Abstract
Aims
The aim of this study is to evaluate the contemporary use of a pulmonary artery catheter (PAC) in acute myocardial infarction‐cardiogenic shock (AMI‐CS).
Methods and results
A retrospective cohort of AMI‐CS admissions using the National Inpatient Sample (2000–2014) was identified. Admissions with concomitant cardiac surgery or non‐AMI aetiology for cardiogenic shock were excluded. The outcomes of interest were in‐hospital mortality, resource utilization, and temporal trends in cohorts with and without PAC use. In the non‐PAC cohort, the use and outcomes of right heart catheterization was evaluated. Multivariable regression and propensity matching was used to adjust for confounding. During 2000–2014, 364 001 admissions with AMI‐CS were included. PAC was used in 8.1% with a 75% decrease during over the study period (13.9% to 5.4%). Greater proportion of admissions to urban teaching hospitals received PACs (9.5%) compared with urban non‐teaching (7.1%) and rural hospitals (5.4%); P < 0.001. Younger age, male sex, white race, higher comorbidity, noncardiac organ failure, use of mechanical circulatory support, and noncardiac support were independent predictors of PAC use. The PAC cohort had higher in‐hospital mortality (adjusted odds ratio 1.07 [95% confidence interval 1.04–1.10]), longer length of stay (10.9 ± 10.9 vs. 8.2 ± 9.3 days), higher hospitalization costs ($128 247 ± 138 181 vs. $96 509 ± 116 060), and lesser discharges to home (36.3% vs. 46.4%) (all P < 0.001). In 6200 propensity‐matched pairs, in‐hospital mortality was comparable between the two cohorts (odds ratio 1.01 [95% confidence interval 0.94–1.08]). Right heart catheterization was used in 12.5% of non‐PAC admissions and was a marker of greater severity but did not indicate worse outcomes.
Conclusions
In AMI‐CS, there was a 75% decrease in PAC use between 2000 and 2014. Admissions receiving a PAC were a higher risk cohort with worse clinical outcomes.
Keywords: Cardiogenic shock, Acute myocardial infarction, Heart failure, Pulmonary artery catheterization, Right heart catheterization, Cardiac intensive care unit, Critical care cardiology
Introduction
Acute myocardial infarction with cardiogenic shock (AMI‐CS) remains associated with nearly 30–40% mortality in the contemporary era despite early revascularization.1, 2, 3, 4, 5, 6, 7, 8, 9 AMI‐CS is associated with pump failure and subsequent vasoplegic shock resulting in a complex haemodynamic picture.5, 10, 11, 12, 13 Given the rapid haemodynamic changes and high vasoactive medication requirements in this population, invasive haemodynamic monitoring with a pulmonary artery catheter (PAC) may have a role in AMI‐CS.10, 14 There are limited data on the use and outcomes of PAC in AMI‐CS in the contemporary era.15, 16, 17 In 2005, the seminal ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial demonstrated no differences in survival and hospitalization outcomes with PAC use in haemodynamically stable patients with heart failure, but this study specifically excluded patients with CS.18 Similar studies from the sepsis and acute respiratory distress syndrome literature showed no differences in outcomes with PAC use.19 With dissemination of these neutral results, the use of a PAC in undifferentiated shock and critically ill patients has decreased.20 However, there are limited epidemiological data on the use of PAC in patients with AMI‐CS, a population not included in these studies. Prior studies from large national databases have focused on only ST elevation or non‐ST elevation populations or alternately have included undifferentiated CS because of a combination of AMI and end‐stage heart failure.21, 22 Using a 15 year nationally representative database, we sought to assess the use of PAC in AMI‐CS in USA. We also sought to understand the use of right heart catheterization (RHC) that is independent of PAC use in this population.
Methods
The Nationwide/National Inpatient Sample (NIS) is the largest all‐payer database of hospitalized inpatients in USA. The NIS contains discharge data from a 20% stratified sample of nonfederal hospitals and is a part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality.23 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 24 secondary diagnoses, and procedural diagnoses. The HCUP‐NIS does not capture individual patients but captures all information for a given admission/hospitalization.
Study population, variables, and outcomes
Using the HCUP‐NIS data from 2000 to 2014, a retrospective cohort study of admissions with AMI‐CS was identified. AMI in the primary procedure field was identified using International Classification of Diseases 9 Clinical Modification (ICD‐9CM) codes for ST elevation MI (ICD‐9CM 410.1×–410.6×, 410.8×, 410.9×) and non‐ST elevation myocardial infarction (ICD‐9CM 410.×).24 CS was identified using ICD‐9CM code 785.51 that has a specificity of 99.3%, sensitivity of 59.8%, positive predictive value of 78.8%, and negative predictive value of 98.1%.25 Patients without in‐hospital mortality data and AMI‐CS admissions that underwent cardiac surgery (coronary artery bypass grafting, valve repair, valve replacement, durable left ventricular assist device, and orthotropic heart transplant) were excluded. Consistent with prior data, we identified PAC use using the ICD‐9CM codes 89.63 (pulmonary artery pressure monitoring), 89.64 (pulmonary artery wedge monitoring), 89.66 (measurement of mixed venous blood gases), 89.67 [monitoring of cardiac output by oxygen consumption technique (Fick method)], and 89.68 [monitoring of cardiac output by other technique (thermodilution indicator)].22, 26, 27 The use of RHC was identified using ICD‐9CM 37.21 and 37.23, which reflect as isolated measurement of pulmonary arterial pressures or concomitant use with a left heart catheterization.15 Though it is possible that the RHC was subsequently converted to PAC monitoring, there is no reliable way to assess in the absence of a concomitant code for PAC placement. An exploratory cohort of admissions receiving invasive haemodynamic monitoring (IHDM), that is, RHC and/or PAC was also evaluated. Therefore, the PAC cohort was restricted conservatively to those with an ICD‐9CM for a PAC only. Demographic characteristics, hospital characteristics, acute organ failure, coronary angiography, percutaneous coronary interventions (PCI), and mechanical circulatory support (MCS) use were identified for all admissions using previously used methodologies from our group.2, 3, 4, 5, 8, 9, 28, 29, 30, 31, 32, 33, 34, 35 Acute noncardiac organ failure was classified as respiratory (acute respiratory failure, other pulmonary insufficiency, acute respiratory distress syndrome, respiratory arrest, and ventilator management), renal (acute kidney injury), and hepatic (acute hepatic failure, hepatic encephalopathy, hepatic infection, and hepatitis unspecified), hematologic (defibrination syndrome, acquired coagulation factor deficiency, coagulation defect, and thrombocytopenia), and neurologic (anoxic brain injury, acute encephalopathy, coma, altered consciousness, and electroencephalogram).3, 4, 5, 9, 30, 32 Similar to prior literature from the HCUP‐NIS, we used the procedure day for RHC/PAC placement to time concomitant coronary angiography, PCI, MCS, and invasive mechanical ventilation.36 The Deyo's modification of the Charlson comorbidity index was used to identify the burden of co‐morbid diseases (Supporting Information, Table S1 ).37
The primary outcome was the temporal trends of PAC use during the 15 year study period. Temporal trend analysis was further stratified by patient characteristics, hospital demographics, and in the years before (2000–2006) and after (2007–2014) the ESCAPE trial. Secondary outcomes included timing of PAC placement, use with RHC/PAC with concomitant cardiac procedures, in‐hospital mortality, length of stay, hospitalization costs, and discharge disposition in admissions with and without PAC use. In the cohort without PAC use, we compared clinical outcomes between patients that received and did not receive a RHC. A supplementary analysis was performed for unadjusted and adjusted temporal trends in the use and in‐hospital mortality for all admissions receiving IHDM.
