Abstract
Aim
Soluble suppression of tumorigenicity‐2 (sST2) is a strong prognostic biomarker in heart failure. The emerging understanding of circadian biology in cardiovascular disease may lead to novel applications in prognosis and diagnosis and may provide insight into mechanistic aspects of the disease–biomarker interaction. So far, it is unknown whether sST2 exhibits a diurnal rhythm. Repeated measurements of sST2 may aid in clinical decision making. The goal of this study was to investigate whether sST2 exhibits diurnal variation in patients with heart failure with reduced ejection fraction (HFrEF) and in control subjects, thereby enhancing its diagnostic and prognostic values.
Methods and results
The study comprised 32 subjects: 16 HFrEF patients and 16 controls. Blood was collected at seven subsequent time points during a 24 h time period. sST2, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), melatonin, and cortisol were measured from serum. Peak values of sST2 clustered at daytime (modal value: 5 p.m.) in 87.6% of all subjects (81.3% of patients, P = 0.021; 93.8% of controls, P = 0.001), and minimum concentrations at night‐time (modal value: 5 a.m.) in 84.4% (87.5% of patients, P = 0.004 81.3% of controls, P = 0.021). A cosinor analysis of mean normalized sST2 values revealed significant cosine shaped 24 h oscillations of patients (P = 0.026) and controls (P = 0.037). NT‐proBNP in contrast did not show a diurnal rhythm, while melatonin and cortisol patterns were intact in all subjects.
Conclusions
sST2 exhibits a diurnal rhythm with lower values in the morning than in the late afternoon. This new insight could lead to refinement of its diagnostic and prognostic values through specified and consistent sampling times with repeated measurements. For example, by measuring sST2 during the afternoon, when levels are at their highest, false negatives on prognosis prediction could be avoided.
Keywords: Circadian rhythm, Diurnal rhythm, Heart failure, Biomarker, sST2, NT‐proBNP
Introduction
Biomarkers are crucial components of clinical decision making as well as monitoring disease state and progression. Natriuretic peptides [B‐type natriuretic peptide (BNP) and N‐terminal pro‐BNP (NT‐proBNP)] are an important part of the guidelines for diagnosing heart failure (HF). Soluble suppression of tumorigenicity‐2 (sST2) recently emerged as a promising prognostic tool for patients already diagnosed with HF, as well as an aid for risk stratification in identifying those at risk of mortality and prehospitalization.1 Owing to its prognostic value, sST2 has been listed in the 2013 American College of Cardiology Foundation/American Heart Association guidelines as an important biomarker for the monitoring of HF patients.2 However, the use of biomarkers comes with different challenges including analytical and biological variability, setting the appropriate cut‐off values, and determining clinically meaningful changes in biomarker concentrations.3
With a cut‐off value set at 35 ng/mL, sST2 utilization has been shown to predict outcomes as well as to monitor and optimize therapy owing to its rapid concentration change with changing disease state.1 An enzyme‐linked immunosorbent assay has been developed by Critical Diagnostics (Presage ST2) for quantitative measurement of sST2,4 which, with coefficient of variation (CV) < 5%, has a high technical precision even when it comes to scarce analyte concentrations. Furthermore, age, elevated body mass index, or impaired renal function do not significantly affect sST2 values, while remaining common confounding situations for natriuretic peptide measurements.
Biological variation has been determined for sST2 values by means of serial measurements within the same subject over prolonged time periods only (e.g. days and months). However, while accumulating evidence has emphasized the importance of the circadian (24 h) clock in human physiology and pathophysiology,5, 6 sST2 variation within a 24 h period is currently unknown. The role of circadian rhythms has also been confirmed in each type of cardiovascular tissues, influencing its normal function (e.g. heart rate and blood pressure) as well as the onset and severity of disease (e.g. myocardial infarction).7 Therefore, sST2 as a marker and possibly mediator of cardiac pathology might also be governed by the circadian clock.
Knowledge of the 24 h pattern in sST2 levels could have important consequences for its diagnostic and prognostic usage in clinic, by pinpointing one or several time points in a 24 h period necessary for valid assessment of disease and treatment. In addition, the existence of such a 24 h pattern may elucidate potential mechanistic aspects of the disease–biomarker interaction.
In this study, to our knowledge for the first time, within‐day variations of sST2 levels are assessed in patients with HF with reduced ejection fraction (HFrEF) and healthy controls. 24 h variations of NT‐proBNP were previously investigated,8 thereby serving as a control biomarker for our cohorts. Melatonin and cortisol were used as a control for circadian rhythms within our groups.9
Methods
Study design and participants
The study was conducted according to the principles of the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO). It was approved by the Medical Ethics Committee of University Medical Centre Utrecht (Study Number 14/471).
Study participants were admitted to the University Medical Centre Utrecht. All provided written informed consent after which the blood withdrawal and biomarker analysis commenced (Figure S1). Furthermore, for each individual patient, health status was established based on electronic health records within a 12 month period after the blood withdrawal. For 1/16 control subject, the first time point (9 a.m.) is missing owing to problems with blood withdrawal.
