as part of the second “Investissements d'Avenir” programmes Fighting Heart Failure (reference: ANR‐15‐RHU‐0004), GEENAGE Impact Lorraine Université d'Excellence and by the Contrat de Plan Etat Lorraine IT2MP and FEDER Lorraine.
Supporting information
Table S1. Changes of echocardiographic parameters during hospitalization in patients with AHF and non‐cardiac dyspnea.
Table S2. Changes of echocardiographic parameters during hospitalization in patients with acute HFPEF (LVEF≥50%) and HFREF (LVEF<40%).
Table S3. Changes of echocardiographic parameters during hospitalization in AHF patients according to below or above median value of total IV furosemide dose.
Table S4. Changes of echocardiographic parameters during hospitalization in AHF patients receiving IV furosemide alone or IV furosemide plus vasodilators.
Table S5. Changes of biomarkers during hospitalization in patients with AHF and non‐cardiac dyspnea.
Table S6. Changes of biomarkers during hospitalization in patients with acute HFPEF (LVEF≥50%) and HFREF (LVEF<40%).
Table S7. Changes of biomarkers during hospitalization in AHF patients according to below or above median value of total IV furosemide dose.
Table S8. Changes of biomarkers during hospitalization in AHF patients receiving IV furosemide only or IV furosemide plus vasodilators.
Acknowledgements
LNLVA is supported by a training grant from the European Society of Cardiology (2015) and a travelling award from the International Society for Heart and Lung Transplantation (August 2015 and 2016). LNLVA gratefully acknowledges the financial support from the Fund for Cardiac Surgery through the Jacqueline Bernheim prize 2015.
Akiyama, E. , Cinotti, R. , Čerlinskaitė, K. , Van Aelst, L. N. L. , Arrigo, M. , Placido, R. , Chouihed, T. , Girerd, N. , Zannad, F. , Rossignol, P. , Badoz, M. , Launay, J.‐M. , Gayat, E. , Cohen‐Solal, A. , Lam, C. S. P. , Testani, J. , Mullens, W. , Cotter, G. , Seronde, M.‐F. , and Mebazaa, A. (2020) Improved cardiac and venous pressures during hospital stay in patients with acute heart failure: an echocardiography and biomarkers study. ESC Heart Failure, 7: 996–1006. 10.1002/ehf2.12645.
References
- 1. Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, Little WC. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001; 344: 17–22. [DOI] [PubMed] [Google Scholar]
- 2. Little WC. Hypertensive pulmonary oedema is due to diastolic dysfunction. Eur Heart J 2001; 22: 1961–1964. [DOI] [PubMed] [Google Scholar]
- 3. Santaguida PL, Don‐Wauchope AC, Oremus M, McKelvie R, Ali U, Hill SA, Balion C, Booth RA, Brown JA, Bustamam A, Sohel N, Raina P. BNP and NT‐proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev 2014; 19: 453–470. [DOI] [PubMed] [Google Scholar]
- 4. Gayat E, Caillard A, Laribi S, Mueller C, Sadoune M, Seronde MF, Maisel A, Bartunek J, Vanderheyden M, Desutter J, Dendale P, Thomas G, Tavares M, Cohen‐Solal A, Samuel JL, Mebazaa A. Soluble CD146, a new endothelial biomarker of acutely decompensated heart failure. Int J Cardiol 2015; 199: 241–247. [DOI] [PubMed] [Google Scholar]
- 5. Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, Manivet P, Rossignol P, Badoz M, Sadoune M, Launay JM, Gayat E, Lam CSP, Cohen‐Solal A, Mebazaa A, Seronde MF. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 2018; 20: 738–747. [DOI] [PubMed] [Google Scholar]
- 6. Seronde MF, Laribi S, Collins SP, Deye N, Logeart D, Plaisance P, Cohen‐Solal A, Mebazaa A. Heart failure diagnosis in acute conditions has high agreement with inpatient diagnosis. Eur J Emerg Med 2016; 23: 179–184. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 8. Strauss MB, Davis RK, Rosenbaum JD, Rossmeisl EC. Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest 1951; 30: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol 2002; 39: 1901–1908. [DOI] [PubMed] [Google Scholar]
- 10. Duarte K, Monnez JM, Albuisson E, Pitt B, Zannad F, Rossignol P. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail 2015; 3: 886–893. [DOI] [PubMed] [Google Scholar]
- 11. Konishi M, Matsuzawa Y, Suzuki H, Akiyama E, Iwahashi N, Maejima N, Endo M, Tsukahara K, Hibi K, Kosuge M, Ebina T, Sakamaki K, Morita S, Umemura S, Kimura K. Higher level at admission and subsequent decline in hemoglobin in patients with acute pulmonary edema. Circ J 2014; 78: 896–902. [DOI] [PubMed] [Google Scholar]
- 12. Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail 2011; 4: 669–675. [DOI] [PubMed] [Google Scholar]
