Abstract
Takotsubo syndrome (TTS), also referred to as stress cardiomyopathy, is characterized by transient left ventricular apical ballooning in the absence of obstructive coronary artery disease. Catecholamine‐induced cardiac injury or vasospasm has been implicated in this pathophysiology. We present a case of a 67‐year‐old man 10 years after heart transplantation diagnosed with TTS. Sympathetic reinnervation could not be detected by iodine‐123 meta iodobenzylguanidine uptake, suggesting that TTS can occur in the absence of functional sympathetic nerve systems reconstruction.
Keywords: Takotsubo syndrome, Heart transplantation, Cardiac sympathetic reinnervation, 123I‐MIBG scintigraphy
Introduction
Takotsubo syndrome (TTS) is an acute cardiac dysfunction that typically represents hypokinesis of the apical segment of the left ventricle (LV) beyond a single coronary artery territory. One common feature is a surge in catecholamines in response to emotional or physical stress1. Therefore, catecholamine‐induced cardiomyocyte injury and epicardial coronary artery or microvascular spasm have been proposed for the pathophysiology.2 Heart transplantation caused cardiac denervation by surgical interruption of the sympathetic and parasympathetic nerve signalling; however, cardiac reinnervation remains a matter of debate. Myocardial uptake of iodine‐123 meta iodobenzylguanidine (123I‐MIBG) reflects sympathetic nerve function3 and is used to assess the extent of cardiac reinnervation after heart transplantation. The present case developed TTS but did not show significant myocardial uptake of 123I‐MIBG, suggesting that cardiac sympathetic innervation persistently disturbed.
Case report
A 67‐year‐old man who had undergone heart transplantation with the biatrial technique for ischaemic cardiomyopathy 10 years earlier was transferred to our hospital with dyspnoea lasting 5 days. He had been in charge of getting a nerve‐fraying project done, having some problems leading a team, and therefore, under emotional and strenuous physical stress in the days before the symptom developed. After the successful heart transplantation, he was regularly followed and had no serious complications except for cryptococcal pneumonia 9 years ago and transient complete atrioventricular block requiring pacemaker implantation 2 years ago. On admission, his blood pressure was 102/60 mmHg, heart rate 87 b.p.m., and peripheral oxygen saturation 90%. A chest X‐ray showed pulmonary oedema. Electrocardiogram showed sinus rhythm with decreased R wave amplitude at inferior leads and non‐significant elevation of ST segment at leads V2, 3 (Figure 1 A). Serum troponin level was elevated at 0.173 ng/mL (normal value <0.014 ng/mL, Table 1). Transthoracic echocardiography was remarkable for severe hypokinesis of the apical portion of the LV and hyperkinesis of the base, with an ejection fraction of 39% (Figure 1 B and Table 1). Coronary angiography revealed non‐obstructive coronary artery disease (Figure 1 C). Based on these findings, he was provisionally diagnosed with myocardial infarction with non‐obstructive coronary arteries (MINOCA) for which the differential diagnosis involved TTS. Endomyocardial biopsy showed mild cellular cardiac allograft rejection (The International Society for Heart and Lung Transplantation acute cellular rejection 2004 classification Grade 1R, Figure 2 A) and cardiac magnetic resonance T2‐weighted imaging did not display increased signal in the left ventricular wall (Figure 2 B). The patient was treated with nitrate and diuretics. Dual‐isotope single‐photon emission computed tomography using thallium‐201 chloride (201TlCl) and iodine‐123 beta‐methyl‐p‐iodophenyl‐pentadecanoic acid (123I‐BMIPP) showed mildly impaired myocardial perfusion at inferior with the concordant defect of 123I‐BMIPP uptake (Figure 2 C). The serial heart‐to‐mediastinal ratio level of 123I‐MIBG showed no significant increase at the 3 months, 2, 3, 5, 7, 8, 9, and 10 years after transplantation (early/delayed heart‐to‐mediastinal ratio: 1.8/1.6, 1.9/1.5, 2.1/1.7, 1.9/1.5, 2.1/1.6, 1.8/1.5, 1.7/1.5, and 1.7/1.4, Figure 3 A–C). His acute heart failure improved over 2 weeks. The follow‐up echocardiography in 4 weeks showed improved left ventricular function with a complete resolution of the wall motion abnormalities (Table 1). He received a definitive diagnosis TTS after fulfilling the recent international expert consensus's diagnostic criteria.4
Figure 1.