Laboratory measurements
At seven subsequent time points (9 a.m., 1 p.m., 5 p.m., 9 p.m., 1 a.m., 5 a.m., and 9 a.m.), SST tubes (BD) were used to collect blood from an intravenous cannula (5 mL per time point). After collection, serum was separated by centrifugation and stored at −80°C until analysis. After all samples from all subjects had been collected, sST2 was quantitatively measured in triplicates by using the Presage® ST2 sandwich monoclonal immunoassay (Critical Diagnostics, San Diego, CA), according to the manufacturer's protocol. NT‐proBNP was measured on a Cobas e411 analyzer using the Elecsys® NT‐proBNP immunoassay (Roche Diagnostics, Indianapolis). Analysis of serum melatonin and cortisol concentrations was performed on liquid chromatography–tandem mass spectrometer.10
Statistical analysis
Differences between the baseline characteristics of the two groups were compared by using Mann–Whitney U test, independent Student's t test, or Fisher's exact test, as appropriate. One‐sample non‐parametric binomial test was used to test the chance of non‐randomness of sST2 and NT‐proBNP concentrations peaking at daytime or night‐time. The range spread (%) was used to describe within‐person difference between maximum and minimum values, calculated by dividing the range (difference between maximum and minimum) by the minimum value. Circadian parameters were calculated with cosinor analysis‐based script in statistics software program R. In this program, a cosinor curve with a period of ~24 h that best fits the provided data is obtained by linear regression.11 Throughout the manuscript, the following indications for significance were used: *P < 0.05 and **P < 0.01. All the analyses were performed in IBM SPSS Statistics (v. 23), and graphs were made in Adobe Illustrator CC (19.2.0).
Results
Patient characteristics
Thirty‐two subjects were enrolled in the study: 16 patients with HFrEF (left ventricular ejection fraction < 40% by echocardiography), and 16 control subjects (Table S1). Baseline characteristics of all study participants are summarized in Table 1. The majority of participants were male (81.3% for patients and 75.0% for controls) with a mean age of 59 ± 13 years for patients and 54 ± 16 for controls. Among the enrolled patients, 75.0% were in New York Heart Association (NYHA) class III and 25.0% in NYHA class II. Outcomes after 1 year are shown in Table S2.
Table 1.
Baseline characteristics of study participants
| Characteristics | All subjects (n = 32) | Patients (n = 16) | Controls (n = 16) | P value | |
|---|---|---|---|---|---|
| Age (years) | 57 ± 15 | 59 ± 13 | 54 ± 16 | *0.310 | |
| Male sex (%) | 78.1 | 81.3 | 75.0 | 1.000 | |
| BMI (kg/m2) | 24.6 ± 3.9 | 24.8 ± 4.5 | 24.5 ± 3.3 | †0.847 | |
| Smoking (%) | 53.1 | 75.0 | 31.1 | 0.032 | |
| Alcohol (IU/week) | 50.0 | 43.8 | 56.3 | 0.724 | |
| CKD‐EPI GFR (mL/min/1.73 m2) | 66 ± 14 | 64± 22 | 69 ± 13 | †0.501 | |
| Comorbidities (%) | Diabetes mellitus | 15.6 | 31.3 | 0.0 | 0.018 |
| Myocardial infarction | 15.6 | 25.0 | 6.3 | 0.600 | |
| Atrial flutter/fibrillation | 18.8 | 37.5 | 0.0 | 0.018 | |
| Medication (%) | ACE inhibitor | 31.3 | 50.0 | 12.5 | 0.054 |
| Angiotensin receptor antagonist | 18.8 | 25.0 | 12.5 | 0.654 | |
| Antimineralocorticoid | 34.4 | 68.8 | 0.0 | <0.001 | |
| Beta‐blocker | 25.0 | 43.8 | 6.3 | 0.037 | |
| Cause of cardiomyopathy (%) | Ischaemic | 44.0 | |||
| Non‐ischaemic (valve, genetics, e.c.i.) | 56.0 | ||||
| Severity of heart failure (%) | NYHA class II | 25.0 | |||
| NYHA class III | 75.0 | ||||
| Ejection fraction (%) | 23.0 ± 7 |
Values are mean ± standard deviation or percentage. Continuous variables were tested for normal distribution using skewness and kurtosis. Differences between groups were studied by the *Mann–Whitney U test, or †independent Student's t test or Fisher's exact test, as appropriate, with P < 0.05 as a cut‐off for significance.
ACE inhibitor, angiotensin‐converting‐enzyme inhibitor; BMI, body mass index; CKD‐EPI GFR, the estimated glomerular filtration rate calculated with the Chronic Kidney Disease Epidemiology Collaboration equation21; IU, international unit; NYHA class, New York Heart Association functional classification of heart failure severity22; pack‐year, 1 year of smoking 20 cigarettes per day.
