Abstract
Aims
Elderly patients with heart failure (HF) are associated with frequent all‐cause readmission or death. The present study sought to develop an accurate and easy‐to‐use model to predict all‐cause readmission or death risk in Chinese elderly patients with HF.
Methods and results
This was a prospective cohort study in patients with HF aged 65 or older. Demographic, co‐morbidity, laboratory, and medication data were collected. A Cox regression model was used to identify factors for the prediction of readmission or death at 30 days and 1 year. A nomogram was developed with bootstrap validation. Of the included 854 patients, the cumulative all‐cause readmission and mortality rates were 10.5% and 11.6% at 30 days and 34.9% and 19.7% at 1 year, respectively. The independent risk factors associated with both 30 day and 1 year readmission or death were older age, stroke, diastolic blood pressure < 60 mmHg, body mass index ≤ 18.5 kg/m2, lower estimated glomerular filtration rate, and BNP > 400 pg/mL (all P < 0.05). Anaemia, abnormal neutrophils, and admission without angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers were the specific independent risk factors of 30 day all‐cause readmission or death (all P < 0.05), whereas serum sodium ≤ 140 mmol/L and admission without beta‐blockers were the specific independent risk factors of 1 year all‐cause readmission or death (all P < 0.05). The C‐index of the 30 day and 1 year diagnosis prediction model was 0.778 [95% confidence interval (CI) 0.693–0.862] and 0.738 (95% CI 0.640–0.836), respectively.
Conclusions
We developed accurate and easy‐to‐use nomograms to predict all‐cause readmission or death in Chinese elderly patients with HF. The nomograms will assist in reducing the all‐cause readmission and mortality rates.
Keywords: Heart failure, Elderly, All‐cause readmission, Mortality, Nomogram, Prognostic model
Introduction
Heart failure (HF) is a quintessential geriatric cardiovascular condition, doubling in prevalence from 6% in those aged 60 to 79 years to >12% in those aged 80 years or older, and its significance is likely to increase in the future owing to an aging population. 1 Despite the effective treatment, unexpectedly, all‐cause readmission and mortality rates of HF have been steadily increasing in the past decade. 2
As many of readmissions and deaths are possibly predictable and preventable, 3 , 4 an easy‐to‐use and accurate model to predict the risk of all‐cause readmission or death has been noted. 5 , 6 , 7 However, it is suboptimal using a one‐size‐fits‐all approach to predict the risk of readmission or death for all patients with HF. First, racial and ethnic disparities have been indicated among patients with HF in some prior studies. 8 HF risk factors vary substantially across world regions, such as China populations having more stroke and lower body mass index (BMI) than have Western populations. 1 , 9 , 10 Second, elderly patients are the majority population of all‐caused HF readmission or death. 11 The burden of noncardiac co‐morbidities is greater, and readmission or death is more frequently driven by noncardiac causes among elderly patients compared with the general population. 12 , 13 Third, the timing of readmission varies among patients with HF. 14 , 15 Although prior studies have suggested that patients are readmitted disproportionately early, no study has rigorously distinguished the weight of risk factors between the precise timing of 30 day and 1 year readmission or death. In addition, prior prediction models using complex methods were only modest discriminative ability. 5 , 6 , 16 Hence, these limitations emphasize the need for a user‐friendly model to accurately target elderly patients with multimorbidity.
Nomogram is designed to provide disease‐specific, clinically relevant prognostic models predictive of outcome measures specific to an individual patient. To date, nomograms derived from risk factors have been widely applied as a straightforward tool to predict survival rate among cancer patients. 17 , 18 To our knowledge, nomogram is also a potentially ideal model to predict readmission or death of patients with HF. 19 However, very few studies specifically focused on the readmission or death of elderly patients with HF using nomograms.
Therefore, to address these gaps mentioned above, we aim to (i) explore both 30 day and 1 year all‐cause readmission or death risk factors and their weights among Chinese elderly patients with HF; (ii) develop and validate accurately models that target Chinese elderly individuals with HF; and (iii) construct simplified and effective nomograms to predict individual all‐cause readmission or death risk using reliable data that can be easily obtained.
Methods
Study population
This was a prospective cohort study of patients hospitalized from the Heart Failure Center of China‐Japan Friendship Hospital between 1 January 2015 and 30 April 2018. All patients 65 years or older admitted to the department with a diagnosis of HF were included in this study. The patient selection process is shown in Figure 1 . Patients with HF were clinically ascertained according to the clinical practice guidelines by the Chinese Society of Cardiology and was briefly based on (i) symptoms, for example, dyspnoea, fatigue, or decreased exercise capacity; (ii) signs, for example, oedema or rales; (iii) B‐type natriuretic peptide (BNP) or N‐terminal pro‐B‐type natriuretic peptide, to differentiate the HF diagnosis for patients with dyspnoea; and (iv) structural and functional abnormalities on echocardiography. 20 The diagnostic criteria were similar to the recommendation of the European Society of Cardiology. Cases lost to follow‐up were excluded. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the China‐Japan Friendship Hospital.
Figure 1.
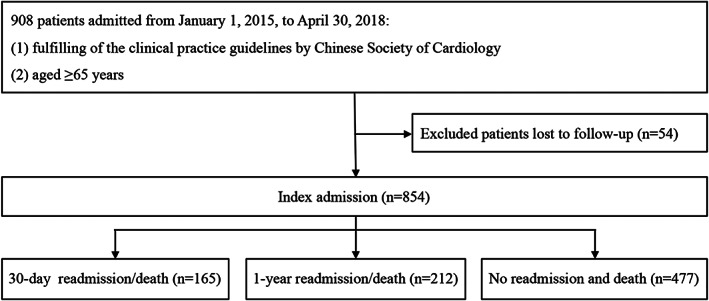
Flow chart of inclusion and exclusion process of patients admitted with heart failure.