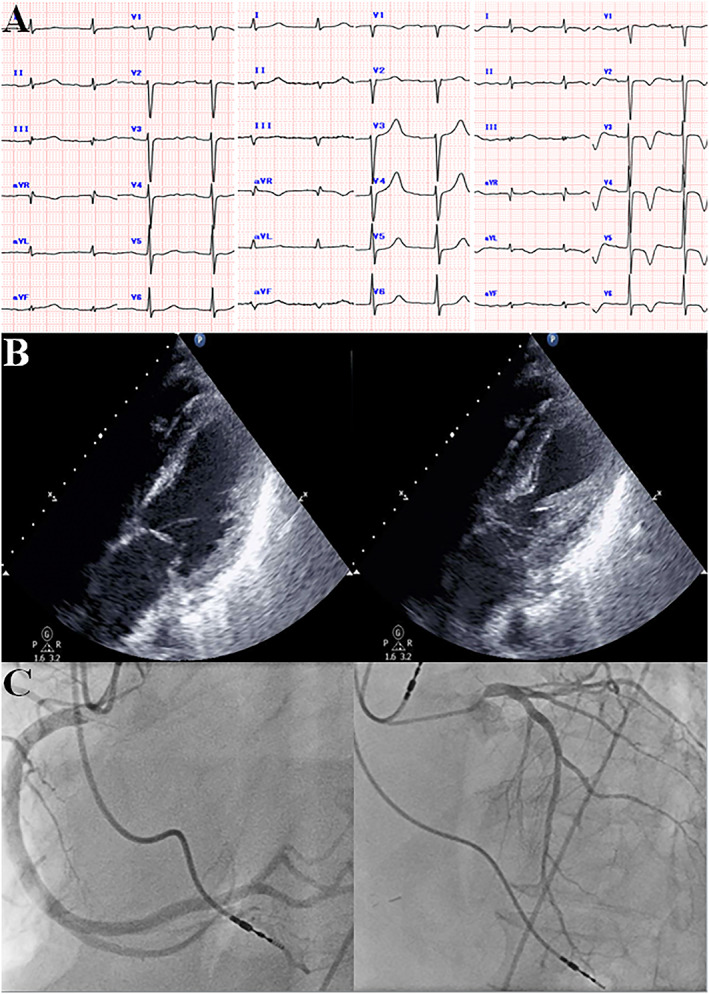
(A) Serial change in electrocardiogram at 4 months before admission (left), admission (middle), and 8 days after admission. (B) Echocardiography in a four‐chamber view at end‐diastole (left) and end‐systole (right) showing severe hypokinesis of the middle and apical portion of the left ventricle. (C) Coronary angiography showed minor luminal irregularities.
Table 1.