Statistical analysis
As recommended by HCUP‐NIS, survey procedures using discharge weights provided with HCUP‐NIS database were used to generate national estimates. Using the trend weights provided by the HCUP‐NIS, samples from 2000 to 2011 were re‐weighted to adjust for the 2012 HCUP‐NIS redesign.38 χ2 and t‐tests were used to compare categorical and continuous variables, respectively. Logistic regression was used to analyse trends over time (referent year 2000). The inherent restrictions of the HCUP‐NIS database related to research design, data interpretation, and data analysis were reviewed and addressed.38 Univariable analysis for trends and outcomes was performed and was represented as odds ratio (OR) with 95% confidence interval (CI). Multivariable logistic regression analysis incorporating age, sex, race, primary payer status, socio‐economic stratum, hospital characteristics, comorbidities, acute organ failure, AMI type, cardiac procedures, and noncardiac procedures was performed for temporal trends of PAC use, temporal trends of in‐hospital mortality, predictors of PAC use, and in‐hospital mortality. For the multivariable modelling, regression analysis with purposeful selection of statistically (liberal threshold of P < 0.20 in univariate analysis) and clinically relevant variables was conducted.
Multiple confirmatory and subgroup analyses were performed to verify the validity of these findings. We performed a propensity‐matched analysis for patient demographics, comorbidities, hospital characteristics, acute organ failure, and acute care interventions between the two cohorts. For the propensity matching, all variables except race had <1% missing variables. For the race category, missing variables were treated as a separate category. Using 1:1 nearest neighbour matching, 6200 matching pairs (12 400 individual admissions) were developed for further use. The propensity‐matched sample had standardized differences <10% for all baseline characteristics. The McNemar χ2 test and paired sample t‐tests were used to compare categorical and continuous variables, respectively, in the propensity‐matched sample. An additional analysis of outcomes was performed in the cohort that did not receive PAC between the admissions that received a RHC vs. those who did not. An exploratory supplementary analysis of temporal trends and in‐hospital mortality in the cohort receiving IHDM vs. those who did not was performed. Two‐tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 25.0 (IBM Corp, Armonk NY).
Results
In the period between 1 January 2000 and 31 December 2014, an estimated 9 747 034 admissions for a primary diagnosis of AMI were identified, of which 444 253 (4.6%) developed CS. Of these admissions with AMI‐CS, 80 252 (18.1%) had concomitant cardiac surgery during the hospital admission and were subsequently excluded. In the final cohort of 364 001 admissions, PAC was used in 29 609 (8.1%). Baseline characteristics of the cohorts with and without PAC use are summarized in Table 1. Admissions that received a PAC had higher rates of prior heart failure (66.5% vs. 53.9%; P < 0.001). During the tertiles of the study period, there was a serial decrease in mean age and increase in comorbidity, proportion of non‐ST segment‐elevation AMI‐CS, and admissions to urban teaching hospitals (Table S2 ). The 15 year unadjusted and adjusted temporal trends of PAC use (Figure 1 ) noted a significant decrease in PAC use in AMI‐CS. Temporal trends in the use of PAC stratified by patient and hospital characteristics demonstrated a decrease in temporal use of PAC in AMI‐CS (Figure 2 ). Greater proportion of admissions to urban teaching hospitals received PACs (9.5%) compared with urban non‐teaching (7.1%) and rural hospitals (5.4%) with a proportionally consistent temporal decline during the study period (Figure 2 ). Compared with those without PAC use, those with PAC use had a higher prevalence of acute organ failure, pulmonary haemorrhage, and noncardiac organ support (Table 2). The PAC cohort received more frequent coronary angiography and MCS support but had lower rates of PCI (Table 2). In a multivariable regression analysis, younger age, male sex, white race, higher comorbidity, noncardiac organ failure, and use of MCS and noncardiac organ support were independent predictors of PAC use (Table 3).
Table 1.
Baseline characteristics of acute myocardial infarction‐cardiogenic shock with and without pulmonary artery catheter use
| Characteristic | PAC (N = 29 609) | No PAC (N = 334 392) | P | |
|---|---|---|---|---|
| AMI type | ST elevation AMI | 70.5 | 70.4 | 0.84 |
| Non‐ST elevation AMI | 29.5 | 29.6 | ||
| Age (years) | 68.3 ± 12.5 | 70.1 ± 13.4 | <0.001 | |
| Female sex | 37.7 | 41.0 | <0.001 | |
| Race | White | 77.8 | 78.1 | <0.001 |
| Black | 6.8 | 7.2 | ||
| Others | 15.4 | 14.7 | ||
| Weekend admission | 27.3 | 27.6 | 0.32 | |
| Primary payer | Medicare | 61.1 | 63.7 | <0.001 |
| Medicaid | 6.6 | 6.0 | ||
| Private | 24.8 | 22.5 | ||
| Uninsured | 5.0 | 5.5 | ||
| Others | 2.5 | 2.3 | ||
| Quartile of median household income for zip code | 0–25th | 17.6 | 23.7 | <0.001 |
| 26th–50th | 24.8 | 26.8 | ||
| 51st–75th | 25.8 | 24.9 | ||
| 75th–100th | 31.8 | 24.6 | ||
| Hospital teaching status and location | Rural | 5.6 | 8.8 | <0.001 |
| Urban non‐teaching | 36.4 | 42.3 | ||
| Urban teaching | 58.0 | 49.0 | ||
| Hospital bed size | Small | 6.6 | 8.5 | <0.001 |
| Medium | 20.1 | 23.3 | ||
| Large | 73.3 | 68.3 | ||
| Hospital region | Northeast | 22.0 | 18.5 | <0.001 |
| Midwest | 21.8 | 23.1 | ||
| South | 31.0 | 38.6 | ||
| West | 25.2 | 19.7 | ||
| Charlson comorbidity index | 0–3 | 22.8 | 23.6 | <0.001 |
| 4–6 | 59.5 | 54.4 | ||
| ≥7 | 17.7 | 22.0 | ||
Represented as percentage or mean ± standard deviation.
