Abstract
Aims
Diabetes mellitus (DM) has a negative impact on prognosis in patients with heart failure (HF). The role impact of DM in HF patients with implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT) devices might differ and remains unclear. The aim of our study was to investigate the impact of DM on periprocedural complications and clinical outcome in HF patients undergoing ICD or CRT implantation.
Methods and results
Within the German Device Registry, data from 50 German centres were collected between January 2007 and February 2014. A retrospective analysis of n = 5329 patients undergoing ICD implantation was conducted. Patients' characteristics, procedural data, periprocedural complications, and post‐procedural clinical outcome, including a composite clinical endpoint of all‐cause mortality, stroke, and myocardial infarction (MACCE), were analysed. Subgroup analysis were performed for ICD and CRT implantations. Median follow‐up was 15.7 (12.9; 20.0) and 16.2 (12.8; 21.2) months in DM and non‐DM patients. Of 5329 patients enrolled, n = 1448 (27.2%) had a diagnosis of DM. Within the cohort, 94% of DM and 90% of non‐DM patients had a diagnosis of HF. Patients with DM were older, had higher body mass index, and higher rate of cardiovascular comorbidities compared with non‐DM patients. Unadjusted and adjusted analyses revealed similar all‐over intrahospital periprocedural complication rates in both groups (4.1% vs 3.9%). Unadjusted Kaplan–Meier survival analysis showed higher all‐cause mortality after 1 year (9.0% vs 6.3%; log‐rank P = 0.001) with higher MACCE rates (10.0% vs 7.3%; P < 0.001) in the DM group versus non‐DM patients. After multivariable adjustment for relevant covariates, the association of DM to MACCE disappeared [HR 1.11 (0.89‐1.38)]. Because chronic kidney disease (CKD) was clearly associated with increased 1 year MACCE after multivariate adjustment [odds ratio (OR) 2.11 (1.68–2.64)], a subgroup analysis was performed showing a strong trend towards more perioperative complications in DM patients with CKD [OR 2.16 (0.9–5.21)], while no effect of DM was observed in patients without CKD [OR 0.73 (0.42–1.28)].
Conclusions
The overall risk of periprocedural complications and short‐term (1 year) clinical outcome in patients with DM and HF undergoing ICD or CRT defibrillator (CRT‐D) implantation was not increased. In contrast, CKD was associated with an increased risk of 1 year MACCE in HF patients undergoing ICD/CRT‐D implantation.
Keywords: Cardiac resynchronization therapy, Implantable cardioverter defibrillator, Heart failure, Diabetes mellitus, Chronic kidney disease, Complication, Outcome, Registry
1. Introduction
Implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT) are well‐established treatment options for primary and secondary prevention of sudden cardiac death, life‐threatening ventricular tachyarrhythmias in patients with heart failure (HF).1, 2, 3 Demographics point towards an older and more complex patient group with multiple comorbidities in the near future. In context of cardiovascular procedures, these comorbidities are thought to be associated with worse prognosis and increased procedure‐related complications, resulting in higher mortality and healthcare costs. Regarding rates of cardiac implantable electronic devices (CIED)‐related complications and associated risk factors, only limited data of few registries are available.4, 5 Additional information regarding the incidence of CIED‐related complications is required to characterize risk factors on CIED patient handling in the daily routine. Diabetes mellitus (DM) is an important risk factor associated with increased infection rates, wound healing problems,6, 7 an elevated risk for HF, sudden cardiac death, and all‐cause mortality.8, 9, 10, 11, 12 Beyond the association with HF, DM does also influence HF progression and response to therapeutic treatments.13 Role of DM in the subgroup of patients being equipped with ICD or CRT devices on clinical outcome remains still unclear.10, 11, 14, 15, 16, 17, 18
We therefore sought to assess periprocedural and post‐procedural outcomes in patients with DM and HF undergoing either an ICD or a CRT implantation based on the large prospective multicentre German Device Registry.
2. Methods
2.1. Participating centres and registry data management
The prospective multicentre German Device Registry is a nationwide database of ICD and CRT implants and revisions initiated by the Stiftung Institut for Herzinfarktforschung (IHF) (Ludwigshafen, Germany), the German Society of Cardiology (Deutsche Gesellschaft für Kardiologie–Herz‐ und Kreislaufforschung e.V.), and the Study Group of Leading Hospital Cardiologists (‘Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte') to analyse and evaluate ICD and CRT under real‐world conditions in the setting of the German Health Care System.
In 50 participating centres over 70 parameters on demographic data, indication for devices, implantation procedures, and perioperative complications were collected at the time of device operation. Laboratory findings were not implemented into the database. Recruitment of patients started in March 2007 until March 2011, then continued as Device II registry, which was terminated in February 2014. After written informed consent, all parameters were entered into an internet‐based electronic case report form by the centres. Case report forms were then transmitted encrypted with a secure socket layer, and the IHF took responsibility for data management and monitoring.
The present study includes HF patients undergoing ICD or CRT operation with a focus on patients with diabetes and chronic kidney disease (CKD). The diagnosis of DM was based on the initial questionnaire (medical history or use of antidiabetic medication). The diagnosis of CKD was based on the past medical history and/or a serum level of creatinine >1.5 mg/dL.
2.2. Complications
Complications were categorized into minor and major complications according to severity. Life‐threatening and all re‐interventions were categorized as major complications and minor, when suitable for treatment on an outpatient basis, involving device reprogramming or requiring exclusive clinical observation. Minor complications included pneumothorax, hematothorax, and pericardial effusion when treated conservatively and lead dislodgements without re‐intervention. Haematomas resulting in a prolonged hospital stay, hospital readmissions, or additional out‐patient visits and wound infections treated with antibiotics were considered as minor complications.
Major complications included lead‐related re‐interventions, local infections requiring re‐intervention, CIED‐related systemic infections, pneumothoraces requiring drainage, cardiac perforations, pocket revisions because of pain, generator‐lead interface problems requiring re‐intervention, haematomas requiring re‐intervention, wound revisions, stroke, myocardial infarctions, and procedure‐related deaths.
2.3. Follow‐up
All patients were contacted 1 year after implantation or revision by telephone. The follow‐up was performed centrally by the IHF. During telephone contact, questions on arrhythmias, cardiac events, complications, medication, and HF symptoms were posed. In case of an ineffective call, further information was gathered from civil registry offices. Major adverse cardiac and cerebrovascular events (MACCE) were defined as occurrence of death (all‐cause), myocardial infarction (MI), or stroke.