Circulating soluble suppression of tumorigenicity‐2 exhibits distinct day and night variations
Peak sST2 values grouped clearly based on sampling time in the majority of subjects (Figure 1): 81.3% (P = 0.021) of patients and 93.8% (P = 0.001) of controls had their maximum sST2 concentrations during the day, while minimum concentrations were usually observed during night‐time for both patients (87.5%; P = 0.004) and controls (81.3%; P = 0.021) (Figure 1A, B). Per subject, range spread of sST2 concentration spanned from a minimum of 7.8% to a maximum of 34.2% for patients (19.4% on average, n = 16) (Table 2), and 9.6% to 60.9% for controls (23.5% on average, n = 16) (data not shown). Taken together, both groups had their highest concentrations during daytime (modal value: 5 p.m. for 13/32 subjects; 1 p.m. for 9/32), declining towards their lowest values during the night (modal value: 5 a.m., for 18/32 subjects). Among patients, of the three (18.8%) that deviated from the normal 24 h distribution of sST2 concentration (i.e. had their peak at night‐time instead of daytime), one was in palliative setting of end‐stage HF, the second recently had an implantable cardioverter–defibrillator (ICD)‐induced shock, and the third was on high‐dose prednisone, which is known to affect the circadian clock.9 These patients were included in all baseline tables and primary data analyses (n = 16), but cosinor analysis for both sST2 and NT‐proBNP was also performed without these three outliers to exclude confounding effects of severe clock disruption (indicated by n = 13).
Figure 1.
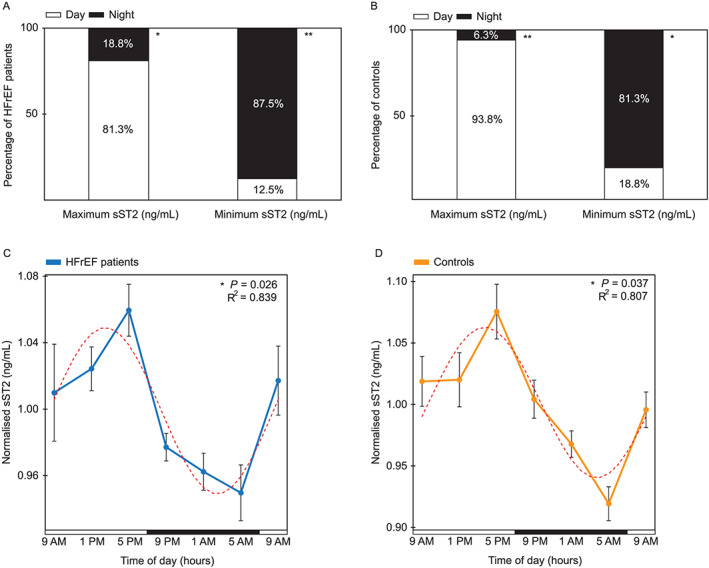
sST2 biomarker exhibits a diurnal rhythm in heart failure patients and controls. (A) Percentage of HFrEF patients (n = 16) who have their maximum and minimum sST2 values (ng/mL) during either the day (9 a.m., 1 p.m., and 5 p.m.) or night (9 p.m., 1 a.m., and 5 a.m.). (B) Percentage of controls (n = 16) who have their maximum and minimum sST2 values (ng/mL) during either the day (9 a.m., 1 p.m., and 5 p.m.) or night (9 p.m., 1 a.m., and 5 a.m.). (C) Cosinor analysis of normalized sST2 (ng/mL) per each HFrEF patient (mean ± SEM), in a period of 24 h. Per subject, each time point was normalized against its mean sST2 value of the entire day (n = 13). (D) Cosinor analysis of normalized sST2 (ng/mL) per each control (mean ± SEM), in a period of 24 h. Per subject, each time point was normalized against its mean sST2 value of the entire day (n = 16). Horizontal bars indicate day (white) and night (black). One‐sample non‐parametric binomial test was used to test the chance of non‐randomness of sST2 concentrations peaking at daytime or night‐time. P < 0.05 was used as a cut‐off for significance. *P < 0.05 and **P < 0.01. R2 indicates the proportion of the variance explained by the 24 h variation. Dashed red line represents a fitted cosine curve. HFrEF, heart failure with reduced ejection fraction; sST2, soluble suppression of tumorigenicity‐2.
Table 2.