Figure 1.
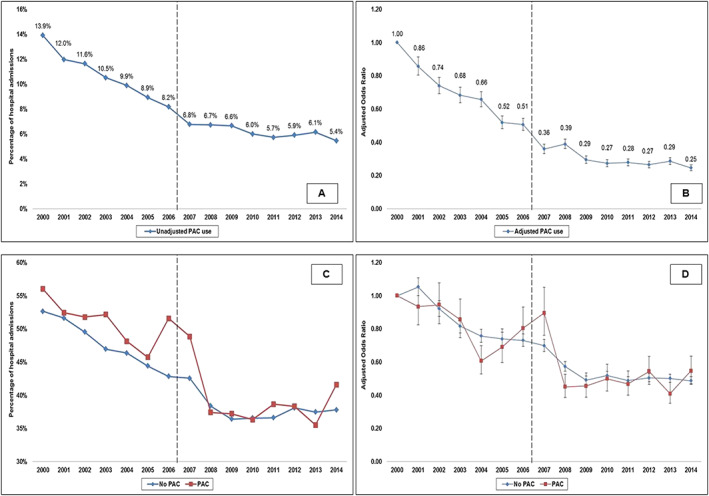
Unadjusted and adjusted 15 year temporal trends of PAC use and in‐hospital mortality in cohorts with and without PAC use in AMI‐CS. Panel (A): Unadjusted temporal trends of PAC use in AMI‐CS (P < 0.001). Panel (B): Adjusted multivariable logistic regression for temporal trends of PAC use with 2000 as referent year; adjusted for age, sex, race, primary payer, socio‐economic status, hospital location/teaching status, hospital bed size, hospital region, comorbidity, acute organ failure, cardiac arrest, coronary angiography, percutaneous coronary intervention, mechanical circulatory support, invasive mechanical ventilation, haemodialysis (P < 0.001); Panel (C): Unadjusted temporal trends of in‐hospital mortality in AMI‐CS stratified by PAC use (P < 0.001); Panel (D): Adjusted multivariable logistic regression for in‐hospital mortality temporal trends with 2000 as referent year; adjusted for age, sex, race, primary payer, socio‐economic status, hospital location/teaching status, hospital bed size, hospital region, comorbidity, acute organ failure, cardiac arrest, coronary angiography, percutaneous coronary intervention, mechanical circulatory support, invasive mechanical ventilation, haemodialysis (P < 0.001). The dotted line demarcates the period before and after the ESCAPE trial. AMI, acute myocardial infarction; CS, cardiogenic shock; PAC, pulmonary artery catheter.
Figure 2.
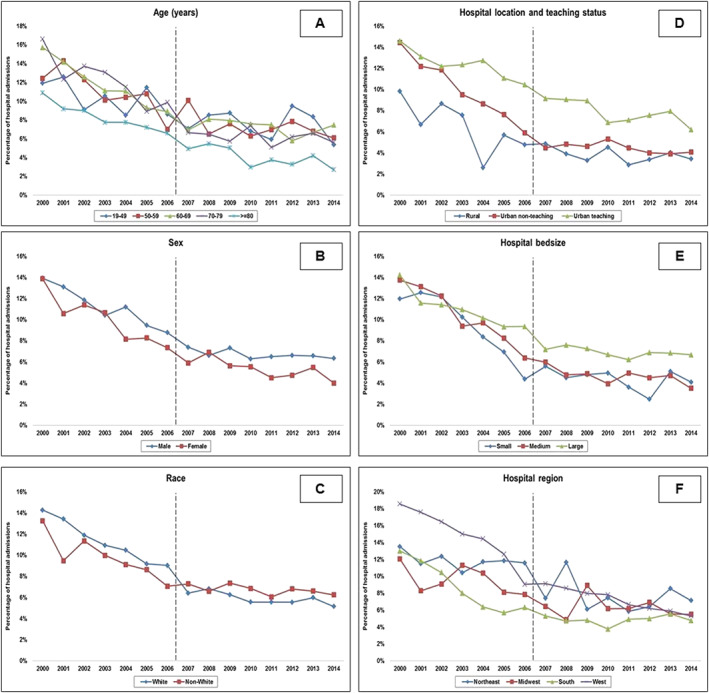
Temporal trends of PAC use in AMI‐CS stratified by patient and hospital characteristics. Fifteen year temporal trends of PAC use in AMI‐CS stratified by patient age groups (Panel A), sex (Panel B), race (Panel C), and hospital location and teaching status (Panel D), hospital bed size (Panel E), and hospital region (Panel F); (all P < 0.001). The dotted line demarcates the period before and after the ESCAPE trial. AMI, acute myocardial infarction; CS, cardiogenic shock; PAC, pulmonary artery catheter.
Table 2.
In‐hospital course and management of acute myocardial infarction‐cardiogenic shock with and without pulmonary artery catheter use
| Characteristic | PAC (N = 29 609) | No PAC (N = 334 392) | P | |
|---|---|---|---|---|
| Acute organ dysfunction | Respiratory | 57.6 | 44.1 | <0.001 |
| Renal | 43.1 | 33.6 | <0.001 | |
| Hepatic | 10.8 | 7.7 | <0.001 | |
| Pulmonary haemorrhage | 1.7 | 1.1 | <0.001 | |
| Cardiac arrest | 18.9 | 19.5 | 0.02 | |
| Coronary angiography | 70.2 | 64.7 | <0.001 | |
| Percutaneous coronary intervention | 48.4 | 53.4 | <0.001 | |
| MCS | IABP | 52.8 | 37.6 | <0.001 |
| Percutaneous MCS | 2.4 | 1.3 | <0.001 | |
| ECMO | 0.6 | 0.3 | <0.001 | |
| Invasive mechanical ventilation | 61.4 | 42.1 | <0.001 | |
| Haemodialysis | 5.8 | 2.9 | <0.001 | |
Represented as percentage.
ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; MCS, mechanical circulatory support.
Table 3.