2.4. Statistical analysis
The statistical computations were performed using SAS, version 9.3 (Cary, North Carolina, USA). Continuous data are presented as median and interquartile range, the distribution of age as mean ± standard deviation. Categorical variables are summarized as counts and percentage. Categorical values were generally compared by χ2 test and rates of infrequent complications by Fisher's exact test. Distributions of continuous variables were compared by two‐tailed Wilcoxon rank sum test. A P‐value ≤ 0.05 was considered significant; all P values were the result of two‐tailed tests. Mortality at exactly 1 year after index discharge was calculated by the Kaplan–Meier estimator and compared between patient groups by log‐rank test. In addition, we computed odds ratios by using multivariable logistic regression models adjusted for age, sex, coronary artery disease, chronic HF, and CKD. In addition, we performed a subgroup analysis for patients with and without diabetes and patients with and without CKD for the endpoints perioperative complication, 1 year MACCE, cardiopulmonary resuscitation/ICD shock/ventricular tachycardia/ablation, and device revision to investigate the impact of DM and CKD on the outcome of ICD and CRT procedures.
3. Results
3.1. Study cohort
During the study period, 5329 patients with ICD or CRT defibrillator (CRT‐D) devices were enrolled; of these, 1448 patients (27.2%) had a diagnosis of DM. Within the cohort, 94% of DM and 90% of non‐DM patients had a diagnosis of HF. With respect to CKD, 920 patients were categorized to CKD and 4409 patients to non‐CKD.
3.2. Baseline characteristics
Patient characteristics are listed in Table 1. Comparing patients with DM versus without DM, we observed multiple significant differences between the groups. Patients with DM were older (68.1 ± 10.0 vs 64.1 ± 13.6 years; P < 0.001) and had higher body mass index (28.1 vs 26.4 kg/m2; P < 0.001). Patients with DM showed a higher prevalence of cardiovascular comorbidities, such as arterial hypertension, history of coronary artery bypass graft or percutaneous coronary intervention, prior MI or stroke, and peripheral artery disease compared with non‐DM patients. In line with these cardiovascular variables, ischemic heart disease was more common in DM, while non‐DM patients showed higher rates of dilated and hypertrophic cardiomyopathy. Presence of atrial fibrillation in the initial electrocardiograph was more prevalent in patients with DM (23.3% and 16.9%; P < 0.001). This difference was mainly driven by the subgroup of ICD patients (24.6% vs 16.2%; P < 0.001; Supporting Information, Table S1 ), while HF patients with CRT showed no difference in atrial fibrillation prevalence (20.8% vs 18.5%; Supporting Information, Table S2 ). The rate of patients with CKD was higher in the DM group.
Table 1.
Baseline demographics: clinical data and characteristics
| Diabetes (n = 1448, 27.2%) | Non‐ diabetes (n = 3881, 72.8%) | P value | |
|---|---|---|---|
| Demographic data | |||
| Male | 81.1% (1174/1448) | 80.9% (3139/3881) | 0.87 |
| Age (year), mean ± SD | 68.1 ± 10.0 | 64.1 ± 13.6 | <0.001 |
| BMI (kg/m2) | 28.1 (25.3–31.2) | 26.4 (23.9–29.7) | <0.001 |
| Indication | |||
| Primary prevention | 67.7% (980/1448) | 56.3% (2185/3881) | <0.001 |
| Secondary prevention | 32.3% (468/1448) | 43.7% (1696/3381) | <0.001 |
| NYHA class | <0.001 | ||
| NYHA I | 9.9% (135/1364) | 20.1% (698/3479) | |
| NYHA II | 38.2% (521/1364) | 38.9% (1355/3479) | |
| NYHA III | 48.3% (659/1364) | 38.1% (1325/3479) | |
| NYHA IV | 3.6% (49/1364) | 2.9% (101/3479) | |
| NYHA III+ | 51.9% (708/1364) | 41.0% (1426/3479) | <0.001 |
| LV‐EF ≤ 30% | 69.4% (980/1412) | 59.3% (2197/3708) | <0.001 |
| Procedure duration, min | 60 (40; 100) | 60 (41; 105) | 0.34 |
| Comorbidities Risk factors (%) | |||
| Diabetes | 100.0% (1448/1448) | 0.0% (0/3881) | |
| Hypertension | 69.5% (1007/1448) | 46.2% (1793/3881) | <0.001 |
| COPD | 4.8% (70/1448) | 3.3% (129/3881) | 0.01 |
| CKD | 25.9% (375/1448) | 14.0% (545/3881) | <0.001 |
| Comorbidities | |||
| Prior PCI | 37.5% (129/344) | 31.3% (275/880) | 0.037 |
| Prior CABG | 25.0% (362/1448) | 14.1% (549/3881) | <0.001 |
| Prior stroke | 5.0% (73/1448) | 3.7% (142/3881) | 0.023 |
| Prior MI | 42.6% (617/1448) | 31.9% (1237/3881) | <0.001 |
| PAD | 5.1% (74/1448) | 2.4% (94/3881) | <0.001 |
| Cardiac disease | |||
| Ischemic | 71.9% (1041/1448) | 56.5% (2191/3881) | <0.001 |
| Dilatative | 31.1% (450/1448) | 33.8% (1312/3880) | 0.059 |
| Hypertrophy | 1.5% (21/1448) | 3.9% (150/3880) | <0.001 |
| Hypertensive | 8.4% (122/1448) | 5.7% (223/3880) | <0.001 |
| Medication at discharge | |||
| Class I antiarrhythmic drugs | 0.8% (12/1444) | 0.9% (35/3866) | 0.80 |
| Class III antiarrhythmic drugs | 14.1% (204/1442) | 13.4% (512/3864) | 0.47 |
| Class IV antiarrhythmic drugs | 6.6% (95/1444) | 4.9% (180/3866) | 0.017 |
| Beta Blockers | 92.5% (1336/1444) | 88.9% (3437/3867) | <0.001 |
| ACE inhibitor/Angiotensin II blocker | 90.7% (1310/1444) | 84.4% (3263/3867) | <0.001 |
| Aldosteron antagonist | 41.8% (603/1444) | 38.5% (1488/3867) | 0.03 |
| Diuretics | 83.9% (1212/1444) | 66.8%(2580/3865) | <0.001 |
| Digitalis | 22.2% (321/1444) | 15.4% (594/3866) | <0.001 |
| Antiplatelet agents | 63.4% (231/344) | 54.7% (478/874) | <0.001 |
| ARVC | 0.1% (2/1448) | 0.6% (22/3880) | 0.038 |
| Electrographic parameters | |||
| Sinusrhythm | 73.6% (1066/1448) | 79.6% (3083/3875) | <0.001 |
| AF | 23.3% (338/1448) | 16.9% (653/3875) | <0.001 |
| PM stimulation | 5.8% (84/1448) | 4.9% (190/3875) | 0.19 |
| LBBB | 37.0% (536/1447) | 30.3% (1172/3870) | <0.001 |
| RBBB | 6.8% (98/1447) | 6.8% (265/3870) | 0.92 |
| Median QRS‐ width (ms) | 120 (100;150) | 114 (100;150) | <0.001 |
| QRS width > 120 ms | 45.3% (653/1142) | 39.8% (1539/3862) | <0.001 |
| Procedure type | |||
| New implant | 87.5% (1264/1445) | 85.5% (3308/3870) | 0.062 |
| System revision | 12.5% (181/1445) | 14.5% (562/3870) | 0.062 |
| Procedure priority | |||
| Emergency | 9.0% (31/344) | 9.2% (81/877) | 0.90 |
| Elective | 91.0% (313/344) | 90.8% (796/877) | 0.90 |
| Device types | |||
| VVI‐ICD | 48.9% (708/1448) | 51.4% (1995/3881) | 0.1 |
| DDD‐ICD | 18.0% (260/1448) | 21.4% (831/3881) | 0.005 |
| CRT‐D | 33.1% (480/1448) | 27.2% (1055/3881) | <0.001 |
AF, atrial fibrillation; ARVC, arrhythmogenic right ventricular cardiomyopathy; BMI, body mass index; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LBBB, left bundle branch block; LV‐EF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PM, pacemaker; RBBB, right bundle branch block.
