Abstract
Aims
Physical frailty screening is more commonly performed at outpatient heart failure (HF) clinics. However, this does not incorporate other common geriatric domains. This study assesses whether a multidomain geriatric assessment, in comparison with HF severity or physical frailty, is associated with short‐term adverse outcomes.
Methods and results
This is a prospective cohort study of 197 patients with HF (mean age 78, 44% female) attending outpatient HF clinics. HF severity was assessed with New York Heart Association class (I‐II versus III‐IV) and N‐terminal pro b‐type natriuretic peptide levels. Physical frailty was assessed with the Fried frailty criteria (not frail, pre‐frail, and frail). The following geriatric domains were assessed: physical function, nutrition, polypharmacy, cognition, and dependency in activities of daily living. Logistic regression analyses adjusted for age, sex, diabetes and kidney function assessed 3 month risk of adverse health outcomes (emergency department visits, hospital admissions, and/or death) according to HF severity, physical frailty, and number of affected domains. Number (%) of patients with HF with no, 1, 2, and ≥3 domains affected were 36 (18%), 61 (31%), 58 (29%), and 42 (21%). Seventy‐four adverse outcomes were experienced in 50 patients at follow‐up. Severity of HF and physical frailty were not significantly associated with an increased risk of adverse health outcomes. However, increasing number of affected domains were significantly associated with an increased risk of adverse outcomes. Compared with no domains affected, odds ratios (95% confidence interval) for 1, 2, and ≥3 domains were 1.8 (0.5–6.5), 4.5 (1.3–15.4), and 7.2 (2.0–26.3) (P‐trend <0.01). Further adjustment for HF severity and frailty status slightly attenuated the effect estimates (P‐trend 0.02).
Conclusions
Having limitations in multiple domains appears more strongly associated with short‐term adverse outcomes than HF severity and physical frailty. This may illustrate the potential added value of a multidomain geriatric assessment in the evaluation and treatment of patients with HF with respect to relevant short‐term health outcomes.
Keywords: Heart failure, Multidomain geriatric assessment, Frailty
Introduction
The incidence and prevalence of heart failure is increasing, affecting over 10% of the older population.1 Older patients with heart failure are likely to have multiple diseases, polypharmacy, and functional limitations, putting them at risk of frailty.2, 3 A systematic review and meta‐analysis including 26 studies in almost 7000 patients estimated nearly half of all patients with heart failure to be frail.4 Major heart failure guidelines recognize that frailty impacts prognosis and treatment success and therefore recommend screening for frailty.5 Yet, similar to the majority of the previously mentioned studies, the focus is laid on physical frailty (e.g. hand grip strength, walking speed, and Fried frailty criteria).6, 7 Next to physical disabilities, however, limitations in other geriatric domains such as malnutrition, multimorbidity, cognitive impairment, and dependency in activities in daily living (ADL) are also highly prevalent in older patients with heart failure.3, 8, 9 A multidomain approach rather than an approach merely focusing on physical frailty may therefore be more strongly associated with adverse health outcomes. Therefore, this study set out to assess whether, compared with heart failure severity [New York Heart Association (NYHA) I‐II versus III‐IV, and N‐terminal pro b‐type natriuretic peptide (NT‐proBNP) tertiles] and physical frailty, the total number of affected geriatric domains is associated with short‐term adverse health outcomes (i.e. emergency department visits, unplanned hospital admissions, and death).
Methods
Design and population
This prospective cohort study included 197 consecutive patients aged ≥60 years previously diagnosed with heart failure (not specified to any underlying cardiac cause) attending outpatient heart failure clinics between January 2018 and March 2019 in two Dutch hospitals, the Amsterdam University Medical Center, location VUmc, Amsterdam, and Amstelland Hospital, Amstelveen. The local medical Ethics Committee approved the research protocol, and all patients gave written informed consent before study entry.
Geriatric domains
This study included the following five domains: physical function, nutrition, polypharmacy, cognition, and ADL dependency. In order to implement the multidomain geriatric assessment into daily clinical practice, we selected easy to use and widely accessible screening tools that require minimal training. The entire assessment can be performed in 20 min by a nurse working at an outpatient heart failure clinic.