Multivariable regression for predictors of pulmonary artery catheter use in acute myocardial infarction‐cardiogenic shock
| Total cohort (N = 364 001) | Odds ratio | 95% confidence interval | P | ||
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Age groups (years) | 19–49 | Reference category | |||
| 50–59 | 0.93 | 0.88 | 0.99 | 0.02 | |
| 60–69 | 0.88 | 0.83 | 0.94 | <0.001 | |
| 70–79 | 0.87 | 0.82 | 0.93 | <0.001 | |
| ≥80 | 0.64 | 0.60 | 0.69 | <0.001 | |
| Female sex | 0.97 | 0.94 | 0.99 | 0.009 | |
| Race | White | Reference category | |||
| Non‐white | 0.96 | 0.93 | 0.99 | 0.002 | |
| Primary payer | Medicare | Reference category | |||
| Medicaid | 0.94 | 0.89 | 0.99 | 0.03 | |
| Private | 0.98 | 0.94 | 1.02 | 0.26 | |
| Uninsured | 0.86 | 0.80 | 0.91 | <0.001 | |
| No charge | 1.10 | 0.91 | 1.34 | 0.32 | |
| Others | 1.04 | 0.96 | 1.12 | 0.39 | |
| Quartile of median household income for zip code | 0–25th | Reference category | |||
| 26th–50th | 1.25 | 1.20 | 1.29 | <0.001 | |
| 51st–75th | 1.35 | 1.29 | 1.40 | <0.001 | |
| 75th–100th | 1.60 | 1.54 | 1.67 | <0.001 | |
| Weekend admission | 1.00 | 0.98 | 1.02 | 0.85 | |
| Hospital teaching status and location | Rural | Reference category | |||
| Urban non‐teaching | 0.97 | 0.92 | 1.03 | 0.34 | |
| Urban teaching | 1.32 | 1.25 | 1.40 | <0.001 | |
| Hospital bed size | Small | Reference category | |||
| Medium | 1.09 | 1.03 | 1.15 | 0.001 | |
| Large | 1.36 | 1.29 | 1.43 | <0.001 | |
| Hospital region | Northeast | Reference category | |||
| Midwest | 0.88 | 0.85 | 0.92 | <0.001 | |
| South | 0.82 | 0.79 | 0.85 | <0.001 | |
| West | 1.25 | 1.20 | 1.30 | <0.001 | |
| Charlson comorbidity index | 0–3 | Reference category | |||
| 4–6 | 1.17 | 1.12 | 1.22 | <0.001 | |
| ≥7 | 0.95 | 0.90 | 1.00 | 0.04 | |
| Acute organ failure | Respiratory | 1.18 | 1.15 | 1.22 | <0.001 |
| Renal | 1.29 | 1.26 | 1.33 | <0.001 | |
| Hepatic | 1.02 | 0.97 | 1.06 | 0.50 | |
| Hematologic | 1.39 | 1.33 | 1.44 | <0.001 | |
| Neurologic | 0.71 | 0.69 | 0.74 | <0.001 | |
| Cardiac arrest | 0.80 | 0.77 | 0.83 | <0.001 | |
| Coronary angiography | 1.24 | 1.19 | 1.28 | <0.001 | |
| Percutaneous coronary intervention | 0.54 | 0.52 | 0.56 | <0.001 | |
| Mechanical circulatory support | 1.94 | 1.88 | 1.99 | <0.001 | |
| Invasive mechanical ventilation | 1.84 | 1.79 | 1.90 | <0.001 | |
| Haemodialysis | 1.28 | 1.20 | 1.35 | <0.001 | |
Compared with those without PAC use, the cohort with PAC use had higher unadjusted in‐hospital all‐cause mortality (42% vs. 46.3%; OR 1.19 [95% CI 1.16–1.22]; P < 0.001). The 15 year unadjusted and adjusted temporal trends of in‐hospital mortality are presented in Figure 1 C and 1 D. Admissions with PAC use had a longer length of stay (10.9 ± 10.9 vs. 8.2 ± 9.3 days), higher hospitalization costs ($128 247 ± 138 181 vs. $96 509 ± 116 060), and less frequent discharges to home (36.3% vs. 46.4%) compared with the cohort that did not receive a PAC (all P < 0.001). In a multivariable logistic regression analysis, PAC use (OR 1.07 [95% CI 1.04–1.10]; P < 0.001) was independently associated with higher in‐hospital mortality in AMI‐CS (Table S3). Using a 1:1 propensity‐matching analysis, 12 400 admissions (6200 pairs) were evaluated (Table S4 ). In the propensity‐matched sample, there were no differences in in‐hospital mortality in the cohorts with and without PAC use (46.4% vs. 46.2%; OR 1.01 [95% CI 0.94–1.08]; P = 0.84). The propensity‐matched cohort receiving PAC was discharged home less frequently (19.5% vs. 23.1%), had longer length of hospital stay (10.8 ± 10.9 vs. 10.4 ± 9.7 days), and had higher hospitalization costs ($137 660 ± 127 881 vs. 131 546 ± 113 261) (all P < 0.001).
In the cohort that did not receive a PAC (N = 334 392), a RHC was performed in 41 719 (12.5%) admissions. Compared with those without a RHC, those who received a RHC were younger, more often male sex, of non‐white race, with lower comorbidity, and higher acuity of illness (Table S5 ). Admissions receiving a RHC had higher use of PCI, MCS, and noncardiac organ support. The RHC cohort had lower unadjusted (35.5% vs. 43%; OR 0.73 [95% CI 0.72–0.75]; P < 0.001) in‐hospital mortality but not adjusted in‐hospital mortality (OR 1.01 [95% CI 0.99–1.04]; P = 0.38). The cohort with RHC use had longer hospital stay and higher hospitalization costs compared with those without a RHC (Table S6 ). Temporal trends in the use and in‐hospital mortality in the cohorts with and without IHDM (RHC and/or PAC) showed trends similar to those with PAC use (Figure S1 ). The cohort that received IHDM had lower in‐hospital mortality than those without IHDM on unadjusted analyses but not on adjusted analyses (40% vs. 43%; unadjusted OR 0.88 [95% CI 0.87–0.90]; P < 0.001; adjusted OR 1.01 [95% CI 0.98–1.05; P = 0.73) (adjusted for covariates listed in Table S3 ).
The timing of PAC/RHC placement was available for 53 247 (74.7%) of the total cohort. RHC/PAC was performed at a median of 0 (interquartile range 0–1) days after admission, with 63% performed the day of admission (Figure 3 A). RHC/PAC was performed concomitantly with coronary angiography, PCI, MCS, and invasive mechanical ventilation in 11.2%, 7.1%, 7.7%, and 5.3% admissions, respectively. In the total cohort, 5.3% of the admissions received coronary angiography (with/without PCI), MCS, and PAC on the same day (Figure 3 B).
Figure 3.