Study design
The study outcomes included all‐cause readmission and death at 30 days and 1 year. Readmission was defined as any unplanned hospital readmission for any cause within the first 30 days after discharge or within the first 1 year after discharge. Only the first readmission occurring at any time after the index admission and throughout the 30 day and 1 year follow‐up period of study was considered to be the qualifying readmission for admission‐based analyses. Patients who were readmitted to other hospitals were also included in the readmission group in this study because study coordinators performed follow‐up interviews regularly and document major events at 30 days and 1 year into a web‐based system (http://data.chinahfc.org).
Predictor variables
Variables used in the present study were demographic, echocardiographic, and biochemical data; cardiac and noncardiac co‐morbidities at admission; and medications at admission and discharge. Patients were categorized into three age groups (65–74, 75–84, and ≥85 years) and four estimated glomerular filtration rate (eGFR) groups (<30, 30–59, 60–89, and ≥90 mL/min/1.73 m2).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as n (%). To identify factors that were associated with 30 day and 1 year readmission, the Kaplan–Meier survival curves and log‐rank test were used for the univariate analysis, and the Cox regression model was used for the multivariate analysis. Hazard ratios (HRs) were presented with 95% confidence interval (CI). In order to facilitate clinical application, continuous variables were divided into categorical variables for analysis according to clinical routine cut‐offs.
A nomogram was constructed based on statistically significant factors identified by the multivariate analysis from the Cox regression model to predict the possibility of readmission. A likelihood ratio test approach for model selection was performed. Nomogram performance was quantified with respect to discrimination and calibration. Discrimination (the ability of a nomogram to separate patients with readmission status) was quantified by the concordance index (C‐index) and 95% CI. Calibration was assessed graphically by plotting the relationship between actual (observed) probabilities and predicted probabilities (calibration plot) by using Hosmer goodness‐of‐fit test. Internal validation of performance was estimated with the bootstrapping method (500 replications). All tests were two‐sided, and P < 0.05 was deemed significant. Statistical analyses were conducted using SPSS for Windows (version 25.0, SPSS Inc., Chicago, IL, USA) and the R programming language and environment version 3.6.0 (http://cran.r-project.org).
Results
Characteristics of elderly patients with heart failure
The baseline characteristics of elderly patients with HF are shown in Table 1 . A total of 854 elderly patients hospitalized with HF were included in the present analysis. For the entire cohort, the mean age was 76.7 ± 6.4 years, with 64.2% being >75 years old. Male constituted 56.2% of the cohort. Clinical characteristics of elderly patients hospitalized with HF by age group were illustrated in Table S1. The cumulative mortality rate at 30 days and 1 year for elderly patients with HF was 11.6% and 19.7%, respectively (Figure S1A). The cumulative readmission rate at 30 days and 1 year for elderly patients with HF was 10.5% and 34.9%, respectively (Figure S1B). The cumulative mortality rate at 30 days and 1 year for elderly patients with HF was 19.3% and 44.2%, respectively.
Table 1.
Baseline characteristics of elderly patients with heart failure
| Variables | Description (n = 854) |
|---|---|
| Age, years | 76.72 ± 6.40 |
| Male, n (%) | 480 (56.21) |
| Heart rate, b.p.m. | 78.04 ± 16.86 |
| SBP, mmHg | 132.42 ± 23.04 |
| DBP, mmHg | 73.90 ± 13.87 |
| BMI, kg/m2 | 24.46 ± 4.11 |
| NYHA class, n (%) | |
| II or under | 336 (39.34) |
| III or IV | 518 (60.66) |
| LVEF, % | 58.03 ± 13.10 |
| ≥50%, n (%) | 545 (63.82) |
| 40–49%, n (%) | 130 (15.22) |
| <40%, n (%) | 179 (20.96) |
| BNP, pg/mL | 488.00 (167.00, 1117.00) |
| Haemoglobin, g/L | 118.48 ± 21.84 |
| eGFR, mL/min/1.73 m2 | 67.90 ± 27.95 |
| Fasting glucose, mmol/L | 7.45 ± 3.36 |
| Serum sodium, mmol/L | 139.56 ± 4.74 |
| Co‐morbidities | |
| Coronary heart disease, n (%) | 571 (66.86) |
| Cardiomyopathy, n (%) | 47 (5.50) |
| Valvular disorders, n (%) | 78 (9.13) |
| Hypertension, n (%) | 677 (79.27) |
| Atrial fibrillation, n (%) | 286 (33.49) |
| Anaemia, n (%) | 342 (40.05) |
| Dyslipidaemia, n (%) | 423 (57.71) |
| Diabetes, n (%) | 338 (39.58) |
| Chronic kidney disease, n (%) | 316 (37.00) |
| Stroke, n (%) | 246 (28.81) |
| Infections, n (%) | 279 (32.67) |
| Medication at admission | |
| ACEIs/ARBs | 491 (57.49) |
| Beta‐blockers | 530 (62.06) |
| MRAs | 351 (41.10) |
| Diuretic | 687 (80.44) |
| Device therapy | |
| Pacemaker | 88 (10.30) |
| ICDs | 4 (0.47) |
| CRT/CRT‐D | 10 (1.17) |
ACEIs, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy with defibrillator. DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; SBP, systolic blood pressure.