Physical function
The physical domain was considered affected when a patient was classified as having low grip strength and/or slow gait speed. Hand grip strength (kg) was measured using an isometric hand dynamometer (Jamar® hand dynamometer, Sammons Preston) in upright standing position, while holding the dynamometer in one hand. Patients were actively encouraged to squeeze with maximal strength. Two trials were performed alternately for each hand. The best performance out of four measurements was used. Low grip strength was defined as <30 kg for men and <20 kg for women.10 Gait speed (m/s) was assessed using a timed 4 m walking test at normal pace from a standing start using a stopwatch. The fastest time of two trials was used for analyses. Slow gait speed was defined as a walking speed <0.8 m/s.11
Nutrition
Nutritional status was assessed using the Mini Nutritional Assessment – Short Form.12 This questionnaire includes six criteria on food intake and appetite, unintentional weight loss, mobility, recent psychological stress or severe disease, dementia, and body mass index. The maximum score is 14 points. In patients at risk of malnutrition or malnourished (Mini Nutritional Assessment – Short Form score <12), the nutrition domain was considered affected.13
Polypharmacy
Polypharmacy (chronic use of ≥5 drugs) is highly prevalent in patients with heart failure as a consequence of guideline‐based care.14 We therefore used chronic use of >10 drugs (hyperpolypharmacy),15 to discriminate in terms of drug use. Evaluation of which drugs were used was based on a patient's current medication listed in the electronic health record.
Cognition
Cognitive functioning was assessed according to the Mini‐Mental State Exam (MMSE), a screening tool with a maximum of 30 points.16 Lower scores indicate a lower level of cognitive functioning. A total score on the MMSE of <26 was used to assess whether the cognitive domain was affected.17
Activities in daily living dependency
The Katz‐ADL questionnaire was used to evaluate whether a patient needed assistance during one of the following six daily activities: bathing, getting dressed, going to the toilet, transferring, eating/feeding, and continence.18 If a patient needed assistance in two or more of these activities, the ADL dependency domain was considered affected.19
Physical frailty
Frailty was assessed according to the Fried frailty criteria.20 This physically oriented frailty tool includes the following five criteria: weight loss, weak grip strength, exhaustion, slow gait speed, and low physical activity. A patient was classified as non‐frail (none of the criteria present), pre‐frail (1 or 2 criteria present), or frail (≥3 criteria present).20
Cardiac assessment, blood pressure, and kidney function
Data on the NYHA classification, left ventricle ejection fraction, NT‐proBNP, blood pressure, and kidney function (creatinine in μmol/L and estimated glomerular filtration rate in mL/min) at time of the baseline visit were derived from the patients' medical records. Patients were categorized as heart failure with reduced ejection fraction, heart failure with mid‐range ejection fraction, and heart failure with preserved ejection fraction if their ejection fraction was <40%, 40–49%, or >50%, respectively.5 Severe renal dysfunction was defined as an estimated glomerular filtration rate <30 mL/min.5
Morbidities
A patient was considered to have diabetes, cardiovascular disease (presence of myocardial infarction, angina pectoris, stroke, transient ischaemic attack, and/or peripheral artery disease), or pulmonary disease (chronic pulmonary disease and asthma) if these co‐morbidities were listed in a patient's medical history in the electronic health record.
Follow‐up
Occurrence of adverse health outcomes was registered at 3 month follow‐up with a questionnaire by telephone. The following adverse health outcomes were addressed: visiting the emergency department, unplanned hospital admission, and/or death. These registered adverse health outcomes were combined in a composite outcome, which scored positive if a patient experienced one or more adverse health outcomes. Data on these adverse health outcomes were verified in the patients' electronic health records. Patients were considered lost to follow‐up if they did not answer the phone after calling them for five times on different days.
Statistical analysis
Baseline characteristics for the entire sample and according to the number of domains affected were assessed. Continuous variables were presented as mean (standard deviation) or median (interquartile range), depending on the distribution of the variable. One‐way analysis of variance, Kruskal–Wallis, and chi‐square tests were used to calculate differences in mean, median, and frequencies.