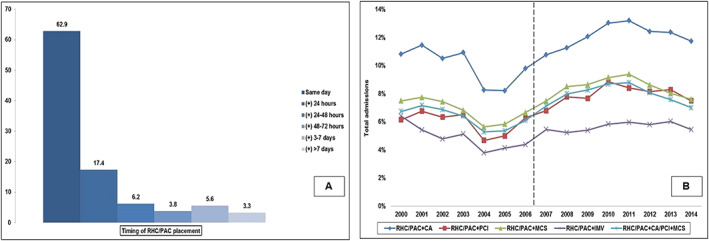
Timing of PAC placement and concomitant cardiac procedures in AMI‐CS. Panel (A): Timing of RHC/PAC during hospital stay (percentage); Panel (B): Fifteen year temporal trends of PAC with concomitant cardiac and noncardiac procedures. The dotted line demarcates the period before and after the ESCAPE trial. AMI, acute myocardial infarction; CA, coronary angiography; CS, cardiogenic shock; IMV, invasive mechanical ventilation; MCS, mechanical circulatory support; PAC, pulmonary artery catheter; PCI, percutaneous coronary intervention; RHC, right heart catheterization.
Discussion
In this nationally representative population of AMI‐CS, we noted a 75% decrease in PAC use between 2000 and 2014 despite a concomitant increase in patient acuity. Younger age, male sex, white race, higher baseline comorbidity, presence of noncardiac organ failure, and use of MCS were independent predictors of PAC use. Admissions receiving a PAC were a higher risk cohort that had higher unadjusted and adjusted in‐hospital mortality, though these differences were not noted in the propensity‐matched sample. The use of a RHC in admissions that did not receive a PAC was a marker of greater acuity and associated with higher resource utilization but no differences in in‐hospital mortality. About 63% of the RHC/PACs were performed on the admission day with nearly 13% performed on the same day as coronary angiography, PCI, MCS, or invasive mechanical ventilation.
This study provides novel insight into management and outcome of a population not previously evaluated. In patients with ST elevation AMI‐CS, Kolte et al.39 noted a declining trend in the use of PAC during 2003–2010. Consistent with these data, we note a similar trend in all AMI‐CS patients over a longer study period (2000–2014). Furthermore, this current study evaluates the use of RHC, either concomitantly with coronary angiography and MCS or in isolation, providing further insights into management of this complex population. The RHC provides similar information without the need for prolonged monitoring; however, the relative role both modalities needs further evaluation. Hernandez et al.22 noted decreased mortality in the CS admissions because of end‐stage heart failure receiving a PAC. In contrast to their data, our study did not observe a similar signal.7, 40 Patients with end‐stage heart failure typically have higher filling pressures, lower ejection fractions, but with lesser end‐organ hypoperfusion that is likely related to the chronicity of the process.40 These differences may justify the greater benefit of PAC in the end‐stage heart failure population to aid in close optimization of filling pressures and fluid status. The results of our study can be readily compared with the substudy from GUSTO IIb and III (Global Use of Strategies To Open occluded coronary arteries in acute coronary syndromes) trials.41 In this study, the cohort with PACs more frequently received coronary revascularization, mechanical circulatory support, and mechanical ventilation consistent with our data. These patients had higher resource utilization and short‐term mortality in the unmatched cohort and higher resource utilization in the propensity‐matched cohort, suggesting that PAC use is a marker for greater illness severity.41, 42 As noted in our study, in admissions within the non‐PAC cohort, RHC was used in those with greater severity, without any differences in outcomes. Smaller studies have shown the use of a PAC to be associated with improved survival in CS because of any aetiology.43, 44 Rossello et al.16 showed the use of PAC to be associated with lower mortality in CS from non‐AMI aetiology, but no differences in those with AMI‐CS. In a subgroup analysis of the CardShock study, Sionis et al.17 noted the use of a PAC to be associated with greater use of aggressive therapies (including inotropes) but without any differences in in‐hospital mortality.
This study noted a declining trend in the use of PAC in AMI‐CS, which is in contrast to similar studies from patients with heart failure.26, 45 These rates of decline started before the ESCAPE trial and continued to show a steady downward trend. These trends of declining PAC use were consistent across all patient and hospital characteristics as noted in a prior study in Medicare beneficiaries with AMI.46 The use of PAC in this study is lower than that noted in prospective registries of patients with AMI‐CS.17, 43 This may be postulated to be because of lesser severity of CS in this nationally representative population, multiple neutral trials in both cardiac and noncardiac patients, the ubiquitous use of echocardiography, and advances in non‐invasive/minimally invasive haemodynamic monitoring.20, 41 This study also identified unique predictors of PAC use. Presence of noncardiac organ failure and use of invasive mechanical ventilation and haemodialysis were strong predictors of PAC use in AMI‐CS. This can be hypothesized to be because of the development of complex haemodynamics in this population with superimposed distributive shock, which needs careful understanding of cardiac haemodynamics and titration of vasoactive medications.10, 12, 13 This study noted significant disparities in the use of PAC in this nationally representative population. We noted greater use of PAC in admissions with higher socio‐economic status and bearing insurance coverage, which may allude to social disparities noted in other AMI populations.47, 48 Furthermore, the use of PAC in large urban hospitals and in patients with MCS demonstrates the paradigm shift in the management of AMI‐CS with the advent of newer percutaneous MCS devices.15, 43, 49, 50 Second, with advanced MCS being available in the contemporary era,6, 7, 28, 29, 51, 52, 53 PAC is being increasingly used to treat patients with higher severity of illness.15, 45 Preliminary data have shown improvements in clinical outcomes with a protocoled approached to AMI‐CS including the use of PAC.54, 55 This is substantiated by the use of greater cardiac and noncardiac organ support in our study and the higher use of MCS and PACs in large, urban teaching hospitals.28, 29 There exist significant disparities in the hospital‐level management and outcomes of AMI‐CS.2 It is possible that these disparities may contribute to the higher use of PAC at large, urban teaching hospitals, or alternately this is representative of the higher acuity of illness in these centres. This crucial selection bias has been previously validated in the ESCAPE registry that noted greater PAC use in heart failure patients with higher severity of illness.56
In this study, we noted nearly 63% of RHC/PAC performed on admission with 13% performed concomitantly with other cardiac and noncardiac procedures. Nalluri et al.15 noted the use of a RHC in AMI‐CS admissions receiving percutaneous MCS to be associated with lower in‐hospital mortality. It is important to note that this study specifically studied RHC with/without concomitant PAC use, because it sought to capture the use of RHC simultaneously with MCS placement. In contrast to their work, this study did not demonstrate any survival benefit from RHC use in the non‐PAC population. The use of RHC/PAC‐derived variables such as cardiac power output (mean arterial pressure X cardiac output/451) has shown significant survival correlations and remains worthy of further dedicated study.43, 57
Limitations
This study has several limitations, despite the HCUP‐NIS database's attempts to mitigate potential errors by using internal and external quality control measures. The ICD‐9CM codes for AMI and CS have been previously validated, reducing the inherent errors in ascertainment in this study.24, 25, 27 However, as noted in the Methods section, the validation study for CS shows moderate sensitivity, and therefore, this study may have missed some admissions with CS. Although we used a strategy consistent with prior work from administrative databases,22, 26, 27 the ICD‐9CM codes for PAC have not been formally validated previously. The clinical consequences of using a haemodynamic tool such as the PAC could not be accurately measured, including data interpretation and treatment changes. Because of the administrative nature of this cohort, we do not have haemodynamic data, biventricular function, pulmonary hypertension, and quantification of vasoactive medications; all of which are known to influence outcomes in patients with shock.12, 58, 59, 60, 61, 62, 63 The declining use of the PAC may have resulted in consequent hesitation in using and/or challenges with interpretation of information.20 The ICD‐9CM codes for a RHC are not validated, and it is possible that those receiving a RHC had a PAC left in situ for continuous monitoring. Admissions receiving durable cardiac replacement therapy such as left ventricular assist devices and cardiac transplantation were excluded from this study, and therefore, our data cannot be extrapolated to this population. The HCUP‐NIS does not provide information on level of care (intensive care unit, step‐down unit, or floor level), so we cannot evaluate the influence of specialized care on the outcomes in this population. Despite these limitations, this study addresses an important knowledge gap highlighting the epidemiology of PAC use in AMI‐CS in a contemporary 15 year period.