p‐value ≤ 0.05
Taken the fact that nearly all patients (>90% of the studied cohort) were suffering from HF, we further analysed HF‐specific variables. The rate of HF patients with severely depressed left ventricular ejection fraction (<30%) was higher in patients with DM. Consistent to the left ventricular ejection fraction distribution, symptoms according to New York Heart Association Classes III and IV were more frequent (51.9%) in the DM group compared with 41.0% in the non‐DM group at the time of the device procedure (P < 0.001). Thirty‐seven percent of patients with DM had a left bundle branch block compared with 30.3% without DM (P < 0.001), which goes along with the higher rate of CRT devices in the DM group.
Indications for ICD therapy were 67.7% primary prevention and 32.3% secondary prevention in DM patients, whereas in the non‐DM group, 56.3% of cases were primary prevention and 43.7% secondary prevention (P < 0.001).
Medication at the time of hospital discharge is summarized in Table 1. Medical therapy regarding antiplatelet therapy with acetylsalicylic acid and regarding HF with use of beta blocker, diuretics, ACE inhibitor, angiotensin II blocker, and aldosterone was significantly different between DM and non‐DM patients (Table 1).
3.3. Device characteristics
Table 1 is listing information regarding different device types implanted and procedural details. In most cases, procedures included single chamber (VVI) ICD systems, followed by CRT‐D systems and dual chamber (DDD) ICD devices. Differences in device types with respect to the presence of DM showed that DM patients were more often equipped with a CRT‐D device compared with non‐DM‐patients (33.1% vs 27.2%; P < 0.001). Furthermore, DDD systems were less frequent implanted in DM versus non‐DM patients (18.0% vs 21.4%; P = 0.005). The majority of patients (86%) underwent a de novo ICD/CRT implantation. In 91% of all cases, the implant procedure was performed in an elective setting (non‐emergency) (Table 1).
3.4. Periprocedural outcome
Table 2 provides a detailed overview of the in‐hospital events including the periprocedural complications. Complication rates were indistinguishable in diabetic compared with non‐diabetic patients (4.1% vs 3.9%; P = 0.79). There were no differences due to periprocedural MACCE events. Analysis of the device procedure‐related complications revealed similar rates for major complications with 2.0% in the DM group and 1.9% in the non‐DM group. Also, minor complications, which included relevant wound infections, haematomas, and device complications, were equally distributed between both groups. Eighty‐seven patients had to undergo an in‐hospital re‐intervention with revision because of device‐related complications. The most common complication resulting in a surgical re‐intervention was lead dysfunction and lead dislodgement counting up for 58% (11/19) and 76% (52/68) of device‐related complications requiring revision in DM and non‐DM patients, respectively.
Table 2.
In‐hospital events
| Variables | Diabetes (n = 1448, 27.2%) | Non‐ diabetes (n = 3881, 72.8%) | P value |
|---|---|---|---|
| Hospital stay (days) | 3 (1;5) | 3 (2;5) | 0.062 |
| In‐hospital complications | 4.1% (45/1098) | 3.9% (116/2981) | 0.79 |
| MACCE | 0.5% (6/1102) | 0.3% (9/2988) | 0.25 |
| New stroke | 0.2% (2/1098) | 0.0% (0/2980) | 0.07 |
| New MI | 0.0% (0/1098) | 0.0% (1/2980) | 1.00 |
| Mortality | 0.4% (6/1448) | 0.3% (10/3877) | 0.40 |
| Complications requiring intervention | 2.0% (29/1442) | 1.9% (73/3859) | 0.82 |
| Pericardial effusion | 0.1% (2/1442) | 0.1% (5/3858) | 1.00 |
| Pneumothorax | 0.2% (3/1442) | 0.6% (22/3859) | 0.11 |
| Hematothorax | 0.2% (3/1442) | 0.1% (3/3858) | 0.35 |
| Pocket hematoma | 1.5% (21/1442) | 1.1% (43/3858) | 0.32 |
| CPR | 0.3% (1/344) | 0.1% (1/878) | 0.48 |
| Complications not requiring intervention | |||
| Pericardial effusion | 0.3% (3/1098) | 0.2% (5/2980) | 0.45 |
| Pneumothorax | 0.1% (1/1098) | 0.3% (8/2981) | 0.46 |
| Hematothorax | 0.0% (0/1098) | 0.1% (2/2980) | 1.00 |
| Wound complication (including infection) | 0.5% (7/1442) | 0.4% (14/3858) | 0.62 |
| Device‐related complications requiring revision | 1.8% (19/1070) | 2.4% (68/2888) | 0.33 |
P values were obtained from Fisher's exact test or Wilcoxon rank‐sum test.
CPR, cardiopulmonary resuscitation; MACCE, major adverse cardiac and cerebrovascular events; MI, Myocardial infarction.