The occurrence of adverse health outcomes (composite score) was assessed according to heart failure severity [NYHA class I‐II versus III‐IV; NT‐proBNP Tertile 1 (0–1120 pmol/L) versus Tertile 2 (1120–3060 pmol/L) and 3 (>3060 pmol/L)], physical frailty (not frail versus pre‐frail or frail according to the Fried frailty criteria), and number of domains affected (0 domains affected versus 1, 2, or ≥3 domains affected). The exact date on which the adverse health outcomes occurred was not available in our dataset. Therefore, we performed logistic regression analyses instead of Cox regression analyses to assess the risk (odds ratio, 95% confidence interval) of experiencing ≥1 adverse health outcome during follow‐up. Initial models were adjusted for age and sex. Secondary models were additionally adjusted for diabetes, creatinine levels, and NT‐proBNP. Model 3 was additionally adjusted for physical frailty (i.e. the Fried frailty criteria). Statistical P‐trend analyses across the heart failure, frailty, and geriatric domain categories were performed to demonstrate the relationship between the categories and occurrence of negative health outcomes.
Results
Baseline characteristics
Table 1 presents the characteristics of the total sample (n = 197) and according to number of domains affected (0, 1, 2, and ≥3). Mean age of the study population was 78 (standard deviation ±8.8) years of age, and 44% was female.
Table 1.
Baseline characteristics in the total study population (n = 197) and according to number of domains affected
| Total (n = 197) | Number of affected domains | P‐value | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | |||
| n = 36 (18%) | n = 61 (31%) | n = 58 (29%) | n = 42 (21%) | |||
| Demographics | ||||||
| Age, years (mean, SD) | 78 (8.8) | 71 (7.4) | 79 (9.1)a | 79 (7.9)a | 81 (7.8)a | <0.01 |
| Gender, female (n, %) | 87 (44%) | 11 (31%) | 31 (51%)a | 22 (38%) | 23 (55%)a | 0.09 |
| Cardiac assessment | ||||||
| NYHA classification | 0.03 | |||||
| Class I (n, %) | 42 (21%) | 14 (39%) | 9 (15%)a | 11 (19%)a | 8 (19%)a | |
| Class II (n, %) | 92 (47%) | 19 (53%) | 33 (54%) | 23 (40%) | 17 (41%) | |
| Class III‐IV (n, %) | 63 (32%) | 3 (8%) | 19 (31%)a | 24 (41%)a | 17 (41%)a | |
| Type of heart failure | 0.24 | |||||
| HFrEF (n, %) | 107 (54%) | 22 (61%) | 35 (57%) | 34 (59%) | 16 (38%)a | |
| HFmEF (n, %) | 52 (26%) | 8 (22%) | 16 (26%) | 11 (19%) | 17 (41%)a | |
| HFpEF (n, %) | 38 (19%) | 6 (17%) | 10 (16%) | 13 (22%) | 9 (21%) | |
| NT‐proBNP, pmol/L (median, IQR) | 2044 (856–4014) | 1062 (347–1838) | 2307 (952–3751)a | 3167 (1327–5575)a | 2545 (937–4642)a | <0.01 |
| Blood pressure, mmHg | ||||||
| Systolic (mean, SD) | 124 (20) | 125 (23) | 124 (17) | 123 (21) | 123 (20) | 0.98 |
| Diastolic (mean, SD) | 69 (10) | 72 (9) | 70 (10) | 69 (12) | 67 (8) | 0.07 |
| Renal function | ||||||
| Creatinine, μmol/L (mean, SD) | 120 (48) | 99 (28) | 114 (43) | 128 (45)a | 138 (63)a | <0.01 |
| eGFR, mL/min/1.73 m2 (mean, SD) | 51 (20) | 65 (18) | 52 (18)a | 46 (18)a | 43 (19)a | <0.01 |
| Severe renal dysfunctionb (n, %) | 29 (15%) | 2 (6%) | 8 (13%) | 9 (16%) | 10 (24%)a | 0.14 |
| Co‐morbidities | ||||||
| Diabetes (n, %) | 48 (24%) | 4 (11%) | 10 (16%) | 20 (35%)a | 14 (33%) | 0.01 |
| Cardiovascular diseasec (n, %) | 174 (88%) | 30 (83%) | 53 (87%) | 52 (90%) | 39 (93%) | 0.58 |
| Pulmonary diseased (n, %) | 64 (33%) | 13 (36%) | 17 (28%) | 23 (40%) | 11 (26%) | 0.40 |
| Drug use | ||||||
| Total number (mean, SD) | 10 (4) | 7 (2) | 8 (3) | 11 (4)a | 12 (4)a | <0.01 |
eGFR, estimated glomerular filtration rate; HFmEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IQR, inter quartile range; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide; NYHA classification, New York Heart Association classification; SD, standard deviation.
Statistically significantly (P‐value ≤0.05) different from patients with 0 domains affected.
eGFR <30 mL/min/1.73 m2.