Twenty‐four‐hour range of soluble suppression of tumorigenicity‐2 concentration (ng/mL) in individual heart failure with reduced ejection fraction patients
| Patient number | Maximum (ng/mL) | Minimum (ng/mL) | Range (ng/mL) | Range spread (%) |
|---|---|---|---|---|
| 1 | 101.9 | 94.5 | 7.4 | 7.8 |
| 2 | 35.9 | 32.5 | 3.4 | 10.3 |
| 3 | 37.1 | 33.2 | 3.9 | 11.7 |
| 4 | 19.2 | 16.9 | 2.3 | 13.9 |
| 5 | 25.6 | 22.5 | 3.1 | 13.9 |
| 6 | 47.8 | 41.9 | 5.9 | 14.1 |
| 7 | 62.7 | 53.9 | 8.9 | 16.5 |
| 8 | 101.9 | 87.4 | 14.5 | 16.6 |
| 9 | 65.9 | 55.6 | 10.2 | 18.4 |
| 10 | 54.6 | 45.5 | 9.1 | 19.9 |
| 11 | 21.6 | 17.7 | 3.9 | 22.0 |
| 12 | 64.2 | 51.5 | 12.8 | 24.8 |
| 13 | 65.4 | 51.1 | 14.3 | 27.9 |
| 14 | 33.2 | 25.8 | 7.4 | 28.6 |
| 15 | 71.4 | 55.3 | 16.1 | 29.2 |
| 16 | 33.1 | 24.7 | 8.4 | 34.2 |
Maximum = highest measured sST2 concentration (ng/mL) in 24 h period. Minimum = lowest measured sST2 concentration (ng/mL) in 24 h period. Range = maximum–minimum. Range spread = range/minimum. Patients in which maximum and minimum sST2 values fluctuate above and below 35 ng/mL cut‐off value are indicated in bold.
HFrEF, heart failure with reduced ejection fraction; sST2, soluble suppression of tumorigenicity‐2.
In order to visualize the overall diurnal patterns of patients with different absolute values, we also analysed normalized sST2 concentrations. Again, we observed a striking gradual decline from daytime values towards the night: a cosinor analysis of serum sST2 levels revealed cosine shaped 24 h oscillations in mean normalized values of HF patients (P = 0.026 for n = 13; P = 0.072 for n = 16) and controls (P = 0.037, n = 16) (Figure 1C, D, and Figure S2).
Circulating N‐terminal pro‐B‐type natriuretic peptide fluctuates randomly within a 24 h period in heart failure with reduced ejection fraction patients and controls
Differences between minimum and maximum values of NT‐proBNP ranged up to 76.5% in HFrEF patients within 24 h (Table S3). NT‐proBNP concentration did not follow a diurnal pattern in either of the two groups, fluctuating randomly within a day (Figure 2). In HFrEF patients, distributions of maximum and minimum values on average were almost equally divided over day and night. During the day, NT‐proBNP concentration reached its peak values in 56.3% and nadir in 43.8% of patients, while a similar peak/nadir prevalence was observed during the night (peak in 43.8% of patients, P = 0.804; and nadir in 56.3%, P = 0.804) (Figure 2A). In controls, peaks were reached in 75.0% during the day and 25.0% during the night (P = 0.077), while nadir values appeared during the day in 31.3% and during the night 68.8% (P = 0.210) (Figure 2B). Random distribution of maximum and minimum values throughout the 24 h period was also noted on mean normalized NT‐proBNP concentrations (pg/mL) where cosinor rhythmicity was assessed using a fitted cosinor model in HFrEF patients and controls and showed no significance (P = 0.091 for n = 13; P = 0.096 for n = 16 patients; P = 0.093 for n = 16 controls) (Figure 2C, 2D, and Figure S3). The non‐circadian, non‐repeating pattern is especially apparent from the very different 9 a.m. values that should be roughly the same in datasets with circadian distribution. Obtained data were in line with previously published studies in which no circadian or diurnal rhythm was found in circulating NT‐proBNP.8
Figure 2.
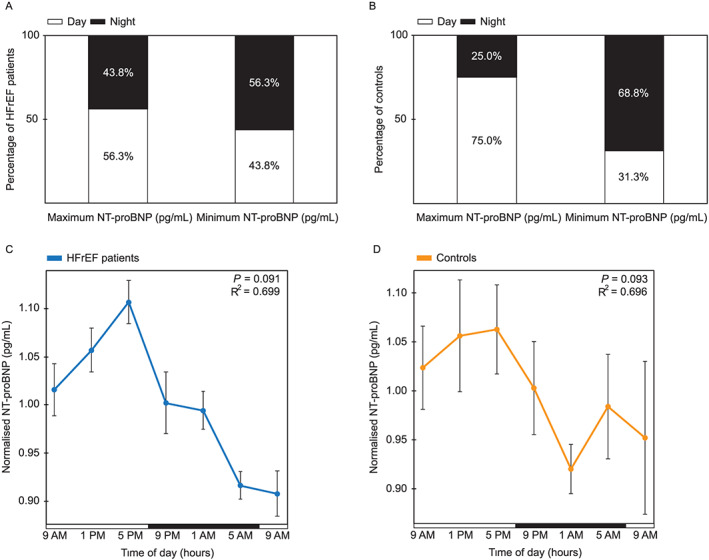
NT‐proBNP biomarker fluctuates randomly within a day in heart failure patients and controls. (A) Percentage of HFrEF patients (n = 16) who have their maximum and minimum NT‐proBNP values (pg/mL) during either the day (9 a.m., 1 p.m., and 5 p.m.) or night (9 p.m., 1 a.m., and 5 a.m.). (B) Percentage of controls (n = 16) who have their maximum and minimum NT‐proBNP values (pg/mL) during either the day (9 a.m., 1 p.m., and 5 p.m.) or night (9 p.m., 1 a.m., and 5 a.m.). (C) Cosinor analysis of normalized NT‐proBNP (pg/mL) per each HFrEF patient (mean ± SEM), in a period of 24 h. Per subject, each time point was normalized against its mean NT‐proBNP value of the entire day (n = 13). (D) Cosinor analysis of normalized NT‐proBNP (pg/mL) per each control (mean ± SEM), in a period of 24 h. Per subject, each time point was normalized against its mean NT‐proBNP value of the entire day (n = 16). Horizontal bars indicate day (white) and night (black). One‐sample non‐parametric binomial test was used to test the chance of non‐randomness of NT‐proBNP concentrations peaking at daytime or night‐time. P < 0.05 was used as a cut‐off for significance. R2 indicates the proportion of the variance explained by the 24 h variation. HFrEF, heart failure with reduced ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Robust circadian markers remain normal in heart failure patients and controls
Serum melatonin and cortisol patterns within the 24 h period were as expected in all of 36 subjects, regardless of the presence or absence of HF (Figure 3). Melatonin was consistently low during daytime in both groups, with the highest concentrations during the night (modal value: 5 a.m.), while cortisol had diametrically opposite values and peaked during the early morning (modal value: 9 a.m.). Distribution of peak times for both hormones is listed in Table S4.