In an additional subgroup analysis of the ICD group, a similar periprocedural complication rate of 3.2% was observed in both DM and non‐DM patients (Supporting Information, Tables S1 and S3 ), while the subgroup of CRT recipients showed a generally higher, compared with the ICD subgroup, but unchanged complication rate of 5.9% (DM) and 5.8% (non‐DM) between the two groups (Supporting Information, Table S2 and S4 ).
3.5. One year clinical outcome
Of 5329 patients enrolled in the device registry, 1 year follow‐up was achieved in 5154 patients (96.7%). Predefined MACCE occurred in 425 patients (8.2%). All results are presented in detail in Table 3. Our initial time‐to‐event analysis revealed a worse outcome of patients with DM with respect to 1 year mortality (log‐rank, P = 0.001; Figure 1 A). Patients with DM showed increased MACCE rates (10.0% vs 7.3%; P < 0.001) and higher 1 year all‐cause mortality (9.0% vs 6.3%; P < 0.001). These results were underlined by higher rehospitalization rates in the group of patients with DM (45.0% vs 40.3%; P = 0.01) resulting in a longer length of hospital stay (10 vs 8 days; P < 0.001) compared with patients without DM. The underlying reason for readmission differed between both groups. In non‐DM patients, device‐related problems were the most frequent reason for readmission, while in the DM group, rehospitalization due to device problems were much less common compared with other reasons (13.0% vs 19.2%; P < 0.001).
Table 3.
Follow‐up at 12 months
| Diabetes (n = 1448, 27.2%) | Non‐diabetes (3881, 72.8%) | P value | |
|---|---|---|---|
| FU duration, months | 15.7 (12,9; 20,0) | 16.2 (12,8; 21,2) | 0.20 |
| FU documented | 96.9% (1403/1448) | 96.7% (3751/3881) | 0.66 |
| MACCE | 10.0% (141/1403) | 7.3% (274/3751) | <0.001 |
| 1 year mortalitya | 9.0% | 6.3% | <0.001 |
| Death, n | 256 | 454 | |
| Alive, n (%) | 1198 (25.9%) | 3422 (74.1%) | |
| Rehospitalization | 45.0% (443/984) | 40.3% (1148/2848) | 0.01 |
| Hospital stay (days) | 10 (6; 21) | 8 (4;18) | 0.001 |
| Cause of readmission | |||
| device | 13.0% (127/980) | 15.1% (428/2837) | 0.1 |
| cardiovascular | 12.7% (124/980) | 10.6% (302/2837) | 0.085 |
| other | 19.2% (188/980) | 14.3% (407/2837) | <0.001 |
| Revision | 7.1% (69/967) | 9.0% (251/2797) | 0.077 |
FU, follow‐up.
Kaplan‐Meier estimates.
Figure 1.
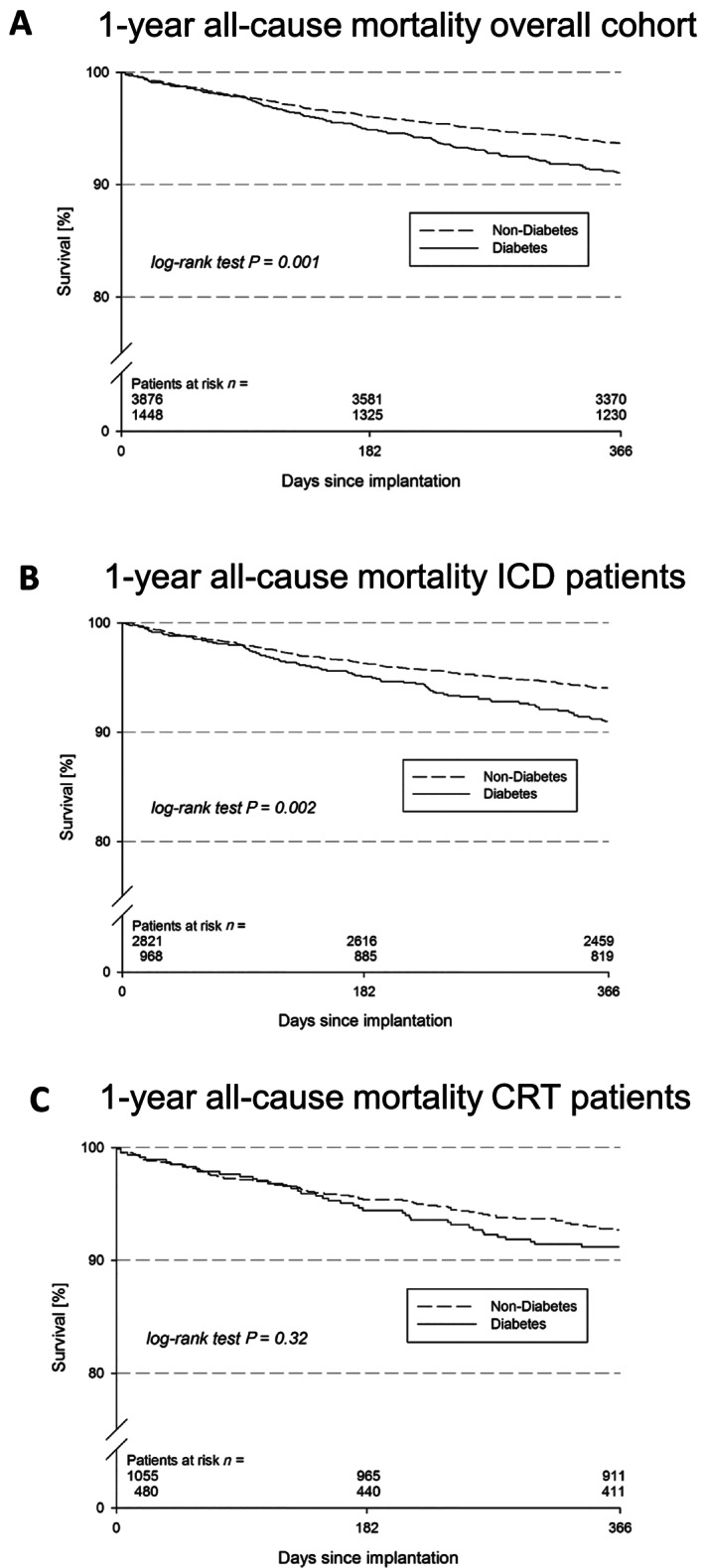
(A) Overall outcome analysis of 1 year mortality, according to presence of diabetes: Kaplan–Meier curve for the time to the first event of all‐cause death after 1 year in patients with diabetes (dashed line) and without diabetes (solid line). Kaplan–Meier survival curves for the time to the first event of all‐cause death after 1 year in diabetes (dashed line) versus non‐diabetes (solid line) patients in the subgroup of (B) implantable cardioverter defibrillator recipients and (C) cardiac resynchronization therapy recipients.