Presence of myocardial infarction, angina pectoris, stroke, transient ischemic attack, and/or peripheral artery disease.
Chronic pulmonary disease and/or asthma.
Compared with patients with 0 domains affected, patients with 1, 2, or ≥3 domains affected were older, more often female, suffered from more severe heart failure, were more likely to have diabetes and renal dysfunction, and took more drugs in total (Table 1).
Heart failure severity, frailty, and multidomain geriatric assessment
Of the 197 patients at baseline, 134 (68%) had less severe heart failure (NYHA class I‐II), and 63 (32%) had severe heart failure (NYHA class III‐IV). Forty‐five (23%) of the patients were classified as non‐frail, 100 (51%) as pre‐frail, and 52 (26%) as frail.
Physical function was affected in the largest number of patients, with a prevalence of 64%. In 37% of the patients scored positive on the nutritional domain, and 32% on the polypharmacy domain.. Cognition and ADL dependency were affected in 21% and 8% of the study population, respectively. The number of patients (prevalence rates) who had 0, 1, 2, and ≥3 affected domains were n = 36 (18%), n = 61 (31%), n = 58 (29%), and n = 42 (21%), respectively.
Adverse health outcomes
Of the 184 patients with heart failure included in the 3 month follow‐up (93% of the study population at baseline), 47 patients (26% of the population included in the 3 month follow‐up) experienced a total of 74 adverse health outcomes. See Figure A1 for a flowchart with details on the experienced adverse health outcomes and patients lost to follow‐up. No differences in baseline characteristics were observed between patients included in follow‐up and patients lost to follow‐up (Table A1).
Figure A1.
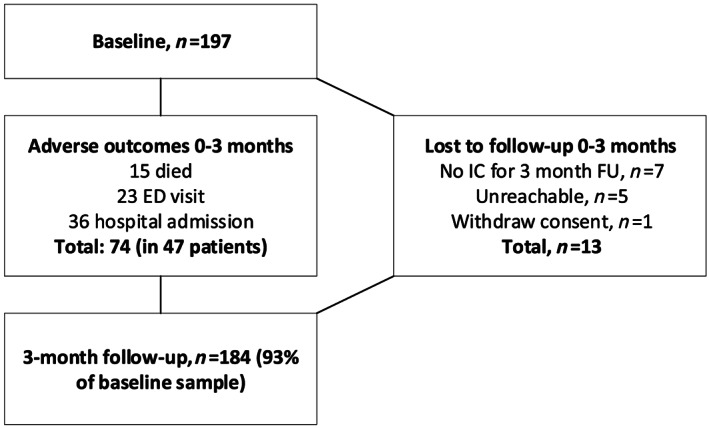
Flowchart 3 month follow‐up (occurrence adverse health outcomes and lost to follow‐up). IC, informed consent; ED, emergency department.
Table A1.
Baseline characteristics in the patients included in the 3 month follow‐up (n = 184, 93% of the population at baseline) and patients lost to follow‐up at 3 month follow‐up (n = 13, 7% of the population at baseline)
| Lost to follow‐up at 3 months | |||
|---|---|---|---|
| No (n = 184) | Yes (n = 13) | P‐value | |
| Demographics | |||
| Age, years (mean, SD) | 77.9 (8.8) | 80.2 (8.5) | 0.35 |
| Gender, female (n, %) | 80 (43%) | 7 (54%) | 0.65 |
| Cardiac assessment | |||
| NYHA classification | 0.47 | ||
| Class I‐II (n, %) | 122 (66%) | 8 (62%) | |
| Class III‐IV (n, %) | 62 (34%) | 5 (38%) | |
| Renal function | |||
| Creatinine, μmol/L (mean, SD) | 121 (48) | 118 (45) | 0.81 |
| Severe renal dysfunctiona (n, %) | 28 (15%) | 1 (7%) | 0.39 |
| Co‐morbidities | |||
| Diabetes (n, %) | 46 (25%) | 2 (15%) | 0.36 |
| Cardiovascular diseaseb (n, %) | 161 (88%) | 13 (100%) | 0.64 |
| Pulmonary diseasec (n, %) | 60 (32%) | 4 (31%) | 0.45 |
| Drug use | |||
| Total number (mean, SD) | 10 (4) | 9 (2) | 0.11 |
NYHA classification, New York Heart Association classification; SD, standard deviation.
eGFR <30 mL/min/1.73 m2.