Figure 3.
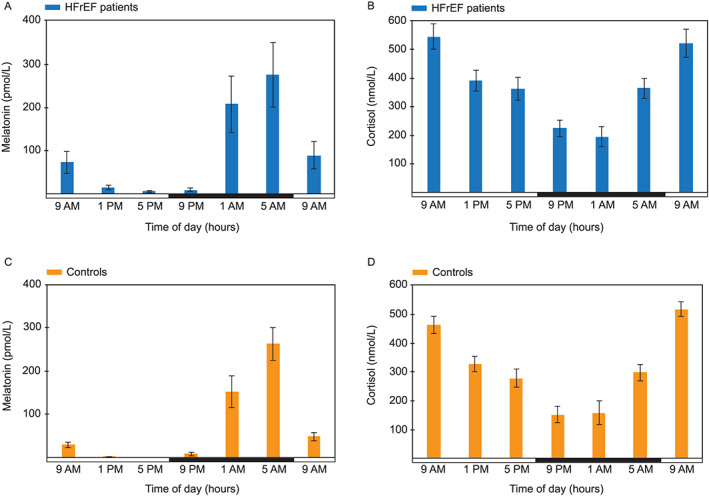
Study subjects exhibit normal melatonin and cortisol patterns. Within‐day distribution of (A) melatonin (pmol/L) and (B) cortisol concentration (nmol/L) in heart failure patients (mean ± SEM). Within‐day distribution of (C) melatonin (pmol/L) and (D) cortisol concentration (nmol/L) in control subjects (mean ± SEM). Horizontal bar indicates day (white) and night (black). HFrEF, heart failure with reduced ejection fraction.
Discussion
In this study, we provide new insights in so far uninvestigated circadian properties of circulating sST2 levels in HF patients. In the vast majority of subjects, sST2 concentration reached its peak in the afternoon, with the lowest levels during the night‐time. Knowledge of this diurnal variation will lead to improved usage of sST2 as a prognostic biomarker.
Circadian rhythms in cardiovascular (patho)physiology
The importance of circadian rhythms in cardiovascular health and disease has been recognized and emphasized in many studies to date. We recently provided an in‐depth explanation of circadian biology and its interplay with cardiovascular function and dysfunction.7 Briefly, functional circadian rhythms have been found in every cell type within the cardiovascular system, influencing its function in many ways; from vascular tone, heart rate, blood pressure, signalling, and cardiac metabolism to thrombus formation, onset of myocardial infarction, and arrhythmias. Therefore, it does not come as a surprise that certain cardiovascular‐related biomarkers are also under direct or indirect influence of the clock and exhibit day and night fluctuations.
The goal of the study was to investigate whether sST2 exhibits circadian variation in patients with HFrEF and controls without cardiovascular disease. While the existence of a circadian rhythm in sST2 serum concentrations was unknown, it has been previously investigated for other biomarkers. For example, cardiac troponin T was shown to exhibit non‐random diurnal variation,12 while predictable daily fluctuations were absent in NT‐proBNP8 and cardiac troponin I13 levels. Serving as a negative control in our study, NT‐proBNP fluctuated randomly throughout the day and indeed did not exhibit any rhythmicity in HF patients or controls. Concentrations of melatonin and cortisol, robust circadian markers, were measured to serve as positive controls of internal circadian rhythms, with cortisol levels peaking during the day and melatonin levels during the night. Observed data were in line with expected rhythmic behaviour of these main endocrine products of the central clock,9 pointing to preserved neurohumoral circadian output in both subject groups.