Univariate and multivariate analyses of factors for 30 day and 1 year readmission or death
In univariate survival analysis, prognostic factors significantly associated with an increased risk of both 30 day and 1 year readmission or death included older age, diastolic blood pressure (DBP) < 60 mmHg, BMI < 18.5, stroke, anaemia, abnormal neutrophils, lower eGFR, serum sodium ≤ 140 mmol/L, BNP > 400 pg/mL, and admission without angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), beta‐blockers, and mineralocorticoid receptor antagonists (MRAs) (all P < 0.05) (Table 2 ).
Table 2.
Univariate and multivariate analyses of 30 day and 1 year death or readmission in elderly patients with heart failure
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| 30 days | 1 year | 30 days | 1 year | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age, years | 0.002 | <0.001 | 0.041 | 0.004 | ||||
| 65–75 | 1.00 | 1.00 | 1.00 | 1.000 | ||||
| 75–85 | 1.17 (0.82–1.67) | 0.379 | 1.41 (1.11–1.79) | 0.004 | 1.13 (0.69–1.82) | 0.633 | 1.30 (0.99–1.72) | 0.060 |
| ≥85 | 2.12 (1.38–3.25) | 0.001 | 2.37 (1.76–3.20) | <0.001 | 1.72 (1.13–2.61) | 0.034 | 1.84 (1.28–2.63) | 0.001 |
| Male | 1.17 (0.86–1.60) | 0.318 | 1.00 (0.82–1.23) | 0.999 | ||||
| SBP > 140 mmHg | 0.69 (0.42–1.13) | 0.140 | 0.55 (0.43–0.71) | <0.001 | ||||
| DBP < 60 mmHg | 2.69 (1.89–3.82) | <0.001 | 2.37 (1.84–3.07) | <0.001 | 2.57 (1.58–4.18) | <0.001 | 1.92 (1.39–2.64) | <0.001 |
| BMI ≤ 18.5 kg/m2 | 3.67 (2.14–6.32) | <0.001 | 1.99 (1.26–3.12) | 0.003 | 3.33 (1.72–6.46) | <0.001 | 1.86 (1.11–3.10) | 0.018 |
| Abnormal NE | 1.99 (1.27–3.11) | 0.003 | 1.68 (1.37–2.06) | <0.001 | 1.85 (1.21–2.82) | 0.005 | ||
| LVEF, % | 0.057 | 0.450 | 0.13 | 0.796 | ||||
| ≥50 | 1.00 | 1.00 | 1.00 | |||||
| 40–49 | 1.28(0.78–2.12) | 0.417 | 0.89(0.61–1.30) | 0.542 | 1.68(0.80–3.55) | 0.47 | 1.10(0.68–1.78) | 0.69 |
| <40 | 1.35(0.92–1.97) | 0.205 | 1.16(0.87–1.54) | 0.313 | 1.73(0.87–3.44) | 0.15 | 1.12(0.78–1.62) | 0.54 |
| eGFR, mL/min/1.73 m2 | 0.001 | <0.001 | 0.009 | 0.001 | ||||
| ≥90 | 1.00 | 1.00 | 1.00 | 1.000 | ||||
| 60–90 | 0.82 (0.53–1.25) | 0.355 | 1.03 (0.78–1.37) | 0.839 | 0.68 (0.37–1.24) | 0.207 | 1.09 (0.77–1.54) | 0.618 |
| 30–60 | 1.27 (0.82–1.96) | 0.289 | 1.47 (1.09–1.98) | 0.011 | 1.62 (0.89–2.95) | 0.114 | 1.55 (1.07–2.23) | 0.019 |
| <30 | 2.38 (1.49–3.79) | <0.001 | 2.83 (2.04–3.93) | <0.001 | 1.63 (1.16–2.28) | 0.028 | 2.17 (1.40–3.38) | 0.001 |
| Serum sodium ≤ 140 mmol/L | 1.46 (1.07–2.00) | 0.019 | 1.49 (1.21–1.84) | <0.001 | 1.33 (1.04–1.70) | 0.027 | ||
| Fasting glucose > 6.9 mmol/L | 1.53 (0.98–2.40) | 0.062 | 1.34 (1.09–1.64) | 0.005 | ||||
| BNP > 400 pg/mL | 3.12 (2.15–4.54) | <0.001 | 1.78 (1.42–2.23) | <0.001 | 2.69 (1.76–4.12) | <0.001 | 1.54 (1.20–1.98) | 0.001 |
| Atrial fibrillation | 1.09 (0.79–1.50) | 0.603 | 1.08 (0.87–1.34) | 0.480 | ||||
| Stroke | 1.49 (1.08–2.04) | 0.014 | 1.56 (1.26–1.92) | <0.001 | 1.77 (1.14–2.73) | 0.010 | 1.60 (1.25–2.06) | <0.001 |
| Anaemia | 1.51 (1.11–2.04) | 0.009 | 1.55 (1.27–1.90) | <0.001 | 1.85 (1.16–2.96) | 0.010 | ||
| Admission without ACEIs/ARBs | 2.43 (1.75–3.36) | <0.001 | 1.77 (1.44–2.17) | <0.001 | 1.79 (1.13–2.81) | 0.012 | ||
| Admission without Beta‐blockers | 1.53 (1.12–2.07) | 0.007 | 1.41(1.15–1.72) | 0.001 | 1.34 (1.04–1.72) | 0.022 | ||
| Admission without MRAs | 1.61 (1.16–2.24) | 0.005 | 1.29(1.04–1.59) | 0.019 | ||||
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; BNP, B‐type natriuretic peptide; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; MRAs, mineralocorticoid receptor antagonists; NE, neutrophils; SBP, systolic blood pressure.