Conclusions
In this large study of 364 001 AMI‐CS admissions, these observational data suggest a 75% decrease in PAC use between 2000 and 2014. Use of a PAC identified a higher risk cohort with greater organ failure and higher in‐hospital mortality and resource utilization. Further research is needed on the role of PAC use in the acute management of AMI‐CS in light to aid in appropriate haemodynamic optimization in this acutely ill cohort.7, 49
Conflict of interest
A.S.J. has been a consultant for Beckman, Abbott, Siemens, ET Healthcare, Sphing6toec, Quidel, Brava, and Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding
S.V. is supported by the Clinical and Translational Science Award (CTSA) Grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author contributions
The study design, literature review, data analysis, and statistical analysis were carried out by S.V., A.S., S.H.P., S.A., and S.V. The data management, data analysis, and drafting of the manuscript were performed by S.V., A.S., S.H.P., S.A., and S.V. S.V., A.S., S.H.P., A.P., M.R.B., J.C.J., S.A., S.V., B.J.G., A.S.J., D.R.H., S.M.D., and G.W.B. have access to the data. The manuscript revision, intellectual revisions, and mentorship were performed by A.P., M.R.B., J.C.J., B.J.G., A.S.J., D.R.H., S.M.D., and G.W.B. The final approval were carried out by S.V., A.S., S.H.P., A.P., M.R.B., J.C.J., S.A., S.V., B.J.G., A.S.J., D.R.H., S.M.D., and G.W.B.
Supporting information
Table S1. Identification of diagnoses and procedures.
Table S2. Characteristics of AMI‐CS with PAC use stratified by time period.
Table S3. Multivariable regression for in‐hospital mortality in AMI‐CS.
Table S4. Propensity‐matched cohorts of AMI‐CS with and without PAC.
Table S5. Baseline characteristics of cohorts with and without RHC use.
Table S6. Clinical outcomes of cohorts with and without RHC use.
Figure S1. Unadjusted and adjusted 15‐year temporal trends of IHDM use and in‐hospital mortality in cohorts with and without IHDM use in AMI‐CS.
Vallabhajosyula, S. , Shankar, A. , Patlolla, S. H. , Prasad, A. , Bell, M. R. , Jentzer, J. C. , Arora, S. , Vallabhajosyula, S. , Gersh, B. J. , Jaffe, A. S. , Holmes, D. R. Jr , Dunlay, S. M. , and Barsness, G. W. (2020) Pulmonary artery catheter use in acute myocardial infarction‐cardiogenic shock. ESC Heart Failure, 7: 1234–1245. 10.1002/ehf2.12652.
References
- 1. Vallabhajosyula S, Barsness GW, Vallabhajosyula S. Multidisciplinary Teams for Cardiogenic Shock. Aging (Albany NY) 2019; 11: 4774–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR Jr, Prasad A. Hospital‐level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol 2019; 124: 491–498. [DOI] [PubMed] [Google Scholar]
- 3. Vallabhajosyula S, Dunlay SM, Kashani K, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Barsness GW. Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int J Cardiol 2019; 285: 6–10. [DOI] [PubMed] [Google Scholar]
- 4. Vallabhajosyula S, Dunlay SM, Murphree DH, Barsness GW, Sandhu GS, Lerman A, Prasad A. Cardiogenic shock in takotsubo cardiomyopathy versus acute myocardial infarction: An 8‐year national perspective on clinical characteristics, management, and outcomes. JACC Heart Fail 2019; 7: 469–476. [DOI] [PubMed] [Google Scholar]
- 5. Vallabhajosyula S, Dunlay SM, Prasad A, Kashani K, Sakhuja A, Gersh BJ, Jaffe AS, Holmes DR Jr, Barsness GW. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol 2019; 73: 1781–1791. [DOI] [PubMed] [Google Scholar]
- 6. Vallabhajosyula S, O'Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Dunlay SM, Holmes DR Jr, Barsness GW. Venoarterial extracorporeal membrane oxygenation with concomitant Impella versus venoarterial extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J 2019. 10.1097/MAT.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 7. Vallabhajosyula S, O'Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Eleid MF, Dunlay SM, Gersh BJ, Rihal CS, Barsness GW. Concomitant intra‐aortic balloon pump use in cardiogenic shock requiring veno‐arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv 2018; 11: e006930. [DOI] [PubMed] [Google Scholar]
- 8. Vallabhajosyula S, Prasad A, Dunlay SM, Murphree DH Jr, Ingram C, Mueller PS, Gersh BJ, Holmes DR Jr, Barsness GW. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: a 15‐year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc 2019; 8: e011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vallabhajosyula S, Ya'Qoub L, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Gersh BJ, Kashani K. Sex disparities in acute kidney injury complicating acute myocardial infarction with cardiogenic shock. ESC Heart Fail 2019; 6: 874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017; 136: e232–e268. [DOI] [PubMed] [Google Scholar]
- 11. Esposito ML, Kapur NK. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000Res 2017; 6: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jentzer JC, Vallabhajosyula S, Khanna AK, Chawla LS, Busse LW, Kashani KB. Management of refractory vasodilatory shock. Chest 2018; 154: 416–426. [DOI] [PubMed] [Google Scholar]
- 13. den Uil CA, Lagrand WK, van der Ent M, Jewbali LS, Cheng JM, Spronk PE, Simoons ML. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2010; 31: 3032–3039. [DOI] [PubMed] [Google Scholar]
- 14. Gidwani UK, Mohanty B, Chatterjee K. The pulmonary artery catheter: a critical reappraisal. Cardiol Clin 2013; 31: 545–565 viii. [DOI] [PubMed] [Google Scholar]
- 15. Nalluri N, Patel NJ, Atti V, Kumar V, Basir MB, O'Neill WW. Temporal trends in utilization of right‐sided heart catheterization among percutaneous ventricular assist device recipients in acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol 2018; 122: 2014–2017. [DOI] [PubMed] [Google Scholar]
- 16. Rossello X, Vila M, Rivas‐Lasarte M, Ferrero‐Gregori A, Sans‐Rosello J, Duran‐Cambra A, Sionis A. Impact of pulmonary artery catheter use on short‐ and long‐term mortality in patients with cardiogenic shock. Cardiology 2017; 136: 61–69. [DOI] [PubMed] [Google Scholar]