Diurnal oscillation of soluble suppression of tumorigenicity‐2 biomarker
To the best of our knowledge, this is the first record of the diurnality of circulating sST2 concentrations, resulting in important implications for clinical usage in terms of prognosis and therapy guidance (Figure 4). Circulating sST2, reflecting stress brought upon myocardial injury and adverse remodelling, recently emerged as a strong predictor of HF outcome. Indeed, within our HFrEF patients, the majority of patients with mean sST2 values higher than 35 ng/mL either died or suffered from another major adverse outcome. For detailed description of sST2 biology and its role in mediating myocardial strain, as well as in the development of vascular disease, the reader is referred to recently published review articles.14, 15 sST2, acting as a decoy receptor for IL‐33 and thereby inhibiting its beneficial cardioprotective effects, is implicated in cardiac and vascular remodelling. As favourable effects of IL‐33 are blocked with the abnormal quantities of sST2 in circulation, measurement of sST2 concentration has proven useful in providing prognostic information for various cardiovascular disorders.
Figure 4.
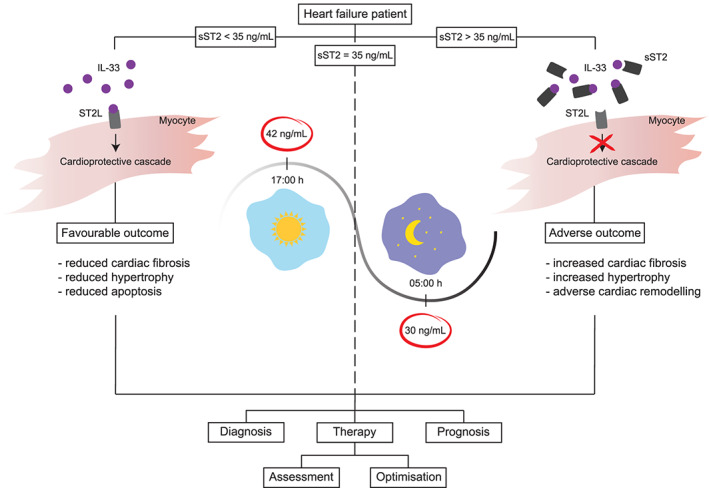
Influence of diurnal rhythms on clinical interpretation of sST2 biomarker. Knowledge about the diurnal variation of sST2 concentration is needed for proper timing of measurements and their interpretation. Prognostic and diagnostic value of sST2 levels, as well as therapy optimization, should be refined by applying specified sampling times and repeated measurements. IL‐33, interleukin‐33; sST2, soluble suppression of tumorigenicity‐2; ST2L, suppression of tumorigenicity‐2 ligand.
Aside from its prognostic value, sST2 could be used to monitor treatment effect and subsequently guide therapy; it was previously shown that patients with reduction of sST2 concentration in response to drug treatment had better outcomes than those with continued sST2 values > 35 ng/mL during the entire study period.16 Notably, biomarkers are increasingly used in clinical HF trials as an inclusion criterion, surrogate endpoint, and target for therapy.17 Thus, a rise or fall in sST2 concentration caused by physiological diurnal variation may lead to misinterpretation of treatment effect, overestimation or underestimation of prognostic warning signs, and confounding of clinical trial outcomes. It is therefore of importance to take into account at what time the blood sample was taken.
Interestingly, while the diurnal pattern of sST2 concentration was remarkably consistent across sex, age, and other baseline characteristics (Table 1), our data indicate that end‐stage HF in palliative setting, recent ICD‐induced shock, and high‐dose prednisone can lead to changes in peak time of sST2 levels. Thus, while the existence of physiological diurnal variation of sST‐2 levels can have an important impact on clinical decision making, a deviating 24 h pattern may also have predictive value.
Clinical outlook
Although sST2 is yet to be implemented in the clinic as a standard of care, it has already proven to be a robust prognostic biomarker for both chronic and acute HFrEF.14 As previously mentioned, the currently applied cut‐off value of sST2 in chronic HF is 35 ng/mL; however, reached consensus did not take into consideration possible biological 24 h variation of its concentration.18 Our study provides the first evidence for circadian rhythmicity of sST2, implying that time of day may be an important variable when determining sST2 levels. Therefore, clinicians are advised to be cautious while interpreting sST2 values, because a result just under 35 ng/mL in the morning may exceed this threshold in the afternoon. In Table 2, we show examples of this phenomenon in two patients.
Because of the diurnal rhythm of sST2, it would be recommended to measure sST2 during the afternoon, when sST2 levels are commonly at their highest, if the aim is to provide maximal negative predictive value and assure patient safety. Future studies re‐evaluating the cut‐off value of sST2 taking sample timing into account may further improve sST2 as a prognostic tool.
Finally, the average range spread of sST2 concentrations found in our patient group was 19.4%, with a maximum range spread of 34.2% in 24 h. Therefore, when using serial sST2 levels to assess the efficacy of initiated HF therapy and refine prognostic value,16 samples should ideally be taken around the same hour or corrected for time of day as much as possible. For example, a first measurement in the afternoon and a follow‐up measurement in the morning (yielding a lower sST2 concentration) may suggest an improvement in the patient's condition and a good therapeutic response, while in fact it demonstrates circadian fluctuation.