In the Cox multivariate analysis, older age [compared with 65–74 and 75–84 years, HR = 1.13 (0.69–1.82), P = 0.034, ≥85 years, HR = 1.72 (1.13–2.61), P = 0.034], DBP < 60 mmHg [HR = 2.57 (1.59–4.18), P < 0.001], BMI < 18.5 kg/m2 [HR = 3.33 (1.72–6.46), P < 0.001], stroke [HR = 1.77 (1.14–2.73), P = 0.010], anaemia [HR = 1.85 (1.16–2.96), P = 0.010], abnormal neutrophils [HR = 1.85 (1.21–2.82), P = 0.005], low eGFR [compared with ≥90 and 60–90 mL/min/1.73 m2, HR = 0.69 (0.37–1.24), P = 0.207; 30–60 mL/min/1.73 m2, HR = 1.62 (0.89–2.95), P = 0.114; <30 mL/min/1.73 m2, HR = 1.63 (1.16–2.28), P = 0.028], BNP > 400 pg/mL [HR = 2.69 (1.76–4.12), P < 0.001], and admission without ACEIs/ARBs [HR = 1.79 (1.13–2.81), P = 0.012] were the independent risk factors associated with increased 30 day all‐cause readmission or death (Table 2 ).
Besides similar risk factors at 30 days including older age, DBP < 60 mmHg, BMI ≤ 18.5 kg/m2, stroke, lower eGFR, BNP > 400 pg/mL, serum sodium < 140 mmol/L [HR = 1.33 (1.04–1.70), P = 0.027], and admission without beta‐blockers [HR = 1.34 (1.04–1.72), P = 0.022] were the specific independent risk factors associated with increased 1 year all‐cause readmission or death (Table 2 ).
In addition, older age, lower eGFR, anaemia, and discharge without ACEI/ARB and MRAs were the independent risk factors associated with increased 1 year HF readmission (Table S2).
Construction of the nomogram for 30 day and 1 year all‐cause readmission or death
Seven variables selected in the Cox model were included to build a nomogram to individualize the risk of readmission or death for 30 days and 1 year separately (Figure 3A and B ). The ratios of the calculated beta were used to decide the proportional prognostic effect of these variables. The projections from total points on the scales below indicated the estimated probability of readmission or death. BMI ≤ 18.5 kg/m2, DBP < 60 mmHg, abnormal neutrophils, eGFR < 60 mL/min/1.73 m2, BNP > 400 pg/mL, and admission without ACEIs/ARBs had higher weights on increased 30 day readmission or death risk (>5 points). Age > 85 years, DBP < 60 mmHg, BMI ≤ 18.5 kg/m2, stroke, eGFR < 30 mL/min/1.73 m2, and BNP > 400 pg/mL had higher weights on increased 1 year readmission or death risk (>5 points) (Figure 2 ).
Figure 3.
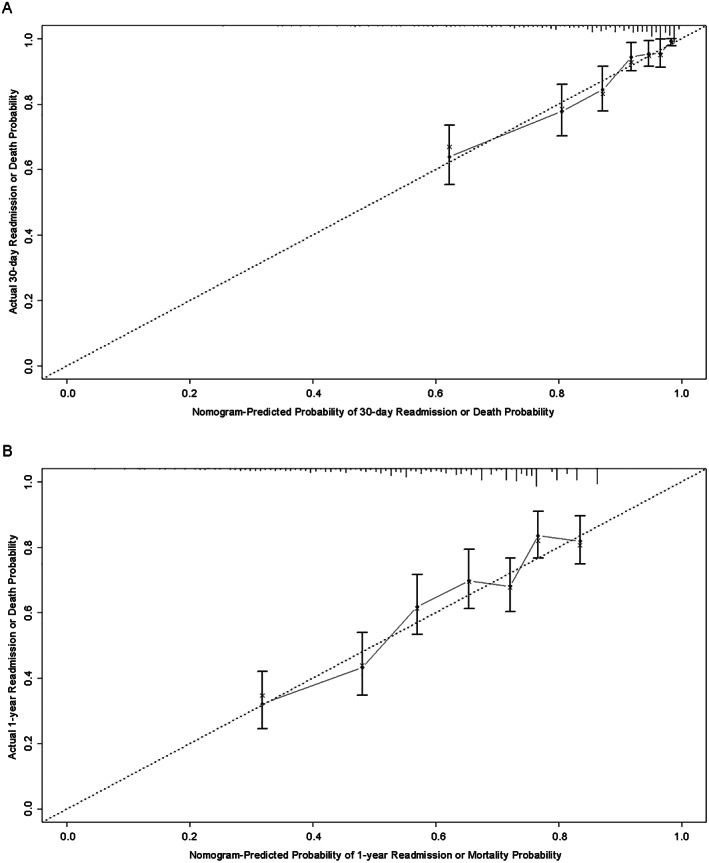
Validation of the nomogram for 30 day and 1 year all‐cause readmission. (A) Calibration plot of observed proportion versus predicted probability of 30 day readmission or death of the novel nomogram. (B) Calibration plot of observed proportion versus predicted probability of 1 year readmission or death of the novel nomogram.
Figure 2.