Temporal changes in echocardiographical parameters, laboratory data, and medications
| On admission | On the 13th day | On the 29th day | |
|---|---|---|---|
| Echocardiography | |||
| LVDd (mm) | 50 | 47 | 46 |
| LVDs (mm) | 33 | 27 | 27 |
| LVEF (%) | 39.2 | 60.4 | 65.0 |
| S′ (cm/s) | 6.3 | 7.2 | 7.6 |
| E/E′ | 12.4 | 6.4 | 10.9 |
| Laboratory data | |||
| Troponin T (ng/mL) | 0.173 | 0.02 | |
| BNP (pg/mL) | 2894 | 409 | 316 |
| Medications | Telmisartan 80 mg | Azosemide 30 mg | |
| Nifedipine 60 mg | Nifedipine 20 mg | ||
| Atorvastatin 10 mg | Atorvastatin 10 mg | ||
| Tacrolimus 0.8 mg | Tacrolimus 0.1 mg | ||
| Everolimus 0.5 mg | Everolimus 0.125 mg | ||
| Fluconazole 200 mg | Fluconazole 200 mg | ||
| Febuxostat 20 mg | Febuxostat 20 mg | ||
| Linagliptin 5 mg | Linagliptin 5 mg | ||
| Levothyroxine 25 μg | Levothyroxine 25 μg | ||
| Rabeprazole 10 mg | Rabeprazole 10 mg |
Tissue Doppler imaging parameters of S′ and E/E′ are expressed as the average of septal and lateral measurements. BNP, B‐type natriuretic peptide; LVDd, left ventricular end‐diastolic diameter; LVDs, left ventricular end‐systolic diameter; LVEF, left ventricular ejection fraction.
Figure 2.
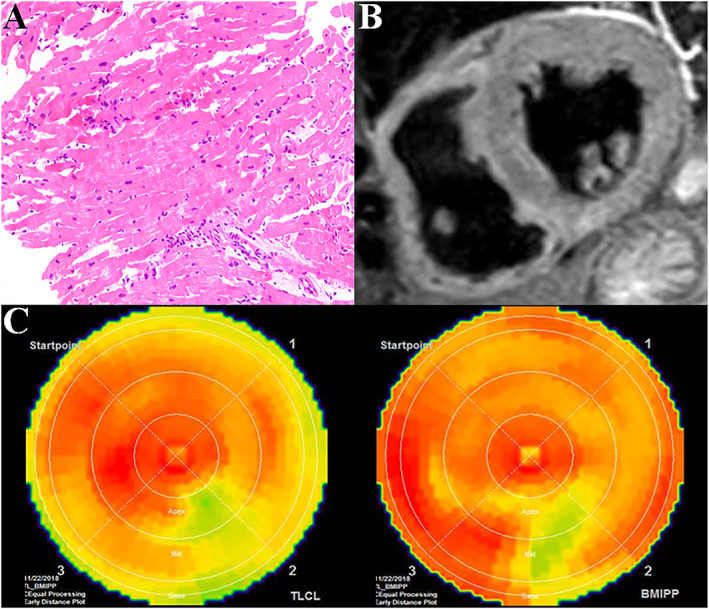
(A) The endomyocardial biopsy specimen showed the International Society for Heart and Lung Transplantation Grade 1R rejection. (B) T2‐weighted short‐axis view showed an absence of increased signal in the mid‐ventricular segment of the left ventricle. (C) Polar maps of dual‐isotope single‐photon emission computed tomography using thallium‐201 chloride (upper) and iodine‐123 beta‐methyl‐p‐iodophenyl‐pentadecanoic acid (123I‐BMIPP, lower) myocardial images showed mild hypoperfusion at inferior.
Figure 3.
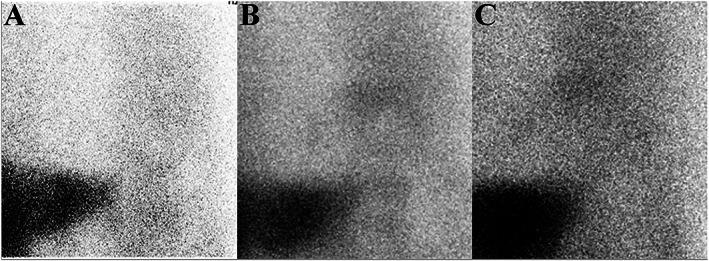
Early planar iodine‐123 meta iodobenzylguanidine showed very low myocardial uptake at 3 months [left, heart‐to‐mediastinal ratio 1.75 (A)], 5 years [middle, heart‐to‐mediastinal ratio 1.9 (B)], and 10 years [right, heart‐to‐mediastinal ratio 1.73 (C)] after heart transplantation.