Study limitations
This study did not make use of standardized conditions for physical activity, meals, light exposure, and sleep duration, as is commonly done for clinical circadian experiments in order to achieve a more comparable circadian rhythm between subjects.12 Nevertheless, our set‐up makes the study clinically more translatable, as patients usually do not follow an externally imposed food/activity regimen. This way, all consistent differences found within daily levels of investigated biomarkers prove to be highly robust, if evident without a controlled environmental setting.
Another limitation of this study lies in the groups assembly, as the majority of the participants were of the male gender (78.1% of total participants). Coglianese et al. found a significant difference in sST2 levels between men and women,19 with higher concentrations noted in men. In our data, we observed similar diurnal patterns in men and women, but the relatively low number of women precludes definite conclusions on comparisons between both sexes. Furthermore, smoking has been shown to increase IL‐33 concentrations in the airway epithelium as well as to alter the ST2 expression pattern in the lung.20 A positive smoking status was reported in 53.1% of our subjects, however, with no obvious effect on the diurnality of sST2. Despite these limitations, the goal of our study, which was to observe diurnal differences within daily sST2 levels, was not affected and even strengthened.
Finally, sample size of the study was relatively low (n = 16 for each group), and three patients were excluded from the cosinor analysis owing to the severe clock disruption. However, this was sufficient to provide the first evidence for a diurnal pattern of sST2 expression. The uniqueness of this study lies in the repeated sST2 measurements during a 24 h period within each study participant. Even though the final number of included patients and controls is modest, sST2 measurements during seven time points per each patient/control provide a resolution that has not been reported before in ST2 and HF research.
Conclusions
Biomarker sST2 concentrations exhibit significant diurnal variation with predictable peak and trough times, a novel finding that has consequences for the clinical interpretation of sST2 levels in HF patients. Besides its impact on clinical management and clinical trials, the within‐day fluctuation of sST2 also gives hints for further investigation into its mechanism of action. Presumably, sST2‐driven inhibition of the cardioprotective IL‐33–ST2L pathway also has a time‐dependent character. While clinical significance is covered within the content of this study, biological importance is yet to be determined, potentially leading towards novel therapeutic strategies.
Funding
This work was supported by the Netherlands Heart Foundation Dekker Grant (2013T056 to LL), Jacob Jongbloed Talent Society Grant (Circulatory Health, UMCU; to S.C.) and by Horizon2020 ERC‐2016‐COG EVICARE (725229 to J.P.G.S.). The authors acknowledge the support from Innovation and the Netherlands CardioVascular Research Initiative (CVON): The Dutch Heart Foundation; Dutch Federation of University Medical Centres; the Netherlands Organization for Health Research and Development (ZonMw); and the Royal Netherlands Academy of Arts and Sciences (Koninklijke Nederlandse Akademie van Wetenschappen).
Conflicts of Interest
S.C., M.P., T.J., L.L., M.M., H.S., M.F., B.P., N.J., C.G., H.K., P.D., and J.S. have no conflict of interest. F.A. was supported by UCL Hospitals NIHR Biomedical Research Centre. L.L. received (related to the current work) NT‐proBNP assays from Roche and ST2 assays from Sopachem BV, in a form of an investigator‐initiated study, and (outside) consultancy fees from Abbott, Vifor, Novartis, and Medtronic, to the UMCU.
Supporting information
Table S1. Inclusion and exclusion criteria for participants.
Table S2. 12‐month outcome of HFrEF patients in relation to average sST2 concentrations (ng/mL).
Table S3. 24‐hour range of NT‐proBNP (pg/mL) in individual HFrEF patients.
Table S4. Distribution of peak times for melatonin and cortisol in HFrEF patients and controls.
Figure S1. Study design overview.
Figure S2. Overall 24‐hour values of sST2 biomarker in heart failure patients.
Figure S3. Overall 24‐hour values of NT‐proBNP biomarker in heart failure patients.
Acknowledgements
The authors would like to thank Walid al Hedni for performing proBNP analysis, Lilian Homsma for organizing clinical data, Leanne Smit and Loes Peters for their help with processing blood samples, and Lena Bosch for helping with patient inclusions.
Crnko, S. , Printezi, M. I. , Jansen, T. P. J. , Leiteris, L. , van der Meer, M. G. , Schutte, H. , van Faassen, M. , du Pré, B. C. , de Jonge, N. , Asselbergs, F. W. , Gaillard, C. A. J. M. , Kemperman, H. , Doevendans, P. A. , Sluijter, J. P. G. , and van Laake, L. W. (2020) Prognostic biomarker soluble ST2 exhibits diurnal variation in chronic heart failure patients. ESC Heart Failure, 7: 1224–1233. 10.1002/ehf2.12673.
Markella I. Printezi and Tijn P. J. Jansen contributed equally.