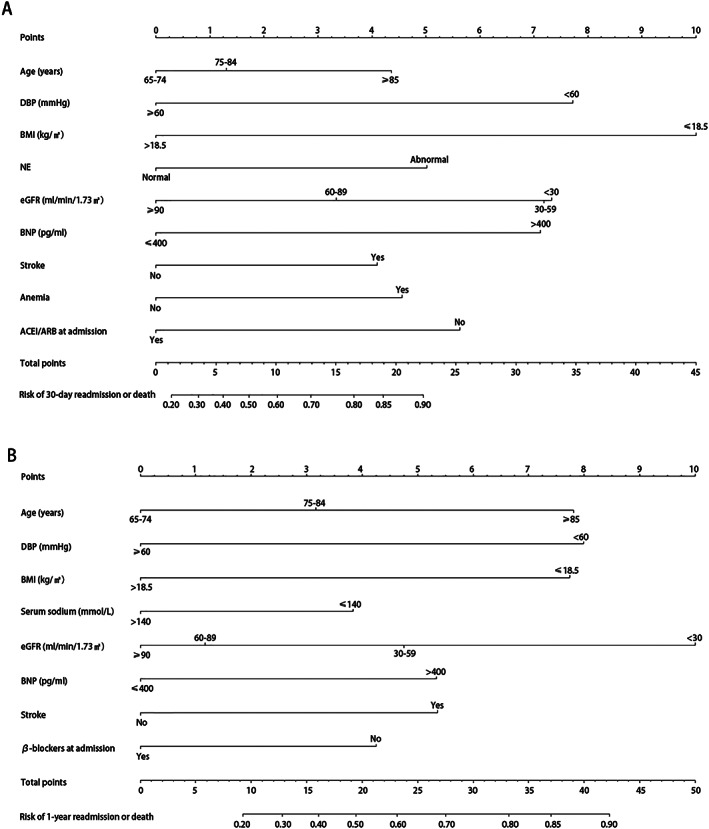
Construction of the nomogram for 30 day and 1 year all‐cause readmission. (A) The nomogram to predict the individual probability of 30 day readmission or death. (B) The nomogram to predict the individual probability of 1 year readmission or death. The nomogram is used by adding up the points identified on the scale of all parameters. The total nomogram point of each patient can be used to predict readmission or death risk on an individual basis. To predict a patient readmission or death risk at 30 days, take the following as an example: a 80‐year‐old female (1.25 points) whose DBP was 70 mmHg (0 points) and BMI was 19.5 kg/m2 (0 points) at admission; had a history of stroke (4.25 points) and anaemia (4.5 points); has an abnormal NE level (5 points); has an eGFR level of 65 mL/min/1.73 m2 (3.25 points); has a BNP level of 800 pg/mL (7 points) and admission without ACEIs/ARBs (5.5 points); and has a total point score of 30.75, corresponding to >90% risk of readmission or death at 30 days. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; BNP, B‐type natriuretic peptide; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; NE, neutrophils.
Validation of the nomogram
The predictive accuracy of the nomogram was measured via 10‐fold cross‐validation and the bootstrap validation method (500 bootstrap resamples). The C‐index for the nomogram of 30 day and 1 year readmission or death risk was 0.778 (95% CI 0.693–0.862) and 0.738 (95% CI 0.640–0.836) with bootstrapping, respectively. As shown in Figure 3A and B , the calibration plot of predicted probabilities against observed readmission or mortality rates indicated excellent concordance.
Discussion
In the present study, this is the first time to develop and validate a user‐friendly and relatively personalized model to predict in 30 day and 1 year all‐cause readmission or death risk specific for Chinese elderly patients with HF. Our constructed nomogram provided an easy‐to‐use and individualized model for prediction of all‐cause readmission or death, which is helpful for optimizing clinical management. Cross‐validation in the split sample confirmed the accuracy of our model in predicting readmission or death risk in elderly patients with HF at 30 days (C‐index = 0.778) and 1 year (C‐index = 0.738). However, this result came from internal verification without support from external data yet. Older age, stroke, DBP < 60 mmHg, BMI < 18.5, lower eGFR, and BNP > 400 pg/mL were the independent risk factors of both 30 day and 1 year all‐cause readmission or death. Anaemia, abnormal neutrophils, and admission without ACEIs/ARBs were the specific independent risk factors of 30 day all‐cause readmission or death, whereas serum sodium ≤ 140 mmol/L and admission without beta‐blockers were the specific independent risk factors of 1 year all‐cause readmission or death of Chinese elderly patients with HF.
Presently, the readmission or death risk prediction models established in previous studies for patients with HF have modest predictive ability. Although medical complex of elderly patients with HF would explain the poor readmission or death risk prediction, overlooking the important risk factors for readmission and death including the heterogeneity between races and noncardiac co‐morbidities was more considerable. 8 , 12 , 21 The HF population in China differs from that in Western countries, including notably more stroke and a lower BMI, 1 , 9 , 22 making data derived from Western populations likely to be not applicable. In addition, previously published studies on HF readmission or death risk model have mainly depended on cardiac conditions, 5 , 6 , 23 whereas they have focused less on the impact of noncardiac factors such as stroke and anaemia, which were the major factors for all‐cause readmission or death in elderly patients with HF.