Presence of myocardial infarction, angina pectoris, stroke, transient ischemic attack, and/or peripheral artery disease.
Chronic pulmonary disease and/or asthma.
Figure 1 presents an overview of the occurrence of adverse health outcomes at 3 month follow‐up, according to heart failure severity (NYHA I‐II versus III‐IV), physical frailty status, and number of domains affected. Although not statistically significant, patients with more severe heart failure and increasing level of frailty were more likely to have experienced ≥1 adverse health outcomes. In patients with severe heart failure and frail patients, approximately a third had experienced ≥1 adverse health outcome at 3 month follow‐up. The number of affected domains was positively correlated with the occurrence of ≥1 adverse health outcomes; the prevalence rates of adverse outcomes in patients with 0, 1, 2, or ≥3 affected domains were 21%, 29%, 49%, and 66%, respectively (P < 0.01).
Figure 1.
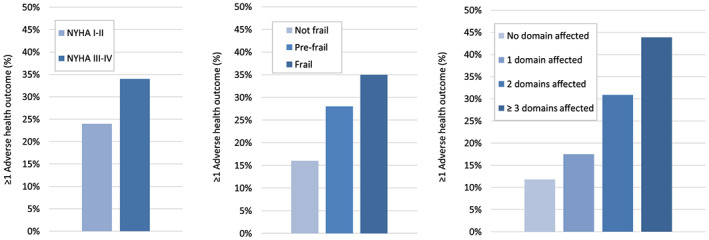
Incidence of adverse health outcomes (composite score of emergency department visit, unplanned hospital admission, and/or death) within 3 months, according to (i) heart failure severity (NYHA I‐II versus III‐IV), (ii) frailty status, and (iii) number of affected domains in older patients with heart failure.
Severity of heart failure was not statistically significantly associated with an increased risk of experiencing ≥1 adverse health outcome; odds ratios (95% confidence intervals) for NYHA III‐IV compared with NYHA I‐II were 1.6 (0.8–3.2) (P‐value for trend 0.15) and for NT‐proBNP Tertiles 2 and 3 compared with NT‐proBNP Tertile 1 were 1.2 (0.5–2.8) and 2.3 (1.0–5.4) (P‐value for trend 0.06) (Table 2, Model 1). Further adjustment for co‐morbidities further attenuated the effect estimates (Table 2, Models 2 and 3).
Table 2.
Risk of experiencing ≥1 adverse health outcome within 3 month follow‐up (composite score of emergency department visit, unplanned hospital admission, and/or death) according to heart failure severity (NYHA class and NT‐proBNP), frailty, and number of domains affected in n = 184 patients (93% of the population at baseline)
| Risk of experiencing ≥1 adverse health outcome at 3 month follow‐up | |||
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Model 1 | Model 2 | Model 3 | |
| Heart failure severity | |||
| NYHA classification | |||
| NYHA I‐II | Ref | Ref | Ref |
| NYHA III‐IV | 1.6 (0.8–3.2) | 1.3 (0.6–2.7) | 1.1 (0.5–2.4) |
| P‐value for trend | 0.15 | 0.51 | 0.78 |
| NT‐proBNP, pmol/L | |||
| Tertile 1 | Ref | Ref | Ref |
| Tertile 2 | 1.2 (0.5–2.8) | 1.0 (0.4–2.6) | 1.1 (0.4–2.7) |
| Tertile 3 | 2.3 (1.0–5.4) | 1.7 (0.6–4.3) | 1.6 (0.6–4.1) |
| P‐value for trend | 0.06 | 0.30 | 0.35 |
| Frailty | |||
| Not frail | Ref | Ref | n.a. |
| Pre‐frail | 2.2 (0.8–5.8) | 1.8 (0.7–5.0) | n.a. |
| Frail | 3.1 (1.1–9.3) | 2.2 (0.7–6.9) | n.a. |
| P‐value for trend | 0.04 | 0.18 | |
| Number of affected domains | |||
| No domain | Ref | Ref | Ref |
| 1 domain | 1.8 (0.5–6.5) | 1.4 (0.4–5.3) | 1.4 (0.4–5.3) |
| 2 domains | 4.5 (1.3–15.4) | 2.9 (0.8–10.7) | 2.8 (0.7–11.4) |
| ≥3 domains | 7.2 (2.0–26.3) | 4.4 (1.1–16.9) | 4.2 (1.0–18.5) |
| P‐value for trend | <0.01 | <0.01 | 0.02 |
A total of 50 patients experienced 74 adverse outcomes (15 died, 23 emergency department visits, and 36 hospital admissions) during the first 3 months of follow‐up. CI, confidence interval; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide; NYHA classification, New York Heart Association classification; OR, odds ratio; Ref, reference group.