- 17. Sionis A, Rivas‐Lasarte M, Mebazaa A, Tarvasmaki T, Sans‐Rosello J, Tolppanen H, Varpula M, Jurkko R, Banaszewski M, Silva‐Cardoso J, Carubelli V, Lindholm MG, Parissis J, Spinar J, Lassus J, Harjola VP, Masip J. Current use and impact on 30‐day mortality of pulmonary artery catheter in cardiogenic shock patients: results from the CardShock study. J Intensive Care Med 2019. 885066619828959. [DOI] [PubMed] [Google Scholar]
- 18. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 19. Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, Boyer A, Brochard L, Teboul JL. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2003; 290: 2713–2720. [DOI] [PubMed] [Google Scholar]
- 20. Marik PE. Obituary: pulmonary artery catheter 1970 to 2013. Ann Intensive Care 2013; 3: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kolte D, Khera S, Dabhadkar KC, Agarwal S, Aronow WS, Timmermans R, Jain D, Cooper HA, Frishman WH, Menon V, Bhatt DL, Abbott JD, Fonarow GC, Panza JA. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non‐ST‐elevation myocardial infarction. Am J Cardiol 2016; 117: 1–9. [DOI] [PubMed] [Google Scholar]
- 22. Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW, Lindenfeld J. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Card Fail. 2019; 25: 364–371. [DOI] [PubMed] [Google Scholar]
- 23. Introduction to the HCUP Nationwide Inpatient Sample 2009. http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_2009_INTRODUCTION.pdf. (18 January 2015).
- 24. Coloma PM, Valkhoff VE, Mazzaglia G, Nielsson MS, Pedersen L, Molokhia M, Mosseveld M, Morabito P, Schuemie MJ, van der Lei J, Sturkenboom M, Trifiro G. Identification of acute myocardial infarction from electronic healthcare records using different disease coding systems: a validation study in three European countries. BMJ Open 2013; 3: e002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lambert L, Blais C, Hamel D, Brown K, Rinfret S, Cartier R, Giguere M, Carroll C, Beauchamp C, Bogaty P. Evaluation of care and surveillance of cardiovascular disease: can we trust medico‐administrative hospital data? Can J Cardiol 2012; 28: 162–168. [DOI] [PubMed] [Google Scholar]
- 26. Khera R, Pandey A, Kumar N, Singh R, Bano S, Golwala H, Kumbhani DJ, Girotra S, Fonarow GC. Variation in hospital use and outcomes associated with pulmonary artery catheterization in heart failure in the United States. Circ Heart Fail 2016; 9: e003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993‐2004. Jama 2007; 298: 423–429. [DOI] [PubMed] [Google Scholar]
- 28. Vallabhajosyula S, Arora S, Lahewala S, Kumar V, Shantha GPS, Jentzer JC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A, Deshmukh AJ. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc 2018; 7: e010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vallabhajosyula S, Arora S, Sakhuja A, Lahewala S, Kumar V, Shantha GPS, Egbe AC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A, Deshmukh AJ. Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol 2019; 123: 489–497. [DOI] [PubMed] [Google Scholar]
- 30. Vallabhajosyula S, Dunlay SM, Barsness GW, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS, Kashani K. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction‐related cardiogenic shock. PLoS One 2019; 14: e0222894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vallabhajosyula S, Prasad A, Gulati R, Barsness GW. Contemporary prevalence, trends, and outcomes of coronary chronic total occlusions in acute myocardial infarction with cardiogenic shock. Int J Cardiol Heart Vasc 2019; 24: 100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vallabhajosyula S, Kashani K, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS, Barsness GW. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000–2014. Ann Intensive Care 2019; 9: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vallabhajosyula S, El Hajj SC, Bell MR, Prasad A, Lerman A, Rihal CS, Holmes DR Jr, Barsness GW. Intravascular ultrasound, optical coherence tomography, and fractional flow reserve use in acute myocardial infarction. Catheter Cardiovasc Interv 2019. 10.1007/s00392-019-01549-0 [DOI] [PubMed] [Google Scholar]
- 34. Vallabhajosyula S, Prasad A, Sandhu GS, Bell MR, Gulati R, Eleid MF, Best PJM, Gersh BJ, Singh M, Lerman A, Holmes DR Jr, Rihal CS, Barsness GW. Mechanical circulatory support‐assisted early percutaneous coronary intervention in acute myocardial infarction with cardiogenic shock: 10‐year national temporal trends, predictors and outcomes. EuroIntervention 2019. 10.4244/EIJ-D-19-00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vallabhajosyula S, Vallabhajosyula S, Bell MR, Prasad A, Singh M, White RD, Jaffe AS, Holmes DR Jr, Jentzer JC. Early vs. delayed in‐hospital cardiac arrest complicating ST‐elevation myocardial infarction receiving primary percutaneous coronary intervention. Resuscitation 2019. 10.1016/j.resuscitation.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 36. Khera R, Cram P, Vaughan‐Sarrazin M, Horwitz PA, Girotra S. Use of mechanical circulatory support in percutaneous coronary intervention in the United States. Am J Cardiol 2016; 117: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 38. Khera R, Krumholz HM. With great power comes great responsibility: big data research from the National Inpatient Sample. Circ Cardiovasc Qual Outcomes 2017; 10: e003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction in the United States. J Am Heart Assoc 2014; 3: e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim HS, Howell N. Cardiogenic shock due to end‐stage heart failure and acute myocardial infarction: characteristics and outcome of temporary mechanical circulatory support. Shock 2018; 50: 167–172. [DOI] [PubMed] [Google Scholar]
- 41. Cohen MG, Kelly RV, Kong DF, Menon V, Shah M, Ferreira J, Pieper KS, Criger D, Poggio R, Ohman EM, Gore J, Califf RM, Granger CB. Pulmonary artery catheterization in acute coronary syndromes: insights from the GUSTO IIb and GUSTO III trials. Am J Med 2005; 118: 482–488. [DOI] [PubMed] [Google Scholar]