Compared with previous reports, uniquely, this study aimed to target population of the predictive model at elderly patients in China and incorporate multiple noncardiac clinical variables, including stroke, anaemia, fasting glucose, and eGFR into a user‐friendly predictive nomogram. The advantage of our nomogram is that all variables are readily available and clinical patient information is accessible, and no advanced mathematical calculation is required. Because the readmission or death risk is known on the date of admission, several of these modifiable variables can direct active interventions to reduce readmission and mortality potentially. Meanwhile, the nomogram provides the prognosis of patients from group level to individual level. Furthermore, the use of 10‐fold cross‐validation substantiates the validity of the model. The 10‐fold cross‐validation was used to validate the model, which divides the raw data into 10 groups (10‐fold); each subset data are used as a verification set, and the remaining K − 1 subset data are used as a training set, so that K models are obtained. 24 Cross‐validation effectively utilizes limited data, making the test results of the model more robust. Unfortunately, this verification has not been carried out using external data, so the external accuracy of the model may require further research to confirm.
In our analysis, several risk factors incorporated in 30 day and 1 year prognostic prediction model warrant clinicians further regard, including stroke, DBP, and anaemia, which were not identified in prior studies. Stroke is a common co‐morbidity in Chinese elderly patients with HF, and the overall prevalence of stroke was 27.6%, noticeably higher than that of the previous trials conducted in Western countries (7–10%). 25 , 26 , 27 The findings of this study may well be relevant to the marked increase in stroke prevalence in China, making it the particularity of our study population. Moreover, in HF patients with stroke, the risk of readmission or death was significantly increased in both 30 days and 1 year. Our study demonstrated that stroke was a potent and persistent independent risk factor for readmission or death among Chinese elderly patients with HF. Contrary to our analyses, previous studies did not identify stroke as a significant predictor of readmission or death in several large cohorts in Western population. 15 , 28 , 29 This discrepancy might be due in part to the racial and ethnic differences. China has the highest current stroke incidence and mortality rates in the world. 30 In addition, the overall prevalence of AF in elderly patients with HF was lower to the rate recently reported in HF patients in elderly HF population in Western countries. 31 , 32 Specifically, in 246 patients with stroke, up to 158 (64.2%) patients were diagnosed with stroke but without AF, indicating that most of stroke was probably noncardioembolic. Similar to other studies, atherosclerosis may be the main cause of stroke in elderly patients with HF in China. 30 As stroke was a clinically important outcome in HF, an optimal approach to stroke prevention and treatment in patients with HF is needed.
Previous reports from prospective cohort studies representative of the general population have demonstrated that higher systolic blood pressure (SBP) and lower DBP are associated with an increased risk for readmission and death in HF, 33 , 34 , 35 whereas our results indicated that low DBP other than SBP was an independent risk factor for readmission or death in elderly patients with HF. Noticeably, low DBP increased not only 30 day but also 1 year readmission risk. A plausible explanation could be that SBP increases with age, whereas DBP remains stable or even decreases spontaneously in older adults, especially in the presence of arterial stiffness, and a declining BP may further decrease an already low DBP, which in turn leads to lower perfusion. 36 At low perfusion pressures, chronic coronary hypoperfusion occurs and associates with myocardial damage, and eventually ventricular function worsens. Additionally, the presence of low perfusion also leads to multiple organ damage, which may eventually worsen HF. Therefore, DBP level < 60 mmHg should alert clinicians to the need for frequent cardiovascular assessment and adjustments in antihypertensive treatment such as ACEIs/ARBs, beta‐blockers, and MRAs.
Anaemia has lately been observed as an imperative noncardiac co‐morbidity in patients with HF. 37 , 38 In our study, we found that 40.0% of the elderly HF patients were diagnosed with anaemia, and more importantly, anaemia was a reliable indicator of unfavourable prognosis and an independent risk factor for 30 day all‐cause readmission or death in elderly patients with HF. These findings were in alignment with several studies that provide evidence of an association between anaemia and adverse outcomes in patients with HF. 39 , 40 Multiple mechanisms appear to contribute to poor outcomes in these anaemic patients. Reduced oxygen delivery to metabolizing tissues in anaemic subjects triggers a host of hemodynamic, neurohormonal, and renal alterations, leading to increased myocardial workload, which could cause adverse outcomes.
Our study has a few limitations. Our study used data from a single centre with an elderly population. However, we have the advantage of systematic data collection and complete follow‐up; hence, we were able to provide more detailed information and to identify HF more accurately than did International Classification of Diseases, 9th Revision. Another limitation is the lack of validation of our predictive model in an external population. Nevertheless, internal validation measured via 10‐fold cross‐validation and the calibration of predicted probabilities against observed readmission and mortality rates indicated good outcome given the strength of our study. Finally, geriatric conditions such as disability, cognitive impairment, and frailty were essential and affect the outcome of elderly patients with HF. Unfortunately, these geriatric conditions were not incorporated in our study.
Conclusions
In the present study, we develop a novel and easy‐to‐use nomogram to rapidly predict all‐cause readmission or death in individual Chinese elderly patients with HF. These nomograms provide individualized all‐cause readmission or death estimation on the date of admission and will assist in clinical decision making. According to the results of the multivariate analysis, the factors associated with the increase both 30 day and 1 year all‐cause readmission or death of elderly patients with HF were older age, stroke, DBP < 60 mmHg, lower eGFR, and BNP > 400 pg/mL. Anaemia, abnormal neutrophils, and admission without ACEIs/ARBs were the specific independent risk factors of 30 day all‐cause readmission or death, whereas serum sodium ≤ 140 mmol/L and admission without beta‐blockers were the specific independent risk factors of 1 year all‐cause readmission or death. Therefore, optimal management, especially for the noncardiac co‐morbidities at admission, may be crucial for reducing the all‐cause readmission and mortality rate.