Model 1: adjusted for age and sex; Model 2: additional adjustment for diabetes, creatinine levels, and NT‐proBNP. Model 3: additional adjustment for physical frailty.
Compared with non‐frail patients, pre‐frail and frail patients had a 2.2 (0.8–5.8) and a 3.1 (1.1–9.3) risk of experiencing ≥1 adverse health outcomes (P‐value for trend 0.04). However, this association was dependent on co‐morbidities (P‐value for trend in Model 2 was 0.18).
For patients with 1, 2, or ≥3 affected domains, the risk of experiencing ≥1 adverse health outcome increased from 1.8 (0.5–6.5) to a 4.5 (1.3–15.4) and 7.2 (2.0–26.3) compared with patients with no affected domains (P‐value for trend <0.01). This was independent of, for example, heart failure severity and physical frailty (P‐value for trend in Model 3 was 0.02).
Of the individual domains, polypharmacy was particularly associated with an increased risk of adverse health outcomes at follow‐up (Table A2).
Table A2.
Risk of experiencing ≥1 adverse health outcome within 3‐month follow‐up (composite score of emergency department visit, unplanned hospital admission, and/or death) according to individual domains in n = 184 patients (93% of the population at baseline)
| Individual domains | Risk of experiencing ≥1 adverse health outcome at 3 month follow‐up | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Model 1 | Model 2 | |
| Physical | 1.9 (0.9–4.2) | 1.4 (0.6–3.3) |
| Nutrition | 1.8 (0.9–3.5) | 1.7 (0.8–3.4) |
| Polypharmacy | 3.1 (1.5–6.1) | 2.0 (0.9–4.4) |
| Cognition | 1.5 (0.7–3.2) | 1.5 (0.6–3.4) |
| ADL dependency | 3.0 (1.0–9.2) | 2.3 (0.6–8.1) |
A total of 50 patients experienced 74 adverse outcomes (15 died, 23 ED visits, 36 hospital admissions) during the first 3 months of follow‐up. ADL, activities of daily living; CI, confidence interval; OR, odds ratio. Model 1: adjusted for age and sex; Model 2: additional adjustment for diabetes, creatinine levels, and NT‐proBNP.
Discussion
This study demonstrates that (i) having limitations in multiple geriatric domains is highly prevalent in older patients with heart failure, and (ii) the number of domains affected appears more strongly associated with short‐term adverse health outcomes than heart failure severity and physical frailty, independent of relevant confounders.
Major guidelines on management of heart failure call for a multidisciplinary approach of heart failure in older patients.5 However, this approach is mainly focused on the presence of co‐morbidities, rather than the limitations in geriatric domains.5 This may explain why a multidomain geriatric assessment has not yet been incorporated into the daily assessment routine at a majority of the outpatient heart failure clinics.21
To date, only a limited number of studies used a multidomain geriatric assessment to assess the multidimensional impact of heart failure in older patients.22, 23, 24 Also, to the best of our knowledge, our study is unique in terms of putting the prevalence of domains into the perspective of heart failure severity and physical frailty, and relating this to adverse health outcomes. The results of our study may suggest the potential added value of a multidomain geriatric assessment; even considering the small number per group, having impairments in multiple geriatric domains appeared more strongly associated with adverse health outcomes than having severe heart failure (higher NYHA class or NT‐proBNP levels) or being physically frail.
Patients with the largest number of domains affected had more advanced heart failure and experienced more adverse health outcomes. Adequate adherence to therapy is thus especially essential in these patients. The European Society of Cardiology guideline for management of heart failure recommends closer contact with a specialist heart failure team, more frequent follow‐up and monitoring, and individualized self‐care support in frail patients.5 However, limitations such as cognitive impairment make self‐management of heart failure and (drug) treatment adherence more difficult.25, 26 By recognizing in which domains a patient experiences impairments could substantially improve prognostication and individualization of care. It may aid in making therapeutic decisions (e.g. whether or not to implant cardiac devices), co‐ordinating care, and in medication management (e.g. deprescribing to reduce polypharmacy).27 Having impairments in multiple domains may signal the need for a transition from a merely ‘cure‐based’ strategy to a more ‘supportive‐care’ based treatment strategy with attention for advanced care planning. This may ultimately lower consumption of care and will increase patient autonomy. However, unanswered questions remain on how to best assess various domains and which interventions result in less negative health outcomes.