Discussion
Takotsubo syndrome is considered as a form of MINOCA, which is a syndrome with different aetiologies, characterized by clinical evidence of myocardial infarction in the absence of obstructive coronary arteries on angiography.5, 6 Although the distinction between TTS and other forms of MINOCA is challenging in some cases, the normalization of LV systolic function in a relatively short period confirms the diagnosis of TTS. TTS commonly occurs in a setting in which the patient is exposed to emotional or physical stress and the resultant surge in catecholamine.1 The underlying pathophysiological mechanisms remain undetermined; however, they include catecholamine‐induced cardiomyocyte injury and microvascular spasm resulting in myocardial stunning. Heart transplantation caused extrinsic cardiac denervation by surgically interrupting the sympathetic and parasympathetic nerve fibres in the heart. Cardiac reinnervation has been shown to occur; however, it is dependent on time after transplantation and does not occur in all recipients.7 Early and late myocardial uptakes of MIBG reflect a distribution of the presynaptic nerves system and neuronal function, respectively, and can be used to study the extent of cardiac innervation after heart transplantation. This patient serially underwent 123I‐MIBG scintigraphy, but no significant myocardial uptake occurred, suggesting that global sympathetic denervation persisted after heart transplantation. In favour of this hypothesis, his chronotropic response to exercise did not significantly improve from 5 months to 2, 3, 5, 6, 7, 8, 9, and 10 years after transplantation (peak increment in heart rate with exercise: 27, 24, 42, 38, 15, 37, 18, 36, and 17 beats, respectively). Possible explanations for the development of TTS in the absence of global regenerating nerves are the following. (i) Cardiac reinnervation partially and heterogeneously might occur8; however, MIBG could not detect this incomplete limited reinnervation. (ii) The transplanted heart might be hypersensitive to circulating catecholamines due to the up‐regulation of β‐adrenergic receptors and have an effect on inotropic responses with increased susceptibility to TTS.9 The recent study proposed a conceptual model describing the potential mechanism responsible for the development of TTS. They reported that patients with TTS had decreased communication between brain regions associated with emotional processing and the autonomic nervous system.10 The impaired neural network communication could handle stress inappropriately and induce an excessive stimulation of the autonomic nervous system that ultimately might make patients subject to TTS. (iii) The absence of parasympathetic reinnervation could potentially lead to an exaggerated response to circulating catecholamines. In support of this hypothesis, the parasympathetic component of heart rate variability, measured by rMSSD (the square root of the mean squared differences of successive normal‐to‐normal intervals), did not improve from 3 months to 10 years after transplantation (9.4 and 10.2 ms, respectively, normal value 27 ± 12 ms).
Several studies suggested that stress‐induced multi‐vessel coronary artery spasm contributed to the pathophysiology of TTS. We could not perform an acetylcholine challenge test for coronary spasm due to chronic renal insufficiency, but we alternatively evaluated myocardial fatty acid metabolism (123I‐BMIPP) combined with myocardial perfusion (201TlCl). The mismatched areas between 201TlCl and 123I‐BMIPP scintigraphy reflect transient ischaemic damage or metabolically stunned myocardium. This case showed that the metabolic abnormality was not larger than that of perfusion, suggesting that post‐ischaemic myocardial stunning caused by multi‐vessel coronary spasm as a causative mechanism of TTS was unlikely.
There are three case reports that spontaneously developed TTS after heart transplantation.11, 12, 13 TTS occurred 6, 9, and 10 years after heart transplantation. It is interesting to note that all these patients including ours did not experience chest pain at the onset of TTS, suggesting the limited sensory afferent reinnervation of their hearts. Our study tried to figure out for the first time about the mechanism of TTS by assessing the cardiac reinnervation status through serial MIBG scintigraphy, heart rate response to physical stress, and heart rate variability. The present case could underline the necessities of further studies to better understand the pathophysiology of TTS.
Takotsubo syndrome can occur in heart transplant recipients with poor sympathetic reinnervation, suggesting that complete cardiac nerve regeneration to connect between the cardiac afferent and efferent fibres is not a prerequisite in the pathophysiology of this disease.