- 13. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray J, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail 2015; 17: 544–558. [DOI] [PubMed] [Google Scholar]
- 14. Arrigo M, Truong QA, Onat D, Szymonifka J, Gayat E, Tolppanen H, Sadoune M, Demmer RT, Wong KY, Launay JM, Samuel JL, Cohen‐Solal A, Januzzi JL Jr, Singh JP, Colombo PC, Mebazaa A. Soluble CD146 is a novel marker of systemic congestion in heart failure patients: an experimental mechanistic and transcardiac clinical study. Clin Chem 2017; 63: 386–393. [DOI] [PubMed] [Google Scholar]
- 15. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 16. Arrigo M, Parissis JT, Akiyama E, Mebazaa A. Understanding acute heart failure: pathophysiology and diagnosis. Eur Heart J Suppl 2016; 18: G11–G18. [Google Scholar]
- 17. O'Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT‐HF registry. J Card Fail 2005; 11: 200–205. [DOI] [PubMed] [Google Scholar]
- 18. Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, Dunlap ME, Ezekowitz JA, Felker GM, Fudim M, Greene SJ, Hernandez AF, O'Connor CM, Schulte P, Starling RC, Teerlink JR, Voors AA, Mentz RJ. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND‐HF trial. JACC Heart Fail 2017; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, DeVore AD, Khazanie P, Redfield MM, Goldsmith SR, Bart BA, Anstrom KJ, Felker GM, Hernandez AF, Stevenson LW. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE‐AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS‐HF). Circ Heart Fail 2015; 8: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, Braunwald E, O'Connor CM, Felker GM. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail 2013; 6: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013; 34: 835–843. [DOI] [PubMed] [Google Scholar]
- 22. Harjola VP, Parissis J, Brunner‐La Rocca HP, Celutkiene J, Chioncel O, Collins SP, De Backer D, Filippatos GS, Gayat E, Hill L, Lainscak M, Lassus J, Masip J, Mebazaa A, Miro O, Mortara A, Mueller C, Mullens W, Nieminen MS, Rudiger A, Ruschitzka F, Seferovic PM, Sionis A, Vieillard‐Baron A, Weinstein JM, de Boer RA, Crespo‐Leiro MG, Piepoli M, Riley JP. Comprehensive in‐hospital monitoring in acute heart failure: applications for clinical practice and future directions for research. A statement from the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2018; 20: 1081–1099. [DOI] [PubMed] [Google Scholar]
- 23. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 24. Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015; 36: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 2008; 51: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 27. Aronson D, Abassi Z, Allon E, Burger AJ. Fluid loss, venous congestion, and worsening renal function in acute decompensated heart failure. Eur J Heart Fail 2013; 15: 637–643. [DOI] [PubMed] [Google Scholar]
- 28. Uthoff H, Breidthardt T, Klima T, Aschwanden M, Arenja N, Socrates T, Heinisch C, Noveanu M, Frischknecht B, Baumann U, Jaeger KA, Mueller C. Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail 2011; 13: 432–439. [DOI] [PubMed] [Google Scholar]
- 29. Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010; 122: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco‐Bacik MA, Chen HH, Felker GM, Hernandez AF, O'Connor CM, Sabbisetti VS, Bonventre JV, Wilson FP, Coca SG, Testani JM. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation 2018; 137: 2016–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Changes of echocardiographic parameters during hospitalization in patients with AHF and non‐cardiac dyspnea.
Table S2. Changes of echocardiographic parameters during hospitalization in patients with acute HFPEF (LVEF≥50%) and HFREF (LVEF<40%).
Table S3. Changes of echocardiographic parameters during hospitalization in AHF patients according to below or above median value of total IV furosemide dose.
Table S4. Changes of echocardiographic parameters during hospitalization in AHF patients receiving IV furosemide alone or IV furosemide plus vasodilators.
Table S5. Changes of biomarkers during hospitalization in patients with AHF and non‐cardiac dyspnea.
Table S6. Changes of biomarkers during hospitalization in patients with acute HFPEF (LVEF≥50%) and HFREF (LVEF<40%).
Table S7. Changes of biomarkers during hospitalization in AHF patients according to below or above median value of total IV furosemide dose.
Table S8. Changes of biomarkers during hospitalization in AHF patients receiving IV furosemide only or IV furosemide plus vasodilators.