In contrast to many other studies assessing physical frailty in patients attending an outpatient heart failure clinic,28, 29 we were able to include a relatively large sample of patients with heart failure. Furthermore, we believe that our patient population is representative of the patients encountered at an average outpatient heart failure clinic. Another strength is that we used a comprehensive and widely used set of assessments with a good balance between ease of administration and assessment of multiple domains. Performing the assessment requires minimal training and can therefore be easily implemented into the routine workup of a heart failure nurse. However, several limitations in the study design must be addressed. First, baseline screening did not include two other important domains, that is, the psychological (e.g. depression and anxiety) and the social domain (e.g. extent of social support at home). Also, data on important co‐morbidities and clinical data on heart failure characteristics, such as anaemia and underlying aetiology, were not structurally assessed for this study. Thirteen patients were lost to follow‐up, which may have influenced our results. However, as assessed in Table A2, no differences in baseline characteristics between patients included and patients not included in the analyses were observed. This implicates that the results of our analyses are applicable to the entire baseline study population. Also, the follow‐up period was short (3 months), and because the total number of adverse events was relatively small (74 adverse events in a total of 50 patients), this may have led to overadjustment of our secondary models. Lastly, because some of the Fried frailty criteria are also evaluated with the physical and nutritional domain, being frail according to the Fried frailty criteria overlaps to some extent with scoring positive on the physical or nutritional domain. Prevalence of cognitive impairment, on the other hand, is likely to have been underestimated, because the MMSE is not a sensitive enough tool to detect mild cognitive impairment.
In conclusion, or study demonstrates that, having limitations in multiple geriatric domains is more strongly associated with poor short‐term prognosis in older patients with heart failure than heart failure severity and physical frailty. This observation may suggest the potential added value of a multidomain geriatric assessment in the evaluation and treatment of older patients with heart failure with respect to relevant short‐term health outcomes.
Conflict of interest
None declared.
Funding
Not applicable.
Acknowledgements
We acknowledge the support from the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018‐28 & 2012‐06 Heart Brain Connection), Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences. We also thank all the students and nurses working at the heart failure clinics of Amsterdam UMC, location VUmc and the Amstelland Hospital for their support and contribution to the data collection.
Kleipool, E. E. F. , Wiersinga, J. H. I. , Trappenburg, M. C. , van Rossum, A. C. , van Dam, C. S. , Liem, S.‐S. , Peters, M. J. L. , Handoko, M. L. , and Muller, M. (2020) The relevance of a multidomain geriatric assessment in older patients with heart failure. ESC Heart Failure, 7: 1264–1272. 10.1002/ehf2.12651.
References
- 1. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 2. Kleipool EE, Hoogendijk EO, Trappenburg MC, Handoko ML, Huisman M, Peters MJ, Muller M. Frailty in older adults with cardiovascular disease: cause, effect or both? Aging Dis 2018; 9: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN, Alexander KP, Geriatric Cardiology Section Leadership Council, American College of Cardiology . Domain management approach to heart failure in the geriatric patient: present and future. J Am Coll Cardiol 2018; 71: 1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denfeld QE, Winters‐Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta‐analysis. Int J Cardiol 2017; 236: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 6. McNallan SM, Singh M, Chamberlain AM, Kane RL, Dunlay SM, Redfield MM, Weston SA, Roger VL. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart failure 2013; 1: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vidan MT, Blaya‐Novakova V, Sanchez E, Ortiz J, Serra‐Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non‐dependent elderly patients with heart failure. Eur J Heart Fail 2016; 18: 869–875. [DOI] [PubMed] [Google Scholar]