Consistent to the all‐over cohort, the subgroup analysis of ICD patients showed a worse prognosis of diabetic compared with non‐diabetic patients (Figure 1 B). In contrast, the smaller subgroup of CRT patients showed no significantly different prognosis with regard to the presence of DM (Figure 1 C).
3.6. Adjusted analysis
In addition, we performed an adjusted analysis to compensate for the covariates age, sex, coronary artery disease, HF, and CKD (Figure 2 ). The results of our adjusted models did confirm a similar risk for periprocedural complications in both groups independent from the presence of DM. In contrast to the unadjusted survival analysis, the adjusted calculations for outcome failed to show a difference between the two groups with respect to 1 year MACCE, the composite of cardiopulmonary resuscitation, ICD shocks, occurrence of ventricular tachycardias or required ablation therapy, and for the endpoint device revision.
Figure 2.
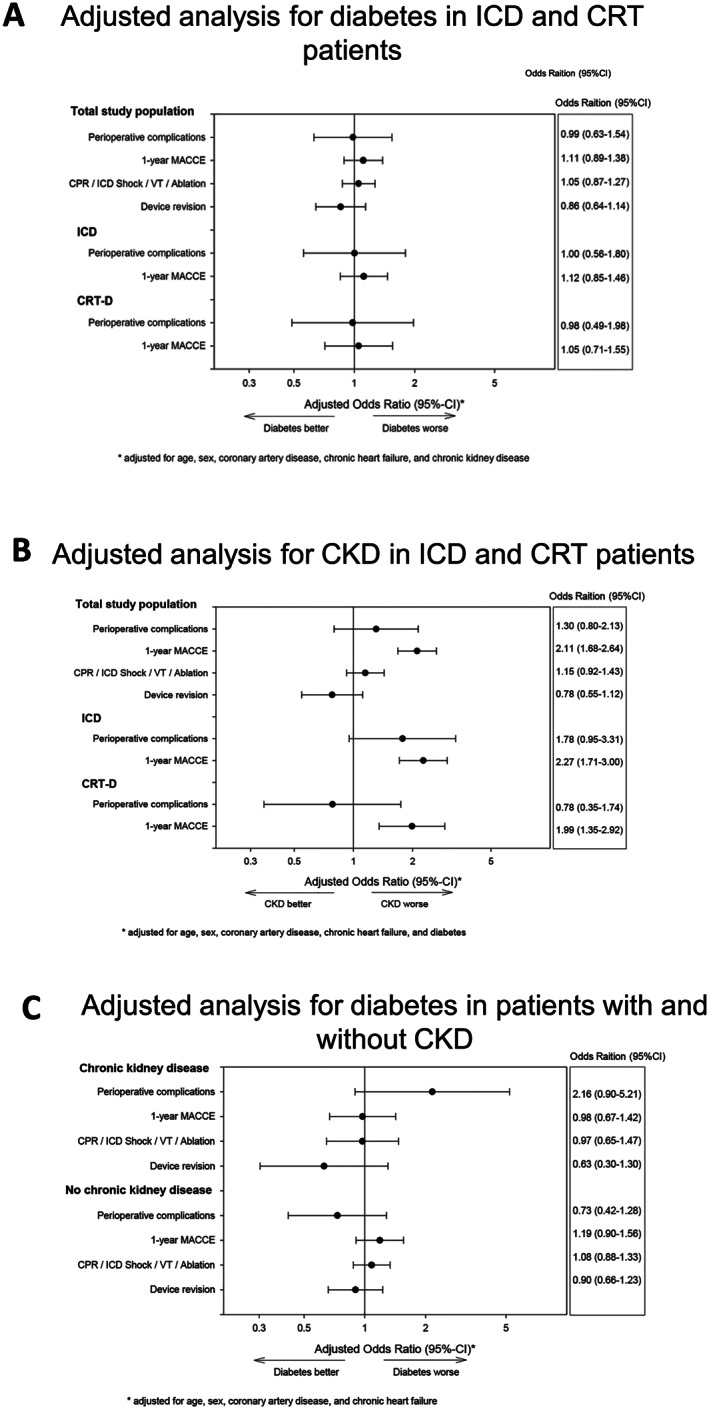
(A) Adjusted analyses of the effect of diabetes in the overall group after multivariable adjustment. A 95% confidence interval adjusted for age, sex, coronary artery disease, chronic heart failure, chronic kidney disease. (B) Adjusted analyses of the effect of chronic kidney disease in the overall group. (C) Adjusted analyses of the effect of diabetes and chronic kidney disease in the overall group. CRT‐D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter defibrillator; MACCE, major adverse cardiac and cerebrovascular events; VT, ventricular tachycardia.
This unchanged outcome was also confirmed in the adjusted analysis of the subgroups with ICDs or CRT‐Ds showing no difference of periprocedural complications or 1 year MACCE rates between the groups.
3.7. Role of chronic kidney disease
Because DM is associated with CKD and CKD is known to be a risk factor for perioperative complications, we performed additional analyses evaluating the role of CKD in this HF cohort of ICD/CRT‐D patients. Adjusted analysis of the overall cohort showed a clear association of CKD with increased risk of 1 year MACCE [odds ratio 2.11 (1.68‐2.64)]; this was consistent for ICD and CRT‐D patients (Figure 2 B). Perioperative complications tended to be increased in CKD patients, but failed significance (Figure 2 B). Furthermore, we performed a subgroup analysis evaluating the role of DM as a risk factor in patients with and without CKD. This subanalysis revealed that in contrast to the overall cohort, there is a strong trend towards increased risk of perioperative complications in DM patients with CKD [odds ratio 2.16 (0.90–5.21)] while in non‐CKD patients there is no difference (Figure 2 C). With respect to clinical outcome, there were no signs of DM being associated with an altered outcome neither in CKD nor in non‐CKD patients (Figure 2 C).
4. Discussion
In this prospective, multicentre registry, we examined the impact of DM on periprocedural and post‐procedural outcome in ICD and CRT recipients with HF. Our results showed a clear association of DM to increased cardiovascular comorbidity in the studied patient cohort. Risk of perioperative complications was not increased in patients with DM undergoing an ICD/CRT device implantation. Clinical outcome analysis showed higher 1 year mortality and MACCE rate in patients with DM after an ICD or CRT‐D device implantation, but unchanged clinical outcome after multivariate adjustment.