References
- 1. Boisot S, Beede J, Isakson S, Chiu A, Clopton P, Januzzi J, Maisel AS, Fitzgerald RL. Serial sampling of ST2 predicts 90‐day mortality following destabilized heart failure. J Card Fail 2008; 14: 732–738. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 3. Miller WL, Jaffe AS. Biomarkers in heart failure: the importance of inconvenient details. ESC Heart Fail 2015; 3: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dieplinger B, Januzzi JL, Steinmair M, Gabriel C, Poelz W, Haltmayer M, Mueller T. Analytical and clinical evaluation of a novel high‐sensitivity assay for measurement of soluble ST2 in human plasma—the Presage ST2 assay. Clin Chim Acta 2009; 409: 33–40. [DOI] [PubMed] [Google Scholar]
- 5. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 2012; 35: 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly RM, Healy U, Sreenan S, McDermott JH, Coogan AN. Clocks in the clinic: circadian rhythms in health and disease. Postgrad Med J 2018; 94: 653–658. [DOI] [PubMed] [Google Scholar]
- 7. Crnko S, Du Pré BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol 2019; 16: 437–447. [DOI] [PubMed] [Google Scholar]
- 8. Goetze JP, Jørgensen HL, Sennels HP, Fahrenkrug J. Diurnal plasma concentrations of natriuretic propeptides in healthy young males. Clin Chem 2012; 58: 789–792. [DOI] [PubMed] [Google Scholar]
- 9. Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E. The functional and clinical significance of the 24‐hour rhythm of circulating glucocorticoids. Endocr Rev 2017; 38: 3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Faassen M, Bischoff R, Kema IP. Relationship between plasma and salivary melatonin and cortisol investigated by LC‐MS/MS. Clin Chem Lab Med 2017; 55: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 11. Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 2007; 38: 275–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klinkenberg LJJ, van Dijk JW, Tan FES, van Loon LJC, van Dieijen‐Visser MP, Meex SJR. Circulating cardiac troponin T exhibits a diurnal rhythm. J Am Coll Cardiol 2014; 63: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 13. van der Linden N, Cornelis T, Klinkenberg LJJ, Kimenai DM, Hilderink JM, Litjens EJR, Kooman JP, Bekers O, van Dieijen‐Visser MP, Meex SJR. Strong diurnal rhythm of troponin T, but not troponin I, in a patient with renal dysfunction. Int J Cardiol 2016; 221: 287–288. [DOI] [PubMed] [Google Scholar]
- 14. McCarthy CP, Januzzi JL. Soluble ST2 in heart failure. Heart Fail Clin 2018; 14: 41–48. [DOI] [PubMed] [Google Scholar]
- 15. Vianello E, Dozio E, Tacchini L, Frati L, Corsi Romanelli MM. ST2/IL‐33 signaling in cardiac fibrosis. Int J Biochem Cell Biol 2019; 116: 105619. [DOI] [PubMed] [Google Scholar]
- 16. van Vark LC, Lesman‐Leegte I, Baart SJ, Postmus D, Pinto YM, Orsel JG, Westenbrink BD, Brunner‐la Rocca HP, van Miltenburg AJM, Boersma E, Hillege HL, Akkerhuis KM, TRIUMPH Investigators . Prognostic value of serial ST2 measurements in patients with acute heart failure. J Am Coll Cardiol 2017; 70: 2378–2388. [DOI] [PubMed] [Google Scholar]
- 17. Ibrahim NE, Gaggin HK, Konstam MA, Januzzi JL Jr. Established and emerging roles of biomarkers in heart failure clinical trials. Circ Heart Fail 2016; 9: e002528. [DOI] [PubMed] [Google Scholar]
- 18. Januzzi JL, Mebazaa A, Di Somma S. ST2 and prognosis in acutely decompensated heart failure: the International ST2 Consensus Panel. Am J Cardiol 2015; 115: 26B–31B. [DOI] [PubMed] [Google Scholar]
- 19. Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, Cheng S, Fradley MG, Kretschman D, Gao W, O'Connor G, Wang TJ, Januzzi JL. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem 2012; 58: 1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kearley J, Silver JS, Sanden C, Liu Z, Berlin AA, White N, Mori M, Pham T‐H, Ward CK, Criner GJ, Marchetti N, Mustelin T, Erjefalt JS, Kolbeck R, Humbles AA. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin‐33‐dependent response to infection. Immunity 2015; 42: 566–579. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and exclusion criteria for participants.
Table S2. 12‐month outcome of HFrEF patients in relation to average sST2 concentrations (ng/mL).
Table S3. 24‐hour range of NT‐proBNP (pg/mL) in individual HFrEF patients.
Table S4. Distribution of peak times for melatonin and cortisol in HFrEF patients and controls.
Figure S1. Study design overview.
Figure S2. Overall 24‐hour values of sST2 biomarker in heart failure patients.
Figure S3. Overall 24‐hour values of NT‐proBNP biomarker in heart failure patients.