Conflict of interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China (81770359 and 81270276 to J.R.), Central Health Research Project of China (W2017BJ30 to J.R.), State Key Laboratory of Molecular Developmental Biology of China (2017‐MDB‐KF‐13 to J.R.), and China‐Japan Friendship Hospital Scientific Research Funds (2017‐1‐QN‐10 to M.Y.).
Author Contributions
Mengxi Yang and Liyuan Tao contributed to the conception and design, analysis and interpretation of data, drafting and critical review of article, and final approval of the version to be published. Hui An contributed to the conception and design, acquisition of data, drafting and critical review of article for important intellectual content, and final approval of the version to be published. Jingyi Ren contributed to the conception and design, acquisition, analysis and interpretation of data, critical review of article for important intellectual content, and final approval of the version to be published. The remaining authors contributed to the conception and design, critical review of article for important intellectual content, and final approval of the version to be published.
Supporting information
Figure S1. The cumulative death rate and readmission rate for elderly patients with HF. (A) The cumulative death rate for elderly patients with HF. (B) The cumulative readmission rate for elderly patients with HF.
Table S1. Clinical characteristics of elderly patients hospitalized with HF by age group
Table S2. Univariate and multivariate analysis of 1‐year HF readmission in elderly patients with HF
Yang, M. , Tao, L. , An, H. , Liu, G. , Tu, Q. , Zhang, H. , Qin, L. , Xiao, Z. , Wang, Y. , Fan, J. , Feng, D. , Liang, Y. , and Ren, J. (2020) A novel nomogram to predict all‐cause readmission or death risk in Chinese elderly patients with heart failure. ESC Heart Failure, 7: 1015–1024. 10.1002/ehf2.12703.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. Khera R, Dharmarajan K, Krumholz HM. Rising mortality in patients with heart failure in the United States: facts versus fiction. JACC Heart Fail 2018; 6: 610–612. [DOI] [PubMed] [Google Scholar]
- 3. Halliday BP, Pennell DJ. Prediction and prevention of heart failure in high‐risk elderly patients. Eur Heart J 2019; 40: 539–541. [DOI] [PubMed] [Google Scholar]
- 4. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis 2016; 58: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Awan SE, Bennamoun M, Sohel F, Sanfilippo FM, Dwivedi G. Machine learning‐based prediction of heart failure readmission or death: implications of choosing the right model and the right metrics. ESC Heart Fail 2019; 6: 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leong KTG, Wong LY, Aung KCY, Macdonald M, Cao Y, Lee S, Chow WL, Doddamani S, Richards AM. Risk stratification model for 30‐day heart failure readmission in a multiethnic South East Asian community. Am J Cardiol 2017; 119: 1428–1432. [DOI] [PubMed] [Google Scholar]
- 7. Fleming LM, Gavin M, Piatkowski G, Chang JD, Mukamal KJ. Derivation and validation of a 30‐day heart failure readmission model. Am J Cardiol 2014; 114: 1379–1382. [DOI] [PubMed] [Google Scholar]
- 8. Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH, Fonarow GC. National differences in trends for heart failure hospitalizations by sex and race/ethnicity. Circ Cardiovasc Qual Outcomes 2017; 10: e003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, Pu C, Jia J, Zhang T, Liu X, Zhang S, Xie P, Fan D, Ji X, Wong K‐SL, Wang L, Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, Pu C, Jia J, Zhang T, Liu X, Zhang S, Xie P, Fan D, Ji X, Wong K‐SL, Wang L, Wei C, Wang Y, Cheng Y, Liu Y, Li X, Dong Q, Zeng J, Peng B, Xu Y, Yang Y, Wang Y, Zhao G, Wang W, Xu Y, Yang Q, He Z, Wang S, You C, Gao Y, Zhou D, He L, Li Z, Yang J, Lei C, Zhao Y, Liu J, Zhang S, Tao W, Hao Z, Wang D, Zhang S. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019; 18: 394–405. [DOI] [PubMed] [Google Scholar]
- 10. Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA, Howard G, Cushman M. Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurol 2019; 16: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN, Alexander KP, Geriatric Cardiology Section Leadership Council ACoC . Domain management approach to heart failure in the geriatric patient: present and future. J Am Coll Cardiol 2018; 71: 1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma A, Zhao X, Hammill BG, Hernandez AF, Fonarow GC, Felker GM, Yancy CW, Heidenreich PA, Ezekowitz JA, DeVore AD. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the Get With The Guidelines‐Heart Failure Registry. Circ Heart Fail 2018; 11: e004646. [DOI] [PubMed] [Google Scholar]
- 13. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012; 59: 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fudim M, O'Connor CM, Dunning A, Ambrosy AP, Armstrong PW, Coles A, Ezekowitz JA, Greene SJ, Metra M, Starling RC, Voors AA, Hernandez AF, Michael Felker G, Mentz RJ. Aetiology, timing and clinical predictors of early vs. late readmission following index hospitalization for acute heart failure: insights from ASCEND‐HF. Eur J Heart Fail 2018; 20: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013; 309: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmad FS, French B, Bowles KH, Sevilla‐Cazes J, Jaskowiak‐Barr A, Gallagher TR, Kangovi S, Goldberg LR, Barg FK, Kimmel SE. Incorporating patient‐centered factors into heart failure readmission risk prediction: a mixed‐methods study. Am Heart J 2018; 200: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong D, Tang L, Li ZY, Fang MJ, Gao JB, Shan XH, Ying XJ, Sun YS, Fu J, Wang XX, Li LM, Li ZH, Zhang DF, Zhang Y, Li ZM, Shan F, Bu ZD, Tian J, Ji JF. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol 2019; 30: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008; 26: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 19. Barlera S, Tavazzi L, Franzosi MG, Marchioli R, Raimondi E, Masson S, Urso R, Lucci D, Nicolosi GL, Maggioni AP, Tognoni G. Predictors of mortality in 6975 patients with chronic heart failure in the gruppo italiano per lo studio della streptochinasi nell'Infarto miocardico‐heart failure trial: proposal for a nomogram. Circ Heart Fail 2013; 6: 31–39. [DOI] [PubMed] [Google Scholar]