- 8. Doehner W, Ural D, Haeusler KG, Celutkiene J, Bestetti R, Cavusoglu Y, Peña‐Duque MA, Glavas D, Iacoviello M, Laufs U, Alvear RM. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail 2018; 20: 199–215. [DOI] [PubMed] [Google Scholar]
- 9. Minamisawa M, Seidelmann SB, Claggett B, Hegde SM, Shah AM, Desai AS, Lewis EF, Shah SJ, Sweitzer NK, Fang JC, Anand IS, O'Meara E, Rouleau JL, Pitt B, Solomon SD. Impact of malnutrition using geriatric nutritional risk index in heart failure with preserved ejection fraction. JACC Heart Failure 2019; 7: 664–675. [DOI] [PubMed] [Google Scholar]
- 10. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003; 95: 1851–1860. [DOI] [PubMed] [Google Scholar]
- 11. BCGuidelines.ca: Frailty in older adults—early identification and management (2017), Gait speed test
- 12. van Bokhorst‐de van der Schueren MA, Guaitoli PR, Jansma EP, de Vet HC. Nutrition screening tools: does one size fit all? A systematic review of screening tools for the hospital setting. Clin Nutr 2014; 33: 39–58. [DOI] [PubMed] [Google Scholar]
- 13. Vellas B, Villars H, Abellan G, Soto ME, Rolland Y, Guigoz Y, Morley JE, Chumlea W, Salva A, Rubenstein LZ, Garry P. Overview of the MNA—its history and challenges. J Nutr Health Aging 2006; 10: 456–463 discussion 63‐5. [PubMed] [Google Scholar]
- 14. Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011; 124: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slater N, White S, Venables R, Frisher M. Factors associated with polypharmacy in primary care: a cross‐sectional analysis of data from The English Longitudinal Study of Ageing (ELSA). BMJ Open 2018; 8: e020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arevalo‐Rodriguez I, Smailagic N, Roque IFM, Ciapponi A, Sanchez‐Perez E, Giannakou A, Pedraza OL, Cosp XB, Cullum S. Mini‐Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 2015; 3: CD010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff‐Radford NR, Petersen RC, Lucas JA. Detecting dementia with the mini‐mental state examination in highly educated individuals. Arch Neurol 2008; 65: 963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hopman‐Rock M. Activities of daily living in older community‐dwelling persons: a systematic review of psychometric properties of instruments. Aging Clin Exp Res 2019; 31: 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallace M, Shelkey M. Hartford Institute for Geriatric N. Katz Index of independence in activities of daily living (ADL). Urol Nurs 2007; 27: 93–94. [PubMed] [Google Scholar]
- 20. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 21. Cannon JA, McMurray JJ, Quinn TJ. ‘Hearts and minds’: association, causation and implication of cognitive impairment in heart failure. Alzheimers Res Ther 2015; 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long‐term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2005; 35: 723–730. [DOI] [PubMed] [Google Scholar]
- 23. Gastelurrutia P, Lupon J, Altimir S, de Antonio M, Gonzalez B, Cabanes R, Rodríguez M, Urrutia A, Domingo M, Zamora E, Díez C. Fragility is a key determinant of survival in heart failure patients. Int J Cardiol 2014; 175: 62–66. [DOI] [PubMed] [Google Scholar]
- 24. Uchmanowicz I, Mlynarska A, Lisiak M, Kaluzna‐Oleksy M, Wleklik M, Chudiak A, Dudek M, Migaj J, Hinterbuchner L, Gobbens R. Heart failure and problems with frailty syndrome: why it is time to care about frailty syndrome in heart failure. Card Fail Rev 2019; 5: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harkness K, Heckman GA, Akhtar‐Danesh N, Demers C, Gunn E, McKelvie RS. Cognitive function and self‐care management in older patients with heart failure. Eur J Cardiovasc Nur: Journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology 2014; 13: 277–284. [DOI] [PubMed] [Google Scholar]
- 26. Cameron J, Worrall‐Carter L, Page K, Riegel B, Lo SK, Stewart S. Does cognitive impairment predict poor self‐care in patients with heart failure? Eur J Heart Fail 2010; 12: 508–515. [DOI] [PubMed] [Google Scholar]
- 27. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano GMC, Seferovic P, Coats AJ. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019; 21: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 28. Abou‐Raya S, Abou‐Raya A. Osteoporosis and congestive heart failure (CHF) in the elderly patient: double disease burden. Arch Gerontol Geriatr 2009; 49: 250–254. [DOI] [PubMed] [Google Scholar]
- 29. Madan SA, Fida N, Barman P, Sims D, Shin J, Verghese J, Piña I, Jorde U, Patel SR. Frailty assessment in advanced heart failure. J Card Fail 2016; 22: 840–844. [DOI] [PubMed] [Google Scholar]