4.1. Prevalence of diabetes in cardiac implantable electronic device patients
Diabetes is a common risk factor in patients undergoing CIED procedures. In the FOLLOWPACE study, the prevalence of DM was only 15%.19 Our study showed a prevalence of 27.2% in the studied cohort of patients undergoing an ICD or CRT‐D procedure within the German Device Registry. This rate is absolutely in line with previous mixed CIED and sole ICD studies showing rates of DM varying from 24% to 29%.20, 21, 22, 23, 24 Therefore, it can be assumed that in this cohort, there was not a large proportion of under‐diagnosed DM patients. These studies together with our own data suggest that nearly a quarter of all ICD/CRT recipients are suffering from DM, which might be a larger proportion than in a sole pacemaker cohort, probably based on the fact that ICD/CRT patients are commonly HF patients.
4.2. Role of diabetes in cardiac implantable electronic device‐related complications
Data regarding complication rates from previous studies on ICD/CRT cohorts are scarce. The Ontario ICD database investigated 3340 patients undergoing a de novo ICD implant and observed an overall complication rate of 7.5% within 45 days after the procedure.25 Data of a nationwide cohort in Denmark4 from the combined Danish pacemaker and ICD registry showed an overall complication rate of 9.5% after 6 months. This study included 1520 ICD and 4398 pacemaker patients. Another study investigating complication rates in subcutaneous versus transvenous ICDs showed all‐over complications of 7–8% after 1 year of follow‐up.26 The all‐over numbers observed in our cohort of 5329 ICD/CRT patients were lower with 4.1% but were only related to the intrahospital period until discharge. Therefore, the absolute rates in our study are hardly comparable with other studies based on the distinct follow‐up period, but would be still consistent to the reported data.
Looking at the role of DM in perioperative complications, previous cardiac device studies demonstrated an association of DM with increased complication rates, specifically infections and a worse outcome.16, 18, 27 Therefore, one hypothesis of our study was that complication rates might be higher in diabetic patients undergoing ICD/CRT‐D procedures. Of note, our analysis did not show any difference in complications between the groups. Our results actually even suggest an identical periprocedual risk of DM patients undergoing an ICD/CRT surgical procedure despite the presence of DM and DM‐associated comorbidity. This is important and in contrast to other device studies and retrospective analyses, which investigated the correlation of DM to infections and wound complications and identified DM as a significant risk factor.23, 24, 28 Infection rates were low in these studies with rates ≤1%.4, 18, 28, 29 Despite the fact that we did not observe a higher periprocedural complication rate in DM patients, the absolute numbers, showing rates of wound complications (including infections) ranging from 0.4% in non‐DM to 0.5% in DM patients, are consistent with these previous reports.
In line with our data, the Ontario ICD database could not also identify DM as a relevant risk factor for complications in univariate and multivariate analyses.25 Taken these results and our data, two of the largest ICD patient cohorts, there is no cumulative evidence of DM being a risk factor for ICD/CRT‐related perioperative complications.
Possible explanations for an unchanged complication risk in diabetic patients in our real‐world data comprise the fact that our outcome data could have potentially been biased by the operator, who may have acted more cautious during the procedure, or by the type of operator and/or patient selected for the procedure. However, these are known limitations of registries and trials.
4.3. Role of diabetes on outcome in heart failure implantable cardioverter defibrillator patients
Based on other previous studies in diabetic patients with HF, which suggest an increase in mortality compared with non‐diabetic patients,30 we also evaluated clinical outcome over 1 year after ICD/CRT‐D implantation with respect to all‐cause mortality and MACCE.
Data from the MADIT trial showed that patients with DM and ICD have higher mortality rates (increase by ~20%), but still benefit from an ICD therapy compared with patients without DM.9, 30 The post hoc analysis of the MADIT trial revealed that patients with DM are more likely to have hypertension, renal dysfunction, increased body mass index, and higher New York Health Association classes. These basic characteristics of DM patients are reflected in the diabetic group of our cohort. The absolute mortality rate of diabetic ICD patients was 25% after 2 years. In our study, the analysis revealed all‐cause mortality rates of 9.0% after 1 year in patients with DM, which represented an increase in mortality of 43% compared with patients without DM. These data match previous observations and further underline the worse prognosis of diabetic HF patients.9 We confirmed these results in the subgroup analysis in patients undergoing an ICD procedure (n = 3794), while this DM‐associated mortality increase could not be observed in the smaller subgroup of the CRT‐D patients (n = 1525). These results could be interpreted towards a larger benefit of CRT therapy versus ICD protection in DM patients and might suggest an association of DM to an HF‐related mode of death rather than to an arrhythmic event. Nevertheless, there was also a trend in the CRT cohort towards a higher event rate in the DM group, which might be due a lack of statistical power based on the lower number of CRT patients compared with the ICD cohort.
To elucidate the sole role of diabetes as a risk factor independent from all DM‐associated comorbidities, we performed an adjusted multivariate analysis for all relevant covariables. In contrast to the unadjusted results, this analysis revealed no difference for the predefined endpoints in association with DM, neither for complication rates nor for MACCE, or any other arrhythmia‐associated outcome. Based on these results, DM alone seems less important than the accumulation of associated cardiovascular and renal diseases contributing to a worse prognosis of DM patients equipped with ICD/CRT devices suggesting, that the protection and/or treatment by ICD/CRT devices might overcome some negative effects of DM probably mediated via arrhythmia events or HF progression.
4.4. Chronic kidney disease and implantable cardioverter defibrillator/cardiac resynchronization therapy‐related complications
Another established important risk factor associated with increased mortality and morbidity in patients with chronic HF is kidney dysfunction.31, 32, 33, 34 We investigated the influence CKD on the outcome of patients undergoing ICD and CRT procedures. In our study, CKD was associated with an increased risk of 1 year MACCE in the overall cohort. This result confirms the plausibility of our data set. In addition, we sought to investigate if DM might be a risk factor in the subgroup of CKD patients. This analysis showed a clear trend in the adjusted analysis that DM favours occurrence of perioperative complications in CKD patients. However, there was no effect on clinical outcome neither in CKD nor in non‐CKD patients. Because renal dysfunction is a frequent comorbidity of patients with diabetes, this observation might be relevant.
4.5. Relevance and potential clinical implications
Based on the lack of data in the literature, results of large trials or cohorts are always required to elucidate critical variables that are important to an operator performing a CIED procedure. Our results clearly suggest that HF patients undergoing an ICD or CRT implantation do not carry a higher procedural risk compared with patients without DM. Therefore, DM alone should not be taken as a reason to withhold an ICD or CRT implantation from the patient.