- 20. Chinese Society of Cardiology , Editorial Board of Chinese Journal of Cardiology . 2014 China guideline for diagnosis and treatment of heart failure. Zhonghua Xin Xue Guan Bing Za Zhi 2014; 42: 98–122. [PubMed] [Google Scholar]
- 21. Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol 2003; 42: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 22. Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, Cairns BJ, Huxley R, Jackson CL, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Collins R, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O'Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Z, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam T‐H, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. The Lancet 2016; 388: 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aizawa H, Imai S, Fushimi K. Factors associated with 30‐day readmission of patients with heart failure from a Japanese administrative database. BMC Cardiovasc Disord 2015; 15: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das KK, Geng X, Brown JW, Morales‐Oyarvide V, Huynh T, Pergolini I, Pitman MB, Ferrone C, Al Efishat M, Haviland D, Thompson E, Wolfgang C, Lennon AM, Allen P, Lillemoe KD, Fields RC, Hawkins WG, Liu J, Castillo CF, Das KM, Mino‐Kenudson M. Cross validation of the monoclonal antibody das‐1 in identification of high‐risk mucinous pancreatic cystic lesions. Gastroenterology 2019; 157: 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P‐HC. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Eur J Heart Fail 2014; 16: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, Demets D, Massie BM. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the irbesartan in heart failure with preserved ejection fraction study (I‐PRESERVE). Circ Heart Fail 2011; 4: 27–35. [DOI] [PubMed] [Google Scholar]
- 27. Remme WJ, Torp‐Pedersen C, Cleland JG, Poole‐Wilson PA, Metra M, Komajda M, Swedberg K, Di Lenarda A, Spark P, Scherhag A, Moullet C, Lukas MA. Carvedilol protects better against vascular events than metoprolol in heart failure: results from COMET. J Am Coll Cardiol 2007; 49: 963–971. [DOI] [PubMed] [Google Scholar]
- 28. Blecker S, Herrin J, Li L, Yu H, Grady JN, Horwitz LI. Trends in hospital readmission of Medicare‐covered patients with heart failure. J Am Coll Cardiol 2019; 73: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Formiga F, Masip J, Chivite D, Corbella X. Applicability of the heart failure readmission risk score: a first European study. Int J Cardiol 2017; 236: 304–309. [DOI] [PubMed] [Google Scholar]
- 30. Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL, Investigators NE‐C . Prevalence, incidence, and mortality of stroke in China: results from a nationwide population‐based survey of 480687 adults. Circulation 2017; 135: 759–771. [DOI] [PubMed] [Google Scholar]
- 31. Daneshvar M, Desai N, Andriulli J, Ortman M, Eno E, Hunter K, Russo AM. Gender differences in presentation, treatment, and in‐hospital outcome of patients admitted with heart failure complicated by atrial fibrillation (from the get with the guidelines‐heart failure [GWTG‐HF] registry). Am J Cardiol 2018; 121: 450–454. [DOI] [PubMed] [Google Scholar]
- 32. Lane DA, Skjoth F, Lip GYH, Larsen TB, Kotecha D. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017; 6: e005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsujimoto T, Kajio H. Low diastolic blood pressure and adverse outcomes in heart failure with preserved ejection fraction. Int J Cardiol 2018; 263: 69–74. [DOI] [PubMed] [Google Scholar]
- 34. Sandesara PB, O'Neal WT, Kelli HM, Topel M, Samman‐Tahhan A, Sperling LS. Diastolic blood pressure and adverse outcomes in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial. J Am Heart Assoc 2018; 7: e007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsimploulis A, Lam PH, Arundel C, Singh SN, Morgan CJ, Faselis C, Deedwania P, Butler J, Aronow WS, Yancy CW, Fonarow GC, Ahmed A. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2018; 3: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res 2019; 124: 1045–1060. [DOI] [PubMed] [Google Scholar]
- 37. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation 2018; 138: 80–98. [DOI] [PubMed] [Google Scholar]
- 38. Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta‐analysis. J Am Coll Cardiol 2008; 52: 818–827. [DOI] [PubMed] [Google Scholar]
- 39. Tang YD, Katz SD. The prevalence of anemia in chronic heart failure and its impact on the clinical outcomes. Heart Fail Rev 2008; 13: 387–392. [DOI] [PubMed] [Google Scholar]
- 40. Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 2006; 113: 2713–2723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The cumulative death rate and readmission rate for elderly patients with HF. (A) The cumulative death rate for elderly patients with HF. (B) The cumulative readmission rate for elderly patients with HF.
Table S1. Clinical characteristics of elderly patients hospitalized with HF by age group
Table S2. Univariate and multivariate analysis of 1‐year HF readmission in elderly patients with HF