Conflict of interest
None declared.
Miyake, R. , Ohtani, K. , Hashimoto, T. , Yada, R. , Sato, T. , Shojima, Y. , Hayashidani, S. , Higo, T. , and Tsutsui, H. (2020) Takotsubo syndrome in a heart transplant recipient with poor cardiac sympathetic reinnervation. ESC Heart Failure, 7: 1145–1149. 10.1002/ehf2.12632.
References
- 1. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352: 539–548. [DOI] [PubMed] [Google Scholar]
- 2. Nef HM, Mollmann H, Akashi YJ, Hamm CW. Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol 2010; 7: 187–193. [DOI] [PubMed] [Google Scholar]
- 3. Sawada H, Oeda T, Yamamoto K, Kitagawa N, Mizuta E, Hosokawa R, Ohba M, Nishio R, Yamakawa K, Takeuchi H, Shimohama S, Takahashi R, Kawamura T. Diagnostic accuracy of cardiac metaiodobenzylguanidine scintigraphy in Parkinson disease. Eur J Neurol 2009; 16: 174–182. [DOI] [PubMed] [Google Scholar]
- 4. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Shams YH, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018; 39: 2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P, Pharmacotherapy WGoC . ESC working group position paper on myocardial infarction with non‐obstructive coronary arteries. Eur Heart J 2017; 38: 143–153. [DOI] [PubMed] [Google Scholar]
- 6. Ali M, Rigopoulos AG, Ali K, Ikonomidis I, Makavos G, Matiakis M, Melnyk H, Abate E, Mammadov M, Pruser JL, de Vecchis R, Wohlgemuth W, Manginas A, Bigalke B, Mavrogeni S, Sedding D, Noutsias M. Advancements in the diagnostic workup, prognostic evaluation, and treatment of Takotsubo syndrome. Heart Fail Rev 2019; 1–15. [DOI] [PubMed] [Google Scholar]
- 7. Beckers F, Ramaekers D, Speijer G, Ector H, Vanhaecke J, Verheyden B, Van Cleemput J, Droogne W, Van de Werf F, Aubert AE. Different evolutions in heart rate variability after heart transplantation: 10‐year follow‐up. Transplantation 2004; 78: 1523–1531. [DOI] [PubMed] [Google Scholar]
- 8. Uberfuhr P, Frey AW, Ziegler S, Reichart B, Schwaiger M. Sympathetic reinnervation of sinus node and left ventricle after heart transplantation in humans: regional differences assessed by heart rate variability and positron emission tomography. J Heart Lung Transplant 2000; 19: 317–323. [DOI] [PubMed] [Google Scholar]
- 9. von Scheidt W, Bohm M, Schneider B, Reichart B, Erdmann E, Autenrieth G. Isolated presynaptic inotropic beta‐adrenergic supersensitivity of the transplanted denervated human heart in vivo. Circulation 1992; 85: 1056–1063. [DOI] [PubMed] [Google Scholar]
- 10. Templin C, Hanggi J, Klein C, Topka MS, Hiestand T, Levinson RA, Jurisic S, Luscher TF, Ghadri JR, Jancke L. Altered limbic and autonomic processing supports brain‐heart axis in Takotsubo syndrome. Eur Heart J 2019; 40: 1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunanan V, Ganesh S, Kleisli T, Barr M. Takotsubo cardiomyopathy in a heart transplant recipient. Chest 2013; 144: 124A. [Google Scholar]
- 12. Behnes M, Baumann S, Borggrefe M, Haghi D. Biventricular Takotsubo cardiomyopathy in a heart transplant recipient. Circulation 2013; 128: e62–e63. [DOI] [PubMed] [Google Scholar]
- 13. Chinali M, Formigari R, Grutter G. Takotsubo cardiomyopathy in a young adult with transplanted heart: what happened to denervation? ESC Heart Fail 2018; 5: 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]