While patients with DM showed higher 1 year mortality, our multivariate analysis showed that this association of DM to an increased MACCE rates disappears after adjustment for relevant covariates. This suggests that DM as such is probably an indirect cause contributing to the accumulation of other cardiovascular comorbidities and that DM alone is not associated with a worse outcome in ICD/CRT patients. This further highlights the very important role of an ICD and CRT in DM patients in addition to the required optimized drug treatment in DM HF patients.
5. Limitations
This trial was based on a retrospective analysis of registry data, which always carries bias of selected data input by the investigator. A limitation of our study is the designation of patients as diabetics based on self‐reporting rather than laboratory results such as serum glucose or glycosylated haemoglobin (HbA1c) levels that constitute the criteria for the diagnosis of diabetes. Nevertheless, the percentage of DM patients was consistent to previous studies investigating HF patients undergoing ICD/CRT‐D implantation.4, 35, 36, 37 Another point is missing data regarding the length of time since diagnosis and the degree of glycemic control and non‐cardiac organ failure.
Standards of follow‐up and data collection do not meet the criteria of randomized controlled trials. There is always the problem of a potential bias of the operator, the kind of treatment performed, and patient selection. Our results are in line and consistent with other studies confirming complication and overall mortality rates, but add additional data into the context of role of ICD/CRT device therapy in DM HF patients presenting one of the largest cohorts of ICD/CRT patients, which were systematically investigated regarding the role of DM on complication and outcome rates.
6. Conclusions
The reported analysis of the German Device Registry, including 5329 patients undergoing an ICD or CRT‐D implantation, sought to investigate the periprocedural and post‐procedural outcome in patients with both DM and HF. Diabetic patients were older and were more likely to have ischemic cardiomyopathy, a lower ejection fraction, chronic kidney disease, and other comorbidities. Despite this pronounced comorbidity, the perioperative complication rate was not increased in patients with DM. There was only a trend towards more perioperative complications in the subgroup of DM patients with CKD. Adjusted outcome analysis showed similar MACCE rates in both groups over time. These results point towards an impact of DM, including arrhythmic events and HF progression, which are addressed by an ICD/CRT therapy and thereby further underline the importance and need of device therapy in DM patients with HF beyond an optimal medical therapy.
Conflict of interests
All authors with reported conflict of interest have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Reza Wakili has received consultant fees, speaking honoraria and travel expenses from Boston Scientific; consultant fees from Biotronik; investigator‐initiated funding for research projects (initiated by him) from Bristol‐Myers Squibb, Pfizer, and Boston Scientific.
Funding
This project was supported by an unrestricted grant from foundation Stiftung Institut for Herzinfarktforschung Ludwigshafen, Germany.
Jochen Senges has received unrestricted sponsoring of the Registry from Medtronic, Biotronik and St. Jude Medical.
Reza Wakili was supported by the German Centre for Cardiovascular Research (DZHK) Partner site Munich (DZHK; 81X1600203, 81X1600204, 81X2600216, 81X2600232, 81X2600234). Reza Wakili received funding from the European Union's Horizon 2020 research and innovation program under grant agreement 633193" [CATCH ME] and the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation – DO 637/23‐1; Projekt 394433254)
Supporting information
Table S1. Subgroup ICD recipients Baseline demographics: clinical data and characteristics.
Table S2. Subgroup CRT recipients' baseline demographics: clinical data and characteristics.
Table S3. Subgroup ICD recipients: In hospital events.
Table S4. Subgroup CRT recipients: In hospital events.
Kaya, E. , Senges, J. , Hochadel, M. , Eckardt, L. , Andresen, D. , Ince, H. , Spitzer, S. G. , Kleemann, T. , Maier, S. S. K. , Jung, W. , Stellbrink, C. , Rassaf, T. , and Wakili, R. (2020) Impact of diabetes on clinical outcome of patients with heart failure undergoing ICD and CRT procedures: results from the German Device Registry. ESC Heart Failure, 7: 984–995. 10.1002/ehf2.12613.
References
- 1. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, American College of Cardiology F, American Heart Association Task Force on Practice G and Heart Rhythm S . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013; 127: e283–e352. [DOI] [PubMed] [Google Scholar]
- 2. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM, Comparison of medical therapy P and defibrillation in heart failure I . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350: 2140–2150. [DOI] [PubMed] [Google Scholar]
- 3. Penn J, Goldenberg I, McNitt S, Polonsky B, Ruwald MH, Zareba W, Moss AJ, Alexis JD. Changes in drug utilization and outcome with cardiac resynchronization therapy: a MADIT‐CRT substudy. J Card Fail 2015; 21: 541–547. [DOI] [PubMed] [Google Scholar]
- 4. Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35: 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kobe J, Andresen D, Maier S, Stellbrink C, Kleemann T, Gonska BD, Reif S, Hochadel M, Senges J, Eckardt L. Complications and 1‐year benefit of cardiac resynchronization therapy in patients over 75 years of age—insights from the German Device Registry. Int J Cardiol 2017; 228: 784–789. [DOI] [PubMed] [Google Scholar]
- 6. Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 2014; 174: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 2000; 101: 2271–2276. [DOI] [PubMed] [Google Scholar]
- 8. George J, Barsheshet A, Moss AJ, Martin D, Ouellet G, McNitt S, Goldenberg I. Effectiveness of cardiac resynchronization therapy in diabetic patients with ischemic and nonischemic cardiomyopathy. Ann Noninvasive Electrocardiol 2012; 17: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wittenberg SM, Cook JR, Hall WJ, McNitt S, Zareba W, Moss AJ and Multicenter Automatic Defibrillator Implantation T . Comparison of efficacy of implanted cardioverter‐defibrillator in patients with versus without diabetes mellitus. Am J Cardiol 2005; 96: 417–419. [DOI] [PubMed] [Google Scholar]
- 10. Martin DT, McNitt S, Nesto RW, Rutter MK, Moss AJ. Cardiac resynchronization therapy reduces the risk of cardiac events in patients with diabetes enrolled in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT‐CRT). Circ Heart Fail 2011; 4: 332–338. [DOI] [PubMed] [Google Scholar]