- 42. Rayes HA, Vallabhajosyula S, Barsness GW, Anavekar NS, Go RS, Patnaik MS, Kashani KB, Jentzer JC. Association between anemia and hematological indices with mortality among cardiac intensive care unit patients. Clin Res Cardiol 2019: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Basir MB, Schreiber T, Dixon S, Alaswad K, Patel K, Almany S, Khandelwal A, Hanson I, George A, Ashbrook M, Blank N, Abdelsalam M, Sareen N, Timmis SBH, O'Neill Md WW. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv 2018; 91: 454–461. [DOI] [PubMed] [Google Scholar]
- 44. Doshi R, Patel K, Patel P, Meraj PM. Trends in the utilization and in‐hospital mortality associated with pulmonary artery catheter use for cardiogenic shock hospitalizations. Indian Heart J 2018; 70: S496–s498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pandey A, Khera R, Kumar N, Golwala H, Girotra S, Fonarow GC. Use of Pulmonary artery catheterization in US patients with heart failure, 2001‐2012. JAMA Intern Med 2016; 176: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ikuta K, Wang Y, Robinson A, Ahmad T, Krumholz HM, Desai NR. National trends in use and outcomes of pulmonary artery catheters among Medicare beneficiaries, 1999‐2013. JAMA Cardiol 2017; 2: 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patel N, Gupta A, Doshi R, Kalra R, Bajaj NS, Arora G, Arora P. In‐hospital management and outcomes after ST‐segment‐elevation myocardial infarction in Medicaid beneficiaries compared with privately insured individuals. Circ Cardiovasc Qual Outcomes 2019; 12: e004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Biswas S, Andrianopoulos N, Duffy SJ, Lefkovits J, Brennan A, Walton A, Chan W, Noaman S, Shaw JA, Ajani A, Clark DJ, Freeman M, Hiew C, Oqueli E, Reid CM, Stub D. Impact of socioeconomic status on clinical outcomes in patients with ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Qual Outcomes 2019; 12: e004979. [DOI] [PubMed] [Google Scholar]
- 49. Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB 3rd, O'Neill W. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol 2018; 72: 1972–1980. [DOI] [PubMed] [Google Scholar]
- 50. Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of temporary percutaneous mechanical circulatory support for cardiogenic shock: an appraisal of contemporary literature. Cardiol Ther 2019; 8: 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vallabhajosyula S, Patlolla SH, Sandhyavenu H, Vallabhajosyula S, Barsness GW, Dunlay SM, Greason KL, Holmes DR Jr, Eleid MF. Periprocedural cardiopulmonary bypass or venoarterial extracorporeal membrane oxygenation during transcatheter aortic valve replacement: a systematic review. J Am Heart Assoc 2018; 7: e009608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vallabhajosyula S, Prasad A, Bell MR, Sandhu GS, Eleid MF, Dunlay SM, Schears GJ, Stulak JM, Singh M, Gersh BJ, Jaffe AS, Holmes DR Jr, Rihal CS, Barsness GW. Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ Heart Fail 2019; 12: e005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vallabhajosyula S, Vallabhajosyula S, Vaidya VR, Patlolla SH, Desai V, Mulpuru SK, Noseworthy PA, Kapa S, Egbe AC, Gersh BJ, Deshmukh AJ. Venoarterial extracorporeal membrane oxygenation support for ventricular tachycardia ablation: a systematic review. ASAIO J 2020. 10.1097/MAT.0000000000001125 [DOI] [PubMed] [Google Scholar]
- 54. O'Neill WW, Grines C, Schreiber T, Moses J, Maini B, Dixon SR, Ohman EM. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J 2018; 202: 33–38. [DOI] [PubMed] [Google Scholar]
- 55. Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, Rosner C, Raja A, Barnett SD, Saulino P. deFilippi CR, Gurbel PA, Murphy CE, O'Connor CM. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol 2019; 73: 1659–1669. [DOI] [PubMed] [Google Scholar]
- 56. Allen LA, Rogers JG, Warnica JW, Disalvo TG, Tasissa G, Binanay C, O'Connor CM, Califf RM, Leier CV, Shah MR, Stevenson LW. High mortality without ESCAPE: the registry of heart failure patients receiving pulmonary artery catheters without randomization. J Card Fail 2008; 14: 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004; 44: 340–348. [DOI] [PubMed] [Google Scholar]
- 58. Kotecha AA, Vallabhajosyula S, Apala DR, Frazee E, Iyer VN. Clinical outcomes of weight‐based norepinephrine dosing in underweight and morbidly obese patients: a propensity‐matched analysis. J Intensive Care Med 2018; 885066618768180. [DOI] [PubMed] [Google Scholar]
- 59. Poterucha JT, Vallabhajosyula S, Egbe AC, Krien JS, Aganga DO, Holst K, Golden AW, Dearani JA, Crow SS. Vasopressor magnitude predicts poor outcome in adults with congenital heart disease after cardiac surgery. Congenit Heart Dis 2019; 14: 193–200. [DOI] [PubMed] [Google Scholar]
- 60. Vallabhajosyula S, Geske JB, Kumar M, Kashyap R, Kashani K, Jentzer JC. Doppler‐defined pulmonary hypertension in sepsis and septic shock. J Crit Care 2019; 50: 201–206. [DOI] [PubMed] [Google Scholar]
- 61. Vallabhajosyula S, Jentzer JC, Kotecha AA, Murphree DH Jr, Barreto EF, Khanna AK, Iyer VN. Development and performance of a novel vasopressor‐driven mortality prediction model in septic shock. Ann Intensive Care 2018; 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vallabhajosyula S, Kumar M, Pandompatam G, Sakhuja A, Kashyap R, Kashani K, Gajic O, Geske JB, Jentzer JC. Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: an 8‐year historical cohort study. Ann Intensive Care 2017; 7: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vallabhajosyula S, Rayes HA, Sakhuja A, Murad MH, Geske JB, Jentzer JC. Global longitudinal strain using speckle‐tracking echocardiography as a mortality predictor in sepsis: a systematic review. J Intensive Care Med 2019; 34: 87–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Identification of diagnoses and procedures.
Table S2. Characteristics of AMI‐CS with PAC use stratified by time period.
Table S3. Multivariable regression for in‐hospital mortality in AMI‐CS.
Table S4. Propensity‐matched cohorts of AMI‐CS with and without PAC.
Table S5. Baseline characteristics of cohorts with and without RHC use.
Table S6. Clinical outcomes of cohorts with and without RHC use.
Figure S1. Unadjusted and adjusted 15‐year temporal trends of IHDM use and in‐hospital mortality in cohorts with and without IHDM use in AMI‐CS.