- 11. Cygankiewicz I, Gillespie J, Zareba W, Brown MW, Goldenberg I, Klein H, McNitt S, Polonsky S, Andrews M, Dwyer EM, Hall WJ, Moss AJ, Investigators MI. Predictors of long‐term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) patients with implantable cardioverter‐defibrillators. Heart Rhythm 2009; 6: 468–473. [DOI] [PubMed] [Google Scholar]
- 12. Bergner DW, Goldberger JJ. Diabetes mellitus and sudden cardiac death: what are the data? Cardiol J 2010; 17: 117–129. [PubMed] [Google Scholar]
- 13. Lind M, Bounias I, Olsson M, Gudbjornsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011; 378: 140–146. [DOI] [PubMed] [Google Scholar]
- 14. Herce B, Nazeyrollas P, Lesaffre F, Sandras R, Chabert JP, Martin A, Tassan‐Mangina S, Bui HT, Metz D. Risk factors for infection of implantable cardiac devices: data from a registry of 2496 patients. Europace 2013; 15: 66–70. [DOI] [PubMed] [Google Scholar]
- 15. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta‐analysis. Europace 2015; 17: 767–777. [DOI] [PubMed] [Google Scholar]
- 16. Bloom H, Heeke B, Leon A, Mera F, Delurgio D, Beshai J, Langberg J. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol 2006; 29: 142–145. [DOI] [PubMed] [Google Scholar]
- 17. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 16‐year trends in the infection burden for pacemakers and implantable cardioverter‐defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011; 58: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 18. Klug D, Balde M, Pavin D, Hidden‐Lucet F, Clementy J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, Barnay C, Grandbastien B, Kacet S, Group PS . Risk factors related to infections of implanted pacemakers and cardioverter‐defibrillators: results of a large prospective study. Circulation 2007; 116: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 19. Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, Moons KG. Incidence and predictors of short‐ and long‐term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012; 9: 728–735. [DOI] [PubMed] [Google Scholar]
- 20. Strimel W, Koplik S, Chen HR, Song J, Huang SK. Safety and effectiveness of primary prevention cardioverter defibrillators in octogenarians. Pacing Clin Electrophysiol 2011; 34: 900–906. [DOI] [PubMed] [Google Scholar]
- 21. Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner S, Baddour LM. Management and outcome of permanent pacemaker and implantable cardioverter‐defibrillator infections. J Am Coll Cardiol 2007; 49: 1851–1859. [DOI] [PubMed] [Google Scholar]
- 22. Uslan DZ, Gleva MJ, Warren DK, Mela T, Chung MK, Gottipaty V, Borge R, Dan D, Shinn T, Mitchell K, Holcomb RG, Poole JE. Cardiovascular implantable electronic device replacement infections and prevention: results from the REPLACE Registry. Pacing Clin Electrophysiol 2012; 35: 81–87. [DOI] [PubMed] [Google Scholar]
- 23. Joy PS, Kumar G, Poole JE, London B, Olshansky B. Cardiac implantable electronic device infections: who is at greatest risk? Heart Rhythm 2017; 14: 839–845. [DOI] [PubMed] [Google Scholar]
- 24. Qintar M, Zardkoohi O, Hammadah M, Hsu A, Wazni O, Wilkoff BL, Tarakji KG. The impact of changing antiseptic skin preparation agent used for cardiac implantable electronic device (CIED) procedures on the risk of infection. Pacing Clin Electrophysiol 2015; 38: 240–246. [DOI] [PubMed] [Google Scholar]
- 25. Lee DS, Krahn AD, Healey JS, Birnie D, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV, and Investigators of the Ontario ICDD . Evaluation of early complications related to De Novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol 2010; 55: 774–782. [DOI] [PubMed] [Google Scholar]
- 26. Brouwer TF, Yilmaz D, Lindeboom R, Buiten MS, Olde Nordkamp LR, Schalij MJ, Wilde AA, van Erven L, Knops RE. Long‐term clinical outcomes of subcutaneous versus transvenous implantable defibrillator therapy. J Am Coll Cardiol 2016; 68: 2047–2055. [DOI] [PubMed] [Google Scholar]
- 27. Lekkerkerker JC, van Nieuwkoop C, Trines SA, van der Bom JG, Bernards A, van de Velde ET, Bootsma M, Zeppenfeld K, Jukema JW, Borleffs JW, Schalij MJ, van Erven L. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart 2009; 95: 715–720. [DOI] [PubMed] [Google Scholar]
- 28. Kirkfeldt RE, Johansen JB, Nielsen JC. Management of cardiac electronic device infections: challenges and outcomes. Arrhythm Electrophysiol Rev 2016; 5: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population‐based cohort study of 46299 consecutive patients. Eur Heart J 2011; 32: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kutyifa V, Beck C, Brown MW, Cannom D, Daubert J, Estes M, Greenberg H, Goldenberg I, Hammes S, Huang D, Klein H, Knops R, Kosiborod M, Poole J, Schuger C, Singh JP, Solomon S, Wilber D, Zareba W, Moss AJ, Committee MSIE. Multicenter automatic defibrillator implantation trial‐subcutaneous implantable cardioverter defibrillator (MADIT S‐ICD): design and clinical protocol. Am Heart J 2017; 189: 158–166. [DOI] [PubMed] [Google Scholar]
- 31. Cruz DN, Schmidt‐Ott KM, Vescovo G, House AA , Kellum JA, Ronco C, McCullough PA. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013; 182: 117–136. [DOI] [PubMed] [Google Scholar]
- 32. Bagshaw SM, Hoste EA, Braam B, Briguori C, Kellum JA, McCullough PA, Ronco C. Cardiorenal syndrome type 3: pathophysiologic and epidemiologic considerations. Contrib Nephrol 2013; 182: 137–157. [DOI] [PubMed] [Google Scholar]
- 33. Tumlin JA, Costanzo MR, Chawla LS, Herzog CA, Kellum JA, McCullough PA, Ronco C. Cardiorenal syndrome type 4: insights on clinical presentation and pathophysiology from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013; 182: 158–173. [DOI] [PubMed] [Google Scholar]
- 34. Mehta RL, Rabb H, Shaw AD, Singbartl K, Ronco C, McCullough PA, Kellum JA. Cardiorenal syndrome type 5: clinical presentation, pathophysiology and management strategies from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013; 182: 174–194. [DOI] [PubMed] [Google Scholar]
- 35. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH, and Defibrillators in non‐ischemic cardiomyopathy treatment evaluation I . Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004; 350: 2151–2158. [DOI] [PubMed] [Google Scholar]
- 36. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, and Multicenter Automatic Defibrillator Implantation Trial III . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 37. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, and Sudden Cardiac Death in Heart Failure Trial I . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Subgroup ICD recipients Baseline demographics: clinical data and characteristics.
Table S2. Subgroup CRT recipients' baseline demographics: clinical data and characteristics.
Table S3. Subgroup ICD recipients: In hospital events.
Table S4. Subgroup CRT recipients: In hospital events.


